A Vital Staining Practice That Discerns Ancestry within Groups of Settling Larvae of a Brooding Coral
Abstract
1. Introduction

2. Materials and Methods
2.1. Experimental Design
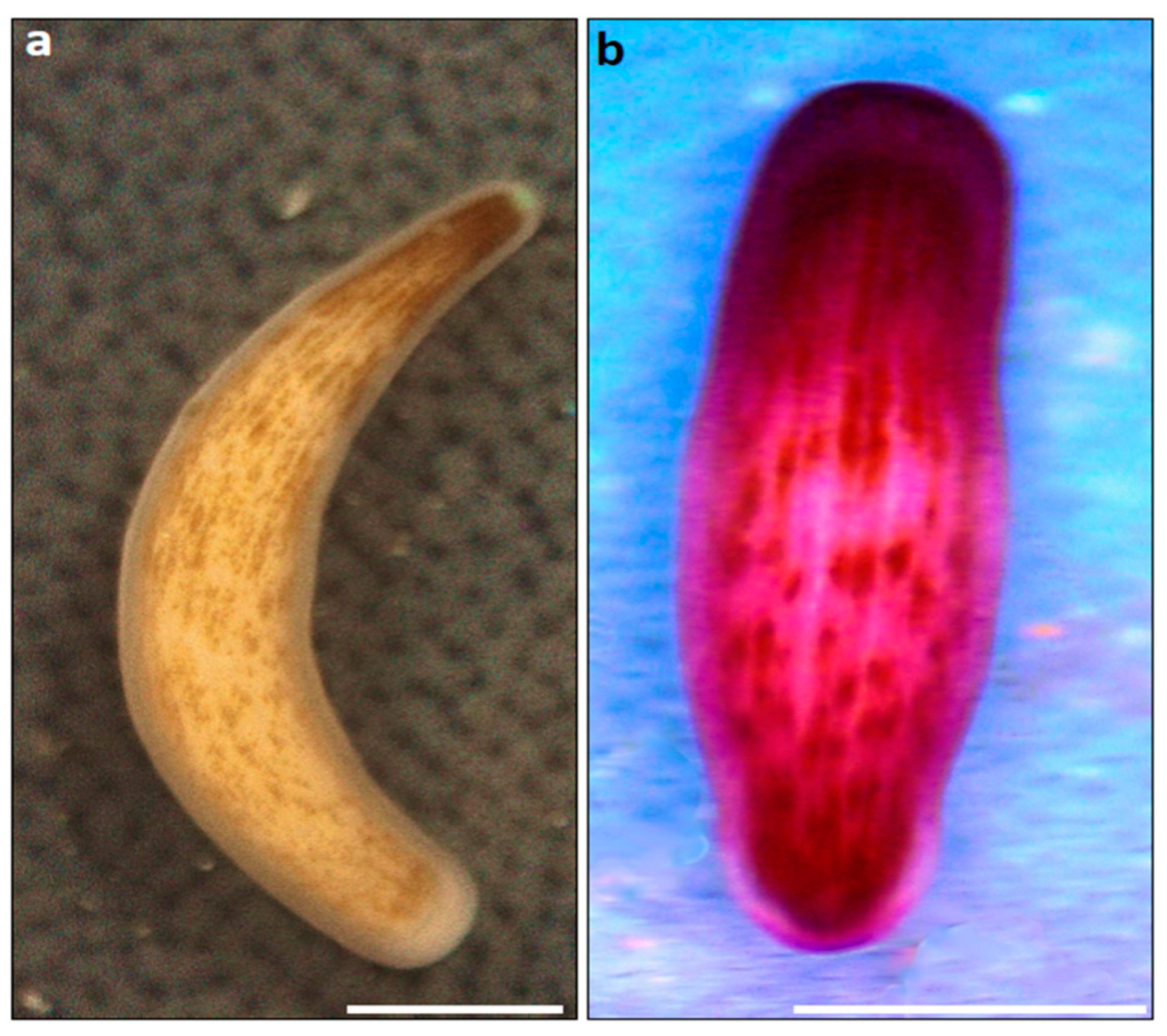
2.2. Staining Procedures
2.3. Statistical Analysis
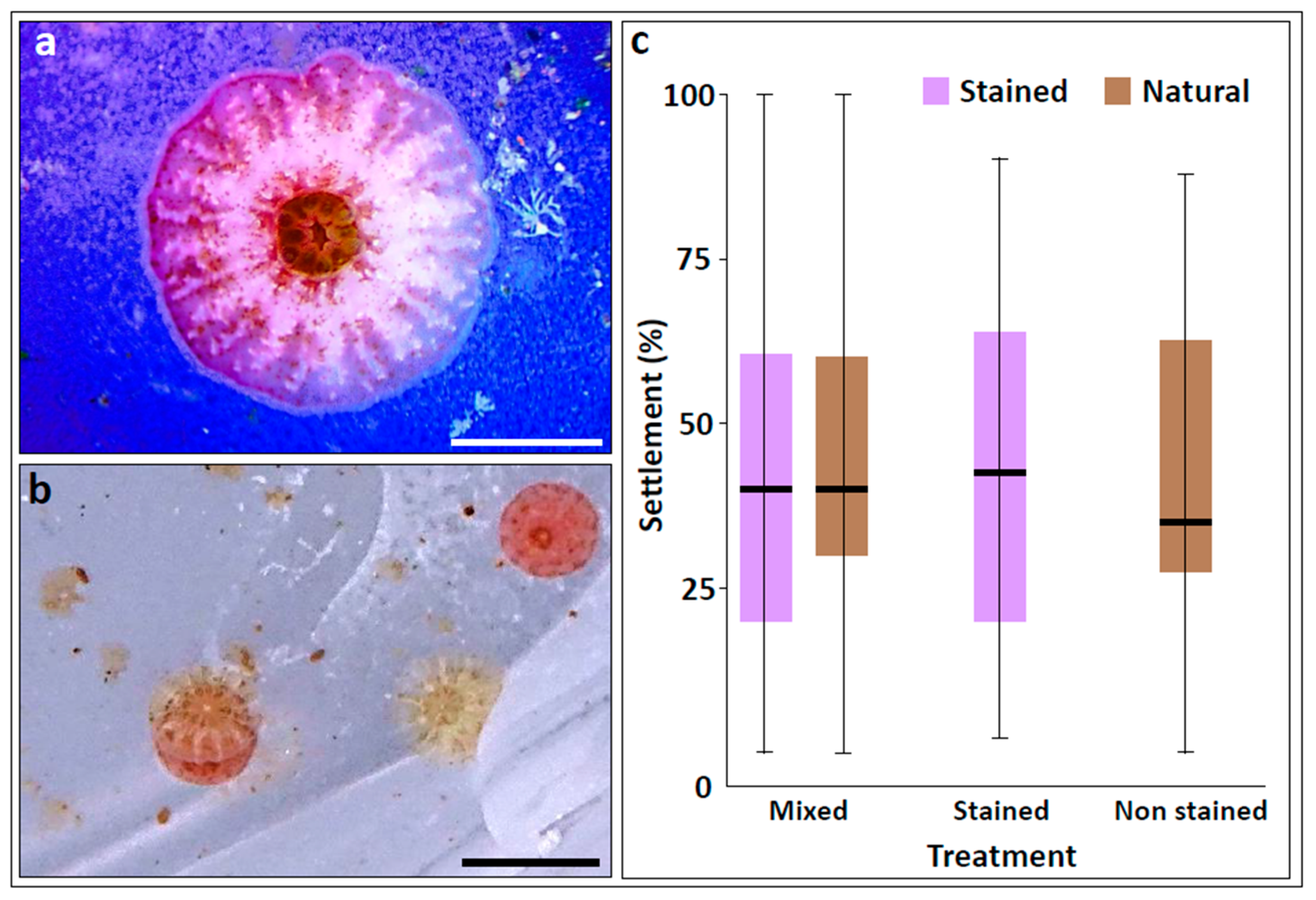
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ritson-Williams, R.; Arnold, S.N.; Fogarty, N.D.; Steneck, R.S.; Vermeij, M.J.A.; Paul, V.J. New perspectives on ecological mechanisms affecting coral recruitment on reefs. Smithson. Contrib. Mar. Sci. 2009, 437–457. [Google Scholar] [CrossRef]
- Tebben, J.; Motti, C.A.; Siboni, N.; Tapiolas, D.M.; Negri, A.P.; Schupp, P.J.; Kitamura, M.; Hatta, M.; Steinberg, P.D.; Harder, T. Chemical mediation of coral larval settlement by crustose coralline algae. Sci. Rep. 2015, 5, 10803. [Google Scholar] [CrossRef] [PubMed]
- Birrell, C.L.; McCook, L.J.; Willis, B.L. Effects of algal turfs and sediment on coral settlement. Mar. Pollut. Bull. 2005, 51, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Richmond, R.H. Reproduction and recruitment in corals: Critical links in the persistence of reefs. In Life Death Coral Reefs; Chapman Hall: New York, NY, USA, 1997; pp. 175–197. [Google Scholar]
- Arnold, S.N.; Steneck, R.S. Settling into an Increasingly Hostile World: The Rapidly Closing “Recruitment Window” for Corals. PLoS ONE 2011, 6, e28681. [Google Scholar] [CrossRef]
- Rinkevich, B. Neglected biological features in Cnidarians self—nonself recognition. In Self-NonSelf Recognition; López-Larrea, C., Ed.; Springer: New York, NY, USA, 2012; pp. 46–59. [Google Scholar]
- Duerden, J.E. Aggregated Colonies in Madreporarian Corals. Am. Nat. 1902, 36, 461–471. [Google Scholar] [CrossRef]
- Goreau, N.I.; Goreau, T.J.; Hayes, R.L. Settling, survivorship and spatial aggregation in planulae and juveniles of the coral Porites porites (Pallas). Bull. Mar. Sci. 1981, 31, 424–435. [Google Scholar]
- Neves, E.G.; Silveira, F.L. Release of planula larvae, settlement and development of Siderastrea stellata Verrill, 1868 (Anthozoa, Scleractinia). CEUR Workshop Proc. 2000, 1621, 36–43. [Google Scholar] [CrossRef]
