Alginate-Sr/Mg Containing Bioactive Glass Scaffolds: The Characterization of a New 3D Composite for Bone Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Bioactive Glasses and Chemical Characterization of Bioactive Glasses and HAp
2.3. Preparation of Alginate/Hydroxyapatite-Bioactive Glass Composite Scaffolds
2.4. PhysicoChemical and Structural Scaffold Characterizations
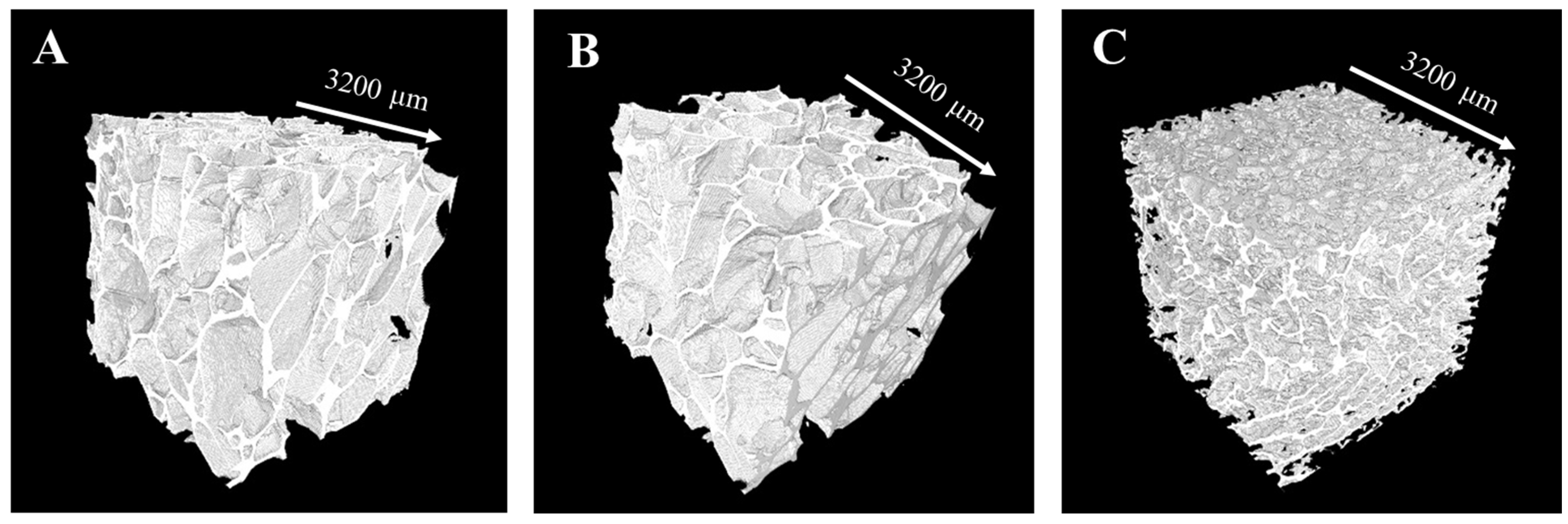
2.4.1. X-ray Microcomputed Tomography Analysis
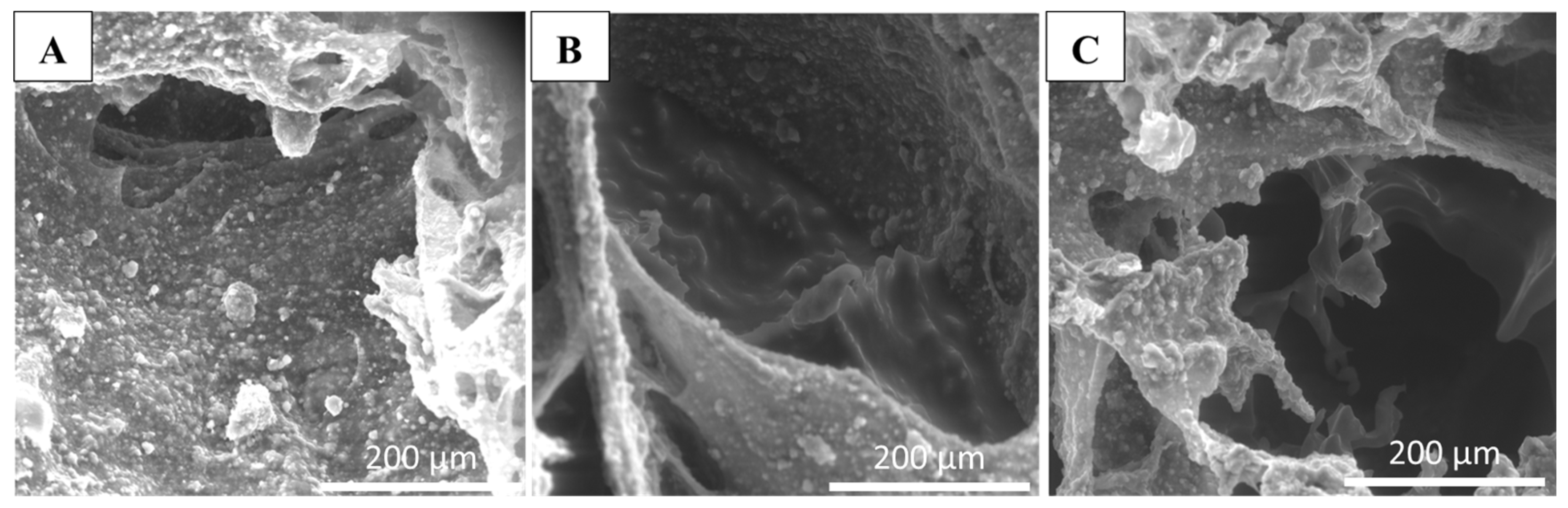
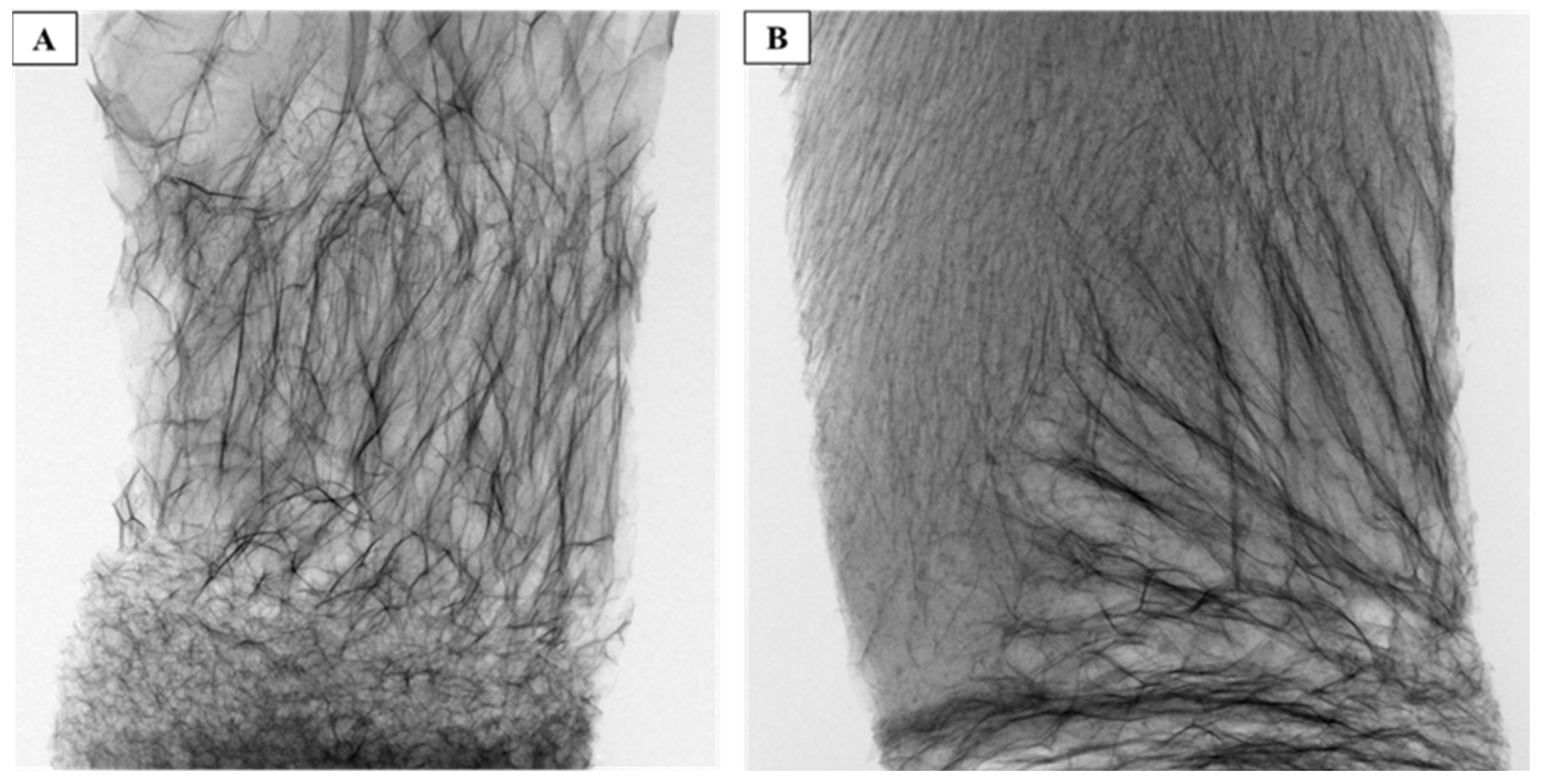
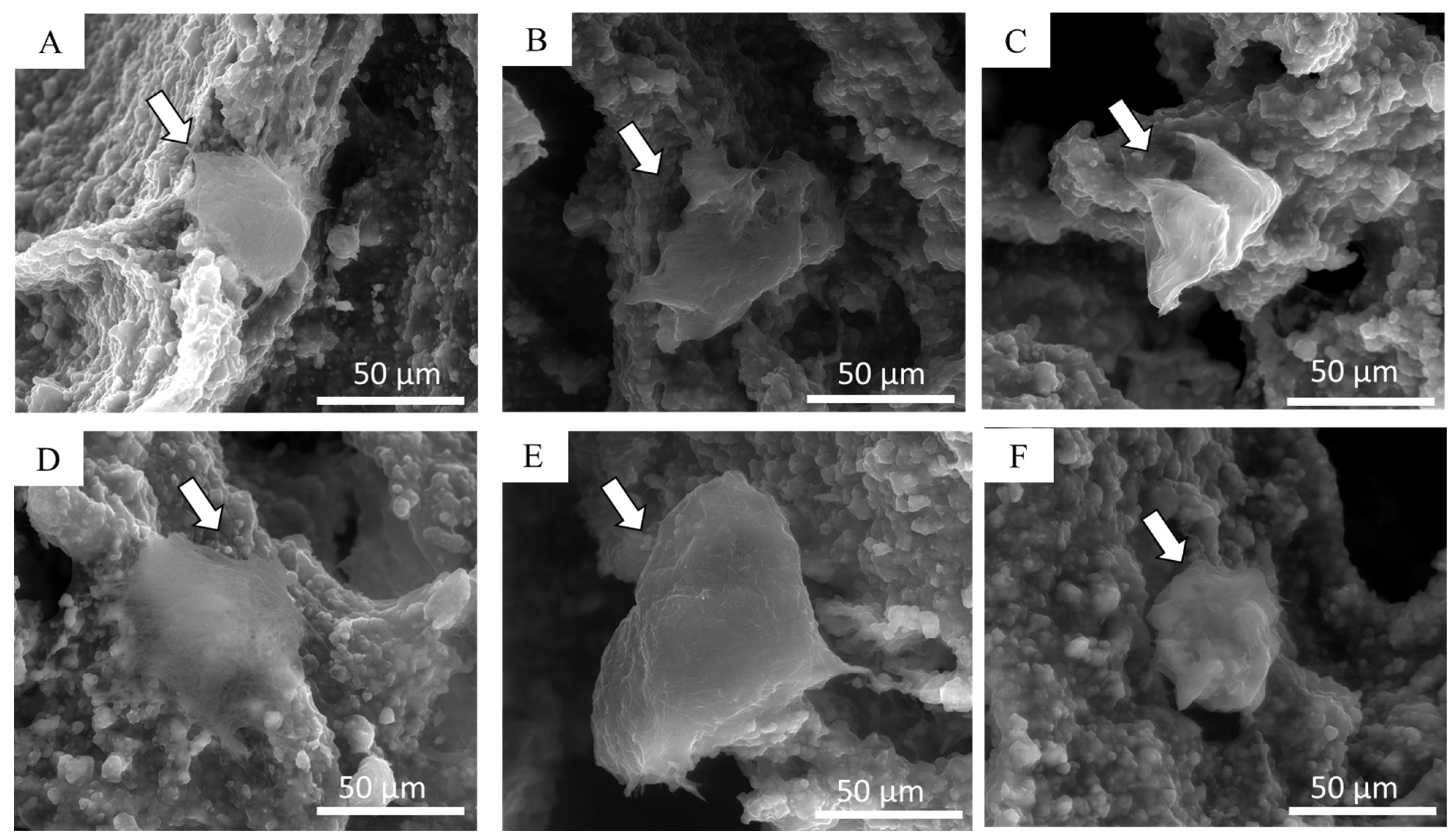
2.4.2. Scanning Electron Microscopy (SEM)
2.4.3. Attenuated Total Reflectance–Fourier Transform Infrared (ATR-FTIR) Spectroscopy
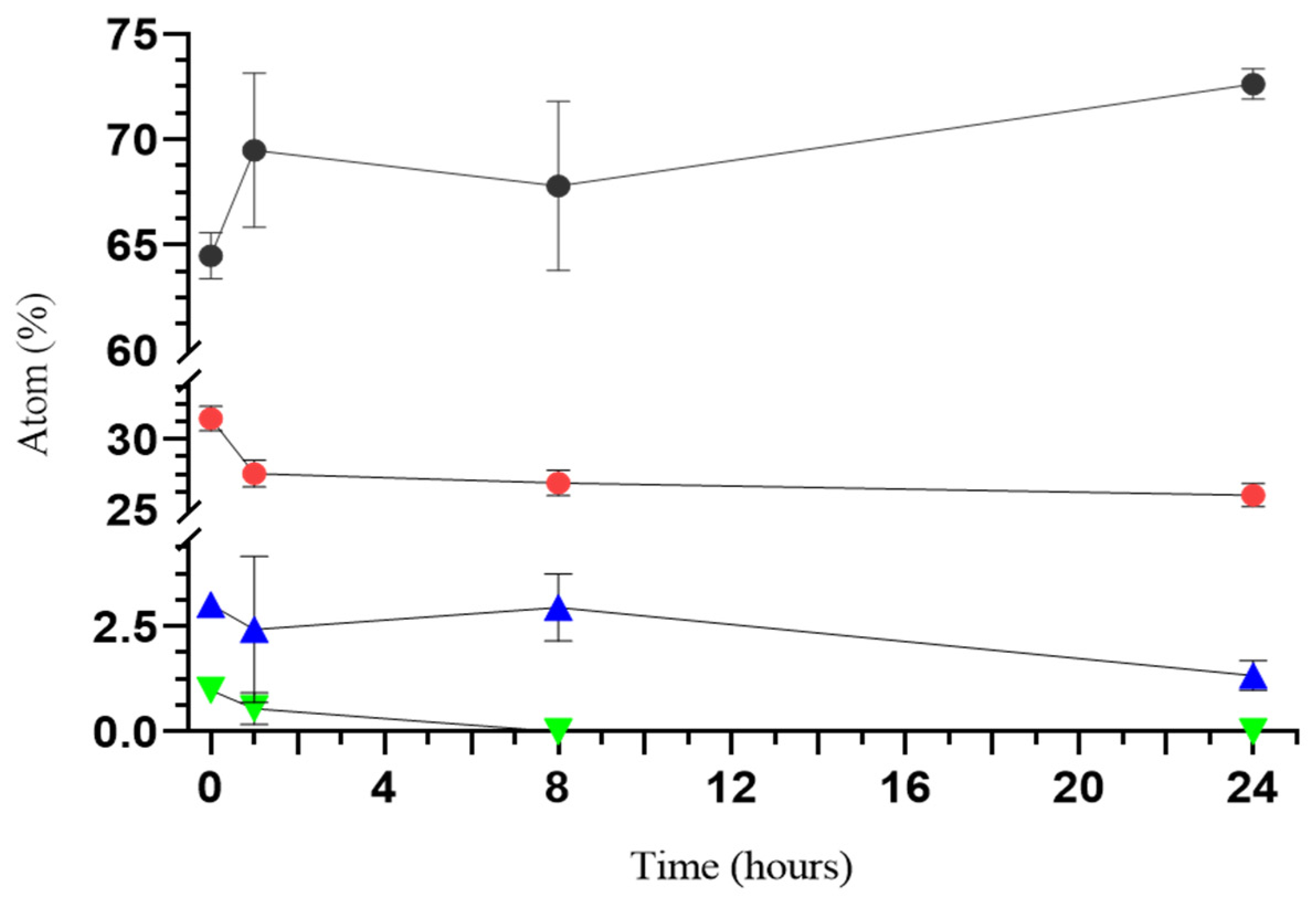
2.4.4. Ion Release Evaluation with Energy-Dispersive Spectroscopy (EDS)
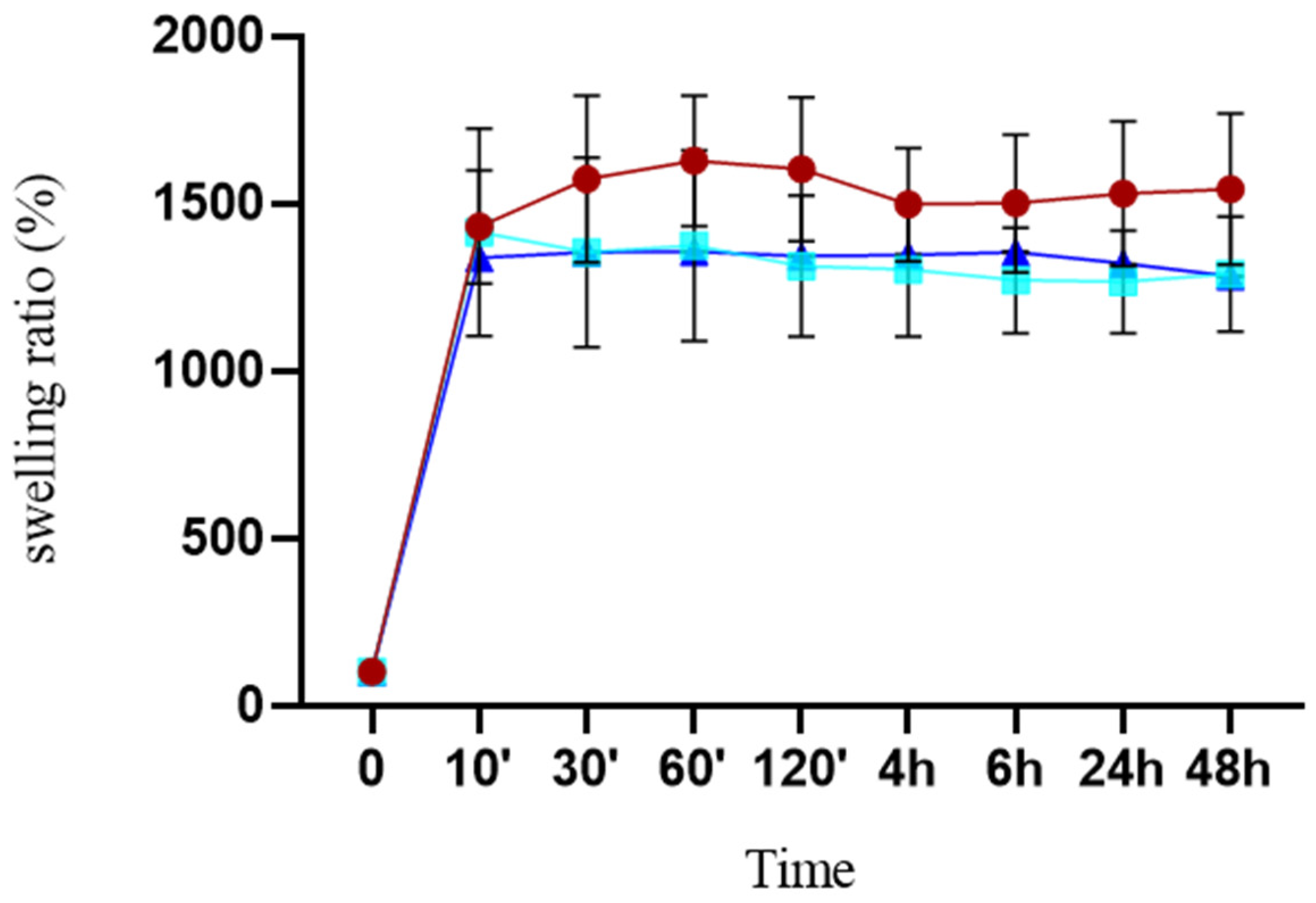
2.5. Swelling Studies
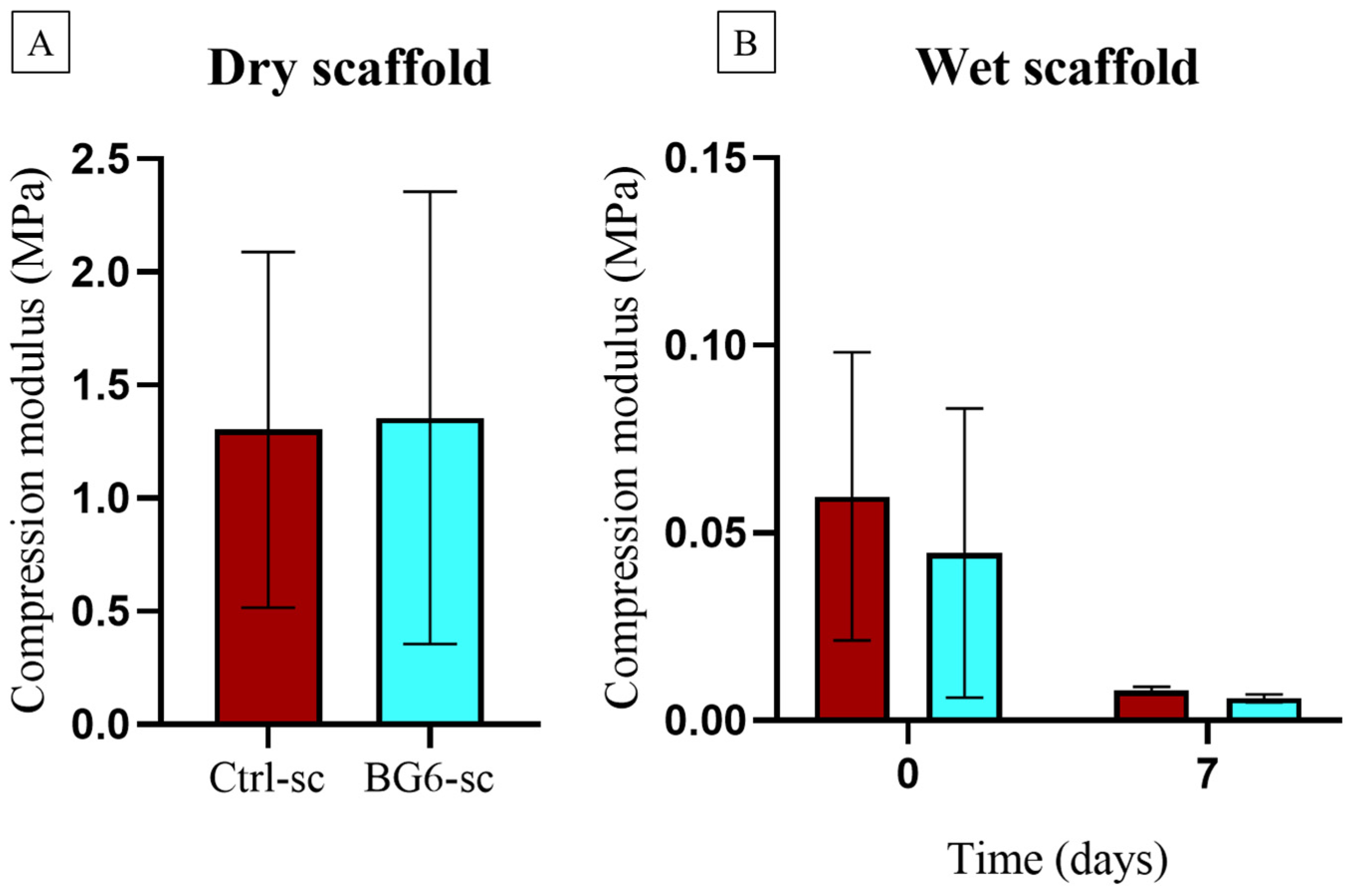
2.6. Uniaxial Compression Tests of Scaffolds
2.7. Cell Culture, Adhesion, and Proliferation on Scaffolds
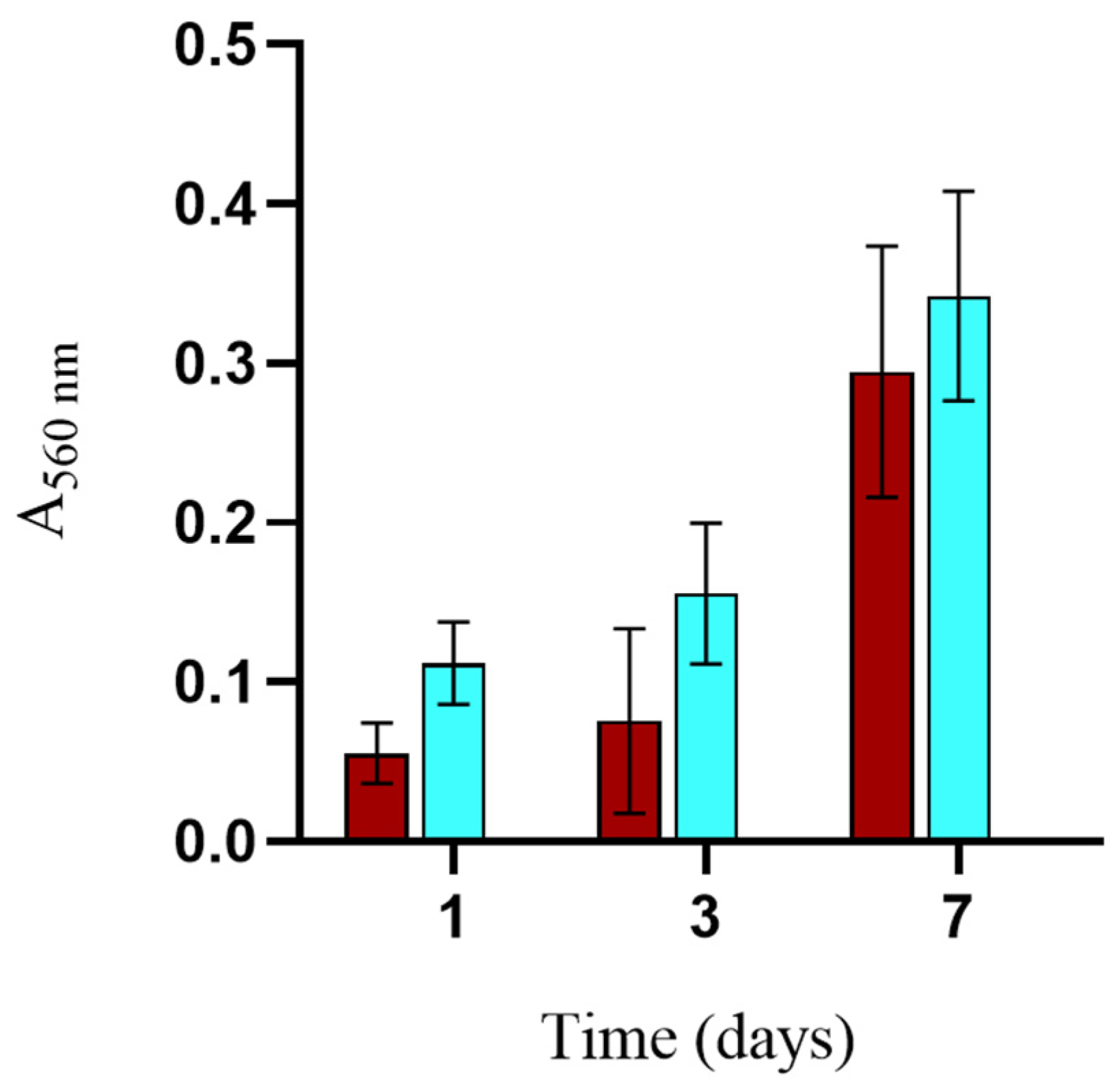
2.7.1. Alamar Blue
2.7.2. MTT Assay
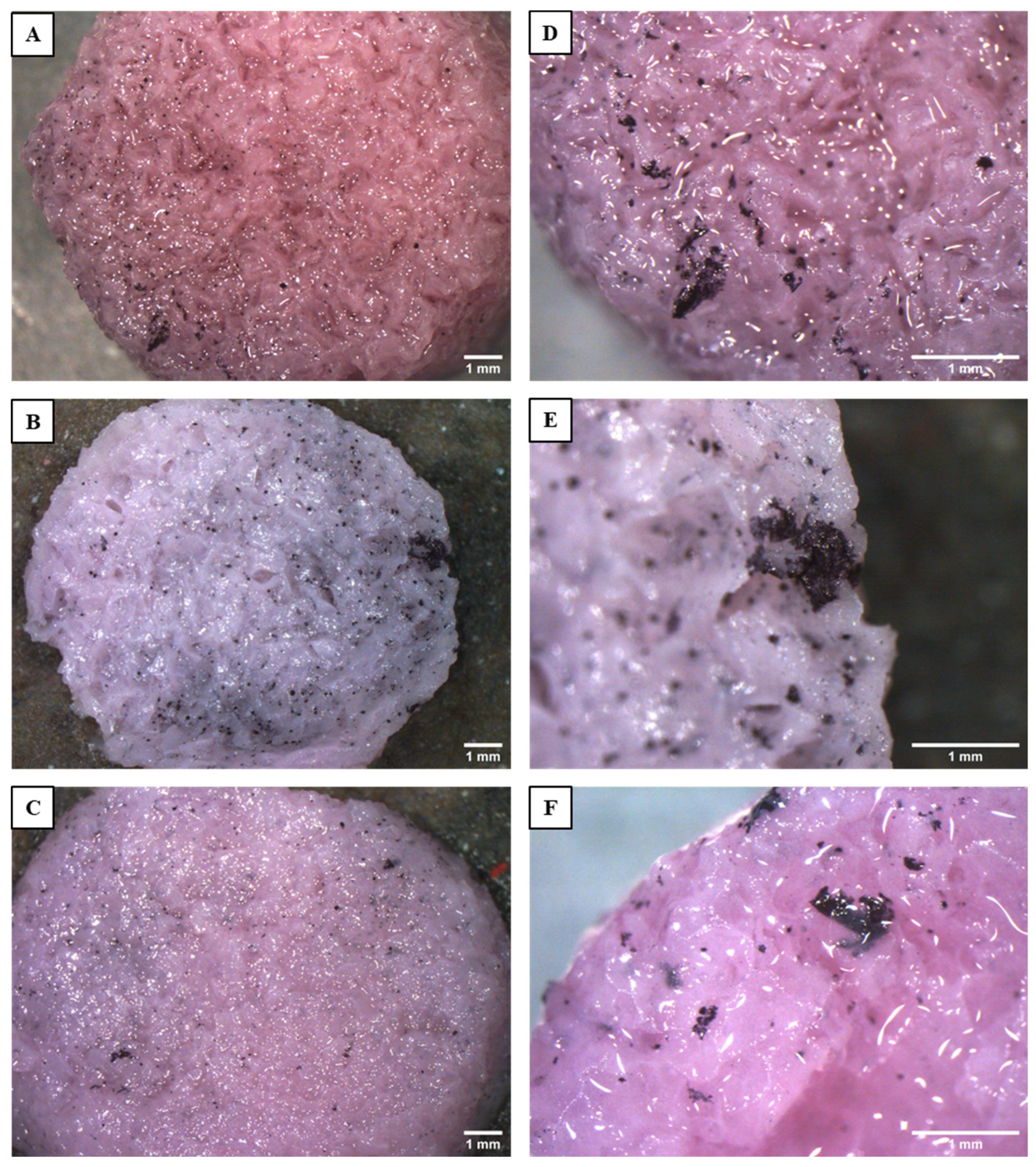
2.7.3. Cell Morphology Study by Stereoscope and eSEM Observation
2.8. Antimicrobial Effects of Composite Scaffolds
2.9. Statistical Analysis
3. Results
3.1. Preparation and Characterization of Composite Scaffolds
3.2. Mechanical Characterization
3.3. Biocompatibility and Cell Morphology on Scaffolds
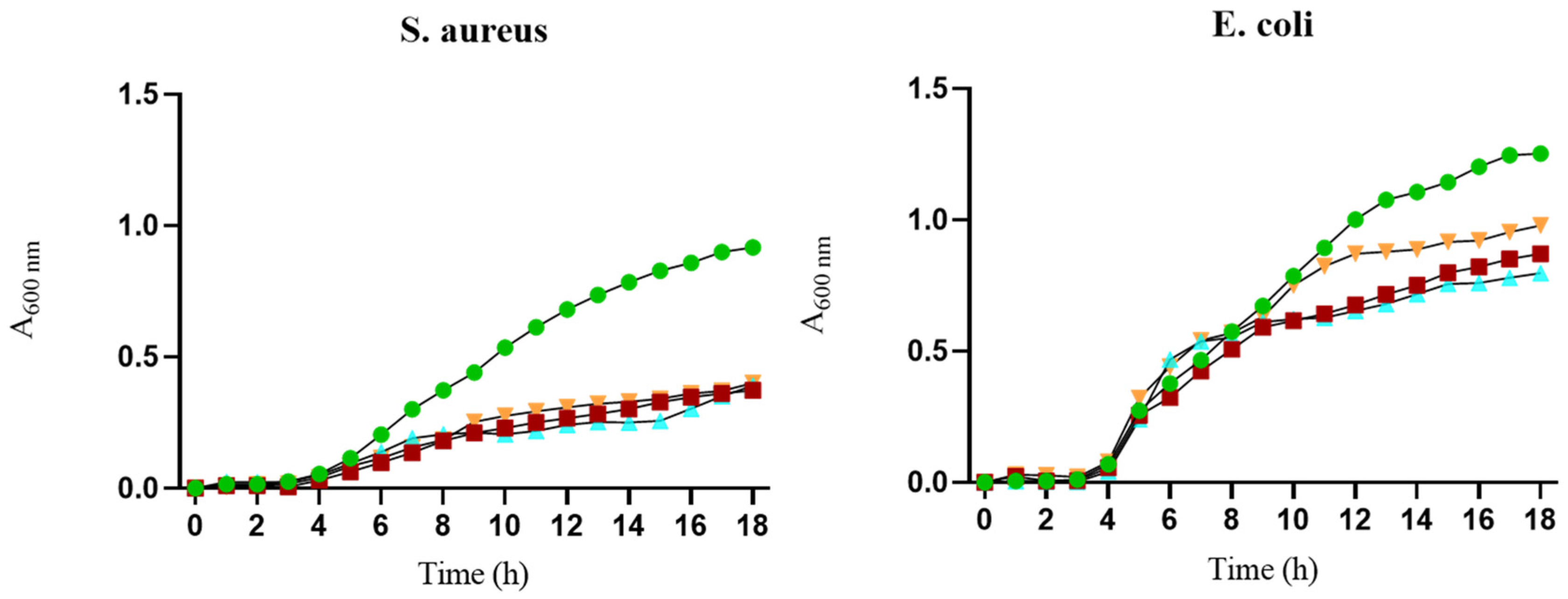
3.4. Antibacterial Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, C.; Liu, Z.; Chen, X.; Gao, Y.; Wang, W.; Zhuang, X.; Zhang, H.; Dong, X. Bone Tissue Engineering Scaffold Materials: Fundamentals, Advances, and Challenges. Chin. Chem. Lett. 2024, 35, 109197. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Biswal, T. Alginate and Its Application to Tissue Engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Iglesias-Mejuto, A.; García-González, C.A. 3D-Printed Alginate-Hydroxyapatite Aerogel Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2021, 131, 112525. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Raus, R.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and Alginate Composites for Biomedical Applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef] [PubMed]
