Mechanical Performance of Nonabsorbable Monofilament Suture Materials Tied with Different Suturing Techniques Under Various Knot Configurations: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample
2.2. Study Variables
2.3. Data Collection Methods
2.4. Statistical Analysis
3. Results
3.1. Effect of Suturing Technique
3.2. Effect of Suture Material
3.3. Effect of Immersion Time
3.4. Effect of Knot Configuration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PTFE | polytetrafluoroethylene |
| N | newton |
| µm | micrometer |
| mm | millimeter |
References
- Meyle, J. Suture Materials and Suture Techniques. Perio 2006, 3, 253–268. [Google Scholar]
- Griffin, T.J.; Hur, Y.; Bu, J. Basic suture techniques for oral mucosa. Clin. Adv. Periodontics 2011, 1, 221–232. [Google Scholar] [CrossRef]
- Laurell, L.; Gottlow, J.; Zybutz, M.; Persson, R. Treatment of intrabony defects by different surgical procedures. A literature review. J. Clin. Periodontol. 1998, 69, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Sentineri, R.; Lombardi, T.; Berton, F.; Stacchi, C. Laurell–Gottlow suture modified by Sentineri for tight closure of a wound with a single line of sutures. Br. J. Oral. Maxillofac. Surg. 2016, 54, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Trikka, D.; Vassilopoulos, S. Periodontal Regeneration with Enamel Matrix Derivative in the Management of Generalized Aggressive Periodontitis: A Case Report with 11-Year Follow-up and Literature Review. J. Int. Soc. Prev. Community Dent. 2019, 9, 13–20. [Google Scholar] [CrossRef]
- Lee, S.J. The sausage technique using collagen membrane without autogenous bone graft: A case report. J. Implantol. Appl. Sci. 2021, 25, 74–83. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Lim, E.L. Alveolar Ridge Preservation after Tooth Extraction and Replacement with Fibre-reinforced Composite Bridge in a Young Patient: A Case Report. Arch. Orofac. Sci. 2022, 17, 119–127. [Google Scholar] [CrossRef]
- Wong, Y.R.; McGrouther, D.A. Biomechanics of surgical knot security: A systematic review. Int. J. Surg. 2023, 109, 481–490. [Google Scholar] [CrossRef]
- Dennis, C.; Sethu, S.; Nayak, S.; Mohan, L.; Morsi, Y.Y.; Manivasagam, G. Suture materials—Current and emerging trends. J Biomed. Mater. Res. A 2016, 104, 1544–1559. [Google Scholar] [CrossRef]
