Effect of Calcium Precursor on the Bioactivity and Biocompatibility of Sol-Gel-Derived Glasses
Abstract
1. Introduction
2. Results
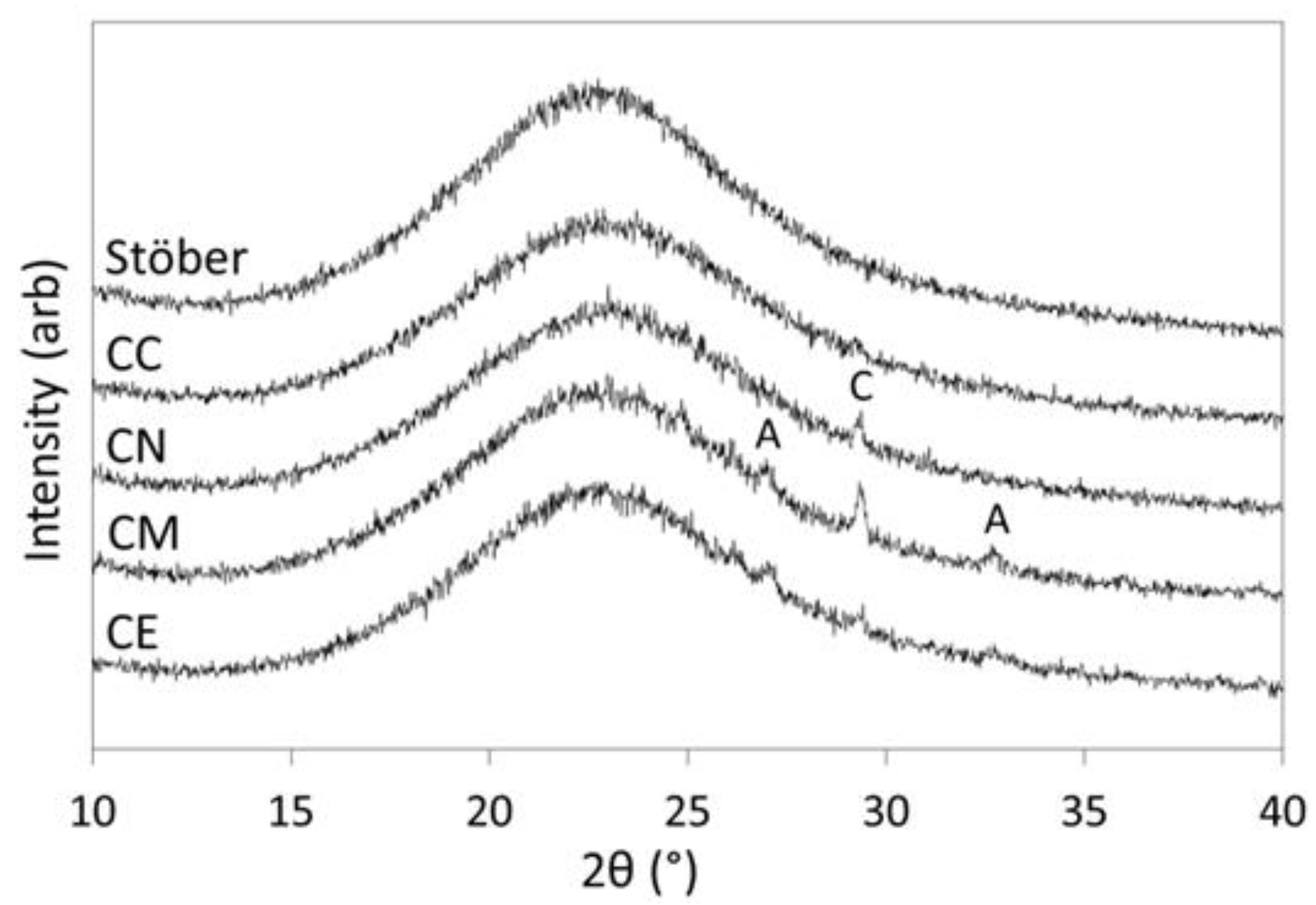
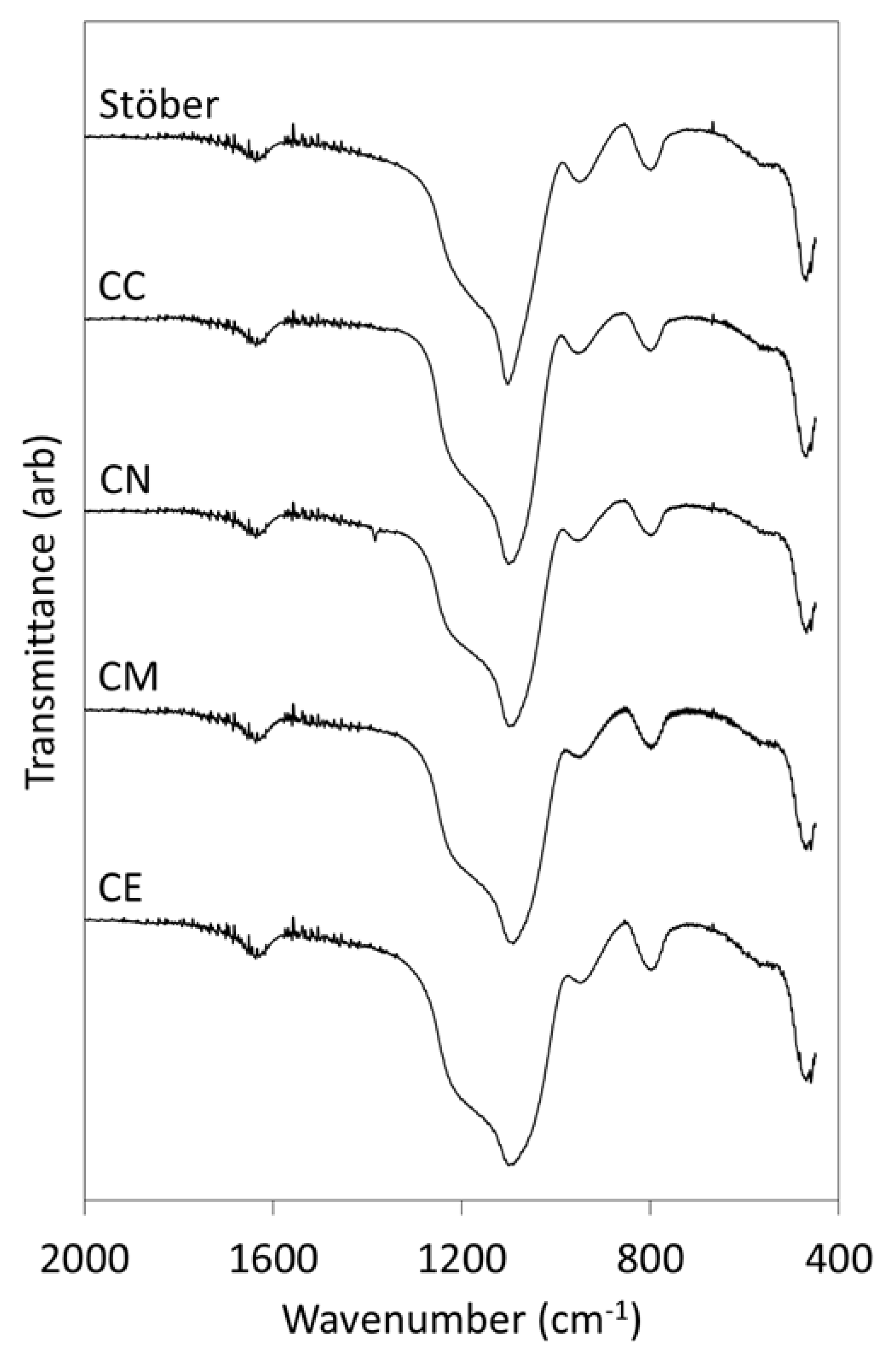
2.1. Materials Characterisation
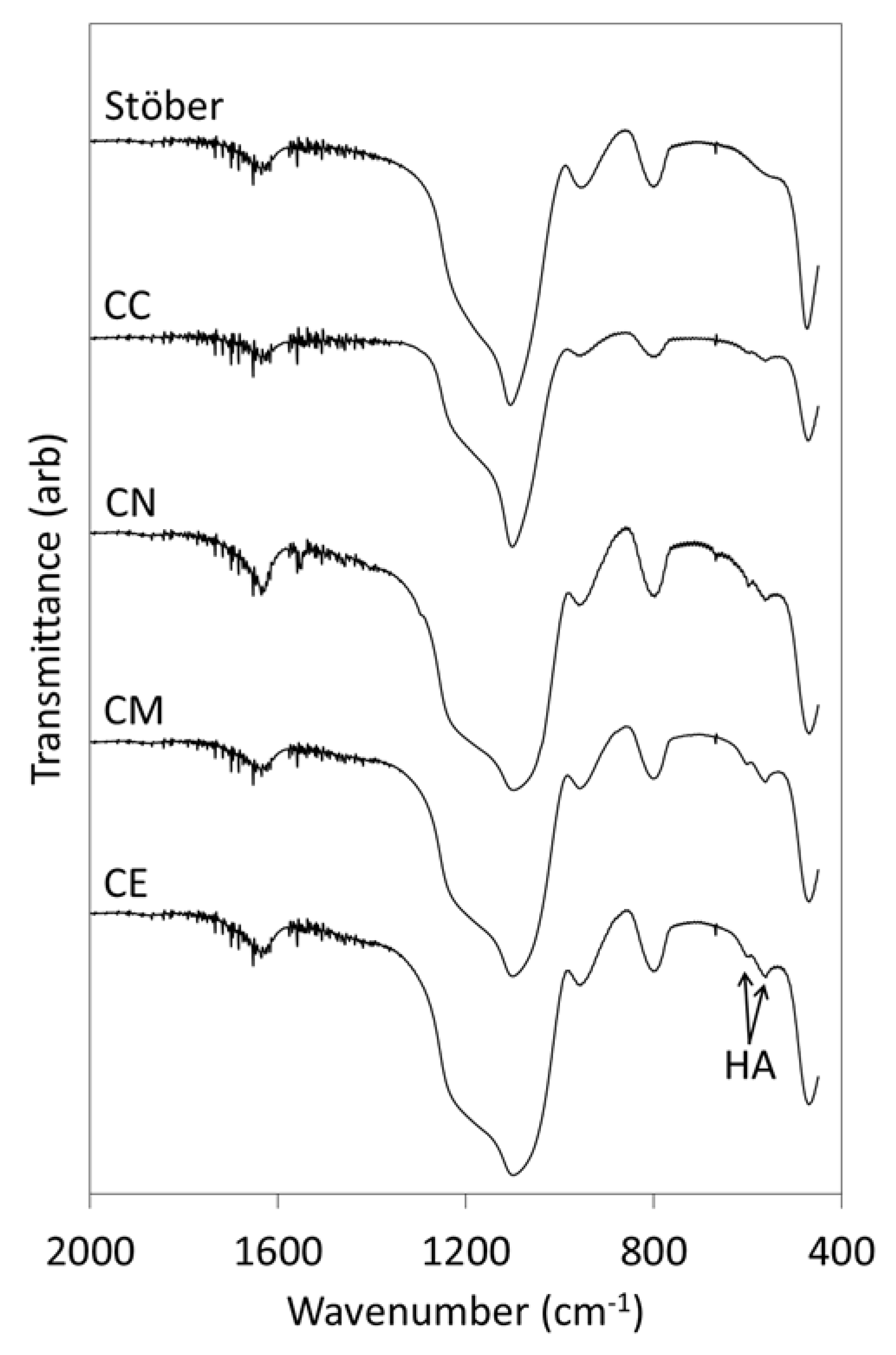
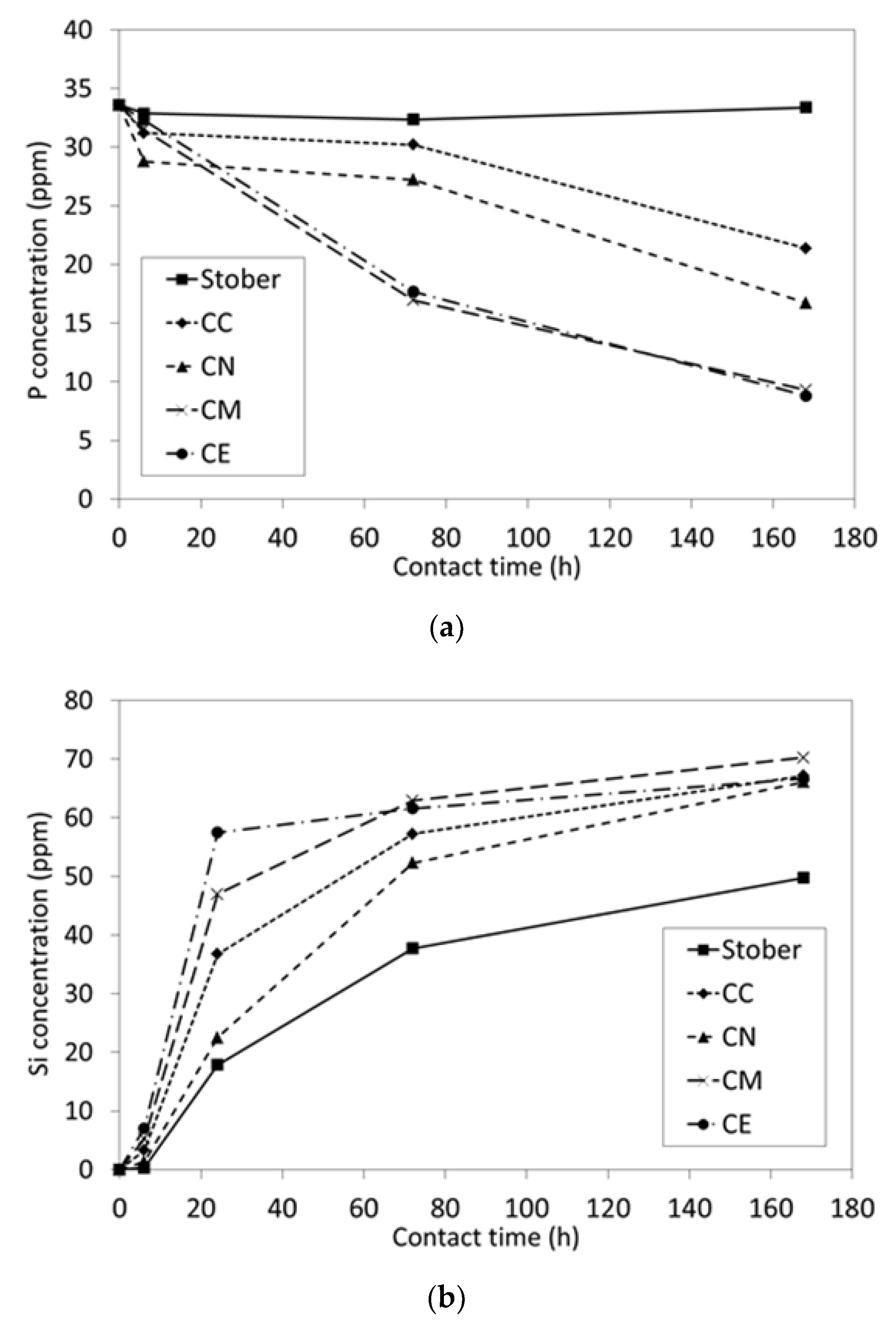
2.2. In Vitro Bioactivity and Dissolution Characteristics
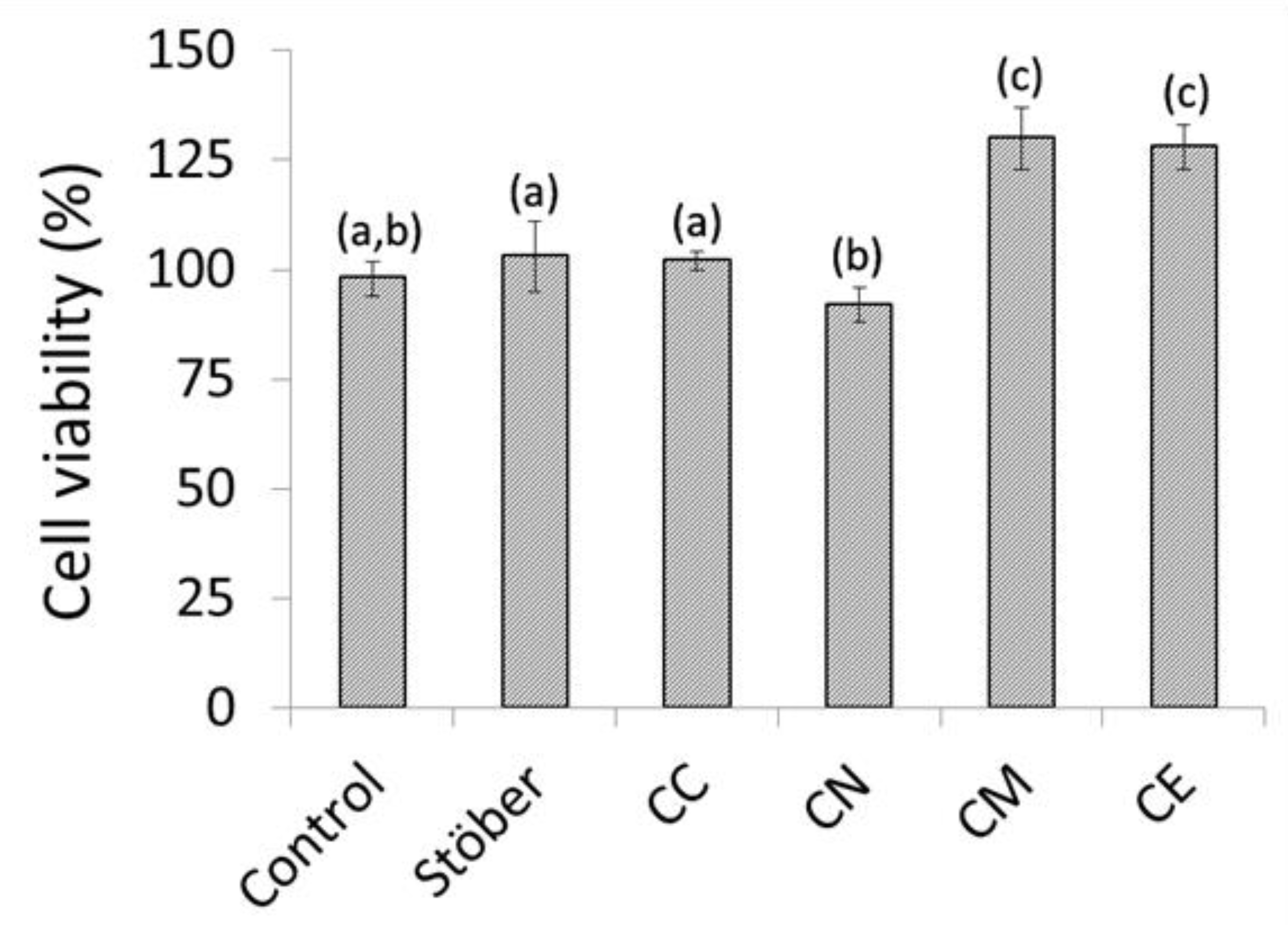
2.3. In Vitro Cytocompatibility of Composite Chitosan-Glass Membranes
3. Discussion
3.1. Pure Silica Stöber Particles
3.2. Calcium-Doped Glasses
4. Materials and Methods
4.1. Preparation and Characterisation of Glasses
4.2. In Vitro Bioactivity Analysis
4.3. In Vitro Cytocompatibility Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vaca-Cornejo, F.; Macías Reyes, H.; Dueñas Jiménez, S.H.; Llamas Velázquez, R.A.; Dueñas Jiménez, J.M. Pilot study using a chitosan-hydroxyapatite implant for guided alveolar bone growth in patients with chronic periodontitis. J. Funct. Biomater. 2017, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Dehnavi, S.S.; Mehdikhani, M.; Rafienia, M.; Bonakdar, S. Preparation and in vitro evaluation of polycaprolactone/PEG/bioactive glass nanopowders nanocomposite membranes for GTR/GBR applications. Mater. Sci. Eng. C 2018, 90, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.; Yuc, N.; Caridade, S.G.; Luz, G.M.; Gomes, M.E.; Reis, R.L.; Jansen, J.A.; Walboomers, F.; Mano, J.F. Chitosan/bioactive glass nanoparticle composite membranes for periodontal regeneration. Acta Biomater. 2012, 87, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Jayasree, R.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Biocompatible alginate/nano bioactive glass ceramic composite scaffolds for periodontal tissue regeneration. Carbohydr. Polym. 2012, 87, 274–283. [Google Scholar] [CrossRef]
