The Effect of Elevated CO2 and Increased Temperature on in Vitro Fertilization Success and Initial Embryonic Development of Single Male:Female Crosses of Broad-Cast Spawning Corals at Mid- and High-Latitude Locations
Abstract
:1. Introduction
2. Material and Methods
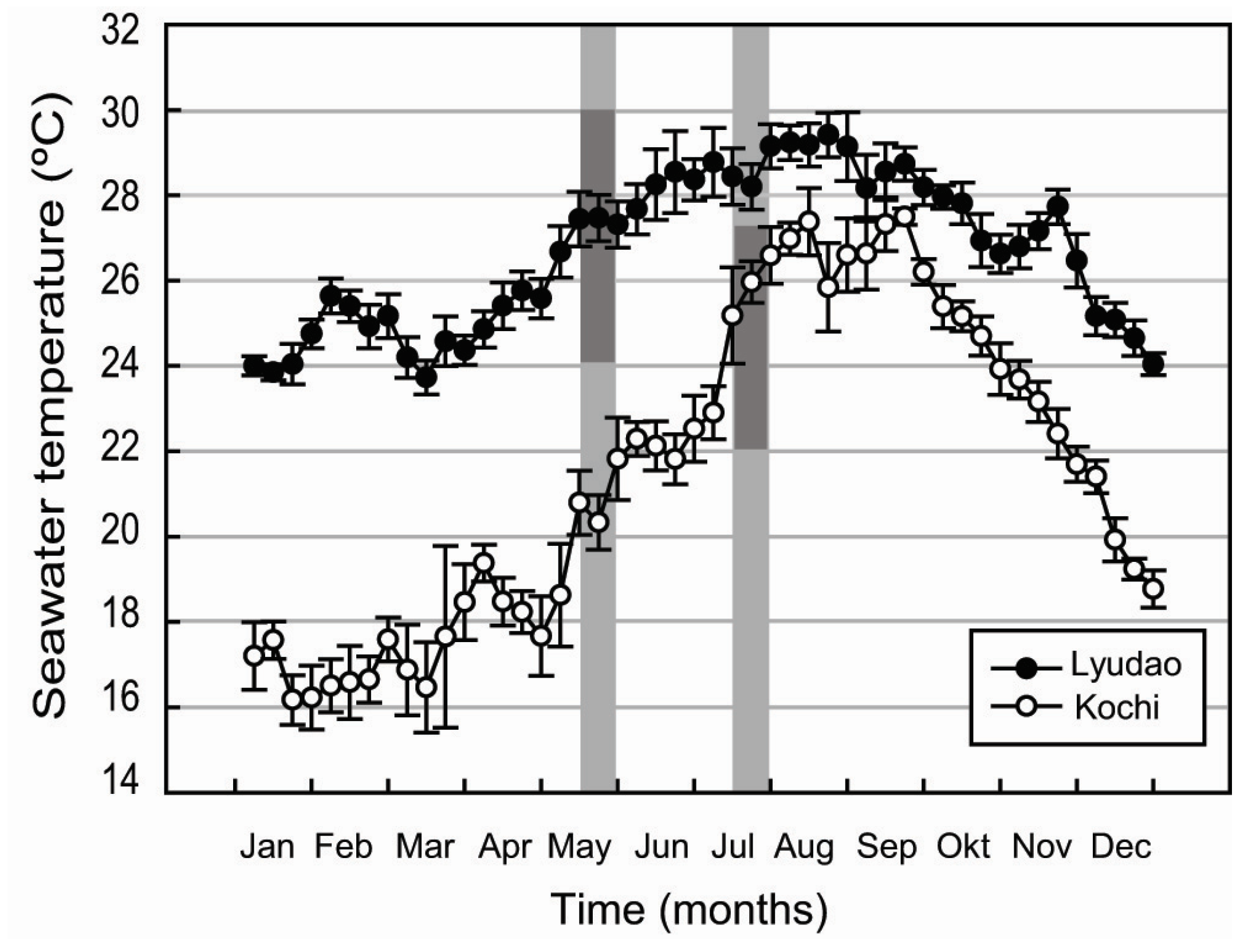
2.1. Study Locations
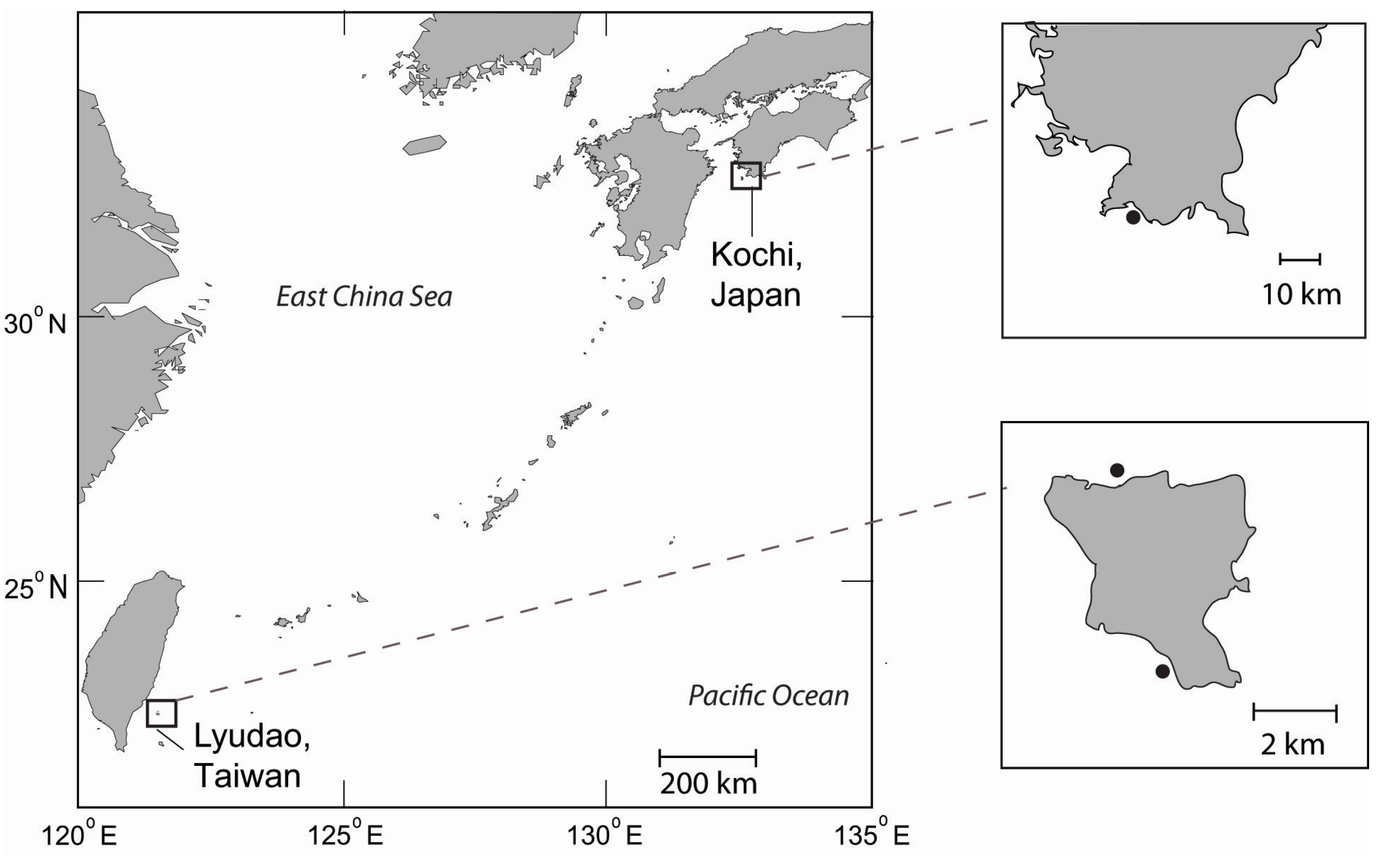
2.2. Gamete Collection
2.3. Experimental Treatments and Seawater Chemistry
2.4. Fertilization Assays
2.5. Analysis of Fertilization and Embryonic Development
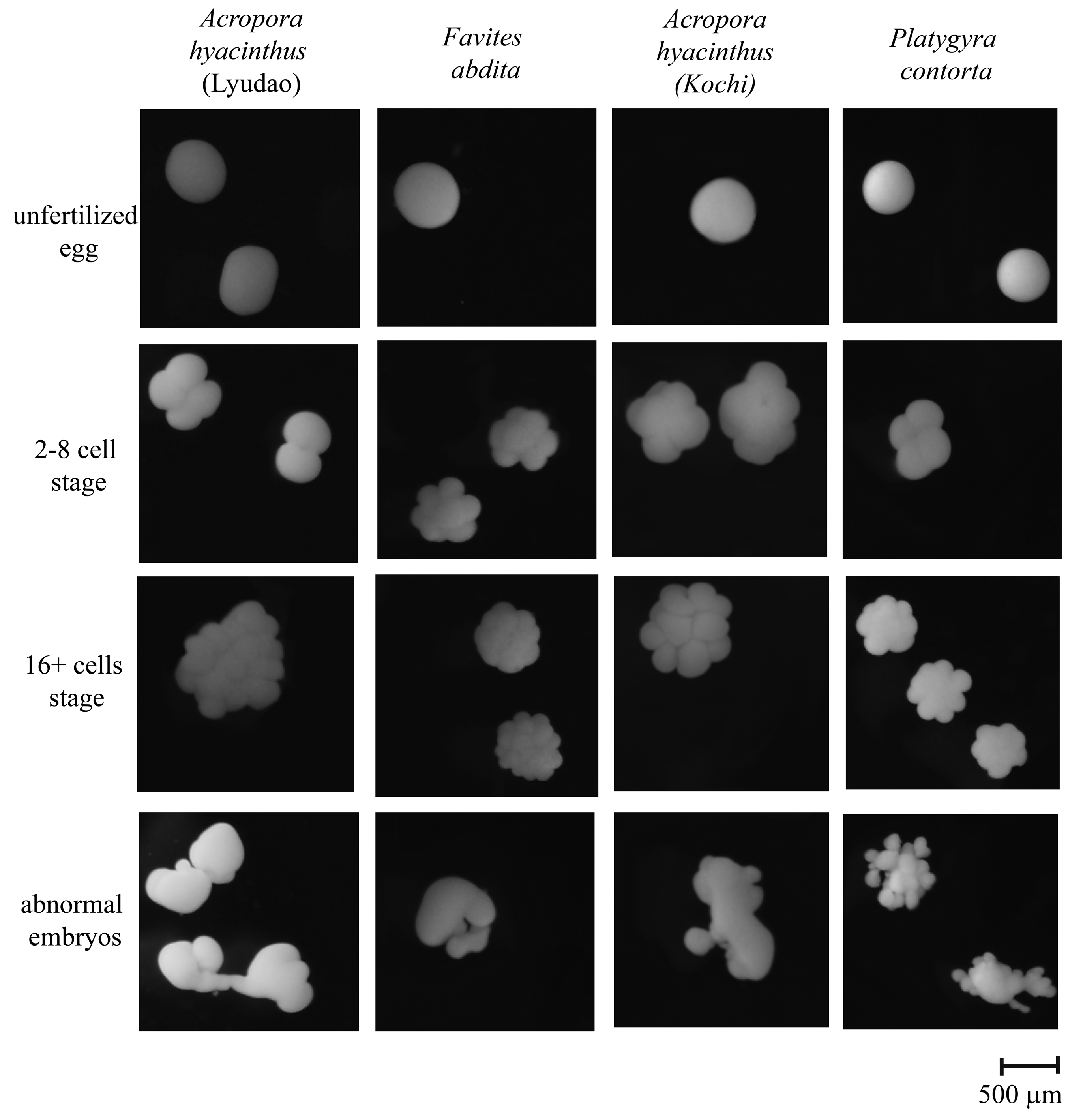
2.6. Data Analysis
3. Results
3.1. Seawater Chemistry
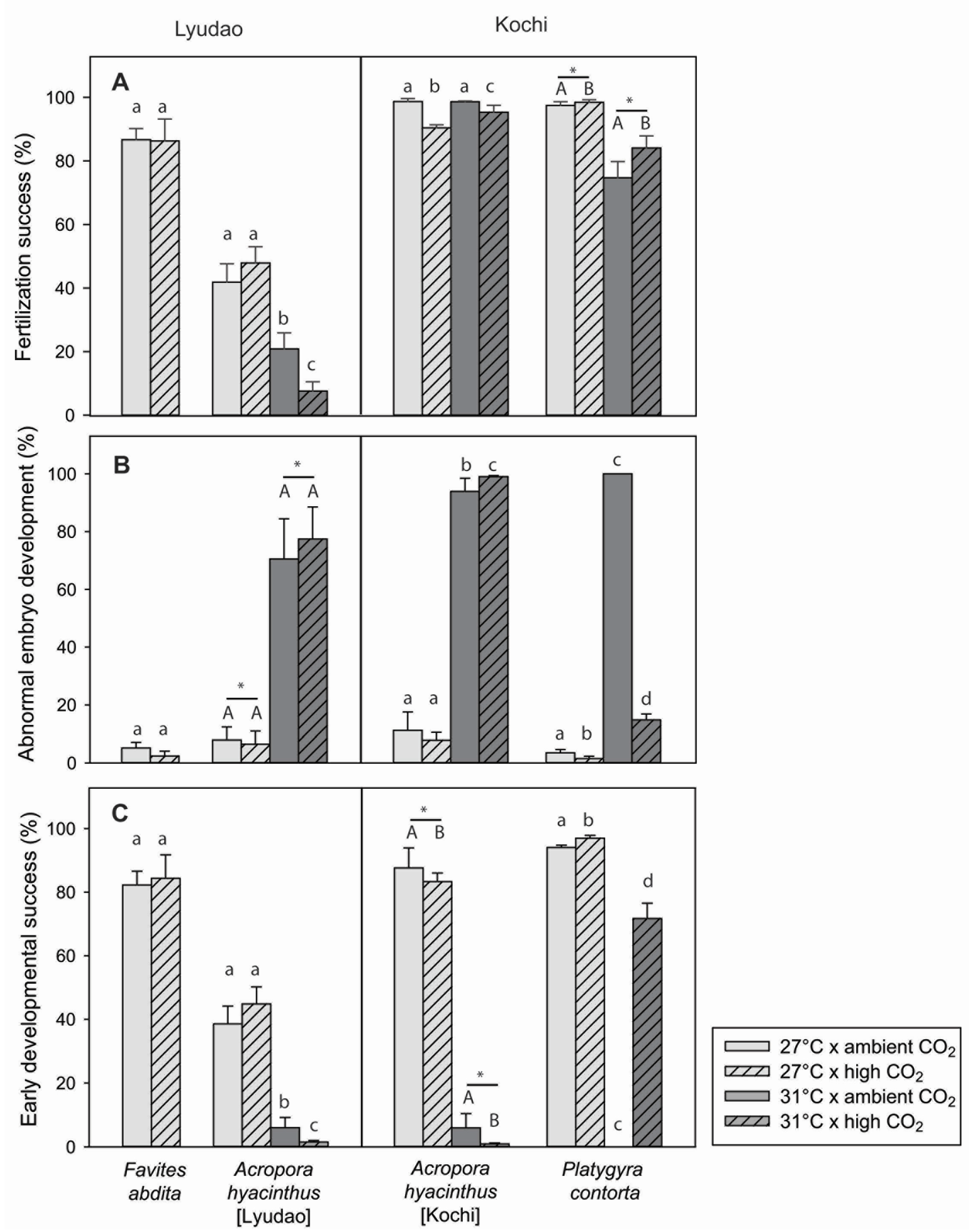
3.2. Ambient Fertilization Success
3.3. Fertilization Success in Response to Climate Change
3.3.1. Response to Climate Change for Each Coral Species
| Fertilization Success | Abnormal Development | Early Developmental Success | ||||
|---|---|---|---|---|---|---|
| p-value | p-value | p-value | ||||
| GLM Per Coral Species | ||||||
| A. hyacinthus (Kochi) | ||||||
| CO2 | 0.49 | NS | 2.10 × 10−3 | ** | 0.19 | NS |
| T | 5.60 × 10−10 | *** | 5.00 × 10−10 | *** | 1.30 × 10−12 | *** |
| CO2:T | 9.40 × 10−5 | *** | 0.25 | NS | 1.20 × 10−3 | ** |
| F. abdita | ||||||
| CO2 | 0.86 | NS | 0.05 | NS | 0.66 | NS |
| A. hyacinthus (Kochi) | ||||||
| CO2 | 4.50 × 10−8 | *** | 0.72 | NS | 0.24 | NS |
| T | 9.30 × 10−4 | *** | 3.30 × 10−15 | *** | 3.20 × 10−15 | *** |
| CO2:T | 0.06 | NS | 4.80 × 10−3 | ** | 0.03 | * |
| P. contorta | ||||||
| CO2 | 3.70 × 10−4 | *** | 2.20 × 10−16 | *** | 9.70 × 10−16 | *** |
| T | 5.60 × 10−11 | *** | 2.20 × 10−16 | *** | 2.20 × 10−16 | *** |
| CO2:T | 0.7 | NS | 1.10 × 10−10 | *** | 4.10 × 10−11 | *** |
| Effect of Temperature | Effect of CO2 | |||||||
|---|---|---|---|---|---|---|---|---|
| At Ambient CO2 | At High CO2 | At 27 °C | At 31 °C | |||||
| Fertilization Success | ||||||||
| A. hyacynthus (Lyudao) | <0.001 | - | <0.001 | - | 0.287 | NS | <0.001 | - |
| F.abdita | ||||||||
| A. hyacynthus (Kochi) | 0.998 | NS | <0.001 | + | <0.001 | - | <0.001 | - |
| P. contorta | ||||||||
| Abnormal Development | ||||||||
| A. hyacynthus (Lyudao) | ||||||||
| F.abdita | ||||||||
| A. hyacynthus (Kochi) | <0.001 | + | <0.001 | + | 0.5816 | NS | <0.05 | + |
| P. contorta | <0.001 | + | <0.001 | + | <0.001 | - | <0.001 | - |
| Early Developmental Success | ||||||||
| A. hyacynthus (Lyudao) | <0.001 | - | <0.001 | - | 0.16 | NS | <0.05 | - |
| F. abdita | ||||||||
| A. hyacynthus (Kochi) | <0.001 | + | <0.001 | + | 0.4791 | NS | <0.05 | + |
| P. contorta | <0.001 | + | <0.001 | + | <0.01 | - | <0.001 | - |
3.3.2. Comparison of A. hyacinthus from Lyudao and Kochi
| Fertilization Success | Abnormal Development | Early Developmental Success | ||||
