Adaptive Responses in Byssal Growth and Shedding: Insights from Pteria penguin Under Thread Trimming and Non-Trimming Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Conditions
2.2. Byssal Thread Counts, Diameter Measure, and Trimming
2.3. Data Analysis
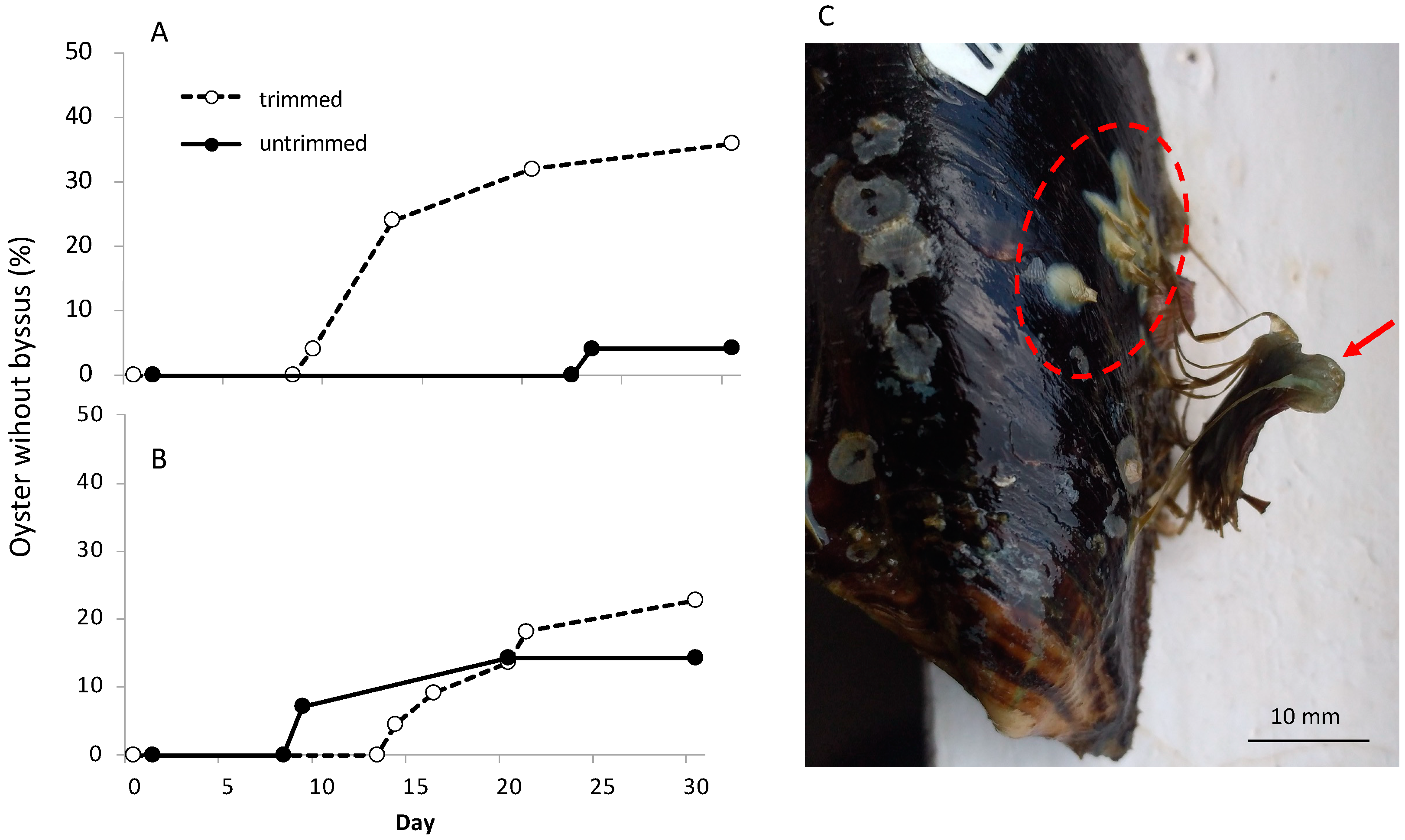
3. Results
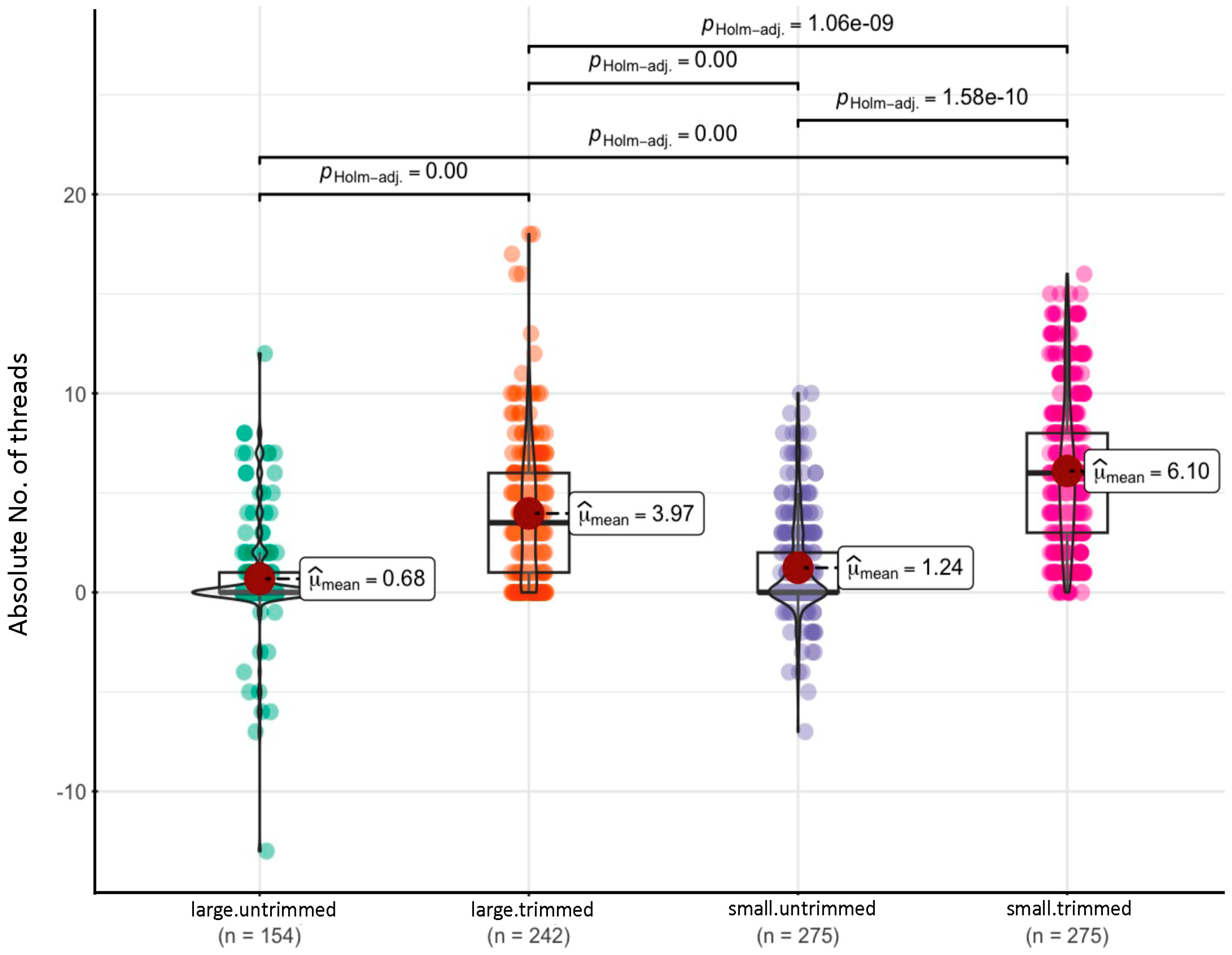
3.1. Survival Rate and Thread Counts in Oysters with Trimmed and Untrimmed Byssus
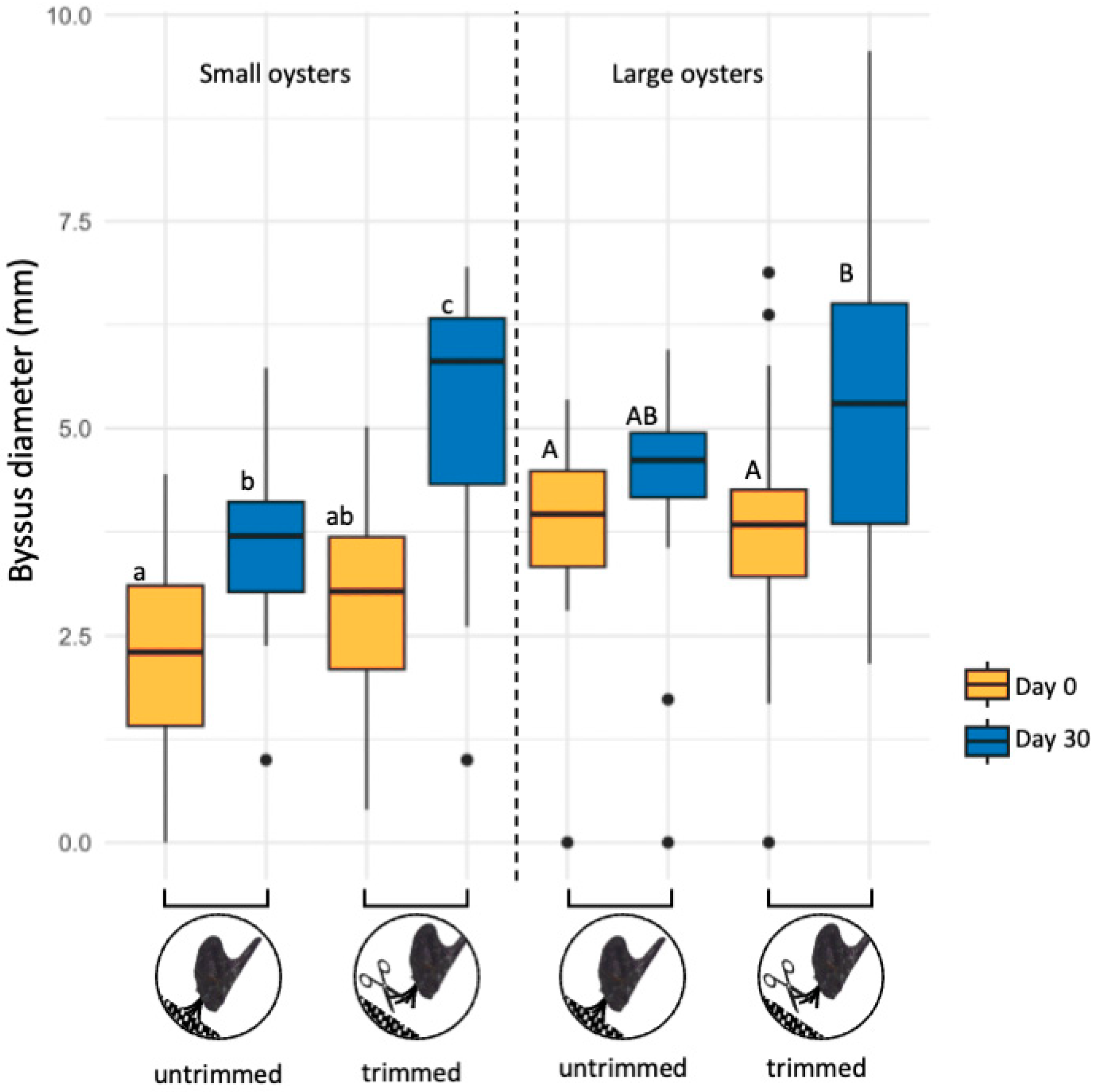
3.2. Byssal Diameter in Small and Large Oysters
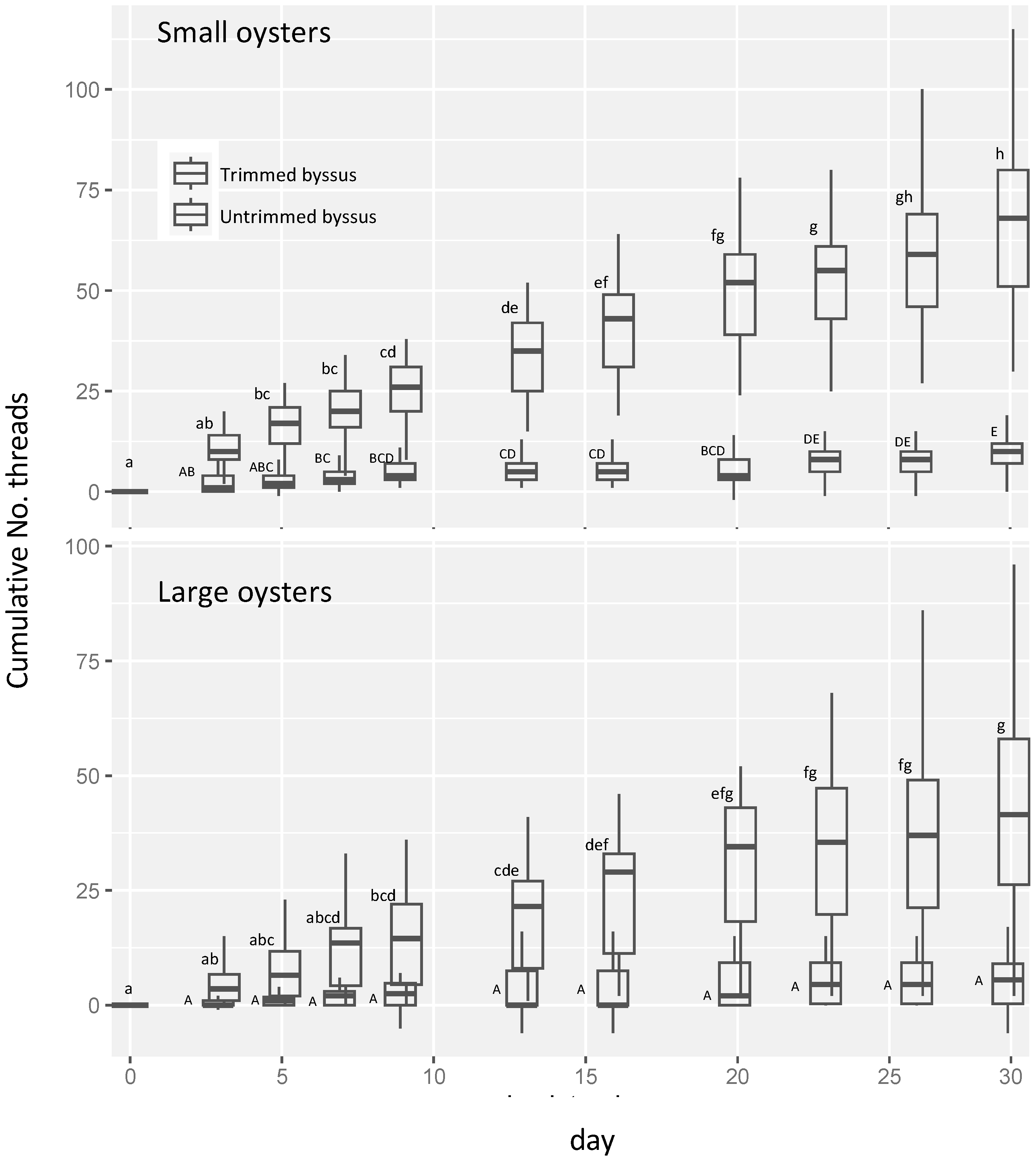
3.3. Byssal Shedding in Small and Large Oysters
4. Discussion
4.1. Thread Production in Oysters with Trimmed and Untrimmed Byssus
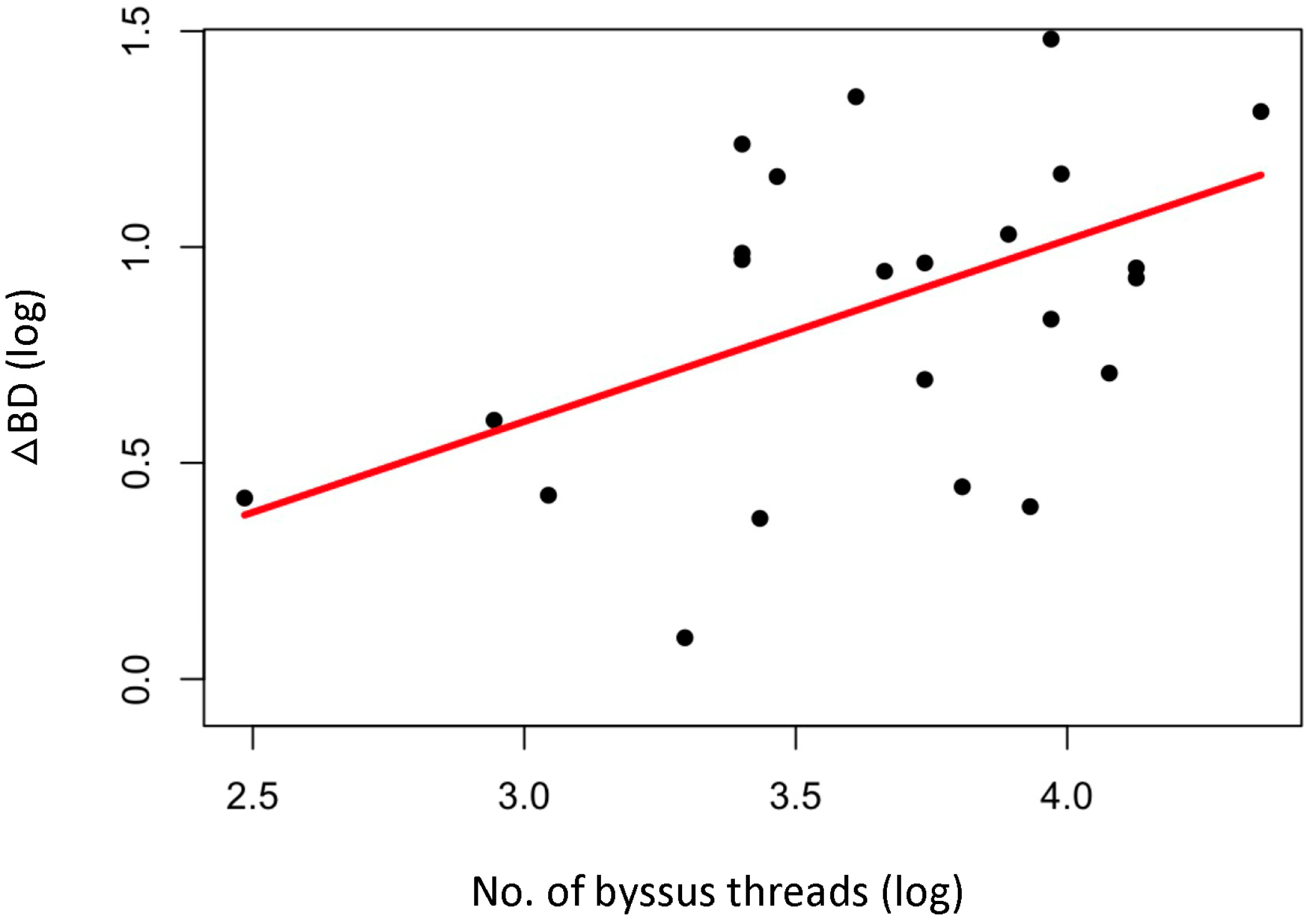
4.2. Byssal Diameter in Oysters with Trimmed and Untrimmed Byssus
4.3. Byssal Shedding in Oysters with Trimmed and Untrimmed Byssus
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inoue, K.; Onitsuka, Y.; Koito, T. Mussel Biology: From the Byssus to Ecology and Physiology, Including Microplastic Ingestion and Deep-Sea Adaptations. Fish. Sci. 2021, 87, 761–771. [Google Scholar] [CrossRef]