- Castro, A.G.B.; Diba, M.; Kersten, M.; Jansen, J.A.; van den Beucken, J.J.J.P.; Yang, F. Development of a PCL-silica nanoparticles composite membrane for guided bone regeneration. Mater. Sci. Eng. C 2018, 85, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Hurt, A.P.; Getti, G.; Coleman, N.J. Bioactivity and biocompatibility of a chitosan-tobermorite composite membrane for guided tissue regeneration. Int. J. Biol. Macromol. 2014, 64, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Tamburaci, S.; Tihminlioglu, F. Diatomite reinforced chitosan composite membrane as potential scaffold for guided bone regeneration. Mater. Sci. Eng. C 2017, 80, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Riccò, R.; Nizzero, S.; Penna, E.; Meneghello, A.; Cretaio, E.; Enrichi, F. Ultra-small dye-doped silica nanoparticles via modified sol-gel technique. J. Nanopart. Res. 2018, 20, 117. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Osaka, A.; Hayakawa, S.; Kanji Tsuru, K.; Fujii, E.; Kawabata, K. Microstructure evolution in Stöber-type silica nanoparticles and their in vitro apatite deposition. J. Sol-Gel. Sci. Technol. 2008, 48, 322–335. [Google Scholar] [CrossRef]
- Rocha de Oliveira, A.A.; de Souza, D.A.; Silveira Dias, L.L.; de Carvalho, S.M.; Mansur, H.S.; Pereira, M.d.M. Synthesis, characterization and cytocompatibility of spherical bioactive glass nanoparticles for potential hard tissue engineering applications. Biomed. Mater. 