Abstract
In cryopreservation technology, the choice of cryoprotectant plays a crucial role in cell survival and function. Different types of cryoprotectants, each with unique protective mechanisms, mitigate cellular damage from ice crystal formation during freezing. This study investigated the effects of different types and concentrations of cryoprotectants on the cryopreservation efficacy of noble scallop Mimachlamys nobilis sperm. Six cryoprotectants were tested, including four permeable cryoprotectants (dimethyl sulfoxide (DMSO), ethylene glycerol (EG), propylene glycerol (PG), methanol (MET)) and two non-permeable cryoprotectants (trehalose (TRE), fetal bovine serum (FBS)). The results showed that permeable cryoprotectants, which penetrate the cell membrane, regulate the osmotic pressure inside and outside cells to reduce dehydration damage. Among them, 10% DMSO provided the best protection, significantly preserving sperm motility, velocity, and morphology. Non-permeable cryoprotectants, although unable to penetrate cells, stabilized the extracellular environment at higher concentrations (such as FBS). Additionally, MET and FBS exhibited enhanced protective effects with increasing concentration, indicating their potential in reducing sperm structural damage at higher concentrations. Morphological observations indicated that freezing caused varying degrees of structural damage to sperm, with flagellar integrity being crucial for motility. Overall, selecting an appropriate cryoprotectant and concentration is essential for the efficient cryopreservation of M. nobilis sperm, providing a valuable reference for conserving germplasm resources of marine species.
1. Introduction
The noble scallop (Mimachlamys nobilis), classified under the phylum Mollusca, class Lamellibranchia, order Pterioida, and family Pectinidae1, is recognized for its relatively large size and swift growth cycle [1]. Its adductor muscle, often referred to as “dried scallop”, holds substantial commercial importance [2]. In China, the cultivation of scallops has become a pillar industry driving coastal economic growth, with scallops standing among the country’s most prominent seafood exports [3]. Geographically, this species is chiefly found in Japan, in Indonesia, and along China’s southeastern shoreline [4]. Remarkably, M. nobilis sperm remain motile for over 40 h after activation by natural seawater, exhibiting high swimming speeds, earning them the designation “marathon-type sperm” [5].
In order to promote effective reproduction and enhance the economic benefits of M. nobilis, research on and the optimization of ultralow-temperature cryopreservation techniques for its sperm are critically important. Cryobiology was initiated in 1949 when Polge and Smith discovered that glycerol could be used for the low-temperature preservation of animal sperm [6]. Cryobiological principles suggest that nearly all biochemical activities in living organisms are enzyme-dependent, with enzyme activity decreasing progressively with temperature reduction [7]. Below a critical temperature, enzyme activity ceases temporarily, halting all metabolic processes and inducing a low-energy dormant state that enables long-term preservation [8]. Upon thawing, these processes can be reactivated. Cryopreservation at ultralow temperatures not only extends the storage time of sperm but also improves the success rate of artificial insemination [9]. However, while most studies have focused on optimal freezing temperatures and cooling rates, the selection and formulation of effective cryoprotectants and their concentrations remain unresolved challenges. Further research is needed to optimize cryoprotectant types and dosages to minimize cellular damage during freezing and thawing, thereby preserving sperm viability and motility.
Cryoprotectants play a crucial role in minimizing cryoinjury during cryopreservation by protecting the structural and functional integrity of cells. Based on their permeability to cell membranes, cryoprotectants are classified as permeating or non-permeating [10]. Permeating cryoprotectants exhibit high membrane penetration capabilities, allowing them to interact with intracellular water, reduce the concentration of electrolytes in unfrozen solutions, lower the freezing point, and inhibit ice crystal formation [11]. Their protective effect generally increases with concentration; however, at higher concentrations, they can exhibit cytotoxicity, necessitating the careful determination of safe concentration ranges [12]. Ideal permeating cryoprotectants possess high permeability and low toxicity. Common permeating cryoprotectants used in the cryopreservation of marine germplasm include dimethyl sulfoxide (DMSO), ethylene glycol (EG), propylene glycol (PG), and methanol (MET) [13]. Among these, DMSO is recognized as an effective cryoprotectant and is commonly used for bivalve sperm cryopreservation at concentrations between 5 and 15% [14].
Non-permeating cryoprotectants, on the other hand, excel at stabilizing cell membranes during freezing. They regulate the movement of intracellular and extracellular contents, maintaining the integrity and functionality of the membrane [15]. Common non-permeating cryoprotectants include trehalose (TRE), fetal bovine serum (FBS), glucose (GLC), polyethylene glycol (PEG), and fructose (FRU) [16]. Sugars interact with lipid structures on cell membranes, stabilizing and protecting their structure, while FBS mitigates the adverse effects of permeating ions. Non-permeating cryoprotectants can be used individually or in combination with permeating cryoprotectants [17]. However, there is limited research on cryoprotectants for M. nobilis sperm.
The effectiveness of cryoprotectant types and concentrations varies across species, which is a major reason for the lack of standardized cryoprotectant protocols. DMSO, MET, and EG are commonly used as effective permeating cryoprotectants in the cryopreservation of Pacific oyster (Crassostrea gigas) sperm [18]. DMSO and PG have been found suitable for the cryopreservation of Kumamoto oyster (Crassostrea sikamea) sperm [19]. A solution comprising 10–15% DMSO, 5% sucrose, and 10% egg yolk has been reported to be effective for the cryopreservation of Yesso scallop (Patinopecten yessoensis) sperm [20]. Additionally, mixtures of DMSO and various non-permeating cryoprotectants (e.g., FBS) have been successfully used in the cryopreservation of black-lip pearl oyster (Pinctada margaritifera) sperm [21].
This study aims to investigate the effects of different cryoprotectants and their concentrations on sperm motility, velocity, and ultrastructure in M. nobilis. By comprehensively assessing these parameters, this research seeks to reveal the physiological state of, damage to, and impact on sperm following ultralow-temperature freezing and thawing. These findings are anticipated to expand our understanding of cryobiology and reproductive biology in marine organisms, while offering scientific and technical support to address reproductive cycle constraints in artificial insemination. Ultimately, this could lead to higher scallop yields and greater economic returns.
2. Materials and Methods
2.1. Materials
In January 2024, M. nobilis specimens were obtained from Xincun Harbor in Lingshui Li Autonomous County, Hainan Province, China. Throughout the study, strict attention was given to stabilizing key environmental parameters—particularly temperature and salinity—to ensure the reliability of the experiment. During specimen collection, the water temperature remained at 25 ± 3 °C, while salinity was held at 35 ± 0.2 ‰, with both measurements continuously tracked by a PR730 series precision digital thermometer (Taian Panran Measurement and Control Technology Co., Ltd., Taian, China) and a SALTTEST11 meter from Eutech™ (Thermo Fisher Scientific, Waltham, MA, USA). An HK-HWJ-7200 aquaculture incubator (Guangzhou Huankong Agricultural Biotechnology Co., Ltd., Guangzhou, China) was used to maintain consistent temperatures throughout the experimental period, thus reducing environmental fluctuation impacts on sperm quality. Additionally, salinity was kept steady during sample processing to preserve uniform conditions.
2.2. Trait Data Collection
From a total of seventy M. nobilis specimens, three males displaying the most pronounced gonadal development were chosen. Their gonads were visibly enlarged, were milky white, and showed branching ducts, indicative of maturity. An examination of sperm development confirmed that these individuals were at an optimal reproductive stage (Table 1).

Table 1.
Experimental animal data.
2.3. Sperm Collection
Among the seventy M. nobilis specimens, three males with the most developed gonads were chosen. To expose these gonads, a sterile scalpel was used to incise one side of the adductor muscle, revealing a visceral mass largely covered by white or pale-yellow gonadal tissue. The gonads were then carefully separated, gently blotted with clean absorbent paper to remove residual seawater or tissue fluids, and subsequently weighed. Prior to sperm collection, all instruments (including collection tubes and pipettes) underwent either high-temperature sterilization or disinfection with 70% ethanol to prevent external contamination. Additionally, work surfaces and equipment were disinfected in a clean laboratory environment to minimize microbial interference.
During sample collection, operators wore disposable gloves and masks to prevent any direct contact between their skin or respiratory system and the specimens. The excised gonads were placed on a 450-mesh sieve cloth and finely minced with scissors before being rinsed in precooled Hank’s solution (supplied by Shantou Xilong Scientific Co., Ltd., Shantou, China; 99% analytical grade). Finally, the samples were maintained at 4 °C to preserve sperm quality and minimize external environmental influences.
Subsequently, the sperm obtained from these three individuals were pooled to form a homogeneous composite sample as the singular experimental unit for all subsequent assays.
2.4. Sperm Density Determination and Evaluation of Fresh Sperm Viability and Motility
During the activation phase, filtered natural seawater (0.25 µm pore size) served as the medium. The sperm suspension was combined with this seawater at a 1:10 ratio in a 15 mL conical tube and then vigorously agitated using a basic vortex oscillator (Thermo Fisher Scientific). A 5 µL aliquot of the resulting mixture was transferred to a sperm counting slide, where sperm activity was monitored via a Beionmed S3 analyzer (Shanghai Beion Medical Ltd., Shanghai, China) until most sperm ceased movement. All procedures were conducted at room temperature (25 ± 2 °C) on a 23 °C temperature-controlled platform. The analyzer provided measurements of sperm density, fresh sperm viability, and motility.
Subsequently, the sperm obtained from these three individuals were pooled to form a homogeneous composite sample as the singular experimental unit for all subsequent assays.
M. nobilis specimens with sperm motility exceeding 50% at 30 s post-activation were selected for further experiments. Each sample underwent three parallel tests, with three reliable data points recorded in each trial.
