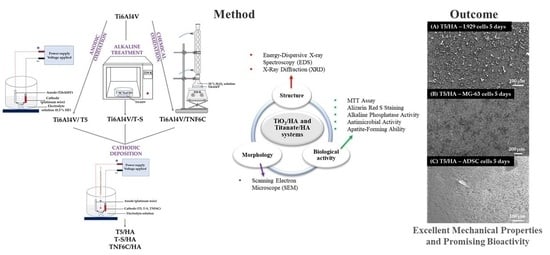
TiO2/HA and Titanate/HA Double-Layer Coatings on Ti6Al4V Surface and Their Influence on In Vitro Cell Growth and Osteogenic Potential
Abstract
1. Introduction
2. Materials and Methods
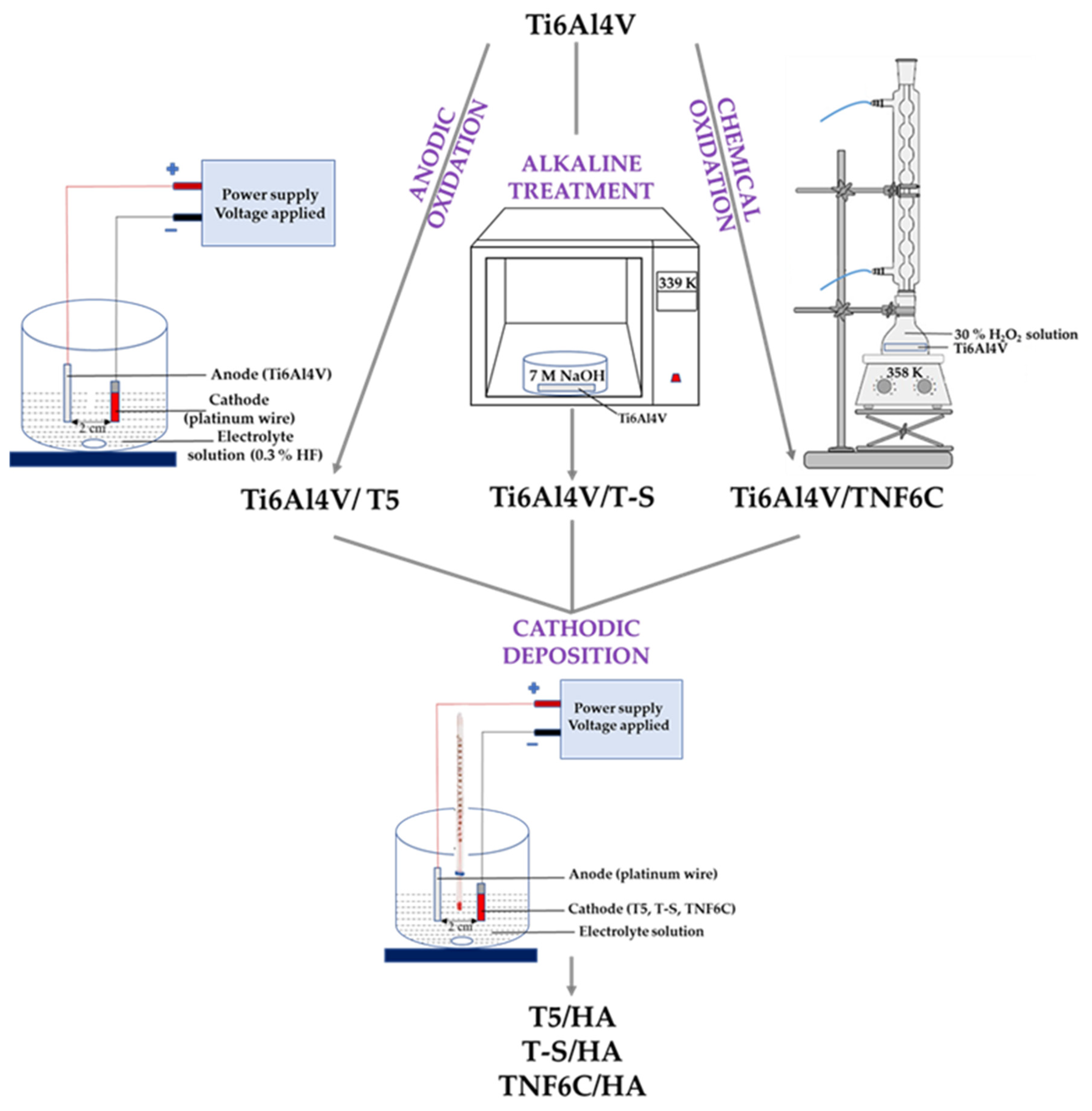
2.1. Synthesis of TiO2/HA and Titanate/HA Double-Layer Coating
2.2. SEM and Element Analysis
2.3. Apatite-Forming Ability
2.4. Cell Culture
2.5. Cell Proliferation Assay
2.6. Analysis of Cells Using Scanning Electron Microscopy
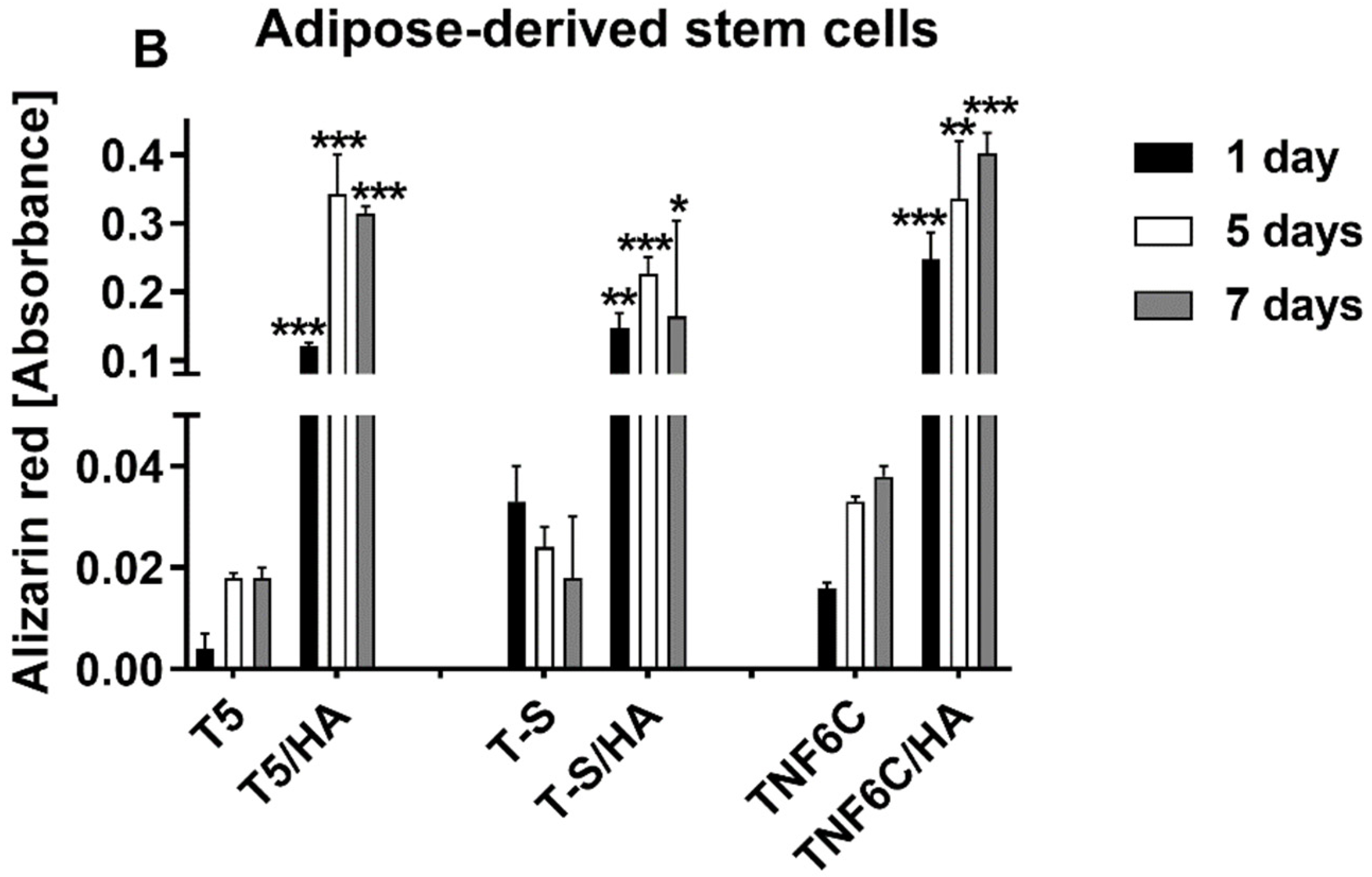
2.7. Alizarin Red S Staining
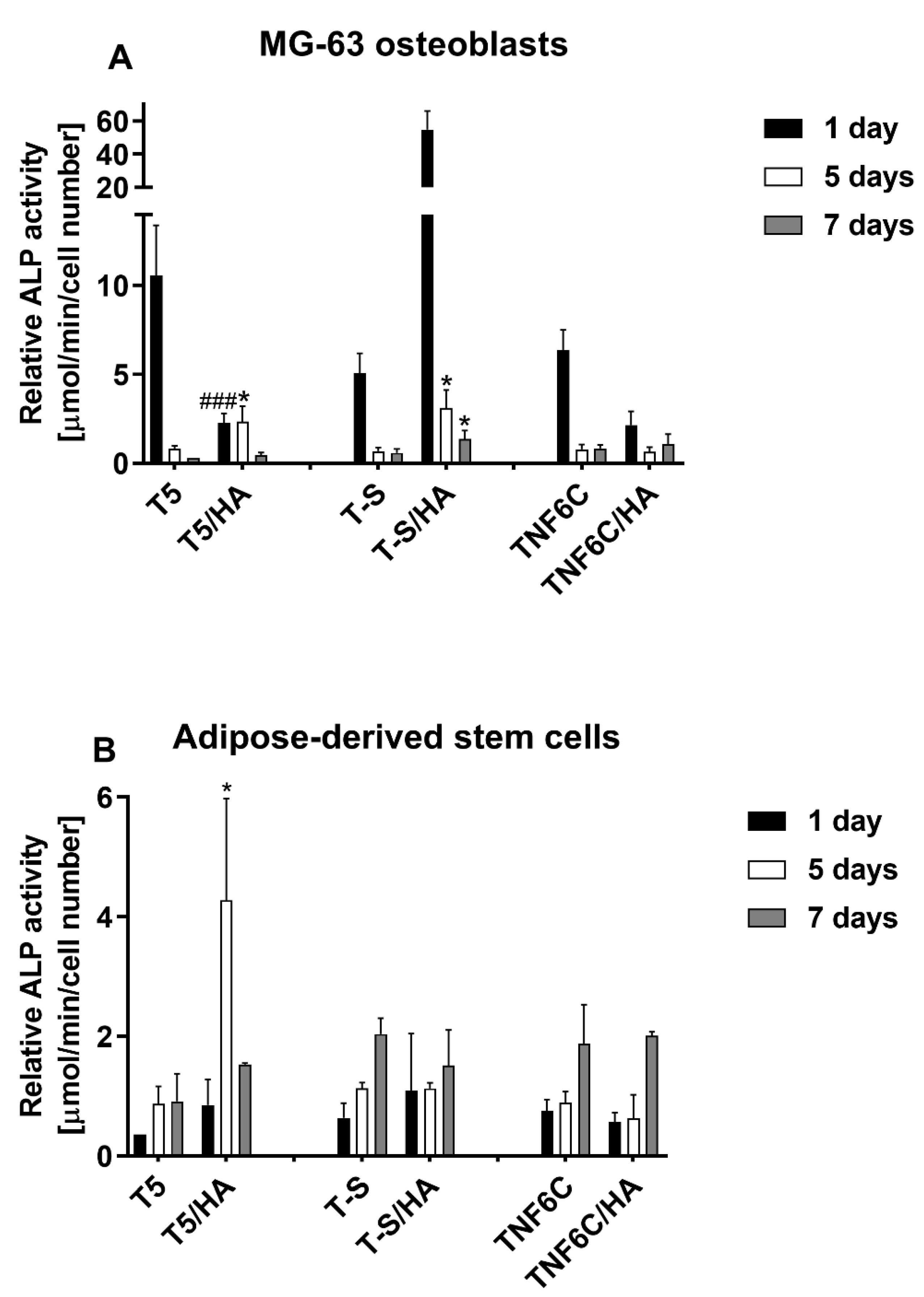
2.8. Alkaline Phosphatase (ALP) Activity
2.9. Antimicrobial Activity
2.10. Statistical Analysis
3. Results
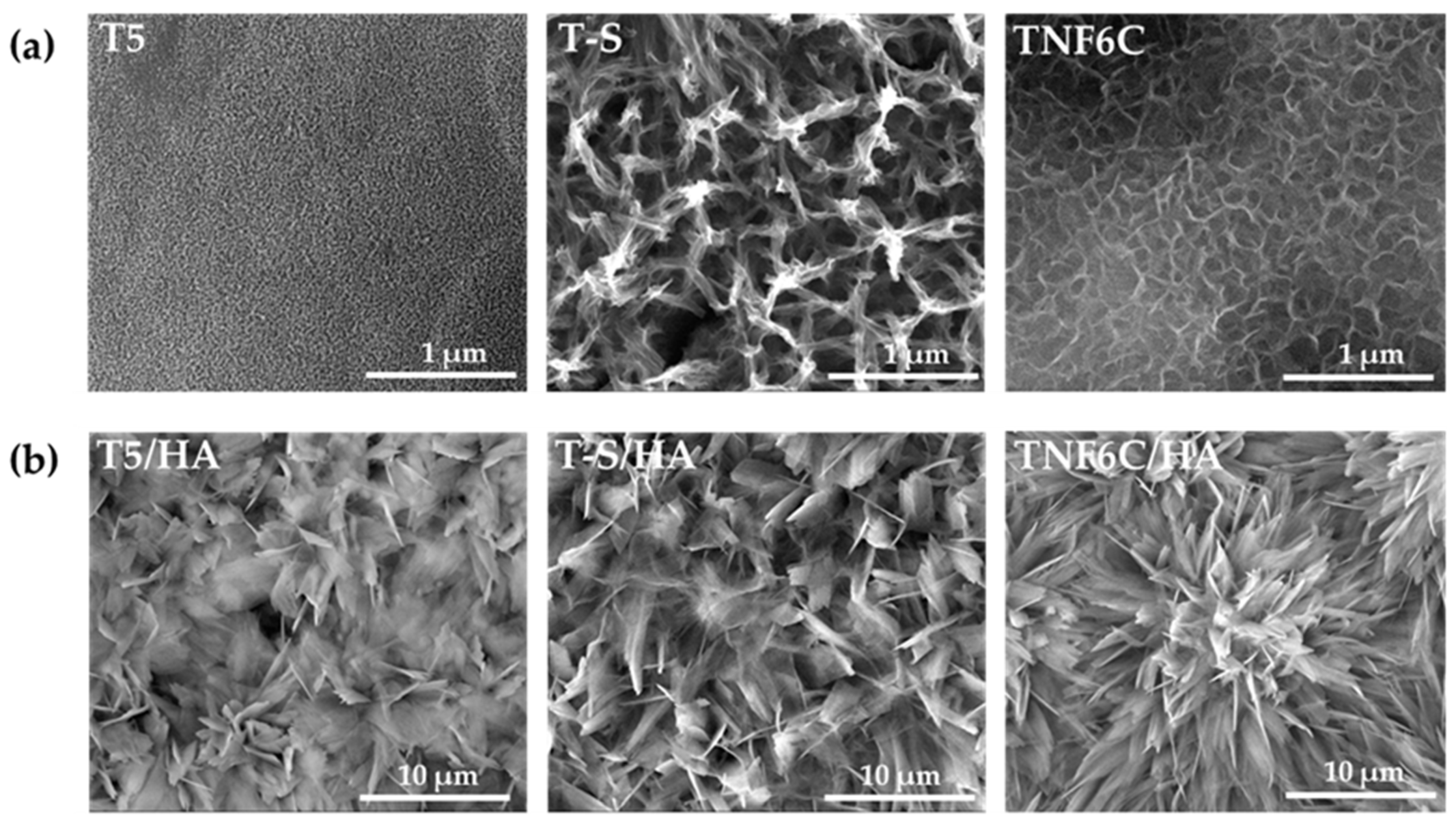
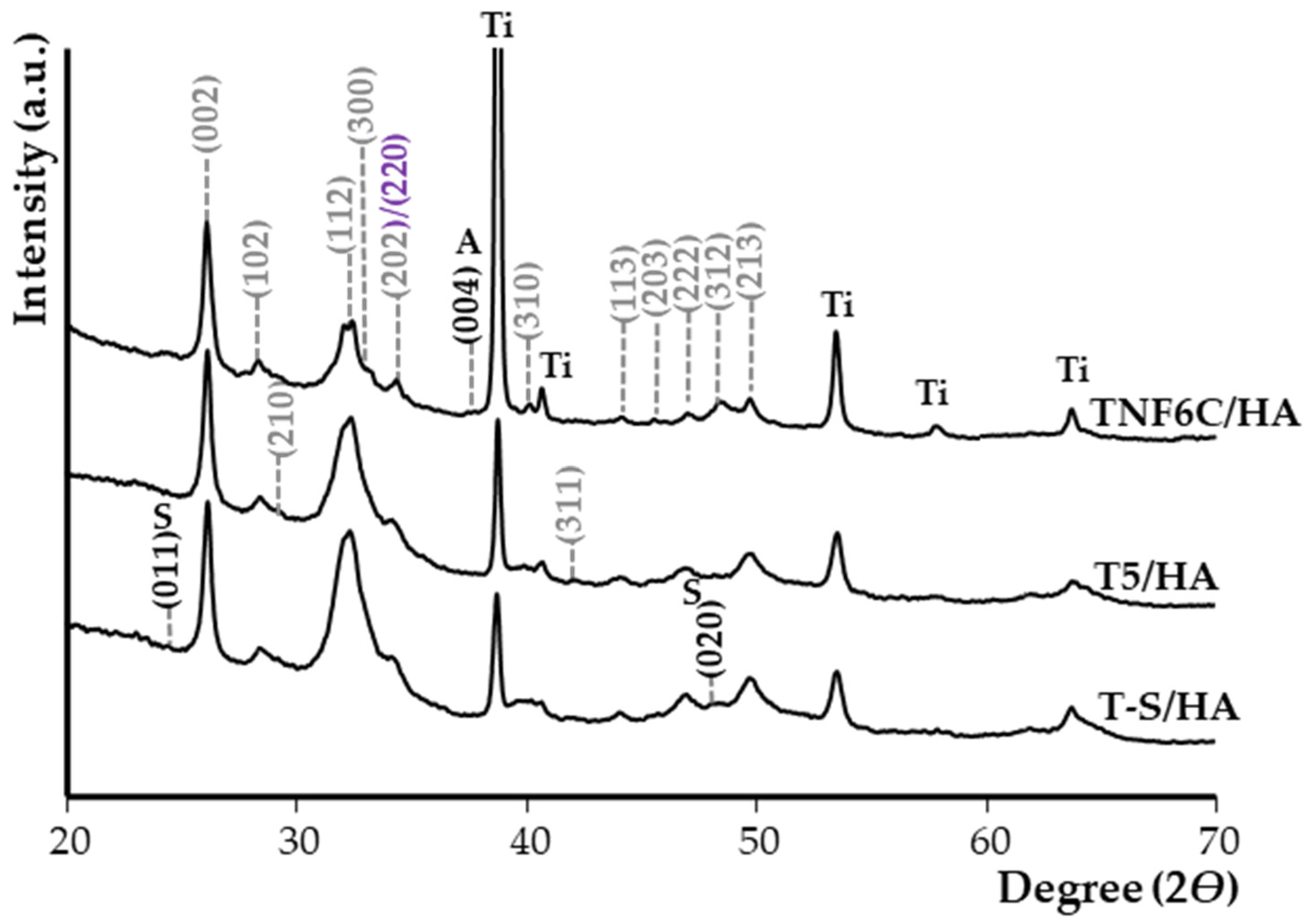
3.1. Surface Morphology of TiO2/HA and Titanate/HA Double-Layer Coating
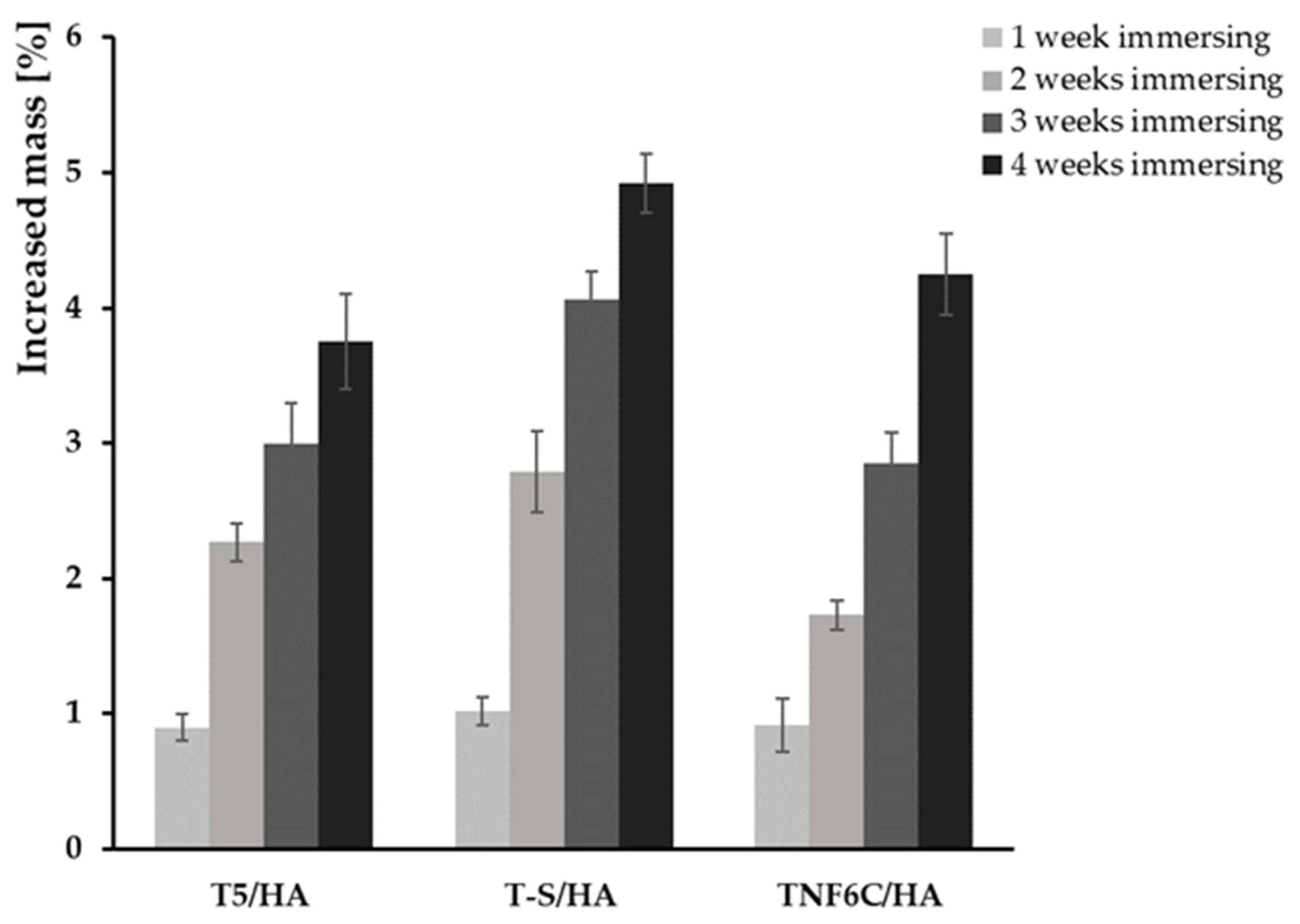
3.2. Electrochemical Cathodic Deposition of HA
3.3. Apatite-Forming Ability
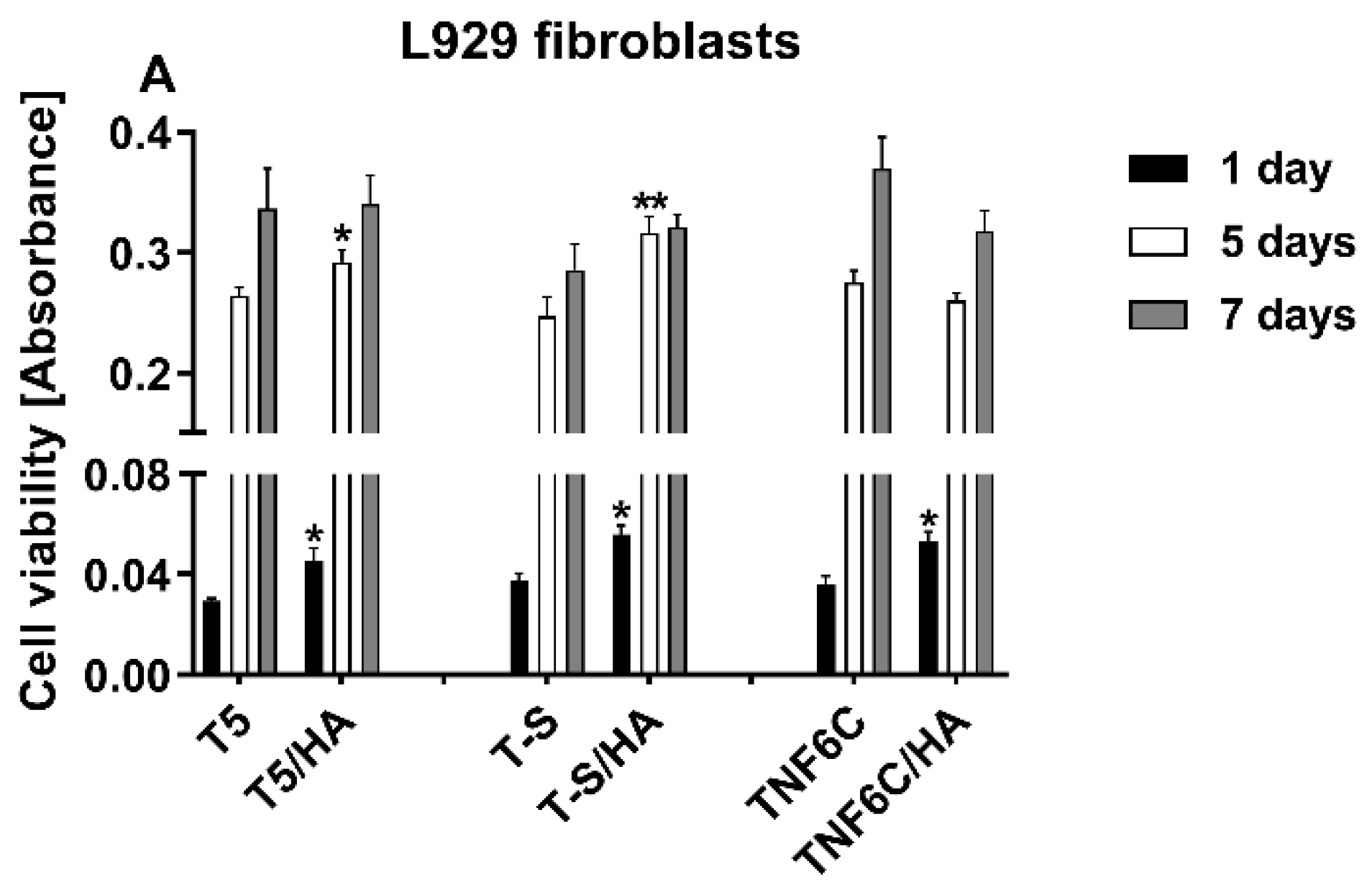
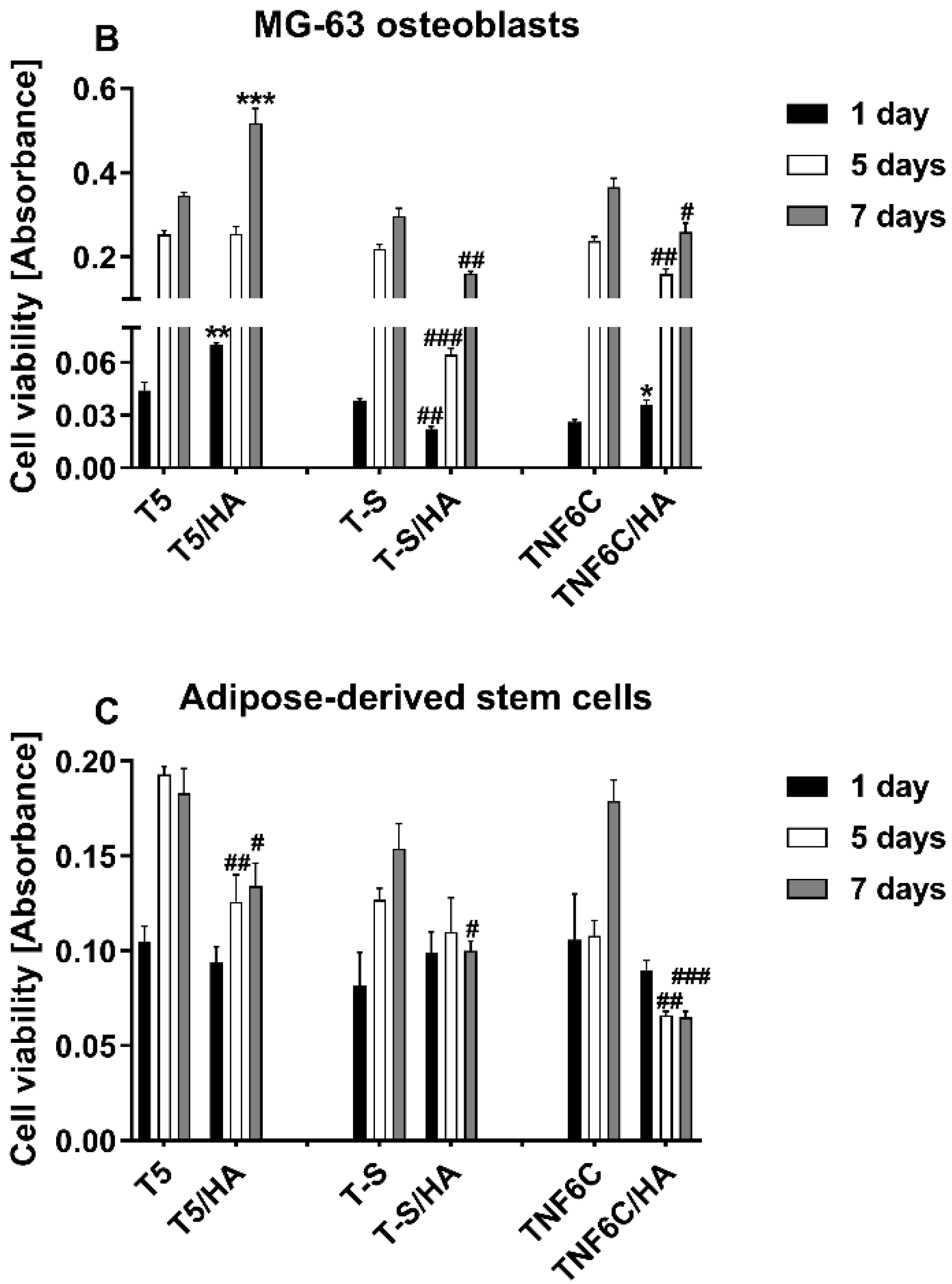
3.4. The Viability of Cells Cultured on the Scaffolds
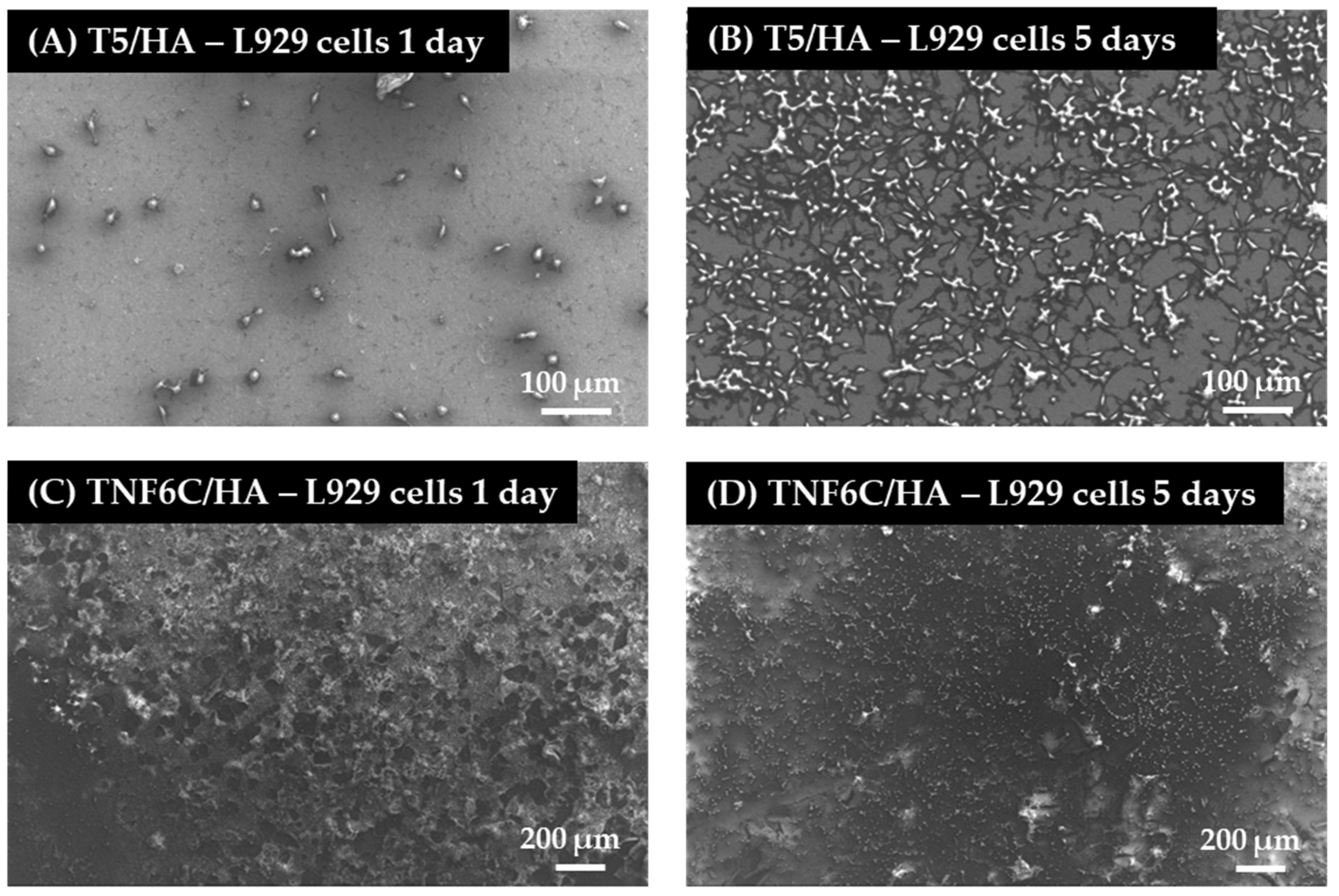
3.5. Cell Proliferation Rate Observed by Scanning Electron Microscopy
3.6. Osteogenic Potential of Cells Cultured on Different Specimens
3.7. Antimicrobial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jain, P.; Kathuria, H.; Dubey, N. Advances in 3D Bioprinting of Tissues/Organs for Regenerative Medicine and in-Vitro Models. Biomaterials 2022, 287, 121639. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Leong, K.W. Scaffolding in Tissue Engineering: General Approaches and Tissue-Specific Considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.L.; Atala, A.; Yoo, J.J. Tissue Engineering: Current Strategies and Future Directions. Chonnam Med. J. 2011, 47, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Todros, S.; Todesco, M.; Bagno, A. Biomaterials and Their Biomedical Applications: From Replacement to Regeneration. Processes 2021, 9, 1949. [Google Scholar] [CrossRef]
- Apablaza, J.A.; Días, F.J.; Sánchez, K.G.; Navarro, P.; Venegas, C.; Fuentes, R. Analysis of the Chemical Composition and Morphological Characterization of Tissue Osseointegrated to a Dental Implant after 5 Years of Function. Int. J. Mol. Sci. 2022, 23, 8882. [Google Scholar] [CrossRef]
- Aminov, L.; Sindilar, E.V.; Pasca, A.S.; Antohi, C.; Decolli, Y.; Stamatin, O.; Costin, L.I.; Bulancea, B.P.; Francu, L.; Mihalas, E.; et al. In Vivo Evaluation of Biocompatibility of Three Biomaterials Used in Endodontics for Prosthetic Purposes in Complex Rehabilitation Treatment. Appl. Sci. 2021, 11, 6519. [Google Scholar] [CrossRef]
- Rubežić, M.; Krstić, A.; Stanković, H.; Ljupković, R.; Ranđelović, M.; Zarubica, A. Different Types of Biomaterials: Structure and Application: A Short Review. Adv. Technol. 2020, 9, 69–79. [Google Scholar] [CrossRef]
- Cacopardo, L. Chapter 18-Biomaterials and Biocompatibility. In Human Orthopaedic Biomechanics; Innocenti, B., Galbusera, F., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 341–359. ISBN 978-0-12-824481-4. [Google Scholar]
- Matusiewicz, H. Potential Release of in Vivo Trace Metals from Metallic Medical Implants in the Human Body: From Ions to Nanoparticles–A Systematic Analytical Review. Acta Biomater. 2014, 10, 2379–2403. [Google Scholar] [CrossRef]