- Makrygiannis, I.H.; Nikolaidis, A.K.; Tilaveridis, I.; Kouvelas, A.D.; Lykakis, I.Ν.; Venetis, G. Coated sutures for use in oral surgery: A comprehensive review. Clin. Oral Investig. 2025, 29, 109. [Google Scholar] [CrossRef]
- Taylor, L.; Saeinasab, M.; Shahbazi, M.A.; Zhang, X.; Zhang, W.; Nair, K.; Sefat, F. Smart sutures. In Advanced Technologies and Polymer Materials for Surgical Sutures; Woodhead Publishing: Cambridge, UK, 2023; pp. 129–148. [Google Scholar]
- Hansen, K.J.; Favreau, J.T.; Guyette, J.P.; Tao, Z.W.; Coffin, S.T.; Cunha-Gavidia, A.; D’AMore, B.; Perreault, L.R.; Fitzpatrick, J.P.; DeMartino, A.; et al. Functional effects of delivering human mesenchymal stem cell-seeded biological sutures to an infarcted heart. BioResearch Open Access 2016, 5, 249–260. [Google Scholar] [CrossRef]
- Faris, A.; Khalid, L.; Hashim, M.; Yaghi, S.; Magde, T.; Bouresly, W.; Hamdoon, Z.; Uthman, A.T.; Marei, H.; Al-Rawi, N. Characteristics of Suture Materials Used in Oral Surgery: Systematic Review. Int. Dent. J. 2022, 72, 278–287. [Google Scholar] [CrossRef]
- Pcheliakov, A.A.; Diachkova, E.Y.; Vasil’ev, Y.L.; Svitich, O.A.; Poddubikov, A.V.; Evlashin, S.A.; Volel, B.A.; Bakhmet, A.A.; Klochkova, S.V.; Velichko, E.V.; et al. Comparative Analysis of Mechanical Properties and Microbiological Resistance of Polyfilament and Monofila-ment Suture Materials Used in the Operation “Tooth Extraction”. Biomimetics 2023, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Muffly, T.M.; Boyce, J.; Kieweg, S.L.; Bonham, A.J. Tensile strength of a surgeon’s or a square knot. J. Surg. Educ. 2010, 67, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Ching, S.S.; Mok, C.W.; Koh, Y.X.; Tan, S.M.; Tan, Y.K. Assessment of surgical trainees’ quality of knot-tying. J. Surg. Educ. 2013, 70, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Avoine, X.; Lussier, B.; Brailovski, V.; Inaekyan, K.; Beauchamp, G. Evaluation of the effect of 4 types of knots on the mechanical properties of 4 types of suture material used in small animal practice. Can. J. Vet. Res. 2016, 80, 162–170. [Google Scholar]
- Abellán, D.; Nart, J.; Pascual, A.; Cohen, R.E.; Sanz-Moliner, J.D. Physical and mechanical evaluation of five suture materials on three knot configurations: An in vitro study. Polymers 2016, 8, 147. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- González-Barnadas, A.; Camps-Font, O.; Espanya-Grifoll, D.; España-Tost, A.; Figueiredo, R.; Valmaseda-Castellón, E. In Vitro Tensile Strength Study on Suturing Technique and Material. J. Oral Implantol. 2017, 43, 169–174. [Google Scholar] [CrossRef]