- Simmons, M.; Horbelt, N.; Sverko, T.; Scoppola, E.; Jackson, D.J.; Harrington, M.J. Invasive Mussels Fashion Silk-like Byssus via Mechanical Processing of Massive Horizontally Acquired Coiled Coils. Proc. Natl. Acad. Sci. USA 2023, 120, e2311901120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mao, F.; Xiao, S.; Yu, H.; Xiang, Z.; Xu, F.; Li, J.; Wang, L.; Xiong, Y.; Chen, M. Comparative Genomics Reveals Evolutionary Drivers of Sessile Life and Left-Right Shell Asymmetry in Bivalves. Genom. Proteom. Bioinform. 2022, 20, 1078–1091. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.T.; Tëmkin, I. Taxonomy and Phylogeny. In The Pearl Oyster; Elsevier: Amsterdam, The Netherlands, 2008; Volume 2008, pp. 37–75. [Google Scholar]
- Gervis, M.H.; Sims, N.A. The Biology and Culture of Pearl Oysters (Bivalvia pteriidae); WorldFish: Penang, Malaysia, 1992; Volume 21, p. 49. [Google Scholar]
- Taylor, J.J.; Rose, R.A.; Southgate, P.C. Byssus Production in Six Age Classes of the Silver-Lip Pearl Oyster, Pinctada maxima (Jameson). J. Shellfish Res. 1997, 16, 97–102. [Google Scholar]
