Abstract
The vast majority of the world’s fishery by-products are utilized in the fish farming industry. However, due to the high cost and unsustainability of these by-products, alternative sources must be found. Marine diatoms produce important amounts of fatty acids, sterols, proteins and carbohydrates. In this work, we assessed the nutritional value of Halamphora coffeaeformis at the exponential growth phase, to determine its suitability for aquafeed. The strain was grown in a photobioreactor at 20 °C for 6 days. The production of proteins, lipids and carbohydrates was determined, and essential fatty acid, sterol and amino acid composition was assessed. The highest values of triacylglycerides (TAG), free and esterified sterols, proteins and carbohydrates were found after 6 days of growth in the photobioreactor. Fatty acid analysis by gas chromatography showed the presence of eicosapentaenoic (EPA) and arachidonic (ARA) in amounts similar to those for fish oils. In terms of sterols, a predominance of stigmasterol was observed, followed by cholesterol. The amino acid composition revealed 50% of them to be among those essential for fish and other aquatic animals. Finally, a suitable amount of carbohydrates was found in H. coffeaeformis cultures. Together, these findings support the use of H. coffeaeformis as an alternative and sustainable source for aquafeed to partially replace the use of fishery by-products.
1. Introduction
In recent years, microalgae have emerged as potential sources of food, pharmaceutical, nutraceutical and cosmetic products, as well as animal feed. It is known that some species of microalgae can be used to enhance the nutritional value of animal feed due to their ability to produce essential high-added-value metabolites [1].
In aquafarming, fishmeal represents the main source of proteins and essential long chain poly-unsaturated omega-3 fatty acids, such as eicosapentaenoic (EPA) and docosahexaenoic (DHA). However, there is increasing interest in reducing the dependence on this resource due to its unsustainability and variable cost and supply [2]. In this context, microalgae have emerged as appropriate candidates due to their accessibility, adaptability and nutrient composition [3]. For aquaculture purposes, the crucial factors are growth rate and essential nutrient composition, both of which can be improved through adjustment of environmental factors and culture strategies, to obtain a sufficient biomass of greater nutritional value [4].
Halamphora coffeaeformis is a marine benthic diatom isolated from Bahía Blanca Estuary, Argentina (Southwestern Atlantic coast). This strain was selected due to its apparent fast growth in culture, its adequate cell size as food for marine planktonic larvae [5] and because it belongs to the Bacillariophyceae class, mostly considered as adequate food for the larvae [6]. The strain was grown under optimal conditions in a photobioreactor in order to obtain basic information on its growth and biochemical composition, with a view to the biotechnological applications of the biomass for aquaculture. The results of this study are expected to provide valuable input on the potential of this species as feedstock for aquaculture.
2. Material and Methods
2.1. Experimental Design
The diatom Halamphora coffeaeformis, isolated from the Bahía Blanca Estuary [7], was maintained in f/2 medium with a salinity of 30, at 20 ± 1 °C and 40 μmol photons∙m−2∙s−1 under a 12:12 h light/dark photoperiod. For experiments, a starting inoculum of 6.82 × 105 cells·mL−1 in f/2 medium was used. The working volume in the cylindrical photobioreactor (Figmay) was 5 liters. Cells were kept continuously agitated (30 rpm) at 20 °C (± 2 °C) for 6 days, and air containing 5% (v/v) CO2 was supplied at 25 cm3∙min−1. Then, cells were centrifuged for 10 min at 800 × g at 20 °C and washed with phosphate saline buffer.
2.2. Growth rate and Biomass
Cells were counted daily in a Sedgwick-Rafter chamber, as described in [7]. The specific growth rate (µ) was estimated by a least square fit to a straight line of the logarithmically transformed data, according to [8]. Biomass dry weight (DW) was determined as in [9,10], in triplicate, using pre-conditioned and pre-weighed filters (Whatman GF/C, Maidstone, UK). The filters containing 20 mL of each sample were washed three times and then dried at 100 °C until constant weight. The samples were subsequently placed in a muffle/ashing furnace at 450 °C for 8 h, cooled in a vacuum desiccator and weighed to obtain ash-free dry weight (AFDW). The percentage of biomass and cell walls or frustules was estimated from the difference between DW and AFDW values.