- Mantripragada, V.P.; Lecka-Czernik, B.; Ebraheim, N.A.; Jayasuriya, A.C. An Overview of Recent Advances in Designing Orthopedic and Craniofacial Implants. J. Biomed. Mater. Res. Part A 2013, 101, 3349–3364. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on Titanium and Titanium Based Alloys as Biomaterials for Orthopaedic Applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Warburton, A.; Girdler, S.J.; Mikhail, C.M.; Ahn, A.; Cho, S.K. Biomaterials in Spinal Implants: A Review. Neurospine 2020, 17, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeu, F.; Dourado, N.; Pereira, F.; Alves, N.; Miranda, G.; Silva, F.S. Additive Manufactured Porous Biomaterials Targeting Orthopedic Implants: A Suitable Combination of Mechanical, Physical and Topological Properties. Mater. Sci. Eng. C 2020, 107, 110342. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-M.; Liu, X. Advancing Biomaterials of Human Origin for Tissue Engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Gupta, H.; Tandan, A. Technical Complications of Implant-Causes and Management: A Comprehensive Review. Natl. J. Maxillofac. Surg. 2015, 6, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Apostu, D.; Lucaciu, O.; Berce, C.; Lucaciu, D.; Cosma, D. Current Methods of Preventing Aseptic Loosening and Improving Osseointegration of Titanium Implants in Cementless Total Hip Arthroplasty: A Review. J. Int. Med. Res. 2018, 46, 2104–2119. [Google Scholar] [CrossRef] [PubMed]
- Shayesteh Moghaddam, N.; Taheri Andani, M.; Amerinatanzi, A.; Haberland, C.; Huff, S.; Miller, M.; Elahinia, M.; Dean, D. Metals for Bone Implants: Safety, Design, and Efficacy. Biomanuf. Rev. 2016, 1, 1. [Google Scholar] [CrossRef]
- Radtke, A.; Ehlert, M.; Jędrzejewski, T.; Bartmański, M. The Morphology, Structure, Mechanical Properties and Biocompatibility of Nanotubular Titania Coatings before and after Autoclaving Process. J. Clin. Med. 2019, 8, 272. [Google Scholar] [CrossRef]
- Hofmann, S.; Stok, K.S.; Kohler, T.; Meinel, A.J.; Müller, R. Effect of Sterilization on Structural and Material Properties of 3-D Silk Fibroin Scaffolds. Acta Biomater. 2014, 10, 308–317. [Google Scholar] [CrossRef]
- Serro, A.P.; Saramago, B. Influence of Sterilization on the Mineralization of Titanium Implants Induced by Incubation in Various Biological Model Fluids. Biomaterials 2003, 24, 4749–4760. [Google Scholar] [CrossRef]
- Guo, T.; Oztug, N.A.K.; Han, P.; Ivanovski, S.; Gulati, K. Influence of Sterilization on the Performance of Anodized Nanoporous Titanium Implants. Mater. Sci. Eng. C 2021, 130, 112429. [Google Scholar] [CrossRef]
- Yamanoglu, R.; Bahador, A.; Kondoh, K. Fabrication Methods of Porous Titanium Implants by Powder Metallurgy. Trans. Indian Inst. Met. 2021, 74, 2555–2567. [Google Scholar] [CrossRef]
- Piszczek, P.; Radtke, A.; Ehlert, M.; Jędrzejewski, T.; Sznarkowska, A.; Sadowska, B.; Bartmański, M.; Erdoğan, Y.K.; Ercan, B.; Jędrzejczyk, W. Comprehensive Evaluation of the Biological Properties of Surface-Modified Titanium Alloy Implants. J. Clin. Med. 2020, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Grodzicka, M.; Ehlert, M.; Muzioł, T.M.; Szkodo, M.; Bartmański, M.; Piszczek, P. Studies on Silver Ions Releasing Processes and Mechanical Properties of Surface-Modified Titanium Alloy Implants. Int. J. Mol. Sci. 2018, 19, 3962. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Yoo, J.J.; Lee, S.J. Chapter 13-Biomaterials in Regenerative Medicine: Challenges in Technology Transfer from Science to Process Development. In Translational Regenerative Medicine; Atala, A., Allickson, J.G., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 151–167. ISBN 978-0-12-410396-2. [Google Scholar]
- Nouri, A.; Rohani Shirvan, A.; Li, Y.; Wen, C. Surface Modification of Additively Manufactured Metallic Biomaterials with Active Antipathogenic Properties. Smart Mater. Manuf. 2022, 1, 100001. [Google Scholar] [CrossRef]
- Kowalski, P.S.; Bhattacharya, C.; Afewerki, S.; Langer, R. Smart Biomaterials: Recent Advances and Future Directions. ACS Biomater. Sci. Eng. 2018, 4, 3809–3817. [Google Scholar] [CrossRef]