- Chen, Y.; Han, C.; Chen, H.; Yan, J.; Zhan, X. The Mechanisms Involved in Byssogenesis in Pteria penguin under Different Temperatures. Sci. Total Environ. 2023, 905, 166894. [Google Scholar] [CrossRef]
- Li, S.; Liu, C.; Zhan, A.; Xie, L.; Zhang, R. Influencing Mechanism of Ocean Acidification on Byssus Performance in the Pearl Oyster Pinctada fucata. Environ. Sci. Technol. 2017, 51, 7696–7706. [Google Scholar] [CrossRef]
- Welladsen, H.M.; Heimann, K.; Southgate, P.C. The Effects of Exposure to Near-Future Levels of Ocean Acidification on Activity and Byssus Production of the Akoya Pearl Oyster, Pinctada Fucata. J. Shellfish Res. 2011, 30, 85–88. [Google Scholar] [CrossRef]
- O’Connor, W.A.; Lawler, N.F. Salinity and Temperature Tolerance of Embryos and Juveniles of the Pearl Oyster, Pinctada imbricata Röding. Aquaculture 2004, 229, 493–506. [Google Scholar] [CrossRef]
- Vasquez, H.E.; Xing, Z.; Zhan, X.; Gu, Z.; Wang, A. Byssal Re-attachment Behavior in the Winged Pearl Oyster Pteria penguin in Response to Low Salinity Levels. J. World Aquac. Soc. 2021, 52, 457–465. [Google Scholar] [CrossRef]