2.3. Lipid Determinations
2.3.1. Quantification of Triacylglycerides (TAG) and Sterols
Lipid extracts, from samples taken every two days, were obtained by applying the method proposed by Folch et al. (1957) [11]. One-dimensional thin-layer chromatography (TLC) was then performed to separate TAG and sterols, as described in detail in [12]. These lipids were quantified spectrophotometrically at a wavelength of 505 nm using TG color GPO/PAP AA and Colestat Enzimático commercial kits from Wiener Lab, Rosario, Argentina; following the manufacturer’s instructions.
2.3.2. Fatty Acid Characterization
TAG extracts from samples taken at day 6 were used to analyze the fatty acid composition according to [12]. Fatty acids methyl esters (FAMEs) were obtained through transesterification using a potassium hydroxide solution. Then, they were analyzed in an HP 4890D gas chromatograph with a capillary SP2560 column (100 m, 0.25 mm, and 0.2 μm) (Supelco Inc., USA), a split/splitless injector and a flame-ionization detector, at 260 °C. Data were processed using the HP3398a GC Chemstation Software (Hewlett Packard, USA), and FAMEs were identified through comparison with the standard certificate material Supelco FAME 10 mix 37 (CRM47885, Sigma-Aldrich), as detailed in [7,12].
2.3.3. Sterol Characterization
Sterol extracts, from day 6, were silanized using SIGMA SIL-A reagent (Sigma-Aldrich, St. Louis, MI, USA). The separation of sterols was carried out in an Agilent 7820A gas chromatograph equipped with a 30 m SE 54 capillary column of 0.25 mm i.d. and 0.2 μm film thickness, after which the sterols were analyzed according to [12].
2.4. Protein Determinations
2.4.1. Total Protein Quantification
Proteins were determined spectrophotometrically through the Bradford method, using a standard of bovine serum albumin (BSA) [13]. For this, samples were taken every two days and lysed using the lysis buffer described in [12].
2.4.2. Amino Acid Characterization
The amino acid composition was determined from protein hydrolysates obtained according to [14,15]. Then, free amino acids were separated through cationic exchange chromatography and were identified by comparing retention times with those of amino acid commercial standards (18AA-Sigma® and individual standards for L-Methionisulfone, L-Norleucine, L-Methioninsulfoxide, L-Tryptophan y L-Ornithine). The resulting amino acids were re-derivatized and were detected spectrophotometrically at 570 and 440 nm and quantified using L-norleucine as internal standard [14,15]. Amino acid composition was determined after 6 days of growing H. coffeaeformis in the photobioreactor.
2.5. Determination of Total Carbohydrates
The phenol-sulfuric acid method was used to determine the total carbohydrate production of H. coffeaeformis in samples taken every two days [16]. Briefly, an aqueous phenolic solution (5% (w/v)) was added to the samples, together with concentrated sulfuric acid obtaining orange-yellow solutions that were quantified spectrophotometrically at 490 nm. A standard curve using glucose (0.1 μg∙μL−1) was also prepared.
2.6. Statistical Analysis
The results are expressed as average values ± standard deviation (SD). Statistical differences between groups were calculated by a two-tailed t test, using the t test function available in Microsoft Excel. p < 0.001 (***), p < 0.01 (**) and p < 0.05 (*) were considered very highly significant, highly significant and statistically significant, respectively.
3. Results
3.1. Growth and Biomass Production of H. coffeaeformis
Cell density and biomass production of Halamphora coffeaeformis cultured in a photobioreactor under favorable conditions are shown in Figure 1. As can be observed, both values increased exponentially with increasing days of culture and were significantly higher at day 6, when cell density and biomass reached values of 7.8 ± 1.05 × 105 cells·mL−1 and 0.18 ± 0.03 g∙L−1, respectively, with a µ of 0.92 div∙d−1. The rate of biomass generation, shown as g of dry weight (DW)∙L−1, was 22 ± 2.9 mg∙L−1∙day−1.
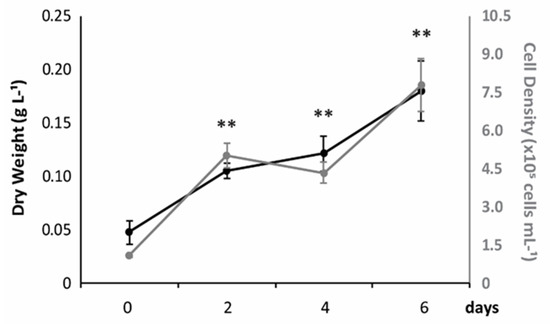
Figure 1.
