Six-Month Color Stability Assessment of Two Calcium Silicate-Based Cements Used in Regenerative Endodontic Procedures
Abstract
:1. Introduction
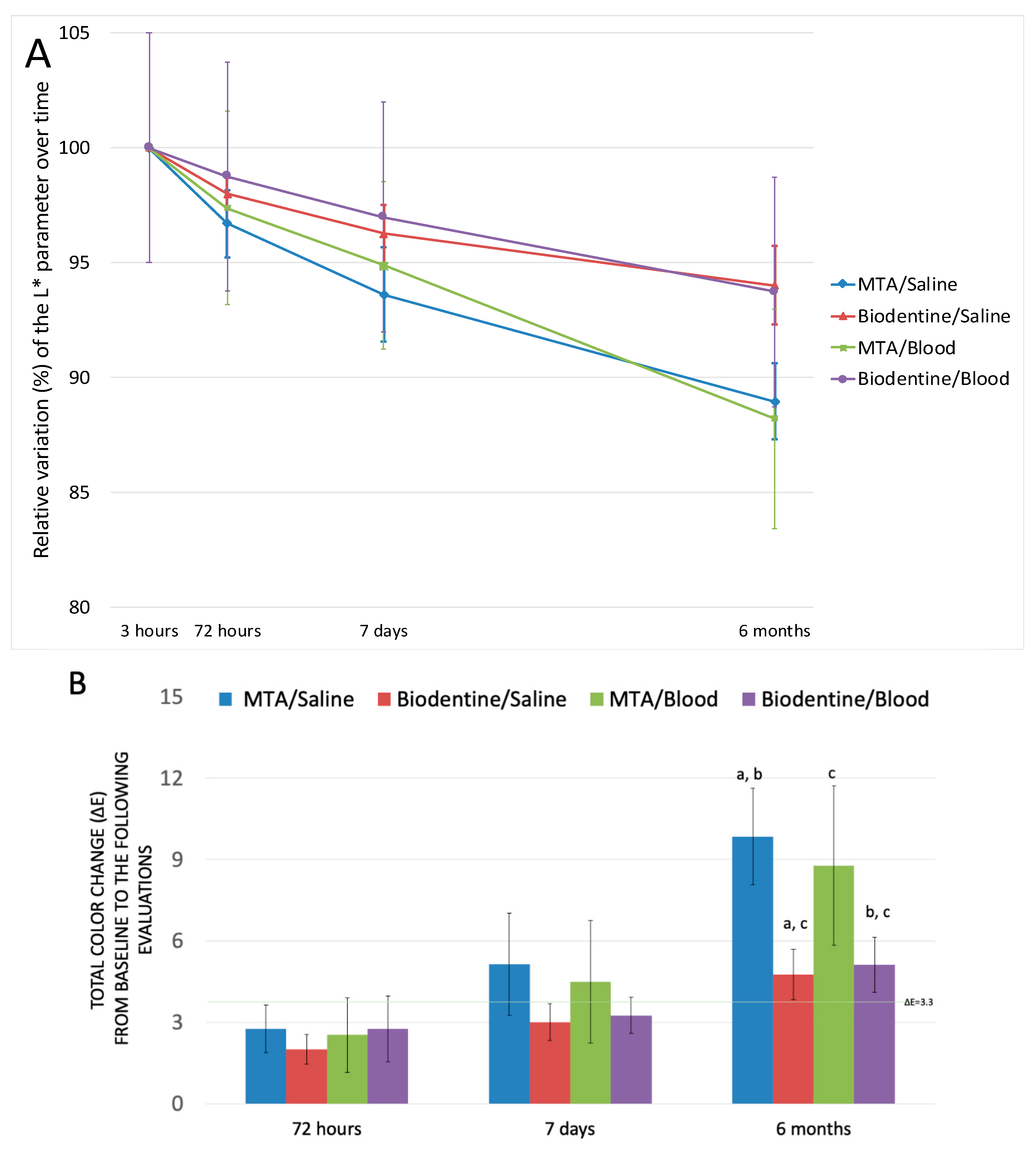
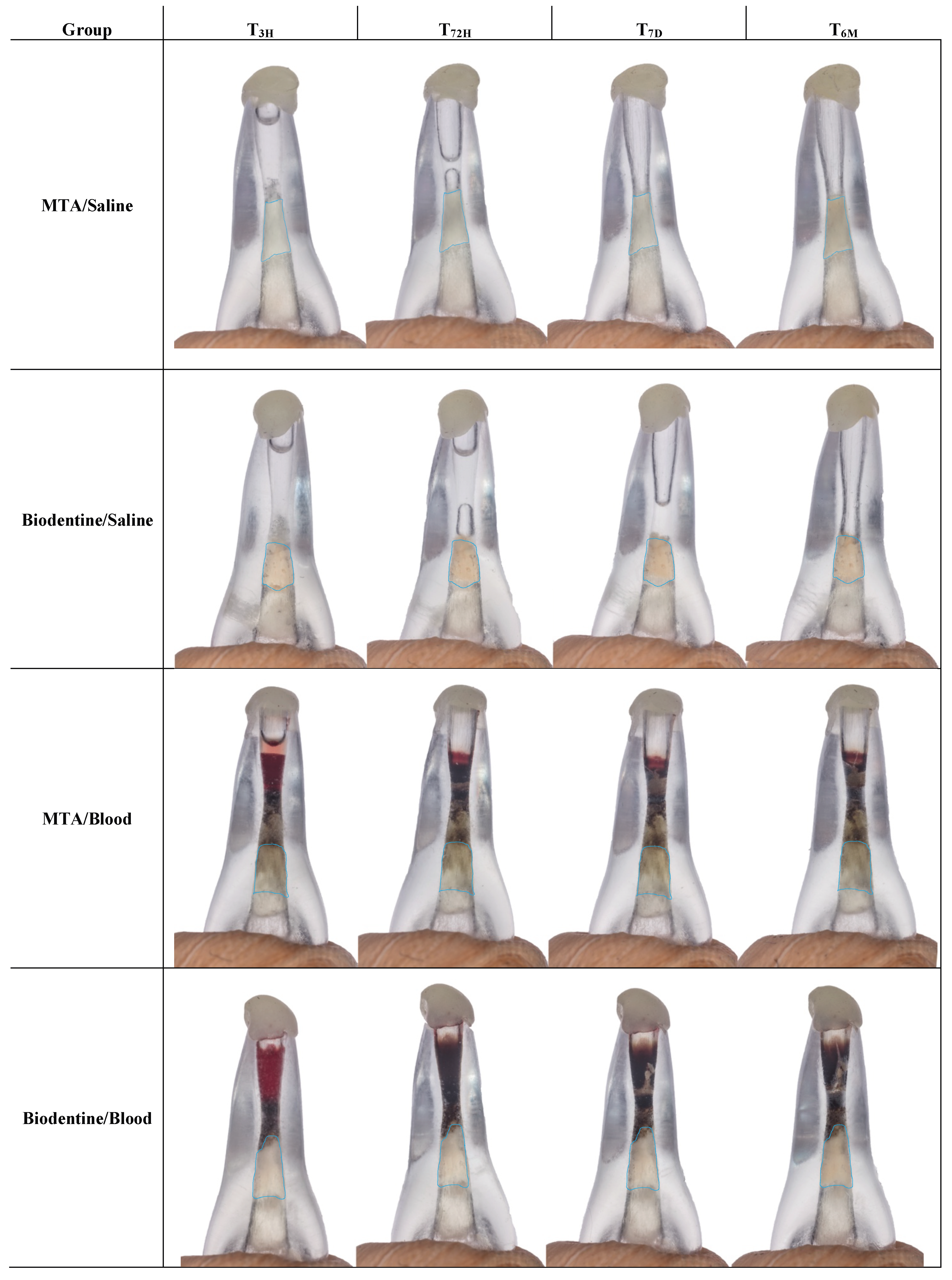
2. Results
3. Discussion
4. Materials and Methods
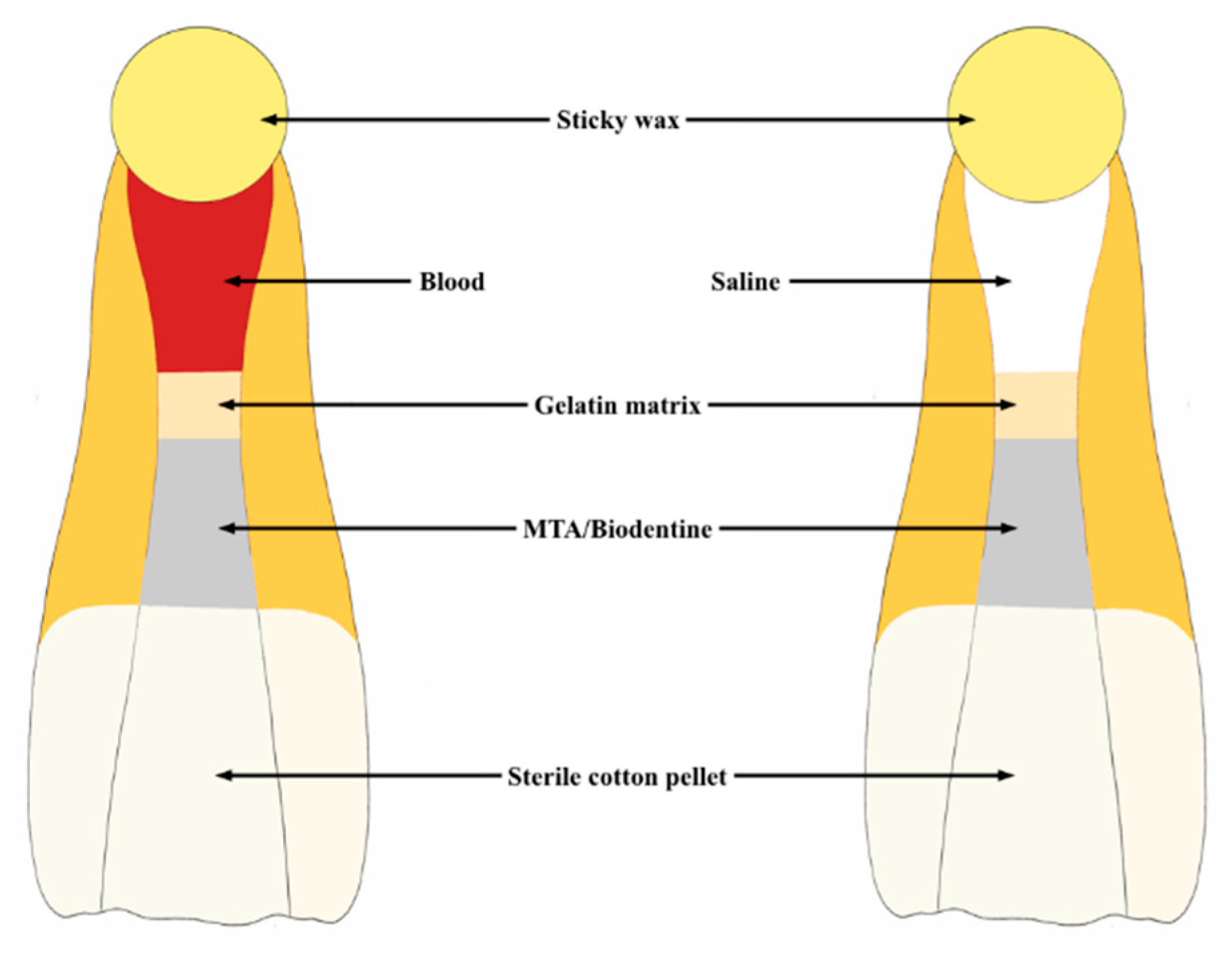
4.1. Experimental Groups
4.2. Sample Preparation
4.3. Photographic Record
4.4. Color Assessment
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hattab, F.N.; Qudeimat, M.A.; al-Rimawi, H.S. Dental discoloration: An overview. J. Esthet. Dent. 1999, 11, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.M.; Abbott, P.V. Discolouration potential of endodontic procedures and materials: A review. Int. Endod. J. 2012, 45, 883–897. [Google Scholar] [CrossRef]
- Rafter, M. Apexification: A review. Dent. Traumatol. 2005, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M. Mineral trioxide aggregate: A comprehensive literature review—Part III: Clinical applications, drawbacks, and mechanism of action. J. Endod. 2010, 36, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Mente, J.; Leo, M.; Panagidis, D.; Ohle, M.; Schneider, S.; Bermejo, J.L.; Pfefferle, T. Treatment outcome of mineral trioxide aggregate in open apex teeth. J. Endod. 2013, 39, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative endodontics: A review of current status and a call for action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic evaluation of regenerative endodontic procedures with the use of chitosan scaffolds in immature dog teeth with apical periodontitis. J. Endod. 2017, 43, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.M.; Geisler, T.; Henry, M.; Wang, Y. Regeneration potential of the young permanent tooth: What does the future hold? J. Endod. 2008, 34, S51–S56. [Google Scholar] [CrossRef]