- Barki, Y.; Gateño, D.; Graur, D.; Rinkevich, B. Soft-coral natural chimerism: A window in ontogeny allows the creation of entities comprised of incongruous parts. Mar. Ecol. Prog. Ser. 2002, 231, 91–99. [Google Scholar] [CrossRef]
- Puill-Stephan, E.; van Oppen, M.J.H.; Pichavant-Rafini, K.; Willis, B.L. High potential for formation and persistence of chimeras following aggregated larval settlement in the broadcast spawning coral, Acropora millepora. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2012, 279, 699–708. [Google Scholar] [CrossRef]
- Amar, K.O.; Chadwick, N.E.; Rinkevich, B. Coral kin aggregations exhibit mixed allogeneic reactions and enhanced fitness during early ontogeny. BMC Evol. Biol. 2008, 8, 126. [Google Scholar] [CrossRef]
- Doropoulos, C.; Evensen, N.R.; Gómez-Lemos, L.A.; Babcock, R.C. Density-dependent coral recruitment displays divergent responses during distinct early life-history stages. R. Soc. Open Sci. 2017, 4, 170082. [Google Scholar] [CrossRef]
- Cameron, K.A.; Harrison, P.L. Density of coral larvae can influence settlement, post-settlement colony abundance and coral cover in larval restoration. Sci. Rep. 2020, 10, 5488. [Google Scholar] [CrossRef]
- Rinkevich, B.; Shashar, N.; Liberman, T. Nontransitive xenogeneic interactions between four common Red Sea sessile invertebrates. Proc. Seventh Int. Coral Reef Symp. 1993, 2, 833–839. [Google Scholar]
- Rinkevich, B. Allorecognition and xenorecognition in reef corals: A decade of interactions. Hydrobiologia 2004, 530, 443–450. [Google Scholar] [CrossRef]
- Rinkevich, B.; Weissman, I.L. Chimeras in colonial invertebrates: A synergistic symbiosis or somatic-cell and germ-cell parasitism? Symbiosis 1987, 4, 117–134. [Google Scholar]
- Guerrini, G.; Shefy, D.; Shashar, N.; Shafir, S.; Rinkevich, B. Morphometric and allometric rules of polyp’s landscape in regular and chimeric coral colonies of the branching species Stylophora pistillata. Dev. Dyn. 2020, 1–17. [Google Scholar] [CrossRef]
- Shefy, D.; Shashar, N.; Rinkevich, B. Exploring traits of engineered coral entities to be employed in reef restoration. J. Mar. Sci. Eng. 2020, 8, 1038. [Google Scholar] [CrossRef]
