Abstract
Suncheonman Bay, Korea’s most representative estuary, is an invasive coastal wetland composed of 22.6 km2 of tidal flats surrounded by the Yeosu and Goheung Peninsulas. In January 2006, this region was registered in the Ramsar Convention list in Korea, representing the first registered wetland. Estuaries are generally known to have high species diversity. In particular, several studies have been conducted on planktonic and epipelic diatoms as primary producers. Suncheonman Bay has already been involved in many biological and geochemical studies, but fossil diatoms have not been evaluated. Therefore, we investigated fossil diatoms in Suncheonman Bay and introduced sub-fossil diatoms recorded in Korea. One sedimentary core has been extracted in 2018. We identified 87 diatom taxa from 52 genera in the SCW03 core sample. Of these, six species represent new records in Korea: Cymatonitzschia marina, Fallacia hodgeana, Navicula mannii, Metascolioneis tumida, Surirella recedens, and Thalassionema synedriforme. These six newly recorded diatom species were examined by light microscopy and scanning electron microscopy. The ecological habitats for all the investigated taxa are presented.
1. Introduction
An estuary can be defined as a semi-enclosed coastal body of water that has a free connection with the open ocean, within which seawater is diluted with freshwater derived from land drainage [1,2]. River mouths, coastal bays, tidal marsh systems, and sounds all fit this definition. Estuaries are transitional zones between freshwater and marine habitats. Due to tides and storms, the water level and salinity vary in estuaries [3]. They are most commonly located in low-relief coastal regions. Estuaries and wetlands are among the most productive aquatic ecosystems, providing a home for both freshwater and marine plants, and a source of nutrients for a variety of animal communities adapted to brackish waters [4,5]. Moreover, they filter out pollutants supplied to the ocean [4,6,7]. Thus, many animals rely on estuaries that have abundant species diversity for food, places to breed, and migration stopovers [8,9] (https://oceanservice.noaa.gov/facts/estuary.html (accessed on 8 February 2021)).
In South Korea, where three sides are surrounded by the sea, there are numerous estuaries such as the Nakdonggang, Keumgang, and Seomjingang. Coastal wetlands cover approximately 2800 km2, which represents approximately 3% of the total land area [10,11,12]. Suncheonman Bay, Korea’s most representative estuary, is an invasive coastal wetland composed of 21.6 km2 of tidal flats and 5.4 km2 of reed fields surrounded by the Yeosu and Goheung Peninsulas [10]. In January 2006, it became the first registered wetland in the Ramsar Convention list in Korea, as it was designated as a “wetland protected area” by the Ministry of Land, Transport and Maritime Affairs in December 2003 [10,13]. In June 2008, Suncheonman Bay was designated as national cultural property, “Myeongseung” number 41, and in 2010, south-western tidal flats in Korea, including Suncheonman Bay (south-western coast tidal flats), were included in the UNESCO World Heritage Tentative List. Such registrations and designations demonstrate the recognition of its ecological and environmental value [14] (https://whc.unesco.org/en/tentativelists/5482 (accessed on 20 April 2021)). In particular, the natural environment and ecosystems within Suncheonman Bay are well preserved, making them a habitat favorable for many species of marine organisms [15]. In these regions, primary producers such as diatoms play a critical role as a food source for large invertebrates and fishes [16].
Diatoms are unicellular algae characterized by a biomineralized (opaline) cell wall that may fossilize and be preserved in the sedimentary record [17,18]. The sub-fossil diatoms herein described consist of Holocene diatom remains not fully involved in the fossilization process. Diatoms thrive in very different environments (e.g., hot springs, polar regions, and fresh, brackish, and marine waters) and are extremely sensitive to physical and chemical changes (e.g., temperature, salinity, and nutrients) in the water [18,19,20,21,22,23]. Therefore, fossil diatoms represent an excellent source of information about past climate change and its effect on aquatic ecosystems. There have been relatively few studies on sub-fossil diatoms along the southern Korean coast. Marine to brackish sub-fossil diatom assemblages were initially studied in the Pohang and Gampo sediments in 1975 [24], then they were extended to the regions of Bukpyeong [25] and Pohang [26,27] in the East Sea and the regions of the Mankyung-Dongin river estuary [28], Dodaecheon River [29], Ilsan estuary [30], Chollipo [31], Isanpo [32], and Sabsi-do and Kunsan in the Yellow Sea [33].
The Suncheonman Bay has been used to study various environmental characteristics, including grain size and organic matter in tidal flat sediments [10], seasonal water quality, pollution, environmental safety [13,34], and other geochemical characteristics, as well as local inhabitants such as benthic invertebrates, plants, fishes, birds, bacteria, and fungi [6,14,35,36,37,38,39]. Among the studies conducted to date, the investigation of phytoplankton communities in the Dong Cheon River and Isa Cheon River stream into Suncheonman bay was the most interesting [40]. In this study, we describe a newly recorded sub-fossil diatom assemblage recovered in the sediments of the Suncheonman Bay.
2. Materials and Methods
2.1. Coring and Sampling of Sediment
Drilling was carried out using a peat core sampler (52 mm diameter; Peat Sampler, Eijkelkamp Soil & Water, Giesbeek, Netherlands). One sediment core (= SCW03 with a length of 6.0 m) was retrieved from Suncheonman Bay in Korea on 11 June 2018 (Figure 1, Table 1). The core was transported to the laboratory after vacuum packing in a plastic bag to prevent drying and oxidation. Sedimentological description and subsampling were performed after the sediment core profile was cut vertically in half [41]. Shell fragments in the SCW03 core were selected for the analysis of chronology and diatoms because the sediment layer was well preserved.
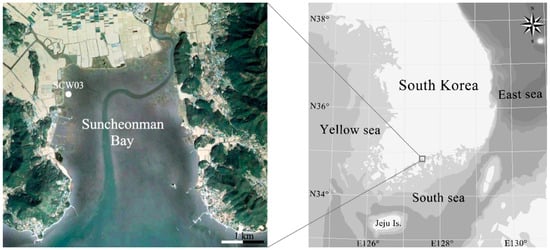
Figure 1.
Sampling sites in Suncheonman Bay.

Table 1.
Information of sampling sites.
2.2. Analysis of Chronology
Age dating was performed using an accelerator mass spectrometer (AMS) at the Korean Institute of Geoscience and Mineral Resources (KIGAM), Korea. The estimated ages were calibrated by the OxCal statistical analysis program (http://c14.arch.ox.ac.uk (accessed on 20 May 2021)).
2.3. Sample Preparation for Diatom Identification
Thirteen samples of diatoms were collected every 0.5 m along the SCW03 core. Their analysis was conducted according to the following steps: 1 g of sediment was dried at 60 °C for 24 h; the siliceous material was boiled with 20 mL of 30% hydrogen peroxide (H2O2) and washed with distilled water to remove organic matter; the treated samples were mounted with Pleurax (Mountmedia, Wako, Japan) and briefly heated using an alcohol lamp for subsequent analysis using a light microscope (LM; Eclipse Ni, Nikon, Tokyo, Japan). Photomicrographs were taken using a digital camera (DS-Ri2, Nikon, Tokyo, Japan). Some remaining peroxide-cleaned samples were filtered using 2.0-μm polycarbonate membrane filters (Nuclepore, Whatman, Maidstone, UK). The membranes were placed on stubs and coated with gold-palladium for analysis using a field emission scanning electron microscope (FE-SEM; MIRA 3, TESCAN, Brno-Kohoutovice, Czech Republic). SEM photomicrographs of all the samples were used to identify the diatoms. Morphological analyses of diatoms were performed using ImageJ v1.32 software (NIS-Elements BR4.50.00, Nikon, Tokyo, Japan) [42]. Taxonomical nomenclature was based on recent taxonomic information guidelines [43].
3. Results
3.1. Sedimentary Facies Analysis
The core SCW03 mostly consists of greenish-grey silty clay and can be divided distinguished into three sedimentary facies according to color, fossils content, and sedimentary structure (Figure 2A,C). Facies A is characterized by yellowish-brown mottling structures. Shell fragments are not observed in this facies. In Facies B, the yellowish-brown mottling structures are less abundant and shell fragments are observed to occur sporadically. The size of shell fragments are about 2 mm in diameter. Facies C is represented by highly concentrated shells and shell fragments in several-centimeters-thick intervals.