- Nosrat, A.; Homayounfar, N.; Oloomi, K. Drawbacks and unfavorable outcomes of regenerative endodontic treatments of necrotic immature teeth: A literature review and report of a case. J. Endod. 2012, 38, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Endodontists AAo. AAE Clinical Considerations for a Regenerative Procedure. 2016. Available online: https://http://www.aae.org/specialty/wp-content/uploads/sites/2/2017/06/currentregenerativeendodonticconsiderations.pdf (accessed on 25 April 2018).
- Galler, K.M.; Krastl, G.; Simon, S.; Van Gorp, G.; Meschi, N.; Vahedi, B.; Lambrechts, P. European Society of Endodontology position statement: Revitalization procedures. Int. Endod. J. 2016, 49, 717–723. [Google Scholar] [CrossRef]
- Torabinejad, M.; Nosrat, A.; Verma, P.; Udochukwu, O. Regenerative Endodontic Treatment or Mineral Trioxide Aggregate Apical Plug in Teeth with Necrotic Pulps and Open Apices: A Systematic Review and Meta-analysis. J. Endod. 2017, 43, 1806–1820. [Google Scholar] [CrossRef] [PubMed]
- Kahler, B.; Rossi-Fedele, G. A Review of Tooth Discoloration after Regenerative Endodontic Therapy. J. Endod. 2016, 42, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Kohli, M.R.; Yamaguchi, M.; Setzer, F.C.; Karabucak, B. Spectrophotometric Analysis of Coronal Tooth Discoloration Induced by Various Bioceramic Cements and Other Endodontic Materials. J. Endod. 2015, 41, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Septodont. Biodentine Active Biosilicate Technology. 2017. Available online: http://www.septodont.co.uk/sites/uk/files/2016-08/brochure Biodentine HD UK.pdf (accessed on 13 June 2017).
- Valles, M.; Roig, M.; Duran-Sindreu, F.; Martinez, S.; Mercade, M. Color Stability of Teeth Restored with Biodentine: A 6-month In Vitro Study. J. Endod. 2015, 41, 1157–1160. [Google Scholar] [CrossRef]