Growth rate and biomass production of H. coffeaeformis. The concentration of cells (grey) and the rate of biomass generation (black) (average ± standard deviation, n = 3) are shown, expressed as cells∙mL−1 and g of dry weight∙L−1, respectively.
3.2. Triglyceride Production and Fatty Acid and Sterol Composition
In terms of biochemical composition, H. coffeaeformis biomass produced a small but detectable amount of TAG at day 0, which proportionally increased in culture, reaching the highest value at day 6 (~18 mg∙L−1) (Figure 2A). In addition, TAG content, considering total biomass (g TAG 100 g∙DW−1), presented the same trend. Analysis of the fatty acids (FA) at day 6 revealed that saturated (SFA) and monounsaturated (MUFA) fatty acids were the predominant lipid classes. A significant percentage of essential poly-unsaturated fatty acids (PUFA) was also produced by H. coffeaeformis, representing ~17.5% of the FA (Figure 2C). Of these PUFA, approximately 9% were omega-3 (ω-3), 5% were omega-6 (ω-6) and 3% were omega-9 (ω-9) (Figure 2D), giving a ω-3/ω-6 ratio of 1.64.

Figure 2.
Lipid profile of H. coffeaeformis. (A) Production of triacylglycerides (TAG) by the marine diatom cultured at 20 °C in the photobioreactor. (B) TAG content (g TAG 100 g∙DW−1) (C) Percentage composition of saturated (SFA), mono-unsaturated (MUFA) and poly-unsaturated (PUFA) fatty acids after 6 days of growing H. coffeaeformis in the photobioreactor. (D) Relative content of essential omega (ω) -3, -6 and -9 fatty acids in H. coffeaeformis after 6 days. The graphics show average values ± their standard deviation (n = 3). * stands for statistically significant (p < 0.05), ** highly significant (p < 0.01) or *** very highly significant differences (p < 0.001) between conditions, respectively.
Furthermore, the FA profile revealed that eicosapentaenoic (C20:5ω3), linoleic (C18:2ω6c) and oleic (C18:1ω9c) acids were the most abundant in the ω-3, ω-6 and ω-9 groups, respectively (Table 1).

Table 1.
Fatty acid profile of H. coffeaeformis. Percentage fatty acid composition of the neutral lipid fraction of H. coffeaeformis. The table shows average values ± standard deviation (n = 3).
As can be observed in Figure 3, the level of non-esterified sterols markedly increased in culture, reaching its highest value at day 6 (Figure 3A). However, when these results were expressed in terms of biomass (g Non-esterified sterols 100 g∙DW−1), a slight decrease was observed (Figure 3B). In the case of esterified sterols, slighter increases in their production (mg∙L−1) were observed after four and six days of culture in the photobioreactor. Their maximal values were also reached at day 6 (Figure 3C), but their contribution to total biomass decreased over time (Figure 3D). Nevertheless, the production of non-esterified sterols was quantitatively more significant than that of esterified sterols, representing ~5 and 3 mg∙L−1, respectively. Analysis of the sterol profile of H. coffeaeformis revealed only four species: stigmasterol, cholesterol, clerosterol and sitostanol (Figure 3E). Stigmasterol was quantitatively the most important sterol, representing more than 65% of the sterol fraction, the other three sterols being minor components (~12%, ~11% and ~9% respectively) (Figure 3E).

Figure 3.
Sterol production and composition of H. coffeaeformis. Non-esterified (A) and esterified (C) sterols (shown as mg of sterols L−1). Non-esterified (B) and esterified (D) sterols expressed in terms of biomass (g 100 g∙DW−1). E) Sterols detected in H. coffeaeformis at day 6 (expressed as % of total sterols). In the graphics, average values ± standard deviation are indicated (n = 3). * stands for statistically significant (p < 0.05), ** highly significant (p < 0.01) or *** very highly significant differences (p < 0.001) between conditions, respectively.