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Greasley, S.L.; Page, S.J.; Sirovica, S.; Chen, S.; Martin, R.A.; Riveiro, A.; Hanna, J.V.; Porter, A.E.; Jones, J.R. Controlling particle size in the Stöber process and incorporation of calcium. J. Colloid Interface Sci. 2016, 469, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, D.K.; Kim, C.W.; Cha, H.G.; Kang, Y.S.; Jo, B.G.; Jeong, J.H. Preparation and antibiotic property of Ag-SiO2 nanoparticle. Mol. Cryst. Liq. Cryst. 2007, 464, 665–673. [Google Scholar] [CrossRef]
- Gonçalves, M.C. Sol-gel silica nanoparticles in medicine: A natural choice. Design, synthesis and products. Molecules 2018, 23, 2021. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Zhao, T.; Gao, B.; Miao, Y.; Zhang, D.; Dong, Y. Guided bone regeneration using chitosan-collagen membranes in dog dehiscence-type defect model. J. Oral Maxillofac. Surg. 2014, 72, 304.e1–304.e14. [Google Scholar] [CrossRef] [PubMed]
- Coleman, N.J.; Bellantone, M.; Nicholson, J.W.; Mendham, A.P. Textural and structural properties of bioactive glasses in the system CaO-SiO2. Ceramics Silikáty 2007, 51, 1–8. [Google Scholar]
- Lee, B.-S.; Lin, H.-P.; Chan, J.C.-C.; Wang, W.-C.; Hung, P.-H.; Tsai, Y.-H.; Lee, Y.-L. A novel sol-gel-derived calcium silicate cement with short setting time for application in endodontic repair of perforations. Int. J. Nanomed. 2018, 13, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Turdean-Ionescu, C.A.; Martin, R.A.; Newport, R.J.; Hanna, J.V.; Smith, M.E.; Jones, J.R. Effect of calcium source on structure and properties of sol-gel derived bioactive glasses. Langmuir 2012, 28, 17465–17476. