Stimuli-Responsive Drug Release from Smart Polymers
Abstract
1. Introduction
2. Chemical Stimuli-Responsive Systems
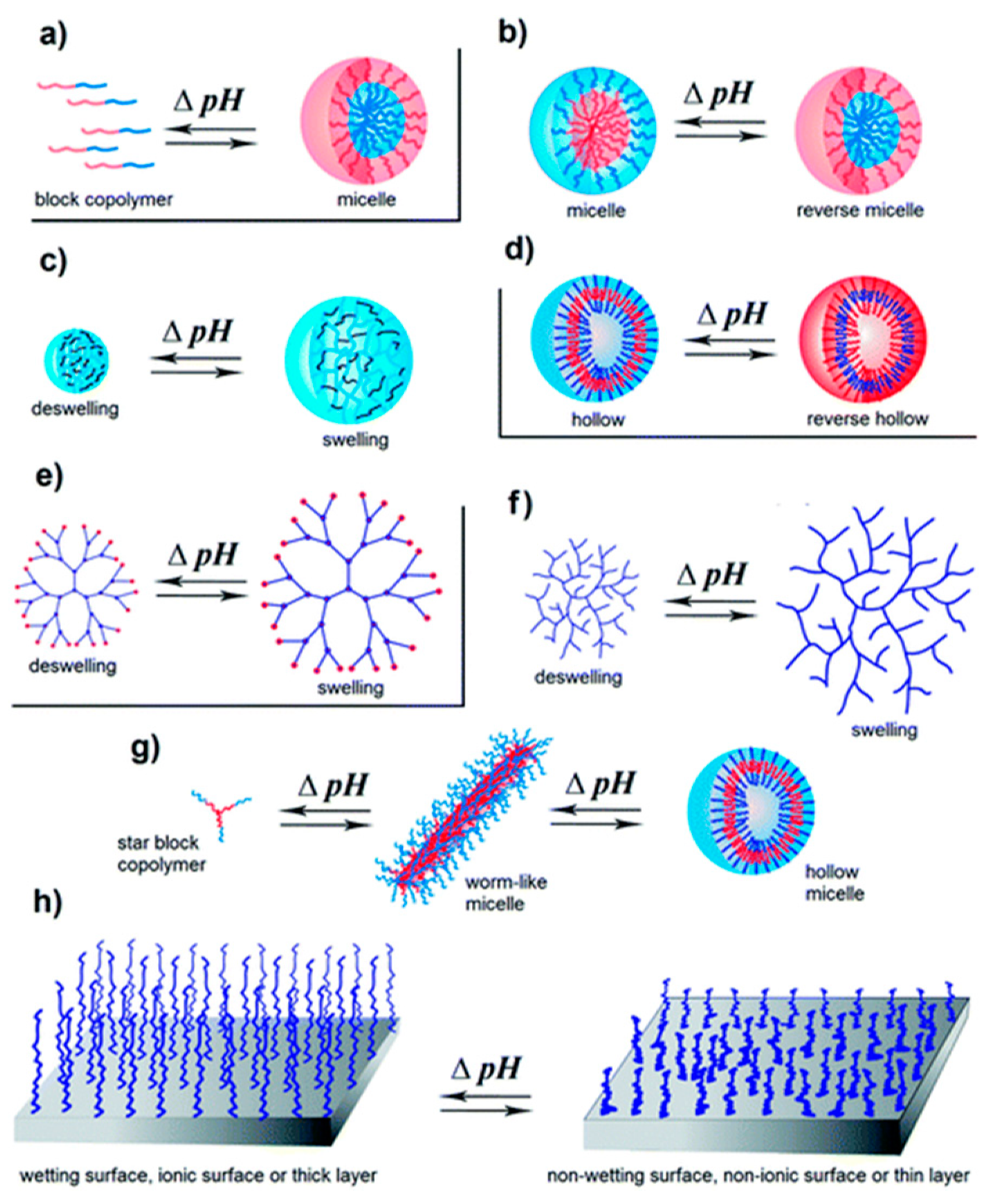
pH-Responsiveness
3. Physical Stimuli-Responsive Systems
3.1. Acoustic-Responsiveness
3.2. Photo-Responsiveness
3.3. Magnetic-Responsiveness
3.4. Electric-Responsiveness
4. Biological Stimuli-Responsive Systems
Enzyme-Responsiveness
5. Multi Stimuli-Responsive Systems
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Contr. Release 2008, 126, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, K.; Cao, W.; Sun, Y.; Sheng, W.; Li, F.; Wu, Y.; Liang, X.J. Ph-responsive biocompatible fluorescent polymer nanoparticles based on phenylboronic acid for intracellular imaging and drug delivery. Nanoscale 2014, 6, 13701–13709. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, Y.; Tang, L. Engineering cancer vaccines using stimuli-responsive biomaterials. Nano Res. 2018, 11, 5355–5371. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Jing, L.; Liang, X.; Li, X.; Yang, Y.; Dai, Z. Covalent attachment of Mn-porphyrin onto doxorubicin-loaded poly (lactic acid) nanoparticles for potential magnetic resonance imaging and pH-sensitive drug delivery. Acta Biomater. 2013, 9, 9434–9441. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017, 58, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Liu, Y.; Chen, Y.; Zhang, Z.; Ding, Y.; Wu, Z.; Yin, J.; Nie, L. Versatile pH-response micelles with high cell-penetrating helical diblock copolymers for photoacoustic imaging guided synergistic chemo-photothermal therapy. Theranostics 2016, 6, 2170. [Google Scholar] [CrossRef] [PubMed]
- Tekade, R.K.; Tekade, M.; Kumar, M.; Chauhan, A.S. Dendrimer-stabilized smart-nanoparticle (DSSN) platform for targeted delivery of hydrophobic antitumor therapeutics. Pharm. Res. 2015, 32, 910–928. [Google Scholar] [CrossRef] [PubMed]
- Unsoy, G.; Yalcin, S.; Khodadust, R.; Mutlu, P.; Onguru, O.; Gunduz, U. Chitosan magnetic nanoparticles for pH responsive Bortezomib release in cancer therapy. Biomed. Pharmacother. 2014, 68, 641–648. [Google Scholar]
- Aycan, D.; Alemdar, N. Development of pH-responsive chitosan-based hydrogel modified with bone ash for controlled release of amoxicillin. Carbohydr. Polym. 2018, 184, 401–407. [Google Scholar] [CrossRef]
- Chung, M.F.; Chia, W.T.; Liu, H.Y.; Hsiao, C.W.; Hsiao, H.C.; Yang, C.M.; Sung, H.W. Inflammation-Induced Drug Release by using a pH-Responsive Gas-Generating Hollow-Microsphere System for the Treatment of Osteomyelitis. Adv. Healthc. Mater. 2014, 3, 1854–1861. [Google Scholar] [CrossRef]
- Li, X.; Fu, M.; Wu, J.; Zhang, C.; Deng, X.; Dhinakar, A.; Huang, W.; Qian, H.; Ge, L. pH-sensitive peptide hydrogel for glucose-responsive insulin delivery. Acta Biomater. 2017, 51, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.V.; Rajput, S.J. Facile synthesis of chitosan capped mesoporous silica nanoparticles: A pH responsive smart delivery platform for raloxifene hydrochloride. AAPS PharmSciTech 2018, 19, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wang, J.; Peng, X.; Li, Y.; Yuan, X.; Ma, Y. Facile Solvothermal synthesis of mesostructured Fe3O4/chitosan nanoparticles as delivery vehicles for pH-responsive drug delivery and magnetic resonance imaging contrast agents. Chem. Asian J. 2014, 9, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Krishnatreya, G.; Gogoi, N.; Thakur, D.; Chowdhury, D. Carbon-dot-coated alginate beads as a smart stimuli-responsive drug delivery system. ACS Appl. Mater. Interfaces 2016, 8, 34179–34184. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Sun, Z.; Wang, D.; Qiao, Y.; Zhu, H.; Ma, X.; Liu, X. Smart release of doxorubicin loaded on polyetheretherketone (PEEK) surface with 3D porous structure. Colloids Surf. B Biointerfaces 2018, 163, 175–183. [Google Scholar] [CrossRef]
