Variable-Stiffness Underwater Robotic Systems: A Review
Abstract
1. Introduction
2. Evolution of Underwater Robotic Systems
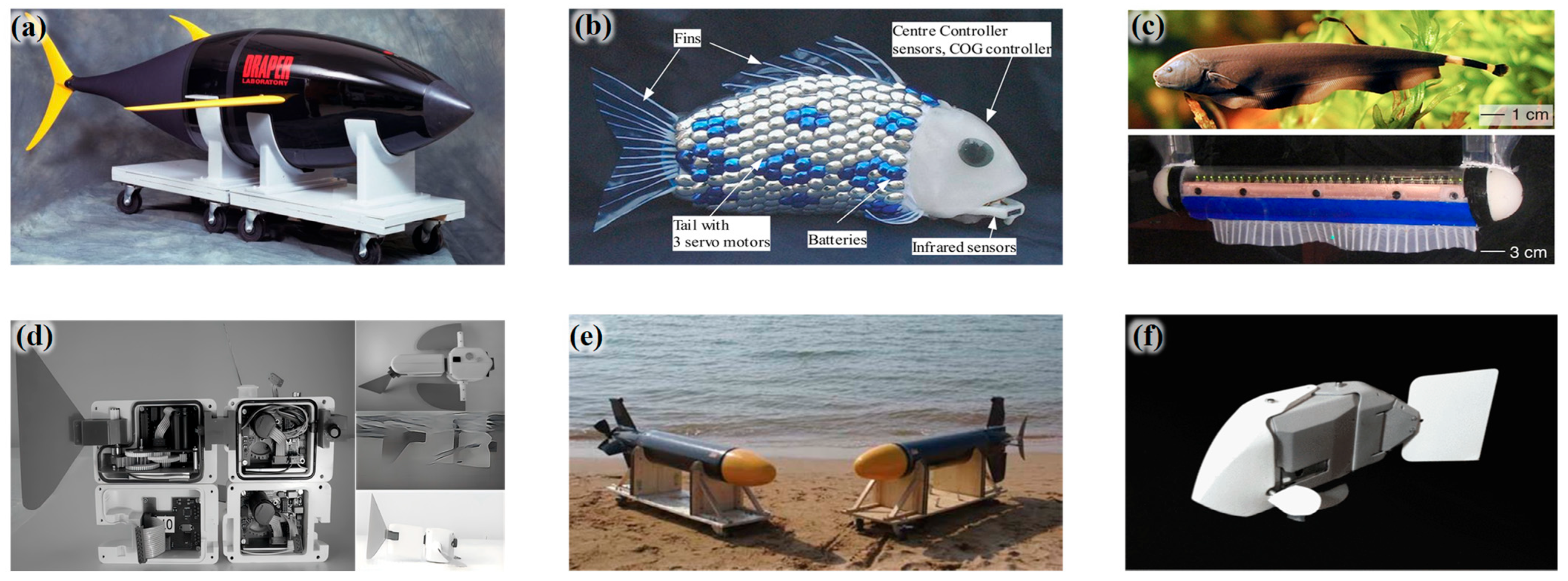
2.1. Rigid-Structured Underwater Robotic Systems
2.2. Flexible-Structured Underwater Robotic Systems

2.3. Variable-Stiffness Underwater Robotic Systems

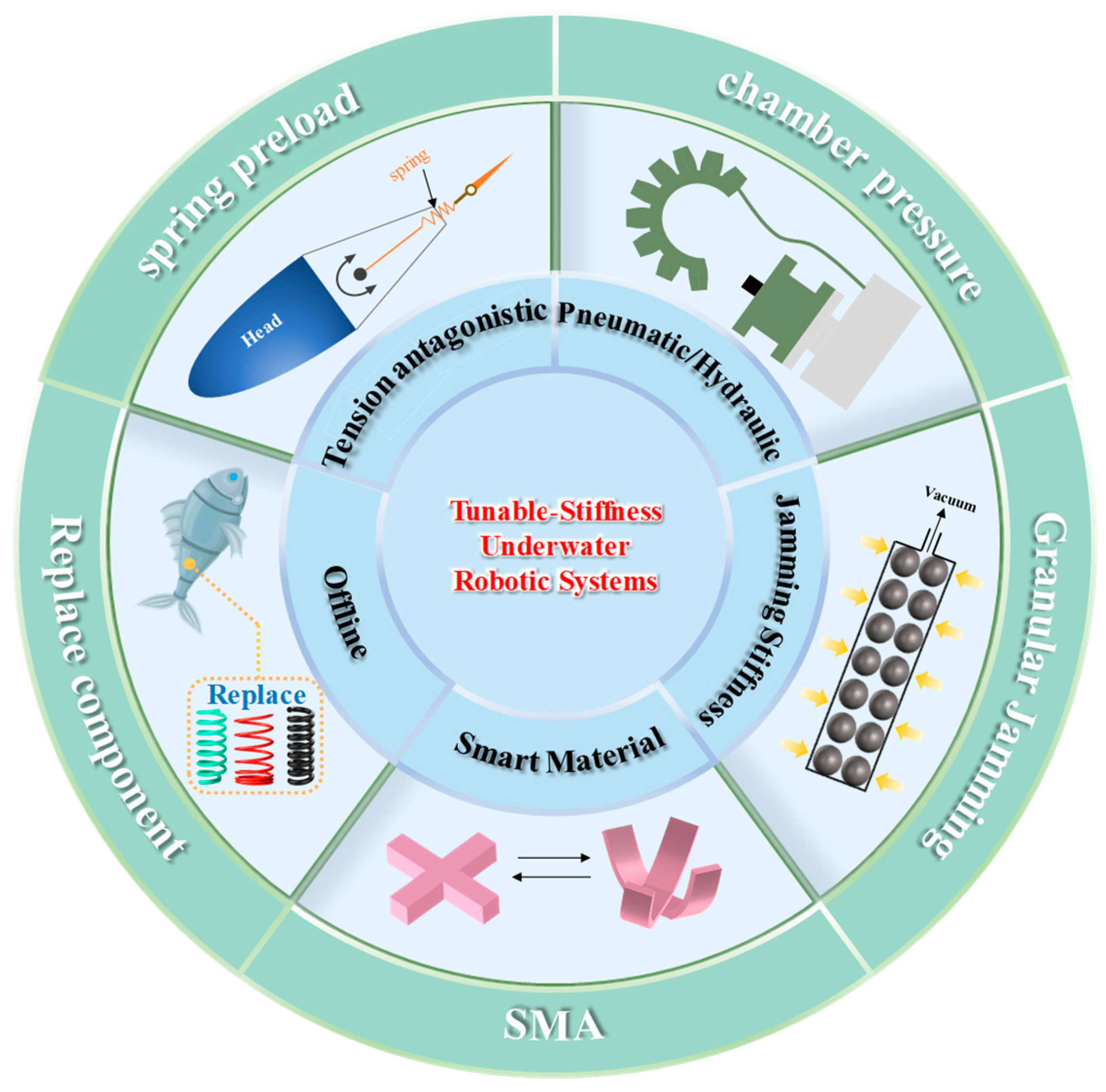
3. Methods for Stiffness Modulation in Underwater Robotic Systems
3.1. Offline Stiffness Modulation
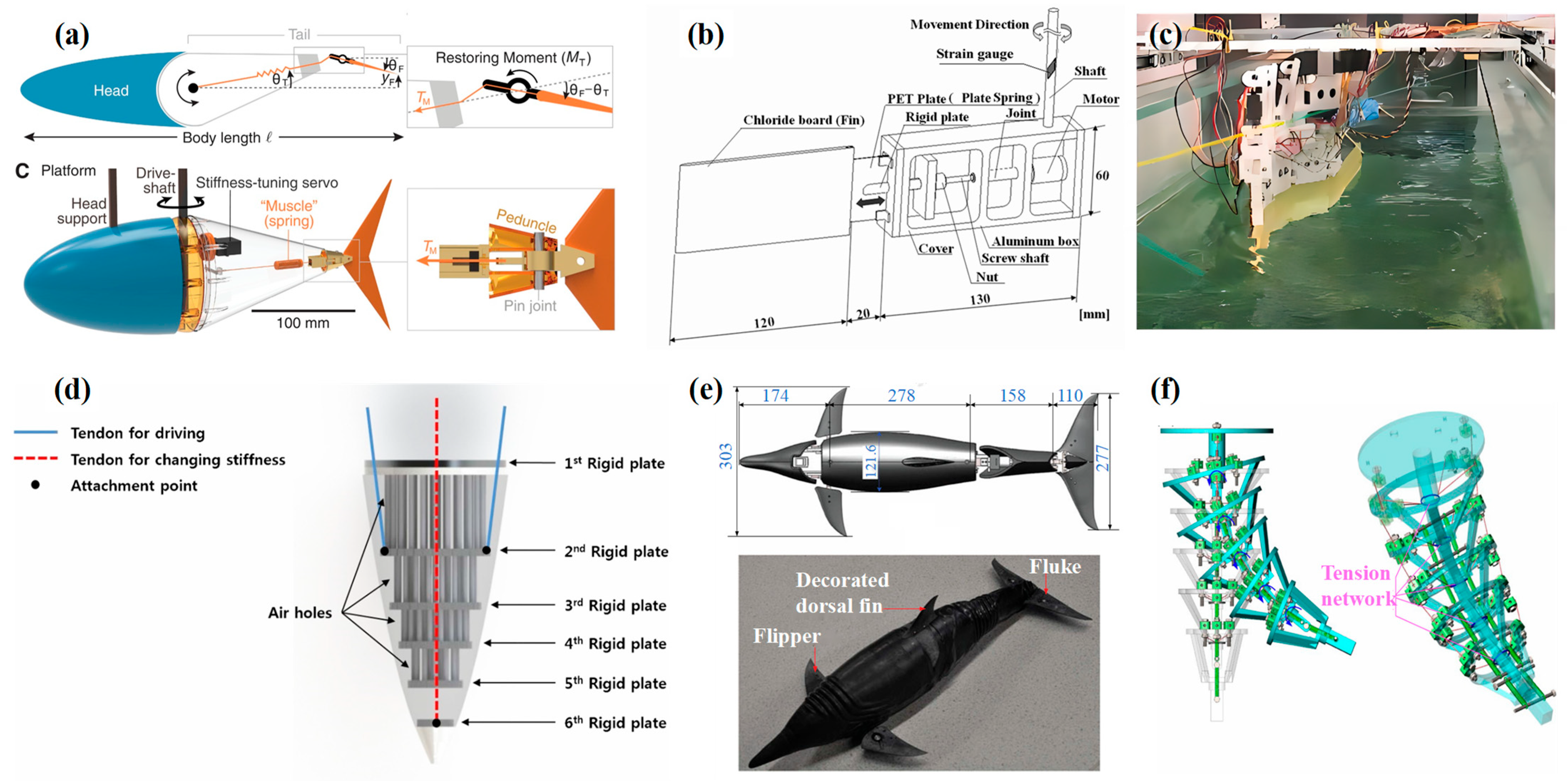
3.2. Tension Against Stiffness Modulation
3.3. Pneumatic/Hydraulic Stiffness Modulation
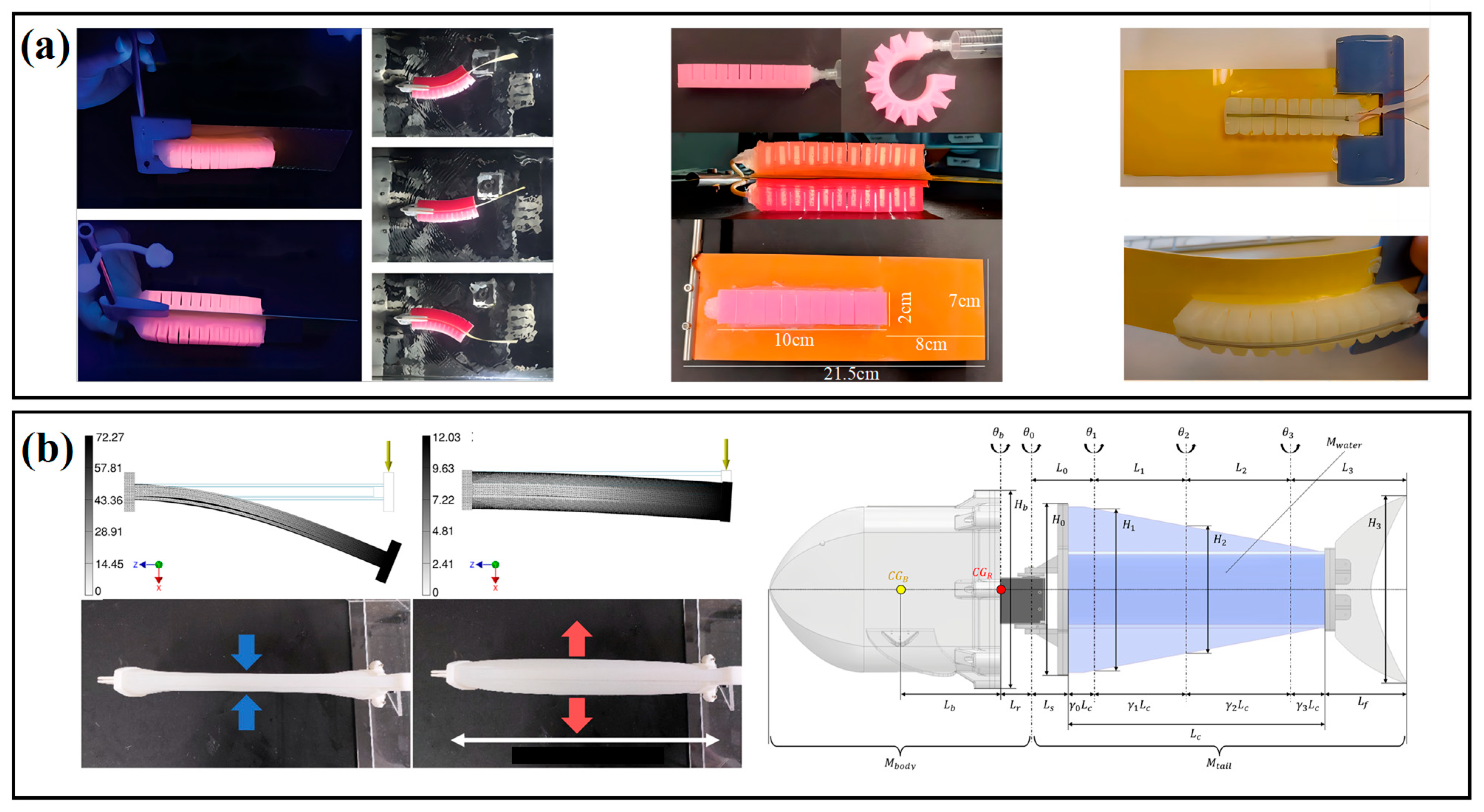
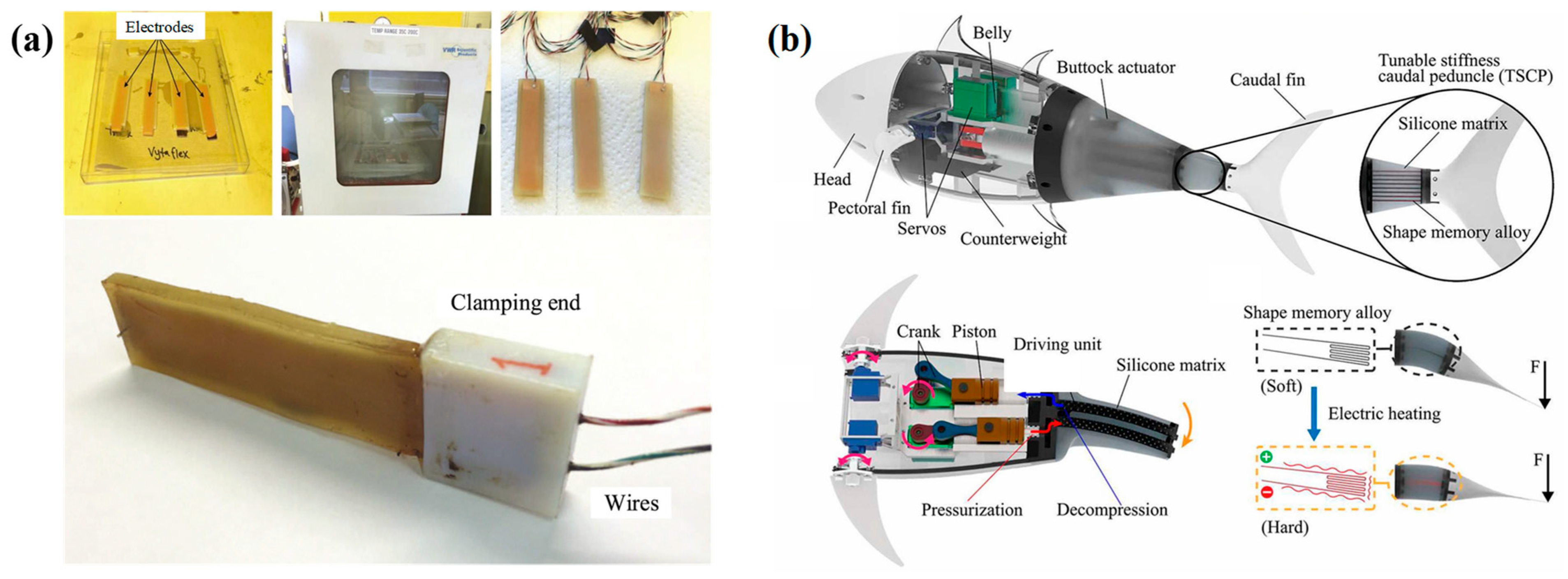
3.4. Smart Material Stiffness Modulation
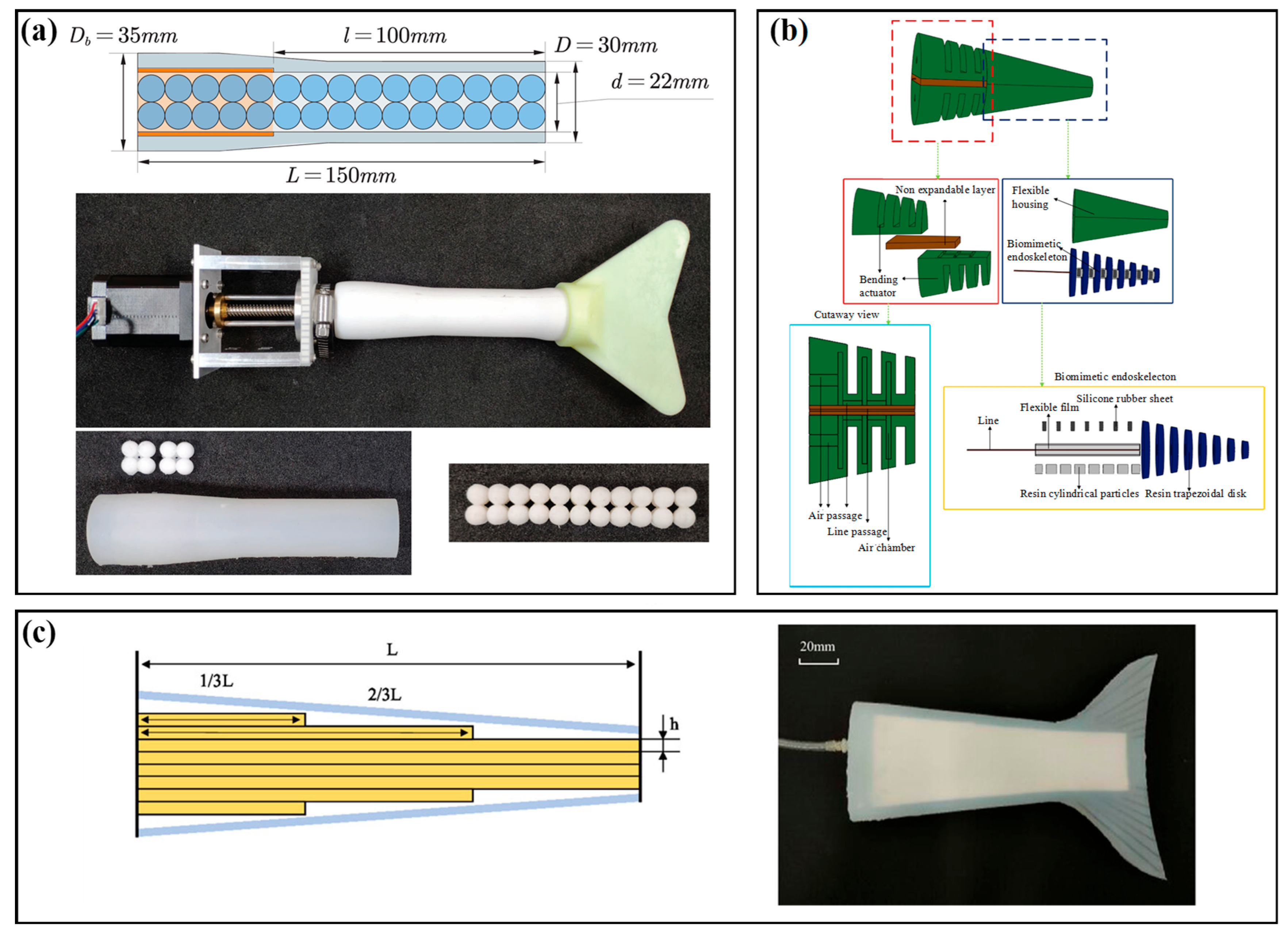
3.5. Jamming Stiffness Modulation
3.6. Performance Comparison of Stiffness Modulation Techniques in Underwater Robotics
4. Critical Challenges and Integrated Solutions for Stiffness Modulation in Underwater Robotic Systems
4.1. Challenges in Adjustable Stiffness
4.2. Innovative Solutions for Stiffness Control and Environmental Adaptability
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirwin, D.J. Metallic Mineral Resources: The Critical Components for a Sustainable Earth. Ore Geol. Rev. 2025, 180, 106578. [Google Scholar] [CrossRef]
- Zhang, F.; Li, M. Land and Water Resources for Food and Agriculture. Agronomy 2024, 14, 880. [Google Scholar] [CrossRef]
- Irigoien, X.; Huisman, J.; Harris, R.P. Global biodiversity patterns of marine phytoplankton and zooplankton. Nature 2004, 429, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Surendhiran, D.; Li, C.Z.; Cui, H.Y.; Lin, L. Marine algae as efficacious bioresources housing antimicrobial compounds for preserving foods—A review. Int. J. Food Microbiol. 2021, 358, 109416. [Google Scholar] [CrossRef]
- Takai, Y.; Tanoue, W.; Qiu, X.C.; Takaku, H.; Kang, I.J.; Shimasaki, Y.; Honjo, T.; Oshima, Y. Effects of Tributyltin and Diazinon on the Intertidal Marine Harpacticoid Copepod Tigriopus japonicus. J. Fac. Agric. Kyushu Univ. 2020, 65, 289–294. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Z.; Xu, K.; Lin, M. Editorial: Marine biodiversity under global climate change. J. Sea Res. 2024, 202, 102555. [Google Scholar] [CrossRef]
- Zhong, R.T.; Wan, X.Z.; Wang, D.Y.; Zhao, C.; Liu, D.; Gao, L.Y.; Wang, M.F.; Wu, C.J.; Nabavid, S.M.; Daglia, M.; et al. Polysaccharides from Marine Enteromorpha: Structure and function. Trends Food Sci. Technol. 2020, 99, 11–20. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhang, D.; Liu, G.M.; Chen, Q.C.; Lu, Z.H. Ameliorative effect of dieckol-enriched extraction from Laminaria japonica on hepatic steatosis induced by a high-fat diet via β-oxidation pathway in ICR mice. J. Funct. Foods 2019, 58, 44–55. [Google Scholar] [CrossRef]
- Deutsch, C.; Penn, J.L.; Lucey, N. Climate, Oxygen, and the Future of Marine Biodiversity. Annu. Rev. Mar. Sci. 2023, 16, 217–245. [Google Scholar] [CrossRef]
- Garcia Perez, P.; Cassani, L.; Garcia Oliveira, P.; Xiao, J.B.; Simal Gandara, J.; Prieto, M.A.; Lucini, L. Algal nutraceuticals: A perspective on metabolic diversity, current food applications, and prospects in the field of metabolomics. Food Chem. 2023, 409, 135295. [Google Scholar] [CrossRef]
- Hong, S.K.; Kim, Y.; Kim, Y.-M. Assessment of REY resource potential in deep-sea sediments with Fe–Mn (oxyhydr)oxides in the Pacific Ocean. J. Geochem. Explor. 2024, 267, 107581. [Google Scholar] [CrossRef]
- Ding, Q.; Li, Z.; Wu, W.; Su, Y.; Sun, N.; Luo, L.; Ma, H.; He, R. Physicochemical and functional properties of dietary fiber from Nannochloropsis oceanica: A comparison of alkaline and ultrasonic-assisted alkaline extractions. LWT Food Sci. Technol. 2020, 133, 110080. [Google Scholar] [CrossRef]
- Jamshidi, A.; Cao, H.; Xiao, J.; Simal Gandara, J. Advantages of techniques to fortify food products with the benefits of fish oil. Food Res. Int. 2020, 137, 109353. [Google Scholar] [CrossRef]
- Muzaffar, H.; Faisal, M.N.; Anwar, H.; Hussain, A.; Khan, J.A.; Muhammad, F.; Aslam, B.; Mahmood, A.; Abdelsadik, A.; Aslam, J. Fish protein intake is a novel dietary approach for managing diabetes-associated complications in diabetic Wistar rat model. Food Sci. Nutr. 2021, 9, 1017–1024. [Google Scholar] [CrossRef]
- Gao, R.; Yu, Q.; Shen, Y.; Chu, Q.; Chen, G.; Fen, S.; Yang, M.; Yuan, L.; McClements, D.J.; Sun, Q. Production, bioactive properties, and potential applications of fish protein hydrolysates: Developments and challenges. Trends Food Sci. Technol. 2021, 110, 687–699. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, G.; Wang, Y.; Rehman, A.; Yu, L.; Zhang, H.; Jin, Q.; Suleria, H.A.R.; Wang, X. Recent developments, challenges, and prospects of dietary omega-3 PUFA-fortified foods: Focusing on their effects on cardiovascular diseases. Food Chem. 2025, 470, 142498. [Google Scholar] [CrossRef]
- Zhao, H. Research on multi-sensor data fusion technology for underwater robots for deep-sea exploration. Appl. Math. Nonlinear Sci. 2024, 9, 1863. [Google Scholar] [CrossRef]
- Matsumoto, N. Development of underwater search and rescue remotely operated vehicles. Adv. Robot. 2002, 16, 561–564. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, F.; Zhou, C.; Yang, X.; Xiao, X.; Yan, B. Technology and Equipment for Underwater Robots. J. Mar. Sci. Eng. 2025, 13, 644. [Google Scholar] [CrossRef]
- Mihaylova, L.; Kakogawa, A.; Ma, S.; Choi, H.R.; Nakamura, T. Editorial: Pipeline inspection robots. Front. Robot. AI 2024, 11, 1497809. [Google Scholar] [CrossRef]
- Ni, Y.; Xiong, Y.; He, W.; Yan, Y.; Xiong, J.; Sun, Y. Pipeline inspection robot design and walking motion research. Phys. Conf. Ser. 2025, 2954, 012063. [Google Scholar] [CrossRef]