- Muffly, T.M.; Kow, N.; Iqbal, I.; Barber, M.D. Minimum number of throws needed for knot security. J. Surg. Educ. 2011, 68, 130–133. [Google Scholar] [CrossRef]
- Muffly, T.M.; Cook, C.; Distasio, J.; Bonham, A.J.; Blandon, R.E. Suture end length as a function of knot integrity. J Surg Educ. 2009, 66, 276–280. [Google Scholar] [CrossRef]
- Bushong, E.E.; Janis, J.E. Knot Security 101: A Comprehensive Practical Review to Optimal Knot Configuration, Pulling Direction, Throw Count, and Tail Length. PRS Global Open 2024, 12, e6047. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.C.; Roberts, A.D.; Hire, J.M.; Mueller, T.L. The Effect of Instrumentation on Suture Tensile Strength and Knot Pullout Strength of Common Suture Materials. J. Surg. Educ. 2016, 3, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Hazar, A.; Üçtaşlı, M.B. Effect of Different MMP Inhibitors on the Bond Strength and Durability of an Etch-and-rinse and a Self-etch Adhesive. ADO J. Clin. Sci. 2024, 13, 453–460. [Google Scholar] [CrossRef]
- Pashley, D.H.; Tay, F.R.; Yiu, C.; Hashimoto, M.; Breschi, L.; Carvalho, R.M.; Ito, S. Collagen degradation by host-derived enzymes during aging. J. Dent. Res. 2004, 83, 216–221. [Google Scholar] [CrossRef]
- Setzen, G.; Williams, E.F., 3rd. Tissue response to suture materials implanted subcutaneously in a rabbit model. Plast. Reconstr. Surg. 1997, 100, 1788–1795. [Google Scholar] [CrossRef]
- Maksoud, M.; Koo, S.; Barouch, K.; Karimbux, N. Popularity of suture materials among residents and faculty members of a postdoctoral periodontology program. J. Investig. Clin. Dent. 2014, 5, 45–50. [Google Scholar] [CrossRef]
- Koyuncuoglu, C.Z.; Yaman, D.; Kasnak, G.; Demirel, K. Preference of Suture Specifications in a Selected Periodontal and Implant Surgeries in Turkey. Eur. J. Dent. 2019, 13, 108–113. [Google Scholar] [CrossRef]
- Gillanders, S.L.; Anderson, S.; Mellon, L.; Heskin, L. A systematic review and meta-analysis: Do absorbable or non-absorbable suture materials differ in cosmetic outcomes in patients requiring primary closure of facial wounds? J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 1682–1692. [Google Scholar] [CrossRef]