|---|---|---|---|---|---|---|
| p-Value | p-Value | p-Value | ||||
| GLM comparing A. hyacinthus from Lyudao with Kochi | ||||||
| location | 2.2 × 10−16 | *** | 3.1 × 10−12 | *** | 2.6 × 10−12 | *** |
| T | 2.2 × 10−14 | *** | 2.2 × 10−16 | *** | 2.2 × 10−16 | *** |
| CO2 | 2.6 × 10−4 | *** | 0.30 | NS | 0.20 | NS |
| location:T | 9.8 × 10−11 | *** | 7.5 × 10−5 | *** | 2.1 × 10−7 | *** |
| location:CO2 | 1.5 × 10−6 | *** | 0.75 | NS | 0.01 | ** |
| T:CO2 | 7.8 × 10−6 | *** | 1.2 × 10−3 | ** | 1.6 × 10−4 | *** |
| location:T:CO2 | 1.2 × 10−3 | ** | 0.16 | NS | 0.89 | NS |
4. Discussion
4.1. Temperature Effect: Physiological Mechanisms
4.2. Effect of CO2 Level: Physiological Mechanisms
4.3. Synergistic Effect of Temperature and CO2 Level
4.4. Ecological Consequences
5. Conclusion
Supplementary Materials
Supplementary File 1Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Baird, A.H.; Guest, J.R.; Willis, B.L. Systematic and Biogeographical Patterns in the Reproductive Biology of Scleractinian Corals. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 551–571. [Google Scholar] [CrossRef]
- Gleason, D.F.; Hofmann, D.K. Coral larvae: From gametes to recruits. J. Exp. Mar. Bio. Ecol. 2011, 408, 42–57. [Google Scholar] [CrossRef]
- Harrison, P.L. Sexual Reproduction of Scleractinian Corals. In Coral Reefs: An Ecosystem in Transition; Dubinsky, Z., Stambler, N., Eds.; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Edmunds, P.J.; Gates, R.D.; Gleason, D.F. The biology of larvae from the reef coral Porites. astreoides, and their response to temperature disturbances. Mar. Biol. 2001, 139, 981–989. [Google Scholar] [CrossRef]
- Bassim, K.M.; Sammarco, P.W.; Snell, T.L. Effects of temperature on success of (self and non-self) fertilization and embryogenesis in Diploria strigosa (Cnidaria, Scleractinia). Mar. Biol. 2002, 140, 479–488. [Google Scholar] [CrossRef]
- Negri, A.P.; Marshall, P.A.; Heyward, A.J. Differing effects of thermal stress on coral fertilization and early embryogenesis in four Indo Pacific species. Coral Reefs 2007, 26, 759–763. [Google Scholar] [CrossRef]
- Randall, C.J.; Szmant, A.M. Elevated temperature affects development, survivorship, and settlement of the elkhorn coral, Acropora palmata (Lamarck 1816). Biol. Bull. 2009, 217, 269–282. [Google Scholar] [PubMed]
- Randall, C.J.; Szmant, A.M. Elevated temperature reduces survivorship and settlement of the larvae of the Caribbean scleractinian coral, Favia fragum (Esper). Coral Reefs 2009, 28, 537–545. [Google Scholar] [CrossRef]
- Fine, M.; Tchernov, D. Scleractinian coral species survive and recover from decalcification. Science 2007, 315, 1811. [Google Scholar] [CrossRef] [PubMed]