- Kocak, G.; Tuncer, C.; Butun, V. Ph-responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Pafiti, K.; Cui, Z.; Adlam, D.; Hoyland, J.; Freemont, A.J.; Saunders, B.R. Hydrogel composites containing sacrificial collapsed hollow particles as dual action pH-responsive biomaterials. Biomacromolecules 2016, 17, 2448–2458. [Google Scholar] [CrossRef] [PubMed]
- Kearney, C.J.; Skaat, H.; Kennedy, S.M.; Hu, J.; Darnell, M.; Raimondo, T.M.; Mooney, D.J. Switchable release of entrapped nanoparticles from alginate hydrogels. Adv. Healthc. Mater. 2015, 4, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Kearney, C.J.; Zhao, X.; Kim, J.; Cezar, C.A.; Suo, Z.; Mooney, D.J. Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. Proc. Natl. Acad. Sci. USA 2014, 111, 9762–9767. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guo, L.; Shi, D.; Duan, S.; Li, J. Biocompatible Chitosan Nanobubbles for Ultrasound-Mediated Targeted Delivery of Doxorubicin. Nanoscale Res. Lett. 2019, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Li, D.; Jin, S.; Ding, J.; Guo, J.; Shi, W.; Wang, C. Stimuli-responsive biodegradable poly (methacrylic acid) based nanocapsules for ultrasound traced and triggered drug delivery system. Biomaterials 2014, 35, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Pan, H.Y.; Haworth, K.; Mahoney, E.; Mercado-Shekhar, K.P.; Lin, C.Y.; Zhang, Z.; Park, Y.C. Multiple-exposure drug release from stable nanodroplets by high-intensity focused ultrasound for a potential degenerative disc disease treatment. Ultrasound Med. Biol. 2018, 45, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, Y.; Yu, T.; Guo, Y.; Liu, F.; Yao, Y.; Li, P.; Wang, D.; Wang, Z.; Chen, Y. Drug release from phase-changeable nanodroplets triggered by low-intensity focused ultrasound. Theranostics 2018, 8, 1327. [Google Scholar] [CrossRef] [PubMed]
- Salgarella, A.R.; Zahoranová, A.; Šrámková, P.; Majerčíková, M.; Pavlova, E.; Luxenhofer, R.; Kronek, J.; Lacík, I.; Ricotti, L. Investigation of drug release modulation from poly (2-oxazoline) micelles through ultrasound. Sci. Rep. 2018, 8, 9893. [Google Scholar] [CrossRef]
- Gai, M.; Frueh, J.; Tao, T.; Petrov, A.V.; Petrov, V.V.; Shesterikov, E.V.; Tverdokhlebov, S.I.; Sukhorukov, G.B. Polylactic acid nano-and microchamber arrays for encapsulation of small hydrophilic molecules featuring drug release via high intensity focused ultrasound. Nanoscale 2017, 9, 7063–7070. [Google Scholar] [CrossRef]
- Park, W.; Bae, B.-c.; Na, K. A highly tumor-specific light-triggerable drug carrier responds to hypoxic tumor conditions for effective tumor treatment. Biomaterials 2016, 77, 227–234. [Google Scholar] [CrossRef]
- Higuchi, A.; Ling, Q.-D.; Kumar, S.S.; Chang, Y.; Kao, T.-C.; Munusamy, M.A.; Alarfaj, A.A.; Hsu, S.-T.; Umezawa, A. External stimulus-responsive biomaterials designed for the culture and differentiation of ES, iPS, and adult stem cells. Prog. Polym. Sci. 2014, 39, 1585–1613. [Google Scholar] [CrossRef]
- Alonso-Cristobal, P.; Oton-Fernandez, O.; Mendez-Gonzalez, D.; Díaz, J.F.; Lopez-Cabarcos, E.; Barasoain, I.; Rubio-Retama, J. Synthesis, characterization, and application in HeLa cells of an NIR light responsive doxorubicin delivery system based on NaYF4: Yb, Tm@ SiO2-PEG nanoparticles. ACS Appl. Mater. Interface 2015, 7, 14992–14999. [Google Scholar]
- Shell, T.A.; Lawrence, D.S. Vitamin B12: A tunable, long wavelength, light-responsive platform for launching therapeutic agents. Acc. Chem. Res. 2015, 48, 2866–2874. [Google Scholar] [CrossRef]
- Yang, G.; Liu, J.; Wu, Y.; Feng, L.; Liu, Z. Near-infrared-light responsive nanoscale drug delivery systems for cancer treatment. Coord. Chem. Rev. 2016, 320, 100–117. [Google Scholar] [CrossRef]
- Bunker, B.; Kim, B.; Houston, J.; Rosario, R.; Garcia, A.; Hayes, M.; Gust, D.; Picraux, S. Direct observation of photo switching in tethered spiropyrans using the interfacial force microscope. Nano Lett. 2003, 3, 1723–1727. [Google Scholar] [CrossRef]
