Optimizing Covalent Immobilization of Glucose Oxidase and Laccase on PV15 Fluoropolymer-Based Bioelectrodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Bioanode Preparation: Covalent Immobilization of Glucose Oxidase (GOx)
2.2.2. Biocathode Preparation: Covalent Immobilization of Laccase
2.2.3. SEM Analysis
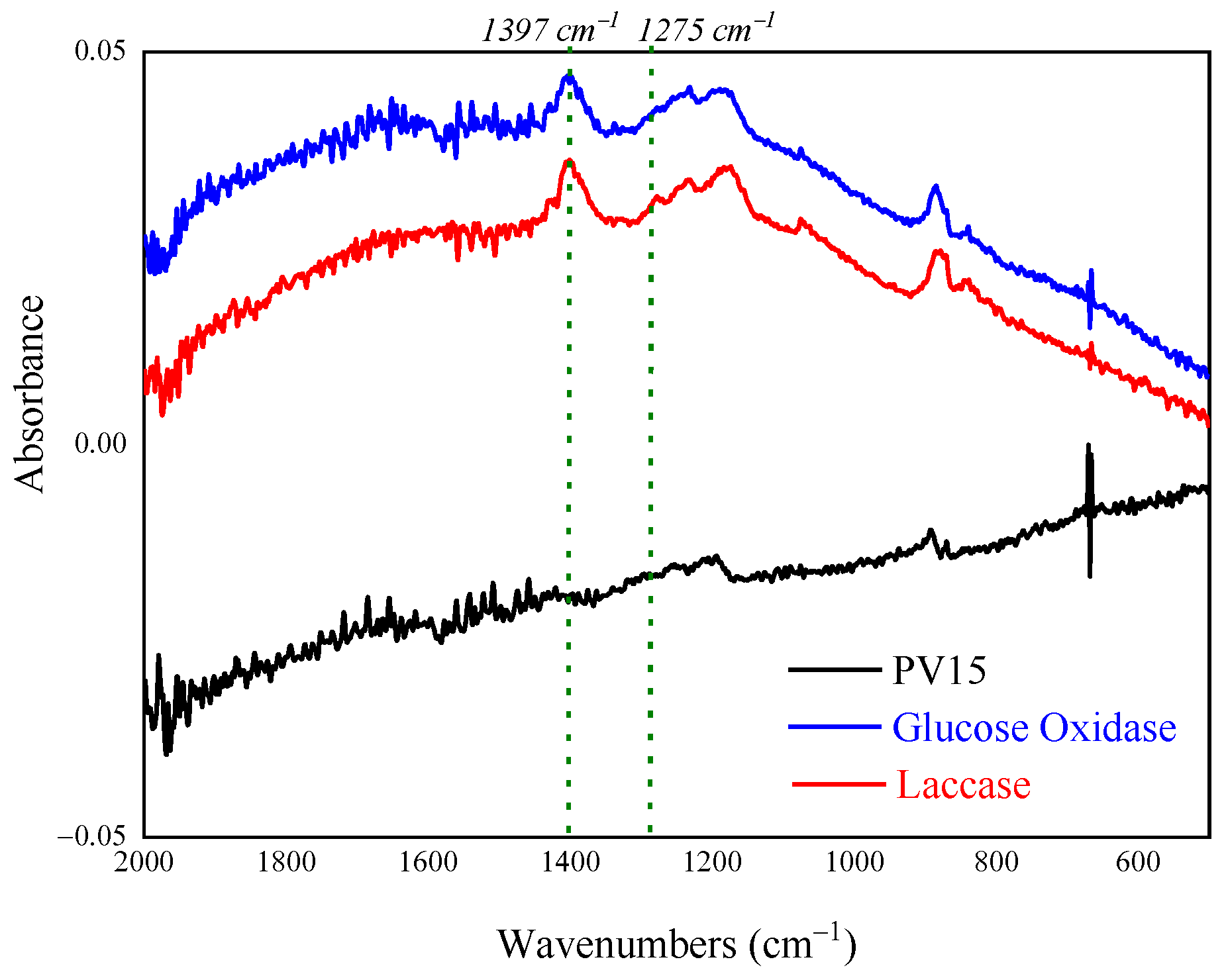
2.2.4. FT-IR/ATR Measurements
2.2.5. GOx Enzymatic Assay
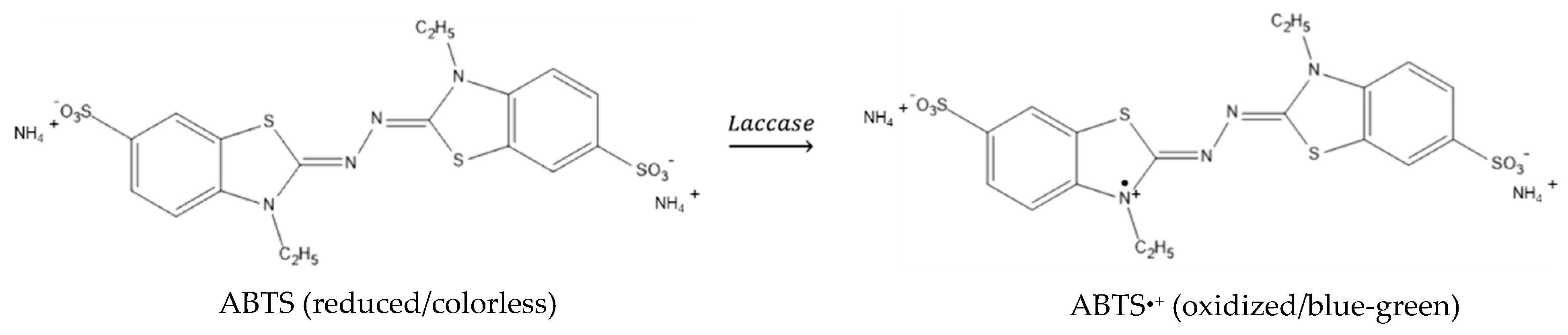
2.2.6. Laccase Enzymatic Assay
2.2.7. Electrochemical Characterization of Bioanode
2.2.8. Electrochemical Characterization of Biocathode
- In phosphate buffer solution after purging nitrogen for half an hour.
- In phosphate buffer solution after purging air for at least half an hour.
- In phosphate buffer solution after purging air and in presence of the catechol mediator.
3. Results and Discussion
3.1. Bioanode Characterization
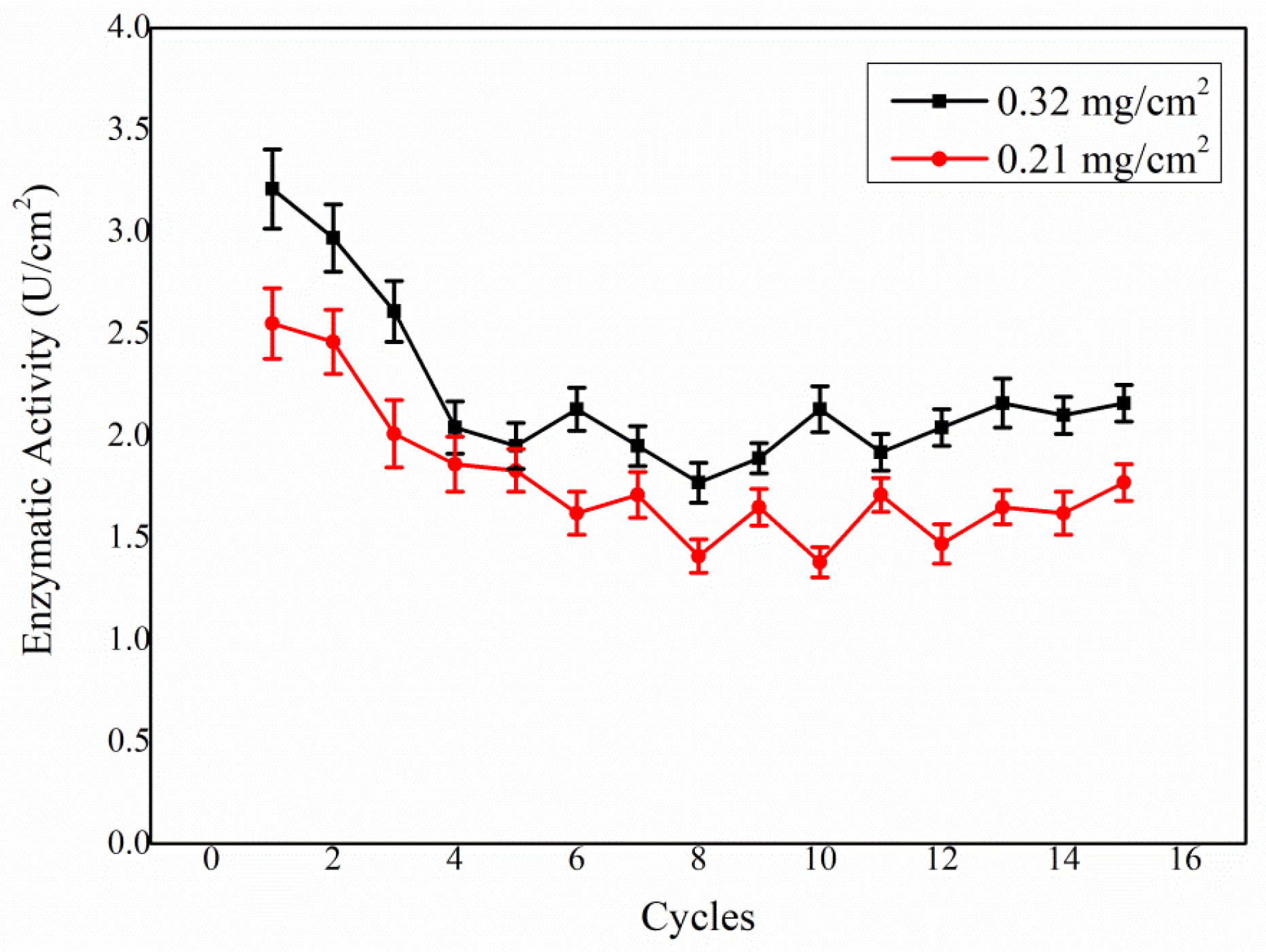
3.2. Biocathode Characterization
3.3. Morphological Characterization
3.4. Electrochemical Analysis
3.4.1. Cyclic Voltammetry
3.4.2. Bioanode Cyclic Voltammetry
3.4.3. Biocathode Cyclic Voltammetry
3.4.4. Chronoamperometry
3.4.5. Open Circuit Voltage Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeon, W.-Y.; Lee, J.-H.; Dashnyam, K.; Choi, Y.-B.; Kim, T.-H.; Lee, H.-H.; Kim, H.-W.; Kim, H.-H. Performance of a Glucose-Reactive Enzyme-Based Biofuel Cell System for Biomedical Applications. Sci. Rep. 2019, 9, 10872. [Google Scholar] [CrossRef] [PubMed]
- Magotra, V.K.; Kumar, S.; Kang, T.W.; Inamdar, A.I.; Aqueel, A.T.; Im, H.; Ghodake, G.; Shinde, S.; Waghmode, D.P.; Jeon, H.C. Compost Soil Microbial Fuel Cell to Generate Power Using Urea as Fuel. Sci. Rep. 2020, 10, 4154. [Google Scholar] [CrossRef] [PubMed]
- Gajda, I.; Greenman, J.; Melhuish, C.; Ieropoulos, I.A. Electricity and Disinfectant Production from Wastewater: Microbial Fuel Cell as a Self-Powered Electrolyser. Sci. Rep. 2016, 6, 25571. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.Q.; Kuis, R.; Narayanan, J.S.; Slaughter, G. Fabrication of Highly Effective Hybrid Biofuel Cell Based on Integral Colloidal Platinum and Bilirubin Oxidase on Gold Support. Sci. Rep. 2018, 8, 16351. [Google Scholar] [CrossRef] [PubMed]
- Barelli, L.; Bidini, G.; Calzoni, E.; Cesaretti, A.; Di Michele, A.; Emiliani, C.; Gammaitoni, L.; Sisani, E. Enzymatic Fuel Cell Technology for Energy Production from Bio-Sources. AIP Conf. Proc. 2019, 2191, 020014. [Google Scholar] [CrossRef]
