Comparative Analysis of Amino Acid Composition, Fatty Acid Profiles, and Genetic Diversity Among Three Populations of Penaeus semisulcatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Comprehensive Nutritional Analysis
2.3. Nutritional and Antioxidant Analysis
2.4. Genetic Diversity
2.5. Data Analysis
3. Results
3.1. Analysis of Overall Nutritional Composition
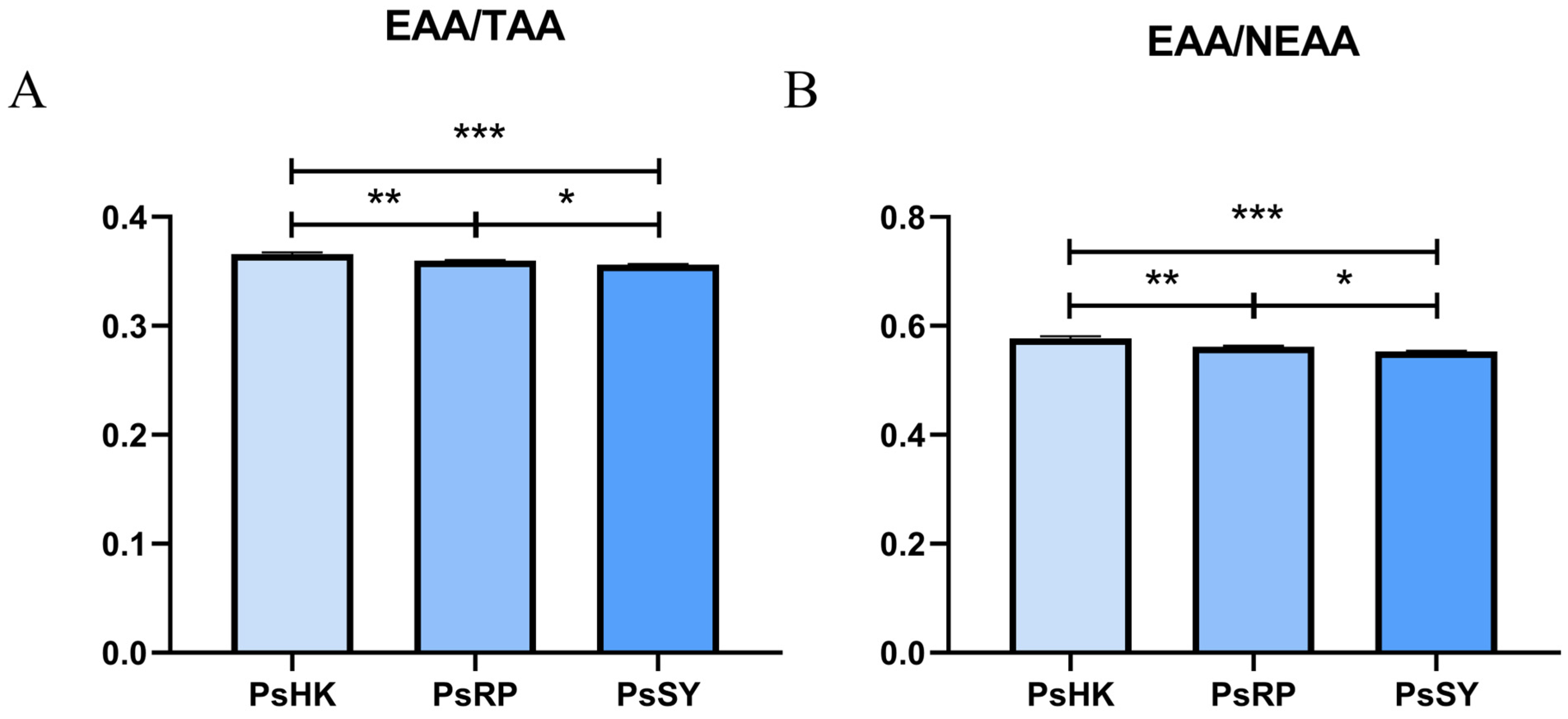
3.2. Analysis of Amino Acids
3.3. Analysis of Fatty Acids
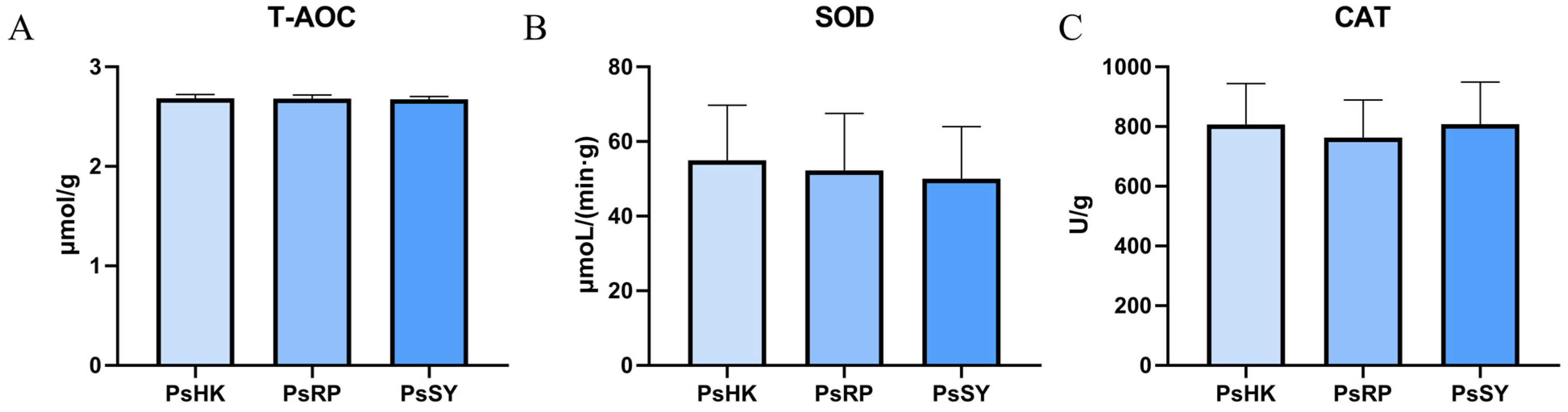
3.4. Analysis of Antioxidant Enzymes
3.5. Genetic Diversity Analysis
4. Discussion
4.1. Comparative Analysis of Fundamental Nutritional Components in P. semisulcatus Populations
4.2. Comparative Analysis of Amino Acid Profiles in P. semisulcatus Populations
4.3. Comparative Analysis of Fatty Acid Profiles Across P. semisulcatus Populations
4.4. Comparative Analysis of Antioxidant Enzyme Activity Across P. semisulcatus Populations
4.5. Genetic Diversity Comparison Among P. semisulcatus Populations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Halim, S.A.A.A.; Othman, A.S.; Akib, N.A.M.; Jamaludin, N.-A.; Esa, Y.; Nor, S.A.M. Mitochondrial Markers Identify a Genetic Boundary of the Green Tiger Prawn (Penaeus semisulcatus) in the Indo-Pacific Ocean. Zool. Stud. 2021, 60, 8. [Google Scholar] [CrossRef]
- Kumlu, M.; Eroldogan, O.T.; Aktas, M. Effects of Temperature and Salinity on Larval Growth, Survival and Development of Penaeus semisulcatus. Aquaculture 2000, 188, 167–173. [Google Scholar] [CrossRef]
- Rajkumar, M.; Pillai, S.L.; Rameshkumar, P.; Saravanan, R.; Thirumalaiselvan, S.; Jose, J.; Sobhana, K.S.; George, M.R. Reproductive Biology of Green Tiger Shrimp Penaeus semisulcatus De Haan, 1844 in Palk Bay, Southeast Coast of India. Reg. Stud. Mar. Sci. 2023, 66, 103161. [Google Scholar]