- Anandkumar, A.; Li, J.; Prabakaran, K.; Zhang, X.J.; Leng, Z.; Nagarajan, R.; Du, D. Accumulation of toxic elements in an invasive crayfish species (Procambarus clarkii) and its health risk assessment to humans. J. Food Compos. Anal. 2020, 88, 103449. [Google Scholar] [CrossRef]
- Hassan, M.M.; Ahmad, W.; Zareef, M.; Rong, Y.; Xu, Y.; Jiao, T.; He, P.; Li, H.; Chen, Q. Rapid detection of mercury in food via rhodamine 6G signal using surface-enhanced Raman scattering coupled multivariate calibration. Food Chem. 2021, 358, 129844. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, X.; Chen, H.; Tian, X. Automatic cruise system for water quality monitoring. Int. J. Agric. Biol. Eng. 2018, 11, 244–250. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.W.; Tahir, H.; Zou, X.; Wang, P. Rapid and wide-range determination of Cd(II), Pb(II), Cu(II) and Hg(II) in fish tissues using light addressable potentiometric sensor (Article). Food Chem. 2017, 221, 541–547. [Google Scholar] [CrossRef]
- Adade, S.Y.S.S.; Lin, H.; Johnson, N.A.N.; Sun, Q.; Nunekpeku, X.; Ahmad, W.; Kwadzokpui, B.A.; Ekumah, J.-N.; Chen, Q. Rapid qualitative and quantitative analysis of benzo(b)fluoranthene (BbF) in shrimp using SERS-based sensor coupled with chemometric models. Food Chem. 2024, 454, 139836. [Google Scholar] [CrossRef]
- Xu, Y.W.; Zhang, W.; Shi, J.Y.; Zou, X.; Li, Y.; Haroon Elrasheid, T.; Huang, X.; Li, Z.; Zhai, X.; Hu, X. Electrodeposition of gold nanoparticles and reduced graphene oxide on an electrode for fast and sensitive determination of methylmercury in fish. Food Chem. 2017, 237, 423–430. [Google Scholar] [CrossRef]
- Han, F.K.; Huang, X.Y.; Mahunu, G.K. Exploratory review on safety of edible raw fish per the hazard factors and their detection methods. Trends Food Sci. Technol. 2017, 59, 37–48. [Google Scholar] [CrossRef]
- Shi, B.; Sreeram, V.; Zhao, D.; Duan, S.; Jiang, J. A wireless sensor network-based monitoring system for freshwater fishpond aquaculture. Biosyst. Eng. 2018, 172, 57–66. [Google Scholar] [CrossRef]
- Wu, W.W.; Ahmad, W.; Hassan, M.M.; Wu, J.Z.; Ouyang, Q.; Chen, Q. An upconversion biosensor based on inner filter effect for dual-role recognition of sulfadimethoxine in aquatic samples. Food Chem. 2024, 437, 137832. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, J.; Li, Y.; Zhang, L.; Bi, N.; Gou, J.; Zhu, T.; Jia, L. A novel intelligently integrated MOF-based ratio fluorescence sensor for ultra-sensitive monitoring of TC in water and food samples. Food Chem. 2023, 405, 134899. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Li, D.; Wu, J.; Jiao, T.; Wei, J.; Chen, X.; Chen, Q.; Chen, Q. Sulfadiazine detection in aquatic products using upconversion nanosensor based on photo-induced electron transfer with imidazole ligands and copper ions. Food Chem. 2024, 456, 139992. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Xu, Y.; Li, Z.; Yu, Z.; Mao, B.; Wang, Y.; Wang, Z.; Fan, Q.; Qian, X.; Zhang, M.; et al. Recent Advances on Underwater Soft Robots. Adv. Intell. Syst. 2024, 6, 2300299. [Google Scholar] [CrossRef]
- Weihs, D.; Lighthill, M.J. A hydrodynamical analysis of fish turning manoeuvres. Proc. R. Soc. B Biol. Sci. 1972, 182, 59–72. [Google Scholar] [CrossRef]
- Lighthill, M.J. Large-amplitude elongated-body theory of fish locomotion. Proc. R. Soc. B Biol. Sci. 1971, 179, 125–138. [Google Scholar] [CrossRef]
- Vandamm, J.P.; Marras, S.; Claireaux, G.; Handelsman, C.A.; Nelson, J.A. Acceleration Performance of Individual European Sea Bass Dicentrarchus labrax Measured with a Sprint Performance Chamber: Comparison with High-Speed Cinematography and Correlates with Ecological Performance. Physiol. Biochem. Zool. 2012, 85, 704–717. [Google Scholar] [CrossRef] [PubMed]
- van Ginneken, V.; Antonissen, E.; Müller, U.K.; Booms, R.; Eding, E.; Verreth, J.; van den Thillart, G. Eel migration to the Sargasso: Remarkably high swimming efficiency and low energy costs. J. Exp. Biol. 2005, 208, 1329–1335. [Google Scholar] [CrossRef]
- Wardle, C.S.; He, P. Burst swimming speeds of mackerel, Scomber scombrus L. J. Fish Biol. 1988, 32, 471–478. [Google Scholar] [CrossRef]
- Linden, P.F.; Turner, J.S. ‘Optimal’ vortex rings and aquatic propulsion mechanisms. Proc. R. Soc. B Biol. Sci. 2004, 271, 647–653. [Google Scholar] [CrossRef]
- Nauen, J.C.; Lauder, G.V. Quantification of the wake of rainbow trout (Oncorhynchus mykiss) using three-dimensional stereoscopic digital particle image velocimetry. J. Exp. Biol. 2002, 205, 3271–3279. [Google Scholar] [CrossRef] [PubMed]
- Wise, T.N.; Schwalbe, M.A.B.; Tytell, E.D. Hydrodynamics of linear acceleration in bluegill sunfish, Lepomis macrochirus. J. Exp. Biol. 2018, 221, jeb190892. [Google Scholar] [CrossRef]
- Maia, A.; Lauder, G.V.; Wilga, C.D. Hydrodynamic function of dorsal fins in spiny dogfish and bamboo sharks during steady swimming. J. Exp. Biol. 2017, 220, 3967–3975. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.J.; Tangorra, J.L.; Flammang, B.E.; Lauder, G.V. A robotic fish caudal fin: Effects of stiffness and motor program on locomotor performance. J. Exp. Biol. 2012, 215, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Borazjani, I.; Sotiropoulos, F. Numerical investigation of the hydrodynamics of anguilliform swimming in the transitional and inertial flow regimes. J. Exp. Biol. 2009, 212, 576–592. [Google Scholar] [CrossRef]
- Borazjani, I.; Sotiropoulos, F. Numerical investigation of the hydrodynamics of carangiform swimming in the transitional and inertial flow regimes. J. Exp. Biol. 2008, 211, 1541–1558. [Google Scholar] [CrossRef]