- Cosnier, S.; Gross, A.J.; Giroud, F.; Holzinger, M. Beyond the Hype Surrounding Biofuel Cells: What’s the Future of Enzymatic Fuel Cells? Curr. Opin. Electrochem. 2018, 12, 148–155. [Google Scholar] [CrossRef]
- Gonzalez-Solino, C.; Lorenzo, M.D. Enzymatic Fuel Cells: Towards Self-Powered Implantable and Wearable Diagnostics. Biosensors 2018, 8, 11. [Google Scholar] [CrossRef]
- Karim, N.A.; Yang, H. Mini-Review: Recent Technologies of Electrode and System in the Enzymatic Biofuel Cell (EBFC). Appl. Sci. 2021, 11, 5197. [Google Scholar] [CrossRef]
- Naina Mohamed, S.; Ajit Hiraman, P.; Muthukumar, K.; Jayabalan, T. Bioelectricity Production from Kitchen Wastewater Using Microbial Fuel Cell with Photosynthetic Algal Cathode. Bioresour. Technol. 2020, 295, 122226. [Google Scholar] [CrossRef]
- Xu, Y.; Fei, Q.; Page, M.; Zhao, G.; Ling, Y.; Stoll, S.B.; Yan, Z. Paper-Based Wearable Electronics. iScience 2021, 24, 102736. [Google Scholar] [CrossRef]
- Yu, H.; Li, N.; Zhao, N. How Far Are We from Achieving Self-Powered Flexible Health Monitoring Systems: An Energy Perspective. Adv. Energy Mater. 2021, 11, 2002646. [Google Scholar] [CrossRef]
- Sharifi, M.; Pothu, R.; Boddula, R.; Bardajee, G.R. Trends of Biofuel Cells for Smart Biomedical Devices. Int. J. Hydrog. Energy 2021, 46, 3220–3229. [Google Scholar] [CrossRef]
- Mishra, P.; Lakshmi, G.B.V.S.; Mishra, S.; Avasthi, D.K.; Swart, H.C.; Turner, A.P.F.; Mishra, Y.K.; Tiwari, A. Electrocatalytic Biofuel Cell Based on Highly Efficient Metal-Polymer Nano-Architectured Bioelectrodes. Nano Energy 2017, 39, 601–607. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Su, B.S.Q.; Song, R.; Zhang, J.-R.; Zhu, J.-J. Enzymatic Biofuel Cell: Opportunities and Intrinsic Challenges in Futuristic Applications. Adv. Energy Sustain. Res. 2021, 2, 2100031. [Google Scholar] [CrossRef]
- Yang, J.; Ghobadian, S.; Goodrich, P.J.; Montazami, R.; Hashemi, N. Miniaturized Biological and Electrochemical Fuel Cells: Challenges and Applications. Phys. Chem. Chem. Phys. 2013, 15, 14147–14161. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Asghary, M.; Fallah, M. Microbial Fuel Cell (MFC): An Innovative Technology for Wastewater Treatment and Power Generation. In Bioremediation of Industrial Waste for Environmental Safety: Volume II: Biological Agents and Methods for Industrial Waste Management; Bharagava, R.N., Saxena, G., Eds.; Springer: Singapore, 2020; pp. 215–235. ISBN 9789811334269. [Google Scholar]