- Niamaimandi, N.; Aziz, A.; Siti Khalijah, D.; Che Roos, S.; Kiabi, B. Reproductive Biology of the Green Tiger Prawn (Penaeus semisulcatus) in Coastal Waters of Bushehr, Persian Gulf. ICES J. Mar. Sci. 2008, 65, 1593–1599. [Google Scholar]
- Alrashada, Y.N.; Tharwat, A.; Boqursain, A. Population Dynamics of the Green Tiger Prawn, Penaeus semisulcatus de Haan, 1844 in the Saudi Coast of the Arabian Gulf. Indian J. Anim. Res. 2019, 1–6. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Jiang, S.; Huang, J.; Jiang, S.; Yang, Q.; Yang, L.; Shi, J.; Zhou, F. A Comprehensive Study on Nutritional Quality, Physiological Enzyme Activity and Genetic Diversity in Six Populations of Penaeus Monodon. Aquacult. Int. 2024, 32, 10141–10157. [Google Scholar] [CrossRef]
- Li, Y.; Cao, S.; Jiang, S.; Huang, J.; Yang, Q.; Jiang, S.; Yang, L.; Zhou, F. Comparative Study of Nutritional Composition, Physiological Indicators, and Genetic Diversity in Litopenaeus vannamei from Different Aquaculture Populations. Biology 2024, 13, 722. [Google Scholar] [CrossRef]
- Meng, X.; Fu, Q.; Luan, S.; Luo, K.; Sui, J.; Kong, J. Genome Survey and High-Resolution Genetic Map Provide Valuable Genetic Resources for Fenneropenaeus chinensis. Sci. Rep. 2021, 11, 7533. [Google Scholar]
- El-Gendy, A.M.; El-Feky, F.; Mahmoud, N.H.; Elsebakhy, G.S. Evaluation of Nutritional Quality of Green Tiger Prawn, Penaeus semisulcatus from Land Fisheries (Alexandria) and Market (India). Egypt. J. Hosp. Med. 2018, 70, 924–934. [Google Scholar] [CrossRef]
- Tamadoni Jahromi, S.; Sofman Othman, A.; Rosazlina, R.; Pourmozaffar, S.; Gozari, M. Population Genetics of Penaeus semisulcatus from Persian Gulf and Oman Sea Using Newly Developed DNA Microsatellite Markers. Iran. J. Fish. Sci. 2021, 20, 157–178. [Google Scholar] [CrossRef]