- Kishore, P.; Hunter, J.; Zeng, C.; Southgate, P.C. The Effects of Different Culture Apparatuses and Current Velocities on Byssus Production by the Black-Lip Pearl Oyster, Pinctada margaritifera. Aquaculture 2014, 434, 74–77. [Google Scholar] [CrossRef]
- Vasquez, H.E.; Zheng, X.; Zhan, X.; Gu, Z.; Wang, A. Byssus Growth in Winged Pearl Oyster Pteria penguin (Röding, 1798). J. Shellfish Res. 2018, 37, 515–519. [Google Scholar] [CrossRef]
- Sivasundarampillai, J.; Youssef, L.; Priemel, T.; Mikulin, S.; Eren, E.D.; Zaslansky, P.; Jehle, F.; Harrington, M.J. A Strong Quick-Release Biointerface in Mussels Mediated by Serotonergic Cilia-Based Adhesion. Science 2023, 382, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Dharmaraj, S.; Kandasamy, D.; Alagarswami, K. Some Aspects of Physiology of Pearl Oyster. CMFRI Bull.-Pearl Cult. 1987, 39, 21–28. [Google Scholar]
- Giraldes, B.W.; Leitão, A.; Smyth, D. The Benthic Sea-Silk-Thread Displacement of a Sessile Bivalve, Pinctada imbricata radiata (Leach, 1819) in the Arabian-Persian Gulf. PLoS ONE 2019, 14, e0215865. [Google Scholar] [CrossRef]