- Mi, A.; Guo, L.; Guo, S.; Wang, L.; Shang, H.; Wang, D.; Zhao, Y.; Zhang, B. Freeze-Casting in Synthetic Porous Materials: Principles, Different Dimensional Building Units and Recent Applications. Sustain. Mater. Technol. 2024, 39, e00830. [Google Scholar] [CrossRef]
- Kotlarz, M.; Melo, P.; Ferreira, A.M.; Gentile, P.; Dalgarno, K. Cell Seeding via Bioprinted Hydrogels Supports Cell Migration into Porous Apatite-Wollastonite Bioceramic Scaffolds for Bone Tissue Engineering. Biomater. Adv. 2023, 153, 213532. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.H.; Mabrouk, M.; Salama, A.A.; Hamzawy, E.M.A. Alginate-Based Composite Scaffolds Loaded with Streptomycin Sulfate for Bone Regeneration: In Vitro Studies. J. Drug Deliv. Sci. Technol. 2024, 94, 105486. [Google Scholar] [CrossRef]
- Turco, G.; Marsich, E.; Bellomo, F.; Semeraro, S.; Donati, I.; Brun, F.; Grandolfo, M.; Accardo, A.; Paoletti, S. Alginate/Hydroxyapatite Biocomposite for Bone Ingrowth: A Trabecular Structure with High and Isotropic Connectivity. Biomacromolecules 2009, 10, 1575–1583. [Google Scholar] [CrossRef]
- Porrelli, D.; Gruppuso, M.; Vecchies, F.; Marsich, E.; Turco, G. Alginate Bone Scaffolds Coated with a Bioactive Lactose Modified Chitosan for Human Dental Pulp Stem Cells Proliferation and Differentiation. Carbohydr. Polym. 2021, 273, 118610. [Google Scholar] [CrossRef]
- Dalavi, P.A.; Prabhu, A.; Shastry, R.P.; Venkatesan, J. Microspheres Containing Biosynthesized Silver Nanoparticles with Alginate-Nano Hydroxyapatite for Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2020, 31, 2025–2043. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Niu, W.; Lei, B.; Boccaccini, A.R. Immunomodulatory Bioactive Glasses for Tissue Regeneration. Acta Biomater. 2021, 133, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, T.; Mesgar, A.S.; Mohammadi, Z. Bioactive Glasses: A Promising Therapeutic Ion Release Strategy for Enhancing Wound Healing. ACS Biomater. Sci. Eng. 2020, 6, 5399–5430. [Google Scholar] [CrossRef] [PubMed]