- Shuhua, T.A.N.; Guizhong, W.; Qiongwu, L.I.N. Genetic Diversity of Two Wild Populations of Penaeus semisulcatus Revealed by RAPD Technique. Acta Ecol. Sin. 2006, 26, 3907–3911. [Google Scholar]
- GB 5009.3-2016; Determination of Moisture in Food. National Standards of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.4-2016; National Food Safety Standard—Determination of Ash in Food. National Standards of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.5-2016; National Standards for Food Safety—Determination of Proteins in Foods. National Standards of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.6-2016; National Food Safety Standard—Determination of Crude Fat in Food. National Standards of the People’s Republic of China: Beijing, China, 2016.
- GB/T 15672-2009; Determination of Total Sugar Content in Edible Fungi. National Standards of the People’s Republic of China: Beijing, China, 2009.
- GB 5009.124-2016; National Food Safety Standard—Determination of Amino Acids in Foods by Hydrochloric Acid Hydrolysis. National Standards of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.168-2016; National Food Safety Standard—Determination of Fatty Acids in Foods by Gas Chromatography. National Standards of the People’s Republic of China: Beijing, China, 2016.
- Jin, X.; Wang, X.; Tse, W.K.F.; Shi, Y. Homeostasis and Physiological Regulation in the Aquatic Animal during Osmotic Stress. Front. Physiol. 2022, 13, 977185. [Google Scholar] [CrossRef]
- Boyd, C.E.; Lawrence, J.M. The Mineral Composition of Several Fresh-Water Algae. 1967. Available online: https://seafwa.org/sites/default/files/journal-articles/BOYD-413.pdf (accessed on 1 March 2025).