2.5. Cryoprotectant Solution Preparation
The diluent used in this experiment was selected through multiple preliminary trials, which identified Hank’s solution as the optimal diluent. As cryoprotectants, dimethyl sulfoxide (DMSO), ethylene glycol (EG), propylene glycol (PG), methanol (MET), trehalose (TRE), and fetal bovine serum (FBS) were chosen [22]. All cryoprotectants used in the experiment, including DMSO, EG, PG, MET, TRE, and FBS, were manufactured by Xilong Scientific Co., Ltd., with a purity of 99% (analytical grade). Each cryoprotectant was mixed with Hank’s solution to form the cryoprotective solution, achieving final concentrations of 5, 10, and 15% for DMSO, EG, PG, MET, TRE, and FBS (Table 2). Based on preliminary experiments that confirmed uniform sperm distribution and optimal cryopreservation performance, the prepared cryoprotectant solution was mixed with the sperm suspension at a consistent ratio of 10:1. Immediately after mixing, the solution was dispensed into 2 mL cryovials (each containing 1.5 mL) with all procedures performed on ice to form the experimental groups. Starting from the time of mixing, samples were equilibrated at 4 °C for 30 min and then transferred into a programmed freezer precooled to 4 °C. Each group was set up with three replicates.

Table 2.
Experimental cryoprotectant solution data.
2.6. Cooling and Thawing Procedures Configuration
In this experiment, a programmable freezing device (model CJ-L35, produced by Haier Biomedical Technology Co., Ltd., Chengdu, China) and an upright high-pressure liquid nitrogen container (model YDZ-125, also from Haier Biomedical Technology Co., Ltd.) were employed to cryopreserve the samples under controlled conditions. Through multiple preliminary tests, the optimal cooling procedure was identified: the temperature was lowered from 4 °C to −25 °C at 5 °C per min, held at −25 °C for 5 min, and then further reduced at 10 °C per min to −80 °C, where it was held for another 5 min. Prior to the loading of the sperm samples (both control and experimental), the freezing device was dried for 30 min and programmed to stabilize at 4 °C. Once the sperm samples reached the target temperature via the programmed protocol, they were swiftly transferred to the liquid nitrogen container.
Preliminary experiments demonstrated that a 38 °C water bath provided optimal thawing results. Therefore, both control and experimental samples were immersed in this 38 °C bath and gently swirled to achieve uniform thawing. After the ice in each cryovial had completely melted, the samples stood for approximately 10 s before being collected for subsequent analysis [23].
2.7. Assessment of Effect of Cryoprotectants on Motility and Kinematic Parameters of Sperm from M. nobilis
After thawing, sperm samples were mixed with sperm activation solution at a 1:10 ratio in a 15 mL conical tube. Subsequently, 5 µL of the mixture was placed in a sperm counting chamber for observation. A computer-assisted sperm analyzer was used to record sperm motility, curvilinear velocity, average path velocity, and straight-line velocity, with each group tested in triplicate. The CASA system settings were as follows: a maximum capture area of 80 im2, a frame rate of 300 f/s, and a sample drift parameter of 4 pm/s, with each field capturing between 300 and 400 sperm. These settings ensured the accurate and consistent measurement of sperm motion parameters for reliable subsequent analysis.
Subsequently, the sperm obtained from these three individuals were pooled to form a homogeneous composite sample as the singular experimental unit for all subsequent assays.
2.8. Assessment of Effect of Cryoprotectants on Morphology and Ultrastructure of Sperm
Based on the effects of different cryoprotectants on M. nobilis sperm, fresh sperm samples, as well as samples from the best and worst experimental conditions, were selected. A 1 mL aliquot of each sample was fixed with a solution of 4% paraformaldehyde and 1% glutaraldehyde (prepared using 50% glutaraldehyde 10 mL, 20 g paraformaldehyde, and 3.029 g Tris buffer, dissolved in 500 mL filtered seawater). The fixed samples were subjected to dehydration in a graded ethanol series, followed by the addition of isoamyl acetate. After critical point drying using a LEICA EM CPD 300 critical point dryer (produced by Leica Microsystems, Beijing, China) and coating with gold using an IB-5uj ion sputter coater, the morphology of the sperm was imaged and observed with a Phenom ProX G6 scanning electron microscope (manufactured by Phenom Scientific Instruments, Shanghai, China).
For ultrastructural observation, fresh sperm samples and those from the most and least favorable experimental conditions were initially rinsed with precooled 0.1 mol/L phosphate buffer at 4 °C. They were then immersed in 2.5% glutaraldehyde fixative under dark conditions at 4 °C for 4 h. After this fixative was discarded, the samples were placed in fresh 2.5% glutaraldehyde solution for overnight preservation at 4 °C. Next, they were rinsed three times in 0.1 mol/L phosphate buffer (15 min each), followed by fixation in 1% osmium tetroxide for 2 h. The osmium tetroxide was then removed, and the samples were again rinsed three times in 0.1 mol/L phosphate buffer (15 min each). Dehydration was performed using a graded ethanol series (30, 50, 70, 80, 90, and 100%) and ethanol–acetone mixtures (3:1, 1:1, and 1:3), and then washed twice in pure acetone (10 min each). After this, the samples were embedded in resin and subjected to high-temperature polymerization once the resin had fully solidified. Ultrathin sections (70–80 nm) were prepared using an ultramicrotome (UC Enuity ultramicrotome, Leica Microsystems, Beijing, China) and double-stained with 5% uranyl acetate and lead citrate. Finally, sperm ultrastructure images were captured using a Spectra Ultra S/TEM transmission electron microscope (Thermo Fisher Scientific).
Subsequently, the sperm obtained from these three individuals were pooled to form a homogeneous composite sample as the singular experimental unit for all subsequent assays.
2.9. Sperm Quality Assessment
After the recovery procedure, M. nobilis sperm were activated using the designated activation medium, and detailed observations were documented. Each sample was then analyzed using a sperm quality analyzer, capturing 400–600 sperm per measurement. Sperm vitality was determined by examining six randomly selected fields of view. Key parameters assessed for sperm quality included sperm vitality (combined percentage of progressive and non-progressive motility), curvilinear velocity (VCL), average path velocity (VAP), and straight-line velocity (VSL) [24].
Subsequently, the sperm obtained from these three individuals were pooled to form a homogeneous composite sample as the singular experimental unit for all subsequent assays.
2.10. Data Analysis
Sperm parameter data collected via the Computer-Assisted Sperm Analysis (CASA) system were analyzed using SPSS 27.0. Prior to the one-way ANOVA, arcsine transformation was applied to ensure adherence to normal distribution assumptions. Distribution tests were also performed to verify normality and the homogeneity of variances, confirming the appropriateness of the variance analysis. All necessary statistical assumptions were met, affirming the validity of the ANOVA results. Significance was set at p < 0.05, with p < 0.01 indicating a highly significant difference, and p < 0.001 denoting an extremely significant difference. Results are expressed as mean ± standard deviation (mean ± S.D). All graphs were created using Origin 2021.
3. Results
3.1. Effects of Cryoprotectants on Sperm Motility in M. nobilis
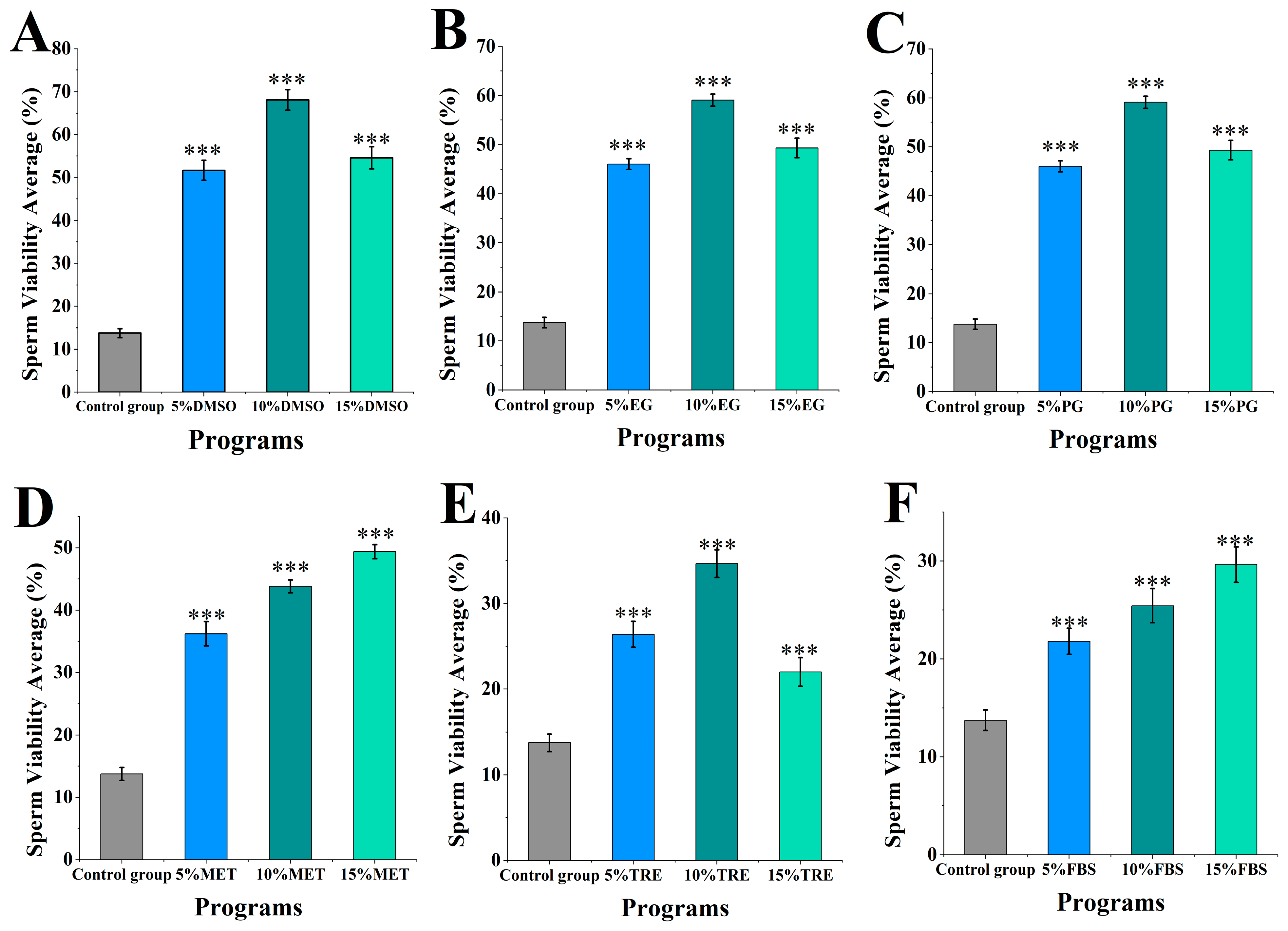
The motility of M. nobilis sperm after cryopreservation and thawing was assessed (Figure 1). In the control group without cryoprotectants, sperm motility was 13.76 ± 1.04%. Among the experimental groups with DMSO added as a cryoprotectant at concentrations ranging from 5 to 15% (Figure 1A), sperm motility was the highest, with 5% DMSO at 51.68 ± 2.36%, 10% DMSO at 69.10 ± 2.37%, and 15% DMSO at 54.59 ± 2.54%. Sperm motility in all DMSO groups was significantly higher than in the control group (p < 0.001) and superior to the other experimental groups. Notably, 10% DMSO achieved the highest motility, minimally affecting sperm compared to fresh sperm motility at 81.32 ± 2.48%.