- Gabrielli, F.; Potenza, C.; Puddu, P.; Sera, F.; Masini, C.; Abeni, D. Suture materials and other factors associated with tissue reactivity, infection, and wound dehiscence among plastic surgery outpatients. Plast. Reconstr. Surg. 2011, 107, 38–45. [Google Scholar] [CrossRef]
- Faulkner, B.C.; Tribble, C.G.; Thacker, J.G.; Rodeheaver, G.T.; Edlich, R.F. Knot performance of polypropylene sutures. J. Biomed. Mater. Res. 1996, 33, 187–192. [Google Scholar] [CrossRef]
- Bloom, B.S.; Goldberg, D.J. Suture material in cosmetic cutaneous surgery. J. Cosmet. Laser. Ther. 2007, 9, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.A.; Clark, R.M. Advances in suture material for obstetric and gynecologic surgery. Rev. Obstet. Gynecol. 2009, 2, 146–158. [Google Scholar] [PubMed]
- Im, J.N.; Kim, J.K.; Kim, H.K.; Lee, K.Y.; Park, W.H. Effect of tying conditions on the knot security of suture materials. J. Appl. Polym. Sci. 2008, 109, 918–922. [Google Scholar] [CrossRef]
- Lawrence, T.M.; Davis, T.R. A biomechanical analysis of suture materials and their influence on a four-strand flexor tendon repair. J. Hand. Surg. Am. 2005, 30, 836–841. [Google Scholar] [CrossRef]
- Polykandriotis, E.; Besrour, F.; Arkudas, A.; Ruppe, F.; Zetzmann, K.; Braeuer, L.; Horch, R.E. Flexor tendon repair with a polytetrafluoroethylene (PTFE) suture material. Arch. Orthop. Trauma Surg. 2019, 139, 429–434. [Google Scholar] [CrossRef]
- Hertweck, S.P.; von Fraunhofer, J.A.; Masterson, B.J. Tensile characteristics of PTFE sutures. Biomaterials 1988, 9, 457–459. [Google Scholar] [CrossRef]
- Marturello, D.M.; McFadden, M.S.; Bennett, R.A.; Ragetly, G.R.; Horn, G. Knot security and tensile strength of suture materials. Vet. Surg. 2014, 43, 73–79. [Google Scholar] [CrossRef]
- Brooks, S.E. Securing extraocular muscles in strabismus surgery: Biomechanical analysis of muscle imbrication and knot tying technique. J. AAPOS 2019, 23, 57–59. [Google Scholar] [CrossRef]
- Dang, M.C.; Thacker, J.G.; Hwang, J.C.; Rodeheaver, G.T.; Melton, S.M.; Edlich, R.F. Some biomechanical considerations of polytetrafluoroethylene sutures. Arch. Surg. 1990, 125, 647–650. [Google Scholar] [CrossRef]
- Alves de Oliveira, M.; Arcanjo, A.; Castro, F.; Fernandes, J.C.H.; Fernandes, G.V.O. Evaluating and Comparing the Tensile Strength and Clinical Behavior of Monofilament Polyamide and Multifilament Silk Sutures: A Systematic Review. Surgeries 2024, 5, 350–366. [Google Scholar] [CrossRef]