3.3. Protein Production and Amino Acid Composition
The amount of proteins significantly increased after 4 days of culture in the photobioreactor (Figure 4A). The highest protein production occurred at day 6, representing ~40 mg∙L−1. On the other hand, the amount of proteins in terms of biomass (g Protein 100 g∙DW−1) showed no significant changes between the values obtained at day 0 and day 6 (Figure 4B). Analysis of the amino acid composition showed total amino acid content to be 22/100 g biomass at day 6. Close to 50% of total amino acids (~11 g 100 g∙DW−1) were essential for fish and other aquatic animals and ~5% of them were conditionally essential (Figure 4C). With the exception of tryptophan, all amino acids considered as essential were present in H. coffeaeformis cultures, leucine and arginine being the most abundant (Table 2).

Figure 4.
Protein production and amino acid composition of H. coffeaeformis. (A) Protein production (mg of proteins∙L−1). (B) Proteins considering total biomass (g Protein 100 g∙DW−1) (C) Essential, conditionally essential and non-essential amino acids. The graphics show average values ± standard deviation (n = 3). * statistically significant (p < 0.05), *** very highly significant differences (p < 0.001) between conditions.

Table 2.
Amino acid profile of H. coffeaeformis. Amino acid composition (% of total amino acids) of H. coffeaeformis after 6 days of culture in the photobioreactor. The table shows average values ± standard deviation (n = 3). The amino acid classification shown was taken from [17], taking into account the nutritional needs of aquatic animals.
3.4. Carbohydrate Production
Finally, to complete the biochemical profile of H. coffeaeformis biomass, the carbohydrate content was determined. The results, expressed as mg of glucose per liter of culture (mg of glucose∙L−1) (Figure 5A) and as g per 100 g of biomass (g Glucose 100 g∙DW−1) (Figure 5B), showed that the amount of carbohydrates increased with the days in culture, reaching maximal values (~50 mg∙L−1 or ~28 g 100 g∙DW−1, respectively) after 6 days of culture in the photobioreactor.
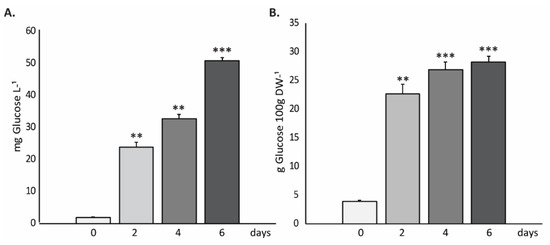
Figure 5.
Total carbohydrate content of H. coffeaeformis cells. The graphics show the carbohydrates (expressed in mg of glucose∙L−1 (A) and also in g Glucose 100 g∙DW−1 (B)) at day 0, and after 2, 4 and 6 days of growing in the photobioreactor. The graphics show an average of the obtained values ± standard deviation (n = 3). ** highly significant (p < 0.01); *** very highly significant differences (p < 0.001) between conditions, respectively.
A summary of the biochemical composition of H. coffeaeformis is shown in Figure 6. Cell walls or frustules represented a significant fraction of the biomass (~25 g 100 g∙DW−1). However, the organic constituents such as TAG, sterols, proteins and carbohydrates were the major components of biomass (~65 g 100 g∙DW−1), carbohydrates being the most abundant (28 g 100 g∙DW−1), followed by proteins (25 g 100 g∙DW−1) and TAG plus sterols (10.5 g 100 g∙DW−1). The remainders would include other components such as pigments, polar lipids and minerals, not analyzed in this study.
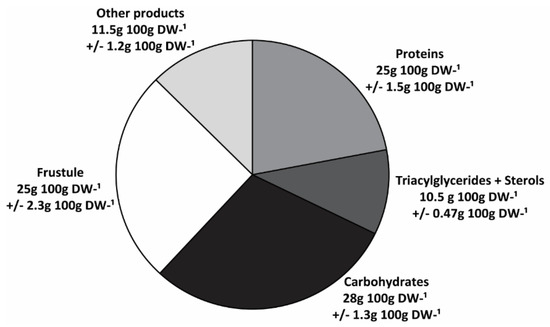
Figure 6.
Biochemical profile of H. coffeaeformis. The graphic summarizes the main components found in H. coffeaeformis, expressed as considering total biomass (g 100 g∙DW−1).