- Bandodkar, A.J.; Wang, J. Wearable Biofuel Cells: A Review. Electroanalysis 2016, 28, 1188–1200. [Google Scholar] [CrossRef]
- Xiao, X.; Xia, H.; Wu, R.; Bai, L.; Yan, L.; Magner, E.; Cosnier, S.; Lojou, E.; Zhu, Z.; Liu, A.; et al. Tackling the Challenges of Enzymatic (Bio) Fuel Cells. Chem. Rev. 2019, 119, 9509–9558. [Google Scholar] [CrossRef]
- Ul Haque, S.; Nasar, A.; Inamuddin; Rahman, M.M. Applications of Chitosan (CHI)-Reduced Graphene Oxide (RGO)-Polyaniline (PAni) Conducting Composite Electrode for Energy Generation in Glucose Biofuel Cell. Sci. Rep. 2020, 10, 10428. [Google Scholar] [CrossRef]
- Nasar, A.; Perveen, R. Applications of Enzymatic Biofuel Cells in Bioelectronic Devices—A Review. Int. J. Hydrog. Energy 2019, 44, 15287–15312. [Google Scholar] [CrossRef]
- Bollella, P.; Lee, I.; Blaauw, D.; Katz, E. A Microelectronic Sensor Device Powered by a Small Implantable Biofuel Cell. ChemPhysChem 2020, 21, 120–128. [Google Scholar] [CrossRef]
- Cosnier, S.; Gross, A.J.; Le Goff, A.; Holzinger, M. Recent Advances on Enzymatic Glucose/Oxygen and Hydrogen/Oxygen Biofuel Cells: Achievements and Limitations. J. Power Sources 2016, 325, 252–263. [Google Scholar] [CrossRef]
- Tsujimura, S. From Fundamentals to Applications of Bioelectrocatalysis: Bioelectrocatalytic Reactions of FAD-Dependent Glucose Dehydrogenase and Bilirubin Oxidase. Biosci. Biotechnol. Biochem. 2019, 83, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Inamuddin; Shakeel, N. Optimization of RGO-PEI/Naph-SH/AgNWs/Frt/GOx Nanocomposite Anode for Biofuel Cell Applications. Sci. Rep. 2020, 10, 8919. [Google Scholar] [CrossRef] [PubMed]
- Barelli, L.; Bidini, G.; Pelosi, D.; Sisani, E. Enzymatic Biofuel Cells: A Review on Flow Designs. Energies 2021, 14, 910. [Google Scholar] [CrossRef]
- Katz, E.; Bu, A.F.; Weg, M. Self-Powered Enzyme-Based Biosensors. J. Am. Chem. Soc. 2001, 123, 10752–10753. [Google Scholar] [CrossRef]
- Zhou, M.; Kuralay, F.; Windmiller, J.R.; Wang, J. DNAzyme Logic-Controlled Biofuel Cells for Self-Powered Biosensors. Chem. Commun. 2012, 48, 3815–3817. [Google Scholar] [CrossRef]