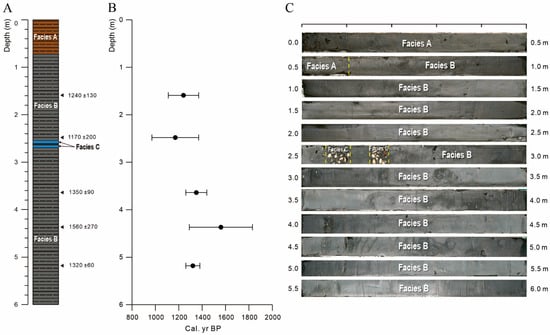
Figure 2.
(A) Stratigraphic section, (B) result of age dating, and (C) photographs of core SCW03.
The sediments of core SCW03 are interpreted as deposited in a tidal flat [44,45]. It is very similar to the current tidal flat environment. Abundant mottling structures in facies A originate from an oxygen-rich environment, indicating that the sea level has gradually decreased slightly in facies B. In the meantime, highly concentrated shells and shell fragments of facies C are interpreted as sedimentation caused by flooding or storm events [45].
3.2. Age Dating
The results of age dating for five samples in the core SCW03 shows a range from 1170 to 1560 cal. yr BP (Table 2 and Figure 2B).

Table 2.
Results of AMS 14C dating and calibrated dates for core SCW03.
3.3. Diatom Assemblages
A total of 87 diatom species belonging to 52 different genera were identified in the sediments from Suncheonman Bay in Korea (Table 3); of these, six species, namely, Cymatonitzschia marina, Fallacia hodgeana, Navicula mannii, Metascolioneis tumida, Surirella recedens, and Thalassionema synedriforme, were never described before in this area. The diatom flora encountered in this survey was composed of 87 taxa, which were classified into 52 genera. We present information on the valve shape, occurrence depth in sediment, and habitat of 87 species, including the six newly recorded species. Information collected about the newly recorded diatoms identified here included taxonomic information, illustrations, basionyms, synonyms, original description references, depth in the core, distribution, and diagnosis (Table 3).

Table 3.
Occurrence (black squares) and habitat of diatom species by depth. A total of 72 diatom species belonging to 52 different genera were identified. Star marks on the specific name are newly recorded species in Korea (6 species: Cymatonitzschia marina, Fallacia hodgeana, Navicula mannii, Metascolioneis tumida, Surirella recedens, and Thalassionema synedriforme).
Cymatonitzschia marina (F.W.Lewis) Simonsen 1974 (Figure 3A,B)
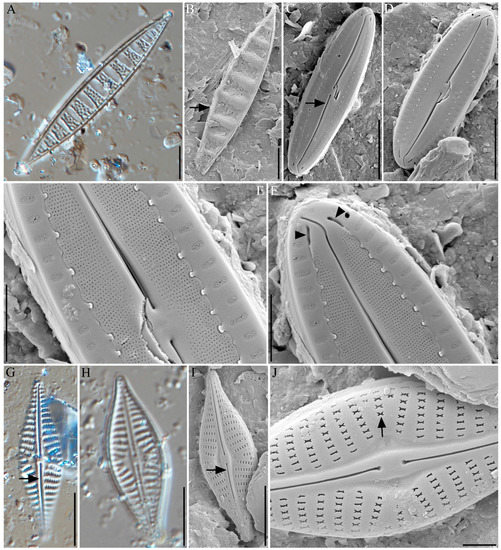
Figure 3.
Light microscope (A,G,H) and field emission scanning electron microscopic (B–F,I,J) photomicrographs of diatoms: (A,B) Cymatonitzschia marina (the arrow points to raphe), (C–F) Fallacia hodgeana (in C the arrow points to raphe; in F the arrow points to the terminal openings), (G–J) Navicula mannii with raphe and ribbon-shaped areola (the arrow points to raphe and ribbon-shaped areola). Scale bar = 10 µm.
Basionym: Cymatopleura marina F.W.Lewis 1861 [107]
Synonym: Cymatopleura marina F.W.Lewis 1861 [107]
Original description: Simonsen 1974: 56, pl. 41: Figures 5–9 [108]
Description: Valves are observed to be solitary, usually lying in the valve view. Valves are linear lanceolate, with very acute ends. Valves are strictly isopolar, and not constricted in the middle. Overall dimensions include a valve length ranges from 58.42 to 67.94 µm and valve width from 9.14 to 11.28 µm. The valve faces have numerous undulations (9–11), with a distance between two undulations in the ranges from 4.81 to 7.97 µm. Undulations are found to have a nearly trapezoidal shape (Figure 3A). The valve surface has irregular punctate on the undulations. A raphe system is observed running around one side of the valve margin. Striae uniseriate are found to be densely spaced, with approximately 28–29 per 10 µm observed, on one side of the valve margin (Figure 3B).
Depth occurrence in the core: 2.0 m.
Distribution: This species is reported from brackish water to marine environments mainly [61,62,63,64,65,66]. This taxon is reported from some estuary, e.g., East River, New York and Long Island Sound [109]. Cymatopleura marina was first recorded from the Indian Ocean [108].
Differential diagnosis: This genus differs from Cymatopleura. The genus Cymatopleura, as a member of the Surirellaceae, has a completely different raphe morphology, which runs along the edge of the valve around the entire margin, whereas in Cymatonitzschia it is, as in Nitzschia, limited to one of the sides [108]
Remarks of raphe: Cymatonitzschia marina has an eccentric keeled raphe placed through the edge of the valve, and it appears on one of the sides [108,110].
Fallacia hodgeana (R.M.Patrick and Freese) Y.H.Li and H.Suzuki 2014 (Figure 3C–F)
Basionym: Navicula hodgeana R.M.Patrick and Freese 1961 [111]
Synonym: Navicula hodgeana R.M.Patrick and Freese 1961 [111]
Original description: Li et al., 2014 in p. 33 [74]
Description: Valves are observed to be solitary, usually lying in the valve view. Valves are naviculoid and linear-elliptic, with bluntly rounded ends. Overall dimensions include length ranges from 14.73 to 15.42 µm and width ranges from 4.21 to 4.61 µm. The valve face is nearly flat with a slightly curved raphe (Figure 3C,D, arrow). Central raphe endings are proximately hooked (Figure 3E,F, arrowheads). Terminal raphe fissures exhibit a sickle-shaped curve in the same direction. Some parts of the striae are covered with a thin siliceous covering, or conopeum, on the external valve surface. Tow slits opening of the canal, present near the terminal raphe fissures, are also observed (Figure 3F, arrowhead). Areolae are found to be curved upward and were directly connected to the mantle. The finely porous conopeum extends outward from the outer edge of the raphe sterna, running through the surface, and connect to the proximal edge of the mantle. Numerous peg-shaped structures are observed in the nonporous margin of the conopeum along the proximal edge of the mantle. The elongated areolae, with an approximate length and width of 0.28–0.42 µm and 0.15–0.18 µm, respectively, are found on the hyaline area of the valve surface with an undulated junction. The elongated areolae were found in groupings of 12 per 5 µm. Peg-shaped structures, in groups of 12 per 5 µm, are also observed, finely porous on the conopeum 12–13 per 1 µm transversely.
Depth occurrence in the core: 6.0 m.
Distribution: This species lives in fresh- to brackish-water environments. It was first reported from scrapings of small rock submerged at the edge of a lagoon as Navicula hodgeana [111]. Li et al. 2014 collected this species from Edogawa River, Japan. This taxon is known to benthic diatom [74,112,113].
Differential diagnosis: Fallacia hodgeana possesses morphological features such as a single H-shaped plastid, depressed lateral sterna interrupting striae that contain round areolae enclosed by hymen; well-developed, finely porous conopeum; and a canal system between the primary silica layer and the conopeum. These features indicate that this species does not belong to the genus Navicula or Pseudofallacia [74]. This species is related to Navicula dissipata. The length-to-breadth ratio is similar, although N. dissipata is a larger taxon. The clear central area is narrower in our taxon, and the striae are composed of many fine puncta instead of a few large ones. The median ends of the raphe are close together as in N. dissipata [111].
Remarks of raphe: Fallacia hodgeana has a slightly curved raphe that terminates at fissures curved in the same way. The distal end fissures were sickle-like and curved in the same direction (Figure 3C arrow). The central endings lie close to each other, and slightly curved slits seem to be promoted from the general valve [74].