4. Discussion
In the last decade, interest in microalgae has increased considerably, due to their potential applications in sewage treatment, bioenergy, biopharmaceutics and food biotechnology [18]. A growing number of studies are being performed to establish whether microalgal-based feed can be used for aquaculture purposes [19]. The growth and biochemical composition of microalgae are two key qualities used to select appropriate species. The growth performance of H. coffeaeformis revealed a higher specific growth rate than the 25 species of benthic diatoms evaluated by Scholz and Liebezeit [20], including four species of the genus Amphora.
In general, neutral lipids are the main lipid fraction found in diatoms [7,21,22,23]. The production of TAG by H. coffeaeformis increased with the number of days in culture in the photobioreactor, and its value (in mg·L−1) was comparable to that reported for another diatom from Bahía Blanca Estuary grown under similar conditions [24]. Moreover, diatom biomass is valuable in terms of its fatty acid composition, as it can contain high levels of essential poly-unsaturated long chain fatty acids (PUFAs) such as eicosapentaenoic (EPA) and arachidonic (ARA) acids [25]. These PUFAs are highly valuable to the aquaculture industry and are currently obtained from fish oils [19]. Diatoms have therefore emerged as a promising alternative source to partially replace fish-oil-based aquaculture feed [26]. In this context, H. coffeaeformis showed an interesting essential long-chain PUFA content, ω-3 FAs being the major ones, followed by ω-6 and ω-9 FAs. The FA profile obtained under the tested culture conditions was similar to that previously reported for this strain [7]. PUFA represented about 17% of the total FA, EPA being the most abundant (9.4%). This finding is consistent with previous studies showing that EPA is quantitatively the major FA of the long-chain PUFA fraction of diatoms [19]. However, although the ω3/ω6 ratio obtained in H. coffeaeformis is acceptable, Ryckebosch et al. [27] reported a higher EPA content than that found in this study in two other diatoms: P. tricornutum and T. pseudonana. Taking into account essential FAs, the level of ARA and EPA found in H. coffeaeformis was even higher than that reported for the fish oils used for aquafeed [28] (Table 3). However, we did not detect DHA FA. As this FA is essential for the growth and survival of marine fish larvae [29], the use of an additional strain containing high levels of DHA to supplement fish diet could be a strategy to successfully replace the use of fish oil for aquaculture purposes.

Table 3.
Main lipid species relevant for aquafeeds. The percentages of lipids found in H. coffeaeformis are compared with those found in fish oils used for aquafeed according to [32].
It is known that diatoms can synthesize different classes of sterols [30]. In H. coffeaeformis cultures we found the main sterol species to be phytosterols, with cholesterol as a minor component. These findings are in agreement with a previous report on Amphora coffeaeformis showing a predominance of a sterol of 29 carbons, the stigmasterol [31]. Our findings are also in agreement with those reported in [32], which establishes that there are distinctive sterols for each diatom cluster, stigmasterol being the typical biomarker of Amphora, Amphiprora and Entomoneis species [32]. Unlike the sterol profile of Nitzchia closterium, consisting mainly of cholesterol [33], the latter was quantitatively the second most abundant sterol found in the studied strain. Although it is not an essential nutrient for fish, it is still an important component of their diet. In fact, it was demonstrated that its deficiency in fish diets stimulated the β-oxidation of FA [34]. Therefore, in terms of sterol composition, H. coffeaeformis shows a suitable profile for aquaculture purposes under the culture conditions tested.
For aquafeed purposes, proteins are an important source of nitrogen, providing essential and conditionally essential amino acids that cannot be synthesized or are only partially synthesized by fish [17,35]. Some of these amino acids are key metabolic regulators of fish growth, reproduction and resistance to environmental stressors [17]. Moreover, an appropriate amount of proteins may help to reduce the level of ammonia, a water pollutant released by fish [36], such that protein and amino acid requirements are important issues to be considered in connection with aquaculture feed. We observed an interesting amount of proteins in H. coffeaeformis, with amino acid composition comparable to that reported for other diatoms [14]. H coffeaeformis proteins provide all the amino acids that are considered essential for aquatic animals, representing 50% of total amino acids, except for tryptophan. In addition, two conditionally essential amino acids, cysteine and proline, were found in the studied strain. In terms of proteins and amino acids, it therefore appears that H. coffeaeformis-based formulated aquafeed could be a valuable non-fishmeal protein source, providing a similar composition to fishmeal [37], the otherwise ideal protein source for aquatic animals.