- Beilke, M.C.; Klotzbach, T.L.; Treu, B.L.; Sokic-Lazic, D.; Wildrick, J.; Amend, E.R.; Gebhart, L.M.; Arechederra, R.L.; Germain, M.N.; Moehlenbrock, M.J.; et al. Enzymatic Biofuel Cells. In Micro Fuel Cells; Zhao, T.S., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 179–241. [Google Scholar] [CrossRef]
- Fokina, O.; Eipper, J.; Kerzenmacher, S.; Fischer, R. Selective Natural Induction of Laccases in Pleurotus Sajor-Caju, Suitable for Application at a Biofuel Cell Cathode at Neutral pH. Bioresour. Technol. 2016, 218, 455–462. [Google Scholar] [CrossRef]
- Fokina, O.; Eipper, J.; Winandy, L.; Kerzenmacher, S.; Fischer, R. Improving the Performance of a Biofuel Cell Cathode with Laccase-Containing Culture Supernatant from Pycnoporus Sanguineus. Bioresour. Technol. 2015, 175, 445–453. [Google Scholar] [CrossRef]
- Miyake, T.; Haneda, K.; Nagai, N.; Yatagawa, Y.; Onami, H.; Yoshino, S.; Abe, T.; Nishizawa, M. Enzymatic Biofuel Cells Designed for Direct Power Generation from Biofluids in Living Organisms. Energy Environ. Sci. 2011, 4, 5008–5012. [Google Scholar] [CrossRef]
- Pankratov, D.; Ohlsson, L.; Gudmundsson, P.; Halak, S.; Ljunggren, L.; Blum, Z.; Shleev, S. Ex Vivo Electric Power Generation in Human Blood Using an Enzymatic Fuel Cell in a Vein Replica. RSC Adv. 2016, 6, 70215–70220. [Google Scholar] [CrossRef]
- Inamuddin; Shakeel, N.; Imran Ahamed, M.; Kanchi, S.; Abbas Kashmery, H. Green Synthesis of ZnO Nanoparticles Decorated on Polyindole Functionalized-MCNTs and Used as Anode Material for Enzymatic Biofuel Cell Applications. Sci. Rep. 2020, 10, 5052. [Google Scholar] [CrossRef]
- Christwardana, M.; Kim, K.J.; Kwon, Y. Fabrication of Mediatorless/Membraneless Glucose/Oxygen Based Biofuel Cell Using Biocatalysts Including Glucose Oxidase and Laccase Enzymes. Sci. Rep. 2016, 6, 30128. [Google Scholar] [CrossRef]
- Ul Haque, S.; Inamuddin; Nasar, A.; Rajender, B.; Khan, A.; Asiri, A.M.; Ashraf, G.M. Optimization of Glucose Powered Biofuel Cell Anode Developed by Polyaniline-Silver as Electron Transfer Enhancer and Ferritin as Biocompatible Redox Mediator. Sci. Rep. 2017, 7, 12703. [Google Scholar] [CrossRef]
- Ben Tahar, A.; Romdhane, A.; Lalaoui, N.; Reverdy-Bruas, N.; Le Goff, A.; Holzinger, M.; Cosnier, S.; Chaussy, D.; Belgacem, N. Carbon Nanotube-Based Flexible Biocathode for Enzymatic Biofuel Cells by Spray Coating. J. Power Sources 2018, 408, 1–6. [Google Scholar] [CrossRef]