- Albright, R.; Mason, B.; Langdon, C. Effect of aragonite saturation state on settlement and post-settlement growth of Porites astreoides larvae. Coral Reefs 2008, 27, 485–490. [Google Scholar] [CrossRef]
- Jokiel, P.L.; Rodgers, K.S.; Kuffner, I.B.; Andersson, A.J.; Cox, E.F.; Mackenzie, F.T. Ocean acidification and calcifying reef organisms: A mesocosm investigation. Coral Reefs 2008, 27, 473–483. [Google Scholar] [CrossRef]
- Kurihara, H. Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar. Ecol. Prog. Ser. 2008, 373, 275–284. [Google Scholar] [CrossRef]
- Morita, M.; Suwa, R.; Iguchi, A.; Nakamura, M.; Shimada, K.; Sakai, K.; Suzuki, A. Ocean acidification reduces sperm flagellar motility in broadcast spawning reef invertebrates. Zygote 2010, 18, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Albright, R.; Mason, B.; Miller, M.; Langdon, C. Ocean acidification compromises recruitment success of the threatened Caribbean coral Acropora palmata. Proc. Natl. Acad. Sci. USA 2010, 107. [Google Scholar] [CrossRef] [PubMed]
- Suwa, R.; Nakamura, M.; Morita, M.; Shimada, K.; Iguchi, A.; Sakai, K.; Suzuki, A. Effects of acidified seawater on early life stages of scleractinian corals (Genus Acropora). Fish. Sci. 2009, 76, 93–99. [Google Scholar] [CrossRef]
- Albright, R. Effects of Ocean Acidification on Early Life History Stages of Caribbean scleractinian corals. Ph.D. Thesis, Rosenstiel School of Marine and Atmospheric Science, University of Miami, Rickenbacker Causeway, Miami, FL, USA, 7 April 2011. [Google Scholar]
- Albright, R.; Langdon, C. Ocean acidification impacts multiple early life history processes of the Caribbean coral Porites astreoides. Glob. Chang. Biol. 2011, 17, 2478–2487. [Google Scholar] [CrossRef]
- Anlauf, H.; D’Croz, L.; O’Dea, A.A. corrosive concoction: The combined effects of ocean warming and acidification on the early growth of a stony coral are multiplicative. J. Exp. Mar. Bio. Ecol. 2010, 397, 13–20. [Google Scholar] [CrossRef]
- Nakamura, M.; Ohki, S.; Suzuki, A.; Sakai, K. Coral Larvae under Ocean Acidification: Survival, Metabolism, and Metamorphosis. PLoS ONE 2011, 6, e14521. [Google Scholar] [PubMed]
- Nakamura, M.; Morita, M. Sperm motility of the scleractinian coral Acropora. digitifera under preindustrial, current, and predicted ocean acidification regimes. Aquat. Biol. 2012, 15, 299–302. [Google Scholar] [CrossRef]
- Albright, R.; Mason, B. Projected near-future levels of temperature and pCO2 reduce coral fertilization success. PLoS ONE 2013, 8, e56468. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.M.; Leggat, W.; Moya, A.; Baird, A.H. Temperature affects the early life history stages of corals more than near future ocean acidification. Mar. Ecol. Prog. Ser. 2013, 475, 85–92. [Google Scholar] [CrossRef]