Carbohydrates are important energy sources for fish and must be provided with the diet in order to avoid the use of lipids and proteins to obtain energy [38]. In this connection, it has been reported that the growth rate of chinook salmon significantly improved with a diet containing 10% carbohydrates [39]. It is thus important to include an appropriate amount of these nutrients in the aquafeed diet. Total carbohydrates found in H. coffeaeformis, representing about 28 g 100 g∙DW−1, were higher than those found in other microalgal species, such as P. tricornutum and Chlorella sp. [40]. This value would appear to be adequate even taking into account the high quantities required for efficient use by fish. However, it should be taken into account that nutritional values can vary according to the species cultivated, due to the anatomical and functional variations occurring within different fish species [41].
Therefore, when H. coffeaeformis is grown in f/2 medium, a good growth performance is observed. In addition, the fact of carrying out experiments lasting six days allows the culture medium not to be depleted of nutrients, thus obtaining significant amounts of proteins and carbohydrates in the biomass. For aquaculture purposes, the culturing strategy requires a compromise between biomass optimization and harvesting to avoid leading cell metabolism towards exclusive lipid synthesis, because carbohydrates are required to avoid fish fat digestion, and proteins containing essential amino acids to obtain good levels of fish growth and reproduction [17,35,41]. In fact, the proximal chemical analysis of H. coffeaeformis revealed protein and lipid contents (g 100 g∙DW−1) similar to that reported for Navicula incerta [42]. There, lipid content was significantly higher during the stationary growth phase than during the exponential phase, whereas protein content was found to be similar in both growth phases. Different to N. incerta, H. coffeaeformis showed a marked increase in carbohydrate content, as was observed in the benthic diatom Proschkinia sp. [42]. On the other hand, in the diatoms Thalassiosira pseudonana [43] and Nitzschia inconspicua [44] both lipid and carbohydrate contents were higher in the stationary phase, while protein content was lower than that observed in the exponential growth phase. These differences in the biochemical composition could be due to variations in both the culture conditions and harvesting time.
In conclusion, the growth performance and nutrient composition of H. coffeaeformis under the tested conditions, shows this strain to have considerable potential for use as feed in aquaculture. These findings provide useful baseline information for the design of larger scale production systems.
Author Contributions
P.G.S.B., L.A.M., C.A.P. and P.I.L. participated in the conceptualization and design of experiments. L.A.M. performed the experiments in the photobioreactor. P.S.B. performed the carbohydrate, protein and lipid biochemical analyses. P.S.B. was involved in writing—original manuscript preparation. V.C. performed amino acid analyses. P.G.S.B., L.A.M., C.A.P., M.D.A. and P.I.L. made the interpretation of the data of the article and discussed their results. All authors made the critical revision of the article contributing with intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica to P.G.S.B. (PICT 2014-0893) and P.I.L. (PICT 2015-0800); Consejo Nacional de Investigaciones Científicas y Técnicas de la República Argentina (CONICET, PIP 112-2015 01-00510) and Universidad Nacional del Sur (PGI 24/B246) to P.I.L.
Acknowledgments
M.D.A. is a Fellow Member of CONICET. P.G.S.B., L.A.M. and P.I.L. are Research Members of CONICET. C.A.P. is a Research Member of Comisión de Investigaciones Científicas de la Provincia de Buenos Aires. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design, execution, interpretation, or writing of the present study.