- Ramos, J.C.; Palma, P.J.; Nascimento, R.; Caramelo, F.; Messias, A.; Vinagre, A.; Santos, J.M. 1-year In vitro evaluation of tooth discoloration induced by 2 calcium silicate-based cements. J. Endod. 2016, 42, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Lenherr, P.; Allgayer, N.; Weiger, R.; Filippi, A.; Attin, T.; Krastl, G. Tooth discoloration induced by endodontic materials: A laboratory study. Int. Endod. J. 2012, 45, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Felman, D.; Parashos, P. Coronal tooth discoloration and white mineral trioxide aggregate. J. Endod. 2013, 39, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, B.M.; Tartari, T.; Marciano, M.A.; Vivan, R.R.; Mondeli, R.F.; Camilleri, J.; Duarte, M.A.H. Color stability, radiopacity, and chemical characteristics of white mineral trioxide aggregate associated with 2 different vehicles in contact with blood. J. Endod. 2015, 41, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Shokouhinejad, N.; Nekoofar, M.H.; Pirmoazen, S.; Shamshiri, A.R.; Dummer, P.M. Evaluation and Comparison of Occurrence of Tooth Discoloration after the Application of Various Calcium Silicate-based Cements: An Ex Vivo Study. J. Endod. 2016, 42, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.J.; Marques, J.A.; Falacho, R.I.; Vinagre, A.; Santos, J.M.; Ramos, J.C. Does Delayed Restoration Improve Shear Bond Strength of Different Restorative Protocols to Calcium Silicate-Based Cements? Materials 2018, 11, 2216. [Google Scholar] [CrossRef] [PubMed]
- Yoldas, S.E.; Bani, M.; Atabek, D.; Bodur, H. Comparison of the Potential Discoloration Effect of Bioaggregate, Biodentine, and White Mineral Trioxide Aggregate on Bovine Teeth: In Vitro Research. J. Endod. 2016, 42, 1815–1818. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, D.; ASeabra, C.; Palma, P.J.; Cardoso, A.; Peça, J.; Santos, J.M. Effects of a New Bioceramic Material on Human Apical Papilla Cells. J. Funct. Biomater. 