- Iguchi, A.; Suzuki, A.; Sakai, K.; Nojiri, Y. Comparison of the effects of thermal stress and CO2-driven acidified seawater on fertilization in coral Acropora digitifera. Zygote 2014, 22. [Google Scholar] [CrossRef] [PubMed]
- Putnam, H.M.; Mayfield, A.B.; Fan, T.Y.; Chen, C.S.; Gates, R.D. The physiological and molecular responses of larvae from the reef-building coral Pocillopora damicornis exposed to near-future increases in temperature and pCO2. Mar. Biol. 2013, 160, 2157–2173. [Google Scholar] [CrossRef]
- Byrne, M.; Soars, N.A.; Ho, M.A.; Wong, E.; McElroy, D.; Selvakumaraswamy, P.; Dworjanyn, S.A.; Davis, A.R. Fertilization in a suite of coastal marine invertebrates from SE Australia is robust to near-future ocean warming and acidification. Mar. Biol. 2010, 157, 2061–2069. [Google Scholar] [CrossRef]
- Byrne, M.; Soars, N.; Selvakumaraswamy, P.; Dworjanyn, S.A.; Davis, A.R. Sea urchin fertilization in a warm, acidified and high pCO2 ocean across a range of sperm densities. Mar. Environ. Res. 2010, 69, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Moulin, L.; Catarino, A.I.; Claessens, T.; Dubois, P. Effects of seawater acidification on early development of the intertidal sea urchin Paracentrotus. lividus (Lamarck 1816). Mar. Pollut. Bull. 2011, 62, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Chown, S.L.; Gaston, K.J.; Robinson, D. Macrophysiology: Large-scale patterns in physiological. Funct. Ecol. 2004, 18, 159–167. [Google Scholar] [CrossRef]
- Addo-Bediako, A.; Chown, S.L.; Gaston, K.J. Thermal tolerance, climatic variability and latitude. Proc. Biol. Sci. 2000, 267, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Coles, S.L.; Jokiel, P.L.; Lewis, C.R. Thermal Tolerance in Tropical Vs Subtropical Pacific Reef Corals. Pac. Sci. 1976, 30, 159–166. [Google Scholar]
- Cook, C.B.; Logan, A.; Ward, J.; Luckhurst, B.E.; Berg, C.J., Jr. Elevated temperatures and bleaching on a high latitude coral reef: The 1998 Bermuda event. Coral Reefs 1990, 9, 45–49. [Google Scholar] [CrossRef]
- Ulstrup, K.; Berkelmans, R.; Ralph, P.; van Oppen, M. Variation in bleaching sensitivity of two coral species across a latitudinal gradient on the Great Barrier Reef: The role of zooxanthellae. Mar. Ecol. Prog. Ser. 2006, 314, 135–148. [Google Scholar] [CrossRef]
- Woolsey, E.S.; Keith, S.A.; Byrne, M.; Schmidt-Roach, S.; Baird, A.H. Latitudinal variation in thermal tolerance thresholds of early life stages of corals. Coral Reefs 2014, 33. [Google Scholar] [CrossRef]
- Meehl, G.A.; Stocker, T.F.; Collins, W.D.; Friedlingstein, P.; Gaye, A.T.; Gregory, J.M.; Kitoh, A.; Knutti, R.; Murphy, J.M.; Noda, A.; et al. Global Climate Projections. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, New York, NY, USA, 2007; pp. 747–845. [Google Scholar]