- Przekora, A. Current Trends in Fabrication of Biomaterials for Bone and Cartilage Regeneration: Materials Modifications and Biophysical Stimulations. Int. J. Mol. Sci. 2019, 20, 435. [Google Scholar] [CrossRef]
- Ehlert, M.; Roszek, K.; Jędrzejewski, T.; Bartmański, M.; Radtke, A. Titania Nanofiber Scaffolds with Enhanced Biointegration Activity—Preliminary In Vitro Studies. Int. J. Mol. Sci. 2019, 20, 5642. [Google Scholar] [CrossRef]
- Ehlert, M.; Radtke, A.; Bartmański, M.; Piszczek, P. Evaluation of the Cathodic Electrodeposition Effectiveness of the Hydroxyapatite Layer Used in Surface Modification of Ti6Al4V-Based Biomaterials. Materials 2022, 15, 6925. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Zhang, H.; Qiao, H.; Zhang, X.; Jia, T.; Han, S.; Gao, Y.; Xiao, H.; Yang, H. Fabrication of Silver- and Strontium-Doped Hydroxyapatite/TiO2 Nanotube Bilayer Coatings for Enhancing Bactericidal Effect and Osteoinductivity. Ceram. Int. 2017, 43, 992–1007. [Google Scholar] [CrossRef]
- Huang, Y.; Hao, M.; Nian, X.; Qiao, H.; Zhang, X.; Zhang, X.; Song, G.; Guo, J.; Pang, X.; Zhang, H. Strontium and Copper Co-Substituted Hydroxyapatite-Based Coatings with Improved Antibacterial Activity and Cytocompatibility Fabricated by Electrodeposition. Ceram. Int. 2016, 42, 11876–11888. [Google Scholar] [CrossRef]
- Cheng, H.; Xiong, W.; Fang, Z.; Guan, H.; Wu, W.; Li, Y.; Zhang, Y.; Alvarez, M.M.; Gao, B.; Huo, K.; et al. Strontium (Sr) and Silver (Ag) Loaded Nanotubular Structures with Combined Osteoinductive and Antimicrobial Activities. Acta Biomater. 2016, 31, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Cockerill, I.; Wang, Y.; Qin, Y.-X.; Chang, L.; Zheng, Y.; Zhu, D. Zinc-Based Biomaterials for Regeneration and Therapy. Trends Biotechnol. 2019, 37, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Ahangarani, S.; Esmailian, M.; Shanaghi, A. Investigation on the Corrosion Behavior and Biocompatibility of Ti-6Al-4V Implant Coated with HA/TiN Dual Layer for Medical Applications. Surf. Coat. Technol. 2020, 397, 126044. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, G.; Jiao Li, J. Advances in Implant Surface Modifications to Improve Osseointegration. Mater. Adv. 2021, 2, 6901–6927. [Google Scholar] [CrossRef]
- Ren, B.; Wan, Y.; Liu, C.; Wang, H.; Yu, M.; Zhang, X.; Huang, Y. Improved Osseointegration of 3D Printed Ti-6Al-4V Implant with a Hierarchical Micro/Nano Surface Topography: An in Vitro and in Vivo Study. Mater. Sci. Eng. C 2021, 118, 111505. [Google Scholar] [CrossRef]
- Radtke, A.; Ehlert, M.; Jędrzejewski, T.; Sadowska, B.; Więckowska-Szakiel, M.; Holopainen, J.; Ritala, M.; Leskelä, M.; Bartmański, M.; Szkodo, M.; et al. Titania Nanotubes/Hydroxyapatite Nanocomposites Produced with the Use of the Atomic Layer Deposition Technique: Estimation of Bioactivity and Nanomechanical Properties. Nanomaterials 2019, 9, 123. [Google Scholar] [CrossRef]
- Koju, N.; Niraula, S.; Fotovvati, B. Additively Manufactured Porous Ti6Al4V for Bone Implants: A Review. Metals 2022, 12, 687. [Google Scholar] [CrossRef]
- Brizuela, A.; Herrero-Climent, M.; Rios-Carrasco, E.; Rios-Santos, J.; Pérez, R.; Manero, J.; Gil Mur, J. Influence of the Elastic Modulus on the Osseointegration of Dental Implants. Materials 2019, 12, 980. [Google Scholar] [CrossRef]
- Bittredge, O.; Hassanin, H.; El-Sayed, M.A.; Eldessouky, H.M.; Alsaleh, N.A.; Alrasheedi, N.H.; Essa, K.; Ahmadein, M. Fabrication and Optimisation of Ti-6Al-4V Lattice-Structured Total Shoulder Implants Using Laser Additive Manufacturing. Materials 2022, 15, 3095. [Google Scholar] [CrossRef]