- Higuchi, A.; Hamamura, A.; Shindo, Y.; Kitamura, H.; Yoon, B.O.; Mori, T.; Uyama, T.; Umezawa, A. Photon-modulated changes of cell attachments on poly (spiropyran-co-methyl methacrylate) membranes. Biomacromolecules 2004, 5, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Shin, E.; Kim, B.-S. Light-responsive micelles of spiropyran initiated hyperbranched polyglycerol for smart drug delivery. Biomacromolecules 2014, 15, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xie, Y.; Shao, H.; Jiang, X. Using azobenzene-embedded self-assembled monolayers to photochemically control cell adhesion reversibly. Angew. Chem. Int. Ed. 2009, 48, 4406–4408. [Google Scholar] [CrossRef] [PubMed]
- Pearson, S.; Vitucci, D.; Khine, Y.Y.; Dag, A.; Lu, H.; Save, M.; Billon, L.; Stenzel, M.H. Light-responsive azobenzene-based glycopolymer micelles for targeted drug delivery to melanoma cells. Eur. Polym. J. 2015, 69, 616–627. [Google Scholar] [CrossRef]
- Hardy, J.G.; Larrañeta, E.; Donnelly, R.F.; McGoldrick, N.; Migalska, K.; McCrudden, M.T.; Irwin, N.J.; Donnelly, L.; McCoy, C.P. Hydrogel-forming microneedle arrays made from light-responsive materials for on-demand transdermal drug delivery. Mol. Pharm. 2016, 13, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; He, Q.; Liu, Y.; Zhang, F.; Yang, X.; Wang, Z.; Lu, N.; Fan, W.; Lin, L.; Niu, G. Light-responsive biodegradable nanomedicine overcomes multidrug resistance via no-enhanced chemosensitization. ACS Appl. Mater. Interfaces 2016, 8, 13804–13811. [Google Scholar] [CrossRef]
- Poelma, S.O.; Oh, S.S.; Helmy, S.; Knight, A.S.; Burnett, G.L.; Soh, H.T.; Hawker, C.J.; de Alaniz, J.R. Controlled drug release to cancer cells from modular one-photon visible light-responsive micellar system. Chem. Commun. 2016, 52, 10525–10528. [Google Scholar] [CrossRef]
- Tian, Y.; Zheng, J.; Tang, X.; Ren, Q.; Wang, Y.; Yang, W. Near-infrared light-responsive nanogels with diselenide-cross-linkers for on-demand degradation and triggered drug release. Part. Part. Syst. Charact. 2015, 32, 547–551. [Google Scholar] [CrossRef]
- Liang, Y.; Gao, W.; Peng, X.; Deng, X.; Sun, C.; Wu, H.; He, B. Near infrared light responsive hybrid nanoparticles for synergistic therapy. Biomaterials 2016, 100, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Ling, M.H.; Wang, K.W.; Lin, Z.W.; Lai, B.H.; Chen, D.H. Near-infrared light-responsive composite microneedles for on-demand transdermal drug delivery. Biomacromolecules 2015, 16, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, S. Red-light-responsive supramolecular valves for photocontrolled drug release from mesoporous nanoparticles. Langmuir 2016, 32, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wagner, M.; Butt, H.J.; Wu, S. Supramolecular hydrogels constructed by red-light-responsive host–guest interactions for photo-controlled protein release in deep tissue. Soft Matter 2015, 11, 7656–7662. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, M.; Gneveckow, U.; Eckelt, L.; Feussner, A.; Waldöfner, N.; Scholz, R.; Deger, S.; Wust, P.; Loening, S.; Jordan, A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int. J. Hyperth. 2005, 21, 637–647. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, P.; Thiesen, B.; Feussner, A.; von Deimling, A.; Waldoefner, N.; Felix, R. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: Results of a feasibility study on patients with glioblastoma multiforme. J. Neurooncol. 2007, 81, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Riedinger, A.; Guardia, P.; Curcio, A.; Garcia, M.A.; Cingolani, R.; Manna, L.; Pellegrino, T. Subnanometer local temperature probing and remotely controlled drug release based on azo-functionalized iron oxide nanoparticles. Nano Lett. 2013, 13, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Guisasola, E.; Baeza, A.; Talelli, M.; Arcos, D.; Moros, M.; de la Fuente, J.s.M.; Vallet-Regí, M. Magnetic-responsive release controlled by hot spot effect. Langmuir 2015, 31, 12777–12782. [Google Scholar] [CrossRef]
- Dias, J.T.; Moros, M.; del Pino, P.; Rivera, S.; Grazú, V.; de la Fuente, J.M. DNA as a molecular local thermal probe for the analysis of magnetic hyperthermia. Angew. Chem. Int. Ed. 2013, 52, 11526–11529. [Google Scholar] [CrossRef]
