Alpha Suppression Is Associated with the Tip-of-the-Tongue (TOT) State Whereas Alpha Expression Is Associated with Knowing That One Does Not Know
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Design
2.3. Stimuli
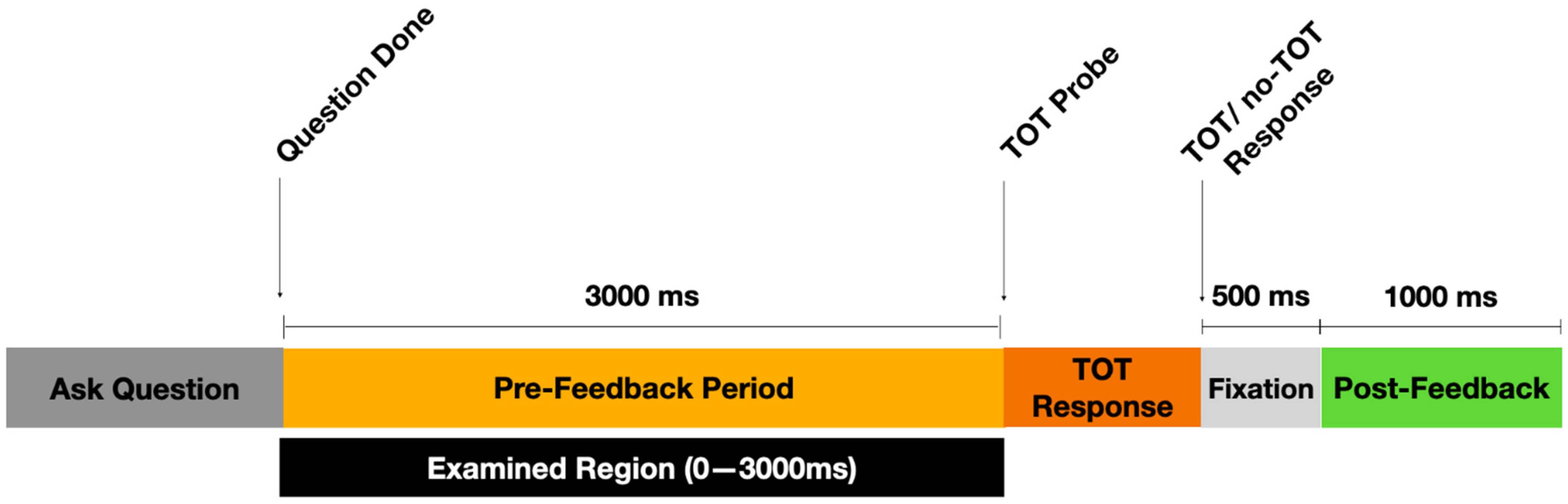
2.4. Procedure
2.5. Response Scoring
2.6. Electroencephalographic (EEG) Recording and Processing
2.7. Spectral Analyses
3. Results
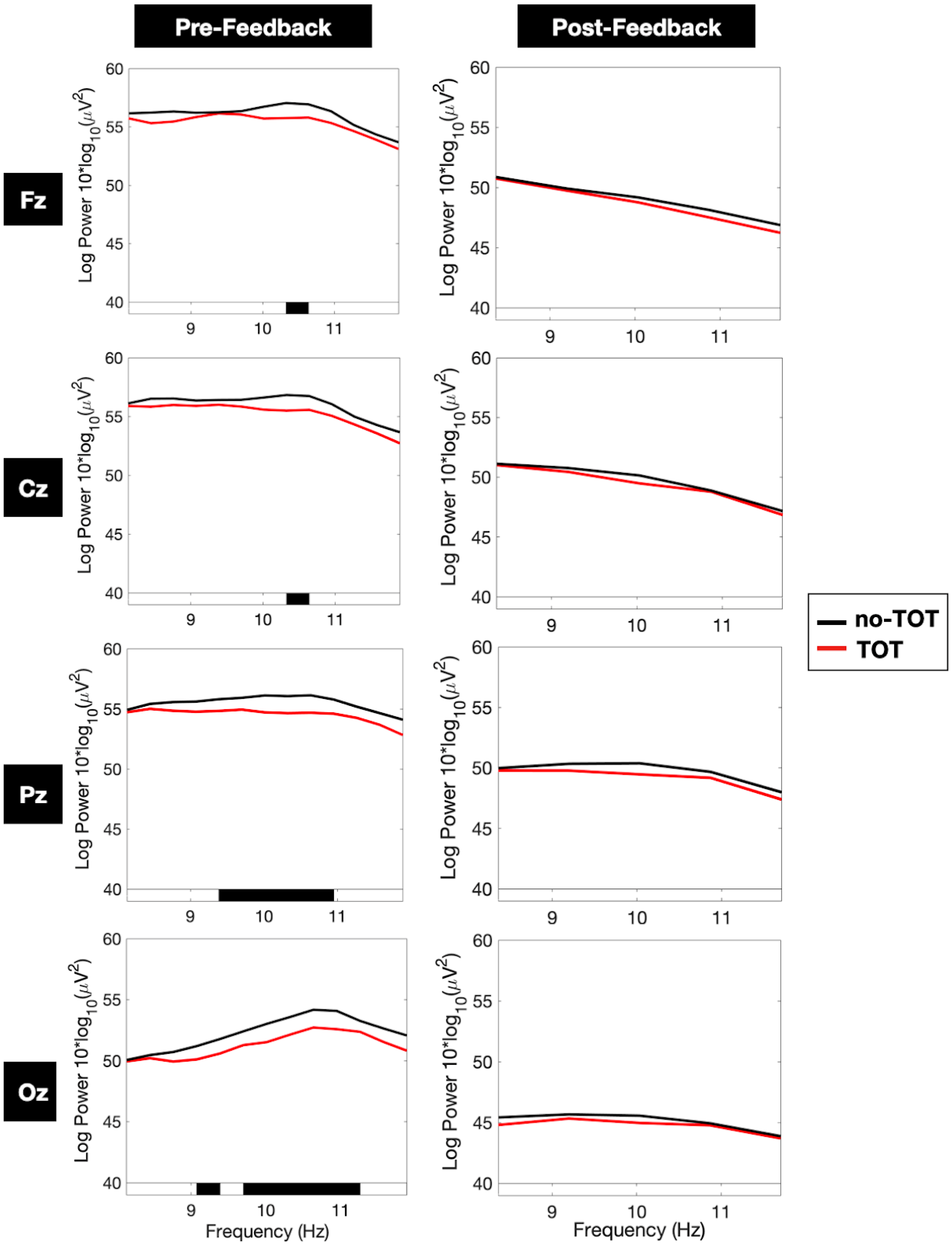
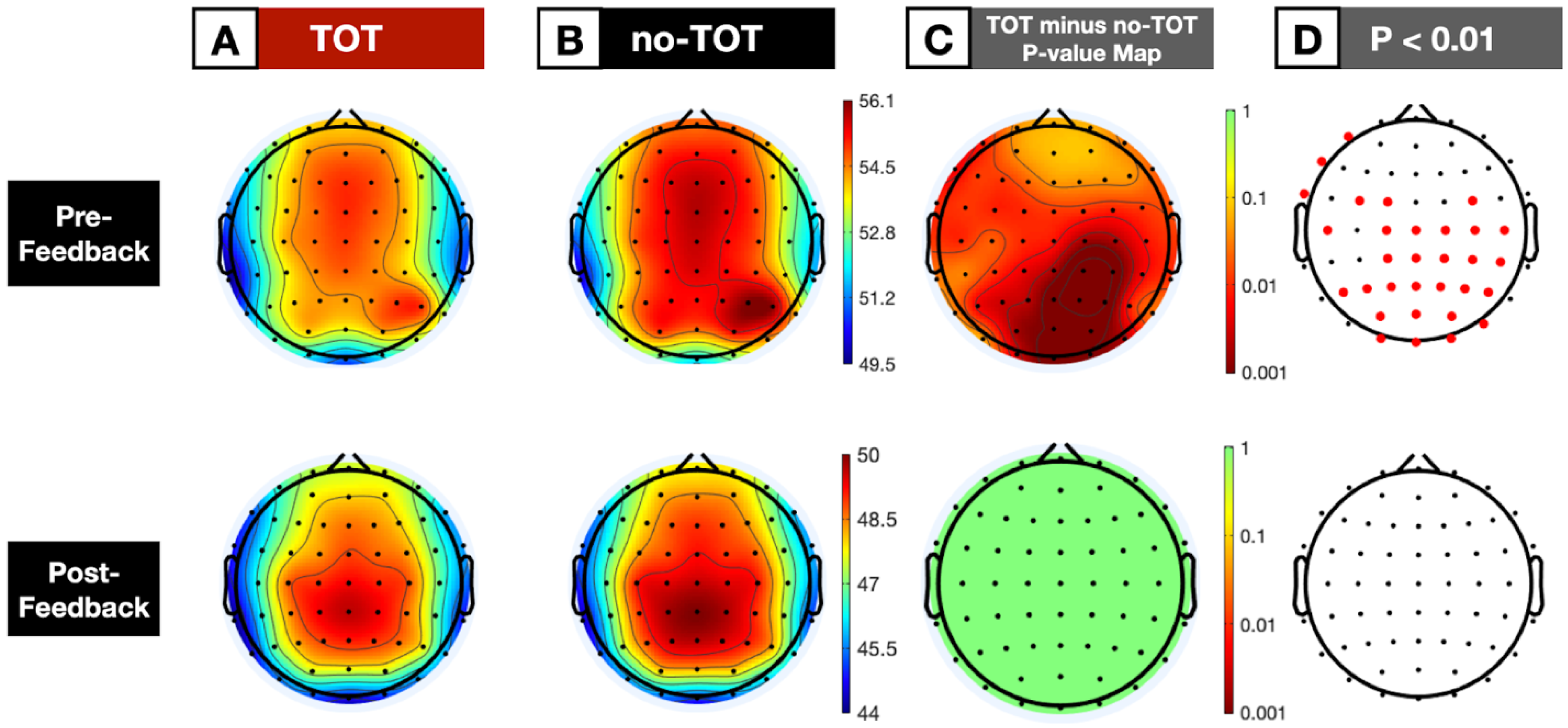
3.1. Full-Period Spectral Decompositions
3.1.1. Individual-Electrode Analysis
3.1.2. Averaged Scalp Topographies
3.1.3. ERSP Analysis
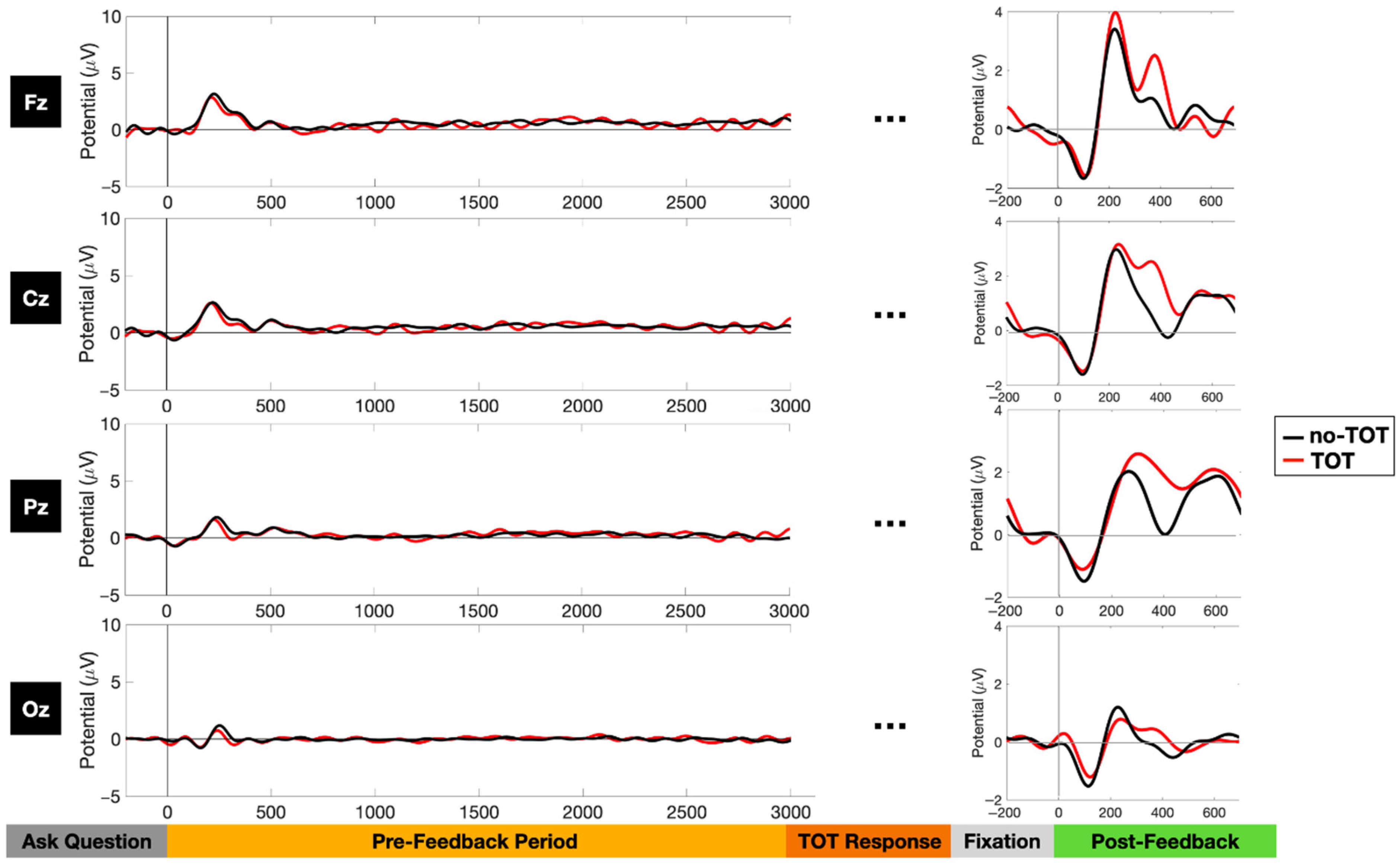
3.2. Event-Related Potential Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
| 1 | Maradona (or maybe Messi). Rigoberta Menchú, for her work for the rights of indigenous peoples and reconciliation between ethnic groups. |
| 2 | This post-feedback analysis was already reported, in detail, in Bloom et al. (2018). We reanalyzed their post-feedback data here because we used different preprocessing software for our other analyses and wanted consistency throughout. |
References
- Ablin, Pierre, Jean-François Cardoso, and Alexandre Gramfort. 2018. Faster independent component analysis by preconditioning with Hessian approximations. IEEE Transactions on Signal Processing 66: 4040–49. Available online: https://arxiv.org/abs/1706.08171 (accessed on 8 May 2021). [CrossRef]
- Ackerman, Rakefet, and Valerie A. Thompson. 2017. Meta-reasoning: Monitoring and control of thinking and reasoning. Trends in Cognitive Sciences 21: 607–17. [Google Scholar] [CrossRef] [PubMed]