- Nicastro, K.; Zardi, G.; McQuaid, C. Movement Behaviour and Mortality in Invasive and Indigenous Mussels: Resilience and Resistance Strategies at Different Spatial Scales. Mar. Ecol. Prog. Ser. 2008, 372, 119–126. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Kong, L.; Yu, H.; Zheng, X. Identifying the True Oysters (Bivalvia: Ostreidae) with Mitochondrial Phylogeny and Distance-based DNA Barcoding. Mol. Ecol. Resour. 2011, 11, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Johnston, W.; Gordon, S.E.; Wingfield, M.; Halafihi, T.; Southgate, P.C. Influence of Production Method on the Profitability of Mabé Pearl Farming Using Traditional and Research-Informed Nucleus Implanting Practices with the Winged Pearl Oyster, Pteria penguin. Aquaculture 2022, 546, 737280. [Google Scholar] [CrossRef]
- Nur, I.; Mushaffa, W.O.; Hamzah, M. Effect of Number of Nuclei and Nucleus Position on Shell Growth and Mabé Pearl Coating in Pteria penguin Cultured in Coastal Waters of Southeast Sulawesi, Indonesia. J. Shellfish Res. 2020, 39, 345. [Google Scholar] [CrossRef]
- Kishore, P.; Southgate, P.C. A Detailed Description of Pearl-Sac Development in the Black-Lip Pearl Oyster, Pinctada margaritifera (Linnaeus 1758). Aquac. Res. 2016, 47, 2215–2226. [Google Scholar] [CrossRef]
- Tëmkin, I. Morphological Perspective on the Classification and Evolution of Recent Pterioidea (Mollusca: Bivalvia). Zool. J. Linn. Soc. 2006, 148, 253–312. [Google Scholar] [CrossRef]
- Vasquez, H.E.; Zheng, X.; Gu, Z.; Wang, A. Relationships between Shell Morphological Traits and the Byssus Dimensions in the Winged Pearl Oyster Pteria penguin (Röding, 1798) Cultivated in Sanya, Hainan Island, China. J. Shellfish Res. 2017, 36, 669–676. [Google Scholar] [CrossRef]
- Patil, I. Visualizations with Statistical Details: The “ggstatsplot” Approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer New York: New York, NY, USA, 2016; ISBN 978-0-387-98140-6. [Google Scholar]
- Ogle, D.; Doll, J.; Wheeler, A.; Dinno, A. Package: FSA, version 0.9.6; FSA: Simple Fisheries Stock Assessment Methods; fishR Core Team: Vienna, Austria, 2025.