- Shibata, Y.; Tanimoto, Y.; Maruyama, N.; Nagakura, M. A Review of Improved Fixation Methods for Dental Implants. Part II: Biomechanical Integrity at Bone–Implant Interface. J. Prosthodont. Res. 2015, 59, 84–95. [Google Scholar] [CrossRef]
- Ehlert, M.; Radtke, A.; Roszek, K.; Jędrzejewski, T.; Piszczek, P. Assessment of Titanate Nanolayers in Terms of Their Physicochemical and Biological Properties. Materials 2021, 14, 806. [Google Scholar] [CrossRef] [PubMed]
- Ehlert, M.; Radtke, A.; Jędrzejewski, T.; Roszek, K.; Bartmański, M.; Piszczek, P. In Vitro Studies on Nanoporous, Nanotubular and Nanosponge-Like Titania Coatings, with the Use of Adipose-Derived Stem Cells. Materials 2020, 13, 1574. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-Scale Modification of Titanium Implant Surfaces to Enhance Osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Srikant, P.V.S.; Manna, I.; Chatterjee, U.K.; Dutta Majumdar, J. Chemical Oxidation of Ti–6Al–4V for Improved Wear and Corrosion Resistance. Surf. Eng. 2008, 24, 442–446. [Google Scholar] [CrossRef]
- Carradò, A.; Perrin-Schmitt, F.; Le, Q.V.; Giraudel, M.; Fischer, C.; Koenig, G.; Jacomine, L.; Behr, L.; Chalom, A.; Fiette, L.; et al. Nanoporous Hydroxyapatite/Sodium Titanate Bilayer on Titanium Implants for Improved Osteointegration. Dent. Mater. 2017, 33, 321–332. [Google Scholar] [CrossRef]
- Kokubo, T.; Yamaguchi, S. Novel Bioactive Titanate Layers Formed on Ti Metal and Its Alloys by Chemical Treatments. Materials 2009, 3, 48–63. [Google Scholar] [CrossRef]
- Gulati, K.; Maher, S.; Findlay, D.M.; Losic, D. Titania Nanotubes for Orchestrating Osteogenesis at the Bone–Implant Interface. Nanomedicine 2016, 11, 1847–1864. [Google Scholar] [CrossRef]
- Tan, A.W.; Pingguan-Murphy, B.; Ahmad, R.; Akbar, S.A. Review of Titania Nanotubes: Fabrication and Cellular Response. Ceram. Int. 2012, 38, 4421–4435. [Google Scholar] [CrossRef]
- Safavi, M.S.; Walsh, F.C.; Surmeneva, M.A.; Surmenev, R.A.; Khalil-Allafi, J. Electrodeposited Hydroxyapatite-Based Biocoatings: Recent Progress and Future Challenges. Coatings 2021, 11, 110. [Google Scholar] [CrossRef]
- Du, M.; Chen, J.; Liu, K.; Xing, H.; Song, C. Recent Advances in Biomedical Engineering of Nano-Hydroxyapatite Including Dentistry, Cancer Treatment and Bone Repair. Compos. Part B Eng. 2021, 215, 108790. [Google Scholar] [CrossRef]
- Fitzpatrick, V.; Martín-Moldes, Z.; Deck, A.; Torres-Sanchez, R.; Valat, A.; Cairns, D.; Li, C.; Kaplan, D.L. Functionalized 3D-Printed Silk-Hydroxyapatite Scaffolds for Enhanced Bone Regeneration with Innervation and Vascularization. Biomaterials 2021, 276, 120995. [Google Scholar] [CrossRef] [PubMed]
- Farnoush, H.; Aghazadeh Mohandesi, J.; Haghshenas Fatmehsari, D.; Moztarzadeh, F. Modification of Electrophoretically Deposited Nano-Hydroxyapatite Coatings by Wire Brushing on Ti–6Al–4V Substrates. Ceram. Int. 2012, 38, 4885–4893. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Z.; Li, W.; Fan, Y.; Li, Z.; Wei, J. Hydroxyapatite Based Materials for Bone Tissue Engineering: A Brief and Comprehensive Introduction. Crystals 2021, 11, 149. [Google Scholar] [CrossRef]
- Thomas, K.A. Hydroxyapatite Coatings. Orthopedics 1994, 17, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-C.; Chen, H.-T.; Shih, W.-J.; Chang, H.-F.; Hon, M.-H.; Hung, I.-M. Crystalline Size, Microstructure and Biocompatibility of Hydroxyapatite Nanopowders by Hydrolysis of Calcium Hydrogen Phosphate Dehydrate (DCPD). Ceram. Int. 2015, 41, 2999–3008. [Google Scholar] [CrossRef]
- Vranceanu, D.M.; Ungureanu, E.; Ionescu, I.C.; Parau, A.C.; Kiss, A.E.; Vladescu, A.; Cotrut, C.M. Electrochemical Surface Biofunctionalization of Titanium through Growth of TiO2 Nanotubes and Deposition of Zn Doped Hydroxyapatite. Coatings 2022, 12, 69. [Google Scholar] [CrossRef]