- Arnau, Stefan, Christoph Löffler, Jan Rummel, Dirk Hagemann, Edmund Wascher, and Anna-Lena Schubert. 2020. Intertrial alpha power indicates mind wandering. Psychophysiology 57: e13581. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupo, Felix, and Steven J. Luck. 2019. Lateralized suppression of alpha-band EEG activity as a mechanism of target processing. The Journal of Neuroscience 39: 900–17. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, Carryl L., Daniel M. Roberts, Daniela Barragan, John D. Lee, Neil Lerner, and James S. Higgins. 2017. Detecting and quantifying mind wandering during simulated driving. Frontiers in Human Neuroscience 11: 406. [Google Scholar] [CrossRef] [PubMed]
- Berlyne, Daniel E. 1954. A theory of human curiosity. British Journal of Psychology 45: 180–91. [Google Scholar] [CrossRef]
- Bloom, Paul A., David Friedman, Judy Xu, Matti Vuorre, and Janet Metcalfe. 2018. Tip-of-the-tongue states predict enhanced feedback processing and subsequent memory. Consciousness and Cognition 101: 221–33. [Google Scholar] [CrossRef]
- Bock, J. Kathryn. 1987. Coordinating words and syntax in speech plans. In Progress in the Psychology of Language. Edited by Albert Ellis. London: Erlbaum, pp. 337–90. [Google Scholar]
- Bowers, Kenneth S., Glenn Regehr, Claude Balthazard, and Kevin Parker. 1990. Intuition in the context of discovery. Cognitive Psychology 22: 72–110. [Google Scholar] [CrossRef]
- Bozhilova, Natali, Ruth Cooper, Jonna Kuntsi, Philip Asherson, and Giorgia Michelini. 2020. Electrophysiological correlates of spontaneous mind wandering in attention-deficit/hyperactivity disorder. Behavioural Brain Research 391: 112632. [Google Scholar] [CrossRef]