- Lin, C-H.; Nozawa, Y. Spawning timing of Scleractinian Corals in Lyudao, Taiwan. Unpublished work. 2015. [Google Scholar]
- Nozawa, Y. Annual variation in the timing of coral spawning in a high-latitude environment: Influence of temperature. Biol. Bull. 2012, 222, 192–202. [Google Scholar] [PubMed]
- Ladner, J.T.; Palumbi, S.R. Extensive sympatry, cryptic diversity and introgression throughout the geographic distribution of two coral species complexes. Mol. Ecol. 2012, 21, 2224–2238. [Google Scholar] [CrossRef] [PubMed]
- Mezaki, T.; Hayashi, T.; Iwase, F.; Nozawa, Y.; Miyamoto, M.; Tominaga, M. Spawning patterns of high latitude scleractinian corals from 2002 to 2006 at Nishidomari, Otsuki, Kochi, Japan. Kuroshio Biosph. 2007, 3, 33–47. [Google Scholar]
- Veron, J.E.N. Corals of the World; Australian Institute of Marine Science: Townsville, Australia, 2000. [Google Scholar]
- Evans, J.P.; Marshall, D.J. Male-by-female interactions influence fertilization success and mediate the benefits of polyandry in the sea urchin Heliocidaris erythrogramma. Evolution 2005, 59, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.; Havenhand, J.N.; Gillings, M.R.; Williamson, J.E. Individual Variability in Reproductive Success Determines Winners and Losers under Ocean Acidification: A Case Study with Sea Urchins. PLoS ONE 2012, 7, 1–8. [Google Scholar] [CrossRef]
- Lewis, E.; Wallace, D.; Allison, L.J. Program developed for CO2 system calculations. Tennessee: Carbon Dioxide Information Analysis Center; Martin, L., Ed.; Energy Research Corporation for the US Department of Energy: New York, NY, USA, 1998. [Google Scholar]
- Mehrbach, C.; Culberson, C.H.; Hawley, J.E.; Pytkowicz, R.M. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 1973, 18, 897–907. [Google Scholar] [CrossRef]
- Dickson, A.G.; Millero, F.J. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res. Part. A Oceanogr. Res. Pap. 1987, 34, 1733–1743. [Google Scholar] [CrossRef]
- Oliver, J.; Babcock, R. Aspects of the Fertilization Ecology of Broadcast Spawning Corals: Sperm Dilution Effects and in situ Measurements of Fertilization. Biol. Bull. 1992, 183, 409–417. [Google Scholar] [CrossRef]
- Ho, M.A.; Price, C.; King, C.K.; Virtue, P.; Byrne, M. Effects of ocean warming and acidification on fertilization in the Antarctic echinoid Sterechinus neumayeri across a range of sperm concentrations. Mar. Environ. Res. 2013, 90, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.M.; Ross, P.M.; O’Connor, W.A. Comparing the effect of elevated pCO2 and temperature on the fertilization and early development of two species of oysters. Mar. Biol. 2010, 157, 2435–2452. [Google Scholar] [CrossRef]