Navicula mannii Hagelstein 1939 (Figure 3G–J)
Synonym: Navicula elegantissima Meister 1935 [114]
Original description: Hagelstein 1939, p. 388, pl. 7, Figures 7 and 8 [115]
Description: Valves are observed to be solitary. Valves are broadly lanceolate, and abruptly constricted toward the ends (Figure 3G–I). Overall dimensions include average length and width ranges from 28.28 to 30.09 µm and from 8.57 to 9.44 µm, respectively. The axial area is narrow, and becomes gradually wider, larger, and rounded toward the central area (Figure 3I). Raphe is observed to be very slightly curved filiform style with a very thickened and hyaline sternum (Figure 3I,J, arrow). Striae are very coarse and of low density (9–11 in 10 µm); they are observed to strongly radiate in the middle and then become parallel towards the ends. The central area striae alternate between longer and shorter forms (longer striae 4 areolae, and shorter striae 2 areolae). Areolae are observed to be ribbon-shaped, and approximately 5–6 are found in 2 μm sections (Figure 3J, arrow).
Depth occurrence in the core: 2.7 cm.
Distribution: Navicula mannii was reported in brackish water or marine environments [70,116,117]. Navarro (1983) reported the taxon in tropical temperate waters from the southwestern coast of Puerto Rico [116]. This species was known to neritic, pantropical, and cosmopolitan [116]. Ohtsuka (2005) collected the species from a muddy tidal flat in the Ariake Sea in south-western Japan [117].
Differential diagnosis: Hagelstein (1939) described that the Navicula mannii have minutely punctate areolae, but we found the ribbon-shaped areolae on the striae based on SEM observation in this study [115]. Ultrastructural studies of this species are rarely performed using a SEM; this study represents the first example of this approach.
Remarks of raphe: Navicula mannii has a straight raphe. Proximal raphe ends have an expanded pore-like shape and bent distal raphe ends (Figure 3G,H,J, arrows). Normally, Navicula spp. have a straight raphe system, unlike the raphe shapes of Navicula cryptocephala and Navicula gregaria, which commonly occur in Korea. N. mannii and N. cryptocephala both have drop-like internal ends, but N. mannii has a more pore-like end than N. cryptocephala [83,118], with a T-shaped structure. In contrast, Navicula gregaria has a different raphe shape than N. mannii, which is bent in the same direction as the raphe and exhibits asymmetrical thickening, beside the proximal raphe ends and beside the raphe rib [118].
Metascolioneis tumida (Brébisson ex Kützing) Blanco and Wetzel 2016 (Figure 4A,B)
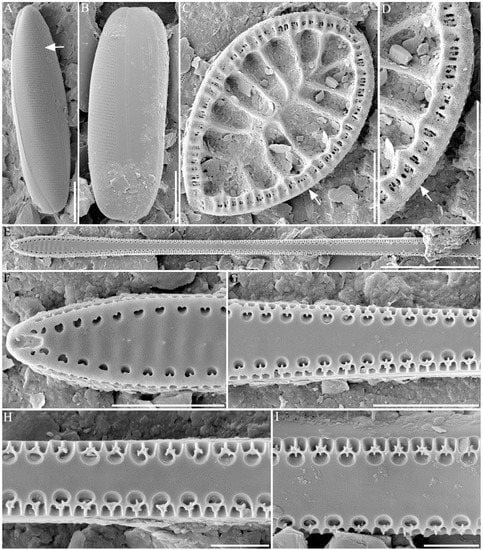
Figure 4.
Field emission scanning electron microscopic photomicrographs of diatoms. (A,B) Metascolioneis tumida (the arrow points to raphe), (C,D) Surirella recedens (the arrow points to raphe), (E–I) Thalassionema synedriforme. Scale bar = 10 µm.
Basionym: Navicula tumida Brébisson ex Kützing 1849 [119]
Synonym: Navicula tumida Brébisson ex Kützing 1849 [119]
Scoliopleura tumida (Brébisson ex Kützing) Rabenhorst 1864 [120]
Microstigma tumida (Brébisson) Meister 1919 [121]
Scoliotropis tumida (Brébisson ex Kützing) R.M.Patrick and Freese 1961 [111]
Scolioneis tumida (Brébisson ex Kützing) D.G.Mann 1990 [122]
Navicula jenneri W.Smith 1853 [84]
Scoliopleura jenneri Grunow 1860 [123]
Original description: Blanco, S. and Wetzel, C.E., 2016, pp. 195–205 [124].
Description: Valves are found to be solitary with one-layered valves. Valves are linear lanceolate with bluntly rounded apices (Figure 4A). Overall dimensions include an average length and width ranges from 41.72 to 52.5 µm and from 6.05 to 6.50 µm, respectively. Cells are usually found in a girdle view and twisted about the apical axis (Figure 4B). The valve mantle is relatively deep, and its face curved moderately into mantles. Striae uniseriate (16–17 in 10 µm) with small poroids (12–15 µm in 5 µm) are observed. The raphe system is twisted and sigmoidal in shape (Figure 4A, arrow). The raphe sternum is generally narrow and slightly wider in thcenterre. The raphe is found to be straight with simple raphe endings and straight terminal fissures extending to the valve margin. The girdle consists of several open bands. The band closest to the valve bears one transverse row of poroids 31–32 at 10 µm.
Depth occurrence in the core: 1.0, 1.5, 2.0 m.
Distribution: This species has been found in marine habitats. Stoermer et al. (1999) presented this taxon in a checklist of diatoms as Navicula tumida from the marine environment in the Laurentian Great Lakes [125]. Vilicic et al. (2002) reported the species as Scoliopleura tumida from the eastern Adriatic Sea [93]. This species was listed to the British marine diatoms as Scoliopleura tumida [126,127]. Méléder et al. (2007) reported the taxon as Scolioneis tumida in a sediment of mudflat from Bourgneuf Bay, France [128].
Differential diagnosis: Formerly included in Scolipleura taxon, but lacking the offset central raphe endings and longitudinal canals of that genus. Distinguishable from Scoliotropis by having fewer plastids, which lie against the valves rather than the girdle, by the simple uniseriate striae and raphe structure [122].
Remarks of raphe: Metascolioneis tumida (syn. Scolioneis tumida, Navicula tumida) has a slightly twisted raphe and raphe sternum that is normally narrow and becomes expanded in the center (Figure 4A, arrow). External central raphe endings have straight fissures along the valve margin. Furthermore, internal central endings are T-shaped and elongated [122].
Surirella recedens A.W.F.Schmidt 1875 (Figure 4C,D)
Homotypic synonym: Surirella fastuosa var. recedens (A.W. F. Schmidt) Cleve 1878 [129]
Surirella fastuosa var. typica f. recedens (A. W. F. Schmidt) Deby 1897 [130]
Original description: Schmidt and Fricke 1875, pls 17–20. [131]
Description: Valves are found to be solitary, strongly silicified, and lying in the valve or girdle view. Valves are heteropolar with a broadly rounded headpole and cuneate footpole. Overall dimensions include a length and width of 35.08 µm and 22.83 µm, respectively. The valve surface has four costae of 10 µm in length. The valve margin includes fibulae, siliceous braces, 7–8 fibulae, 10 µm. The raphe system runs around the entire valve margin and is located within a canal (Figure 4C,D; arrows). The canal is raised above the valve’s surface. One or more potulae are located between the two fibulae. Four or more fibulae are located between the two costae.
Occurring depth in Core: 2.7, 5.5 m.
Distribution: This taxon was known to marine species [95,96]. López-Fuerte and Siqueiros-Beltrones (2016) reported the species as a benthic diatom from coastal waters in Mexico [132] and the Nanaura mudflat in Ariake Sea, Japan [117]. However, Surirella recedens was found in brackish waters from Cochin Backwater south in the Indian Ocean [133].
Differential diagnosis: S. recedens is composed of a heteropolar valve, whereas Surirella fastuosa has an isopolar valve [134]. S. recedens is smaller overall, in comparison to S. fastuosa, and more lanceolate in shape. In addition, S. fastuosa has more noticeable apices than S. recedens [135]. Goldman (1990) identified the Surirella cf. fastuosa based on the outline, length, infundibula, and circular pattern of the valve [136].
Remarks of raphe: Surirella recedens (Syn. Surirella fastuosa var. recedens, Surella fastuosa var. typical f. recedens) Surirella sp. has a raphe that is located along the margin of the valve (Figure 4D, arrow). A raphe positioned within a canal might be elevated above the valve surface in several species [137]. S. recedens shows a representative Surirellaceae raphe system, positioned along the margin of the valve (Figure 4D, arrow).