- Brennen, Tim, Anne Vikan, and Ragnhild Dybdahl. 2007. Are tip-of-the-tongue states universal? Evidence from an Unwritten Language Memory 15: 167–76. [Google Scholar]
- Brooks, Gregory, Haopei Yang, and Stefan Köhler. 2021. Feeling-of-knowing experiences breed curiosity. Memory 29: 153–67. [Google Scholar] [CrossRef]
- Brown, Alan S. 2012. Tip of the Tongue State. New York: Psychology Press. [Google Scholar]
- Brown, Roger, and David McNeill. 1966. The “tip of the tongue” phenomenon. Journal of Verbal Learning & Behavior 5: 325–37. [Google Scholar]
- Buján, Ana, Santiago Galdo-Álvarez, Mónica Lindín, and Fernando Diaz. 2012. An event-related potentials study of face naming: Evidence of phonological retrieval deficit in the tip-of-the-tongue state. Psychophysiology 49: 980–90. [Google Scholar] [CrossRef]
- Burke, Deborah M., Donald G. MacKay, Joanna S. Worthley, and Elizabeth Wade. 1991. On the tip of the tongue: What causes word finding failures in young and older adults? Journal of Memory and Language 30: 542–79. [Google Scholar] [CrossRef]
- Carruthers, Peter. 1989. Brute experience. The Journal of Philosophy 86: 258–69. [Google Scholar] [CrossRef]
- Cleary, Anne M. 2019. The biasing nature of the tip-of-the-tongue experience: When decisions bask in the glow of the tip-of-the-tongue state. Journal of Experimental Psychology: General 148: 1178–91. [Google Scholar] [CrossRef] [PubMed]
- Cleary, Anne M., and Alexander B. Claxton. 2015. The tip-of-the-tongue heuristic: How tip-of-the-tongue states confer perceptibility on inaccessible words. Journal of Experimental Psychology: Learning, Memory, and Cognition 41: 1533–39. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cleary, Anne M., Katherine L. McNeely-White, Shaylyn A. Russell, Andrew M. Huebert, and Hannah Hausman. 2021. The tip-of-the-tongue state as a form of access to information: Use of tip-of-the-tongue states for strategic adaptive test-taking. Journal of Applied Research in Memory and Cognition 10: 131–42. [Google Scholar] [CrossRef]
- Cooper, Nicholas R., Rodney J. Croft, Samuel J. Dominey, Adrian P. Burgess, and John H. Gruzelier. 2003. Paradox lost? Exploring the role of alpha oscillations during externally vs. internally directed attention and the implications for idling and inhibition hypotheses. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology 47: 65–74. [Google Scholar] [CrossRef]
- Delorme, Arnaud, and Scott Makeig. 2004. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods 134: 9–21. [Google Scholar] [CrossRef]
- Diaz, Fernando, Mónica Lindín, Santiago Galdo-Alvarez, David Facal, and Onésimo Juncos-Rabadán. 2007. An event-related potentials study of face identification and naming: The tip-of-the-tongue state. Psychophysiology 44: 50–68. [Google Scholar] [CrossRef] [PubMed]
- Fastrich, Greta M., Tyson Kerr, Alan D. Castel, and Kou Murayama. 2018. The role of interest in memory for trivia questions: An investigation with a large-scale database. Motivation Science 4: 227–50. [Google Scholar] [CrossRef] [PubMed]
- Fink, Andreas, and Mathias Benedek. 2014. EEG alpha power and creative ideation. Neuroscience and Biobehavioral Reviews 44: 111–23. [Google Scholar] [CrossRef]
- FitzGibbon, Lily, Johnny King L. Lau, and Kou Murayama. 2021. The seductive lure of curiosity: Information as a motivationally salient reward. Current Opinion in Behavioral Sciences 35: 21–27. [Google Scholar] [CrossRef]