- Lall, S.P.; Kaushik, S.J. Nutrition and Metabolism of Minerals in Fish. Animals 2021, 11, 2711. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z. Foundations for the Study of Structure and Function of Proteins. In Basics of Bioinformatics; Jiang, R., Zhang, X., Zhang, M.Q., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 303–336. ISBN 978-3-642-38950-4. [Google Scholar]
- Alhotan, R.A.; Pesti, G.M.; Billard, L. The Linear Relationship between True Protein and Nitrogen Contents of Feed and Food Ingredients: Calculating True Protein from a New Conversion Factor. Cogent Food Agric. 2024, 10, 2428821. [Google Scholar] [CrossRef]
- Ahmed, I.; Jan, K.; Fatma, S.; Dawood, M.A.O. Muscle Proximate Composition of Various Food Fish Species and Their Nutritional Significance: A Review. Anim. Physiol. Nutr. 2022, 106, 690–719. [Google Scholar] [CrossRef] [PubMed]
- Kainz, M.; Brett, M.T.; Arts, M.T. (Eds.) Lipids in Aquatic Ecosystems; Springer: New York, NY, USA, 2009; ISBN 978-0-387-88607-7. [Google Scholar]
- dos Santos Carvalho, C.; Fernandes, M.N. Effect of Copper on Liver Key Enzymes of Anaerobic Glucose Metabolism from Freshwater Tropical Fish Prochilodus lineatus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 437–442. [Google Scholar] [CrossRef]
- Dos Santos Carvalho, C.; Fernandes, M.N. Effects of Copper Toxicity at Different pH and Temperatures on the in Vitro Enzyme Activity in Blood and Liver of Fish, Prochilodus lineatus. Mol. Biol. Rep. 2019, 46, 4933–4942. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Li, H.; Jiang, X.; Ji, L.; Liu, T.; Sun, Y. Chemical and Quality Evaluation of Pacific White Shrimp Litopenaeus vannamei: Influence of Strains on Flesh Nutrition. Food Sci. Nutr. 2021, 9, 5352–5360. [Google Scholar] [CrossRef]
- Xu, L.; Yan, B. Nutritional Component Analysis and Quality Evaluation of Penaeus Japonicus. Food Sci. 2011, 32, 297–301. [Google Scholar]
- Li, Y.; Chen, J.; Jiang, S.; Yang, Q.; Yang, L.; Huang, J.; Shi, J.; Zhang, Y.; Lu, Z.; Zhou, F. A Comprehensive Assessment of Nutritional Value, Antioxidant Potential, and Genetic Diversity in Metapenaeus ensis from Three Different Populations. Biology 2024, 13, 838. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Cao, S.; Jiang, Z.; Jiang, S.; Yang, Q.; Yang, L.; Huang, J.; Shi, J.; Ma, Z. A Comprehensive Assessment of the Nutritional Value, Antioxidant Potential, and Genetic Diversity of Fenneropenaeus merguiensis from Three Different Regions in China. Biology 2024, 13, 1002. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Q.; Zhang, D.; Wei, S.; Sun, Q.; Xia, Q.; Shi, W.; Ji, H.; Liu, S. Comparison of the Proximate Composition and Nutritional Profile of Byproducts and Edible Parts of Five Species of Shrimp. Foods 2021, 10, 2603. [Google Scholar] [CrossRef]
- Wang, J. Comparision of Nutritional Composition in Muscle of Penaeus chinensis, Penaeus vannamei Boone and Penaeus japonicuss Bate. Food Sci. Technol. 2013, 38, 145–150. [Google Scholar]
- Li, P.; Yin, Y.-L.; Li, D.; Kim, S.W.; Wu, G. Amino Acids and Immune Function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar]