- Rinkevich, B. Coral chimerism as an evolutionary rescue mechanism to mitigate global climate change impacts. Glob. Chang. Biol. 2019, 25, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.P.A.; Lee, D. First report of allogeneic fusion and allorecognition in tabulate corals. J. Paleontol. Soc. 1991, 65, 69–74. [Google Scholar] [CrossRef]
- Puill-Stephan, E.; Willis, B.L.; van Herwerden, L.; van Oppen, M.J.H. Chimerism in wild adult populations of the broadcast spawning coral Acropora millepora on the Great Barrier Reef. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Maier, E.; Buckenmaier, A.; Tollrian, R.; Nürnberger, B. Intracolonial genetic variation in the scleractinian coral Seriatopora hystrix. Coral Reefs 2012, 31, 505–517. [Google Scholar] [CrossRef]
- Schweinsberg, M.; Weiss, L.C.; Striewski, S.; Tollrian, R.; Lampert, K.P. More than one genotype: How common is intracolonial genetic variability in scleractinian corals? Mol. Ecol. 2015, 24, 2673–2685. [Google Scholar] [CrossRef] [PubMed]
- Rinkevich, B.; Shaish, L.; Douek, J.; Ben-Shlomo, R. Venturing in coral larval chimerism: A compact functional domain with fostered genotypic diversity. Sci. Rep. 2016, 6, 19493. [Google Scholar] [CrossRef]
- Giordano, B.; Bramanti, L. First report of chimerism in Mediterranean red coral (Corallium rubrum ). Mediterr. Mar. Sci. 2021, 22, 157–160. [Google Scholar] [CrossRef]
- Guérard, M.; Zeller, A.; Singer, T.; Gocke, E. In Vitro genotoxicity of neutral red after photo-activation and metabolic activation in the Ames test, the micronucleus test and the comet assay. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 746, 15–20. [Google Scholar] [CrossRef]
- LaManna, J.C.; Mccracken, K.A. The use of Neutral Red as an intracellular pH Indicator in rat brain cortex in vivo. Analyt 1984, 142, 117–125. [Google Scholar] [CrossRef]
- New, J.G. Dyes for studying the movements of small mammals. Am. Soc. Mammal. 1958, 39, 416–429. [Google Scholar] [CrossRef]
- Freeman, G. The role of polarity in the development of the hydrozoan planula larva. Roux’s Arch. Dev. Biol. 1981, 190, 168–184. [Google Scholar] [CrossRef]