- Funnell, Margaret, Janet Metcalfe, and Kyrana Tsapkini. 1996. In the mind but not in the tongue: Feeling of knowing in anomic patient H.W. In Implicit Memory and Metacognition. Edited by Lynne M. Reder. Hillsdale: Erlbaum, pp. 171–94. [Google Scholar]
- Galdo-Alvarez, Santiago, Mónica Lindín, and Fernando Díaz. 2009. The effect of age on event-related potentials (ERP) associated with face naming and the tip-of-the-tongue (TOT) state. Biological Psychology 81: 14–23. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, Fohn M., Fergus I. M. Craik, and Fraser A. Bleasdale. 1973. Retrieval difficulty and subsequent recall. Memory & Cognition 1: 213–16. [Google Scholar]
- Gollan, Tamar H., and Alan S. Brown. 2006. From tip-of-the-tongue (TOT) data to theoretical implications in two steps: When more TOTs means better retrieval. Journal of Experimental Psychology: General 135: 462–83. [Google Scholar] [CrossRef]
- Gruber, Matthias J., Bernard D. Gelman, and Charan Ranganath. 2014. States of curiosity modulate hippocampus-dependent learning via the dopaminergic circuit. Neuron 84: 486–96. [Google Scholar] [CrossRef]
- Heine, Marilyn K., Beth A. Ober, and Gregory K. Shenaut. 1999. Naturally occurring and experimentally induced tip-of-the-tongue experiences in three adult age groups. Psychology and Aging 14: 445–57. [Google Scholar] [CrossRef]
- Jacobs, William J., and Janet Metcalfe. Forthcoming. The role of Curiosity1 and Curiosity2 in the emergence of insight. In The Emergence of Insight. Edited by Stephen M. Smith, Carola Salvi Salvi and Jennifer Wiley. Cambridge: Cambridge University Press.