Figure 1.
Sperm quality in different cryoprotectants at different concentrations. (A) Dimethyl sulfoxide (DMSO) at concentrations of 5%, 10%, and 15%; (B) Ethylene glycol (EG) at concentrations of 5%, 10%, and 15%; (C) Propylene glycol (PG) at concentrations of 5%, 10%, and 15%; (D) Methanol (MET) at concentrations of 5%, 10%, and 15%; (E) Trehalose (TRE) at concentrations of 5%, 10%, and 15%; (F) Fetal bovine serum (FBS) at concentrations of 5%, 10%, and 15% (*** p < 0.001).
In the groups with MET and FBS as cryoprotectants (Figure 1D,F), sperm motility displayed a positive correlation with increasing concentration. In the MET group, motility was 36.19 ± 1.95% for 5% MET, 43.81 ± 1.02% for 10% MET, and 49.38 ± 1.14% for 15% MET. As MET concentration increased, sperm motility significantly rose, with the 15% MET group displaying motility notably higher than the 5 and 10% groups (p < 0.001). In the FBS group, motility was 25.43 ± 1.33% for 5% FBS, 25.43 ± 1.76% for 10% FBS, and 29.65 ± 1.82% for 15% FBS. At 15% FBS, sperm motility was significantly higher than at 5 and 10% FBS (p < 0.001). Although MET and FBS provided varying protective effects, both demonstrated increased motility with rising concentrations.
In the other cryoprotectant groups (ethylene glycol, propylene glycol, and trehalose), sperm motility at all concentrations was also significantly higher than in the control group (p < 0.001) (Figure 1B,C,E), though cryopreservation effectiveness across these concentrations did not match that of DMSO. Overall, the effects of different cryoprotectants on sperm motility varied, with 10% DMSO being the most effective and minimally impacting sperm motility.
3.2. Impact of Cryoprotectants on Curvilinear Velocity of M. nobilis Sperm
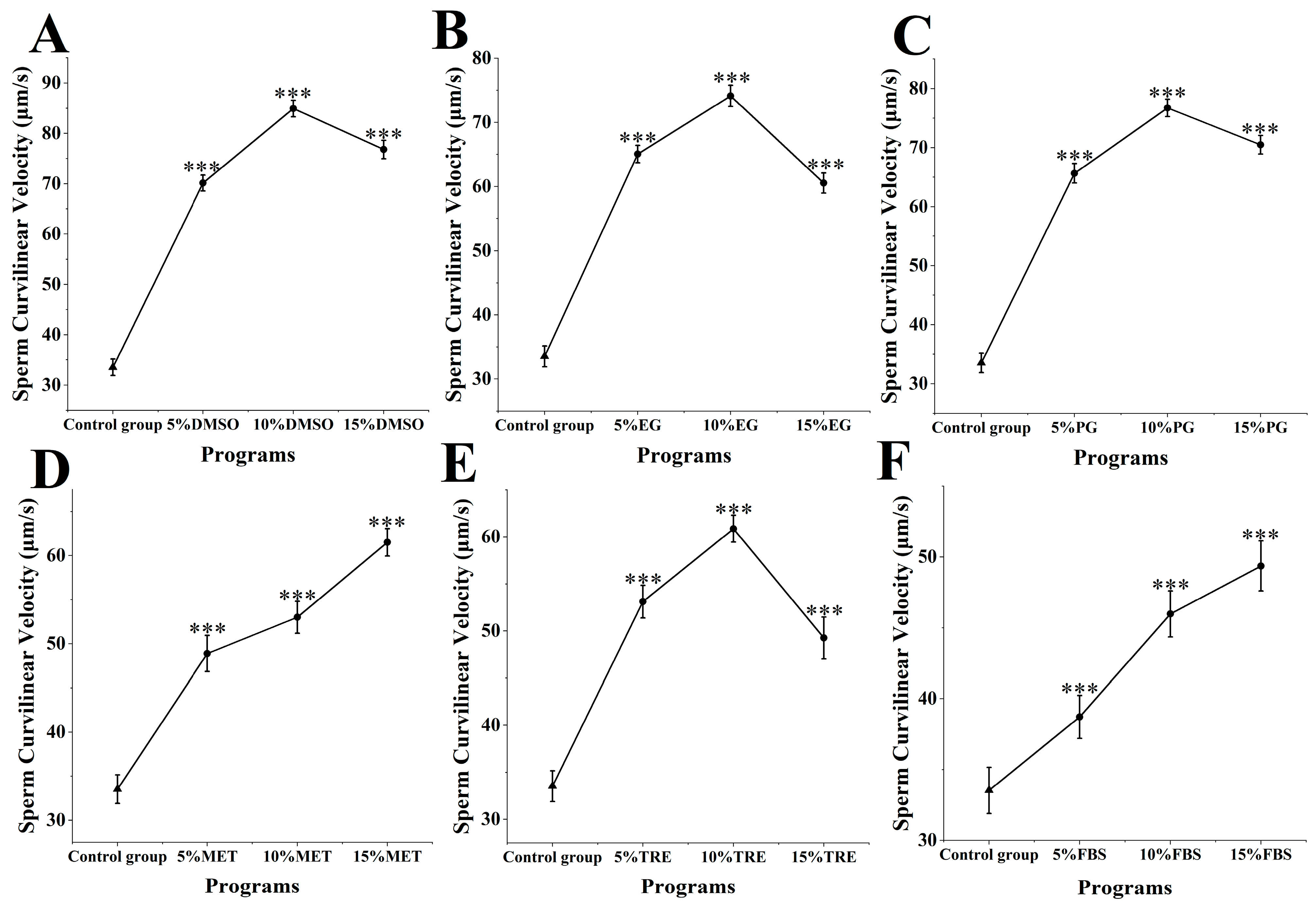
The curvilinear velocity of M. nobilis sperm after cryopreservation and thawing was assessed (Figure 2). In the control group without cryoprotectants, the curvilinear velocity was 33.53 ± 1.63 μm/s. When DMSO was introduced as a cryoprotectant, the sperm’s curvilinear velocity increased significantly, outperforming all other experimental groups. In particular, sperm treated with 10% DMSO exhibited the highest curvilinear velocity at 84.94 ± 1.64 μm/s, significantly higher than that of the control group (p < 0.001). This rate closely approximates the motility of fresh, untreated sperm at 103.64 ± 2.85 μm/s, indicating minimal impact on curvilinear velocity. Other DMSO concentrations also showed effective preservation, with 5 and 15% DMSO resulting in velocities of 70.13 ± 1.57 and 76.81 ± 1.82 μm/s, respectively, both significantly higher than those of the control group (p < 0.001).
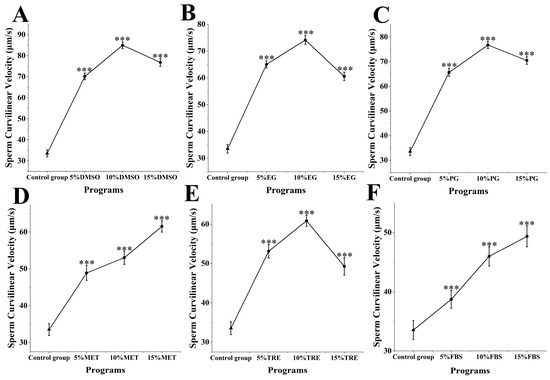
Figure 2.
Impact of cryoprotectants on curvilinear velocity of sperm from M. nobilis. (A) Dimethyl sulfoxide (DMSO) at concentrations of 5%, 10%, and 15%; (B) Ethylene glycol (EG) at concentrations of 5%, 10%, and 15%; (C) Propylene glycol (PG) at concentrations of 5%, 10%, and 15%; (D) Methanol (MET) at concentrations of 5%, 10%, and 15%; (E) Trehalose (TRE) at concentrations of 5%, 10%, and 15%; (F) Fetal bovine serum (FBS) at concentrations of 5%, 10%, and 15% (*** p < 0.001).
In the experimental groups with MET and FBS as cryoprotectants (Figure 2D,F), increased concentrations of MET and FBS were associated with proportional increases in curvilinear velocity. In the MET group, 5% MET achieved a velocity of 48.9 ± 2.05 μm/s, 10% MET reached 53.26 ± 1.81 μm/s, and 15% MET reached 61.52 ± 1.55 μm/s. As MET concentration increased, curvilinear velocity improved significantly, with the 15% MET group showing a significantly higher velocity than the 5 and 10% groups (p < 0.05). In the FBS group, the curvilinear velocities were 38.72 ± 1.52 μm/s for 5% FBS, 42.98 ± 1.68 μm/s for 10% FBS, and 49.37 ± 1.79 μm/s for 15% FBS, with the 15% FBS group significantly outperforming the 5 and 10% groups (p < 0.05). While the protective effects of MET and FBS varied, both demonstrated an upward trend in velocity with increasing concentration.
In the other cryoprotectant groups (ethylene glycol, propylene glycol, and trehalose), sperm curvilinear velocity at all concentrations was also significantly higher than in the control group (p < 0.001) (Figure 2B,C,E), though none of these concentrations matched the effectiveness of DMSO. Overall, the impact of different cryoprotectants on sperm curvilinear velocity varied, with 10% DMSO exhibiting the most notable effect and minimal impact on velocity.