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2021. Available online: https://www.R-Project.org/ (accessed on 1 December 2024).
- Pasche, D.; Horbelt, N.; Marin, F.; Motreuil, S.; Fratzl, P.; Harrington, M.J. Self-Healing Silk from the Sea: Role of Helical Hierarchical Structure in Pinna nobilis Byssus Mechanics. Soft Matter 2019, 15, 9654–9664. [Google Scholar] [CrossRef]
- Harrington, M.J.; Waite, J.H. Holdfast Heroics: Comparing the Molecular and Mechanical Properties of Mytilus californianus Byssal Threads. J. Exp. Biol. 2007, 210, 4307–4318. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, A.; Bertinetti, L.; Fratzl, P.; Harrington, M.J. Cooperative Behavior of a Sacrificial Bond Network and Elastic Framework in Providing Self-Healing Capacity in Mussel Byssal Threads. J. Struct. Biol. 2016, 196, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xia, Z.; Chen, Y.; Gao, Y.; Zhan, A. Byssus Structure and Protein Composition in the Highly Invasive Fouling Mussel Limnoperna fortunei. Front. Physiol. 2018, 9, 418. [Google Scholar] [CrossRef]
- Xu, X.; Yang, K.; Liu, Y.; Deng, Y.; Zhao, L. Heatwaves Hinder Mussel Invasion by Weakening Byssus Production. Front. Mar. Sci. 2023, 10, 1239801. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Ni, J.-Y.; Huang, S.-H.; Cui, Q.-W.; Wang, Y.-Q.; Gu, Z.-Q.; Li, Y.-F. Hyposalinity Stress Reduces Mussel Byssus Secretion but Does Not Cause Detachment. Sci. Total Environ. 2024, 930, 172561. [Google Scholar] [CrossRef]
- Selin, N.I. The Byssus Morphology and Strength of Attachment to Substrate in the Bivalve Mollusk Arca boucardi Jousseaume, 1894 (Arcidae). Russ. J. Mar. Biol. 2021, 47, 403–406. [Google Scholar] [CrossRef]
- Ni, J.-Y.; Zhou, Y.; Wang, Y.-Q.; Huang, S.-H.; Cui, Q.-W.; Wang, W.-Y.; Yang, X.-Y.; Power, D.M.; Li, Y.-F. Examination of the Effects of Excess Microalgae Availability on the Disruption of Mussel Byssus Secretion. Aquaculture 2024, 590, 741106. [Google Scholar] [CrossRef]
- Padin, X.A.; Babarro, J.M.F.; Silva, E.; Longa Portabales, M.Á.; Calvo, S.; Nolasco, R. Variability in Strength of Byssus Attachment and Index Condition of Subtidal Mussels during the Maximum Growth Stage. Aquac. Res. 2021, 52, 3485–3497. [Google Scholar] [CrossRef]
- Rajagopal, S.; Velde, G.; Jenner, H.A.; Gaag, M.; Kempers, A.J. Effects of Temperature, Salinity and Agitation on Byssus Thread Formation of Zebra Mussel Dreissena polymorpha. Neth. J. Aquat. Ecol. 1996, 30, 187–195. [Google Scholar] [CrossRef]
- Roberts, E.A.; Newcomb, L.A.; McCartha, M.M.; Harrington, K.J.; LaFramboise, S.A.; Carrington, E.; Sebens, K.P. Resource Allocation to a Structural Biomaterial: Induced Production of Byssal Threads Decreases Growth of a Marine Mussel. Funct. Ecol. 2021, 35, 1222–1239. [Google Scholar] [CrossRef]
- Young, G.A. Byssus-Thread Formation by the Mussel Mytilus edulis: Effects of Environmental Factors. Mar. Ecol. Prog. Ser. 1985, 24, 261–271. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Han, C.; Ou, H.; Zhan, X. The Structure and Proteomic Analysis of Byssus in Pteria penguin: Insights into Byssus Evolution and Formation. J. Proteom. 2024, 307, 105267. [Google Scholar] [CrossRef]
- McCartney, M.A. Structure, Function and Parallel Evolution of the Bivalve Byssus, with Insights from Proteomes and the Zebra Mussel Genome. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200155. [Google Scholar] [CrossRef]
- Waite, J.H.; Harrington, M.J. Following the Thread: Mytilus Mussel Byssus as an Inspired Multi-Functional Biomaterial. Can. J. Chem. 2022, 100, 197–211. [Google Scholar] [CrossRef]