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-Y.; Huang, H.-L.; Chen, Y.-C.; Hsu, J.-T.; Shieh, T.-M.; Ming-Tzu Tsai, M.-T. Biological Characteristics of the MG-63 human osteosarcoma cells on composite tantalum carbide/amorphous carbon films. PLoS ONE 2014, 9, e95590. [Google Scholar] [CrossRef] [PubMed]
- Turco, G.; Porrelli, D.; Marsich, E.; Vecchies, F.; Lombardi, T.; Stacchi, C.; Di Lenarda, R. Three-dimensional bone substitutes for oral and maxillofacial surgery: Biological and structural characterization. J. Funct. Biomater. 2018, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Papale, F. Preparation, characterization, and biological properties of organic-inorganic nanocomposite coatings on titanium substrates prepared by sol-gel. J. Biomed. Mater. Res. Part A 2014, 102, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Qasim, S.B.; Delaine-Smith, R.M.; Fey, T.; Rawlinson, A.; Rehman, I.U. Freeze gelated porous membranes for periodontal tissue regeneration. Acta Biomater. 2015, 23, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Hurt, A.P.; Kotha, A.K.; Trivedi, V.; Coleman, N.J. Bioactivity, biocompatibility and antimicrobial properties of a chitosan-mineral composite for periodontal tissue regeneration. Polimeros 2015, 25, 311–316. [Google Scholar] [CrossRef]
- Staehlke, S.; Rebl, H.; Nebe, B. Phenotypic stability of the human MG-63 osteoblastic cell line at different passages. Cell Biol. Int. 2019, 43, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Brinker, J.; Scherer, G.W. Sol-Gel Science. The Physics and Chemistry of Sol-Gel Processing; Academic Press: Boston, MA, USA, 1990. [Google Scholar]