- Okubo, N.; Motokawa, T. Embryogenesis in the reef-building coral Acropora. spp. Zool. Sci. 2007, 24, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Okubo, N.; Mezaki, T.; Nozawa, Y.; Nakano, Y.; Lien, Y.-T.; Fukami, H.; Hayward, D.C.; Ball, E.E. Comparative embryology of eleven species of stony corals (Scleractinia). PLoS ONE 2013, 8, e84115. [Google Scholar]
- Keshavmurthy, S.; Fontana, S.; Mezaki, T.; González, L.D.C.; Chen, C.A. Doors are closing on early development in corals facing climate change. Sci. Rep. 2014, 4, 18–23. [Google Scholar] [CrossRef]
- Szmant-Froelich, A.; Yevich, P.; Pilson, M.E.Q. Gametogenesis and early development of the temperate coral Astrangia danae (Anthozoa: Scleractinia). Biol. Bull. 1980, 158, 257–269. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biometrical J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Iguchi, A.; Morita, M.; Nakajima, Y.; Nishikawa, A.; Miller, D. In vitro fertilization efficiency in coral Acropora digitifera. Zygote 2009, 17, 225–227. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.I.; Bruno, J.F.; Gaines, S.D.; Halpern, B.S.; Lester, S.E.; Kinlan, B.P.; Weiss, J.M. Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc. Natl. Acad. Sci. USA 2007, 104, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Heyward, A.J.; Negri, A.P. Plasticity of larval pre-competency in response to temperature: Observations on multiple broadcast spawning coral species. Coral Reefs 2010, 29, 631–636. [Google Scholar] [CrossRef]
- Oliver, T.A.; Palumbi, S.R. Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 2011, 30, 429–440. [Google Scholar] [CrossRef]
- Reuter, K.E.; Lotterhos, K.E.; Crim, R.N.; Thompson, C.A.; Harley, C.D.G. Elevated pCO2 increases sperm limitation and risk of polyspermy in the red sea urchin Strongylocentrotus franciscanus. Glob. Chang. Biol. 2011, 17, 163–171. [Google Scholar] [CrossRef]
- Kurihara, H.; Kato, S.; Ishimatsu, A. Effects of increased seawater pCO2 on early development of the oyster Crassostrea gigas. Aquat. Biol. 2007, 1, 91–98. [Google Scholar] [CrossRef]
- Desrosiers, R.R.; Désilets, J.; Dubé, F. Early developmental events following fertilization in the giant scallop Placopecten magellanicus. Can. J. Fish. Aquat. Sci. 1996, 53, 1382–1392. [Google Scholar] [CrossRef]
- Dupont, S.; Havenhand, J.; Thorndyke, W.; Peck, L.; Thorndyke, M. Near-future level of CO2-driven ocean acidification radically affects larval survival and development in the brittlestar Ophiothrix fragilis. Mar. Ecol. Prog. Ser. 2008, 373, 285–294. [Google Scholar] [CrossRef]
- Parker, L.M.; Ross, P.M.; O’Connor, W.A. The effect of ocean acidification and temperature on the fertilization and embryonic development of the Sydney rock oyster Saccostrea glomerata (Gould 1850). Glob. Chang. Biol. 2009, 15, 2123–2136. [Google Scholar] [CrossRef]