2018, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Marin, P.D.; Bartold, P.M.; Heithersay, G.S. Tooth discoloration by blood: An in vitro histochemical study. Endod. Dent. Traumatol. 1997, 13, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Widbiller, M.; Ducke, S.; Eidt, A.; Buchalla, W.; Galler, K.M. A training model for revitalization procedures. Int. Endod. J. 2018, 51 (Suppl. 4), e301–e308. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Montesin, F.E.; Brady, K.; Sweeney, R.; Curtis, R.V.; Ford, T.R. The constitution of mineral trioxide aggregate. Dent. Mater. 2005, 21, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Parirokh, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part II: Other clinical applications and complications. Int. Endod. J. 2018, 51, 284–317. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Kang, M.; Ahn, S.; Kim, S.; Kim, W.; Kim, Y.; Kim, E. Tooth discoloration after the use of new pozzolan cement (Endocem) and mineral trioxide aggregate and the effects of internal bleaching. J. Endod. 2013, 39, 1598–1602. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J. Color stability of white mineral trioxide aggregate in contact with hypochlorite solution. J. Endod. 2014, 40, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Marciano, M.A.; Costa, R.M.; Camilleri, J.; Mondelli, R.F.; Guimaraes, B.M.; Duarte, M.A. Assessment of color stability of white mineral trioxide aggregate angelus and bismuth oxide in contact with tooth structure. J. Endod. 2014, 40, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Marciano, M.A.; Duarte, M.A.; Camilleri, J. Dental discoloration caused by bismuth oxide in MTA in the presence of sodium hypochlorite. Clin. Oral Investig. 2015, 19, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Valles, M.; Mercade, M.; Duran-Sindreu, F.; Bourdelande, J.L.; Roig, M. Influence of light and oxygen on the color stability of five calcium silicate-based materials. J. Endod. 2013, 39, 525–528. [Google Scholar] [CrossRef]