- Stocco, T.D.; Rodrigues, P.J.G.; de Almeida Filho, M.A.; Lobo, A.O. Nanohydroxyapatite Electrodeposition onto Electrospun Nanofibers: Technique Overview and Tissue Engineering Applications. Bioengineering 2021, 8, 151. [Google Scholar] [CrossRef]
- Alonso-Goulart, V.; Carvalho, L.N.; Marinho, A.L.G.; de Oliveira Souza, B.L.; de Aquino Pinto Palis, G.; Lage, H.G.D.; de Lima, I.L.; Guimarães, L.D.; Peres, L.C.; Silveira, M.M.; et al. Biomaterials and Adipose-Derived Mesenchymal Stem Cells for Regenerative Medicine: A Systematic Review. Materials 2021, 14, 4641. [Google Scholar] [CrossRef]
- Liu, T.; Xu, J.; Pan, X.; Ding, Z.; Xie, H.; Wang, X.; Xie, H. Advances of Adipose-Derived Mesenchymal Stem Cells-Based Biomaterial Scaffolds for Oral and Maxillofacial Tissue Engineering. Bioact. Mater. 2021, 6, 2467–2478. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, Z.; Zhang, H.; Lu, W.; Liu, S.; Huang, X.; Luo, H.; Jin, Y. Expansion and Delivery of Adipose-Derived Mesenchymal Stem Cells on Three Microcarriers for Soft Tissue Regeneration. Tissue Eng. Part A 2011, 17, 2981–2997. [Google Scholar] [CrossRef]
- Merceron, C.; Vinatier, C.; Clouet, J.; Colliec-Jouault, S.; Weiss, P.; Guicheux, J. Adipose-Derived Mesenchymal Stem Cells and Biomaterials for Cartilage Tissue Engineering. Jt. Bone Spine 2008, 75, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Tuli, R.; Huang, X.; Laquerriere, P.; Tuan, R.S. Multilineage Differentiation of Human Mesenchymal Stem Cells in a Three-Dimensional Nanofibrous Scaffold. Biomaterials 2005, 26, 5158–5166. [Google Scholar] [CrossRef] [PubMed]
- Kobolak, J.; Dinnyes, A.; Memic, A.; Khademhosseini, A.; Mobasheri, A. Mesenchymal Stem Cells: Identification, Phenotypic Characterization, Biological Properties and Potential for Regenerative Medicine through Biomaterial Micro-Engineering of Their Niche. Methods 2016, 99, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Zuo, Y.; Li, J.; Ma, S.; Cheng, L. Biocompatibility and Osteogenesis of Biomimetic Nano-Hydroxyapatite/Polyamide Composite Scaffolds for Bone Tissue Engineering. Biomaterials 2007, 28, 3338–3348. [Google Scholar] [CrossRef] [PubMed]
- Barry, F.P.; Murphy, J.M. Mesenchymal Stem Cells: Clinical Applications and Biological Characterization. Int. J. Biochem. Cell Biol. 2004, 36, 568–584. [Google Scholar] [CrossRef]
- Pountos, I.; Giannoudis, P.V. Biology of Mesenchymal Stem Cells. Injury 2005, 36, S8–S12. [Google Scholar] [CrossRef]
- Kamat, P.; Frueh, F.S.; McLuckie, M.; Sanchez-Macedo, N.; Wolint, P.; Lindenblatt, N.; Plock, J.A.; Calcagni, M.; Buschmann, J. Adipose Tissue and the Vascularization of Biomaterials: Stem Cells, Microvascular Fragments and Nanofat—A Review. Cytotherapy 2020, 22, 400–411. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Systematic Review: Adipose-Derived Mesenchymal Stem Cells, Platelet-Rich Plasma and Biomaterials as New Regenerative Strategies in Chronic Skin Wounds and Soft Tissue Defects. Int. J. Mol. Sci. 2021, 22, 1538. [Google Scholar] [CrossRef]
- Walker, J.; Flynn, L. Chapter 15-Biomaterial Control of Adipose-Derived Stem/Stromal Cell Differentiation. In Scientific Principles of Adipose Stem Cells; Kokai, L., Marra, K., Rubin, J.P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 313–346. ISBN 978-0-12-819376-1. [Google Scholar]