- Huang, M.; Gao, Q.; Yang, X.; Jiang, W.; Hao, L.; Yu, Y.; Tian, Y. Free Amino Acids in Response to Salinity Changes in Fishes: Relationships to Osmoregulation. Fish Physiol. Biochem. 2023, 49, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Functional Amino Acids in Nutrition and Health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef]
- Niu, J.; Hu, X.L.; Ip, J.C.; Ma, K.Y.; Tang, Y.; Wang, Y.; Qin, J.; Qiu, J.-W.; Chan, T.F.; Chu, K.H. Multi-Omic Approach Provides Insights into Osmoregulation and Osmoconformation of the Crab Scylla paramamosain. Sci. Rep. 2020, 10, 21771. [Google Scholar] [PubMed]
- Huo, D.; Zhang, L.; Yang, H.; Sun, L. Adaptation to Hypoxic Stress Involves Amino Acid Metabolism: A Case in Sea Cucumber. Environ. Pollut. 2023, 330, 121766. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.; Hildebrandt, T.M. The Role of Amino Acid Metabolism During Abiotic Stress Release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, H.; Zou, Z.; Yao, H.; Lin, Z.; Dong, Y. Effects of Medium-and Long-Term High-Salinity Environments on Free Amino Acid Content and Related Genes of Sinonovacula constricta. Front. Mar. Sci. 2024, 11, 1368952. [Google Scholar] [CrossRef]
- Houten, S.M.; Violante, S.; Ventura, F.V.; Wanders, R.J.A. The Biochemistry and Physiology of Mitochondrial Fatty Acid β-Oxidation and Its Genetic Disorders. Annu. Rev. Physiol. 2016, 78, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X.; Weylandt, K.H. Modulation of Inflammatory Cytokines by Omega-3 Fatty Acids. In Lipids in Health and Disease; Quinn, P.J., Wang, X., Eds.; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2008; Volume 49, pp. 133–143. ISBN 978-1-4020-8830-8. [Google Scholar]
- Fritsche, K.L. The Science of Fatty Acids and Inflammation. Adv. Nutr. 2015, 6, 293S–301S. [Google Scholar] [PubMed]
- Rashid, M.A.; Haque, M.; Akbar, M. Role of Polyunsaturated Fatty Acids and Their Metabolites on Stem Cell Proliferation and Differentiation. In The Benefits of Natural Products for Neurodegenerative Diseases; Essa, M.M., Akbar, M., Guillemin, G., Eds.; Advances in Neurobiology; Springer International Publishing: Cham, Switzerland, 2016; Volume 12, pp. 367–380. ISBN 978-3-319-28381-4. [Google Scholar]
- Cao, X.; Xia, J.; Zhou, Y.; Wang, Y.; Xia, H.; Wang, S.; Liao, W.; Sun, G. The Effect of Mufa-Rich Food on Lipid Profile: A Meta-Analysis of Randomized and Controlled-Feeding Trials. Foods 2022, 11, 1982. [Google Scholar] [CrossRef]
- Calder, P.C. N-3 Fatty Acids, Inflammation and Immunity: New Mechanisms to Explain Old Actions. Proc. Nutr. Soc. 2013, 72, 326–336. [Google Scholar] [PubMed]
- Lee-Okada, H.-C.; Xue, C.; Yokomizo, T. Recent Advances on the Physiological and Pathophysiological Roles of Polyunsaturated Fatty Acids and Their Biosynthetic Pathway. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2025, 1870, 159564. [Google Scholar]
- Zhang, L.; Zhao, M.; Wang, Y. A Review on Superoxide Dismutases of Hydrobios. 2012, pp. 800–804. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20123388727 (accessed on 1 March 2025).
