First Report of the Marine Benthic Dinoflagellate Bysmatrum subsalsum from Korean Tidal Pools
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Culture
2.2. Morphological Observation
2.3. DNA Extraction and Sequencing
2.4. Phylogenetic Analysis
3. Results
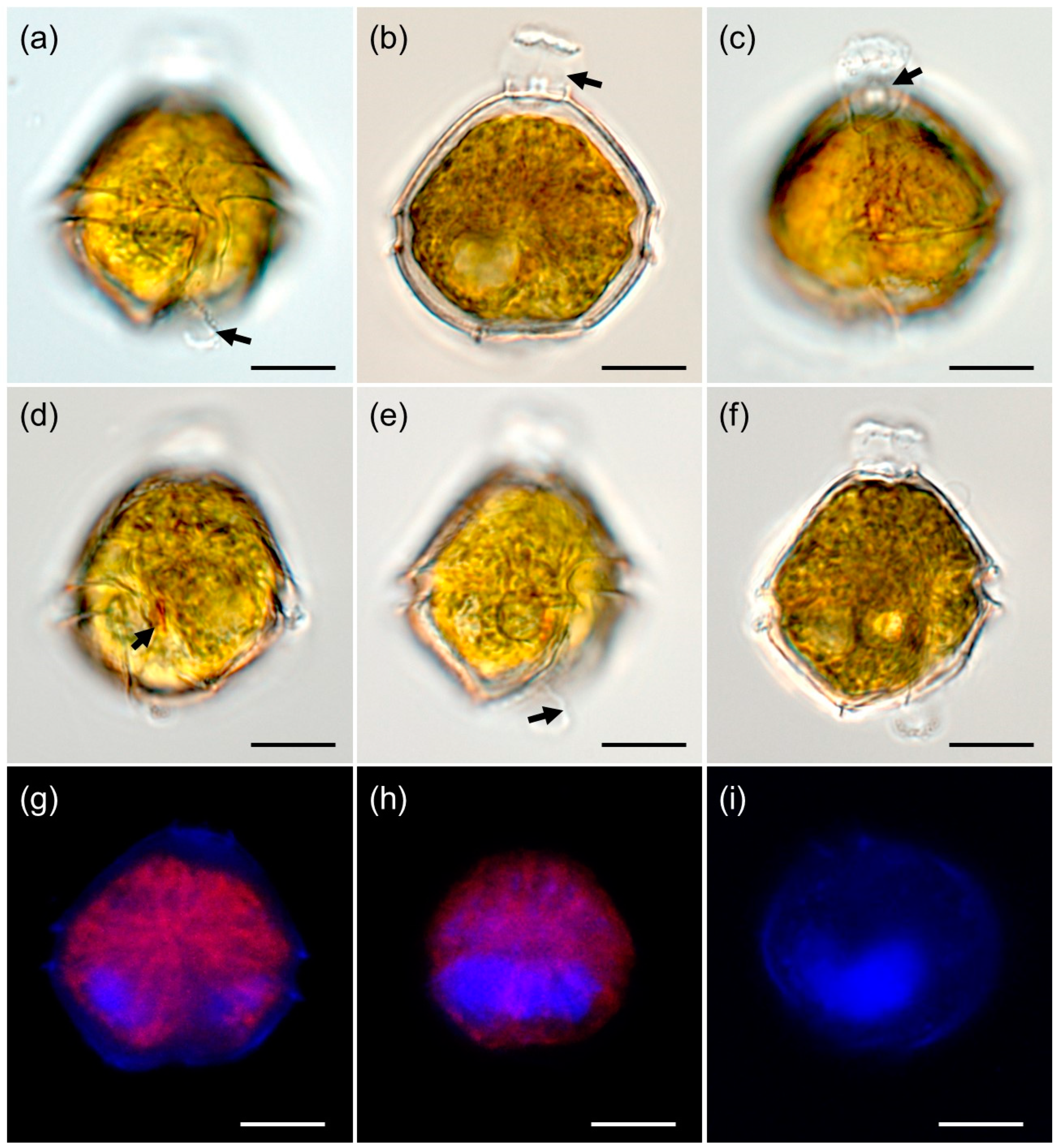
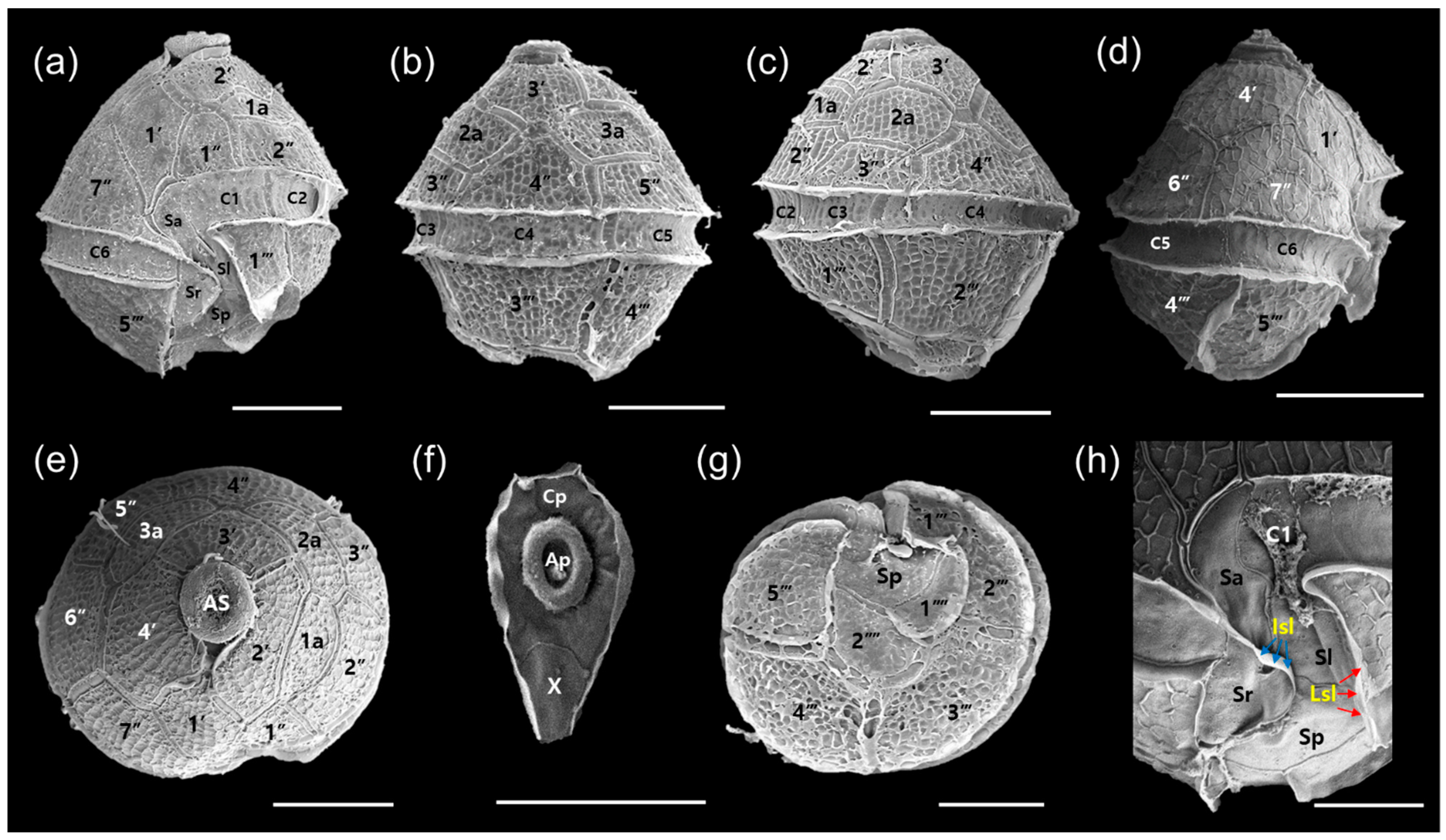
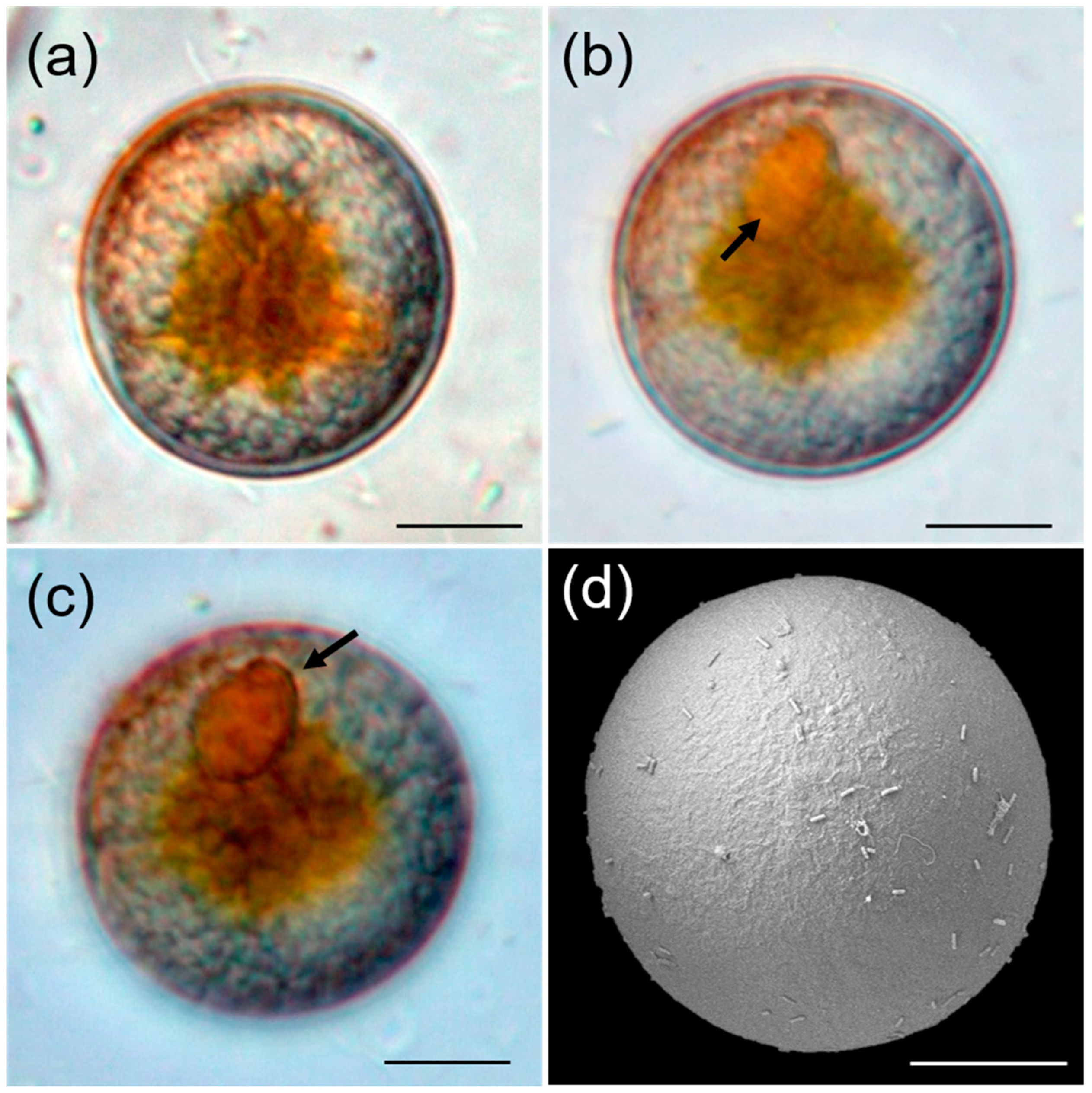
3.1. Morphology of Vegetative Cell and Resting Cyst of Bysmatrum subsalsum
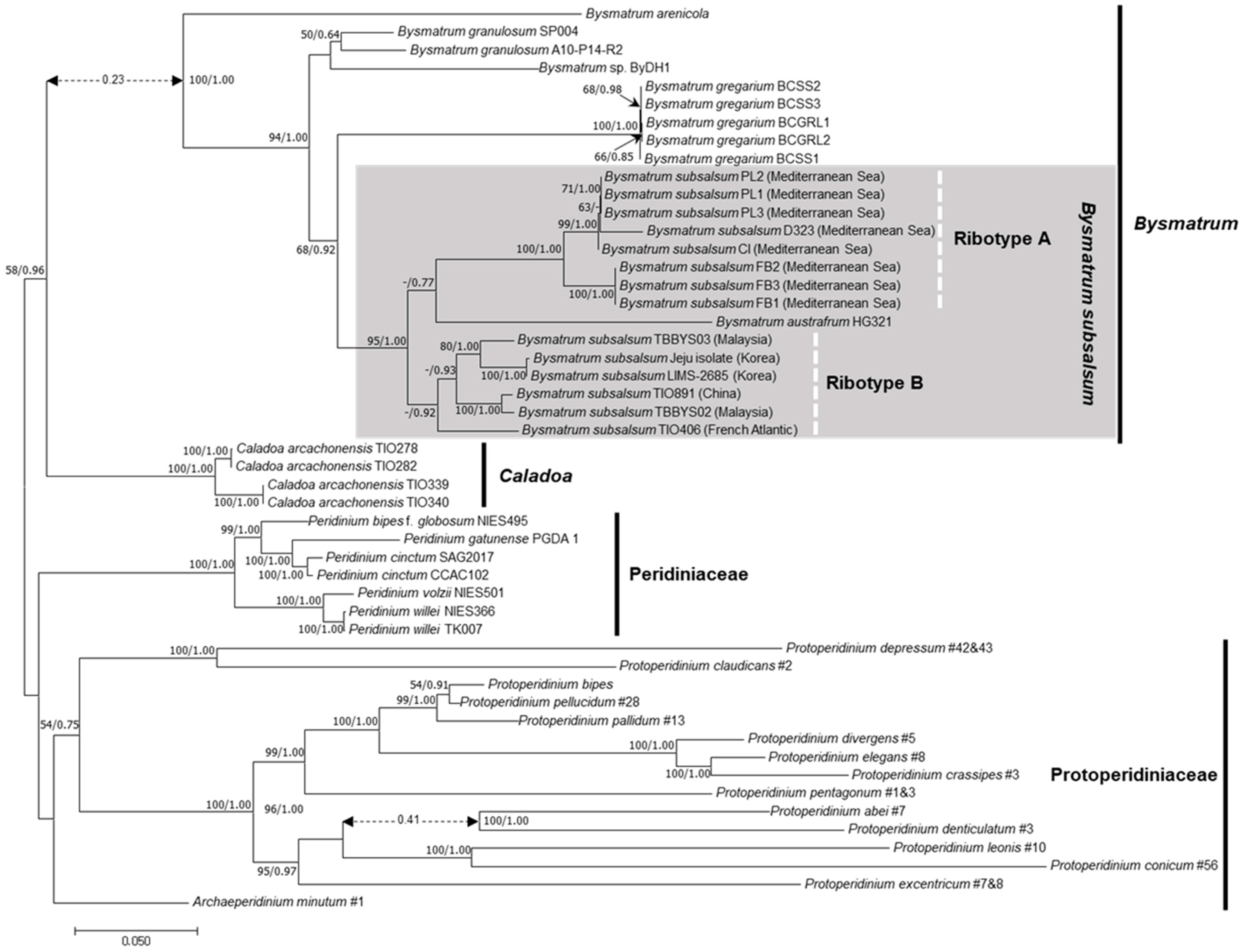
3.2. Molecular Phylogeny
4. Discussion
4.1. Morphological Comparisons of Korean Isolates of Bysmatrum subsalsum with Other Isolates of B. subsalsum, and B. austrafrum
4.2. Morphology of Bysmatrum subsalsum Cyst
4.3. Phylogenetic Position of Bysmatrum subsalsum
4.4. Environmental Conditions in Relation to the Growth of Bysmtrum subsalsum
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faust, M.A.; Steidinger, K.A. Bysmatrum gen. nov. (Dinophyceae) and three new combinations for benthic scrippsielloid species. Phycologia 1998, 37, 47–52. [Google Scholar] [CrossRef]
- Horiguchi, T.; Pienaar, R.N. Validation of Bysmatrum arenicola Horiguchi et Pienaar, sp. nov. (Dinophyceae). J. Phycol. 2000, 36, 237. [Google Scholar] [CrossRef]