- Li, P.J.; Ohtsuki, C.; Kokubo, T.; Nakanishi, K.; Soga, N.; de Groot, K. The role of hydrated silica, titania, and alumina in inducing apatite on implants. J. Biomed. Mater. Res. 1994, 28, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Lutz-Christian Gerhardt, L.-C.; Boccaccini, A.R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [PubMed]
- Fiume, E.; Barberi, J.; Verné, E.; Baino, F. Bioactive glasses: From parent 45S5 composition to scaffold-assisted tissue-healing therapies. J. Funct. Biomater. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Diab, R.; Canilho, N.; Pavel, I.A.; Haffner, F.B.; Girardon, M.; Pasc, A. Silica-based systems for oral delivery of drugs, macromolecules and cells. Adv. Colloid Interface Sci. 2017, 249, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Labbaf, S.; Tsigkou, O.; Mueller, K.H.; Stevens, M.M.; Porter, A.E.; Jones, J.R. Spherical bioactive glass particles and their interaction with human mesenchymal stem cells in vitro. Biomaterials 2011, 32, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Ionescu, C.; Pike, K.J.; Smith, M.E.; Jones, J.R. Nanostructure evolution and calcium distribution in sol-gel derived bioactive glass. J. Mater. Chem. 2009, 19, 1276–1282. [Google Scholar] [CrossRef]
- Valliant, E.M.; Turdean-Ionescu, C.A.; Hanna, J.V.; Smith, M.E.; Jones, J.R. Role of pH and temperature on silica network formation and calcium incorporation into sol–gel derived bioactive glasses. J. Mater. Chem. 2012, 22, 1613–1619. [Google Scholar] [CrossRef]