Thalassionema synedriforme (Greville) G.R.Hasle 1999 (Figure 4E–I)
Basionym: Asterionella synedriformis Greville 1865 [138]
Synonym: Asterionella synedriformis Greville 1865 [138]
Thalassionema javanicum Grunow Hasle in Hasle and Syvertsen 1996 [139]
Depth occurrence in the core: Hasle, G.R. 1999, pp. 54–59, 23 figures [140].
Description: The valves are heteropolar spatulate, linear, long, slightly wider in the middle, and constricted towards the head pole, rather than towards the foot pole (Figure 4E). Valve width ranges from 2.66 to 2.87 µm in the middle part of the valve and from 4.03 to 4.40 µm in the middle part of the footpole. An apical spine is located in the head pole, not the foot-pole part of the valve. The valve face is flat, with a wide sternum and slight undulation (5–6 in 5 µm) at the foot pole (Figure 4F). Areolae are placed within the valve face and valve mantle. Areolae are heart-shaped (5–6 in 5 µm) near the foot pole part, but similar to Y- or flower-shaped occlusions (5–6 in 5 µm) toward the middle part of the valve (Figure 4F–I). Labiate processes are placed at each pole of the valve. An opening of the labiate process places the external apex in the foot pole [141].
Depth occurrence in the core: 3.0 m.
Distribution: Thalassionema synedriforme was known to marine species. Hasle (2001) mentioned the species is restricted in tropical and subtropical waters [98]. This species was recorded for the first time from Argentinean coastal waters [142].
Differential diagnosis: Asterionella synedriformis Greville is the basionym of Thalassionema synedriforme [98]. Frenguelli (1941) mentioned that he found valves with 9–10 areolae within 10 μm and illustrated a linear, fragmentary specimen that, according to the valve outline and areola density, might also be attributed to Thalassionema frauenfeldii, but not to T. synedriforme [143]. Additionally, in the case of Thalassiothrix javanica (Grunow), Hustedt and Frenguelli illustrated a specimen that was slightly heteropolar with 6–7 areolae within 10 μm, which differs in areolae density and valve outline from Thalassionema synedriforme (12–16 areolae in 10 μm, according to Hasle 2001) [98].
4. Discussion
4.1. Diatoms in SCW03
In this study, the analysis of sub-fossil diatoms among core samples obtained from Suncheon Bay, Korea, was carried out. Within the SCW03 core sample, a total of 52 genera and 87 species of sub-fossil diatoms were identified, with locations ranging from the surface to within 6 m of the basement, and among them, six species of newly recorded sub-fossil diatom never recorded before were found. At a depth of 4.0 m, the maximum variety of diatom samples was observed, with 23 genera 34 species, while depths of 0.5 m and 3.5 m revealed the lowest variety of diatoms, with 12 genera 14 species and 10 genera 14 species, respectively (Table 3). The highest and lowest taxonomic richness occur at depths of 4.0 m (23 genera, 34 species) and 3.5 m (10 genera, 14 species), respectively (Table 3).
Among the diatoms observed, Amphora sp., Auliscus sculptus, and Fragilaria capucina were found only at 0.1 m depths; therefore, they are hypothesized to have only recently entered the Suncheonman Bay (Table 3). Cymatosira lorenziana, Fallacia hodgeana, Pleurosigma sp., Semiorbis sp., and Trachyneis aspera are no longer observed since they appeared to only occur at 6.0 m depth. It is hypothesized that this is due to climate, environmental, or topographical changes in the Suncheonman Bay habitat. Intriguingly, C. lorenziana is mainly found in warm waters, whereas T. aspera is mainly distributed in the Antarctic area, with almost opposite habitat characteristics; however, the causative environmental changes in Suncheonman Bay could not be identified in this study [144,145,146]. Nevertheless, identifying past environmental changes in Suncheon Bay is an important source of information for the prediction of future environmental changes, and should be investigated further.
Most of the marine and brackish species occur at a depth of 5.0 to 6.0 m of the SCW03 core sample, and the emergence of freshwater species gradually increased within depth ranges of 3.0 to 4.5 m. Interestingly, only marine and brackish species appeared at the 2.5 m section (Table 3). Marine and some freshwater species appeared within occur the range of 1.0 m to 2.0 m depths, while only marine and brackish species appeared at 0.5 m depth; finally, freshwater species increased again at 0.1 m depth. These changes in the flora of diatoms assemblage composition observed suggest that the sediments recovered from SCW03 were deposited in a marine area that was scarcely influenced by freshwater inflow in the past (3.0–4.5 m depth; about 1260–1830 yr BP) and experienced a renewed influx of fresh water in recent times (0.1 m depth; about 1340 ± 20 yr BP) (Table 2 and Table 3, Figure 2) [147,148]. More accurate results and solid interpretations of the triggers responsible for such environmental changes require further studies of comprehensive environmental change, including a quantitative analysis of diatoms and a chronological analysis of core samples.
4.2. New Recorded Taxa from Korea
In this study, we identified six species newly recorded in Suncheonman Bay area: Cymatonitzschia marina, Fallacia Hodgeana, Navicula mannii, Metascolioneis tumida, Surirella recedens, and Thalassionema synedriforme. N. mannii was first identified by light microscopy in 2005; however, in this study, we observed the ultrastructure of N. mannii and discovered a ribbon-shaped areolae by Fe-SEM [117]. The ribbon-shaped areolae of N. mannii are the first to ever be recorded.
Unrecorded sub-fossil diatoms in Suncheonman Bay were discovered at 1.0 m depths. This study is therefore meaningful because if we study the modern composition of diatoms in Suncheonman Bay or Korea, we would estimate six previously unrecorded diatoms were extinct in this area.
Author Contributions
Data curation, formal analysis, writing—original draft, and writing—review and editing, M.P., D.K., and S.D.L.; funding acquisition, S.D.L.; field investigation, J.-Y.L. and J.-M.C.; writing—review and editing, M.P., S.D.L., H.L., J.-Y.L., D.K., and J.-M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Nakdonggang National Institute of Biological Resources (NNIBR) (NNIBR202101108) projects.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Thanks to Seung Won Nam, Suk Min Yun, and Pyo Yun Cho for helping with the coring of sediment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pritchard, D.W. What Is an Estuary: Physical Viewpoint; American Association for the Advancement of Science: Washington, DC, USA, 1967. [Google Scholar]
- Smol, J.P.; Stoermer, E.F. The Diatoms: Applications for the Environmental and Earth Sciences; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Ralston, D.K.; Geyer, W.R. Response to channel deepening of the salinity intrusion, estuarine circulation, and stratification in an urbanized estuary. J. Geophys. Res. Ocean. 2019, 124, 4784–4802. [Google Scholar] [CrossRef]
- Selleslagh, J.; Amara, R. Environmental factors structuring fish composition and assemblages in a small macrotidal estuary (eastern English Channel). Estuar. Coast. Shelf Sci. 2008, 79, 507–517. [Google Scholar] [CrossRef]
- Elliott, M.; O’reilly, M.G.; Taylor, C.J.L. The forth estuary: A nursery and overwintering area for North Sea fishes. Hydrobiologia 1990, 195, 89–103. [Google Scholar] [CrossRef]