- Kang, S.; Yoo, K.S.; Chung, Y.; Kwon, Y. Cathodic Biocatalyst Consisting of Laccase and Gold Nanoparticle for Improving Oxygen Reduction Reaction Rate and Enzymatic Biofuel Cell Performance. J. Ind. Eng. Chem. 2018, 62, 329–332. [Google Scholar] [CrossRef]
- Li, X.; Lv, P.; Yao, Y.; Feng, Q.; Mensah, A.; Li, D.; Wei, Q. A Novel Single-Enzymatic Biofuel Cell Based on Highly Flexible Conductive Bacterial Cellulose Electrode Utilizing Pollutants as Fuel. Chem. Eng. J. 2020, 379, 122316. [Google Scholar] [CrossRef]
- Zhong, Z.; Qian, L.; Tan, Y.; Wang, G.; Yang, L.; Hou, C.; Liu, A. A High-Performance Glucose/Oxygen Biofuel Cell Based on Multi-Walled Carbon Nanotube Films with Electrophoretic Deposition. J. Electroanal. Chem. 2018, 823, 723–729. [Google Scholar] [CrossRef]
- Pelosi, D.; Barelli, L.; Montegiove, N.; Calzoni, E.; Cesaretti, A.; Di Michele, A.; Emiliani, C.; Gammaitoni, L. Immobilizing Enzymes on a Commercial Polymer: Performance Analysis of a GOx-Laccase Based Enzymatic Biofuel Cell Assembly. Energies 2022, 15, 2182. [Google Scholar] [CrossRef]
- Lv, J.; Cheng, Y. Fluoropolymers in Biomedical Applications: State-of-the-Art and Future Perspectives. Chem. Soc. Rev. 2021, 50, 5435–5467. [Google Scholar] [CrossRef]
- Chung, Y.; Ji, J.; Kwon, Y. Performance Evaluation of Enzymatic Biofuel Cells Using a New Cathodic Catalyst Containing Hemin and Poly Acrylic Acid Promoting the Oxygen Reduction Reaction. J. Mater. Chem. C 2019, 7, 11597–11605. [Google Scholar] [CrossRef]
- Ghosh, B.; Saha, R.; Bhattacharya, D.; Mukhopadhyay, M. Laccase and Its Source of Sustainability in an Enzymatic Biofuel Cell. Bioresour. Technol. Rep. 2019, 6, 268–278. [Google Scholar] [CrossRef]
- Kipf, E.; Sané, S.; Morse, D.; Messinger, T.; Zengerle, R.; Kerzenmacher, S. An Air-Breathing Enzymatic Cathode with Extended Lifetime by Continuous Laccase Supply. Bioresour. Technol. 2018, 264, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Calzoni, E.; Cesaretti, A.; Tacchi, S.; Caponi, S.; Pellegrino, R.M.; Luzi, F.; Cottone, F.; Fioretto, D.; Emiliani, C.; Di Michele, A. Covalent Immobilization of Proteases on Polylactic Acid for Proteins Hydrolysis and Waste Biomass Protein Content Valorization. Catalysts 2021, 11, 167. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Montegiove, N.; Di Michele, A.; Emiliani, C. Enhanced Stability of Long-Living Immobilized Recombinant β-d-N-Acetyl-Hexosaminidase A on Polylactic Acid (PLA) Films for Potential Biomedical Applications. J. Funct. Biomater. 2021, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Worthington, C.C. Enzyme and Related Biochemical. In Enzyme Manual; Worthington Biochemical Co.: Lakewood, NJ, USA, 1988; pp. 155–158. [Google Scholar]