- Fiume, E.; Migneco, C.; Kargozar, S.; Verné, E.; Baino, F. Processing of Bioactive Glass Scaffolds for Bone Tissue Engineering. In Bioactive Glasses and Glass-Ceramics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 119–145. ISBN 978-1-119-72419-3. [Google Scholar]
- Fiume, E.; Barberi, J.; Verné, E.; Baino, F. Bioactive Glasses: From Parent 45S5 Composition to Scaffold-Assisted Tissue-Healing Therapies. J. Funct. BIomater. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass®-Derived Glass–Ceramic Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, A.; Leśniak, M.; Kochanowicz, M.; Żmojda, J.; Miluski, P.; Dorosz, D. Crystallization Kinetics and Structural Properties of the 45S5 Bioactive Glass and Glass-Ceramic Fiber Doped with Eu3+. Materials 2020, 13, 1281. [Google Scholar] [CrossRef] [PubMed]
- Flores-Jacobo, A.; Aguilar-Reyes, E.A.; León-Patiño, C.A. Effect of Dopants on the Physical, Mechanical, and Biological Properties of Porous Scaffolds for Bone Tissue Engineering. Biomed. Mater. Devices 2023, 1, 234–255. [Google Scholar] [CrossRef]
- Pantulap, U.; Arango-Ospina, M.; Boccaccini, A.R. Bioactive Glasses Incorporating Less-Common Ions to Improve Biological and Physical Properties. J. Mater. Sci. Mater. Med. 2021, 33, 3. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Cannillo, V. A Novel Bioactive Glass Containing Strontium and Magnesium with Ultra-High Crystallization Temperature. Mater. Lett. 2018, 213, 67–70. [Google Scholar] [CrossRef]
- Farzadi, A.; Bakhshi, F.; Solati-Hashjin, M.; Asadi-Eydivand, M.; abu Osman, N.A. Magnesium Incorporated Hydroxyapatite: Synthesis and Structural Properties Characterization. Ceram. Int. 2014, 40, 6021–6029. [Google Scholar] [CrossRef]
- Lijuan, X.; Liuyun, J.; Lixin, J.; Chengdong, X. Synthesis of Mg-Substituted Hydroxyapatite Nanopowders: Effect of Two Different Magnesium Sources. Mater. Lett. 2013, 106, 246–249. [Google Scholar] [CrossRef]
- Tao, Z.-S.; Zhou, W.-S.; He, X.-W.; Liu, W.; Bai, B.-L.; Zhou, Q.; Huang, Z.-L.; Tu, K.; Li, H.; Sun, T.; et al. A Comparative Study of Zinc, Magnesium, Strontium-Incorporated Hydroxyapatite-Coated Titanium Implants for Osseointegration of Osteopenic Rats. Mater. Sci. Eng. C 2016, 62, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Salvatori, R.; Giannatiempo, J.; Anesi, A.; Bortolini, S.; Cannillo, V. A New Bioactive Glass/Collagen Hybrid Composite for Applications in Dentistry. Materials 2019, 12, 2079. [Google Scholar] [CrossRef] [PubMed]