- Thekkethil, N.; Sharma, A.; Agrawal, A. Unified hydrodynamics study for various types of fishes-like undulating rigid hydrofoil in a free stream flow. Phys. Fluids 2018, 30, 077107. [Google Scholar] [CrossRef]
- Xu, Y.G.; Wan, D.C. Numerical Simulation of Fish Swimming with Rigid Pectoral Fins. J. Hydrodyn. 2012, 24, 263–272. [Google Scholar] [CrossRef]
- Li, N.; Liu, H.; Su, Y. Numerical study on the hydrodynamics of thunniform bio-inspired swimming under self-propulsion. PLoS ONE 2017, 12, e0174740. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, R.; Wang, S.; Tan, M.; Yu, J. Underwater Bioinspired Propulsion: From Inspection to Manipulation. IEEE Trans. Ind. Electron. 2020, 67, 7629–7638. [Google Scholar] [CrossRef]
- Raj, A.; Thakur, A. Fish-inspired robots: Design, sensing, actuation, and autonomy—A review of research. Bioinspir. Biomim. 2016, 11, 031001. [Google Scholar] [CrossRef]
- Salazar, R.; Fuentes, V.; Abdelkefi, A. Classification of biological and bioinspired aquatic systems: A review. Ocean Eng. 2018, 148, 75–114. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, Z.; Du, R. Robot fish with two-DOF pectoral fins and a wire-driven caudal fin. Adv. Robot. 2018, 32, 25–36. [Google Scholar] [CrossRef]
- Pfeifer, R.; Lungarella, M.; Iida, F. Self-Organization, Embodiment, and Biologically Inspired Robotics. Science 2007, 318, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Palagi, S.; Fischer, P. Bioinspired microrobots. Nat. Rev. Mater. 2018, 3, 113–124. [Google Scholar] [CrossRef]
- Rus, D.; Tolley, M.T. Design, fabrication and control of soft robots. Nature 2015, 521, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.J.; Godaba, H.; Chen, G.; Tan, S.T.M.; Wan, G.; Li, G.; Lee, P.M.; Cai, Y.; Li, S.; Shepherd, R.F.; et al. A transparent, self-healing and high-κ dielectric for low-field-emission stretchable optoelectronics. Nat. Mater. 2020, 19, 182–188. [Google Scholar] [CrossRef]
- Trivedi, D.; Rahn, C.D.; Kier, W.M.; Walker, I.D. Soft Robotics: Biological Inspiration, State of the Art, and Future Research. Appl. Bionics Biomech. 2008, 5, 520417. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, C.; Zhang, Y.; Yang, L.; Tan, W.; Qin, H.; Wang, F.; Liu, L. Fast-Swimming Soft Robotic Fish Actuated by Bionic Muscle. Soft Robot. 2024, 11, 845–856. [Google Scholar] [CrossRef]
- Li, Z.; Chu, X.; Hu, X.; Zhang, Z.; Li, N.; Li, J. Variable stiffness methods for robots: A review. Smart Mater. Struct. 2024, 33, 063002. [Google Scholar] [CrossRef]
- Zuo, C.; Zhou, J.H. A study of the planar serial-parallel mechanism with various stiffness for a biotic compliant fish. In Proceedings of the ASME 2013 International Mechanical Engineering Congress and Exposition, San Diego, CA, USA, 15–21 November 2013; p. V03AT03A044. [Google Scholar]
- Behbahani, S.B.; Tan, X.B. Design and dynamic modeling of electrorheological fluid-based variable-stiffness fin for robotic fish. Smart Mater. Struct. 2017, 26, 085014. [Google Scholar] [CrossRef]
- Nakabayashi, M.; Kobayashi, R.; Kobayashi, S.; Morikawa, H. A novel propulsion mechanism using a fin with a variable-effective-length spring. In Proceedings of the IEEE International Conference on Robotics and Biomimetics, Bangkok, Thailand, 22–25 February 2009; pp. 1515–1521. [Google Scholar]
- Jusufi, A.; Vogt, D.M.; Wood, R.J.; Lauder, G.V. Undulatory swimming performance and body stiffness modulation in a soft robotic fish-inspired physical model. Soft Robot. 2017, 4, 202–210. [Google Scholar] [CrossRef]
- Li, K.; Jiang, H.; Wang, S.; Yu, J. A soft robotic fish with variable-stiffness decoupled mechanisms. J. Bionic Eng. 2018, 15, 599–609. [Google Scholar] [CrossRef]
- Xu, D.; Zeng, H.; Peng, X.; Zhao, Z.; Liu, J. A stiffness adjustment mechanism based on negative work for high-efficient propulsion of robotic fish. J. Bionic Eng. 2018, 15, 270–282. [Google Scholar] [CrossRef]
- Song, Z.; Fu, Z.; Romano, D.; Dario, P.; Dai, J.S. A novel biomimetic compliant structural skin based on composite materials for biorobotics applications. Soft Robot. 2022, 9, 440–450. [Google Scholar] [CrossRef]
- Brown, E.; Rodenberg, N.; Amend, J.; Mozeika, A.; Steltz, E.; Zakin, M.R.; Lipson, H.; Jaeger, H.M. Universal robotic gripper based on the jamming of granular material. Proc. Natl. Acad. Sci. USA 2010, 107, 18809–18814. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.; Chen, Y.; Wang, Q.; Chi, C.; Zhang, H.; Gao, Q.; Lan, Y.; Li, Z.; Mu, Z. Design and control of a highly redundant rigid-flexible coupling robot to assist the COVID-19 oropharyngeal-swab sampling. IEEE Robot. Autom. Lett. 2021, 7, 1856–1863. [Google Scholar] [CrossRef]
- George Thuruthel, T.; Ansari, Y.; Falotico, E.; Laschi, C. Control strategies for soft robotic manipulators: A survey. Soft Robot. 2018, 5, 149–163. [Google Scholar] [CrossRef]
- Iacoponi, S.; Vuuren, G.J.V.; Santaera, G.; Mankovskii, N.; Zhilin, I.; Renda, F.; Stefanini, C.; Masi, G.D. H-SURF: Heterogeneous Swarm of Underwater Robotic Fish. In Proceedings of the OCEANS 2022, Hampton Roads, VA, USA, 17–20 October 2022; pp. 1–5. [Google Scholar]
- Salazar, J.; Cai, L.; Cook, B.; Rus, D. Multi-Robot Visual Control of Autonomous Soft Robotic Fish. In Proceedings of the 2022 IEEE/OES Autonomous Underwater Vehicles Symposium (AUV), Singapore, 19–21 September 2022; pp. 1–6. [Google Scholar]
- Zhang, T.; Wang, R.; Wang, S.; Wang, Y.; Cheng, L.; Zheng, G.; Tan, M. Autonomous Skill Learning of Water Polo Ball Heading for a Robotic Fish: Curriculum and Verification. IEEE Trans. Cogn. Dev. Syst. 2023, 15, 865–876. [Google Scholar] [CrossRef]