- Thirunavukkarasu, G.K.; Cherukula, K.; Lee, H.; Jeong, Y.Y.; Park, I.-K.; Lee, J.Y. Magnetic field-inducible drug-eluting nanoparticles for image-guided thermo-chemotherapy. Biomaterials 2018, 180, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.C.; Shieh, D.B.; Hsiao, H.T.; Wang, J.C.F.; Lin, Y.C.; Liu, Y.C. Magnetic field distribution modulation of intrathecal delivered ketorolac iron-oxide nanoparticle conjugates produce excellent analgesia for chronic inflammatory pain. J. Nanobiotechnol. 2018, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Dolati, S.; Sadreddini, S.; Rostamzadeh, D.; Ahmadi, M.; Jadidi-Niaragh, F.; Yousefi, M. Utilization of nanoparticle technology in rheumatoid arthritis treatment. Biomed. Pharmacother. 2016, 80, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Dong, J.; Zhang, T.; Su, Z.; Ding, J.; Zhang, Y.; Mao, X. Polyethyleneimine-functionalized iron oxide nanoparticles for systemic siRNA delivery in experimental arthritis. Nanomedicine 2014, 9, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Ahmed, H.; Barr, B.; LeVine, D.; Pace, L.; Mohapatra, A.; Morshed, B.; Bumgardner, J.D.; Jennings, J.A. Magnetic stimuli-responsive chitosan-based drug delivery biocomposite for multiple triggered release. Int. J. Biol. Macromol. 2017, 104, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.; Harris, M.A.; LeVine, D.; Ghimire, M.; Jennings, J.A.; Morshed, B.I.; Haggard, W.O.; Bumgardner, J.D.; Mishra, S.R.; Fujiwara, T. Magnetic stimulus responsive vancomycin drug delivery system based on chitosan microbeads embedded with magnetic nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2169–2176. [Google Scholar] [CrossRef]
- Pillay, V.; Tsai, T.S.; Choonara, Y.E.; du Toit, L.C.; Kumar, P.; Modi, G.; Naidoo, D.; Tomar, L.K.; Tyagi, C.; Ndesendo, V.M. A review of integrating electroactive polymers as responsive systems for specialized drug delivery applications. J. Biomed. Mater. Res. Part A 2014, 102, 2039–2054. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Atoufi, Z.; Zarrintaj, P.; Motlagh, G.H.; Amiri, A.; Bagher, Z.; Kamrava, S.K. A novel bio electro active alginate-aniline tetramer/agarose scaffold for tissue engineering: Synthesis, characterization, drug release and cell culture study. J. Biomater. Sci. Polym. Ed. 2017, 28, 1617–1638. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Zawisza, P.; Herman, A.P.; Turczyn, R.; Boncel, S.; Zak, J.K. An electrically controlled drug delivery system based on conducting poly (3, 4-ethylenedioxypyrrole) matrix. Bioelectrochemistry 2016, 108, 13–20. [Google Scholar] [CrossRef]
- Mongkolkitikul, S.; Paradee, N.; Sirivat, A. Electrically controlled release of ibuprofen from conductive poly (3-methoxydiphenylamine)/crosslinked pectin hydrogel. Eur. J. Pharm. Sci. 2018, 112, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hong, W.; Jeon, S.; Choi, Y.; Cho, Y. Electroactive polypyrrole nanowire arrays: Synergistic effect of cancer treatment by on-demand drug release and photothermal therapy. Langmuir 2015, 31, 4264–4269. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Sun, G.; Wang, M.; Zhou, B.; Fu, J. Voltage/pH-driven mechanized silica nanoparticles for the multimodal controlled release of drugs. ACS Appl. Mater. Interfaces 2015, 7, 21295–21304. [Google Scholar] [CrossRef] [PubMed]
- Perrier, D.L.; Rems, L.; Boukany, P.E. Lipid vesicles in pulsed electric fields: Fundamental principles of the membrane response and its biomedical applications. Adv. Colloid Interface Sci. 2017, 249, 248–271. [Google Scholar] [CrossRef] [PubMed]