- Angioni, D.; Orrù, R.; Cao, G.; Garroni, S.; Bellucci, D.; Cannillo, V. Recent Advances on Innovative Bioactive Glass-Hydroxyapatite Composites for Bone Tissue Applications: Processing, Mechanical Properties, and Biological Performance. J. Eur. Ceram. Soc. 2023, 43, 7688–7696. [Google Scholar] [CrossRef]
- Haider, A.; Waseem, A.; Karpukhina, N.; Mohsin, S. Strontium- and Zinc-Containing Bioactive Glass and Alginates Scaffolds. Bioengineering 2020, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Ma, Y.; Cui, X.; Jiang, L.; Wu, M.; Hu, Y.; Luo, Y.; Pan, H.; Ruan, C. 13-93 Bioactive Glass/Alginate Composite Scaffolds 3D Printed under Mild Conditions for Bone Regeneration. RSC Adv. 2017, 7, 11880–11889. [Google Scholar] [CrossRef]
- Manoochehri, H.; Ghorbani, M.; Moosazadeh Moghaddam, M.; Nourani, M.R.; Makvandi, P.; Sharifi, E. Strontium Doped Bioglass Incorporated Hydrogel-Based Scaffold for Amplified Bone Tissue Regeneration. Sci. Rep. 2022, 12, 10160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, S.; Zhu, Y.; Huang, Y.; Zhu, M.; Tao, C.; Zhang, C. Three-Dimensional Printing of Strontium-Containing Mesoporous Bioactive Glass Scaffolds for Bone Regeneration. Acta Biomater. 2014, 10, 2269–2281. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; You, D.; Fei, T.; Wu, Y.; Shao, Y.; Xie, Y.; Xu, M.; Hu, Y.; Zhang, J.; Yu, M. The Role and Application of Metal Ions in Maxillofacial Bone Defect. Chem. Eng. J. 2024, 493, 152317. [Google Scholar] [CrossRef]
- Atkinson, I.; Anghel, E.M.; Predoana, L.; Mocioiu, O.C.; Jecu, L.; Raut, I.; Munteanu, C.; Culita, D.; Zaharescu, M. Influence of ZnO Addition on the Structural, in Vitro Behavior and Antimicrobial Activity of Sol–Gel Derived CaO–P2O5–SiO2 Bioactive Glasses. Ceram. Int. 2016, 42, 3033–3045. [Google Scholar] [CrossRef]
- Ranga, N.; Poonia, E.; Jakhar, S.; Sharma, A.K.; Kumar, A.; Devi, S.; Duhan, S. Enhanced Antimicrobial Properties of Bioactive Glass Using Strontium and Silver Oxide Nanocomposites. J. Asian Ceram. Soc. 2019, 7, 75–81. [Google Scholar] [CrossRef]