- James, William. 1890. The Principles of Psychology. New York: Holt. [Google Scholar]
- Jauk, Emanuel, Mathias Benedek, and Aljoscha C. Neubauer. 2012. Tackling creativity at its roots: Evidence for different patterns of EEG alpha activity related to convergent and divergent modes of task processing. International Journal of Psychophysiology 84: 219–25. [Google Scholar] [CrossRef]
- Jin, Christina Yi, Jelmer P. Borst, and Marieke K. Van Vugt. 2019. Predicting task-general mind-wandering with EEG. Cognitive, Affective, and Behavioral Neuroscience 19: 1059–73. [Google Scholar] [CrossRef] [PubMed]
- Kang, Min Jeong, Ming Hsu, Ian M. Krajbich, George Loewenstein, Samuel M. McClure, Joseph Tao-yi Wang, and Colin F. Camerer. 2009. The wick in the candle of learning: Epistemic curiosity activates reward circuitry and enhances memory. Psychological Science 20: 963–73. [Google Scholar] [CrossRef] [PubMed]
- Kikyo, Hideyuki, Kenichi Ohki, and Kensuke Sekihara. 2001. Temporal characterization of memory retrieval processes: An fMRI study of the “tip of the tongue” phenomenon. European Journal of Neuroscience 14: 887–92. [Google Scholar] [CrossRef]
- Klimesch, Wolfgang. 1997. EEG-alpha rhythms and memory processes. International Journal of Psychophysiology 26: 319–40. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, Wolfgang. 1999. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Research Reviews 29: 169–95. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, Wolfgang. 2012. Alpha-band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences 16: 607–17. [Google Scholar] [CrossRef]
- Koriat, Asher. 2000. The feeling of knowing: Some metatheoretical implications for consciousness and control. Consciousness and Cognition 9: 149–71. [Google Scholar] [CrossRef]
- Koriat, Asher, and Israel Lieblich. 1974. What does a person in a “TOT” state know that a person in a “don’t know” state doesn’t know. Memory & Cognition 2: 647–55. [Google Scholar]
- Koriat, Asher, and Israel Lieblich. 1977. A study of memory pointers. Acta Psychologica 41: 151–64. [Google Scholar] [CrossRef]
- Kothe, Christian, Makoto Miyakoshi, and Antoine Delorme. 2019. clean_rawdata (Version 2.7) [Computer Software]. Available online: https://github.com/sccn/clean_rawdata/wiki (accessed on 8 May 2021).
- Kuhbandner, Christof, Philipp Spachtholz, and Bernhard Pastötter. 2016. Bad things come easier to the mind but harder to the body: Evidence from brain oscillations. Cognitive Affective Behavioral Neuroscience 16: 768–78. [Google Scholar] [CrossRef]
- Kuhbandner, Christof, Simon Hanslmayr, Markus A. Maier, Reinhard Pekrun, Bernhard Spitzer, Bernhard Pastötter, and Karl-Heinz Bäuml. 2009. Effects of mood on the speed of conscious perception: Behavioural and electrophysiological evidence. Social Cognitive and Affective Neuroscience 4: 286–93. [Google Scholar] [CrossRef] [PubMed]
- Lau, Johnny King L., Hiroki Ozono, Kei Kuratomi, Asuka Komiya, and Kou Murayama. 2020. Shared striatal activity in decisions to satisfy curiosity and hunger at the risk of electric shocks. Nature Human Behaviour 4: 531–43. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, George. 1994. The psychology of curiosity: A review and reinterpretation. Psychological Bulletin 116: 75. [Google Scholar] [CrossRef]
- Loewenstein, George. 2007. Exotic Preferences. New York: Oxford University Press. [Google Scholar]
- MacKay, Donald G., and Deborah M. Burke. 1990. Cognition and Aging: A Theory of New Learning and the Use of Old Connections. In Advances in Psychology. Edited by F. Gordon A. Stone and Robert West. Amsterdam: North-Holland, vol. 71, pp. 213–63. [Google Scholar] [CrossRef]
- Magazzini, Lorenzo, Philipp Ruhnau, and Nathan Weisz. 2016. Alpha suppression and connectivity modulations in left temporal and parietal cortices index partial awareness of words. NeuroImage 133: 279–87. [Google Scholar] [CrossRef]
- Maril, Anat, Anthony D. Wagner, and Daniel L. Schacter. 2001. On the tip of the tongue: An event-related fMRI study of semantic retrieval failure and cognitive conflict. Neuron 31: 653–60. [Google Scholar] [CrossRef]
- Maril, Anat, Jon S. Simons, Josh J. Weaver, and Daniel L. Schacter. 2005. Graded recall success: An event-related fMRI comparison of tip of the tongue and feeling of knowing. NeuroImage 24: 1130–38. [Google Scholar] [CrossRef]
- Marvin, Caroline B., Ellen Tedeschi, and Daphna Shohamy. 2020. Curiosity as the impulse to know: Common behavioral and neural mechanisms underlying curiosity and impulsivity. Current Opinion in Behavioral Sciences 35: 92–98. [Google Scholar] [CrossRef]