- Attia, H.G.; El-Morshedy, S.M.; Nagy, A.M.; Ibrahim, A.M.; Aleraky, M.; Abdelrahman, S.S.; Osman, S.M.; Alasmari, S.M.; El Raey, M.A.; Abdelhameed, M.F. Citrus Clementine Peel Essential Oil Ameliorates Potassium Dichromate-Induced Lung Injury: Insights into the PI3K/AKT Pathway. Metabolites 2024, 14, 68. [Google Scholar] [CrossRef]
- Silvestrini, A.; Meucci, E.; Ricerca, B.M.; Mancini, A. Total Antioxidant Capacity: Biochemical Aspects and Clinical Significance. Int. J. Mol. Sci. 2023, 24, 10978. [Google Scholar] [CrossRef]
- Carmo De Carvalho E Martins, M.D.; Martins; Da Silva Santos Oliveira, A.S.; Da Silva, L.A.A.; Primo, M.G.S.; De Carvalho Lira, V.B. Biological Indicators of Oxidative Stress [Malondialdehyde, Catalase, Glutathione Peroxidase, and Superoxide Dismutase] and Their Application in Nutrition. In Biomarkers in Nutrition; Patel, V.B., Preedy, V.R., Eds.; Biomarkers in Disease: Methods, Discoveries and Applications; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–25. ISBN 978-3-030-81304-8. [Google Scholar]
- Tan, S.H.; Deng, X.Y.; Jiang, W.M.; He, F.L. Effects of High Level Chromium on Antioxidant Enzyme System in Gill and Hepatopancreas of Procambarus Clarkii. J. Agro-Environ. Sci. 2007, 23, 24–34. [Google Scholar]
- O’Connell, M.; Wright, J.M. Microsatellite DNA in Fishes. Rev. Fish Biol. Fish. 1997, 7, 331–363. [Google Scholar] [CrossRef]
- Matukumalli, L.K.; Lawley, C.T.; Schnabel, R.D.; Taylor, J.F.; Allan, M.F.; Heaton, M.P.; O’Connell, J.; Moore, S.S.; Smith, T.P.; Sonstegard, T.S. Development and Characterization of a High Density SNP Genotyping Assay for Cattle. PLoS ONE 2009, 4, e5350. [Google Scholar]
- Luo, W.; Luo, C.; Wang, M.; Guo, L.; Chen, X.; Li, Z.; Zheng, M.; Folaniyi, B.S.; Luo, W.; Shu, D. Genome Diversity of Chinese Indigenous Chicken and the Selective Signatures in Chinese Gamecock Chicken. Sci. Rep. 2020, 10, 14532. [Google Scholar] [PubMed]

| Population | Body Length (mm) | Weight (g) | Location |
|---|---|---|---|
| Haikou, Hainan Province (PsHK) | 155.4 ± 13.6 | 26.95 ± 5.46 | 20°03′ N, 110°37′ E |
| Raoping, Guangdong Province (PsRP) | 140.8 ± 12.3 | 24.37 ± 6.52 | 23°66′ N, 117°00′ E |
| Sanya, Hainan Province (PsSY) | 135.6 ± 16.7 | 22.39 ± 6.43 | 18°25′ N, 109°51′ E |
| General Nutrition Content | PsHK | PsRP | PsSY |
|---|---|---|---|
| Moisture (g/100 g) | 74.07 ± 0.71 a | 74.50 ± 0.70 a | 74.57 ± 0.55 a |