3.3. Impact of Cryoprotectants on Average Path Velocity of M. nobilis Sperm
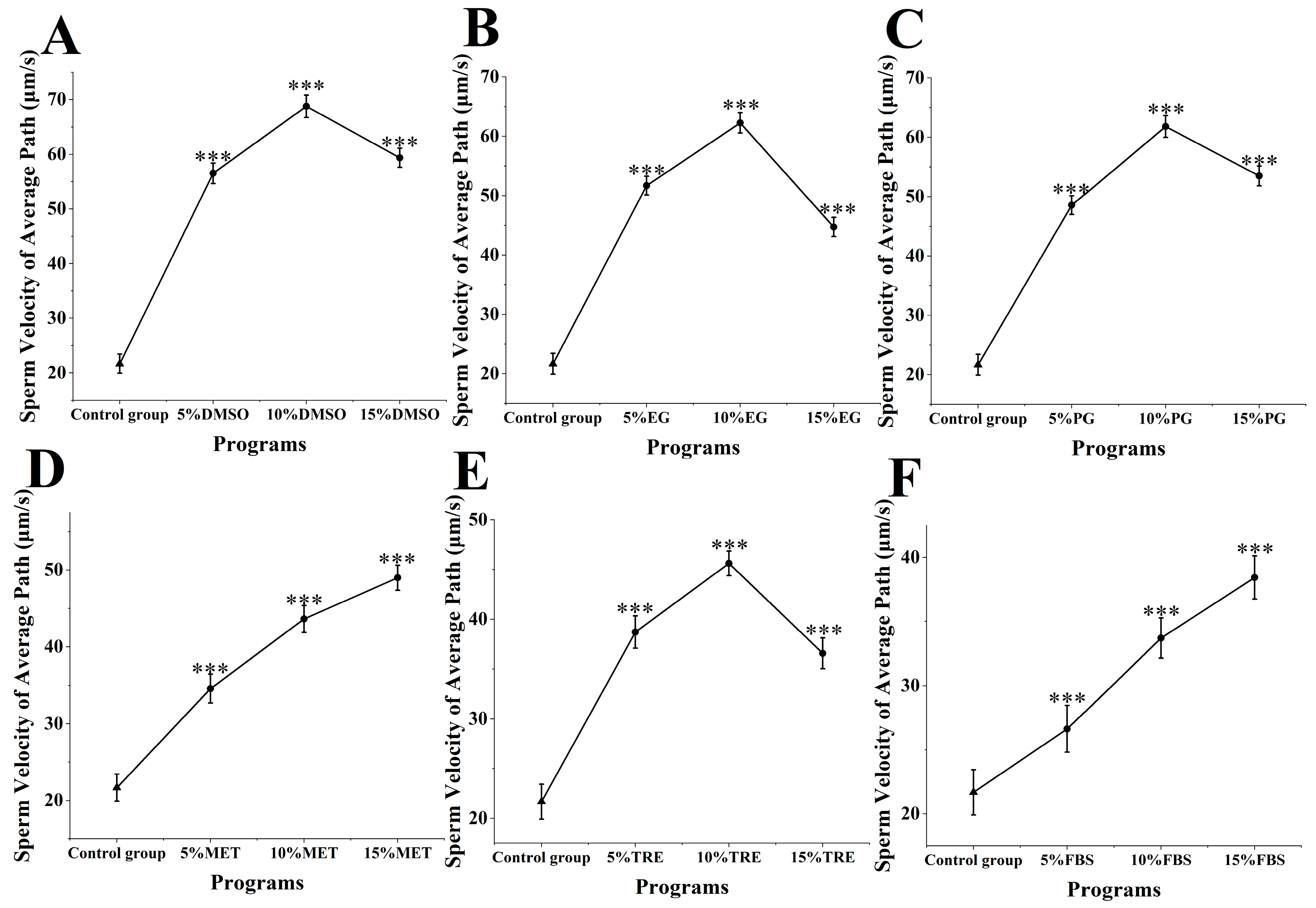
The average path velocity of M. nobilis sperm was measured following cryopreservation and thawing (Figure 3). In the control group, which lacked cryoprotectants, the sperm’s average path velocity was 21.68 ± 1.76 μm/s. When DMSO was added as a cryoprotectant (Figure 3A), the average path velocity of the sperm increased markedly, outperforming all other groups. Notably, 10% DMSO resulted in the highest average path velocity at 68.77 ± 2.05 μm/s, significantly surpassing the control group (p < 0.001). This velocity was close to that of fresh, untreated sperm at 81.35 ± 2.36 μm/s, indicating minimal reduction in velocity. Other concentrations of DMSO also provided strong protection, with velocities of 56.53 ± 1.87 μm/s for 5% DMSO and 59.36 ± 1.75 μm/s for 15% DMSO, both significantly higher than those of the control group (p < 0.001).

Figure 3.
Impact of cryoprotectants on average path velocity of sperm from M. nobilis. (A) Dimethyl sulfoxide (DMSO) at concentrations of 5%, 10%, and 15%; (B) Ethylene glycol (EG) at concentrations of 5%, 10%, and 15%; (C) Propylene glycol (PG) at concentrations of 5%, 10%, and 15%; (D) Methanol (MET) at concentrations of 5%, 10%, and 15%; (E) Trehalose (TRE) at concentrations of 5%, 10%, and 15%; (F) Fetal bovine serum (FBS) at concentrations of 5%, 10%, and 15% (*** p < 0.001).
For the MET and FBS groups (Figure 3D,F), the average path velocity showed a positive relationship with increasing concentrations of these cryoprotectants. In the MET group, the average velocities were 34.59 ± 1.88 μm/s for 5% MET, 46.63 ± 1.75 μm/s for 10% MET, and 54.01 ± 1.63 μm/s for 15% MET. As methanol concentration increased, the sperm’s average path velocity significantly improved, with the 15% MET group showing a substantially higher velocity than the 5 and 10% groups (p < 0.05). In the FBS group, the average path velocities were 26.64 ± 1.92 μm/s for 5% FBS, 32.71 ± 1.57 μm/s for 10% FBS, and 39.43 ± 1.75 μm/s for 15% FBS, with 15% FBS notably exceeding the lower concentrations (p < 0.05). Although MET and FBS showed different protective effects, both followed an upward trend in velocity as concentration increased.
In the remaining cryoprotectant groups (ethylene glycol, propylene glycol, and trehalose), the average path velocities for all concentrations were significantly greater than in the control group (p < 0.001) (Figure 3B,C,E). However, these cryoprotectants were generally less effective than DMSO. Overall, the influence of different cryoprotectants on the sperm’s average path velocity varied, with 10% DMSO providing the most significant protective effect, resulting in the smallest reduction in sperm velocity.
3.4. Impact of Cryoprotectants on Straight-Line Velocity of M. nobilis Sperm
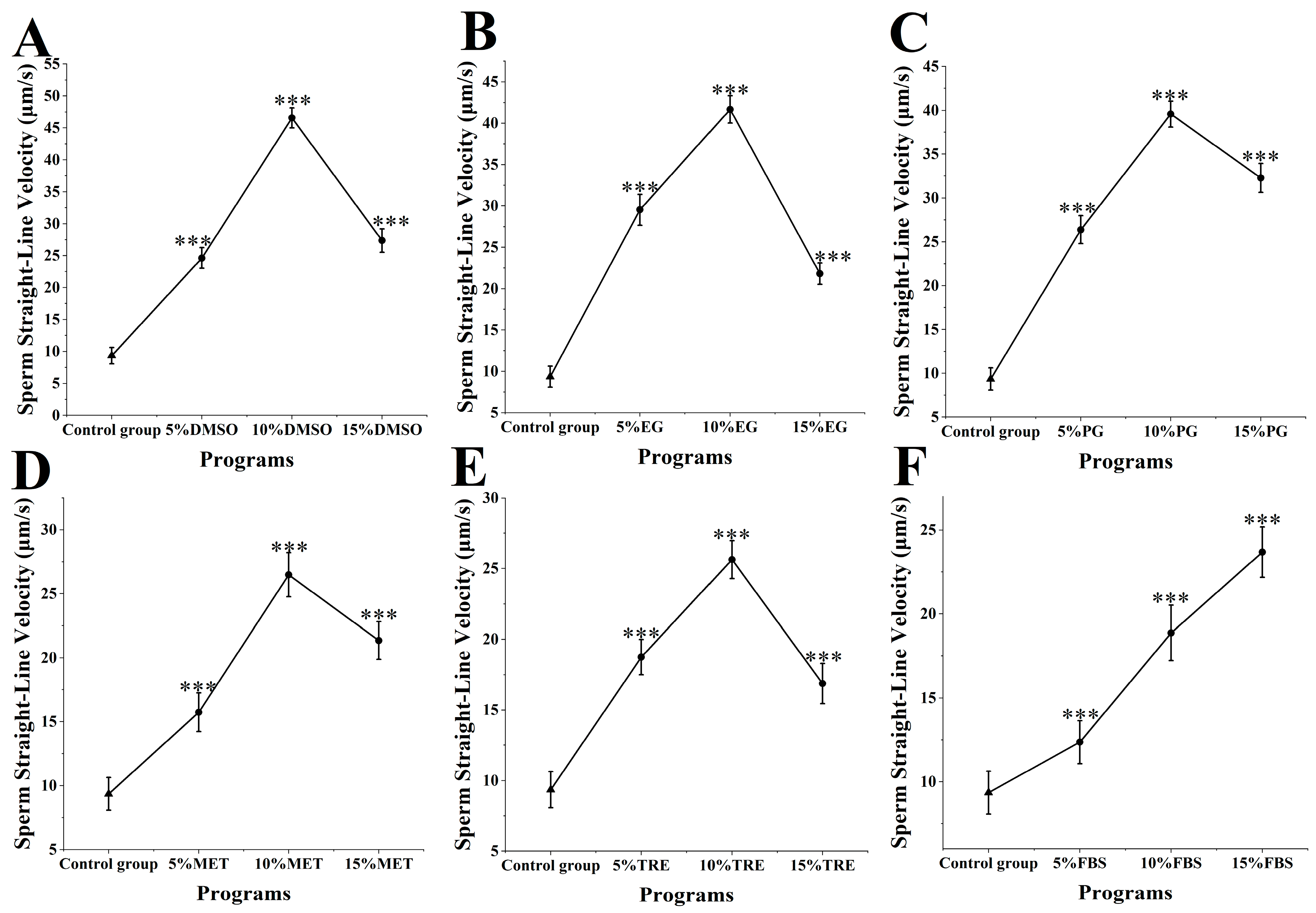
The straight-line velocity of M. nobilis sperm post-cryopreservation and thawing was analyzed (Figure 4). In the control group, which did not include any cryoprotectants, sperm straight-line velocity was 9.35 ± 1.28 μm/s. When DMSO was applied as a cryoprotectant (Figure 4A), the sperm’s straight-line velocity increased significantly compared to all other groups. Specifically, treatment with 10% DMSO yielded the highest velocity at 46.57 ± 1.57 μm/s, substantially surpassing the control group (p < 0.001). This rate was notably close to that of fresh sperm at 58.27 ± 1.58 μm/s, showing minimal impact on straight-line velocity. Other DMSO concentrations also provided effective protection, with velocities of 24.63 ± 1.62 μm/s for 5% DMSO and 27.36 ± 1.78 μm/s for 15% DMSO, both significantly higher than those of the control group (p < 0.001).