- Agrawal, K.; Chaturvedi, V.; Verma, P. Fungal Laccase Discovered but yet Undiscovered. Bioresour. Bioprocess. 2018, 5, 4. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- More, S.S.; Renuka, P.S.; Pruthvi, K.; Swetha, M.; Malini, S.; Veena, S.M. Isolation, Purification, and Characterization of Fungal Laccase from Pleurotus sp. Enzym. Res. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Xu, F.J.; Cai, Q.J.; Li, Y.L.; Kang, E.T.; Neoh, K.G. Covalent Immobilization of Glucose Oxidase on Well-Defined Poly(Glycidyl Methacrylate)−Si(111) Hybrids from Surface-Initiated Atom-Transfer Radical Polymerization. Biomacromolecules 2005, 6, 1012–1020. [Google Scholar] [CrossRef]
- Zhang, D.-H.; Yuwen, L.-X.; Peng, L.-J. Parameters Affecting the Performance of Immobilized Enzyme. J. Chem. 2013, 2013, 946248. [Google Scholar] [CrossRef]
- Parker, F.S. Near-Infrared Spectroscopy. In Applications of Infrared Spectroscopy in Biochemistry, Biology, and Medicine; Parker, F.S., Ed.; Springer: Boston, MA, USA, 1971; pp. 25–40. ISBN 978-1-4684-1872-9. [Google Scholar]
- Bourbonnais, R.; Leech, D.; Paice, M.G. Electrochemical Analysis of the Interactions of Laccase Mediators with Lignin Model Compounds. Biochim. Biophys. Acta Gen. Subj. 1998, 1379, 381–390. [Google Scholar] [CrossRef]
- Olszewski, B.; Stolarczyk, K. Laccase-Catalyzed Reduction of Oxygen at Electrodes Modified by Carbon Nanotubes with Adsorbed Promazine or Acetosyringone. Catalysts 2018, 8, 414. [Google Scholar] [CrossRef]
- Zimbardi, A.L.R.L.; Camargo, P.F.; Carli, S.; Neto, S.A.; Meleiro, L.P.; Rosa, J.C.; De Andrade, A.R.; Jorge, J.A.; Furriel, R.P.M. A High Redox Potential Laccase from Pycnoporus Sanguineus RP15: Potential Application for Dye Decolorization. Int. J. Mol. Sci. 2016, 17, 672. [Google Scholar] [CrossRef]
- Tortolini, C.; Rea, S.; Carota, E.; Cannistraro, S.; Mazzei, F. Influence of the Immobilization Procedures on the Electroanalytical Performances of Trametes Versicolor Laccase Based Bioelectrode. Microchem. J. 2012, 100, 8–13. [Google Scholar] [CrossRef]