- Ten-Hage, L.; Quod, J.-P.; Turquet, J.; Coute, A. Bysmatrum granulosum sp. nov., a new benthic dinoflagellate from the southwestern Indian Ocean. Eur. J. Phycol. 2001, 36, 129–135. [Google Scholar] [CrossRef]
- Murray, S.; Hoppenrath, M.; Larsen, J.; Patterson, D.J. Bysmatrum teres sp. nov., a new sand-dwelling dinoflagellate from north-western Australia. Phycologia 2006, 45, 161. [Google Scholar] [CrossRef]
- Hoppenrath, M.; Murray, S.A.; Chomérat, N.; Horiguchi, T. Marine Benthic Dinoflagellates-Unveiling Their Worldwide Biodiversity; E. Schweizerbartsche Verlagsbuchhandlung: Stuttgart, Germany, 2014; 276p. [Google Scholar]
- Dawut, M.; Sym, S.D.; Suda, S.; Horiguchi, T. Bysmatrum austrafrum sp. nov. (Dinophyceae), a novel tidal pool dinoflagellate from South Africa. Phycologia 2018, 57, 169–178. [Google Scholar] [CrossRef]
- Anglès, S.; Reñé, A.; Garcés, E.; Lugliè, A.; Sechi, N.; Camp, J.; Satta, C.T. Morphological and molecular characterization of Bysmatrum subsalsum (Dinophyceae) from the western Mediterranean Sea reveals the existence of cryptic species. J. Phycol. 2017, 53, 833–847. [Google Scholar] [CrossRef]
- Luo, Z.; Lim, Z.F.; Mertens, K.N.; Gurdebeke, P.; Bogus, K.; Carbonell-Moore, M.C.; Vrielinck, H.; Leaw, C.P.; Lim, P.T.; Chomérat, N.; et al. Morpho-molecular diversity and phylogeny of Bysmatrum (Dinophyceae) from the South China Sea and France. Eur. J. Phycol. 2018, 53, 318–335. [Google Scholar] [CrossRef]