- Bulysheva, A.; Hornef, J.; Edelblute, C.; Jiang, C.; Schoenbach, K.; Lundberg, C.; Malik, M.A.; Heller, R. Coalesced thermal and electrotransfer mediated delivery of plasmid DNA to the skin. Bioelectrochemistry 2019, 125, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Schroeder, B.; Sun, C.; Loufakis, D.N.; Cao, Z.; Sriranganathan, N.; Lu, C. Electroporation-based delivery of cell-penetrating peptide conjugates of peptide nucleic acids for antisense inhibition of intracellular bacteria. Integr. Biol. 2014, 6, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Denzi, A.; Della Valle, E.; Apollonio, F.; Breton, M.; Mir, L.M.; Liberti, M. Exploring the applicability of nano-poration for remote control in smart drug delivery systems. Integr. Biol. 2014, 6, 973–978. [Google Scholar] [CrossRef]
- van Rijt, S.H.; Bölükbas, D.A.; Argyo, C.; Datz, S.; Lindner, M.; Eickelberg, O.; Königshoff, M.; Bein, T.; Meiners, S. Protease-mediated release of chemotherapeutics from mesoporous silica nanoparticles to ex vivo human and mouse lung tumors. ACS Nano 2015, 9, 2377–2389. [Google Scholar] [CrossRef]
- Upreti, M.; Jyoti, A.; Sethi, P. Tumor microenvironment and nanotherapeutics. Transl. Cancer Res. 2013, 2, 309. [Google Scholar]
- De La Rica, R.; Aili, D.; Stevens, M.M. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv. Drug Deliv. Rev. 2012, 64, 967–978. [Google Scholar] [CrossRef]
- Hu, Q.; Katti, P.S.; Gu, Z. Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale 2014, 6, 12273–12286. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.Y.; Feng, J.; Rong, L.; Jia, H.Z.; Chen, S.; Liu, X.J.; Luo, G.F.; Zhuo, R.X.; Zhang, X.Z. Theranostic GO-Based Nanohybrid for Tumor Induced Imaging and Potential Combinational Tumor Therapy. Small 2014, 10, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Z.; Liu, Z.; Shi, P.; Dong, K.; Ju, E.; Ren, J.; Qu, X. A multi-stimuli responsive gold nanocage–hyaluronic platform for targeted photothermal and chemotherapy. Biomaterials 2014, 35, 9678–9688. [Google Scholar] [CrossRef] [PubMed]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef] [PubMed]
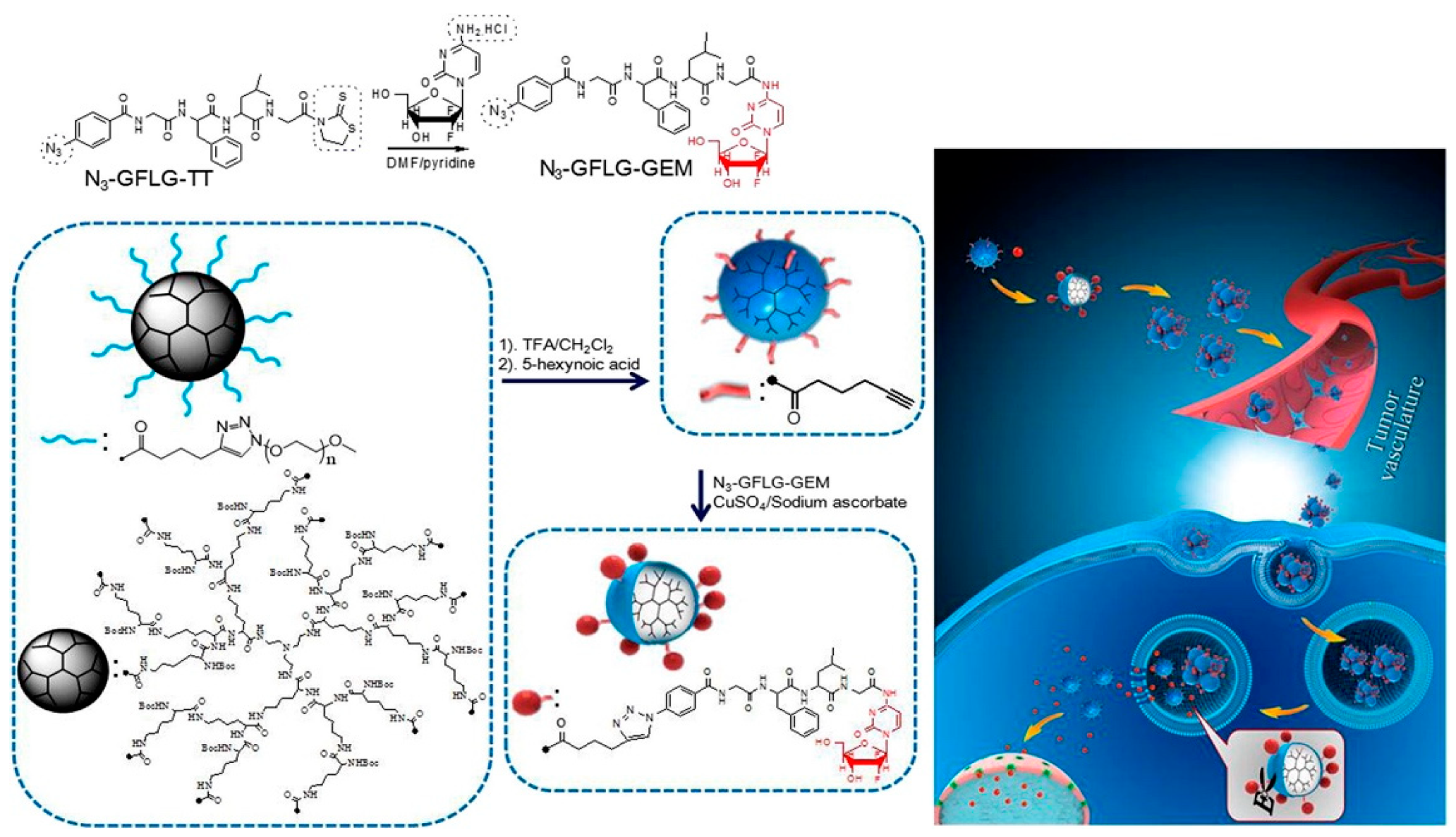
- Zhang, C.; Pan, D.; Li, J.; Hu, J.; Bains, A.; Guys, N.; Zhu, H.; Li, X.; Luo, K.; Gong, Q. Enzyme-responsive peptide dendrimer-gemcitabine conjugate as a controlled-release drug delivery vehicle with enhanced antitumor efficacy. Acta Biomater. 2017, 55, 153–162. [Google Scholar] [CrossRef]
- Randolph, L.M.; Chien, M.-P.; Gianneschi, N.C. Biological stimuli and biomolecules in the assembly and manipulation of nanoscale polymeric particles. Chem. Sci. 2012, 3, 1363–1380. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, G.; Liu, S. Enzyme-responsive polymeric assemblies, nanoparticles and hydrogels. Chem. Soc. Rev. 2012, 41, 5933–5949. [Google Scholar] [CrossRef]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. In Nanoscience And Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 239–250. [Google Scholar]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef]
- Gao, L.; Zheng, B.; Chen, W.; Schalley, C.A. Enzyme-responsive pillar [5] arene-based polymer-substituted amphiphiles: Synthesis, self-assembly in water, and application in controlled drug release. Chem. Commun. 2015, 51, 14901–14904. [Google Scholar] [CrossRef]
- Brancato, V.; Gioiella, F.; Profeta, M.; Imparato, G.; Guarnieri, D.; Urciuolo, F.; Melone, P.; Netti, P.A. 3D tumor microtissues as an in vitro testing platform for microenvironmentally-triggered drug delivery systems. Acta Biomater. 2017, 57, 47–58. [Google Scholar] [CrossRef]
- Wang, B.; Liu, H.; Sun, L.; Jin, Y.; Ding, X.; Li, L.; Ji, J.; Chen, H. Construction of high drug loading and enzymatic degradable multilayer films for self-defense drug release and long-term biofilm inhibition. Biomacromolecules 2017, 19, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Shu, Q.; Wang, L.; Wu, H.; Wang, A.Y.; Mao, H. Layer-by-layer assembled milk protein coated magnetic nanoparticle enabled oral drug delivery with high stability in stomach and enzyme-responsive release in small intestine. Biomaterials 2015, 39, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Van Hove, A.H.; Beltejar, M.-J.G.; Benoit, D.S. Development and in vitro assessment of enzymatically-responsive poly (ethylene glycol) hydrogels for the delivery of therapeutic peptides. Biomaterials 2014, 35, 9719–9730. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ko, J.H.; Mansfield, K.M.; Nauka, P.C.; Bat, E.; Maynard, H.D. Glucose-Responsive Trehalose Hydrogel for Insulin Stabilization and Delivery. Macromol. Biosci. 2018, 18, 1700372. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lin, E.-W.; Lau, U.Y.; Hedrick, J.L.; Bat, E.; Maynard, H.D. Trehalose glycopolymers as excipients for protein stabilization. Biomacromolecules 2013, 14, 2561–2569. [Google Scholar] [CrossRef]