- Ania, C.O.; Gomis-Berenguer, A.; Dentzer, J.; Vix-Guterl, C. Nanoconfinement of Glucose Oxidase on Mesoporous Carbon Electrodes with Tunable Pore Sizes. J. Electroanal. Chem. 2018, 808, 372–379. [Google Scholar] [CrossRef]
- Gonçales, V.R.; Colombo, R.N.P.; Minadeo, M.A.O.S.; Matsubara, E.Y.; Rosolen, J.M.; Córdoba de Torresi, S.I. Three-Dimensional Graphene/Carbon Nanotubes Hybrid Composites for Exploring Interaction between Glucose Oxidase and Carbon Based Electrodes. J. Electroanal. Chem. 2016, 775, 235–242. [Google Scholar] [CrossRef]
- Grosse, W.; Champavert, J.; Gambhir, S.; Wallace, G.G.; Moulton, S.E. Aqueous Dispersions of Reduced Graphene Oxide and Multi Wall Carbon Nanotubes for Enhanced Glucose Oxidase Bioelectrode Performance. Carbon 2013, 61, 467–475. [Google Scholar] [CrossRef][Green Version]
- Lv, C.; Li, S.; Liu, L.; Zhu, X.; Yang, X. Enhanced Electrochemical Characteristics of the Glucose Oxidase Bioelectrode Constructed by Carboxyl-Functionalized Mesoporous Carbon. Sensors 2020, 20, 3365. [Google Scholar] [CrossRef]
- Mazar, F.M.; Alijanianzadeh, M.; Molaeirad, A.; Heydari, P. Development of Novel Glucose Oxidase Immobilization on Graphene/Gold Nanoparticles/Poly Neutral Red Modified Electrode. Process Biochem. 2017, 56, 71–80. [Google Scholar] [CrossRef]
- Gross, A.J.; Robin, M.P.; Nedellec, Y.; O’Reilly, R.K.; Shan, D.; Cosnier, S. Robust Bifunctional Buckypapers from Carbon Nanotubes and Polynorbornene Copolymers for Flexible Engineering of Enzymatic Bioelectrodes. Carbon 2016, 107, 542–547. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, C.; Pita, M.; Vaz-Domínguez, C.; Shleev, S.; De Lacey, A.L. Gold Nanoparticles as Electronic Bridges for Laccase-Based Biocathodes. J. Am. Chem. Soc. 2012, 134, 17212–17220. [Google Scholar] [CrossRef]
- Giroud, F.; Minteer, S.D. Anthracene-Modified Pyrenes Immobilized on Carbon Nanotubes for Direct Electroreduction of O2 by Laccase. Electrochem. Commun. 2013, 34, 157–160. [Google Scholar] [CrossRef]
- Rincón, R.A.; Lau, C.; Luckarift, H.R.; Garcia, K.E.; Adkins, E.; Johnson, G.R.; Atanassov, P. Enzymatic Fuel Cells: Integrating Flow-through Anode and Air-Breathing Cathode into a Membrane-Less Biofuel Cell Design. Biosens. Bioelectron. 2011, 27, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Reuillard, B.; Abreu, C.; Lalaoui, N.; Le Goff, A.; Holzinger, M.; Ondel, O.; Buret, F.; Cosnier, S. One-Year Stability for a Glucose/Oxygen Biofuel Cell Combined with pH Reactivation of the Laccase/Carbon Nanotube Biocathode. Bioelectrochemistry 2015, 106, 73–76. [Google Scholar] [CrossRef] [PubMed]