- Jeong, H.J.; Jang, S.H.; Kang, N.S.; Yoo, Y.D.; Kim, M.J.; Lee, K.H.; Yoon, E.Y.; Potvin, E.; Hwang, Y.J.; Kim, J.I.; et al. Molecular characterization and morphology of the photosynthetic dinoflagellate Bysmatrum caponii from two solar saltons in western Korea. Ocean Sci. J. 2012, 47, 1–18. [Google Scholar] [CrossRef]
- Karen, A.S.; Tangen, K. Dinoflagellates. In Identifying Marine Diatoms and Dinoflagellates; Tomas, C.R., Ed.; Academic Press: San Diego, CA, USA, 1996; pp. 387–584. [Google Scholar]
- Ki, J.-S.; Han, M.-S. Molecular analysis of complete SSU to LSU rDNA sequence in the harmful dinoflagellate Alexandrium tamarense (Korean isolate, HY970328M). Ocean Sci. J. 2005, 40, 43–54. [Google Scholar] [CrossRef]
- Orsini, L.; Sarno, D.; Procaccini, G.; Poletti, R.; Dahlmann, J.; Montresor, M. Toxic Pseudo-nitzschia multistriata (Bacillariophyceae) from the Gulf of Naples: Morphology, toxin analysis and phylogenetic relationships with other Pseudo-nitzschia species. Eur. J. Phycol. 2002, 37, 247–257. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Balech, E. Tercera contribución al conocimiento del género Peridinium Museo Argentino de ciencias naturales ‘Bernadino Rivadavia’e Instituto nacional de investigacion de las ciencias naturales. Rev. Hydrobiol. 1964, 4, 179–195. [Google Scholar]