- Naleway, S.E.; Lear, W.; Kruzic, J.J.; Maughan, C.B. Mechanical properties of suture materials in general and cutaneous surgery. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Gulihar, A.; Whitehead-Clarke, T.; Hajipour, L.; Dias, J.J. A Comparison of Two Monofilament Suture Materials for Repair of Partial Flexor Tendon Lacerations: A Controlled In-vitro Study. J. Hand. Surg. Asian Pac. 2017, 22, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.A.; Pellowski, D.M.; Rawdon, E.J. All monofilament knots assume sliding conformation in vivo. Dermatol. Surg. 2013, 39, 729–733. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, Y.K.; Lim, B.S.; Rhee, S.H.; Yang, H.C. Comparison of tensile and knot security properties of surgical sutures. J. Mater. Sci.-Mater. Med. 2007, 18, 2363–2369. [Google Scholar] [CrossRef]
- Al-Shawabkeh, A.F. Thermodynamic characteristics of the aliphatic polyamide crystal structures: Enhancement of nylon 66α, 610α and 77γ polymers. Heliyon 2023, 9, e21042. [Google Scholar] [CrossRef]
- Terwisscha-Dekker, H.; Hogenelst, T.; Bliem, R.; Weber, B.; Bonn, D. Why Teflon is so slippery while other polymers are not. Phy. Rev. E 2023, 107, 024801. [Google Scholar] [CrossRef]
- Zhou, P.; Tian, J.; Li, C.; Tang, Z. Comparative Study of Durability Behaviors of Thermoplastic Polypropylene and Thermosetting Epoxy Exposed to Elevated Temperature, Water Immersion and Sustained Bending Loading. Polymers 2022, 14, 2953. [Google Scholar] [CrossRef]
- Ferguson, R.E., Jr.; Schuler, K.; Thornton, B.P.; Vasconez, H.C.; Rinker, B. The effect of saliva and oral intake on the tensile properties of sutures: An experimental study. Ann. Plast. Surg. 2007, 58, 268–272. [Google Scholar] [CrossRef]
- Tullis, J.L. Influence of Suture Technique on Ridge Dimensions and Keratinized Tissue After Alveolar Ridge Preservation: A Pilot Study. Doctoral Dissertation, University of Missouri-Kansas City, Kansas City, MO, USA, 2020. [Google Scholar]
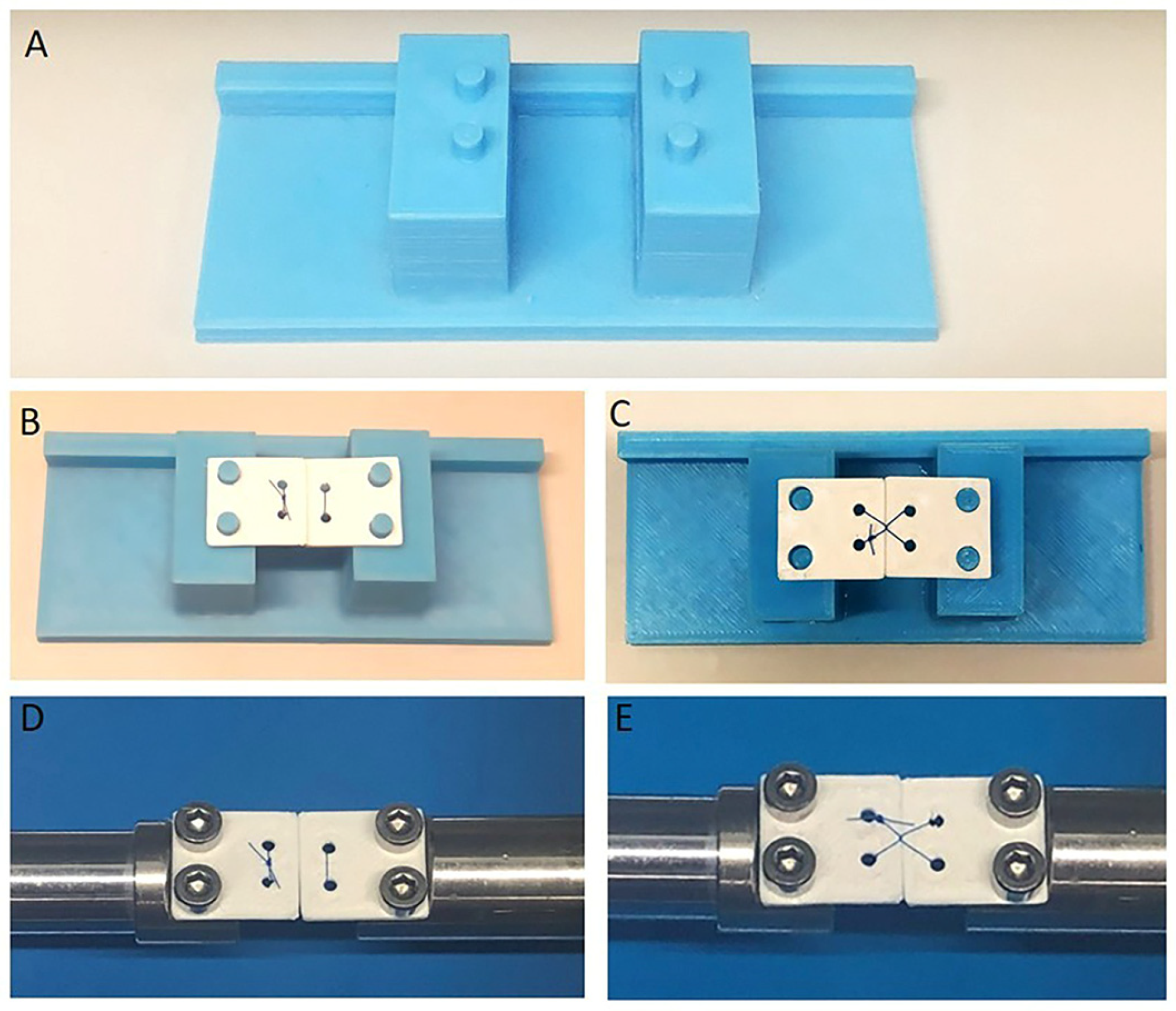
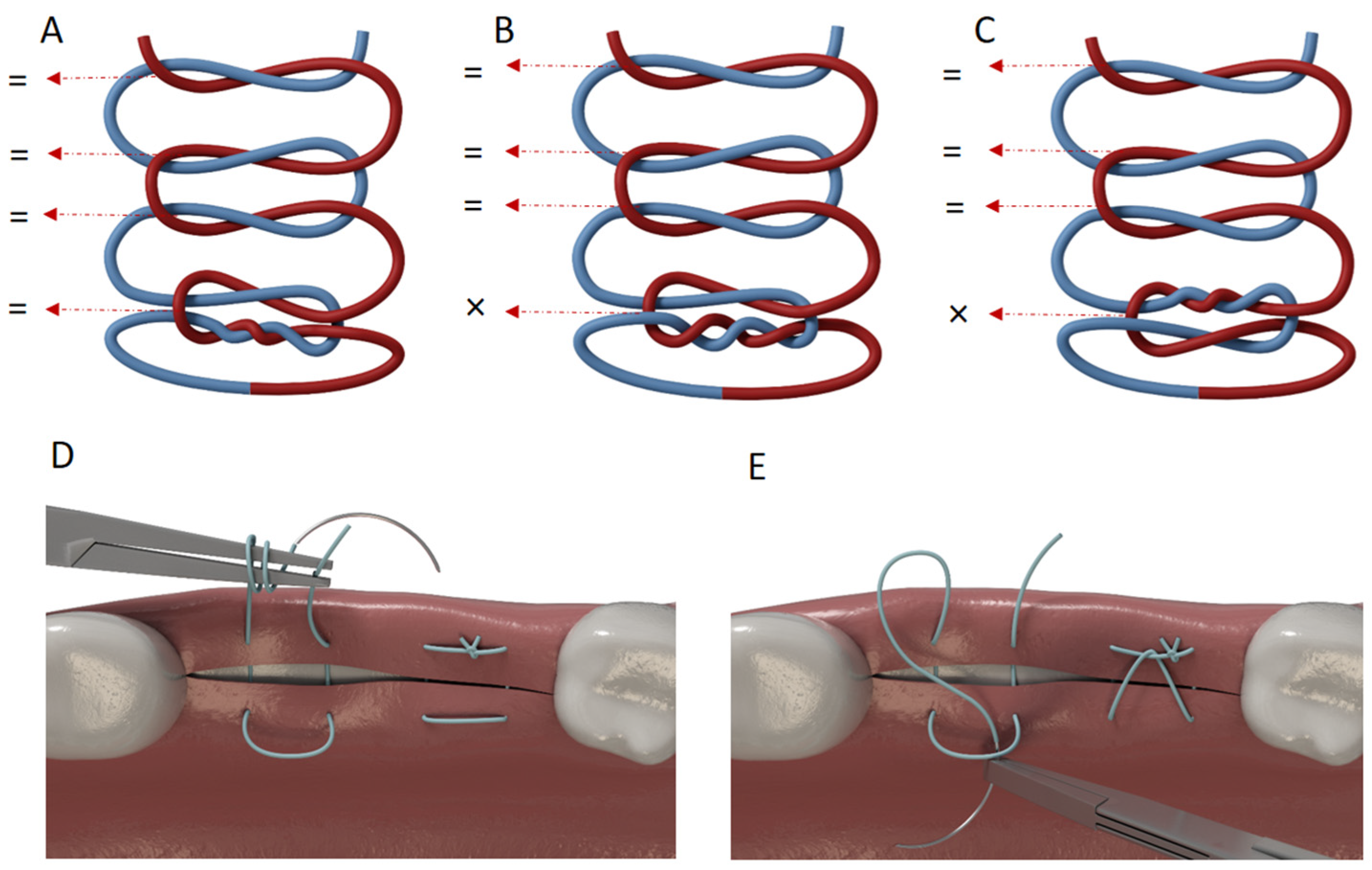
| Suture Material Type and Size | Brand Name | Manufacturer (Lot Number; Expiration Dates) | Suggested Throw Sequence by Manufacturer |
|---|---|---|---|