References
- Borowitzka, M.A. High-value products from microalgae-their development and commercialization. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Senroy, S.; Pal, R. Microalgae in aquaculture: A review with special references to nutritional value and fish dietetics. Proc. Zool. Soc. Lond. 2015, 68, 1–8. [Google Scholar] [CrossRef]
- Sirakov, I.; Velichkova, K.; Stoyanova, S.; Staykov, Y. The importance of microalgae for aquaculture industry. Review. Int. J. Fish. Aquat. Stud. 2015, 2, 81–84. [Google Scholar]
- Muller-Feuga, A. The role of microalgae in aquaculture: Situation and trends. J. Appl. Phycol. 2000, 12, 527–534. [Google Scholar] [CrossRef]
- Brown, M.R.; Jeffrey, S.W.; Volkman, J.K.; Dunstan, G.A. Nutritional properties of microalgae for mariculture. Aquaculture. 1997, 151, 315–331. [Google Scholar] [CrossRef]
- D’Souza, F.M.L.; Lorenagan, N.R. Effects of monospecific and mixed-algae diets, on survival, development and fatty acid composition of penaeid prawn (Penaeus spp.) larvae. Mar. Biol. 1999, 133, 621–633. [Google Scholar] [CrossRef]
- Martín, L.A.; Popovich, C.A.; Martinez, A.M.; Damiani, M.C.; Leonardi, P.I. Oil assessment of Halamphora coffeaeformis diatom growing in a hybrid two-stage system for biodiesel production. Renew. Energy 2016, 92, 127–135. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Kilham, P.; Jackson, T.A. Kinetics of silicon-limited growth in the marina diatom Thalassiosira pseudonana. J. Phycol. 1973, 9, 233–237. [Google Scholar]
- Barnech Bielsa, G.; Popovich, C.; Rodríguez, M.C.; Martínez, A.; Martín, L.A.; Matulewicz, M.C.; Leonardi, P.I. Simultaneous production assessment of triacylglycerols for biodiesel and exopolysaccharides as valuable co-products in Navicula cincta. Algal Res. 2016, 15, 120–128. [Google Scholar] [CrossRef]
- Martín, L.A.; Popovich, C.A.; Martinez, A.M.; Scodelaro Bilbao, P.G.; Damiani, M.C.; Leonardi, P.I. Hybrid two-stage culture of Halamphora coffeaeformis for biodiesel production: Growth phases, nutritional stages and biorefinery approach. Renew. Energy 2018, 118, 984–992. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Scodelaro Bilbao, P.G.; Damiani, M.C.; Salvador, G.A.; Leonardi, P.I. Haematococcus pluvialis as a source of fatty acids and phytosterols: Potential nutritional and biological implications. J. Appl. Phycol. 2016, 28, 3283–3294. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Brown, M.R. The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 1991, 145, 79–99. [Google Scholar] [CrossRef]
- Simpson, R.J.; Neuberger, M.R.; Liu, T.Y. Complete amino acid analysis of proteins from a single hydrolysate. J. Biol. Chem. 1976, 251, 1936–1940. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 2009, 37, 43–53. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Chowdhury, K.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in aquafeeds for a sustainable aquaculture industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- Scholz, B.; Liebezeit, G. Growth responses of 25 benthic marine Wadden Sea diatoms isolated from the Solthörn tidal flat (southern North Sea) in relation to varying culture conditions. Diatom Res. 2012, 27, 65–73. [Google Scholar] [CrossRef]
- Yi, Z.; Xu, M.; Di, X.; Brynjolfsson, S.; Fu, W. Exploring Valuable Lipids in Diatoms. Front. Mar. Sci. 2017, 4, 17. [Google Scholar] [CrossRef]
- Yang, Y.J.; Du, L.; Hosokawa, M.; Miyashita, K.; Kokubun, Y.; Arai, H.; Taroda, H. Fatty acid and lipid class composition of the microalga Phaeodactylum tricornutum. J. Oleo Sci. 2017, 66, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Zulu, N.N.; Zienkiewicz, K.; Vollheyde, K.; Feussner, I. Current trends to comprehend lipid metabolism in diatoms. Progr. Lipid Res. 2018, 70, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Almeyda, M.D.; Scodelaro Bilbao, P.G.; Popovich, C.A.; Constenla, D.; Leonardi, P.I. Enhancement of polyunsaturated fatty acid production under low-temperature stress in Cylindrotheca closterium. J. Appl. Phycol. 2020. [Google Scholar] [CrossRef]