- Mancini, R.J.; Lee, J.; Maynard, H.D. Trehalose glycopolymers for stabilization of protein conjugates to environmental stressors. J. Am. Chem. Soc. 2012, 134, 8474–8479. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lee, J.; Mansfield, K.M.; Ko, J.H.; Sallam, S.; Wesdemiotis, C.; Maynard, H.D. Trehalose glycopolymer enhances both solution stability and pharmacokinetics of a therapeutic protein. Bioconjug. Chem. 2017, 28, 836–845. [Google Scholar] [CrossRef]
- Messina, M.S.; Ko, J.H.; Yang, Z.; Strouse, M.J.; Houk, K.; Maynard, H.D. Effect of trehalose polymer regioisomers on protein stabilization. Polym. Chem. 2017, 8, 4781–4788. [Google Scholar] [CrossRef]
- Pelegri-O’Day, E.M.; Paluck, S.J.; Maynard, H.D. Substituted polyesters by thiol–ene modification: Rapid diversification for therapeutic protein stabilization. J. Am. Chem. Soc. 2017, 139, 1145–1154. [Google Scholar] [CrossRef]
- Chen, J.; Glaus, C.; Laforest, R.; Zhang, Q.; Yang, M.; Gidding, M.; Welch, M.J.; Xia, Y. Gold nanocages as photothermal transducers for cancer treatment. Small 2010, 6, 811–817. [Google Scholar] [CrossRef]
- Moon, G.D.; Choi, S.-W.; Cai, X.; Li, W.; Cho, E.C.; Jeong, U.; Wang, L.V.; Xia, Y. A new theranostic system based on gold nanocages and phase-change materials with unique features for photoacoustic imaging and controlled release. J. Am. Chem. Soc. 2011, 133, 4762–4765. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Cai, H.; Zhang, H.; Chen, K.; Li, N.; Xu, Z.; Gong, Q.; Luo, K. PEGylated Multistimuli-Responsive Dendritic Prodrug-Based Nanoscale System for Enhanced Anticancer Activity. ACS Appl. Mater. Interfaces 2018, 10, 35770–35783. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.; Singh, N.; Surnar, B.; Jayakannan, M. Enzyme and Thermal Dual Responsive Amphiphilic Polymer Core–Shell Nanoparticle for Doxorubicin Delivery to Cancer Cells. Biomacromolecules 2015, 17, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Hervault, A.; Dunn, A.E.; Lim, M.; Boyer, C.; Mott, D.; Maenosono, S.; Thanh, N.T. Doxorubicin loaded dual pH-and thermo-responsive magnetic nanocarrier for combined magnetic hyperthermia and targeted controlled drug delivery applications. Nanoscale 2016, 8, 12152–12161. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, K.; Williams, G.R.; Wu, J.; Wu, J.; Wang, H.; Niu, S.; Zhu, L.-M. Dual temperature and pH responsive nanofiber formulations prepared by electrospinning. Colloids Surf. B Biointerfaces 2018, 171, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Davaran, S.; Ghamkhari, A.; Alizadeh, E.; Massoumi, B.; Jaymand, M. Novel dual stimuli-responsive ABC triblock copolymer: RAFT synthesis,“schizophrenic” micellization, and its performance as an anticancer drug delivery nanosystem. J. Colloid Interface Sci. 2017, 488, 282–293. [Google Scholar] [CrossRef] [PubMed]
- de Solorzano, I.O.; Alejo, T.; Abad, M.; Bueno-Alejo, C.; Mendoza, G.; Andreu, V.; Irusta, S.; Sebastian, V.; Arruebo, M. Cleavable and thermo-responsive hybrid nanoparticles for on-demand drug delivery. J. Colloid Interface Sci. 2019, 533, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Wang, Y.; Wang, C.; Xiao, J.; Zhang, Q.; Cheng, Y. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials 2016, 81, 114–124. [Google Scholar] [CrossRef] [PubMed]
| Stimuli | Polymer | Major Result(s) | Ref(s) |
|---|---|---|---|
| pH | N-carboxyethyl chitosan/dibezaldehyde-terminated poly(ethylene glycol) | pH changes promote chemical and physical modifications that swell the system inducing cargo release | Qu et al. [6] |