- Steidinger, K.A.; Balech, E. Scrippsiella subsalsa (Ostenfeld) comb. nov. (Dinophyceae) with a discussion on Scrippsiella. Phycologia 1977, 16, 69–73. [Google Scholar] [CrossRef]
- Faust, M.A. Morphology and ecology of the marine benthic dinoflagellate Scrippsiella subsalsa (Dinophyceae). J. Phycol. 1996, 32, 669–675. [Google Scholar] [CrossRef]
- Gottschling, M.; Soehner, S.; Zinssmeister, C.; John, U.; Plötner, J.; Schweikert, M.; Aligizaki, K.; Elbrächter, M. Delimitation of the Thoracosphaeraceae (Dinophyceae), including the calcareous dinoflagellates, based on large amounts of ribosomal RNA sequence data. Protist 2012, 163, 15–24. [Google Scholar] [CrossRef]
- Luo, Z.; Mertens, K.N.; Nézan, E.; Gu, L.; Pospelova, V.; Thoha, H.; Gu, H. Morphology, ultrastructure and molecular phylogeny of cyst-producing Caladoa arcachonensis gen. et sp. nov. (Peridiniales, Dinophyceae) from France and Indonesia. Eur. J. Phycol. 2019, 54, 235–248. [Google Scholar] [CrossRef]
- Moestrup, Ø.; Daugbjerg, N. On dinoflagellate phylogeny and classification. In Unravelling the Algae: The past, Present, and Future of Algal Systematics; Brodie, J., Lewis, J., Eds.; CRC press: Boca Raton, FL, USA, 2007; pp. 215–230. [Google Scholar]