- Liu, J.; Rawlinson, S.C.F.; Hill, R.G.; Fortune, F. Strontium-Substituted Bioactive Glasses In Vitro Osteogenic and Antibacterial Effects. Dent. Mater. 2016, 32, 412–422. [Google Scholar] [CrossRef]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Doube, M.; Kłosowski, M.M.; Arganda-Carreras, I.; Cordelières, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and Extensible Bone Image Analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- De Long, W.G.J.; Einhorn, T.A.; Koval, K.; McKee, M.; Smith, W.; Sanders, R.; Watson, T. Bone Grafts and Bone Graft Substitutes in Orthopaedic Trauma Surgery: A Critical Analysis. J. Bone Jt. Surg. Am. 2007, 89, 649–658. [Google Scholar] [CrossRef]
- Ingar Draget, K.; Østgaard, K.; Smidsrød, O. Homogeneous Alginate Gels: A Technical Approach. Carbohydr. Polym. 1990, 14, 159–178. [Google Scholar] [CrossRef]
- Gavinho, S.R.; Pádua, A.S.; Holz, L.I.V.; Sá-Nogueira, I.; Silva, J.C.; Borges, J.P.; Valente, M.A.; Graça, M.P.F. Bioactive Glasses Containing Strontium or Magnesium Ions to Enhance the Biological Response in Bone Regeneration. Nanomaterials 2023, 13, 2717. [Google Scholar] [CrossRef]
- Silva, A.V.; Gomes, D.d.S.; Victor, R.d.S.; Santana, L.N.d.L.; Neves, G.A.; Menezes, R.R. Influence of Strontium on the Biological Behavior of Bioactive Glasses for Bone Regeneration. Materials 2023, 16, 7654. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Chen, Q.; Lefebvre, L.; Gremillard, L.; Chevalier, J. Sintering, Crystallisation and Biodegradation Behaviour of Bioglass®-Derived Glass–Ceramics. Faraday Discuss. 2007, 136, 27–44. [Google Scholar] [CrossRef]
- Bretcanu, O.; Chatzistavrou, X.; Paraskevopoulos, K.; Conradt, R.; Thompson, I.; Boccaccini, A.R. Sintering and Crystallisation of 45S5 Bioglass® Powder. J. Eur. Ceram. Soc. 2009, 29, 3299–3306. [Google Scholar] [CrossRef]
- Anesi, A.; Ferretti, M.; Salvatori, R.; Bellucci, D.; Cavani, F.; Di Bartolomeo, M.; Palumbo, C.; Cannillo, V. In-Vivo Evaluations of Bone Regenerative Potential of Two Novel Bioactive Glasses. J. Biomed. Mater. Res. A 2023, 111, 1264–1278. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. B 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Pandey, O.P.; Singh, K.; Homa, D.; Scott, B.; Pickrell, G. A Review of Bioactive Glasses: Their Structure, Properties, Fabrication and Apatite Formation. J. Biomed. Mater. Res. A 2014, 102, 254–274. [Google Scholar] [CrossRef]