- Marconyak, L.J., Jr.; Kirkpatrick, T.C.; Roberts, H.W.; Roberts, M.D.; Aparicio, A.; Himel, V.T.; Sabey, K.A. A Comparison of Coronal Tooth Discoloration Elicited by Various Endodontic Reparative Materials. J. Endod. 2016, 42, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.; Palma, P.J.; Ramos, J.C.; Cabrita, A.S.; Friedman, S. Periapical inflammation subsequent to coronal inoculation of dog teeth root filled with resilon/epiphany in 1 or 2 treatment sessions with chlorhexidine medication. J. Endod. 2014, 40, 837–841. [Google Scholar] [CrossRef] [PubMed]
| Groups | Coordinates | T3H | T72H | T7D | T6M | p |
|---|---|---|---|---|---|---|
| MTA/Saline | L* | 78.52 ± 1.58 | 75.95 ± 1.79 | 73.51 ± 2.43 | 69.86 ± 1.88 | <0.01 * |
| a* | −0.68 ± 0.10 | −0.56 ± 0.14 | 0.05 ± 0.22 | 0.78 ± 0.78 | <0.01 * | |
| b* | 5.15 ± 0.36 | 5.53 ± 0.78 | 5.51 ± 0.59 | 8.37 ± 3.28 | 0.016 * | |
| Biodentine/Saline | L* | 73.87 ± 1.37 | 72.39 ± 0.84 | 71.11 ± 1.42 | 69.46 ± 1.92 | <0.01 * |
| a* | 2.88 ± 0.16 | 3.12 ± 0.25 | 3.22 ± 0.27 | 3.36 ± 0.29 | 0.003 * | |
| b* | 11.12 ± 0.92 | 11.89 ± 1.01 | 11.90 ± 1.13 | 12.21 ± 0.98 | 0.0046 | |
| MTA/Blood | L* | 72.79 ± 5.70 | 70.86 ± 4.81 | 69.07 ± 4.06 | 64.22 ± 4.95 | <0.001 * |
| a* | 0.20 ± 0.63 | −0.02 ±0.037 | 0.41 ± 0.49 | 0.41 ± 0.64 | 0.047 | |
| b* | 7.14 ± 2.78 | 7.44 ± 2.73 | 7.80 ± 2.92 | 7.65 ± 2.24 | 0.593 | |
| Biodentine/Blood | L* | 71.21 ± 3.22 | 70.33 ± 3.98 | 69.07 ± 4.10 | 66.75 ± 3.38 | <0.01 * |
| a* | 2.92 ± 0.23 | 2.48 ± 0.48 | 2.69 ± 0.55 | 2.88 ± 0.37 | 0.128 | |
| b* | 9.32 ± 0.93 | 8.77 ± 2.56 | 9.33 ± 1.78 | 11.05 ±1.65 | 0.035 * |