- Li, Z.; Mertens, K.N.; Gottschling, M.; Gu, H.; Söhner, S.; Price, A.M.; Marret, F.; Pospelova, V.; Smith, K.F.; Carbonell-Moore, C.; et al. Taxonomy and Molecular Phylogenetics of Ensiculiferaceae, fam. nov. (Peridiniales, Dinophyceae), with Consideration of their Life-history. Protist 2020, 171, 125759. [Google Scholar] [CrossRef]
- Limoges, A.; Mertens, K.N.; Ruíz-Fernández, A.-C.; de Vernal, A. First report of fossilized cysts produced by the benthic Bysmatrum subsalsum (Dinophyceae) from a shallow Mexican lagoon in the Gulf of Mexico. J. Phycol. 2015, 51, 211–215. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Gobler, C.J. The toxic dinoflagellate Cochlodinium polykrikoides (Dinophyceae) produces resting cysts. Harmful Algae 2012, 20, 71–80. [Google Scholar] [CrossRef]
- Li, Z.; Han, M.-S.; Matsuoka, K.; Kim, S.-Y.; Shin, H.H. Identification of the resting cyst of Cochlodinium polykrikoides Margalef (Dinophyceae, Gymnodiniales) in Korean coastal sediments. J. Phycol. 2015, 51, 204–210. [Google Scholar] [CrossRef]
- Shin, H.H.; Li, Z.; Yoon, Y.H.; Oh, S.J.; Lim, W.-A. Formation and germination of temporary cysts of Cochlodinium polykrikoides Margalef (Dinophyceae) and their ecological role in dense blooms. Harmful Algae 2017, 66, 57–64. [Google Scholar] [CrossRef]