- Zhou, Q. Chemical pollution and transport of organic dyes in water–soil–crop systems of the chinese coast. Bull. Environ. Contam. Toxicol. 2001, 66, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.A. A review of methods for labeling and tracking marine invertebrate larvae. Ophelia 1990, 32, 115–144. [Google Scholar] [CrossRef]
- Rinkevich, B.; Loya, Y. The reproduction of the Red Sea coral Stylophora pistillata. I. Gonads and planulae. Mar. Ecol. Prog. Ser. 1979, 1, 145–152. [Google Scholar] [CrossRef]
- Rinkevich, B.; Loya, Y. The reproduction of the Red Sea coral Stylophora pistillata. II. Synchronization in breeding and seasonality of planulae shedding. Mar. Ecol. Prog. Ser. 1979, 1, 133–144. [Google Scholar] [CrossRef]
- Shefy, D.; Shashar, N.; Rinkevich, B. The reproduction of the Red Sea coral stylophora pistillata from Eilat: 4-decade perspective. Mar. Biol. 2018, 165, 1–10. [Google Scholar] [CrossRef]
- Putnam, H.M.; Edmunds, P.J.; Fan, T.-Y. Effect of temperature on the settlement choice and photophysiology of larvae from the reef coral Stylophora pistillata. Biol. Bull. 2008, 215, 135–142. [Google Scholar] [CrossRef]
- Baird, A.H.; Morse, A.N.C. Induction of metamorphosis in larvae of the brooding corals Acropora palifera and Stylophora pistillata. Mar. Freshw. Res. 2004, 55, 469–472. [Google Scholar] [CrossRef]
- Tamir, R.; Eyal, G.; Cohen, I.; Loya, Y. Effects of light pollution on the early life stages of the most abundant northern Red Sea coral. Microorganisms 2020, 8, 193. [Google Scholar] [CrossRef]
- Chadwick-Furman, N.; Rinkevich, B. A complex allorecognition system in a reef-building coral: Delayed responses, reversals and nontransitive hierarchies. Coral Reefs 1994, 13, 57–63. [Google Scholar] [CrossRef]
- Baird, A.H. The Ecology of Coral Larvae: Settlement Patterns, Habitat Selection and the Length of the Larval Phase. Ph.D. Thesis, James Cook University of North Queensland, Queensland, Australia, 2001. [Google Scholar]
- R Core Team R: A Language and Environment for Statistical Computing. 2014. Available online: https://www.r-project.org/ (accessed on 29 May 2021).