- Björkvik, L.; Wang, X.; Hupa, L. Dissolution of Bioactive Glasses in Acidic Solutions with the Focus on Lactic Acid. Int. J. Appl. Glass Sci. 2016, 7, 154–163. [Google Scholar] [CrossRef]
- LeRoux, M.A.; Guilak, F.; Setton, L.A. Compressive and Shear Properties of Alginate Gel: Effects of Sodium Ions and Alginate Concentration. J. Biomed. Mater. Res. 1999, 47, 46–53. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, W.; Fu, X.; Xie, W.; Chen, X. Fabrication and Characterization of Bioactive Glass/Alginate Composite Scaffolds by a Self-Crosslinking Processing for Bone Regeneration. RSC Adv. 2016, 6, 91201–91208. [Google Scholar] [CrossRef]
- Niu, Y.; Du, T.; Liu, Y. Biomechanical Characteristics and Analysis Approaches of Bone and Bone Substitute Materials. J. Funct. BIomater. 2023, 14, 212. [Google Scholar] [CrossRef]
- Prasad, A. State of Art Review on Bioabsorbable Polymeric Scaffolds for Bone Tissue Engineering. Mater. Today Proc. 2021, 44, 1391–1400. [Google Scholar] [CrossRef]
- Misch, C.E.; Qu, Z.; Bidez, M.W. Mechanical Properties of Trabecular Bone in the Human Mandible: Implications for Dental Implant Treatment Planning and Surgical Placement. J. Oral. Maxillofac. Surg. 1999, 57, 700–706. [Google Scholar] [CrossRef]
- Patel, H.; Bonde, M.; Srinivasan, G. Biodegradable Polymer Scaffold for Tissue Engineering. Trends Biomater. Artif. Organs 2011, 25, 20–29. [Google Scholar]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Miao, Y.; Tan, H.; Zhou, T.; Ling, Z.; Chen, Y.; Xing, X.; Hu, X. Injectable Alginate/Hydroxyapatite Gel Scaffold Combined with Gelatin Microspheres for Drug Delivery and Bone Tissue Engineering. Mater. Sci. Eng. C 2016, 63, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Dai, L.; Shi, H.; Xiong, S.; Zhou, C. In Vitro Evaluation of Alginate/Halloysite Nanotube Composite Scaffolds for Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Mattioli-Belmonte, M.; Chiono, V.; Ferretti, C.; Baino, F.; Tonda-Turo, C.; Vitale-Brovarone, C.; Pashkuleva, I.; Reis, R.L.; Ciardelli, G. Bioactive Glass/Polymer Composite Scaffolds Mimicking Bone Tissue. J. Biomed. Mater. Res. A 2012, 100, 2654–2667. [Google Scholar] [CrossRef] [PubMed]