| pH | Poly(lactic acid)-poly(ethyleneimine) | Burst release of doxorubicin (DOX) as pH shifted from 7.4 to 5.4 | Li et al. [2] |
| pH | Poly(lactic-co-glycolic acid) (PLGA) | Morphological change induces drug release | Chung et al. [11] |
| pH | Poly(acrylamide) | Drug release at pH > 4.0 | Pafiti et al. [18] |
| Ultrasound | Poly(ethylene glycol) | Led to a six-fold increase in the cumulative release | Kearney et al. [19] |
| Ultrasound | Alginate | Pulsed stimulation outperformed constant stimulation | Huebsch et al. [20] |
| Ultrasound | Chitosan | Significant release compared to no stimulus | Zhou et al. [21] |
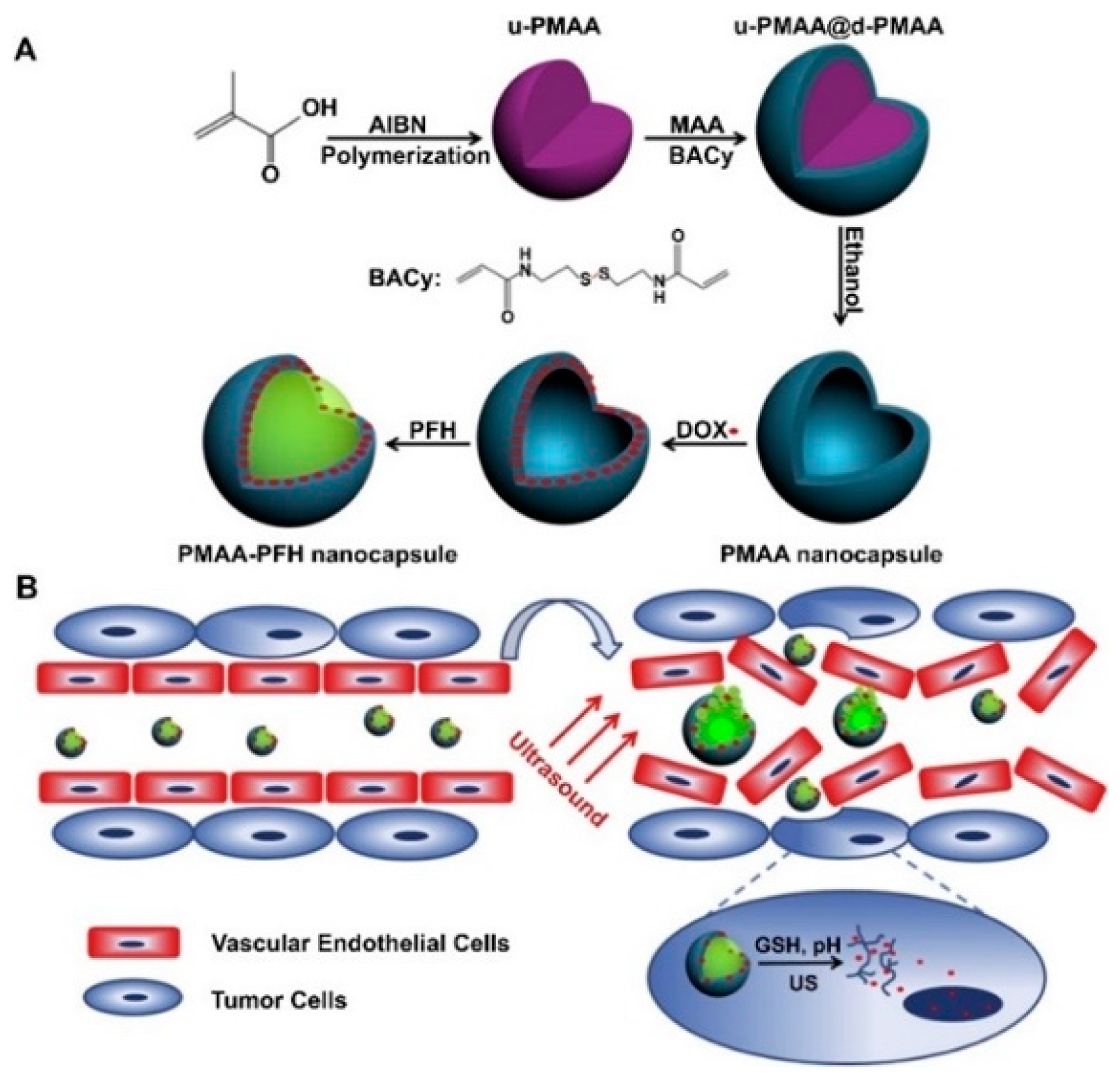
| Ultrasound | Poly(methacrylic acid) (PMAA) | Design a three in one theranostic nanoplatform for imaging and release | Yang et al. [22] |
| Ultrasound | Poly(2-oxazoline) micelles | Possible carrier with increased release | Salgarella et al. [25] |
| Ultrasound | polylactic acid (PLA) | Long-term encapsulation of small hydrophilic molecules and four times the release profile with HIFU | Gai et al. [26] |
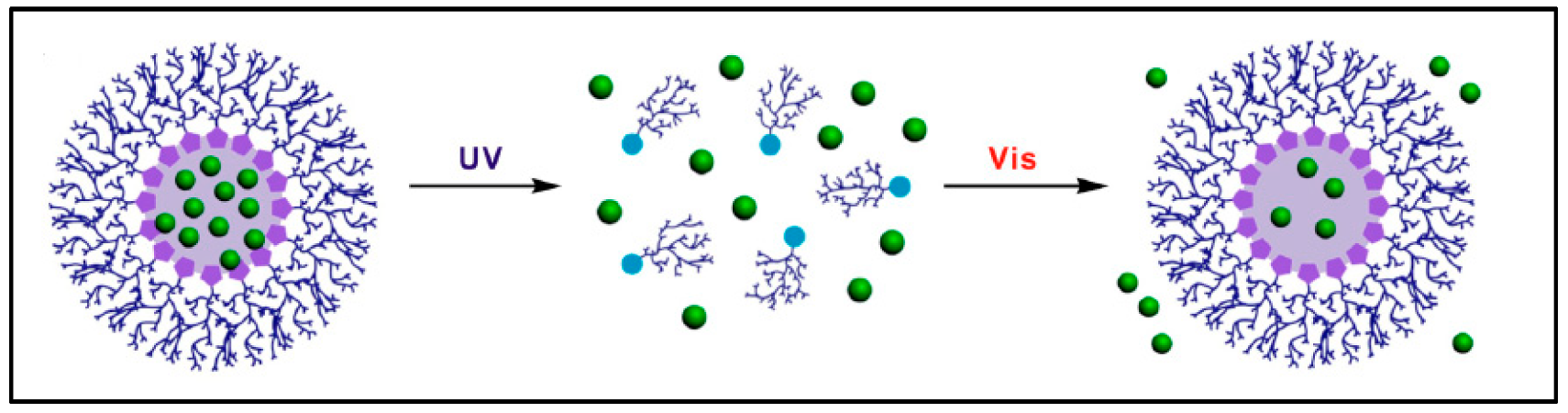
| UV | Spiropyran-hyperbranched polyglycerol micelle | Assembly and disassembly of micelle induced by UV light exposure controls the drug release. Superior biocompatibility with cells in the absence of UV | Son et al. [34] |
| UV | Azobenzene-β-galactose micelle | Short UV exposure (2 min) to release drug; low cytotoxicity of unloaded micelles | Pearson et al. [36] |
| UV | 2-hydroxyethyl methacrylate and ethylene glycol dimethacrylate | Deliver multiple doses of drug upon UV exposure over a prolonged period of time (≤160 h) | Hardy et al. [37] |
| UV | mPEG-PLGA nanoparticle | Reverse multidrug resistance of tumor cells; enhance chemosensitization of cells to DOX therapy | Fan et al. [38] |
| NIR | Diselenide-cross-linked poly(methacrylic acid) | Controlled illumination with specific number of irradiation times allowed for on-demand controlled drug release and nanogel degradation. Rapid internalization by HeLa cell and cytotoxic under NIR irradiation | Tian et al. [40] |
| NIR | Β-cylcodextrin | Anticancer activity in vitro and in vivo against breast cancer, with accelerated drug release upon NIR exposure | Liang et al. [41] |
| NIR | Polycaprolactone | On-demand, stepwise drug-release after multiple cycles of NIR exposure with low off-state leakage. | Chen et al. [42] |
| Red light | Tetra-ortho-methoxy-substituted azobenzene & β-cyclodextrin | Responsive to red light instead of UV. Deeper tissue penetration depth | Wang et al. [44] |
| AMF | Aminosilan-type shell | EMF stimulation of SPIONS can maintain elevated temperatures of approximately 45 °C in glioblastoma multiforme tumors | Maier-Hauff et al. [47] |
| AMF | Polyethylene glycol w/azo drug linker | SPION local temperature can increase up to 50 °C without inducing significant temperature increases in media at sufficiently low concentrations | Riedinger et al. [48] |
| AMF | (N-isopropylacrylamide)-(N-hydroxymethyl) acrylamide | SPION stimulation can trigger PNIPAM critical temperature transition without increasing temperature of surrounding media | Guisasola et al. [49] |
| AMF | Poly(maleic anhydride-alt-1-octadecene) | Distance from the nanoparticle surface can be used to control temperature dependent effects during AMF stimulation | Dias et al. [50] |
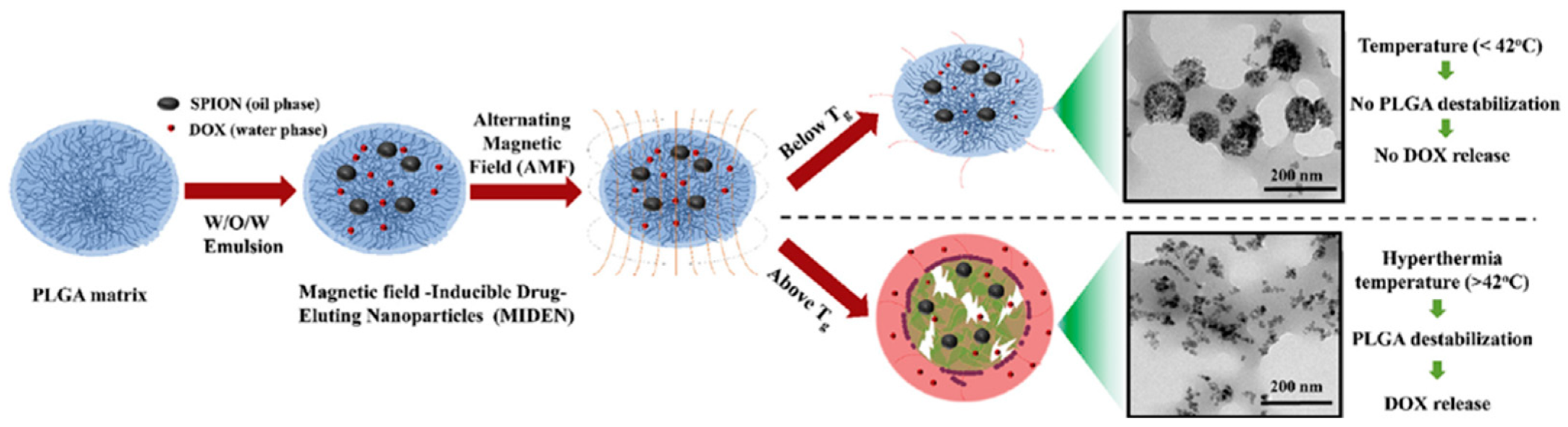
| AMF | PLGA | SPION stimulation induced drug release by increasing temperature above the glass transition of PLGA | Thirunavukkarasu et al. [51] |
| Permanent magnet | Tetramethylazanium hydroxide | Intrathecally delivered SPIONS loaded with NSAIDS produced magnetic field dependent reductions in pain and inflammatory markers in a murine model | Wu et al. [52] |
| Permanent magnet | Polyethyleneimine | External magnetic guidance improved accumulation of SPIONS in arthritic joints in a rat model | Duan et al. [54] |