- Metcalfe, Janet. 2021. The Region of Proximal Learning and Curiosity. In In Their Own Words: What Scholars Want You to Know about Why and How to Apply the Science of Learning in Your Academic Setting. Edited by Catherine E. Overson, Christopher M. Hakala, Lauren L. Kordonowy and Victor A. Benassi. Washington, DC: American Psychological Association’s Division 2 [Society for the Teaching of Psychology]. [Google Scholar]
- Metcalfe, Janet, and Nate Kornell. 2005. A Regional of Proximal Learning model of metacognitively guided study-time allocation. Journal of Memory and Language 52: 463–77. [Google Scholar] [CrossRef]
- Metcalfe, Janet, and William J. Jacobs. 2021. The two faces of curiosity in creative cognition: Curiosity1, Curiosity2 (and their interaction). In International Handbook of Creative Cognition. Edited by Linden J. Ball and Frederic Vallee-Tourangeau. London: Routledge. [Google Scholar]
- Metcalfe, Janet, Bennett L. Schwartz, and Paul A. Bloom. 2017. The Tip-of-the-tongue (TOT) State and curiosity. Cognitive Research: Principles and Implications 2: 31. [Google Scholar]
- Metcalfe, Janet, Bennett L. Schwartz, and Teal S. Eich. 2020. Epistemic curiosity in the Region of Proximal Learning. Current Opinion in Behavioral Sciences 35: 40–47. [Google Scholar] [CrossRef]
- Metcalfe, Janet, Matti Vuorre, Emily Towner, and Teal S. Eich. 2022. Curiosity: The effects of feedback and confidence on the desire to know. Journal of Experimental Psychology: General. Advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Miozzo, Michele, and Alfonso Caramazza. 1997. Retrieval of lexical–syntactic features in tip-of-the tongue states. Journal of Experimental Psychology: Learning, Memory, and Cognition 23: 1410–23. [Google Scholar] [CrossRef] [PubMed]
- Murayama, Kou, Lily FitzGibbon, and Michiko Sakaki. 2019. Process account of curiosity and interest: A reward learning model of knowledge acquisition. Educational Psychology Review 31: 875–95. [Google Scholar] [CrossRef]
- Nagel, Thomas. 1974. What is it like to be a bat? The Philosophical Review 83: 435–50. [Google Scholar] [CrossRef]
- Nelson, Thomas O., and Louis Narens. 1980. Norms of 300 general-information questions: Accuracy of recall, latency of recall, and feeling-of-knowing ratings. Journal of Verbal Learning and Verbal Behavior 19: 338–68. [Google Scholar] [CrossRef]
- Neuper, Christa, and Gert Pfurtscheller. 2001. Event-related dynamics of cortical rhythms: Frequency-specific features and functional correlates. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology 43: 41–58. [Google Scholar] [CrossRef]
- Nordmann, Emily, Alexandra A. Cleland, and Rebecca Bull. 2013. Cat Got Your Tongue? Using the Tip-of-the- Tongue state to investigate fixed expressions. Cognitive Science 37: 1553–64. [Google Scholar] [CrossRef]
- Peirce, Jonathan W. 2007. PsychoPy—Psychophysics software in Python. Journal of Neuroscience Methods 162: 8–13. [Google Scholar] [CrossRef]
- Perfect, Timothy J., and J. Richard Hanley. 1992. The tip-of-the-tongue phenomenon: Do experimenter-presented interlopers have any effect? Cognition 45: 55–75. [Google Scholar] [CrossRef]
- Pfurtscheller, Gert. 1999. EEG Event-Related Desynchronization (ERD) and Event-Related Synchronization (ERS). In Electroencephalography: Basic Principles, Clinical Applications and Related Fields, 4th ed. Edited by Ernst Niedermeyer and F. H. Lopes da Silva. Baltimore: Williams and Wilkins, pp. 958–67. [Google Scholar]
- Pfurtscheller, Gert, and F. H. Lopes da Silva. 1999. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology 110: 1842–57. [Google Scholar] [CrossRef]
- Polanyi, Michael. 1974. Genius in Science. In Methodological and Historical Essays in the Natural and Social Sciences. Boston Studies in the Philosophy of Science. Edited by Robert S. Cohen and Marx W. Wartofsky. Dordrecht: Springer, vol. 14. [Google Scholar] [CrossRef]
- Resnik, Karmen, David Bradbury, Gareth R. Barnes, and Alex P. Leff. 2014. Between thought and expression, a magnetoencephalography study of the “tip-of-the-tongue” phenomenon. Journal of Cognitive Neuroscience 26: 2210–23. [Google Scholar] [CrossRef] [PubMed]
- Ryals, Anthony J., Megan E. Kelly, and Anne M. Cleary. 2021. Increased pupil dilation during tip-of-the-tongue states. Consciousness and Cognition 92: 103152. [Google Scholar] [CrossRef] [PubMed]
- Ryan, Michael P., C. Raymond Petty, and Richard M. 1982. Motivated remembering efforts during tip-of-the-tongue states. Acta Psychologica 51: 137–47. [Google Scholar] [CrossRef]
- Schooler, Jonathan. Forthcoming. The case for curious mind wandering (mind wondering). In The Emergence of Insight. Edited by Stephen M. Smith, Carola Salvi Salvi and Jennifer Wiley. Cambridge: Cambridge Press.