- Staehlke, S.; Rebl, H.; Nebe, B. Phenotypic Stability of the Human MG-63 Osteoblastic Cell Line at Different Passages. Cell Biol. Int. 2019, 43, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Liang, Z.; Yang, Y.; Liu, H.; Ji, J.; Fan, Y. A Resazurin-Based, Nondestructive Assay for Monitoring Cell Proliferation during a Scaffold-Based 3D Culture Process. Regen. Biomater. 2020, 7, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Birru, B.; Mekala, N.K.; Parcha, S.R. Mechanistic Role of Perfusion Culture on Bone Regeneration. J. Biosci. 2019, 44, 23. [Google Scholar] [CrossRef]
- Phatai, P.; Futalan, C.M.; Kamonwannasit, S.; Khemthong, P. Structural Characterization and Antibacterial Activity of Hydroxyapatite Synthesized via Sol-Gel Method Using Glutinous Rice as a Template. J. Sol-Gel Sci. Technol. 2019, 89, 764–775. [Google Scholar] [CrossRef]
- Lamkhao, S.; Phaya, M.; Jansakun, C.; Chandet, N.; Thongkorn, K.; Rujijanagul, G.; Bangrak, P.; Randorn, C. Synthesis of Hydroxyapatite with Antibacterial Properties Using a Microwave-Assisted Combustion Method. Sci. Rep. 2019, 9, 4015. [Google Scholar] [CrossRef]
- Souad, G.; Baghdadi, C. Effect of Calcium Phosphate Synthesis Conditions on Its Physico-Chemical Properties and Evaluation of Its Antibacterial Activity. Mater. Res. Express 2020, 7, 015040. [Google Scholar] [CrossRef]
- Niazvand, F.; Sharifianjazi, F.; Esmaeilkhanian, A.; Ahmadi, E.; Moradigharibvand, N.; Rabiee, N.; Seifalian, A.; Ghiasvand, A.; Hojjati, M. Sol-Gel Derived Bioactive Glasses Containing Boron and Strontium: Bioactivity, Biocompatibility, and Antibacterial Properties. J. Non-Cryst. Solids 2024, 631, 122909. [Google Scholar] [CrossRef]
- Rundegren, J.; Sjödin, T.; Petersson, L.; Hansson, E.; Jonsson, I. Delmopinol Interactions with Cell Walls of Gram-Negative and Gram-Positive Oral Bacteria. Oral Microbiol. Immunol. 1995, 10, 102–109. [Google Scholar] [CrossRef] [PubMed]



| Alginate | Hydroxyapatite | 45S5® | BGMS10 | NaCl | |