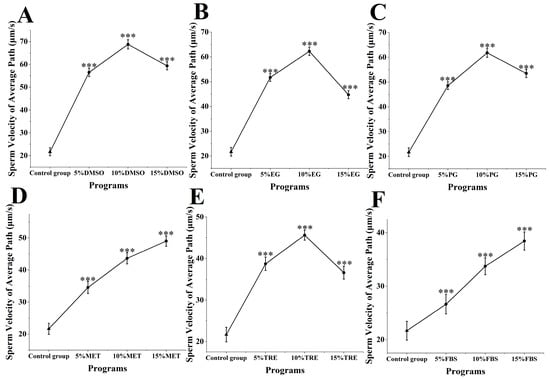
Figure 4.
Impact of cryoprotectants on straight-line velocity of Sperm from M. nobilis. (A) Dimethyl sulfoxide (DMSO) at concentrations of 5%, 10%, and 15%; (B) Ethylene glycol (EG) at concentrations of 5%, 10%, and 15%; (C) Propylene glycol (PG) at concentrations of 5%, 10%, and 15%; (D) Methanol (MET) at concentrations of 5%, 10%, and 15%; (E) Trehalose (TRE) at concentrations of 5%, 10%, and 15%; (F) Fetal bovine serum (FBS) at concentrations of 5%, 10%, and 15% (*** p < 0.001).
In groups using MET and FBS as cryoprotectants (Figure 4D,F), a positive correlation was observed between cryoprotectant concentration and straight-line velocity. In the MET group, 5% MET yielded a velocity of 15.75 ± 1.52 μm/s, 10% MET yielded 27.48 ± 1.92 μm/s, and 15% MET reached 21.35 ± 1.78 μm/s. Increasing the MET concentration significantly raised sperm straight-line velocity, with the 15% MET group surpassing both the 5% and 10% groups (p < 0.05). In the FBS group, the velocities were 12.36 ± 1.28 μm/s for 5% FBS, 17.87 ± 1.65 μm/s for 10% FBS, and 23.68 ± 1.51 μm/s for 15% FBS, with the 15% concentration showing a significant improvement over the lower concentrations (p < 0.05). Despite differing protective effects, both MET and FBS showed a trend of enhanced straight-line velocity with increasing concentrations.
In other experimental groups using ethylene glycol, propylene glycol, and trehalose, sperm straight-line velocity at all concentrations was also significantly higher than in the control group (p < 0.001) (Figure 4B,C,E), though the protective effects across concentrations did not reach the levels achieved by DMSO. Overall, the influence of various cryoprotectants on straight-line velocity varied, with 10% DMSO providing the most prominent protective effect and having the least impact on straight-line velocity.
3.5. Effects of Cryoprotectants on Sperm Morphology and Ultrastructure in M. nobilis
Based on its superior performance—yielding a sperm motility of 69.10 ± 2.37%, curvilinear velocity of 84.94 ± 1.64 μm/s, average path velocity of 68.77 ± 2.05 μm/s, and straight-line velocity of 46.57 ± 1.57 μm/s—compared to other cryoprotectants, 10% DMSO proved to be the best cryoprotectant. Therefore, we selected samples from fresh sperm, sperm cryopreserved with 10% DMSO, and a control group without cryoprotectants to examine sperm morphology and ultrastructure.
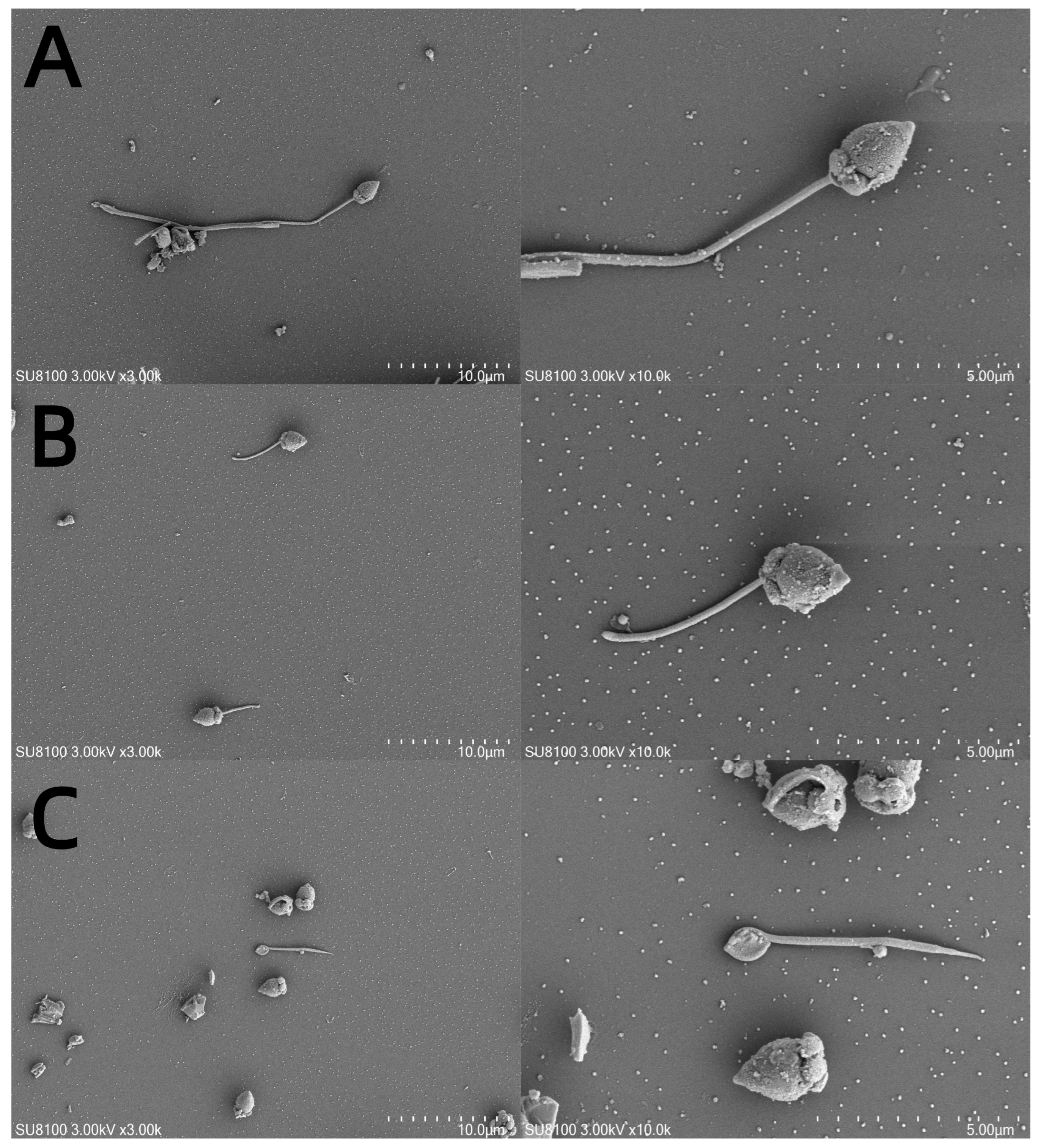
The M. nobilis sperm are flagellated (Figure 5A) and measure approximately 60–70 μm in length, consisting of three main parts: the head, midpiece, and tail. The head, which is about 4.2–5.0 μm long and conical in shape, contains the acrosome and nucleus. The acrosome, approximately 0.7 μm long and conical, is situated at the very tip of the head. The nucleus is cylindrical, narrower at the top and wider at the base, with a length of around 3.6 μm. The midpiece is short, approximately 0.9–1.0 μm in length, while the tail is elongated, consisting of an axoneme and plasma membrane, with the axoneme extending from the distal centriole. After cryopreservation with 10% DMSO, partial fragmentation of the flagellum was observed in M. nobilis sperm (Figure 5B). In contrast, in the control group without cryoprotectants, the sperm flagella exhibited complete breakage (Figure 5C).
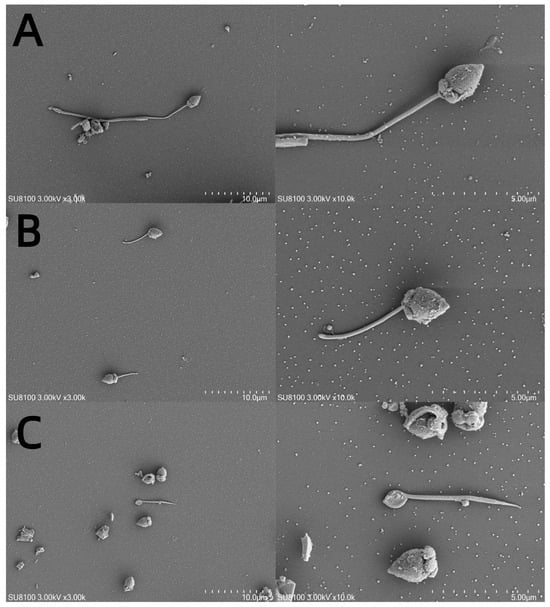
Figure 5.