- Ay, M.; Korkmaz, D.; Ozmen Koca, G.; Bal, C.; Akpolat, Z.H.; Bingol, M.C. Mechatronic Design and Manufacturing of the Intelligent Robotic Fish for Bio-Inspired Swimming Modes. Electronics 2018, 7, 118. [Google Scholar] [CrossRef]
- Dai, S.; Wu, Z.; Wang, J.; Tan, M.; Yu, J. Barrier-Based Adaptive Line-of-Sight 3-D Path-Following System for a Multijoint Robotic Fish With Sideslip Compensation. IEEE Trans. Cybern. 2023, 53, 4204–4217. [Google Scholar] [CrossRef]
- Omari, M.; Ghommem, M.; Romdhane, L.; Hajj, M.R. Performance analysis of bio-inspired transformable robotic fish tail. Ocean Eng. 2022, 244, 110406. [Google Scholar] [CrossRef]
- Verma, S.; Xu, J.X. Analytic Modeling for Precise Speed Tracking of Multilink Robotic Fish. IEEE Trans. Ind. Electron. 2018, 65, 5665–5672. [Google Scholar] [CrossRef]
- Chen, L.; Bi, S.; Cai, Y.; Qiu, H. Design and Hydrodynamic Experiment Research on Novel Biomimetic Pectoral Fins of a Ray-Inspired Robotic Fish. Machines 2022, 10, 606. [Google Scholar] [CrossRef]
- Marcin, M.; Adam, S.; Jerzy, Z.; Marcin, M. Fish-like shaped robot for underwater surveillance and reconnaissance—Hull design and study of drag and noise. Ocean Eng. 2020, 217, 107889. [Google Scholar] [CrossRef]
- Aracri, S.; Giorgio Serchi, F.; Suaria, G.; Sayed, M.E.; Nemitz, M.P.; Mahon, S.; Stokes, A.A. Soft robots for ocean exploration and offshore operations: A perspective. Soft Robot. 2021, 8, 625–639. [Google Scholar] [CrossRef]
- Fang, J.; Zhuang, Y.; Liu, K.; Chen, Z.; Liu, Z.; Kong, T.; Xu, J.; Qi, C. A shift from efficiency to adaptability: Recent progress in biomimetic interactive soft robotics in wet environments. Adv. Sci. 2022, 9, 2104347. [Google Scholar] [CrossRef]
- Chu, W.-S.; Lee, K.-T.; Song, S.-H.; Han, M.-W.; Lee, J.-Y.; Kim, H.-S.; Kim, M.-S.; Park, Y.-J.; Cho, K.-J.; Ahn, S.-H. Review of biomimetic underwater robots using smart actuators. Int. J. Precis. Eng. Manuf. 2012, 13, 1281–1292. [Google Scholar] [CrossRef]
- Barrett, D.; Grosenbaugh, M.; Triantafyllou, M. The optimal control of a flexible hull robotic undersea vehicle propelled by an oscillating foil. In Proceedings of the Symposium on Autonomous Underwater Vehicle Technology, Monterey, CA, USA, 2–6 June 1996; pp. 1–9. [Google Scholar]
- Techet, A.H.; Hover, F.S.; Triantafyllou, M.S. Separation and Turbulence Control in Biomimetic Flows. Flow Turbul. Combust. 2003, 71, 105–118. [Google Scholar] [CrossRef]
- Yu, J.; Chen, E.; Wang, S.; Tan, M. Research evolution and analysis of biomimetic robot fish. Control Theory Appl. 2003, 20, 485–491. [Google Scholar] [CrossRef]
- Anderson, J.M.; Chhabra, N.K. Maneuvering and Stability Performance of a Robotic Tuna. Integr. Comp. Biol. 2002, 42, 118–126. [Google Scholar] [CrossRef]
- Hirata, K.; Takimoto, T.; Tamura, K. Study on turning performance of a fish robot. In Proceedings of the 1st International Symposium on Aqua Bio-Mechanisms, Honolulu, HI, USA, 27–30 August 2000; pp. 287–292. [Google Scholar]
- Hu, H. Biologically inspired design of autonomous robotic fish at Essex. In Proceedings of the IEEE SMC UK-RI Chapter Conference, on Advanced Cybernetics Systems, Taipei, Taiwan, 8–11 October 2006; pp. 3–8. [Google Scholar]
- Hu, H.S.; Liu, J.D.; Dukes, I.; Francis, G. Design of 3D swim patterns for autonomous robotic fish. In Proceedings of the 2006 IEEE/RSJ International Conference on Intelligent Robots and Systems, Beijing, China, 9–15 October 2006; pp. 2406–2411. [Google Scholar]
- Liu, J.; Hu, H. A Methodology of Modelling Fish-like Swim Patterns for Robotic Fish. In Proceedings of the 2007 International Conference on Mechatronics and Automation, Harbin, China, 5–8 August 2007; pp. 1316–1321. [Google Scholar]
- Curet, O.M.; Patankar, N.A.; Lauder, G.V.; MacIver, M.A. Mechanical properties of a bio-inspired robotic knifefish with an undulatory propulsor. Bioinspir. Biomim. 2011, 6, 026004. [Google Scholar] [CrossRef] [PubMed]
- Lachat, D.; Crespi, A.; Auke Jan, I. BoxyBot: A swimming and crawling fish robot controlled by a central pattern generator. In Proceedings of the BioRob, Pisa, Italy, 20–22 February 2006; pp. 643–648. [Google Scholar]
- Hu, T.; Shen, L.; Lin, L.; Xu, H. Biological inspirations, kinematics modeling, mechanism design and experiments on an undulating robotic fin inspired by Gymnarchus niloticus. Mech. Mach. Theory 2009, 44, 633–645. [Google Scholar] [CrossRef]
- Heo, S.; Wiguna, T.; Park, H.C.; Goo, N.S. Effect of an artificial caudal fin on the performance of a biomimetic fish robot propelled by piezoelectric actuators. J. Bionic. Eng. 2007, 4, 151–158. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, Y.; Wang, L. Construction and central pattern generator-based control of a flipper-actuated turtle-like underwater robot. Adv. Robot. 2009, 23, 19–43. [Google Scholar] [CrossRef]
- Liang, J.H.; Wang, T.M.; Wei, H.X. Research and Development of Underwater Robofish I—Development of a Small Experimental Robofish. Robot 2002, 24, 107–111. [Google Scholar] [CrossRef]
- Liang, J.H.; Wang, T.M.; Wei, H.X.; Tao, W. Researchful Development of Underwater Robofish II: Development of a Small Experimental Robofish. Robot 2002, 24, 234–238. [Google Scholar] [CrossRef]
- Liang, J.H.; Wang, T.M.; Wen, L. Development of a two-joint robotic fish for real-world exploration. J. Field Robot. 2011, 28, 70–79. [Google Scholar] [CrossRef]
- Clapham, R.J.; Hu, H. iSplash: Realizing fast carangiform swimming to outperform a real fish. In Robot Fish; Springer: Berlin, Heidelberg, 2015; pp. 193–218. [Google Scholar]
- Otake, M.; Kagami, Y.; Inaba, M.; Inoue, H. Motion design of a starfish-shaped gel robot made of electro-active polymer gel. Robot. Auton. Syst. 2002, 40, 185–191. [Google Scholar] [CrossRef]