|---|---|---|---|---|---|
| Ctrl-sc | 40 | 60 | - | - | - |
| BG6-sc | 40 | 54 | 6 | ||
| BG12-sc | 40 | 48 | 12 | ||
| 45S5®6-sc | 40 | 54 | 6 | ||
| BG6d-sc | 36.3 | 49.1 | - | 5.5 | 9.1 |
| Ctrl-sc | BG6-sc | BG12-sc | |
|---|---|---|---|
| Porosity | 84.6 ± 0.3 | 80.2 ± 1.1 | 70.2 ± 0.6 |
| Mean Tb.Th (µm) | 43.9 ± 1.2 | 53.7 ± 10.1 | 61.5 ± 11.1 |
| Mean Tb.Sp (µm) | 328 ± 19.9 | 335.2 ± 29.1 | 217.7 ± 93.3 |
| Conn.D (µm−3) | 5.90 × 10−8 ± 0.54 × 10−8 | 3.72 × 10−8 ± 0.70 × 10−8 | 3.50 × 10−8 ± 0.40 × 10−8 |
| DA | 0.34 ± 0.1 | 0.41 ± 0.09 | 0.45 ± 0.1 |
| Ctrl-sc | BG6-sc | |||||
|---|---|---|---|---|---|---|
| Dry | Wet (T0) | 1 Week | Dry | Wet (T0) | 1 Week | |
| E (MPa) | 1.30 ± 0.48 | 0.05 ± 0.03 | 0.008 ± 0.001 | 1.35 ± 1.02 | 0.04 ± 0.03 | 0.005 ± 0.001 |
| σucs (MPa) | 0.20 ± 0.02 | 0.008 ± 0.001 | 0.003 ± 0.001 | 0.19 ± 0.05 | 0.011 ± 0.006 | 0.003 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guagnini, B.; Medagli, B.; Zumbo, B.; Cannillo, V.; Turco, G.; Porrelli, D.; Bellucci, D. Alginate-Sr/Mg Containing Bioactive Glass Scaffolds: The Characterization of a New 3D Composite for Bone Tissue Engineering. J. Funct. Biomater. 2024, 15, 183. https://doi.org/10.3390/jfb15070183
Guagnini B, Medagli B, Zumbo B, Cannillo V, Turco G, Porrelli D, Bellucci D. Alginate-Sr/Mg Containing Bioactive Glass Scaffolds: The Characterization of a New 3D Composite for Bone Tissue Engineering. Journal of Functional Biomaterials. 2024; 15(7):183. https://doi.org/10.3390/jfb15070183
Chicago/Turabian StyleGuagnini, Benedetta, Barbara Medagli, Bianca Zumbo, Valeria Cannillo, Gianluca Turco, Davide Porrelli, and Devis Bellucci. 2024. "Alginate-Sr/Mg Containing Bioactive Glass Scaffolds: The Characterization of a New 3D Composite for Bone Tissue Engineering" Journal of Functional Biomaterials 15, no. 7: 183. https://doi.org/10.3390/jfb15070183
APA StyleGuagnini, B., Medagli, B., Zumbo, B., Cannillo, V., Turco, G., Porrelli, D., & Bellucci, D. (2024). Alginate-Sr/Mg Containing Bioactive Glass Scaffolds: The Characterization of a New 3D Composite for Bone Tissue Engineering. Journal of Functional Biomaterials, 15(7), 183. https://doi.org/10.3390/jfb15070183