Scanning electron micrographs of sperm subjected to different cryoprotectants. (A) Fresh sperm, (B) cryopreserved sperm, and (C) control group without cryoprotectant. Note: variation in structure of head and tail in all tested samples.
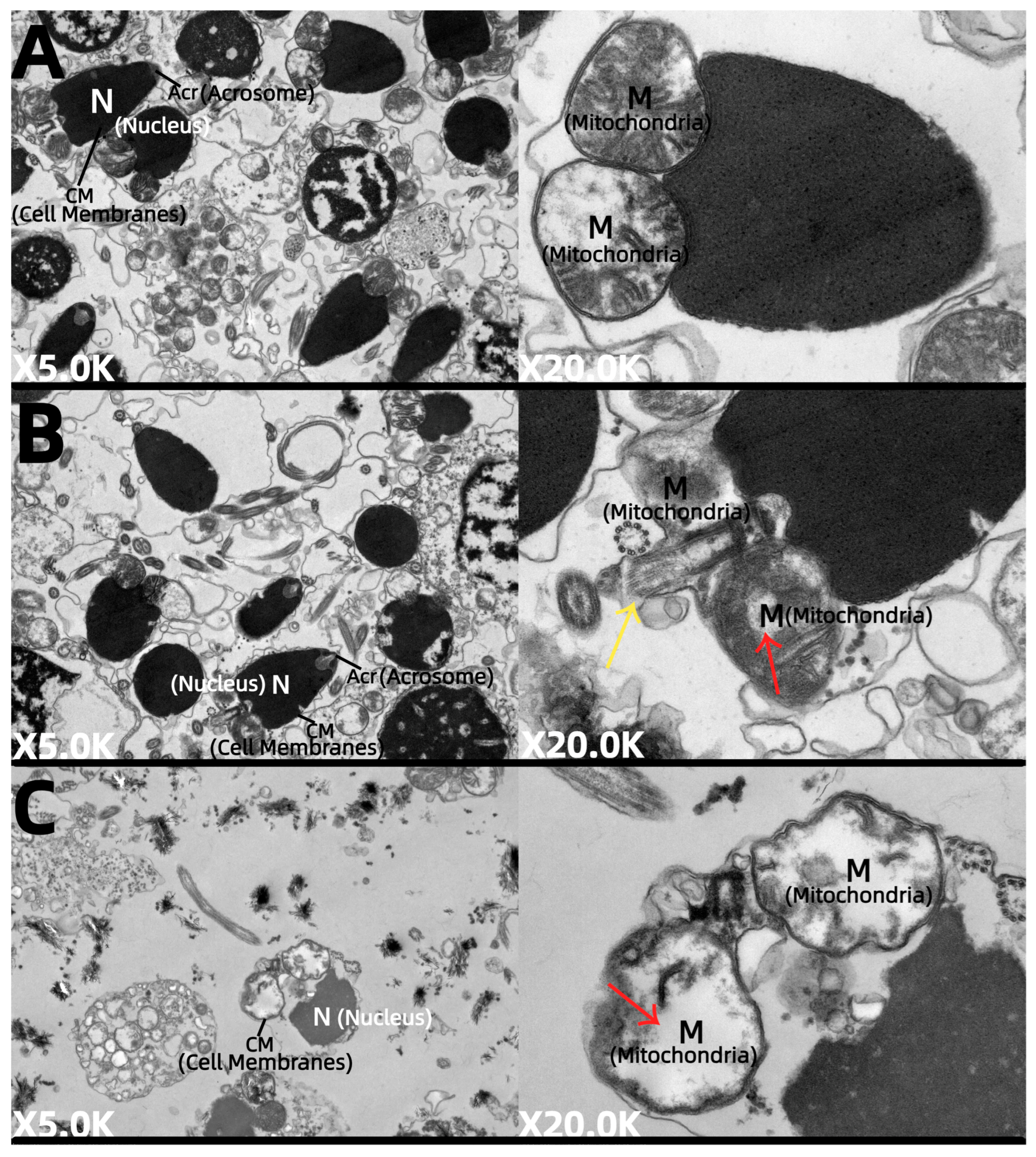
Ultrastructural examination revealed that fresh M. nobilis sperm were abundant, exhibiting no detectable swelling or structural damage (Figure 6A). The cell membranes were intact, continuous, and sharply defined, with densely packed cytoplasm and smoothly outlined organelles. The nuclei maintained a distinct conical shape, characterized by uniformly dispersed chromatin. The mitochondria were well developed, showing a clear, structurally organized matrix and intact, neatly arranged cristae. Moreover, the acrosome structure was fully preserved and clearly demarcated, and the microtubules within the tail region were prominently visible and structurally sound, indicating overall excellent sperm ultrastructure.

Figure 6.
Transmission electron micrographs of sperm subjected to different cryoprotectants. (A) Fresh sperm, (B) cryopreserved sperm, and (C) control group without cryoprotectant. Note: variation in cellular organelles in different treatments. Nucleus (N); acrosome (Ac); mitochondria (M).
In contrast, sperm samples treated with 10% DMSO (Figure 6B) maintained relatively high sperm counts but showed moderate ultrastructural damage. Although the majority of the cell membranes remained intact, certain regions appeared slightly blurred or compromised, accompanied by a visible decrease in cytoplasmic density. The organelles displayed evident swelling, particularly the mitochondria, which showed signs of membrane disruption and exhibited an irregular matrix structure with a slight deformation or loss of cristae (indicated by red arrows). The nucleus generally retained its conical form with evenly dispersed chromatin, indicating minimal nuclear damage. The acrosome was mostly intact, although microtubule structures appeared somewhat indistinct or obscured (marked by yellow arrows), suggesting mild impairment of the sperm tail structure.
In the control group lacking cryoprotectants (Figure 6C), the number of sperm was reduced, and they exhibited severe swelling. The cell membranes were compromised and partially dissolved, while the cytoplasm displayed extensive low-electron-density areas, indicative of pronounced organelle swelling. Although the nuclei appeared irregular, the chromatin was uniformly distributed. Mitochondria showed marked swelling and vacuolization, with blurred or dissolved membranes, widespread matrix degradation, and a significant loss or disruption of cristae (red arrows). Microtubules were barely detectable.
4. Discussion
Cryoprotective agents are compounds that, during ultralow-temperature freezing, help narrow dangerous temperature ranges and reduce freezing damage, thereby protecting sperm. However, they can cause cellular damage, and different animals respond to cryoprotectants with varying mechanisms, leading to differences in effectiveness [25]. Based on their mode of action, cryoprotectants are classified as permeable or non-permeable. Among the factors influencing the efficacy of sperm cryopreservation, selecting the appropriate type and concentration of cryoprotectant is critical, as it significantly affects preservation outcomes [26]. In this study, six cryoprotectants were used, both permeable (DMSO, EG, PG and MET) and non-permeable (TRE and FBS). Permeable agents are generally small, neutral molecules that easily bind water molecules in solution, promoting hydration and increasing solution viscosity, which reduces ice crystal formation and protects cells [27]. Adding permeable cryoprotectants during freezing also dilutes the solute concentration, reducing cellular salt intake as the cryoprotectant replaces it [28]. However, due to their inherent toxicity, permeable cryoprotectants can cause harm at higher concentrations, as toxic effects may outweigh cryoprotective benefits, necessitating a balance to optimize their protective effects [29]. The results from this study indicate that DMSO provided the best cryoprotection for M. nobilis sperm, preserving motility, sperm velocity, and morphology. This effectiveness likely results from DMSO’s ability to quickly penetrate sperm cell membranes and interact with phospholipids [30], aligning with findings in prior studies on Australian flat oyster (Ostrea angasi) sperm cryopreservation [31]. Research has shown that using 10% DMSO as a cryoprotectant in Eastern oyster (Crassostrea virginica) sperm cryopreservation resulted in a 70% post-thaw survival rate [32]. In another study, using sperm motility and fertilization rate as parameters, the researchers found that sperm cryopreserved with a 1:3 mixture of 10% DMSO and seawater achieved over 40% motility and a 20% fertilization rate, with a portion of fertilized eggs successfully developing to the D-larval stage [33]. Additionally, a study on Chinese pearl oyster (Pinctada martensii) sperm reported post-thaw survival rates exceeding 60% and fertilization rates up to 80% when cryopreserved with a 10% DMSO–seawater solution [34]. Thus, species-specific physiological characteristics and biological specificity should be considered in cryopreservation studies.
Non-permeable cryoprotectants create an osmotic pressure gradient around cells, facilitating water efflux and reducing intracellular ice formation during freezing. These cryoprotectants dissolve in water but do not penetrate cells, enabling the solution to remain supercooled and lowering solute concentration at specific temperatures to protect cells [35]. In this study, TRE and FBS served as non-permeable cryoprotectants, though their effects on M. nobilis sperm motility and swimming speed post-freezing were suboptimal. This is likely because TRE and FBS cannot penetrate cell membranes, thus failing to prevent intracellular ice crystal formation, which is essential for cell survival [36]. Furthermore, they do not alleviate the effects of extreme extracellular environmental changes, such as osmotic pressure shifts and ice crystal damage, leading to cell structure and function loss [37]. Research indicates that adding 0.45 mol/L trehalose to Japanese pearl oyster (Pinctada fucata martensii) sperm cryoprotectant significantly improved motility and fertilization rates post-thaw compared to untreated groups [38]. In green-lipped mussel (Perna canaliculus) sperm cryopreservation, a cryoprotectant mixture of 12% DMSO and 0.2 mol/L trehalose yielded effective results [39]. Another study on Japanese pearl oyster sperm cryopreservation compared 10% methanol, dimethylformamide, and 10% DMSO with a diluent of 80% seawater and 20% bovine serum, finding that methanol provided superior post-thaw motility [40]. Using mixed cryoprotectants from different classes may provide enhanced protection, though the specific mechanisms require further exploration [41].