- Ye, S.J.; Jeong, J.M.; Kim, H.J.; Park, J.M.; Huh, S.H.; Baeck, G.W. Fish assemblage in the tidal creek of Sangnae-ri Suncheon, Korea. Korean J. Ichthyol. 2014, 26, 74–80. [Google Scholar]
- Kecinski, M.; Messer, K.D.; Peo, A.J. When cleaning too much pollution can be a bad thigns: A field experiment of consumer demand for oysters. Ecol. Econ. 2018, 146, 686–695. [Google Scholar] [CrossRef]
- Canham, R.; Flemming, S.A.; Hope, D.D.; Drever, M.C. Sandpipers go with the flow: Correlations between estuarine conditions and shorebird abundance at an important stopover on the Pacific Flyway. Ecol. Evol. 2021, 11, 2828–2841. [Google Scholar] [CrossRef] [PubMed]
- Laprise, R.; Dodson, U.J. Environmental variability as a factor controlling spatial patterns in distribution and species diversity of zooplankton in the St. Lawrence Estuary. Mar. Ecol. Prog. Ser. 1994, 107, 67–81. [Google Scholar] [CrossRef]
- Jang, S.G.; Cheong, C.J. Characteristics of grain size and organic matters in the tidal flat sediments of the Suncheon Bay (Korean). J. Korean Soc. Mar. Environ. Energy. 2010, 13, 198–205. [Google Scholar]
- Du, G.; Son, M.; Yun, M.; An, S.; Chung, I.K. Microphytobenthic biomass and species composition in intertidal flats of the Nakdong River estuary, Korea. Estuar. Coast. Shelf Sci. 2009, 82, 663–672. [Google Scholar] [CrossRef]
- Kim, T.I.; Choi, B.H.; Lee, S.W. Hydrodynamics and sedimentation induced by large-scale coastal developments in the Keum River Estuary, Korea. Estuar. Coast. Shelf Sci. 2006, 68, 515–528. [Google Scholar] [CrossRef]
- Jang, S.G.; Cheong, C.J. Seasonal characteristics of seawater quality in the Suncheon Bay. Ecotoxicol. Environ. Saf. 2010, 12, 47–57. [Google Scholar]
- Kim, K.; Lee, K.J.; Han, B.H. Environmental ecological status of Suncheon bay and its application to the criteria of UNESCO world nature heritage. Korean J. Environ. Eco. 2013, 27, 625–641. [Google Scholar] [CrossRef]
- Park, H.J.; Kwak, J.H.; Kang, C.K. Trophic consistency of benthic invertebrates among diversified vegetational habitats in a temperate coastal wetland of Korea as determined by stable isotopes. Estuar. Coast. 2015, 38, 599–611. [Google Scholar] [CrossRef]
- Kim, H.K.; Kwon, Y.S.; Kim, Y.J.; Kim, B.H. Distribution of epilithic diatoms in estuaries of the Korean Peninsula in relation to environmental variables. Water 2015, 7, 6702–6718. [Google Scholar] [CrossRef]
- Leterme, S.C.; Prime, E.; Mitchel, J.; Brown, M.H.; Ellis, A.V. Diatom adaptability to environmental change: A case study of two Cocconeis species from high-salinity areas. Diatom Res. 2013, 28, 29–35. [Google Scholar] [CrossRef]
- Cho, A.; Cheong, D.; Kim, J.C.; Shin, S.; Park, Y.H.; Katsuki, K. Delta formation in the Nakdong River, Korea, during the Holocene as inferred from the diatom assemblage. J. Coast. Res. 2017, 33, 67–77. [Google Scholar] [CrossRef]
- Laugaste, R.; Pork, M. Diatoms of lake peipsi-pihkva: A floristic and ecological review. Hydrobiologia 1996, 338, 63–76. [Google Scholar] [CrossRef]
- Ryu, E.; Lee, S.J.; Yang, D.Y.; Kim, J.Y. Paleoenvironmental studies of the Korean peninsula inferred from diatom assemblages. Quat. Int. 2008, 176, 36–45. [Google Scholar] [CrossRef]
- Lee, S.D.; Lee, H.; Park, J.; Yun, S.M.; Lee, J.-Y.; Lim, J.; Park, M.; Kwon, D. Late Holocene diatoms in sediment cores from the Gonggeomji wetland in Korea. Diatom Res. 2020, 35, 195–229. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.D.; Lee, J.-Y.; Lim, J.; Kwon, D.; Park, M. Holocene paeoenvironmental changes and characteristic of diatom distribution in Upo Wetland of Korea. Korean J. Environ. Ecol. 2020, 53, 109–137. [Google Scholar] [CrossRef]
- Oh, S.H.; Koh, C.H. Distribution of diatoms in the surficial sediments of the Mangyung-Dongjin tidal flat, west coast of Korea (Eastern Yellow Sea). Mar. Bio 1995, 122, 487–496. [Google Scholar] [CrossRef]
- Lee, Y.G. Neogene diatoms of Pohang and Gampo areas, Kyongsangbug-do, Korea. J. Geological. Soc. Korea 1975, 11, 99–113. [Google Scholar]
- Lee, Y.G. On the fossil diatoms in the Bukpyeong Formation, Bukpyeong area, Gangweon-do, Korea. J. Geological. Soc. Korea 1977, 13, 23–40. [Google Scholar]
- LEE, Y.G. Micropaleontological study of Neogene strata of southeastern Korea and adjacent sea floor. J. Paleontol. Soc. Korea 1986, 2, 83–113. [Google Scholar]
- Lee, Y.G. Neogene paleotemperature oscillations in the Pohang Basin, Korea. JKESS 1988, 9, 203–216. [Google Scholar]
- Lee, Y.G.; Park, Y.A.; Choi, J.Y. Sedimentary facies and micropaleontological study of tidal sediments off the Mankyung-Dongjin River estuary, west coast of Korea. J.Korean Soc. Oceanogr. 1995, 30, 77–90. [Google Scholar]
- Hwang, S.I.; Yoon, S.O.; Jo, W.R. The change of the depositional environment on Dodaecheon River basin during the Middle Holocene. J. Geol. Soc. Korea 1997, 32, 403–420. [Google Scholar]
- Hwang, S. The Holocene depositional environment and sea-level change at Ilsan area. J. Geol. Soc. Korea 1998, 33, 143–163. [Google Scholar]
- Ryu, E.; Nahm, W.H.; Yang, D.Y.; Kim, J.Y. Diatom floras of a western coastal wetland in Korea: Implication for late Quaternary paleoenvironment. J. Geol. Soc. Korea 2005, 41, 227–239. [Google Scholar]
- Yi, S.; Ryu, E.; Kim, J.Y.; Nahm, W.H.; Yang, D.Y.; Shin, S.C. Late Holocene paleoenvironmental changes inferred from palynological and diatom assemblages in Isanpo area, Ilsan, Gyeonggi-do, Korea. J. Geol. Soc. Korea 2005, 41, 295–322. [Google Scholar]
- Bak, Y.S. Mid-Holocene sea-level fluctuation inferred from diatom analysis from sediments on the west coast of Korea. Quat. Int. 2015, 384, 139–144. [Google Scholar] [CrossRef]
- Park, T.M.; Kweon, D.H. Study of the heavy metal pollution of the Suncheon Bay tidal flat. J. Korea Soc. Environ. Adm. 2001, 7, 467–472. [Google Scholar]
- Kamimura, S.; Itoh, H.; Ozeki, S.; Kojima, S. Molecular diversity of Cerithidea gastropods inhabiting Suncheon Bay, and the Japanese and Ryukyu Islands. Plankton Benthos Res. 2010, 5, 250–254. [Google Scholar] [CrossRef]
- Park, Y.K.; Yoo, M.L.; Heo, H.S.; Lee, H.W.; Park, S.H.; Jung, S.C.; Park, S.S.; Seo, S.G. Wild reed of Suncheon Bay: Potential bio-energy source. Renew Energy 2012, 42, 168–172. [Google Scholar] [CrossRef]
- Choi, S.C.; Choi, D.G.; Hwang, J.S.; Kim, J.G.; Choo, Y.S. Solute patterns of four halophytic plant species at Suncheon Bay in Korea. J. Ecol. Environ. 2014, 37, 131–137. [Google Scholar] [CrossRef]
- You, Y.H.; Park, J.M.; Lee, M.-C.; Kim, J.G. Phylogenetic analysis and diversity of marine bacteria isolated from rhizosphere soils of halophyte in Suncheon Bay. Microbiol. Biotechnol. Lett. 2015, 43, 65–78. [Google Scholar] [CrossRef]
- You, Y.H.; Yoon, H.; Kang, S.M.; Shin, J.H.; Choo, Y.S.; Lee, I.J.; Lee, J.M.; Kim, J.G. Fungal diversity and plant growth promotion of endophytic fungi from six halophytes in Suncheon Bay. J. Microbiol. Biotechnol. 2012, 22, 1549–1556. [Google Scholar] [CrossRef]
- Noh, K.H.; Kim, J.H.; Chung, Y.C. Species composition and dynamics of phytoplankton community in Dong Cheon and Isa Cheon flowed into Suncheon Bay. Korean J. Limnol. 1991, 24, 153–163. [Google Scholar]
- Lee, H.; Yun, S.M.; Lee, J.-Y.; Lee, S.D.; Lim, J.; Cho, P.Y. Late Holocene climate changes from diatom records in the historical Reservoir Gonggeomji, Korea. J. Appl. Phycol. 2018, 30, 3205–3219. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to imageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Guiry, M.D.; Guiry, G.M. AlgaeBase [online]. World-Wide Electronic Publication, National University of Ireland: Galway, Ireland, 2020. Available online: https://www.algaebase.org (accessed on 8 December 2020).