- Suleman, A.; Crawford, C. Design and testing of a biomimetic tuna using shape memory alloy induced propulsion. Comput. Struct. 2008, 86, 491–499. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Li, J.; Hang, G. A micro biomimetic manta ray robot fish actuated by SMA. In Proceedings of the IEEE International Conference on Robotics and Biomimetics (ROBIO), Guilin, China, 19–23 December 2009; pp. 19–23. [Google Scholar]
- Su, Y.D.; Ye, X.F.; Guo, S.X. An Autonomous Micro Robot Fish Based on IPMC Actuator. Robot 2010, 32, 262–270. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, L.; Liu, B.; Yang, J.; Zhang, S. A novel implementation of a flexible robotic fin actuated by shape memory alloy. J. Bionic Eng. 2012, 9, 156–165. [Google Scholar] [CrossRef]
- Clapham, R.J.; Hu, H. iSplash-MICRO: A 50mm robotic fish generating the maximum velocity of real fish. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems, Chicago, IL, USA, 14–18 September 2014; pp. 287–293. [Google Scholar]
- Marchese, A.D.; Onal, C.D.; Rus, D. Autonomous Soft Robotic Fish Capable of Escape Maneuvers Using Fluidic Elastomer Actuators. Soft Robot. 2014, 1, 75–87. [Google Scholar] [CrossRef]
- Huang, C.; Lv, J.A.; Tian, X.; Wang, Y.; Yu, Y.; Liu, J. Miniaturized swimming soft robot with complex movement actuated and controlled by remote light signals. Sci. Rep. 2015, 5, 17414. [Google Scholar] [CrossRef]
- Sfakiotakis, M.; Kazakidi, A.; Tsakiris, D. Octopus-inspired multi-arm robotic swimming. Bioinspir. Biomim. 2015, 10, 035005. [Google Scholar] [CrossRef]
- Kazakidi, A.; Vavourakis, V.; Tsakiris, D.; Ekaterinaris, J.A. A numerical investigation of flow around octopus-like arms: Near-wake vortex patterns and force development. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 1321–1339. [Google Scholar] [CrossRef]
- Jin, H.; Dong, E.; Alici, G.; Mao, S.; Min, X.; Liu, C.; Low, K.; Yang, J. A starfish robot based on soft and smart modular structure (SMS) actuated by SMA wires. Bioinspir. Biomim. 2016, 11, 056012. [Google Scholar] [CrossRef] [PubMed]
- Shintake, J.; Cacucciolo, V.; Shea, H.; Floreano, D. Soft biomimetic fish robot made of dielectric elastomer actuators. Soft Robot. 2018, 5, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Christianson, C.; Goldberg, N.N.; Deheyn, D.D.; Cai, S.; Tolley, M.T. Translucent soft robots driven by frameless fluid electrode dielectric elastomer actuators. Sci. Robot. 2018, 3, eaat1893. [Google Scholar] [CrossRef]
- Christianson, C.; Bayag, C.; Li, G.; Jadhav, S.; Giri, A.; Agba, C.; Li, T.; Tolley, M.T. Jellyfish-inspired soft robot driven by fluid electrode dielectric organic robotic actuators. Front. Robot. AI 2019, 6, 126. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yang, X.; Wu, Y.; Zhou, Z.; Wang, J.; Zhang, B.; Luo, Y.; Chepinskiy, S.A.; Zhilenkov, A.A. A novel underwater bipedal walking soft robot bio-inspired by the coconut octopus. Bioinspir. Biomim. 2021, 16, 046007. [Google Scholar] [CrossRef]
- Li, G.; Chen, X.; Zhou, F.; Liang, Y.; Xiao, Y.; Cao, X.; Zhang, Z.; Zhang, M.; Wu, B.; Yin, S.; et al. Self-powered soft robot in the Mariana Trench. Nature 2021, 591, 66–71. [Google Scholar] [CrossRef]
- Matharu, P.S.; Gong, P.; Guntaka, K.P.R.; Almubarak, Y.; Jin, Y.; Tadesse, Y.T. Jelly-Z: Swimming performance and analysis of twisted and coiled polymer (TCP) actuated jellyfish soft robot. Sci. Rep. 2023, 13, 11086. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yuan, Z.; Guo, Q.; Wang, W.; Liu, H.; Liu, A.; Ge, Z.; Yu, H.; Yang, W. An Underwater Bionic Snake Soft Robot with Tunable Deformation and Motion Based on Composite Materials. Adv. Mater. Technol. 2023, 8, 2202012. [Google Scholar] [CrossRef]
- Quinn, D.; Lauder, G. Tunable stiffness in fish robotics: Mechanisms and advantages. Bioinspir. Biomim. 2022, 17, 011002. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Zhu, J.; Fish, F.E.; Kerr, S.J.; Downs, A.M.; Bart-Smith, H.; Quinn, D.B. Tunable stiffness enables fast and efficient swimming in fish-like robots. Sci. Robot. 2021, 6, eabe4088. [Google Scholar] [CrossRef]
- Wang, T.; Ren, Z.; Hu, W.; Li, M.; Sitti, M. Effect of body stiffness distribution on larval fish–like efficient undulatory swimming. Sci. Adv. 2021, 7, eabf7364. [Google Scholar] [CrossRef]
- Nakashima, M.; Tokuo, K.; Kaminaga, K.; Ono, K. Experimental study of a self-propelled two-joint dolphin robot. In Proceedings of the 9th International Offshore and Polar Engineering Conference, Brest, France, 30 May–4 June 1999; pp. I-99–I-179. [Google Scholar]
- Long, J.H., Jr.; Koob, T.J.; Irving, K.; Combie, K.; Engel, V.; Livingston, N.; Lammert, A.; Schumacher, J. Biomimetic evolutionary analysis: Testing the adaptive value of vertebrate tail stiffness in autonomous swimming robots. J. Exp. Biol. 2006, 209, 4732–4746. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, M.; Kobayashi, R.; Kobayashi, S.; Morikawa, H. Bioinspired propulsion mechanism using a fin with a dynamic variable-effective-length spring-evaluation of thrust characteristics and flow around a fin in a uniform flow. J. Biomech. Sci. Eng. 2009, 4, 82–93. [Google Scholar] [CrossRef]
- Mutlu, R.; Alici, G. Artificial muscles with adjustable stiffness. Smart Mater. Struct. 2010, 19, 045004. [Google Scholar] [CrossRef]
- Tangorra, J.L.; Lauder, G.V.; Hunter, I.W.; Mittal, R.; Madden, P.G.A.; Bozkurttas, M. The effect of fin ray flexural rigidity on the propulsive forces generated by a biorobotic fish pectoral fin. J. Exp. Biol. 2010, 213, 4043–4054. [Google Scholar] [CrossRef]