- Trubiani, O.; Marconi, G.D.; Pierdomenico, S.D.; Piattelli, A.; Diomede, F.; Pizzicannella, J. Human Oral Stem Cells, Biomaterials and Extracellular Vesicles: A Promising Tool in Bone Tissue Repair. Int. J. Mol. Sci. 2019, 20, 4987. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How Useful Is SBF in Predicting in Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- ISO/FDIS 23317:2007(E) Standards; Implants for Surgery—In Vitro Evaluation for Apatite-Forming Ability of Implant Materials. ISO: Geneva, Switzerland, 2007. Available online: https://www.iso.org/standard/41446.html (accessed on 5 March 2019).
- Yen, S.K.; Lin, C.M. Cathodic Reactions of Electrolytic Hydroxyapatite Coating on Pure Titanium. Mater. Chem. Phys. 2003, 77, 70–76. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, J.; Pang, Y.; Wang, W.; Wang, T. Electrochemical Deposition of Hydroxyapatite Coatings on Titanium. Trans. Nonferrous Met. Soc. China 2006, 16, 633–637. [Google Scholar] [CrossRef]
- Drevet, R.; Benhayoune, H. Electrodeposition of Calcium Phosphate Coatings on Metallic Substrates for Bone Implant Applications: A Review. Coatings 2022, 12, 539. [Google Scholar] [CrossRef]
- Casagrande, T.; Lawson, G.; Li, H.; Wei, J.; Adronov, A.; Zhitomirsky, I. Electrodeposition of Composite Materials Containing Functionalized Carbon Nanotubes. Mater. Chem. Phys. 2008, 111, 42–49. [Google Scholar] [CrossRef]
- Narayanan, R.; Kwon, T.-Y.; Kim, K.-H. Direct Nanocrystalline Hydroxyapatite Formation on Titanium from Ultrasonated Electrochemical Bath at Physiological PH. Mater. Sci. Eng. C 2008, 28, 1265–1270. [Google Scholar] [CrossRef]
- Song, Y.W.; Shan, D.Y.; Han, E.H. Electrodeposition of Hydroxyapatite Coating on AZ91D Magnesium Alloy for Biomaterial Application. Mater. Lett. 2008, 62, 3276–3279. [Google Scholar] [CrossRef]
- Parcharoen, Y.; Kajitvichyanukul, P.; Sirivisoot, S.; Termsuksawad, P. Hydroxyapatite Electrodeposition on Anodized Titanium Nanotubes for Orthopedic Applications. Appl. Surf. Sci. 2014, 311, 54–61. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, J.; Wang, L.; He, P.; Wang, T. HA Coating on Titanium with Nanotubular Anodized TiO2 Intermediate Layer via Electrochemical Deposition. Trans. Nonferrous Met. Soc. China 2008, 18, 631–635. [Google Scholar] [CrossRef]
- Wei, D.; Zhou, Y.; Yang, C. Characteristic, Cell Response and Apatite-Induction Ability of Microarc Oxidized TiO2-Based Coating Containing P on Ti6Al4V before and after Chemical-Treatment and Dehydration. Ceram. Int. 2009, 35, 2545–2554. [Google Scholar] [CrossRef]
- Cao, J.; Lian, R.; Jiang, X. Magnesium and Fluoride Doped Hydroxyapatite Coatings Grown by Pulsed Laser Deposition for Promoting Titanium Implant Cytocompatibility. Appl. Surf. Sci. 2020, 515, 146069. [Google Scholar] [CrossRef]
- Wandiyanto, J.V.; Truong, V.K.; Al Kobaisi, M.; Juodkazis, S.; Thissen, H.; Bazaka, O.; Bazaka, K.; Crawford, R.J.; Ivanova, E.P. The Fate of Osteoblast-Like MG-63 Cells on Pre-Infected Bactericidal Nanostructured Titanium Surfaces. Materials 2019, 12, 1575. [Google Scholar] [CrossRef] [PubMed]
- Thibault, R.A.; Scott Baggett, L.; Mikos, A.G.; Kasper, F.K. Osteogenic Differentiation of Mesenchymal Stem Cells on Pregenerated Extracellular Matrix Scaffolds in the Absence of Osteogenic Cell Culture Supplements. Tissue Eng Part A 2010, 16, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Bloise, N.; Waldorff, E.I.; Montagna, G.; Bruni, G.; Fassina, L.; Fang, S.; Zhang, N.; Jiang, J.; Ryaby, J.T.; Visai, L. Early Osteogenic Marker Expression in HMSCs Cultured onto Acid Etching-Derived Micro- and Nanotopography 3D-Printed Titanium Surfaces. Int. J. Mol. Sci. 2022, 23, 7070. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.-C.; Yue, B. Approaches to Promoting Bone Marrow Mesenchymal Stem Cell Osteogenesis on Orthopedic Implant Surface. World J. Stem Cells 2020, 12, 545–561. [Google Scholar] [CrossRef]