- Lim, J.; Lee, J.-Y.; Hong, S.; Park, S.; Lee, E.; Yi, S. Holocene coastal environmental change and ENSO-driven hydroclimatic variability in East Asia. Quat. Sci. Rev. 2019, 220, 75–86. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.-Y.; Shin, S. Middle Holocene Coastal Environmental and Climate Change on the Southern Coast of Korea. Appl. Sci. 2021, 11, 230. [Google Scholar] [CrossRef]
- Hoppenrath, M. A revised checklist of planktonic diatoms and dinoflagellates from Helgoland (North Sea, German Bight). Helgol. Mar. Res. 2004, 58, 243–251. [Google Scholar] [CrossRef]
- Vilbaste, S. Benthic diatom communities in Estonian rivers. Boreal. Environ. Res. 2001, 6, 191–203. [Google Scholar]
- Tsoy, I.; Prushkovskaya, I.; Aksentov, K.; Astakhov, A. Environmental changes in the Amur Bay (Japan/East Sea) during the last 150 years revealed by examination of diatoms and silicoflagellates. Ocean. Sci. J. 2015, 50, 433–444. [Google Scholar] [CrossRef]
- Jahn, R.; Schmid, A.M.M. Revision of the brackish-freshwater diatom genus Bacillaria Gmelin (Bacillariophyta) with the description of a new variety and two new species. Eur. J. Phycol. 2007, 42, 295–312. [Google Scholar] [CrossRef]
- Myklestad, S. Production of carbohydrates by marine planktonic diatoms. I. Comparison of nine different species in culture. J. Exp. Mar. Biol. Ecol. 1974, 15, 261–274. [Google Scholar] [CrossRef]
- Tabassum, A.; Saifullah, S. The planktonic diatom of the genus Chaetoceros Ehrenberg from northwestern Arabian Sea bordering Pakistan. Pak. J. Bot. 2010, 42, 1137–1151. [Google Scholar]
- Lewis, J.; Harris, A.; Jones, K.; Edmonds, R. Long-term survival of marine planktonic diatoms and dinoflagellates in stored sediment samples. J. Plankton Res. 1999, 21, 343–354. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.H.; Khim, J.S. The identity of ‘Berkeleya scopulorum’from Northeast Asia: Report on Climaconeis mabikii sp. nov. from temperate marine waters with notes on biogeography of the genus. Ocean. Sci. J. 2016, 51, 591–598. [Google Scholar] [CrossRef]
- Tang, T.; Qu, X.; Li, D.; Liu, R.; Xie, Z.; Cai, Q. Benthic algae of the Xiangxi river, China. J. Freshw. Ecol. 2004, 19, 597–604. [Google Scholar] [CrossRef]
- Sumper, M. A phase separation model for the nanopatterning of diatom biosilica. Science 2002, 295, 2430–2433. [Google Scholar] [CrossRef]
- Choudhury, A.K.; Pal, R. Phytoplankton and nutrient dynamics of shallow coastal stations at Bay of Bengal, Eastern Indian coast. Aquat. Ecol. 2010, 44, 55–71. [Google Scholar] [CrossRef]
- Al-Harbi, S.M. Phytoplankton composition of *ROPME Sea Area (Arabian Gulf). Mar. Scienes 2005, 16, 105–114. [Google Scholar] [CrossRef]
- Romero, O.E.; Thunell, R.C.; Astor, Y.; Varela, R. Seasonal and interannual dynamics in diatom production in the Cariaco Basin, Venezuela. Deep Sea Res. Part. I Oceanogr. Res. Pap. 2008, 56, 571–581. [Google Scholar] [CrossRef]
- Lacerda, S.; Koening, M.; Neumann-Leitão, S.; Flores-Montes, M. Phytoplankton nyctemeral variation at a tropical river estuary (Itamaracá-Pernambuco-Brazil). Braz. J. Biol. 2004, 64, 81–94. [Google Scholar] [CrossRef]
- Schlegel, I.; Scheffler, W. Seasonal development and morphological variability of Cyclotella ocellata (Bacillariophyceae) in the eutrophic Lake Dagow (Germany). Int. Rev. Hydrobiol. 1999, 84, 469–478. [Google Scholar]
- Felício-Fernandes, G.; de Souza-Mosimann, R.M. Diatomáceas no sedimento do manguezal de Itacorubi-Florianópolis, Santa Catarina, Brasil. Insul. Rev. Botânica 1994, 23, 149–215. [Google Scholar]
- Lam, N.N.; Hai, D.; Ho, T. Species composition of diatoms (Bacillariophyceae) in Van Phong-Ben Goi Bay, Centrol Viet Nam. Collect. Mar. Res. Work. 1999, 9, 179–195. [Google Scholar]
- Lewis, F.W. Original communications: Notes on new and rarer species of diatomaceæ of the United States sea board. J. Cell Sci. 1862, 2, 155–161. [Google Scholar] [CrossRef]
- Licea, S.; Moreno Ruiz, J.; Luna, R. Checklist of diatoms (Bacillariophyceae) from the Southern Gulf of Mexico: Data-Base (1979–2010) and new records. J. Biodivers. Endanger. Species 2016, 4, 1–7. [Google Scholar]
- Liu, R.; Liu, J.Y. Checklist of Biota of Chinese Seas; Institute of Oceanology, Chinese Academy of Sciences: Beijing, China, 2008; pp. 1–1267. [Google Scholar]
- Natilj, L.; Damsiri, Z.; Chaouti, A.; Loudiki, M.; Khalil, K.; Elkalay, K. Spatio-temporal patterns of the microphytoplankton community structure and distribution in a North African lagoon. J. Mater. Environ. Sci. 2016, 7, 4419–4434. [Google Scholar]
- McGee, D.; Laws, R.A.; Cahoon, L.B. Live benthic diatoms from the upper continental slope: Extending the limits of marine primary production. Mar. Ecol. Prog. Ser. 2008, 356, 103–112. [Google Scholar] [CrossRef]
- Gómez, F.; Wang, L.; Hernández-Becerril, D.U.; Lisunova, Y.O.; Lopes, R.M.; Lin, S. Molecular phylogeny suggests transfer of Hemidiscus into Actinocyclus (Coscinodiscales, Coscinodiscophyceae). Diatom Res. 2017, 32, 21–28. [Google Scholar] [CrossRef]
- Akar, B.; Şahin, B. Diversity and ecology of benthic diatoms in Karagöl lake in Karagöl-Sahara National Park (Şavşat, Artvin, Turkey). Turk. J. Fish. Aquat. Sci. 2017, 17, 15–24. [Google Scholar] [CrossRef]
- Park, J.; Khim, J.S.; Ohtsuka, T.; Araki, H.; Witkowski, A.; Koh, C.H. Diatom assemblages on Nanaura mudflat, Ariake Sea, Japan: With reference to the biogeography of marine benthic diatoms in Northeast Asia. Bot. Stud. 2012, 53, 105–124. [Google Scholar]
- Saros, J.; Anderson, N. The ecology of the planktonic diatom Cyclotella and its implications for global environmental change studies. Biol. Rev. 2015, 90, 522–541. [Google Scholar] [CrossRef]
- Gómez, F.; Wang, L.; Lin, S. Molecular phylogeny suggests the affinity of the planktonic diatoms Climacodium and Bellerochea (Lithodesmiales, Mediophyceae). Diatom Res. 2018, 33, 349–354. [Google Scholar] [CrossRef]
- Battegazzore, M.; Gallo, L.; Lucadamo, L.; Morisi, A. Quality of the main watercourses in the Pollino National Park (Apennine Mts, S Italy) on the basis of the diatom benthic communities. Studi Trent. Sci. Nat. Acta Biol. 2003, 80, 89–93. [Google Scholar]
- Li, Y.; Suzuki, H.; Nagumo, T.; Tanaka, J. Morphology and Ultrastructure of Fallacia hodgeana (Bacillariophyceae). J. JAP. Bot. 2014, 89, 27–34. [Google Scholar]
- Lewis, R.J.; Jensen, S.I.; DeNicola, D.M.; Miller, V.I.; Hoagland, K.D.; Ernst, S.G. Genetic variation in the diatomFragilaria capucina (Fragilariaceae) along a latitudinal gradient across North America. Plant Syst. Evol. 1997, 204, 99–108. [Google Scholar] [CrossRef]
- Dickman, M.D.; Peart, M.R.; Wai-Shu Yim, W. Benthic diatoms as indicators of stream sediment concentrationin Hong Kong. Int. Rev. Hydrobiol. 2005, 90, 412–421. [Google Scholar] [CrossRef]
- Round, F.E. The ecology of benthic algae. In Algae and Man; Springer: Boston, MA, USA, 1964; pp. 138–184. [Google Scholar]
- Underwood, G.; Phillips, J.; Saunders, K. Distribution of estuarine benthic diatom species along salinity and nutrient gradients. Eur. J. Phycol. 1998, 33, 173–183. [Google Scholar] [CrossRef]
- Reid, G.; Williams, D.M. Systematics of the Gyrosigma balticum complex (Bacillariophyta), including three new species. Physiol. Res. 2003, 51, 126–142. [Google Scholar] [CrossRef]
- Stepanek, J.G.; Kociolek, J.P. Several new species of Amphora and Halamphora from the western USA. Diatom Res. 2013, 28, 61–76. [Google Scholar] [CrossRef]
- Rowland, S.; Allard, W.; Belt, S.; Massé, G.; Robert, J.M.; Blackburn, S.; Frampton, D.; Revill, A.; Volkman, J. Factors influencing the distributions of polyunsaturated terpenoids in the diatom, Rhizosolenia setigera. Phytochemistry 2001, 58, 717–728. [Google Scholar] [CrossRef]