| MTA/Saline | Biodentine/Saline | MTA/Blood | Biodentine/Blood | Two-Way ANOVA | |||
|---|---|---|---|---|---|---|---|
| Factor Material | Factor Treatment | Interaction (Material * Treatment) | |||||
| ΔE T3H − T72H | 2.76 ± 1.43 | 2.00 ± 0.88 | 2.53 ± 2.23 | 2.76 ± 1.96 | 0.625 | 0.629 | 0.364 |
| ΔE T3H − T7D | 5.14 ± 3.03 | 3.01 ± 1.10 | 4.49 ± 3.65 | 3.26 ± 1.07 | 0.040 * | 0.798 | 0.568 |
| ΔE T3H − T6M | 9.84 ± 2.67 | 4.76 ± 1.48 | 8.77 ± 4.73 | 5.12 ± 1.63 | <0.01 * | 0.708 | 0.452 |
| Friedman test | <0.01 * | 0.01 * | <0.01 * | 0.082 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma, P.J.; Marques, J.A.; Falacho, R.I.; Correia, E.; Vinagre, A.; Santos, J.M.; Ramos, J.C. Six-Month Color Stability Assessment of Two Calcium Silicate-Based Cements Used in Regenerative Endodontic Procedures. J. Funct. Biomater. 2019, 10, 14. https://doi.org/10.3390/jfb10010014
Palma PJ, Marques JA, Falacho RI, Correia E, Vinagre A, Santos JM, Ramos JC. Six-Month Color Stability Assessment of Two Calcium Silicate-Based Cements Used in Regenerative Endodontic Procedures. Journal of Functional Biomaterials. 2019; 10(1):14. https://doi.org/10.3390/jfb10010014
Chicago/Turabian StylePalma, Paulo J., Joana A. Marques, Rui I. Falacho, Eder Correia, Alexandra Vinagre, João Miguel Santos, and João C. Ramos. 2019. "Six-Month Color Stability Assessment of Two Calcium Silicate-Based Cements Used in Regenerative Endodontic Procedures" Journal of Functional Biomaterials 10, no. 1: 14. https://doi.org/10.3390/jfb10010014
APA StylePalma, P. J., Marques, J. A., Falacho, R. I., Correia, E., Vinagre, A., Santos, J. M., & Ramos, J. C. (2019). Six-Month Color Stability Assessment of Two Calcium Silicate-Based Cements Used in Regenerative Endodontic Procedures. Journal of Functional Biomaterials, 10(1), 14. https://doi.org/10.3390/jfb10010014