| Ash (g/100 g) | 1.77 ± 0.06 a | 2.07 ± 0.06 b | 1.80 ± 0.10 a |
| Crude Fat (g/100 g) | 0.83 ± 0.06 a | 0.80 ± 0.10 a | 0.83 ± 0.06 a |
| Crude Protein (g/100 g) | 22.37 ± 0.9 a | 21.77 ± 0.85 a | 22.13 ± 0.47 a |
| Total Sugar (%) | 30.92 ± 2.47 a | 30.70 ± 3.69 a | 29.92 ± 1.72 a |
| Content (g/100 g) | PsHK | PsRP | PsSY |
|---|---|---|---|
| Thr * | 0.58 ± 0.01 a | 0.58 ± 0.02 a | 0.54 ± 0.02 a |
| Val * | 0.73 ± 0.01 a | 0.72 ± 0.03 a | 0.65 ± 0.03 b |
| Met * | 0.35 ± 0.01 a | 0.33 ± 0.02 a b | 0.31 ± 0.01 b |
| Ile * | 0.67 ± 0.01 a | 0.66 ± 0.03 a b | 0.60 ± 0.03 b |
| Leu * | 1.22 ± 0.01 a | 1.20 ± 0.04 a b | 1.13 ± 0.05 b |
| Phe * | 0.62 ± 0.01 a | 0.59 ± 0.02 a | 0.55 ± 0.02 b |
| Lys * | 1.32 ± 0.02 a | 1.27 ± 0.05 a b | 1.21 ± 0.04 b |
| EAA | 5.49 ± 0.05 a | 5.36 ± 0.19 a b | 4.99 ± 0.18 b |
| Asp @ | 1.54 ± 0.02 a | 1.51 ± 0.06 a | 1.39 ± 0.05 b |
| Glu @ | 2.30 ± 0.03 a | 2.24 ± 0.08 a | 2.07 ± 0.08 b |
| Gly @ | 1.13 ± 0.01 a | 1.42 ± 0.05 b | 1.47 ± 0.06 b |
| Ala @ | 0.91 ± 0.01 a | 0.90 ± 0.03 a | 0.83 ± 0.03 b |
| DAA | 5.89 ± 0.06 a | 6.08 ± 0.22 a | 5.77 ± 0.21 a |
| His & | 0.30 ± 0.01 a | 0.29 ± 0.02 a | 0.26 ± 0.01 b |
| Arg & | 1.65 ± 0.03 a | 1.68 ± 0.05 a | 1.63 ± 0.05 a |
| SEAA | 1.95 ± 0.03 a | 1.98 ± 0.07 a | 1.89 ± 0.06 a |
| Ser | 0.49 ± 0.01 a | 0.48 ± 0.01 a | 0.46 ± 0.03 a |
| Cys | 0.11 ± 0.01 a | 0.11 ± 0.02 a | 0.10 ± 0.01 a |
| Tyr | 0.30 ± 0.01 a | 0.24 ± 0.01 b | 0.28 ± 0.01 a |
| Pro | 0.80 ± 0.01 a | 0.63 ± 0.02 b | 0.51 ± 0.02 c |
| NEAA | 9.51 ± 0.15 a | 9.54 ± 0.31 a | 9.01 ± 0.32 a |
| TAA | 15.00 ± 0.20 a | 14.90 ± 0.50 a | 14.00 ± 0.50 a |
| Content (mg/100 g) | PsHK | PsRP | PsSY |
|---|---|---|---|
| C16:0 | 70.57 ± 2.99 a | 57.00 ± 2.17 b | 52.73 ± 2.66 b |
| C17:0 | 15.17 ± 0.67 a | 12.67 ± 0.51 b | 12.07 ± 0.64 b |
| C18:0 | 108.00 ± 4.55 a | 88.27 ± 3.31 b | 94.13 ± 4.74 b |
| C20:0 | 9.63 ± 0.35 a | 7.83 ± 0.31 b | 8.13 ± 0.40 b |
| C22:0 | 19.13 ± 0.81 a | 10.90 ± 0.36 b | 12.13 ± 0.58 b |
| Saturated fatty acids | 222.50 ± 9.37 a | 176.70 ± 6.65 b | 179.80 ± 9.03 b |
| C16:1 | 18.47 ± 0.76 a | 13.13 ± 0.50 b | 13.60 ± 0.70 b |
| C18:1n9c | 72.80 ± 3.06 a | 50.60 ± 1.87 b | 45.50 ± 2.54 b |
| Monounsaturated fatty acids | 91.27 ± 3.82 a | 63.73 ± 2.37 b | 59.10 ± 2.95 b |
| C18:2n6c | 11.23 ± 0.45 a | 8.67 ± 0.35 b | 6.80 ± 0.26 c |
| C20:2 | 4.70 ± 0.26 a | 4.60 ± 0.26 a | 3.40 ± 0.01 b |
| C20:4n6 | 43.70 ± 1.87 a | 35.80 ± 1.74 b | 41.50 ± 2.08 a |