- Long, J.H., Jr.; Krenitsky, N.M.; Roberts, S.F.; Hirokawa, J.; de Leeuw, J.; Porter, M.E. Testing biomimetic structures in bioinspired robots: How vertebrae control the stiffness of the body and the behavior of fish-like swimmers. Integr. Comp. Biol. 2011, 51, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Huh, T.M.; Park, D.; Cho, K.J. Design of a variable-stiffness flapping mechanism for maximizing the thrust of a bio-inspired underwater robot. Bioinspir. Biomim. 2014, 9, 036002. [Google Scholar] [CrossRef]
- Yu, J.; Su, Z.; Wu, Z.; Tan, M. Development of a fast-swimming dolphin robot capable of leaping. IEEE-ASME Trans. Mechatron. 2016, 21, 2307–2316. [Google Scholar] [CrossRef]
- Behbahani, S.B.; Tan, X. Bio-inspired flexible joints with passive feathering for robotic fish pectoral fins. Bioinspir. Biomim. 2016, 11, 036009. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, H. Swimming performance of a tensegrity robotic fish. Soft Robot. 2019, 6, 520–531. [Google Scholar] [CrossRef]
- Chen, D.; Wu, Z.; Dong, H.; Tan, M.; Yu, J. Exploration of swimming performance for a biomimetic multi-joint robotic fish with a compliant passive joint. Bioinspir. Biomim. 2020, 16, 026007. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zhou, C.; Wang, J.; Tan, M. A Wire-Driven Dual Elastic Fishtail With Energy Storing and Passive Flexibility. IEEE-ASME Trans. Mechatron. 2023, 29, 1914–1925. [Google Scholar] [CrossRef]
- Ding, F.; Chen, W.; Zhang, J.; Chen, B. A high-frequency oscillating tensegrity robotic fish with wide-ranging online body stiffness adjustability. Ocean Eng. 2025, 328, 121063. [Google Scholar] [CrossRef]
- Mohammadi, M.; Kouzani, A.Z.; Bodaghi, M.; Zolfagharian, A. 3D-printed programmable bistable mechanisms for customized wearable devices in tremor attenuation. J. Mech. Behav. Biomed. Mater. 2025, 168, 107006. [Google Scholar] [CrossRef]
- Zolfagharian, A.; Demoly, F.; Lakhi, M.; Rolfe, B.; Bodaghi, M. Bistable mechanisms 3D printing for mechanically programmable vibration control. Adv. Eng. Mater. 2025, 2402233. [Google Scholar] [CrossRef]
- Mohammadi, M.; Kouzani, A.Z.; Bodaghi, M.; Zolfagharian, A. Inverse design of adaptive flexible structures using physical-enhanced neural network. Virtual Phys. Prototyp. 2025, 20, e2530732. [Google Scholar] [CrossRef]
- Sayah Irani, M.; Ranjbar, S.; Lakhi, M.; Zolfagharian, A. Enhancing Damage Tolerance of Structures Using 3D/4D Printing Technologies. Adv. Mater. Technol. 2025, e00535. [Google Scholar] [CrossRef]
- Ziegler, M.; Hoffmann, M.; Carbajal, J.P.; Pfeifer, R. Varying body stiffness for aquatic locomotion. In Proceedings of the IEEE International Conference on Robotics and Automation, Shanghai, China, 9–13 May 2011; pp. 2705–2712. [Google Scholar]
- Wolf, Z.; Jusufi, A.; Vogt, D.; Lauder, G. Fish-like aquatic propulsion studied using a pneumatically-actuated soft-robotic model. Bioinspir. Biomim. 2020, 15, 046008. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.; Vogt, D.M.; Wood, R.J.; Jusufi, A. Soft sensors for curvature estimation under water in a soft robotic fish. In Proceedings of the 2019 2nd IEEE International Conference on Soft Robotics (RoboSoft), Seoul, Republic of Korea, 14–18 April 2019; pp. 367–371. [Google Scholar]
- Leftwich, M.C.; Tytell, E.D.; Cohen, A.H.; Smits, A.J. Wake structures behind a swimming robotic lamprey with a passively flexible tail. J. Exp. Biol. 2012, 215, 416–425. [Google Scholar] [CrossRef]
- Zou, Q.; Lu, B.; Fu, Y.; Liao, X.; Zhang, Z.; Zhou, C. Dynamic Modeling and Optimization of Robotic Fish Based on Passive Flexible Mechanism. In Proceedings of the 2021 IEEE International Conference on Mechatronics and Automation (ICMA), Takamatsu, Japan, 8–11 August 2021; pp. 622–627. [Google Scholar]
- Zou, Q.; Zhou, C.; Lu, B.; Liao, X.; Zhang, Z. Tail-stiffness optimization for a flexible robotic fish. Bioinspir. Biomim. 2022, 17, 066003. [Google Scholar] [CrossRef]
- Chen, D.; Wu, Z.; Zhang, P.; Tan, M.; Yu, J. Performance improvement of a high-speed swimming robot for fish-like leaping. IEEE Robot. Autom. Lett. 2022, 7, 1936–1943. [Google Scholar] [CrossRef]
- Liao, X.; Zhou, C.; Cheng, L.; Wang, J.; Fan, J.; Zhang, Z. A Fast Online Elastic-Spine-Based Stiffness Adjusting Mechanism for Fishlike Swimming. Soft Robot. 2024, 11, 935–945. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, H. Body stiffness variation of a tensegrity robotic fish using antagonistic stiffness in a kinematically singular configuration. IEEE Trans. Robot. 2021, 37, 1712–1727. [Google Scholar] [CrossRef]
- Ju, I.; Yun, D. Hydraulic variable stiffness mechanism for swimming locomotion optimization of soft robotic fish. Ocean Eng. 2023, 286, 115551. [Google Scholar] [CrossRef]
- Yao, J.; Sun, Y.; Wang, Y.; Fu, Q.; Xiong, Z.; Liu, Y. Magnet-induced aligning magnetorheological elastomer based on ultra-soft matrix. Compos. Sci. Technol. 2018, 162, 170–179. [Google Scholar] [CrossRef]
- Malaeke, H.; Moeenfard, H.; Ghasemi, A.H.; Baqersad, J. Vibration Suppression of MR Sandwich Beams Based On Fuzzy Logic. In Shock & Vibration, Aircraft/Aerospace, Energy Harvesting, Acoustics & Optics; Springer: Cham, Switzerland, 2017; Volume 9, pp. 227–238. [Google Scholar]
- Bai, J.; Fu, J.; Lai, J.; Liao, G.; Yu, M. Time-delay analysis of a magnetorheological elastomer actuator for semi-active control. In Proceedings of the 2017 29th Chinese Control And Decision Conference (CCDC), Chongqing, China, 28–30 May 2017; pp. 366–370. [Google Scholar]
- Tu, F.; Liu, X.; Mao, Y.; Hu, L. Recent progress and application of electrorheological fluids. Mater. Rep. 2014, 28, 66–68. [Google Scholar]
- Li, J.; Yin, H.; Tan, Y. A novel variable stiffness soft finger actuated by shape memory alloy. Int. J. Appl. Electromagn. Mech. 2017, 53, 727–733. [Google Scholar] [CrossRef]