| Film | Free Laccase (mg/mL) | Administered Laccase (mg) | Immobilized Laccase (mg/cm2) | Immobilization Yield | Specific Activity (mU/mg) | Recovered Activity | Enzymatic Activity (mU/cm2) |
|---|---|---|---|---|---|---|---|
| 1 | 17.5 | 8.8 | 3.7 ± 0.1 | 42% | 1.1 ± 0.1 | 0.11% | 4.1 ± 0.5 |
| 2 | 8.75 | 4.4 | 2.1 ± 0.2 | 48% | 2.3 ± 0.1 | 0.23% | 4.9 ± 0.7 |
| 3 | 2.0 | 1.0 | 0.80 ± 0.04 | 80% | 13.9 ± 0.2 | 1.4% | 11.1 ± 0.7 |
| 4 | 1.0 | 0.5 | 0.47 ± 0.05 | 94% | 35.3 ± 0.4 | 3.6% | 17 ± 2 |
| 5 | 0.40 | 0.20 | 0.18 ± 0.01 | 90% | 128 ± 4 | 12.9% | 23 ± 2 |
| 6 | 0.10 | 0.05 | 0.050 ± 0.003 | 93% | 167 ± 3 | 16.9% | 7.7 ± 0.6 |
| 7 | 0.040 | 0.02 | 0.019 ± 0.003 | 94% | 360 ± 50 | 36.8% | 7 ± 1 |
| 8 | 0.020 | 0.01 | 0.010 ± 0.001 | 96% | 450 ± 40 | 45.2% | 4.8 ± 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montegiove, N.; Calzoni, E.; Pelosi, D.; Gammaitoni, L.; Barelli, L.; Emiliani, C.; Di Michele, A.; Cesaretti, A. Optimizing Covalent Immobilization of Glucose Oxidase and Laccase on PV15 Fluoropolymer-Based Bioelectrodes. J. Funct. Biomater. 2022, 13, 270. https://doi.org/10.3390/jfb13040270
Montegiove N, Calzoni E, Pelosi D, Gammaitoni L, Barelli L, Emiliani C, Di Michele A, Cesaretti A. Optimizing Covalent Immobilization of Glucose Oxidase and Laccase on PV15 Fluoropolymer-Based Bioelectrodes. Journal of Functional Biomaterials. 2022; 13(4):270. https://doi.org/10.3390/jfb13040270
Chicago/Turabian StyleMontegiove, Nicolò, Eleonora Calzoni, Dario Pelosi, Luca Gammaitoni, Linda Barelli, Carla Emiliani, Alessandro Di Michele, and Alessio Cesaretti. 2022. "Optimizing Covalent Immobilization of Glucose Oxidase and Laccase on PV15 Fluoropolymer-Based Bioelectrodes" Journal of Functional Biomaterials 13, no. 4: 270. https://doi.org/10.3390/jfb13040270
APA StyleMontegiove, N., Calzoni, E., Pelosi, D., Gammaitoni, L., Barelli, L., Emiliani, C., Di Michele, A., & Cesaretti, A. (2022). Optimizing Covalent Immobilization of Glucose Oxidase and Laccase on PV15 Fluoropolymer-Based Bioelectrodes. Journal of Functional Biomaterials, 13(4), 270. https://doi.org/10.3390/jfb13040270