- Li, Z.; Matsuoka, K.; Shin, H.H. Revision of the life cycle of the harmful dinoflagellate Margalefidinium polykrikoides (Gymnodiniales, Dinophyceae) based on isolates from Korean coastal waters. J. Appl. Phycol. 2020, 32, 1863–1873. [Google Scholar] [CrossRef]
- Matsuoka, K.; Fukuyo, Y. Technical Guide for Modern Dinoflagellate Cyst Study; Japan Society for the Promotion of Science: Tokyo, Japan, 2000; pp. 1–29.
- Iwataki, M.; Kawami, H.; Mizushima, K.; Mikulski, C.M.; Doucette, G.J.; Relox, J.R.; Anton, A.; Fukuyo, Y.; Matsuoka, K. Phylogenetic relationships in the harmful dinoflagellate Cochlodinium polykrikoides (Gymnodiniales, Dinophyceae) inferred from LSU rDNA sequences. Harmful Algae 2008, 7, 271–277. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. Transport of toxic dinoflagellates via ships’ ballast water: Bioeconomic risk assessment and efficacy of possible ballast water management strategies. Mar. Ecol. Prog. Ser. 1998, 168, 297–309. [Google Scholar] [CrossRef]
- López-Flores, R.; Garcés, E.; Boix, D.; Badosa, A.; Brucet, S.; Masó, M.; Quintana, X.D. Comparative composition and dynamics of harmful dinoflagellates in Mediterranean salt marshes and nearby external marine waters. Harmful Algae 2006, 5, 637–648. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.S.; Li, Z.; Kim, H.J.; Kim, K.H.; Lee, K.W.; Youn, J.Y.; Kwak, K.Y.; Shin, H.H. First Report of the Marine Benthic Dinoflagellate Bysmatrum subsalsum from Korean Tidal Pools. J. Mar. Sci. Eng. 2021, 9, 649. https://doi.org/10.3390/jmse9060649
Park JS, Li Z, Kim HJ, Kim KH, Lee KW, Youn JY, Kwak KY, Shin HH. First Report of the Marine Benthic Dinoflagellate Bysmatrum subsalsum from Korean Tidal Pools. Journal of Marine Science and Engineering. 2021; 9(6):649. https://doi.org/10.3390/jmse9060649
Chicago/Turabian StylePark, Joon Sang, Zhun Li, Hyun Jung Kim, Ki Hyun Kim, Kyun Woo Lee, Joo Yeon Youn, Kyeong Yoon Kwak, and Hyeon Ho Shin. 2021. "First Report of the Marine Benthic Dinoflagellate Bysmatrum subsalsum from Korean Tidal Pools" Journal of Marine Science and Engineering 9, no. 6: 649. https://doi.org/10.3390/jmse9060649
APA StylePark, J. S., Li, Z., Kim, H. J., Kim, K. H., Lee, K. W., Youn, J. Y., Kwak, K. Y., & Shin, H. H. (2021). First Report of the Marine Benthic Dinoflagellate Bysmatrum subsalsum from Korean Tidal Pools. Journal of Marine Science and Engineering, 9(6), 649. https://doi.org/10.3390/jmse9060649