- Le, J.; Zhongqun, L.; Zhaoyan, W.; Yijun, S.; Yingjin, W.; Yaojie, W.; Yanan, J.; Zhanrong, J.; Chunyang, M.; Fangli, G.; et al. Development of Methods for Detecting the Fate of Mesenchymal Stem Cells Regulated by Bone Bioactive Materials. Bioact. Mater. 2021, 6, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Wang, H.; Wang, W.; Tong, L.; Pan, H.; Ruan, C.; Ma, Q.; Liu, M.; Yang, H.; Zhang, L.; et al. Antibacterial Effects and Biocompatibility of Titanium Surfaces with Graded Silver Incorporation in Titania Nanotubes. Biomaterials 2014, 35, 4255–4265. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, M.; Oda, Y.; Kato, T.; Okuda, K. Influence of Surface Modifications to Titanium on Antibacterial Activity in Vitro. Biomaterials 2001, 22, 2043–2048. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Y.; Jiang, W.; Bai, H.; Liu, H.; Wang, J. In Vivo Antibacterial Efficacy of Nanopatterns on Titanium Implant Surface: A Systematic Review of the Literature. Antibiotics 2021, 10, 1524. [Google Scholar] [CrossRef]
- Das, K.; Bose, S.; Bandyopadhyay, A.; Karandikar, B.; Gibbins, B.L. Surface Coatings for Improvement of Bone Cell Materials and Antimicrobial Activities of Ti Implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87B, 455–460. [Google Scholar] [CrossRef]
- Li, B.; Xia, X.; Guo, M.; Jiang, Y.; Li, Y.; Zhang, Z.; Liu, S.; Li, H.; Liang, C.; Wang, H. Biological and Antibacterial Properties of the Micro-Nanostructured Hydroxyapatite/Chitosan Coating on Titanium. Sci. Rep. 2019, 9, 14052. [Google Scholar] [CrossRef]
- Karunakaran, G.; Cho, E.-B.; Kumar, G.S.; Kolesnikov, E.; Janarthanan, G.; Pillai, M.M.; Rajendran, S.; Boobalan, S.; Sudha, K.G.; Rajeshkumar, M.P. Mesoporous Mg-Doped Hydroxyapatite Nanorods Prepared from Bio-Waste Blue Mussel Shells for Implant Applications. Ceram. Int. 2020, 46, 28514–28527. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Bandyopadhyay, A.; Bose, S. Plasma Sprayed Fluoride and Zinc Doped Hydroxyapatite Coated Titanium for Load-Bearing Implants. Surf. Coat. Technol. 2022, 440, 128464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, H.; Xiao, L.; Li, Z.; Ma, C.; Xu, W.; Wang, Y. Chitosan Regulated Electrochemistry for Dense Hydroxyapatite/MgO Nanocomposite Coating with Antibiosis and Osteogenesis on Titanium Alloy. Colloid Interface Sci. Commun. 2022, 48, 100616. [Google Scholar] [CrossRef]
- Kumar, A.; Nune, K.C.; Misra, R.D.K. Biological Functionality of Extracellular Matrix-Ornamented Three-Dimensional Printed Hydroxyapatite Scaffolds. J. Biomed. Mater. Res. Part A 2016, 104, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Thein-Han, W.W.; Misra, R.D.K. Three-Dimensional Chitosan-Nanohydroxyapatite Composite Scaffolds for Bone Tissue Engineering. JOM 2009, 61, 41–44. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium Phosphate-Based Osteoinductive Materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef]
- Gu, Y.-X.; Du, J.; Zhao, J.-M.; Si, M.-S.; Mo, J.-J.; Lai, H.-C. Characterization and Preosteoblastic Behavior of Hydroxyapatite-Deposited Nanotube Surface of Titanium Prepared by Anodization Coupled with Alternative Immersion Method. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 2122–2130. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, K.; Que, K.; Hou, S.; Chen, Z.; Li, Y.; Wang, Y.; Song, Y.; Guan, B.; Zhang, W.; et al. Surface Modification of Titanium with Hydroxyapatite Layer Induced by Phase-Transited Lysozyme Coating. Mater. Sci. Eng. C 2018, 92, 206–215. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, Y.; Hu, R.; Wang, X.; Lai, Y.; Rui, G.; Lin, C. Hydroxyapatite-Modified Micro/Nanostructured Titania Surfaces with Different Crystalline Phases for Osteoblast Regulation. Bioact. Mater. 2021, 6, 1118–1129. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, W.; Zhang, X.; Liu, X.; Xu, Z.; Han, S.; Su, Z.; Liu, H.; Gao, Y.; Yang, H. A Prospective Material for Orthopedic Applications: Ti Substrates Coated with a Composite Coating of a Titania-Nanotubes Layer and a Silver-Manganese-Doped Hydroxyapatite Layer. Ceram. Int. 2018, 44, 5528–5542. [Google Scholar] [CrossRef]