- Luz, G.M.; Mano, J.F. Preparation and characterization of bioactive glass nanoparticles prepared by sol–gel for biomedical applications. Nanotechnology 2011, 22, 494014. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Chang, J. Preparation and characterization of nano-bioactive-glasses (NBG) by a quick alkali-mediated sol-gel method. Mater. Lett. 2007, 61, 3251–3253. [Google Scholar] [CrossRef]
- Itala, A.; Ylanen, H.O.; Yrjans, J.; Heino, T.; Hentunen, T.; Hupa, M.; Aro, H.T. Characterization of microrough bioactive glass surface: Surface reactions and osteoblast responses in vitro. J. Biomed. Mater. Res. 2002, 62, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Gough, J.E.; Notingher, I.; Hench, L.L. Osteoblast attachment and mineralized nodule formation on rough and smooth 45S5 bioactive glass monoliths. J. Biomed. Mater. Res. 2004, 68, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Tolb, E.; Müller, W.E.G.; Abd El-Hady, B.M.; Neufurth, M.; Wurm, F.; Wang, S.; Schröder, H.C.; Wang, X. High biocompatibility and improved osteogenic potential of amorphous calcium carbonate/vaterite. J. Mater. Chem. B 2016, 4, 376–386. [Google Scholar] [CrossRef]
- CRC Handbook of Chemistry and Physics, 87th ed.; Lide, D.R., Ed.; National Institute of Standards and Technology, CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2006; ISBN 0-8493-0487-3. [Google Scholar]
- Lee, E.-J.; Shin, D.-S.; Kim, H.-E.; Kim, H.-W.; Koh, Y.-H.; Jang, J.-H. Membrane of hybrid chitosan-silica xerogel for guided bone regeneration. Biomaterials 2009, 30, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M. Synthesis and characterization of macroporous chitosan/calcium phosphate composite scaffolds for tissue engineering. J. Biomed. Mater. Res. 2001, 55, 304–312. [Google Scholar] [CrossRef]