| AMF | Chitosan-polyethylene glycol | SPION loaded microbeads can respond to multiple stimuli and increase drug release to efficacious levels as the carrier nears exhaustion | Mohapatra et al. [56] |
| Electric | Agarose/alginate-aniline tetramer | Conductive tetramers improve hydrogel biocompatability with neural cells and enables repeat stimuli responsive drug release | Atoufi et al. [59] |
| Electric | Poly(3,4-ethylenedioxypyrrole) | Stimulation induces rapid release of ionically bound ibuprofen but not ibuprofen physically entrapped in the matrix during electrochemical polymerization | Krukiewicz et al. [60] |
| Electric | Poly(3-methoxydiphenylamine)/Pectin blend | Stimulation increased hydrogel mesh pore size allowing increased drug elution | Mongkolkitikul et al. [61] |
| Electric | Polypyrrole | Sacrificial templates can be used to create electrically responsive nanowires | Lee et al. [62] |
| Electric | Monoferrocene functionalized β-cyclodextrin | Stimulus-induced conformational changes can be used to control polymeric ‘gates’ for on/off delivery using mesoporous particles | Wang et al. [63] |
| Enzyme | PEGylated alkynylated peptide dendrimer | Minimal release in the absence of Cathepsin B | Zhang et al. [75] |
| Enzyme | Polydimethylsiloxane, polyethylenimine | Release in the presence of HAS, E. coli, or S. aureus | Wang et al. [82] |
| Enzyme | Poly(maleic acid) | No release until exposure to intestine protease trypsin | Huang et al. [83] |
| Enzyme | Poly(ethylene glycol) | Peptide cleaving at desired sites | Van Hove et al. [84] |
| Enzyme | Poly(styrenyl ether trehalose), poly(ethylene glycol) | Ability to withstand elevated temperatures with cargo intact | Lee et al. [85] |
| Enzyme, NIR | Poly(vinyl pyrrolidone) | Minimal release in the absence of hyaluronidase, NIR promoting more release | Wang et al. [73] |
| Enzyme, pH | Poly(ethylene glycol) | Release rate increase at pH 5.4 in presence of cathepsin B and glutathione | Duan et al. [93] |
| Enzyme, Thermal | 3-pentadecylphenol, oligoethylene glycol acrylate | Proposed release at tissue based on temperature with intracellular release concurrent with enzyme exposure | Kashyap et al. [94] |
| pH, Thermal | Poly(ethylene glycol) methyl ether methacrylate | pH and temperature greatly influence the release of DOX | Hervault et al. [95] |
| pH, Thermal | Poly(N-vinylcaprolactam), ethyl cellulose, Eudagrit L100 | Most pronounced release occurred at 25 °C and pH 7.4 | Li et al. [96] |
| pH, Thermal | Poly(2-succinyloxyethyl methacrylate)-b-(N-isopropylacrylamide)-b-[(N-4-vinylbenzyl),N,N-diethylamine]], [P(SEMA-b-NIPAAm-b-VEA)] | Greatest DOX release observed at 37 °C and pH 4, increase in temperature led to decrease in DOX release | Davaran et al. [97] |
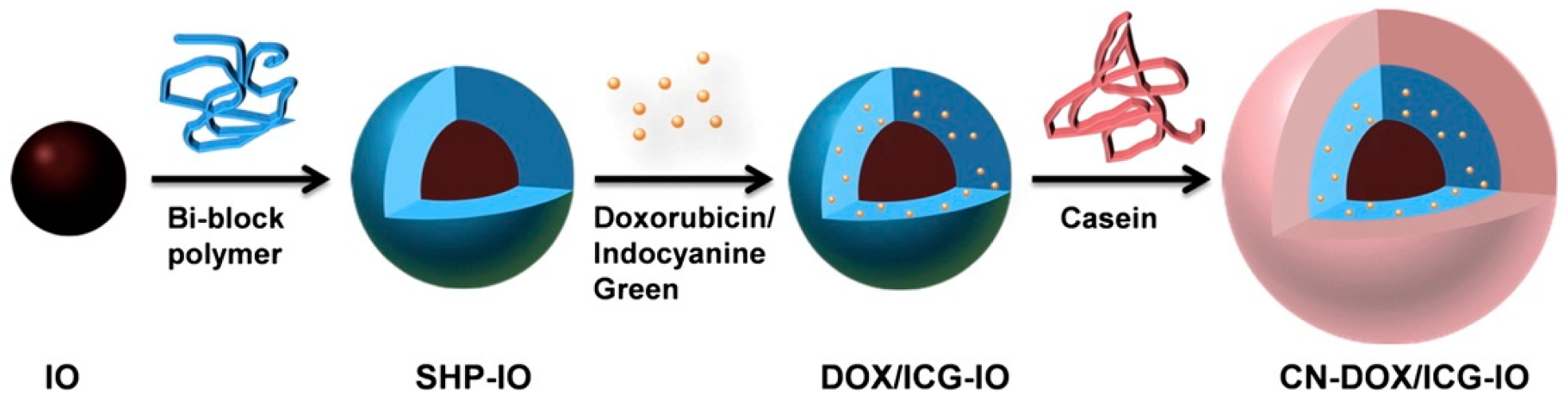
| NIR, Thermal | Poly(ethylene glycol) methyl ether methacrylate, poly(vinyl pyrrolidone) | Release was higher at 45 °C with a burst increase synonymous with NIR irradiation | Ortiz de Solorzano et al. [98] |
| NIR, pH, Redox | Poly(ethylene glycol), poly(dopamine) | NIR irradiation release is function of exposure time, pH and redox release greatest at pH 7.4 | Wang et al. [99] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-Responsive Drug Release from Smart Polymers. J. Funct. Biomater. 2019, 10, 34. https://doi.org/10.3390/jfb10030034
Wells CM, Harris M, Choi L, Murali VP, Guerra FD, Jennings JA. Stimuli-Responsive Drug Release from Smart Polymers. Journal of Functional Biomaterials. 2019; 10(3):34. https://doi.org/10.3390/jfb10030034
Chicago/Turabian StyleWells, Carlos M., Michael Harris, Landon Choi, Vishnu Priya Murali, Fernanda Delbuque Guerra, and J. Amber Jennings. 2019. "Stimuli-Responsive Drug Release from Smart Polymers" Journal of Functional Biomaterials 10, no. 3: 34. https://doi.org/10.3390/jfb10030034
APA StyleWells, C. M., Harris, M., Choi, L., Murali, V. P., Guerra, F. D., & Jennings, J. A. (2019). Stimuli-Responsive Drug Release from Smart Polymers. Journal of Functional Biomaterials, 10(3), 34. https://doi.org/10.3390/jfb10030034