The optimal concentration of cryoprotectants is closely related to species specificity. Cells in different species vary significantly in membrane permeability, sensitivity to freezing stress, metabolic activity, and solute and water distribution inside and outside cells [42], all of which determine species-specific cryoprotectant concentration requirements. In this experiment, a 10% concentration of each cryoprotectant (DMSO, EG, PG, TRE, and FBS) provided superior motility and velocity for M. nobilis sperm compared to 5 and 15% concentrations. A 15% concentration, though increasing cryoprotection, led to higher cellular toxicity, reducing sperm motility and sperm velocity. This may be due to the high concentration of DMSO causing a drastic increase in extracellular osmotic pressure and an excessive amount of DMSO entering the cells, which disrupts the integrity of the cell membrane, leading to cell dehydration and intracellular imbalance [43]. Studies have shown that during cryopreservation, cobia (Rachycentron canadum) sperm exhibited a 16% greater loss of motility under 15% DMSO compared to a 10% concentration [44]. Consequently, protective effects are often offset by toxic effects, resulting in reduced sperm activity and viability. Meanwhile, 5% was too low to offer effective protection. Notably, sperm cryopreserved with MET and FBS showed a positive correlation between cryoprotectant concentration and cryoprotective efficacy, likely due to MET’s low cytotoxicity and freezing point, which minimizes ice formation and effectively reduces sample freezing temperature [45]. MET’s rapid action and easy removal from cells minimize long-term chemical effects, while its dehydrating properties help maintain cell structure [46]. The protein molecules in FBS stabilize the extracellular environment, reducing osmotic damage during freezing and protecting cell membranes through specific interactions with cell surfaces [47]. FBS also provides nutritional support and promotes cell adhesion [48]. Consequently, MET and FBS are more suitable for preserving high bioactivity and cell integrity in sensitive biological samples at high concentrations, whereas other cryoprotectants may be less effective in providing added protection at elevated concentrations [49]. Studies show that 10% methanol as a cryoprotectant resulted in the highest post-thaw motility and longest swimming duration for zebrafish (Danio rerio) sperm [50]. Additionally, bovine serum protein outperformed trehalose in cryopreserving sea bream (Sparus aurata) sperm, as trehalose’s protective mechanism relies on forming an ordered “glassy” state to prevent extracellular ice crystal formation, physically stabilizing the environment around cells [51]. This mechanism depends on trehalose concentration and freeze–thaw control; otherwise, it can reduce sperm motility [52]. For Tambaqui (Colossoma macropomum) sperm, trehalose had the greatest impact on survival rate, supporting these findings [53]. Further research on the effects of cryoprotectant concentration on sperm cryopreservation should incorporate species-specific characteristics and biochemical mechanisms.
In the morphological and ultrastructural observation of cryopreserved M. nobilis sperm, we found that cryopreservation induces similar types of damage across different species, specifically affecting the sperm membrane, acrosome, mitochondria, and flagellum. These damages disrupt membrane integrity and functional proteins [54]. In this experiment, we focused on sperm samples under fresh, optimal (10% DMSO), and control (no cryoprotectant) conditions. Observations showed varying degrees of morphological damage in cryopreserved sperm, primarily concentrated in the flagellum, a critical structure for sperm motility. Flagellar integrity directly impacts sperm motility and viability [55], and partial flagellar breakage likely results from membrane stress responses during thawing, negatively affecting sperm motility to some extent [56]. Under 10% DMSO, flagellar breakage was minimal, preserving some motility. In contrast, complete flagellar breakage in the control group led to significant declines in sperm motility and may have impaired fertilization potential, aligning with this experiment’s findings on sperm motility and sperm velocity. Research on red abalone (Haliotis rufescens) sperm cryopreservation found that cryopreserved sperm exhibited damage to the flagellum or plasma membrane, leading to significant declines in motility and fertilization rates compared to fresh sperm [57]. Additionally, in European eel (Anguilla anguilla) sperm cryopreservation, non-motile sperm could still fertilize eggs and induce the acrosome reaction, suggesting that sperm with flagellar detachment or membrane damage may retain fertilization potential [58]. In this study, ultrastructural examinations revealed multiple types of damage, including varying degrees of membrane swelling, enlarged intermembrane spaces, ruptured or absent acrosomes, the disintegration of mitochondria, distorted cristae, and the separation of the double membrane. Swelling, fractures, and even detachment of flagella were also commonly observed. During freezing and thawing, ice crystals may mechanically damage cell membranes to different extents [59], while low temperatures induce membrane phase transitions that reduce elasticity and increase the likelihood of rupture. This leads to membrane protein loss, protein conformational changes, and cytoplasmic alterations [60].
Cryopreservation-induced damage to the sperm tail disrupts the axonemal structure, which diminishes motility and ultimately impairs sperm function [61]. During the freezing process, cryoprotectants that penetrate cells raise intracellular osmotic pressure above extracellular levels, causing extracellular water influx, further swelling, and membrane rupture, especially in the sperm tail [62]. Studies on grouper (Epinephelus coioides) sperm cryopreservation showed that damage to sperm membranes and a loss of or alteration in membrane proteins disrupted the interaction required for sperm–egg recognition, thereby blocking sperm penetration of the egg [63]. Further research is needed to elucidate the specific mechanisms by which cryoprotectants impact sperm integrity.
5. Conclusions
This study demonstrates that the type and concentration of cryoprotectant are crucial for the cryopreservation efficacy of M. nobilis sperm. Permeable cryoprotectants, such as DMSO, significantly enhance the motility and morphological preservation of M. nobilis sperm at optimal concentrations, with 10% DMSO showing the best results. Meanwhile, non-permeable cryoprotectants like FBS also exhibit stabilizing effects on the extracellular environment at higher concentrations. As concentration increases, both MET and FBS provide progressively enhanced protection, further reducing damage to the sperm. Additionally, damage incurred during the freezing process directly affects the motility of M. nobilis sperm, with 10% DMSO preserving high integrity and motility. These findings underscore the importance of optimizing cryoprotectant selection and concentration, providing key insights for the germplasm preservation of marine species.
Author Contributions
Conceptualization, M.L., Z.F. and Z.M.; methodology, M.L.; software, M.L.; validation, M.L., Z.F. and Z.M.; formal analysis, M.L.; investigation, M.L. and Z.F.; resources, G.Y. and Z.M.; data curation, M.L.; writing—original draft preparation, M.L.; writing—review and editing, M.L., G.Y. and Z.M.; visualization, M.L.; supervision, G.Y. and Z.M.; project administration, G.Y. and Z.M.; funding acquisition, Z.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Central Public-interest Scientific Institution Basal Research Fund, CAFS [grant number 2023TD58], the earmarked fund for CARS [grant number CARS-49], and the Financial Fund of Ministry of Agriculture and Rural affairs of China [grant number NHYYSWZZZYKZX2020].
Institutional Review Board Statement
The noble scallop Mimachlamys nobilis is not a vertebrate nor a regulated invertebrate, so no ethical conduct approval is required.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| mm | Millimeter |
| μm | Micron |
| L | Liter |
| mL | Milliliter |
| μL | Microliter |
| min | Minute |
| s | Second |
| g | Gram |
| °C | Centigrade |
| DMSO | Dimethyl Sulfoxide |
| EG | Ethylene Glycol |
| PG | Propylene Glycol |
| MeOH | Methanol |
| TRE | Trehalose |
| FBS | Fetal Bovine Serum |
| CASA | Computer-Assisted Sperm Analysis |
| Hanks’s | Hank’s Balanced Salt Solution |
| SEM | Scanning Electron Microscopy |
| TEM | Transmission Electron Microscope |
References
- Xu, C.; Wu, X.; Qiu, J.; Ye, J.; Lin, Q.; Deng, J.; Zeng, Y.; Wang, W.; Zhang, H.; Zheng, H. Genome-wide identification of gap junction gene family and their expression profiles under low temperature stress in noble scallop Chlamys nobilis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 52, 101310. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, J.; Yang, R.; Fu, Z.; Yu, G.; Ma, Z. Effects of ammonia concentration on sperm vitality, motility rates, and morphology in three marine bivalve species: A comparative study of the noble scallop Mimachlamys nobilis, Chinese pearl oyster Pinctada fucata martensii, and small rock oyster Saccostrea mordax. Biology 2024, 13, 589. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, Y.; Wen, J.; Xu, B.; Zhu, W.; Zhang, H.; Liu, X.; Lichu, L.; Zheng, H. Effects of chronic cold stress on tissue structure, antioxidant response, and key gene expression in the warm-water bivalve Chlamys nobilis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 50, 101225. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, C.; Tan, K.; Wang, B.; Huang, R.; Wen, J.; Xu, B.; Liu, X.; Lichu, L.; Zheng, H. Variation of lipids and fatty acids in noble scallop Chlamys nobilis under low temperature stress. Aquaculture 2022, 554, 738121. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, S.; Yang, R.; Qin, J.; Wang, A.; Gu, Z.; Ma, Z. Sperm cryopreservation of the noble scallop Chlamys nobilis by a programmable freezing method: Effect of cryoprotectant. Aquac. Res. 2019, 50, 1678–1686. [Google Scholar] [CrossRef]