- Pattnaik, S.; Nethala, S.; Tripathi, A.; Saravanan, S.; Moorthi, A.; Selvamurugan, N. Chitosan scaffolds containing silicon dioxide and zirconia nanoparticles for bone tissue engineering. Int. J. Biol. Macromol. 2011, 49, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.R.; Alves, N.M.; Mano, J.F. Biomimetic polysaccharide/bioactive glass nanoparticles multilayer membranes for guided tissue regeneration. RSC Adv. 2016, 6, 75988–75999. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Papagerakis, S.; Faulk, D.; Badylak, S.F.; Zhao, Y.M.; Ge, L.H.; Qin, M.; Papagerakis, P. Extracellular matrix membrane induces cementoblastic/osteogenic properties of human periodontal ligament stem cells. Front. Physiol. 2018, 9, 942. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Bhattacharjee, M.; Nair, L.S.; Laurencin, C.T. Musculoskeletal tissue regeneration: The role of the stem cells. Regen. Eng. Transl. Med. 2017, 3, 133–165. [Google Scholar] [CrossRef]
- Carvalho, S.M.; Oliveira, A.A.R.; Jardim, C.A.; Melo, C.B.S.; Gomes, D.A.; de Fátima Leite, M.; Pereira, MM. Characterization and induction of cementoblast cell proliferation by bioactive glass nanoparticles. J. Tissue Eng. Regen. Med. 2012, 6, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Perkin, F.M.; Pratt, L. Action of alcohols on metallic calcium. J. Chem. Soc. Trans. 1909, 95, 159–165. [Google Scholar] [CrossRef]

| Sol-Gel Glass | Molar Ca:Si Ratio 1 |
|---|---|
| Ideal glass with 5 mol % Ca2+ | 0.05 |
| Stöber | 0.00 a |
| CC | 0.036 ± 0.002 b |
| CN | 0.029 ± 0.002 c |
| CM | 0.024 ± 0.014 b,c |
| CE | 0.037 ± 0.003 b |