- Albright, R. Reviewing the Effects of Ocean Acidification on Sexual Reproduction and Early Life History Stages of Reef-Building Corals. J. Mar. Biol. 2011, 2011, 1–14. [Google Scholar] [CrossRef]
- Riebesell, U.; Fabry, V.J.; Hansson, L.; Gattuso, J.-P. (Eds.) Guide to Best Practices for Ocean Acidification Research and Data Reporting; Publications Office of the European Union: Luxembourg, Luxembourg, 2010; p. 258.
- Havenhand, J.; Buttler, F.; Thorndyke, M. Near-future levels of ocean acidification reduce fertilization success in a sea urchin. Curr. Biol. 2008, 18, 651–652. [Google Scholar] [CrossRef]
- Caldwell, G.; Fitzer, S.; Gillespie, C.; Pickavance, G.; Turnbull, E.; Bentley, M. Ocean acidification takes sperm back in time. Invertebr. Reprod. Dev. 2011, 55, 1–5. [Google Scholar] [CrossRef]
- Solzin, J.; Helbig, A.; Van, Q.; Brown, J.E.; Hildebrand, E.; Weyand, I.; Kaupp, U.B. Revisiting the role of H+ in chemotactic signaling of sperm. J. Gen. Physiol. 2004, 124, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Nishikawa, A.; Nakajima, A.; Iguchi, A.; Sakai, K.; Takemura, A.; Okuno, M. Eggs regulate sperm flagellar motility initiation, chemotaxis and inhibition in the coral Acropora digitifera, A. gemmifera and A. tenuis. J. Exp. Biol. 2006, 209, 4574–4579. [Google Scholar]
- Darszon, A.; Guerrero, A.; Galindo, B.E.; Nishigaki, T.; Wood, C.D. Sperm-activating peptides in the regulation of ion fluxes, signal transduction and motility. Int. J. Dev. Biol. 2008, 52, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Kühl, M.; Cohen, Y.; Dalgaard, T.; JØrgensen, B.B.; Revsbech, N.P. Microenvironment and photosynthesis of zooxanthellae in scleractinian corals studied pH and light with microsensors for O2, pH and light. Mar. Ecol. Prog. Ser. 1995, 117, 159–172. [Google Scholar] [CrossRef]
- Al-Horani, F.A.; Al-Moghrabi, S.M.; de Beer, D. Microsensor study of photosynthesis and calcification in the scleractinian coral, Galaxea fascicularis: Active internal carbon cycle. J. Exp. Mar. Bio. Ecol. 2003, 288, 1–15. [Google Scholar] [CrossRef]
- Al-Horani, F.A.; Ferdelman, T.; Al-Moghrabi, S.M.; De Beer, D. Spatial distribution of calcification and photosynthesis in the scleractinian coral Galaxea fascicularis. Coral Reefs 2005, 24, 173–180. [Google Scholar] [CrossRef]
- Baums, I.B. A restoration genetics guide for coral reef conservation. Mol. Ecol. 2008, 17, 2796–2811. [Google Scholar] [CrossRef] [PubMed]
- Baird, A.H.; Gilmour, J.P.; Kamiki, T.M.; Nonaka, M.; Pratchett, M.S.; Yamamoto, H.; Yamasaki, H. Temperature Tolerance of Symbiotic And Non-Symbiotic Coral Larvae. In Proceedings of the 10th International Coral Reef Symposium, Okinawa, Japan, 28 June–2 July 2006; pp. 38–42.