- Hidaka, M.; Yurugi, K.; Sunagawa, S.; Kinzie, R.A. Contact reactions between young colonies of the coral Pocillopora damicornis. Coral Reefs 1997, 16, 13–20. [Google Scholar] [CrossRef]
- Nozawa, Y.; Loya, Y. Genetic relationship and maturity state of the allorecognition system affect contact reactions in juvenile Seriatopora corals. Mar. Ecol. Prog. Ser. 2005, 286, 115–123. [Google Scholar] [CrossRef]
- Amar, K.O.; Rinkevich, B. Mounting of erratic histoincompatible responses in hermatypic corals: A multi-year interval comparison. J. Exp. Biol. 2010, 213, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Wijayanti, D.P.; Hidaka, M. Is genetic involve in the outcomes of contact reactions between parent and offspring and between siblings of the coral Pocillopora damicornis? ILMU Kelaut. Indones. J. Mar. Sci. 2018, 23, 69. [Google Scholar] [CrossRef]
- Frank, U.; Oren, U.; Loya, Y.; Rinkevich, B. Alloimmune maturation in the coral Stylophora pistillata is achieved through three distinctive stages, 4 months post-metamorphosis. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1997, 264, 99–104. [Google Scholar] [CrossRef]
- Doropoulos, C.; Ward, S.; Roff, G.; González-Rivero, M.; Mumby, P.J. Linking demographic processes of juvenile corals to benthic recovery trajectories in two common reef habitats. PLoS ONE 2015, 10, e0128535. [Google Scholar] [CrossRef] [PubMed]
- Vermeij, M.J.A.; Sandin, S.A. Density-dependent settlement and mortality structure the earliest life phases of a coral population. Ecology 2008, 89, 1994–2004. [Google Scholar] [CrossRef] [PubMed]
- Rinkevich, B. Ecological engineering approaches in coral reef restoration. ICES J. Mar. Sci. 2020, 2100. [Google Scholar] [CrossRef]
- Rinkevich, B. The active reef restoration toolbox is a vehicle for coral resilience and adaptation in a changing world. J. Mar. Sci. Eng. 2019, 7, 201. [Google Scholar] [CrossRef]
- Linden, B.; Rinkevich, B. Elaborating an eco-engineering approach for stock enhanced sexually derived coral colonies. J. Exp. Mar. Biol. Ecol. 2017, 486, 314–321. [Google Scholar] [CrossRef]
- Cruz, D.W.D.; Harrison, P.L. Enhanced larval supply and recruitment can replenish reef corals on degraded reefs. Sci. Rep. 2017, 7, 13985. [Google Scholar] [CrossRef]
- Linden, B.; Vermeij, M.J.A.; Rinkevich, B. The coral settlement box: A simple device to produce coral stock from brooded coral larvae entirely in situ. Ecol. Eng. 2019, 132, 115–119. [Google Scholar] [CrossRef]
- Amar, K.O.; Rinkevich, B. A floating mid-water coral nursery as larval dispersion hub: Testing an idea. Mar. Biol. 2007, 151, 713–718. [Google Scholar] [CrossRef]
- Tanner, J.E. Interspecific competition reduces fitness in scleractinian corals. J. Exp. Mar. Biol. Ecol. 1997, 214, 19–34. [Google Scholar] [CrossRef]
- Rinkevich, B.; Loya, Y. Intraspecific competitive networks in the Red Sea coral Stylophora pistillata. Coral Reefs 1983, 1, 161–172. [Google Scholar] [CrossRef]
- Frank, U.; Brickner, I.; Rinkevich, B.; Loya, Y.; Bak, R.P.M.; Achituv, Y.; Ilan, M. Allogeneic and xenogeneic interactions in reef-building corals may induce tissue growth without calcification. Mar. Ecol. Prog. Ser. 1995, 124, 181–188. [Google Scholar] [CrossRef]
- Buss, L.W.; Jackson, J.B.C. Competitive networks: Nontransitive competitive relationships in cryptic coral reef environments. Am. Nat. 1979, 113, 223–234. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shefy, D.; Shashar, N.; Rinkevich, B. A Vital Staining Practice That Discerns Ancestry within Groups of Settling Larvae of a Brooding Coral. J. Mar. Sci. Eng. 2021, 9, 616. https://doi.org/10.3390/jmse9060616
Shefy D, Shashar N, Rinkevich B. A Vital Staining Practice That Discerns Ancestry within Groups of Settling Larvae of a Brooding Coral. Journal of Marine Science and Engineering. 2021; 9(6):616. https://doi.org/10.3390/jmse9060616
Chicago/Turabian StyleShefy, Dor, Nadav Shashar, and Baruch Rinkevich. 2021. "A Vital Staining Practice That Discerns Ancestry within Groups of Settling Larvae of a Brooding Coral" Journal of Marine Science and Engineering 9, no. 6: 616. https://doi.org/10.3390/jmse9060616
APA StyleShefy, D., Shashar, N., & Rinkevich, B. (2021). A Vital Staining Practice That Discerns Ancestry within Groups of Settling Larvae of a Brooding Coral. Journal of Marine Science and Engineering, 9(6), 616. https://doi.org/10.3390/jmse9060616