- Dayala, V.; Salas, P.; Sujatha, C. Spatial and seasonal variations of phytoplankton species and their relationship to physicochemical variables in the Cochin estuarine waters, Southwest coast of India. Indian J. Mar. Sci. 2014, 43, 943–953. [Google Scholar]
- de Vijver, B.V.; Jarlman, A.; Lange-Bertalot, H. Four new Navicula (Bacillariophyta) species from Swedish rivers. Cryptogam. Algol. 2010, 31, 355–367. [Google Scholar]
- Smith, W.; West, T. A Synopsis of the British Diatomaceæ: With Remarks on Their Structure, Functions and Distribution; and Instructions for Collecting and Preserving Specimens; Smith and Beck, Pub.: London, UK, 1853; Volume 1, p. 1853. [Google Scholar]
- McQuoid, M.R.; Hobson, L.A. Assessment of palaeoenvironmental conditions on southern Vancouver Island, British Columbia, Canada, using the marine tychoplankter Paralia sulcata. Diatom Res. 1998, 13, 311–321. [Google Scholar] [CrossRef]
- Kawamura, T.; Hirano, R. Seasonal changes in benthic diatom communities colonizing glass slides in Aburatsubo Bay, Japan. Diatom Res. 1992, 7, 227–239. [Google Scholar] [CrossRef]
- Teanpisut, K.; Patarajinda, S. Species diversity of marine planktonic diatoms around Chang Islands, Trat Province. Kasetsart J. 2007, 41, 114–124. [Google Scholar]
- Al-Handal, A.Y.; Thomas, E.W.; Pennesi, C. Marine benthic diatoms in the newly discovered coral reefs, off Basra coast, Southern Iraq. Phytotaxa 2018, 372, 111–152. [Google Scholar] [CrossRef]
- Hagerthey, S.E.; Defew, E.C.; Paterson, D.M. Influence of Corophium volutator and Hydrobia ulvae on intertidal benthic diatom assemblages under different nutrient and temperature regimes. Mar. Ecol. Prog. Ser. 2002, 245, 47–59. [Google Scholar] [CrossRef]
- Siqueiros Beltrones, D.A.; Martínez, Y.J. Prospective floristics of epiphytic diatoms on Rhodophyta from the Southern Gulf of Mexico. CICIMAR Ocean. 2017, 32, 35–49. [Google Scholar] [CrossRef]
- Chen, Y.C. Immobilization of twelve benthic diatom species for long-term storage and as feed for post-larval abalone Haliotis diversicolor. Aquaculture 2007, 263, 97–106. [Google Scholar] [CrossRef]
- Hillebrand, H.; Sommer, U. Nitrogenous nutrition of the potentially toxic diatom Pseudonitzschia pungens f. multiseries Hasle. J. Plankton Res. 1996, 18, 295–301. [Google Scholar] [CrossRef]
- Viličić, D.; Marasović, I.; Mioković, D.J.A.B.C. Checklist of phytoplankton in the eastern Adriatic Sea. Acta Bot. Croat. 2002, 61, 57–91. [Google Scholar]
- Szczepocka, E.; Rakowska, B. Diatoms in the biological assessment of the ecological state of waters using the Czarna Staszowska River as an example. Oceanol. Hydrobiol. St. 2015, 44, 254. [Google Scholar] [CrossRef]
- Villac, M.C.; Kaczmarska, I.; Ehrman, J.M. Diatoms from ship ballast sediments (with consideration of a few additional species of special interest). In Diatom Monographs; Koeltz Botanical Books: Glashütten, Germany, 2016; Volume 18, p. 557. [Google Scholar]
- Foged, N. Diatoms in Alaska. Bibl. Phycol. 1981, 53, 1–318. [Google Scholar]
- Burns, D. Distribution of planktonic diatoms in Pelorus Sound, South Island, New Zealand. N. Z. J. Mar. Freshwater Res. 1977, 11, 275–295. [Google Scholar] [CrossRef]
- Hasle, G.R. The marine, planktonic diatom family Thalassionemataceae: Morphology, taxonomy and distribution. Diatom Res. 2001, 16, 1–82. [Google Scholar] [CrossRef]
- Hasle, G.R. The biogeography of some marine planktonic diatoms. Deep Sea Res. Oceanogr. Abstr. 1976, 23, 319-IN6. [Google Scholar] [CrossRef]
- Barron, J.A. Planktonic marine diatom record of the past 18 my: Appearances and extinctions in the Pacific and Southern Oceans. Diatom Res. 2003, 18, 203–224. [Google Scholar] [CrossRef]
- Palmisano, A.C.; SooHoo, J.B.; White, D.C.; Smith, G.A.; Stanton, G.R.; Burckle, L.H. Shade Adapted Benthic Diatoms Beneath Antarctic Sea Ice. J. Phycol. 1985, 21, 664–667. [Google Scholar] [CrossRef]
- Fernandes, L.; de Souza-Mosimann, R. Triceratium moreirae sp. nov. and Triceratium dubium (Triceratiaceae-Bacillariophyta) from estuarine environments of Southern Brazil, with comments on the genus Triceratium CG Ehrenberg. Rev. Bras. Biol. 2001, 61, 159–170. [Google Scholar] [CrossRef][Green Version]
- Suphan, S.; Peerapornpisal, Y. Fifty three new record species of benthic diatoms from Mekong River and its tributaries in Thailand. Chiang. Mai. J. Sci. 2010, 37, 326–343. [Google Scholar]
- Álvarez-Blanco, I.; Blanco, S. Benthic Diatoms from Mediterranean Coasts; Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 2014; Volume 60, pp. 3–4. [Google Scholar]
- Petrov, A.; Nevrova, E. Database on Black Sea benthic diatoms (Bacillariophyta): Its use for a comparative study of diversity pecularities under technogenic pollution impacts. Ocean. Biodivers. Inform. 2007, 202, 153–165. [Google Scholar]
- Prasad, A.; Nienow, J.; Livingston, R. The marine diatom genus Tryblioptychus Hendey (Thalassiosiraceae, Coscinodiscophyceae): Fine structure, taxonomy, systematics and distribution. Diatom Res. 2002, 17, 291–308. [Google Scholar] [CrossRef]
- Lewis, F.W. Notes on New and Rarer Species of Diatomaceae of the United States Seaboard; Merrihew, Thompson: Philadelphia, PA, USA, 1861. [Google Scholar]
- Simonsen, R. The diatom plankton of the Indian Ocean Expedition of R/V “Meteor” 1964–1965. GebruÌ der Borntraeger 1974, 19, 1–107. [Google Scholar]
- Boyer, C.S. Diatomaceae of Philadelphia and Vicinity; Press of J. B. Lippincott Company: Philadelphia, PA, USA, 1916. [Google Scholar]
- Al-Yamani, F.Y.; Saburova, M.A. Marine phytoplankton of Kuwait’s waters volume 2 Diatoms. Kuwait Inst. Sci. Res. 2019, 351, 1–336. [Google Scholar]
- Patrick, R.M.; Freese, L.R. Diatoms (Bacillariophyceae) from Northern Alaska. Proc. Acad. Nat. Sci. USA 1961, 112, 129–293. [Google Scholar]
- Li, Y.; Suzuki, H.; Nagumo, T.; Tanaka, J.; Sun, Z.; Xu, K. Three new species of Fallacia from intertidal sediments in Japan. Diatom Res. 2019, 34, 75–83. [Google Scholar] [CrossRef]
- Witkowski, A.; Lange-Bertalot, H.; Metzeltin, D. Diatom flora of marine coasts. In Iconographia Diatomologica: Annotated Diatom Micrographs; Lange-Bertalot, H., Ed.; Gantner Verlag: Oxford, UK, 2000; Volume 7. [Google Scholar]
- Meister, Berichte der Schweizerischen Botanischen Gesellschaft. Und Neue Kieselalgen; Seltene, Kommissionsverlag von Rascher & Co.: Zurich, Switzerland, 1935; Volume 44, pp. 87–108. [Google Scholar]
- Hagelstein, R. The Diatomaceae of Porto Rico and the Virgin Islands. Sci. Surv. Porto Rico. Virgin. Isl. 1939, 8, 313–450. [Google Scholar]
- Navarro, J. A survey of the marine diatoms of Puerto Rico VI. suborder Raphidineae: Family Naviculaceae (Genera Haslea, Mastogloia and Navicula). Bot. Mar. 1983, 26, 119–136. [Google Scholar] [CrossRef]
- Ohtsuka, T. Epipelic diatoms blooming in Isahaya Tidal Flat in the Ariake Sea, Japan, before the drainage following the Isahaya-Bay Reclamation Project. Phycol. Res. 2005, 53, 138–148. [Google Scholar] [CrossRef]
- Cox, E.J. Studies on the diatom genus Navicula Bory. VII. The identity and typification of Navicula gregaria DonKin, N. Cryptocephala Kutz. and related taxa. Diatom Res. 1995, 10, 91–111. [Google Scholar] [CrossRef]
- Kützing, F.T. Species Alagrum; F. A. Brockhaus: Lipsiae, Germany, 1849. [Google Scholar]
- Rabenhorst, L. Flora Europaea Algarum Aquae Dulcis et Submarinae; Apud E. Kummerum: Lipsiae, Germany, 1864; Volume 1, pp. 1864–1868. [Google Scholar]
- Meister, F. Zur Pflanzengeographie der Schweizerischen Bacillariacee. In Botanische Jahrbücher für Syster Matik, Pflanzengeschichte und Pflanzengeographie; Switzerland, 1919; Volume 55, pp. 125–159. Available online: https://www.biodiversitylibrary.org/item/136876#page/137/mode/1up (accessed on 28 May 2021).