- Yakovleva, I.; Baird, A.H.; Yamamoto, H.H.; Bhagooli, R.; Nonaka, M.; Hidaka, M. Algal symbionts increase oxidative damage and death in coral larvae at high temperatures. Mar. Ecol. Prog. Ser. 2009, 378, 105–112. [Google Scholar] [CrossRef]
- Coles, S.L. The Effects of Elevated Temperature on Reef Coral Planula Settlement as Related to Power Station Entrainrnent. In Proceedings of the 5th International Coral Reef Symposium, Tahiti, 27 May–1 June 1985; pp. 171–176.
- Nozawa, Y.; Harrison, P.L. Larval settlement patterns, dispersal potential and the effect of temperature on settlement of larvae of the reef coral, Platygyra daedalea, from the Great Barrier Reef. In Proceedings of the 9th International Coral Reef Symposium, Bali Convention Center, Bali, Indonesia, 23–27 October 2000; pp. 409–415.
- Nozawa, Y.; Harrison, P.L. Effects of elevated temperature on larval settlement and post-settlement survival in scleractinian corals, Acropora solitaryensis and Favites chinensis. Mar. Biol. 2007, 152, 1181–1185. [Google Scholar] [CrossRef]
- Muehllehener, N.; Edmunds, P.J. Effects of ocean acidification and increased temperatures on skeletal growth of two scleractinian corals, Pocillopora meandrina and Porites rus. In Proceedings of the 11th International Coral Reef Symposium, Fort Lauderdale, FL, USA, 7–11 July 2008; pp. 57–61.
- Doropoulos, C.; Ward, S.; Diaz-Pulido, G.; Hoegh-Guldberg, O.; Mumby, P.J. Ocean acidification reduces coral recruitment by disrupting intimate larval-algal settlement interactions. Ecol. Lett. 2012, 15, 338–346. [Google Scholar] [CrossRef]
- Doropoulos, C.; Diaz-Pulido, G. High CO2 reduces the settlement of a spawning coral on three common species of crustose coralline algae. Mar. Ecol. Prog. Ser. 2013, 475, 93–99. [Google Scholar] [CrossRef]
- Dufault, A.M.; Cumbo, V.R.; Fan, T.-Y.; Edmunds, P.J. Effects of diurnally oscillating pCO2 on the calcification and survival of coral recruits. Proc. R. Soc. B Biol. Sci. 2012, 279, 2951–2958. [Google Scholar] [CrossRef]
- Putnam, H.M. Resilience and Acclimatization Potential of Reef Corals under Predicted Climate Change Stressors. Ph.D. Thesis, University of Hawai’I, Manoa, HI, USA, December 2012. [Google Scholar]
- Fan, T.; Dai, C. Reproductive plasticity in the reef coral Echinopora lamellosa. Mar. Ecol. Prog. Ser. 1999, 190, 297–301. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schutter, M.; Nozawa, Y.; Kurihara, H. The Effect of Elevated CO2 and Increased Temperature on in Vitro Fertilization Success and Initial Embryonic Development of Single Male:Female Crosses of Broad-Cast Spawning Corals at Mid- and High-Latitude Locations. J. Mar. Sci. Eng. 2015, 3, 216-239. https://doi.org/10.3390/jmse3020216
Schutter M, Nozawa Y, Kurihara H. The Effect of Elevated CO2 and Increased Temperature on in Vitro Fertilization Success and Initial Embryonic Development of Single Male:Female Crosses of Broad-Cast Spawning Corals at Mid- and High-Latitude Locations. Journal of Marine Science and Engineering. 2015; 3(2):216-239. https://doi.org/10.3390/jmse3020216
Chicago/Turabian StyleSchutter, Miriam, Yoko Nozawa, and Haruko Kurihara. 2015. "The Effect of Elevated CO2 and Increased Temperature on in Vitro Fertilization Success and Initial Embryonic Development of Single Male:Female Crosses of Broad-Cast Spawning Corals at Mid- and High-Latitude Locations" Journal of Marine Science and Engineering 3, no. 2: 216-239. https://doi.org/10.3390/jmse3020216
APA StyleSchutter, M., Nozawa, Y., & Kurihara, H. (2015). The Effect of Elevated CO2 and Increased Temperature on in Vitro Fertilization Success and Initial Embryonic Development of Single Male:Female Crosses of Broad-Cast Spawning Corals at Mid- and High-Latitude Locations. Journal of Marine Science and Engineering, 3(2), 216-239. https://doi.org/10.3390/jmse3020216