- Schwartz, Bennett L. 1999. Sparkling at the end of the tongue: The etiology of tip-of-the-tongue phenomenology. Psychonomic Bulletin and Review 6: 379–93. [Google Scholar] [CrossRef]
- Schwartz, Bennett L. 2008. Working memory load differentially affects tip-of-the-tongue states and feeling-of-knowing judgment. Memory & Cognition 36: 9–19. [Google Scholar]
- Schwartz, Bennett L., and Ali Pournaghdali. 2020. Tip of the tongue states: Past and future. In Memory Quirks. New York: Routledge, chp. 13. [Google Scholar] [CrossRef]
- Schwartz, Bennett L., and Anne M. Cleary. 2016. Tip-of-the-tongue states, déjà vu and other metacognitive oddities. In Oxford Handbook of Metamemory. Edited by John Dunlosky and Sean Tauber. Oxford: Oxford University Press, pp. 95–108. [Google Scholar]
- Schwartz, Bennett L., and Janet Metcalfe. 2011. Tip-of-the-tongue (TOT) states: Retrieval, behavior, and experience. Memory & Cognition 39: 737–49. [Google Scholar]
- Schwartz, Bennett L., and Janet Metcalfe. 2014. Tip-of-the-tongue (TOT) states: Mechanisms and metacognitive control. In Tip-of-the-Tongue States and Related Phenomena. Edited by Bennett L. Schwartz and Alan S. Brown. Cambridge: Cambridge University Press. [Google Scholar]
- Schwartz, Bennett L., and Leslie D. Frazier. 2005. Tip-of-the-tongue states and aging: Contrasting psycholinguistic and metacognitive perspectives. Journal of General Psychology 132: 377–91. [Google Scholar] [CrossRef]
- Schwartz, Bennett L., and Steven M. Smith. 1997. The Retrieval of Related Information Influences Tip-of- the-Tongue States. Journal of Memory and Language 36: 68–86. [Google Scholar] [CrossRef]
- Schwartz, Bennett L., Donald M. Travis, Anthony M. Castro, and Steven M. Smith. 2000. The phenomenology of real and illusory tip-of-the-tongue states. Memory & Cognition 28: 18–27. [Google Scholar]
- Smith, Steven M. 1994. Frustrated feelings of imminent recall: On the tip-of-the tongue. In Metacognition: Knowing about Knowing. Edited by Janet Metcalfe and Arthur P. Shimamura. Cambridge: MIT Press, pp. 27–46. [Google Scholar]
- Wade, Shirlene, and Celeste Kidd. 2019. The role of prior knowledge and curiosity in learning. Psychonomic Bulletin & Review 26: 1377–87. [Google Scholar]
- Wilson, Robert C., Andra Geana, John M. White, Elliot A. Ludvig, and Jonathan D. Cohen. 2014. Humans use directed and random exploration to solve the explore–exploit dilemma. Journal of Experimental Psychology: General 143: 2074–81. [Google Scholar] [CrossRef] [PubMed]
- Xu, Judy, and Janet Metcalfe. 2016. Studying in the region of proximal learning reduces mind wandering. Memory & Cognition 44: 681–95. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, E.Q.-L.; Friedman, D.; Bloom, P.A.; Metcalfe, J. Alpha Suppression Is Associated with the Tip-of-the-Tongue (TOT) State Whereas Alpha Expression Is Associated with Knowing That One Does Not Know. J. Intell. 2022, 10, 121. https://doi.org/10.3390/jintelligence10040121
Shen EQ-L, Friedman D, Bloom PA, Metcalfe J. Alpha Suppression Is Associated with the Tip-of-the-Tongue (TOT) State Whereas Alpha Expression Is Associated with Knowing That One Does Not Know. Journal of Intelligence. 2022; 10(4):121. https://doi.org/10.3390/jintelligence10040121
Chicago/Turabian StyleShen, Edmund Qian-Long, David Friedman, Paul Alexander Bloom, and Janet Metcalfe. 2022. "Alpha Suppression Is Associated with the Tip-of-the-Tongue (TOT) State Whereas Alpha Expression Is Associated with Knowing That One Does Not Know" Journal of Intelligence 10, no. 4: 121. https://doi.org/10.3390/jintelligence10040121
APA StyleShen, E. Q.-L., Friedman, D., Bloom, P. A., & Metcalfe, J. (2022). Alpha Suppression Is Associated with the Tip-of-the-Tongue (TOT) State Whereas Alpha Expression Is Associated with Knowing That One Does Not Know. Journal of Intelligence, 10(4), 121. https://doi.org/10.3390/jintelligence10040121