| Sol-Gel Glass | H2O Bend (cm−1) | Si-O-Si Network (cm−1) | Max. width Si-O-Si Band (cm−1) | Si-OH asym str (cm−1) | Si-O sym (cm−1) |
|---|---|---|---|---|---|
| Stöber | 1638 | 1104 | 310 | 950 | 799 |
| CC | 1631 | 1099 | 320 | 951 | 799 |
| CN | 1636 | 1098 | 330 | 951 | 802 |
| CM | 1631 | 1089 | 363 | 950 | 798 |
| CE | 1638 | 1092 | 360 | 947 | 799 |
| Sample Name | Calcium Reagent | Mass of Calcium Reagent (mg) | Molar Ratio Ca:Si |
|---|---|---|---|
| Stöber | – | – | 0.00 |
| CC | CaCl2·2H2O | 73.5 | 0.05 |
| CN | Ca(NO3)2·4H2O | 118.1 | 0.05 |
| CM | Ca(OCH3)2 | 51.1 | 0.05 |
| CE | Ca(OC2H5)2 | 65.1 | 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Clavijo, A.; Hurt, A.P.; Kotha, A.K.; Coleman, N.J. Effect of Calcium Precursor on the Bioactivity and Biocompatibility of Sol-Gel-Derived Glasses. J. Funct. Biomater. 2019, 10, 13. https://doi.org/10.3390/jfb10010013
Ruiz-Clavijo A, Hurt AP, Kotha AK, Coleman NJ. Effect of Calcium Precursor on the Bioactivity and Biocompatibility of Sol-Gel-Derived Glasses. Journal of Functional Biomaterials. 2019; 10(1):13. https://doi.org/10.3390/jfb10010013
Chicago/Turabian StyleRuiz-Clavijo, Alejandra, Andrew P. Hurt, Arun K. Kotha, and Nichola J. Coleman. 2019. "Effect of Calcium Precursor on the Bioactivity and Biocompatibility of Sol-Gel-Derived Glasses" Journal of Functional Biomaterials 10, no. 1: 13. https://doi.org/10.3390/jfb10010013
APA StyleRuiz-Clavijo, A., Hurt, A. P., Kotha, A. K., & Coleman, N. J. (2019). Effect of Calcium Precursor on the Bioactivity and Biocompatibility of Sol-Gel-Derived Glasses. Journal of Functional Biomaterials, 10(1), 13. https://doi.org/10.3390/jfb10010013