- Hines, L.; Arabagi, V.; Sitti, M. Shape memory polymer-based flexure stiffness control in a miniature flapping-wing robot. IEEE Trans. Robot. 2012, 28, 987–990. [Google Scholar] [CrossRef]
- Chenal, T.P.; Case, J.C.; Paik, J.; Kramer, R.K. Variable stiffness fabrics with embedded shape memory materials for wearable applications. In Proceedings of the 2014 IEEE/RSJ International Conference on Intelligent Robots and Systems, Chicago, IL, USA, 14–18 September 2014; pp. 2827–2831. [Google Scholar]
- Liu, S.; Liu, C.; Liang, Y.; Ren, L.; Ren, L. Tunable Stiffness Caudal Peduncle Leads to Higher Swimming Speed Without Extra Energy. IEEE Robot. Autom. Lett. 2023, 8, 5886–5893. [Google Scholar] [CrossRef]
- Zhu, C.; Deng, L.; Wang, X.; Yin, Z.; Zhou, C. Design and modeling of elastic variable stiffness robotic fish tail. In Proceedings of the 2022 IEEE International Conference on Mechatronics and Automation (ICMA), Guilin, China, 7–10 August 2022; pp. 1251–1256. [Google Scholar]
- Zhang, Y.; Wang, N.; Zhao, W.; Peng, L.; Luo, J. Development and Performance Analysis of Pneumatic Variable Stiffness Imitation Dolphin Tail Actuator. J. Bionic Eng. 2024, 21, 2271–2290. [Google Scholar] [CrossRef]
- Hong, Z.; Wu, Z.; Wang, Q.; Li, J.; Zhong, Y. A Novel Variable-Stiffness Tail Based on Layer-Jamming for Robotic Fish. Adv. Intell. Syst. 2024, 6, 2400189. [Google Scholar] [CrossRef]
- Zangeneh, R.; Musa, S.M. Hydrodynamic Analysis of Biomimetic Robot Fish Using OpenFOAM. In Proceedings of the IEEE Conference on Technologies for Sustainability (SusTech), Irvine, CA, USA, 22–24 April 2021; pp. 1–5. [Google Scholar]
- Katzschmann, R.K.; DelPreto, J.; MacCurdy, R.; Rus, D. Exploration of underwater life with an acoustically controlled soft robotic fish. Sci. Robot. 2018, 3, eaar3449. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, L.; Li, B. Design, manufacturing and performance study of flexible drive and stiffness adjustable structure/function integration for minimally invasive surgical operation arm. Chin. J. Mech. Eng. En. 2018, 54, 53–61. [Google Scholar] [CrossRef]
- Li, B.; Cai, Y.; Jiang, L.; Liu, L.; Zhao, Z.; Chen, G. A flexible morphing wing by soft wing skin actuation utilizing dielectric elastomer: Experiments and electro-aerodynamic model. Smart Mater. Struct. 2019, 29, 015031. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Li, Y.; Hong, J.; Wang, M.Y. Electrostatic layer jamming variable stiffness for soft robotics. IEEE-ASME Trans. Mechatron. 2019, 24, 424–433. [Google Scholar] [CrossRef]
- Ou, J.; Yao, L.; Tauber, D.; Steimle, J.; Niiyama, R.; Ishii, H. jamSheets: Thin interfaces with tunable stiffness enabled by layer jamming. In Proceedings of the TEI’14: Proceedings of the 8th International Conference on Tangible, Embedded and Embodied Interaction, Munich, Germany, 16–19 February 2014; pp. 65–72. [Google Scholar]
- Narang, Y.S.; Degirmenci, A.; Vlassak, J.J.; Howe, R.D. Transforming the dynamic response of robotic structures and systems through laminar jamming. IEEE Robot. Autom. Lett. 2017, 3, 688–695. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Hassan, M.M.; Rong, Y.W.; Liu, R.; Li, H.H.; Ouyang, Q.; Chen, Q.S. An upconversion nanosensor for rapid and sensitive detection of tetracycline in food based on magnetic-field-assisted separation. Food Chem. 2022, 373, 131497. [Google Scholar] [CrossRef] [PubMed]
- Persson, B.N.; Guo, J. Electroadhesion for soft adhesive pads and robotics: Theory and numerical results. Soft Matter 2019, 15, 8032–8039. [Google Scholar] [CrossRef]
- Li, D.; Guo, Y.; Gao, F. Structure design and positive kinematics analysis of medical pneumatic soft robot. In Proceedings of the Advances in Mechanical Design ICMD2017; Springer: Singapore, 2018; pp. 1257–1271. [Google Scholar]
- Fan, J.Z.; Zhang, W.; Kong, P.C.; Cai, H.-G.; Liu, G.-F. Design and dynamic model of a frog-inspired swimming robot powered by pneumatic muscles. Chin. J. Mech. Eng. Eng. 2017, 30, 1123–1132. [Google Scholar] [CrossRef]
- Lokhande, S.; Malarkodi, A.; Latha, G.; Srinivasan, S. Autonomous detection, localization and tracking of ships by underwater acoustic sensing using vector sensor array. Appl. Ocean Res. 2025, 154, 104389. [Google Scholar] [CrossRef]
- Lin, H.; Yan, S.; Song, B.T.; Wang, Z.; Sun, L. Discrimination of aged rice using colorimetric sensor array combined with volatile organic compounds. J. Food Process Eng. 2019, 42, e13037. [Google Scholar] [CrossRef]
- Han, F.K.; Huang, X.Y.; Teye, E. Novel prediction of heavy metal residues in fish using a low-cost optical electronic tongue system based on colorimetric sensors array. J. Food Process Eng. 2019, 42, e12983. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.; Shen, C.; Liu, H.; Huang, J.; Tian, K.; Tang, Z. Review of the field environmental sensing methods based on multi-sensor information fusion technology. Int. J. Agric. Biol. Eng. 2024, 17, 1–13. [Google Scholar] [CrossRef]
- Chen, K.W.; Li, T.; Yan, T.J.; Xie, F.; Feng, Q.C.; Zhu, Q.Z.; Zhao, C.J. A Soft Gripper Design for Apple Harvesting with Force Feedback and Fruit Slip Detection. Agriculture 2022, 12, 1802. [Google Scholar] [CrossRef]
- Zhang, H.W.; Ji, W.; Xu, B.; Yu, X.W. Optimizing Contact Force on an Apple Picking Robot End-Effector. Agriculture 2024, 14, 996. [Google Scholar] [CrossRef]
- Zhou, K.; Xia, L.; Liu, J.; Qian, M.; Pi, J. Design of a flexible end-effector based on characteristics of tomatoes. Int. J. Agric. Biol. Eng. 2022, 15, 13–24. [Google Scholar] [CrossRef]
- Wang, H.; Gu, J.; Wang, M. A review on the application of computer vision and machine learning in the tea industry. Front. Sustain. Food Syst. 2023, 7, 1172543. [Google Scholar] [CrossRef]