- Sayanova, O.; Mimouni, V.; Ulmann, L.; Morant-Manceau, A.; Pasquet, V.; Schoefs, B.; Napier, J.A. Modulation of lipid biosynthesis by stress in diatoms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160407. [Google Scholar] [CrossRef]
- Benemann, J.R. Microalgae aquaculture feeds. J. Appl. Phycol. 1992, 4, 233–245. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Bruneel, C.; Termote-Verhalle, R.; Goiris, K.; Muylaert, K.; Fouberta, I. Nutritional evaluation of microalgae oils rich in omega-3 long chain polyunsaturated fatty acids as an alternative for fish oil. Food Chem. 2014, 160, 393–400. [Google Scholar] [CrossRef] [PubMed]
- De Silva, S.; Francis, D.S.; Tacon, A.G.J. Fish oil in aquaculture: In retrospect. In Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds; Turchini, G.M., Ng, W.K., Tocher, D.R., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 1–20. [Google Scholar]
- Watanabe, T.; Tamiya, T.; Oka, A.; Hirata, M.; Kitajima, C.; Fujita, S. Improvement of dietary value of live foods for fish larvae by feeding them on ω-3 highly unsaturated fatty acids and fat-soluble vitamins. NIPPON SUISAN GAKKAISHI 1983, 49, 471–479. [Google Scholar] [CrossRef][Green Version]
- Volkman, J.K. Sterols in microalgae. In The Physiology of Microalgae. Developments in Applied Phycology; Borowitzka, M., Beardall, J., Raven, J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 6, pp. 485–505. [Google Scholar] [CrossRef]
- Gladu, P.K.; Patterson, G.W.; Wikfors, G.H.; Chitwood, D.J.; Lusby, W.R. Sterols of some diatoms. Phytochemistry 1991, 30, 2301–2303. [Google Scholar] [CrossRef]
- Rampen, S.W.; Abbas, B.A.; Schouten, S.; Sinninghe Damsté, J.S. A comprehensive study of sterols in marine diatoms (Bacillariophyta): Implications for their use as tracers for diatom productivity. Limnol. Oceanogr. 2010, 55, 91–105. [Google Scholar] [CrossRef]
- Barrett, S.M.; Volkman, J.K.; Dunstan, G.A.; LeRoi, J.-M. Sterols of 14 species of marine diatoms (Bacillariophyta). J. Phycol. 1995, 31, 360–369. [Google Scholar] [CrossRef]
- Norambuena, F.; Lewis, M.; Hamid, N.K.A.; Hermon, K.; Donald, J.A.; Turchini, G.M. Fish oil replacement in current aquaculture feed: Is cholesterol a hidden treasure for fish nutrition? PLoS ONE 2013, 8, e81705. [Google Scholar] [CrossRef] [PubMed]
- Cowey, C.B. Amino acid requirements of fish: A critical appraisal of present values. Aquaculture 1994, 124, 1–11. [Google Scholar] [CrossRef]
- Brunty, J.L.; Bucklin, R.A.; Davis, J.; Baird, C.D.; Nordstedt, R.A. The influence of feed protein intake on tilapia ammonia production. Aquacult. Eng. 1997, 16, 161–166. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018. [Google Scholar]
- Wilson, R.P. Utilization of dietary carbohydrate by fish. Aquaculture 1994, 124, 67–80. [Google Scholar] [CrossRef]
- Buhler, D.R.; Halver, J.E. Nutrition of salmonoid fishes. Carbohydrate requirements of chinook salmon. J. Nutr. 1961, 74, 307–318. [Google Scholar] [CrossRef]
- Tibbetts, S.; Milley, J.; Lall, S. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J. Appl. Phycol. 2014, 27, 1109–1119. [Google Scholar] [CrossRef]
- Krogdahl, A.; Hemre, G.-I.; Mommsen, T.P. Carbohydrates in fish nutrition: Digestion and absorption in postlarval stages. Aquac. Nutr. 2005, 11, 103–122. [Google Scholar] [CrossRef]
- Courtois de Viçose, G.; Porta, A.; Viera, M.P.; Fernández-Palacios, H.; Izquierdo, M.S. Effects of density on growth rates of four benthic diatoms and variations in biochemical composition associated with growth phase. J. Appl. Phycol. 2012, 24, 1427–1437. [Google Scholar] [CrossRef]
- Brown, M.R.; Dunstan, G.A.; Norwood, S.J.; Miller, K.A. Effects of harvest stage and light on the biochemical composition of the diatom Thalassiosira pseudonana. J. Phycol. 1996, 32, 64–73. [Google Scholar] [CrossRef]
- Chu, W.-L.; Phang, S.-M.; Goh, S.-H. Environmental effects on growth and biochemical composition of Nitzschia inconspicua Grunow. J. Appl. Phycol. 1996, 8, 389–396. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).