- Basso, L.; Vázquez-Luis, M.; García-March, J.R.; Deudero, S.; Alvarez, E.; Vicente, N.; Duarte, C.M.; Hendriks, I.E. The Pen Shell, Pinna nobilis. In Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 71, pp. 109–160. ISBN 978-0-12-803305-0. [Google Scholar]
- Pearce, T.; LaBarbera, M. Biomechanics of Byssal Threads Outside the Mytilidae: Atrina rigida and Ctenoides mitis. J. Exp. Biol. 2009, 212, 1449–1454. [Google Scholar] [CrossRef]
- Cheung, S.G.; Tong, P.Y.; Yip, K.M.; Shin, P.K.S. Chemical Cues from Predators and Damaged Conspecifics Affect Byssus Production in the Green-Lipped Mussel Perna viridis. Mar. Freshw. Behav. Physiol. 2004, 37, 127–135. [Google Scholar] [CrossRef]
- O’Donnell, M.J.; George, M.N.; Carrington, E. Mussel Byssus Attachment Weakened by Ocean Acidification. Nat. Clim. Change 2013, 3, 587–590. [Google Scholar] [CrossRef]
- Li, S. Dietary Exposure to nTiO2 Reduces Byssus Performance of Mussels under Ocean Warming. Sci. Total Environ. 2023, 881, 163499. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Quan, F.; Zhang, Y.; Cao, Y.; Zhang, K.; Li, W.; Luo, H.; Jia, Y.; Liao, Z.; Liu, X. Effect of Low-Salt on the Survival of Mussel Mytilus coruscus and Its Molecular Responses to Chronic Prolonged Low-Salt Stress. Aquaculture 2024, 585, 740689. [Google Scholar] [CrossRef]
- Harrington, M.J.; Gupta, H.S.; Fratzl, P.; Waite, J.H. Collagen Insulated from Tensile Damage by Domains That Unfold Reversibly: In Situ X-Ray Investigation of Mechanical Yield and Damage Repair in the Mussel Byssus. J. Struct. Biol. 2009, 167, 47–54. [Google Scholar] [CrossRef]
- Krauss, S.; Metzger, T.H.; Fratzl, P.; Harrington, M.J. Self-Repair of a Biological Fiber Guided by an Ordered Elastic Framework. Biomacromolecules 2013, 14, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Bayne, B.L. Evolution. In Developments in Aquaculture and Fisheries Science; Elsevier: Amsterdam, The Netherlands, 2017; Volume 41, pp. 47–87. [Google Scholar]
- Lurman, G.J.; Hilton, Z.; Ragg, N.L.C. Energetics of Byssus Attachment and Feeding in the Green-Lipped Mussel Perna canaliculus. Biol. Bull. 2013, 224, 79–88. [Google Scholar] [CrossRef]
- Roberts, E.A.; Carrington, E. Byssal Thread Attachment and Growth Are Not Correlated across Gradients of Temperature and Food Availability for Two Congeneric Mussel Species. Mar. Ecol. Prog. Ser. 2023, 704, 35–54. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasquez, H.E.; Wei, S.; Yang, G.; Wang, L.; Yu, P.; Dong, M.; Yuan, C.; Zheng, X. Adaptive Responses in Byssal Growth and Shedding: Insights from Pteria penguin Under Thread Trimming and Non-Trimming Conditions. J. Mar. Sci. Eng. 2025, 13, 874. https://doi.org/10.3390/jmse13050874
Vasquez HE, Wei S, Yang G, Wang L, Yu P, Dong M, Yuan C, Zheng X. Adaptive Responses in Byssal Growth and Shedding: Insights from Pteria penguin Under Thread Trimming and Non-Trimming Conditions. Journal of Marine Science and Engineering. 2025; 13(5):874. https://doi.org/10.3390/jmse13050874
Chicago/Turabian StyleVasquez, Hebert Ely, Shangkun Wei, Guoliang Yang, Lingfeng Wang, Peixuan Yu, Mingyue Dong, Chao Yuan, and Xing Zheng. 2025. "Adaptive Responses in Byssal Growth and Shedding: Insights from Pteria penguin Under Thread Trimming and Non-Trimming Conditions" Journal of Marine Science and Engineering 13, no. 5: 874. https://doi.org/10.3390/jmse13050874
APA StyleVasquez, H. E., Wei, S., Yang, G., Wang, L., Yu, P., Dong, M., Yuan, C., & Zheng, X. (2025). Adaptive Responses in Byssal Growth and Shedding: Insights from Pteria penguin Under Thread Trimming and Non-Trimming Conditions. Journal of Marine Science and Engineering, 13(5), 874. https://doi.org/10.3390/jmse13050874