| Polypropylene with USP 4-0 caliber | Propilen® | Dogsan Inc., Trabzon, Turkey. (060719; February 2026) | undefined |
| Polyamide 6-6.6 (Nylon) with USP 4-0 caliber | ResolonTM | Resorba Medical GmbH, Nürnberg, Germany. (R00052331; 18 November 2025) | 1. single throw forward 2. double throw forward 3. single throw reverse |
| Polytetrafluoroethylene (PTFE) with USP 4-0 caliber | CytoplastTM | Osteogenics, Biomaterials, Lubbock, TX, USA. (RC16670A; June 2025) | 1. double throw forward 2. single throw forward 3. single throw reverse |
| Effect | df | F | p |
|---|---|---|---|
| (Intercept) | 1 | 84,559.29 | <0.001 |
| Material | 2 | 6948.05 | <0.001 |
| Technique | 1 | 6445.26 | <0.001 |
| Configuration | 2 | 104.76 | <0.001 |
| Time | 1 | 71.86 | <0.001 |
| Material × Technique | 2 | 642.32 | <0.001 |
| Material × Configuration | 4 | 38.67 | <0.001 |
| Technique × Configuration | 2 | 14.17 | <0.001 |
| Material × Time | 2 | 137.28 | <0.001 |
| Technique × Time | 1 | 45.67 | <0.001 |
| Configuration × Time | 2 | 39.79 | <0.001 |
| Material × Technique × Configuration | 4 | 20.62 | <0.001 |
| Material × Technique × Time | 2 | 5.61 | 0.004 |
| Material × Configuration × Time | 4 | 14.81 | <0.001 |
| Technique × Configuration × Time | 2 | 6.36 | 0.002 |
| Material × Technique × Configuration × Time | 4 | 1.32 | 0.263 |
| Residuals | 324 |
| Effect | df | F | p |
|---|---|---|---|
| (Intercept) | 1 | 36,381.42 | <0.001 |
| Material | 2 | 3627.83 | <0.001 |
| Technique | 1 | 1194.99 | <0.001 |
| Configuration | 2 | 8.09 | <0.001 |
| Time | 1 | 83.17 | <0.001 |
| Material × Technique | 2 | 350.77 | <0.001 |
| Material × Configuration | 4 | 18.30 | <0.001 |
| Technique × Configuration | 2 | 10.97 | <0.001 |
| Material × Time | 2 | 22.66 | <0.001 |
| Technique × Time | 1 | 23.88 | <0.001 |
| Configuration × Time | 2 | 2.76 | 0.065 |
| Material × Technique × Configuration | 4 | 4.33 | 0.002 |
| Material × Technique × Time | 2 | 22.64 | <0.001 |
| Material × Configuration × Time | 4 | 13.26 | <0.001 |
| Technique × Configuration × Time | 2 | 1.10 | 0.333 |
| Material × Technique × Configuration × Time | 4 | 15.94 | <0.001 |
| Residuals | 324 |
| Baseline | Day 7 | |||
|---|---|---|---|---|
| Factor | Level | Mean ± SD | Mean ± SD | p (Baseline vs. Day 7) |
| Material × Time | ||||
| Propilen | 32.31 ± 17.64 b | 30.02 ± 17.61 b | <0.001 | |