- Mo, A.; Liao, J.; Xu, W.; Xian, S.; Li, Y.; Bai, S. Preparation and Antibacterial Effect of Silver–Hydroxyapatite/Titania Nanocomposite Thin Film on Titanium. Appl. Surf. Sci. 2008, 255, 435–438. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, Z.; Zhang, X.; Chang, X.; Zhang, X.; Li, Y.; Ye, T.; Han, R.; Han, S.; Gao, Y.; et al. Nanotube-Formed Ti Substrates Coated with Silicate/Silver Co-Doped Hydroxyapatite as Prospective Materials for Bone Implants. J. Alloy. Compd. 2017, 697, 182–199. [Google Scholar] [CrossRef]
- Trujillo, N.A.; Floreani, R.; Ma, H.; Bryers, J.D.; Williams, J.D.; Popat, K.C. Antibacterial Effects of Silver-Doped Hydroxyapatite Thin Films Sputter Deposited on Titanium. Mater. Sci. Eng. C 2012, 32, 2135–2144. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, B.; Huang, C.; Gao, S.; Li, B.; Cao, R.; Cheng, J.; Li, R.; Yu, Z.; Xie, X. Biocompatibility and Antibacterial Properties of Pure Titanium Surfaces Coated with Yttrium-Doped Hydroxyapatite. J. Mech. Behav. Biomed. Mater. 2019, 100, 103363. [Google Scholar] [CrossRef]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial Coatings on Titanium Implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91B, 470–480. [Google Scholar] [CrossRef]





| Ca/P (Mole Ratio) of HA Layer | |||||
|---|---|---|---|---|---|
| Sample | Time: | Time: | Time: | Time: | Time: |
| 0 | 1 week | 2 weeks | 3 weeks | 4 weeks | |
| T5/HA | 1.58 | 1.54 | 1.63 | 1.68 | 1.87 |
| T-S/HA | 1.69 | 1.56 | 1.61 | 1.80 | 1.83 |
| TNF6C/HA | 1.76 | 1.66 | 1.57 | 1.56 | 1.83 |
| Material | Microorganisms | ||||
|---|---|---|---|---|---|
| E. coli ATCC 8739 | E. coli ATCC 25922 | S. aureus ATCC 6538 | S. aureus ATCC 25923 | C. albicans ATCC 10231 | |
| Reduction index (R) | |||||
| T5/HA | 0.34 * | 0.38 * | 0.04 | 1.73 | 0.08 |
| T-S/HA | 0.14 * | 0.25 * | 1.28 | 1.90 | 0.25 |
| TNF6C/HA | 0.43 * | 0.37 * | 0.06 | 0.18 * | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehlert, M.; Radtke, A.; Forbot, N.; Jędrzejewski, T.; Roszek, K.; Golińska, P.; Trykowski, G.; Piszczek, P. TiO2/HA and Titanate/HA Double-Layer Coatings on Ti6Al4V Surface and Their Influence on In Vitro Cell Growth and Osteogenic Potential. J. Funct. Biomater. 2022, 13, 271. https://doi.org/10.3390/jfb13040271
Ehlert M, Radtke A, Forbot N, Jędrzejewski T, Roszek K, Golińska P, Trykowski G, Piszczek P. TiO2/HA and Titanate/HA Double-Layer Coatings on Ti6Al4V Surface and Their Influence on In Vitro Cell Growth and Osteogenic Potential. Journal of Functional Biomaterials. 2022; 13(4):271. https://doi.org/10.3390/jfb13040271
Chicago/Turabian StyleEhlert, Michalina, Aleksandra Radtke, Natalia Forbot, Tomasz Jędrzejewski, Katarzyna Roszek, Patrycja Golińska, Grzegorz Trykowski, and Piotr Piszczek. 2022. "TiO2/HA and Titanate/HA Double-Layer Coatings on Ti6Al4V Surface and Their Influence on In Vitro Cell Growth and Osteogenic Potential" Journal of Functional Biomaterials 13, no. 4: 271. https://doi.org/10.3390/jfb13040271
APA StyleEhlert, M., Radtke, A., Forbot, N., Jędrzejewski, T., Roszek, K., Golińska, P., Trykowski, G., & Piszczek, P. (2022). TiO2/HA and Titanate/HA Double-Layer Coatings on Ti6Al4V Surface and Their Influence on In Vitro Cell Growth and Osteogenic Potential. Journal of Functional Biomaterials, 13(4), 271. https://doi.org/10.3390/jfb13040271