- Round, F.E.; Crawford, R.; Mann, D. The Diatoms: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990; pp. 1–747. [Google Scholar]
- Grunow, A. Über Neue Oder Ungenügend Bekannte Algen, Erste Folge, Diatomeen, Familie Naviculaceen; Verhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft in Wein: Austria, 2021; Volume 10, pp. 503–582. Available online: https://www.zobodat.at/pdf/VZBG_10_0503-0582.pdf (accessed on 27 May 2021).
- Blanco, S.; Wetzel, C.E. Replacement names for botanical taxa involving algal genera. Phytotaxa 2016, 266, 195–205. [Google Scholar] [CrossRef]
- Stoermer, E.F.; Russell, G.; Kreis, J.; Andresen, N.A. Checklist of Diatoms from the Laurentian Great Lakes. II. J. Great Lakes Res. 1999, 25, 515–566. [Google Scholar] [CrossRef]
- Hendey, N.I. A preliminary check-list of British marine diatoms. J. Mar. Biol. Ass. UK 1954, 33, 537–560. [Google Scholar] [CrossRef]
- Hendey, N.I. A revised check-list of British marine diatoms. J. Mar. Biol. Ass. UK 1974, 54, 277–300. [Google Scholar] [CrossRef]
- Méléder, V.; Rincé, Y.; Barillé, L.; Gaudin, P.; Rosa, P. Spatiotemporal changes in microphytobenthos assemblages in a macrotidal flat (Bourgneuf Bay, France) 1. J. Phycol. 2007, 43, 1177–1190. [Google Scholar] [CrossRef]
- Cleve, P.T. Diatoms from the West. Indian Archipelago; Norstedt: Stockholm, Sweden, 1878; Volume 5. [Google Scholar]
- Deby, J. Le genre Surirella. Travail posthume, traduit, mis en ordre et publié par M. le docteur Henri Van Heurck. In Annales de la Société Belge de Microscopie; A. Manceaux, Libraire-editeur: Belgium, 1897; Volume 9, pp. 147–177. [Google Scholar]
- Schmidt, A.; Fricke, F. Atlas der Diatomaceen-Kunde; CH Kain: O. R. Reisland, Germany, 1875; Volume 1. [Google Scholar]
- López-Fuerte, F.O.; Siqueiros-Beltrones, D.A. A checklist of marine benthic diatoms (Bacillariophyta) from Mexico. Phytotaxa 2016, 283, 201–258. [Google Scholar] [CrossRef]
- Gopinathan, C. On new distributional records of plankton diatoms from the Indian Seas. J. Mar. Biol. Ass. India 1975, 17, 223–240. [Google Scholar]
- Watanabe, T.; Mayama, S.; Idei, M. Overlooked Heteropolarity in Surella cf. fastuosa (Bacillariophyta) and Relationships between Valve Morphogenesis and Auxospore Development. J. Phycol. 2012, 48, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Al-Handal, A.Y.; Compere, P.; Riaux-Gobin, C. Marine benthic diatoms in the coral reefs of Reunion and Rodrigues Islands, West Indian Ocean. Micronesica 2016, 2016, 1–77. [Google Scholar]
- Goldman, N.; Paddock, T.B.B.; Shaw, K.M. Quantitative Analysis of Shape Variation in Populations of Surirella fastuosa. Diatom Res. 1990, 5, 25–42. [Google Scholar] [CrossRef]
- Spaulding, S.; Edlund, M. Surirella. In Diatoms of North America. Available online: https://diatoms.org/genera/surirella (accessed on 2 March 2021).
- Greville, R.K. Transactions of The Microscopical Society. In Descriptions of New and Rare Diatoms. Series XIV; Wiley: Hoboken, NJ, USA, 1865; Volume 13, pp. 1–10. [Google Scholar]
- Hasle, G.R.; Syvertsen, E.E. Marine diatoms. In Identifying Marine Diatoms and Dinoflagellates; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Hasle, G. Thalassionema synedriforme comb. nov. and Thalassiothrix spathulata sp. nov., two marine, planktonic diatoms from warm waters. Phycologia 1999, 38, 54–59. [Google Scholar] [CrossRef]
- Sugie, K.; Suzuki, K. A new marine araphid diatom, Thalassionema kuroshioensis sp. nov., from temperate Japanese coastal waters. Diatom Res. 2015, 30, 237–245. [Google Scholar] [CrossRef]
- Sar, E.A.; Sunesen, I.; Fernández, P.V. Marine diatoms from Buenos Aires coastal waters (Argentina). II. Thalassionemataceae and Rhaphoneidaceae. Rev. Chil. Hist. Nat. 2007, 80, 63–79. [Google Scholar] [CrossRef][Green Version]
- Frenguelli, J. XVI Contribución al conocimiento de las diatomeas argentinas. In Diatomeas del Río de La Plata; Uniccersida Nacional de la Plata/ Instituto de Meseo: La Plata, Argentina, 1941; Volume 3, pp. 213–334. [Google Scholar]
- Kaleli, M.A.; Kociolek, J.P.; Solak, C.N. Taxonomy and distribution of diatoms on the Turkish Mediterranean Coast, Dalyan (Muğla). Mediterr. Mar. Sci. 2020, 21, 201–215. [Google Scholar] [CrossRef]
- Sutherland, D.L. Surface-associated diatoms from marine habitats at Cape Evans, Antarctica, including the first record of living Eunotogramma marginopunctatum. Polar Bio 2008, 31, 879–888. [Google Scholar] [CrossRef]
- Rivkin, R.B.; Putt, M. Photosynthesis and cell division by Antarctic microalgae: Comparison of benthic, planktonic and ice algae. J. Phycol. 1987, 23, 223–229. [Google Scholar] [CrossRef]
- Lee, Y.G.; Kim, S.; Jeong, D.U.; Kim, J.K.; Woo, H.J. Effects of Heavy Rainfall on Sedimentation in the Tidal Salt Marsh of Suncheon Bay, South Korea. J. Coast. Res. 2013, 29, 566–578. [Google Scholar] [CrossRef]
- Muylaert, K.; Sabbe, K.; Vyverman, W. Spatial and Temporal Dynamics of Phytoplankton Communities in a Freshwater Tidal Estuary (Schelde, Belgium). Estuar. Coast. Shelf Sci. 2000, 50, 673–682. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).