| PTFE | 11.77 ± 6.44 a | 10.69 ± 6.29 a | 0.162 | |
| Resolon | 37.54 ± 17.86 c | 35.38 ± 18.08 c | <0.001 | |
| Technique × Time | ||||
| HM | 33.77 ± 17.06 a | 31.72 ± 17.18 a | 0.225 | |
| LG | 21.82 ± 15.18 b | 19.83 ± 14.98 b | <0.001 | |
| Configuration × Time | ||||
| A | 30.71 ± 17.65 b | 28.67 ± 17.47 b | 0.018 | |
| B | 28.09 ± 17.46 a | 26.12 ± 17.44 a | <0.001 | |
| C | 27.31 ± 16.43 a | 24.44 ± 13.82 a | <0.001 | |
| Baseline | Day 7 | |||
|---|---|---|---|---|
| Factor | Level | Mean ± SD | Mean ± SD | p (Baseline vs. Day 7) |
| Material × Time | ||||
| Propilen | 10,049 ± 3839 b | 9615 ± 3902 b | 0.001 | |
| PTFE | 7990 ± 3782 a | 7660 ± 3801 a | <0.001 | |
| Resolon | 12,105 ± 3404 c | 11,564 ± 3527 c | 0.071 | |
| Technique × Time | ||||
| HM | 11,493 ± 3566 b | 11,050 ± 3624 b | 0.003 | |
| LG | 8800 ± 3415 a | 8333 ± 3413 a | <0.001 | |
| Configuration × Time | ||||
| A | 10,424 ± 3844 a | 10,039 ± 3921 b | <0.001 | |
| B | 9570 ± 3631 a | 9131 ± 3625 a | <0.001 | |
| C | 4512 ± 2317 a | 4160 ± 2363 ab | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taysi, N.M.; Erten Taysi, A.; Ercal, P.; Sismanoglu, S. Mechanical Performance of Nonabsorbable Monofilament Suture Materials Tied with Different Suturing Techniques Under Various Knot Configurations: An In Vitro Study. J. Funct. Biomater. 2025, 16, 428. https://doi.org/10.3390/jfb16120428
Taysi NM, Erten Taysi A, Ercal P, Sismanoglu S. Mechanical Performance of Nonabsorbable Monofilament Suture Materials Tied with Different Suturing Techniques Under Various Knot Configurations: An In Vitro Study. Journal of Functional Biomaterials. 2025; 16(12):428. https://doi.org/10.3390/jfb16120428
Chicago/Turabian StyleTaysi, Nuri Mert, Aysegul Erten Taysi, Pinar Ercal, and Soner Sismanoglu. 2025. "Mechanical Performance of Nonabsorbable Monofilament Suture Materials Tied with Different Suturing Techniques Under Various Knot Configurations: An In Vitro Study" Journal of Functional Biomaterials 16, no. 12: 428. https://doi.org/10.3390/jfb16120428
APA StyleTaysi, N. M., Erten Taysi, A., Ercal, P., & Sismanoglu, S. (2025). Mechanical Performance of Nonabsorbable Monofilament Suture Materials Tied with Different Suturing Techniques Under Various Knot Configurations: An In Vitro Study. Journal of Functional Biomaterials, 16(12), 428. https://doi.org/10.3390/jfb16120428