| C20:5n3 (EPA) | 52.43 ± 2.05 a | 43.53 ± 1.72 b | 47.10 ± 2.29 b |
| C22:6n3 (DHA) | 70.73 ± 2.87 a | 61.73 ± 2.24 b | 56.63 ± 3.03 b |
| DHA + EPA | 123.20 ± 4.92 a | 105.3 ± 3.95 b | 103.70 ± 5.31 b |
| n-6ΣPUFA | 54.93 ± 2.31 a | 44.47 ± 2.08 b | 48.30 ± 2.34 b |
| Polyunsaturated fatty acids | 182.80 ± 7.33 a | 154.30 ± 6.37 b | 153.20 ± 9.60 b |
| C22:1n9 | 10.13 ± 0.55 a | 10.90 ± 0.72 a,b | 12.07 ± 0.64 b |
| Total fatty acids | 506.70 ± 20.92 a | 405.60 ± 15.99 b | 403.50 ± 22.21 b |
| Population | SNP Density (SNP/Kb) | Nucleotide (π) | Polymorphism Information Content (PIC) | Observed Heterozygosity (Ho) | Inbreeding Coefficient (FHOM) |
|---|---|---|---|---|---|
| PsHK | 2.109 | 4.50 × 10−4 ± 5.99 × 10−4 | 0.118 ± 0.114 | 0.120 ± 0.157 | 6.42 × 10−2 ± 5.67 × 10−2 |
| PsRP | 1.986 | 4.49 × 10−4 ± 5.92 × 10−4 | 0.129 ± 0.114 | 0.130 ± 0.157 | 9.20 × 10−2 ± 5.16 × 10−2 |
| PsSY | 1.541 | 3.37 × 10−4 ± 4.45 × 10−4 | 0.094 ± 0.096 | 0.118 ± 0.095 | 6.21 × 10−2 ± 3.99 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, J.; Jiang, Z.; Jiang, S.; Yang, Q.; Yang, L.; Huang, J.; Shi, J.; Ding, Y.; Liu, T.; et al. Comparative Analysis of Amino Acid Composition, Fatty Acid Profiles, and Genetic Diversity Among Three Populations of Penaeus semisulcatus. J. Mar. Sci. Eng. 2025, 13, 655. https://doi.org/10.3390/jmse13040655
Li Y, Chen J, Jiang Z, Jiang S, Yang Q, Yang L, Huang J, Shi J, Ding Y, Liu T, et al. Comparative Analysis of Amino Acid Composition, Fatty Acid Profiles, and Genetic Diversity Among Three Populations of Penaeus semisulcatus. Journal of Marine Science and Engineering. 2025; 13(4):655. https://doi.org/10.3390/jmse13040655
Chicago/Turabian StyleLi, Yundong, Juan Chen, Ziyi Jiang, Song Jiang, Qibin Yang, Lishi Yang, Jianhua Huang, Jianzhi Shi, Yangyang Ding, Tianmi Liu, and et al. 2025. "Comparative Analysis of Amino Acid Composition, Fatty Acid Profiles, and Genetic Diversity Among Three Populations of Penaeus semisulcatus" Journal of Marine Science and Engineering 13, no. 4: 655. https://doi.org/10.3390/jmse13040655
APA StyleLi, Y., Chen, J., Jiang, Z., Jiang, S., Yang, Q., Yang, L., Huang, J., Shi, J., Ding, Y., Liu, T., & Zhou, F. (2025). Comparative Analysis of Amino Acid Composition, Fatty Acid Profiles, and Genetic Diversity Among Three Populations of Penaeus semisulcatus. Journal of Marine Science and Engineering, 13(4), 655. https://doi.org/10.3390/jmse13040655