- Yan, B. A Real-Time Apple Targets Detection Method for Picking Robot Based on Improved YOLOv5. Remote Sens. 2021, 13, 1619. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Zou, X.; Shi, J.; Zhai, X.; Liu, L.; Li, Z.; Holmes, M.; Gong, Y.; Povey, M.; et al. A visual indicator based on curcumin with high stability for monitoring the freshness of freshwater shrimp, Macrobrachium rosenbergii. J. Food Eng. 2021, 292, 110290. [Google Scholar] [CrossRef]
- Tang, Z.Q.; Heung, H.L.; Tong, K.Y.; Li, Z. Model-based online learning and adaptive control for a “human-wearable soft robot” integrated system. Int. J. Robot Res. 2021, 40, 256–276. [Google Scholar] [CrossRef]
- Caldwell, D.G.; Medrano-Cerda, G.A.; Goodwin, M. Control of pneumatic muscle actuators. IEEE Contr. Syst. Mag. 1995, 15, 40–48. [Google Scholar] [CrossRef]
- Ibarz, J.; Tan, J.; Finn, C.; Kalakrishnan, M.; Pastor, P.; Levine, S. How to train your robot with deep reinforcement learning: Lessons we have learned. Int. J. Robot Res. 2021, 40, 698–721. [Google Scholar] [CrossRef]
- Chen, T.; Yang, X.; Zhang, B.; Li, J.; Pan, J.; Wang, Y. Scale-inspired programmable robotic structures with concurrent shape morphing and stiffness variation. Sci. Robot. 2024, 9, eadl0307. [Google Scholar] [CrossRef]
- Zhang, F.; Teng, S.; Wang, Y.F.; Guo, Z.J.; Wang, J.J.; Xu, R.L. Design of bionic goat quadruped robot mechanism and walking gait planning. Int. J. Agric. Biol. Eng. 2020, 13, 32–39. [Google Scholar] [CrossRef]
- Pi, J.; Liu, J.; Zhou, K.H.; Qian, M.Y. An Octopus-Inspired Bionic Flexible Gripper for Apple Grasping. Agriculture 2021, 11, 1014. [Google Scholar] [CrossRef]
- Zolfagharian, A.; Picken, P.; Bodaghi, M.; Fard, M.; Rolfe, B. Additive Manufacturing of Composite Foam Metamaterial Springs for Vibration Isolation. Adv. Eng. Mater. 2023, 25, 2300356. [Google Scholar] [CrossRef]
- Zolfagharian, A.; Jarrah, H.R.; Xavier, M.S.; Rolfe, B.; Bodaghi, M. Multimaterial 4D printing with a tunable bending model. Smart Mater. Struct. 2023, 32, 065001. [Google Scholar] [CrossRef]
- Zolfaghari, A.; Purrouhani, M.R.; Zolfagharian, A. A response surface methodology study on 4D printing for layered PLA/TPU structures. Prog. Addit. Manuf. 2025, 10, 159–170. [Google Scholar] [CrossRef]
| Year of Publication | Regulation Mode | Stiffness Control Range | Impact of Propulsion Performance |
|---|---|---|---|
| 1999 [120] | Replace the springs | 3.1 times | Swim speed increased by about 39% |
| 2006 [121] | Replace the bionic tail chord | 4.7 times | Swim speed increased by about 60% |
| 2010 [123] | Add a limiter | 2.2 times | Displacement decreased by about 62% |
| 2011 [125] | Increase vertebrae | 5.3 times | Swim speed increased by about 70% |
| 2012 [140] | Replace the nylon inserts | 1.9 times | Thrust increased by about 96% |
| 2018 [64] | MACCEPA | 16 times | Swim speed increased by about 5.8 times |
| 2019 [129] | Preset spring tightness | ~ | Swim speed increased by about 59% |
| 2020 [130] | Replace the springs | ~ | Energy efficiency improved by about 89% |
| 2021 [141] | Replace the spring blades | 91 times | Swim speed increased by about 1.7 times |
| 2022 [142] | Replace the spring steel | 78.8 times | Swim speed increased by about 3.3 times |
| 2022 [143] | Replace the tail fin assembly | 35.1 times | Swim speed increased by about 87% |
| Maximum Pitch Angle | Fixed-Stiffness Velocity | Dynamic Stiffness Velocity | Fixed-Stiffness Propulsive Efficiency | Dynamic Stiffness Propulsive Efficiency | Velocity Improvement Magnitude | Efficiency Improvement Magnitude |
|---|---|---|---|---|---|---|
| 30° | 134 mm/s | 146 mm/s | 26.2% | 26.9% | 9.1% | 2.7% |
| 37.5° | 131 mm/s | 142 mm/s | 29.2% | 30.8% | 8.4% | 5.5% |
| 45° | 86.3 mm/s | 127 mm/s | 20.8% | 34.5% | 47.2% | 65.9% |
| Stiffness Modulation Method | Advantages | Disadvantages | Range of Stiffness Modulation |
|---|---|---|---|
| Offline Stiffness Modulation | Simplicity and reliability | Lack of real-time adaptability | Varies significantly depending on the components replaced, up to 91 times |
| Tension Against Stiffness | Continuous stiffness adjustment | Less precise at high frequencies Mechanical wear over time | About 3 to 5 times |
| Pneumatic/Hydraulic Stiffness | High environmental adaptability Rapid response time | Requires external power supply | Pneumatic: about 2 times Hydraulic: up to 30 times |
| Smart Material Stiffness Modulation | Lightweight and compact High energy efficiency | Limited stiffness range | Relatively narrow, between 1.5 and 3 times |
| Jamming Stiffness Modulation | High-stiffness range | Susceptibility to mechanical wear | Significant increase, up to 56 times |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, P.; Dong, B.; Gao, X.; Zhang, F.; Song, Y.; Liu, Z.; Zhang, Z. Variable-Stiffness Underwater Robotic Systems: A Review. J. Mar. Sci. Eng. 2025, 13, 1805. https://doi.org/10.3390/jmse13091805
Lu P, Dong B, Gao X, Zhang F, Song Y, Liu Z, Zhang Z. Variable-Stiffness Underwater Robotic Systems: A Review. Journal of Marine Science and Engineering. 2025; 13(9):1805. https://doi.org/10.3390/jmse13091805
Chicago/Turabian StyleLu, Peiwen, Busheng Dong, Xiang Gao, Fujian Zhang, Yunyun Song, Zhen Liu, and Zhongqiang Zhang. 2025. "Variable-Stiffness Underwater Robotic Systems: A Review" Journal of Marine Science and Engineering 13, no. 9: 1805. https://doi.org/10.3390/jmse13091805
APA StyleLu, P., Dong, B., Gao, X., Zhang, F., Song, Y., Liu, Z., & Zhang, Z. (2025). Variable-Stiffness Underwater Robotic Systems: A Review. Journal of Marine Science and Engineering, 13(9), 1805. https://doi.org/10.3390/jmse13091805