- Barbas, J.P.; Mascarenhas, R.D. Cryopreservation of domestic animal sperm cells. Cell Tissue Bank. 2009, 10, 49–62. [Google Scholar] [CrossRef]
- Tambovsky, M.A.; Aimaletdinov, A.M.; Zakirova, E.Y. Current trends in the application of stem cells and their derivatives in animal sperm cryopreservation. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2023, 17, 243–248. [Google Scholar] [CrossRef]
- Chelewani, A.P.; Takahashi, E.; Nishimura, T.; Fujimoto, T. Optimizing the post-thaw quality of cryopreserved masu salmon (Oncorhynchus masou) sperm: Evaluating the effects of antioxidant-supplemented extender. Aquaculture 2024, 593, 741332. [Google Scholar] [CrossRef]
- Sciorio, R.; Cantatore, C.; D’Amato, G.; Smith, G.D. Cryopreservation, cryoprotectants, and potential risk of epigenetic alteration. J. Assist. Reprod. Genet. 2024, 41, 2953–2967. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D.; Wang, Q.; Ruan, Q.; Hua, S.; Zhang, W.; Yang, S.; Meng, Z. Addition of cryoprotectant DMSO reduces damage to spermatozoa of yellow catfish (Pelteobagrus fulvidraco) during cryopreservation: Ultrastructural damage, oxidative damage and DNA damage. Animals 2024, 14, 2652. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Sriket, C.; Nalinanon, S.; Visessanguan, W.; Benjakul, S. Gelatin hydrolyzed by papaya latex enzymes as an alternative cryoprotectant for frozen raw Pacific white shrimp (Penaeus vannamei). Food Biosci. 2024, 60, 104199. [Google Scholar] [CrossRef]
- Anjos, C.; Duarte, D.; Fatsini, E.; Matias, D.; Cabrita, E. Comparative transcriptome analysis reveals molecular damage associated with cryopreservation in Crassostrea angulata D-larvae rather than to cryoprotectant exposure. BMC Genom. 2024, 25, 591. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Cao, S.; Zhu, Z.; Hu, B.; Chen, H.; Tu, M.; Tan, Z.; Du, M.; Li, T. Characterization and the mechanism underlying the cryoprotective activity of a peptide from large yellow croaker (Pseudosciaena crocea). Food Chem. 2024, 435, 137512. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Salmon, H.; Jerry, D.R.; Southgate, P.C. Effects of cryoprotectant agents and freezing protocol on motility of black-lip pearl oyster (Pinctada margaritifera L.) spermatozoa. Cryobiology 2007, 54, 13–18. [Google Scholar] [CrossRef]
- Ishiguro, H.; Rubinsky, B. Influence of fish antifreeze proteins on the freezing of cell suspensions with cryoprotectant penetrating cells. Int. J. Heat Mass Transf. 1998, 41, 1907–1915. [Google Scholar] [CrossRef]
- Upton, R.; Clulow, S.; Colyvas, K.; Mahony, M.; Clulow, J. Paradigm shift in frog sperm cryopreservation: Reduced role for non-penetrating cryoprotectants. Reproduction 2023, 165, 583–592. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, X.; Han, Y.; Wang, Z.; Jia, Z.; Chen, D.; Qiao, Z.; Gao, X.; Zhao, C.; Shen, Y. Optimal conditions for cryopreservation by vitrification of largemouth bass (Micropterus salmoides) embryos. Anim. Reprod. Sci. 2024, 270, 107613. [Google Scholar] [CrossRef]
- Gupta, S.; Fletcher, G.C.; Taylor, R.; Hedderley, D.; Cruz, C.D. Effect of air blast freezing and frozen storage on inactivation of Vibrio vulnificus in Pacific oysters (Crassostrea gigas). Food Control 2024, 163, 110467. [Google Scholar] [CrossRef]
- Yan, L.; Li, Y.; Wang, Z.; Su, J.; Yu, R.; Yan, X.; Ma, P.; Cui, Y. Stress response to low temperature: Transcriptomic characterization in Crassostrea sikamea × Crassostrea angulata hybrids. Aquac. Res. 2018, 49, 3374–3385. [Google Scholar] [CrossRef]
- Jiang, P.; Qin, X.; Fan, X.; Zhang, C.; Chen, D. The impact of anhydrous storage and transportation at ice temperatures on the post-capture viability and quality of Patinopecten yessoensis. J. Agric. Food Res. 2024, 18, 101277. [Google Scholar] [CrossRef]
- Lauren, L.; Dean, R.J.; Paul, C.S. Cryopreservation of black-lip pearl oyster (Pinctada margaritifera L.) spermatozoa: Effects of cryoprotectants on spermatozoa motility. J. Shellfish Res. 2005, 24, 1187–1190. [Google Scholar] [CrossRef]
- Claudet, P.V.; Narasimman, S.; Natesan, M. Effect of cryoprotectants and cooling rates on fertility potential of sperm in the giant freshwater prawn, Macrobrachium rosenbergii (De Man). Anim. Reprod. Sci. 2016, 171, 49–57. [Google Scholar] [CrossRef]
- Seshoka, M.M.; Mphaphathi, M.L.; Nedambale, T.L. Comparison of four different permitting and combination of two best cryoprotectants on freezing Nguni sperm evaluated with the aid of computer aided sperm analysis. Cryobiology 2016, 72, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Shaliutina-Kolešová, A.; Cosson, J.; Lebeda, I.; Gazo, I.; Shaliutina, O.; Dzyuba, B.; Linhart, O. The influence of cryoprotectants on sturgeon (Acipenser ruthenus) sperm quality, DNA integrity, antioxidant responses, and resistance to oxidative stress. Anim. Reprod. Sci. 2015, 159, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Abinawanto, A.; Vardini, N.; Kristanto, A.H.; Lestari, R.; Bowolaksono, A. Effect of egg yolk of free-range chicken and methanol as a cryoprotective agent for the sperm preservation of cyprinid fish, Neolissochilus soroides (Valenciennes, 1842). Heliyon 2021, 7, e08158. [Google Scholar] [CrossRef]
- Lee, Y.H.; Park, J.Y.; Lee, I.Y.; Zidni, I.; Lim, H.K. Effects of cryoprotective agents and treatment methods on sperm cryopreservation of stone flounder, Kareius bicoloratus. Aquaculture 2021, 531, 735969. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Park, J.Y.; Lim, H.K. Effects of different diluents, cryoprotective agents, and freezing rates on sperm cryopreservation in Epinephelus akaara. Cryobiology 2018, 83, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, Z.H.; Dzyuba, B.; Hulak, M.; Rodina, M.; Linhart, O. Evaluating the impacts of osmotic and oxidative stress on common carp (Cyprinus carpio L.) sperm caused by cryopreservation techniques. Biol. Reprod. 2010, 83, 852–858. [Google Scholar] [CrossRef]
- Vacquier, V.D.; Hamdoun, A. Cold storage and cryopreservation methods for spermatozoa of the sea urchins Lytechinus pictus and Strongylocentrotus purpuratus. Dev. Dyn. 2024, 253, 781–790. [Google Scholar] [CrossRef]
- Gwo, J.; Arnold, C.R. Cryopreservation of Atlantic croaker spermatozoa: Evaluation of morphological changes. J. Exp. Zool. 1992, 264, 444–453. [Google Scholar] [CrossRef]
- Hassan, M.M.; Li, X.; Liu, Y.; Qin, J.G. Sperm cryopreservation in the spermcasting Australian flat oyster Ostrea angasi by a programmable freezing method. Cryobiology 2017, 76, 119–124. [Google Scholar] [CrossRef]
- Yang, H.; Huo, Y.; Yee, J.C.; Rikard, S.; Walton, W.C.; Saillant, E. Sperm repository for a breeding program of the eastern oyster Crassostrea virginica: Sample collection, processing, cryopreservation, and data management plan. Animals 2021, 11, 2836. [Google Scholar] [CrossRef]
- Riesco, M.F.; Félix, F.; Matias, D.; Joaquim, S.; Suquet, M.; Cabrita, E. Comparative study on cellular and molecular responses in oyster sperm revealed different susceptibilities to cryopreservation. Aquaculture 2019, 498, 223–229. [Google Scholar] [CrossRef]
- Zheng, X.; Gu, Z.; Huang, Z.; Ding, H.; Vasquez, H.E.; Liu, Y.; Shi, Y.; Wang, A. The effects of cryoprotectants on sperm motility of the Chinese pearl oyster, Pinctada fucata martensii. Cryobiology 2018, 82, 64–69. [Google Scholar] [CrossRef]
- Kawamoto, T.; Narita, T.; Isowa, K.; Aoki, H.; Hayashi, M.; Ohta, H.; Komaru, A. Effects of cryopreservation methods on post-thaw motility of spermatozoa from the Japanese pearl oyster, Pinctada fucata martensii. Cryobiology 2007, 54, 19–26. [Google Scholar] [CrossRef]
- Arita, K.; Takamatsu, S.; Isowa, K.; Aoki, H.; Ohta, H. Development of a novel non-programmable cryopreservation method capable of accurate cooling rate manipulation. Aquaculture 2018, 484, 145–151. [Google Scholar] [CrossRef]
- Suquet, M.; Gourtay, C.; Donval, A.; Le Goïc, N.; Quere, C.; Malo, F.; Le Grand, J.; Ratiskol, D.; Mingant, C.; Fauvel, C. The quality of great scallop (Pecten maximus) sperm after thawing. Gen. Comp. Endocrinol. 2016, 229, 127–131. [Google Scholar] [CrossRef]
- Narita, T.; Kawamoto, T.; Isowa, K.; Aoki, H.; Hayashi, M.; Ohta, H.; Komaru, A. Effects of cryopreservation on sperm structure in Japanese pearl oyster Pinctada fucata martensii. Fish. Sci. 2008, 74, 1069–1074. [Google Scholar] [CrossRef]
- Rusk, A.B.; Alfaro, A.C.; Young, T.; Watts, E.; Adams, S.L. Development stage of cryopreserved mussel (Perna canaliculus) larvae influences post-thaw impact on shell formation, organogenesis, neurogenesis, feeding ability and survival. Cryobiology 2020, 93, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Fu, Z.; Lin, S.; Yang, R.; Wang, A.; Gu, Z.; Ma, Z. Which is the major trigger in aquatic environment for pearl oyster Pinctada fucata martensii sperm from gonad: Ammonia ion or pH? Aquaculture 2020, 520, 734673. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, Y.; Wang, M.; Li, Y.; Dai, T.; Qin, W.; Zhu, Y.; Zhang, C.; Zhou, Y.; Qin, Q.; et al. Study on sperm cryopreservation of hybrid fish derived from Carassius cuvieri (♀) × Carassius auratus red var. (♂). Reprod. Breed. 2024, 4, 234–242. [Google Scholar] [CrossRef]
- Superio, J.; Resseguier, J.; Nobrega, R.H.; Grebstad, C.M.; Fakriadis, I.; Foss, A.; Hagen, Ø.; Zhang, M.; Del Pilar García-Hernández, M.; Galindo-Villegas, J. Unravelling spermatogenesis in spotted wolffish: Insights from the ultrastructure of juvenile male testes to the cryopreservation of broodstock sperm. Aquaculture 2024, 592, 741214. [Google Scholar] [CrossRef]
- Abdissa, B.; Getahun, A.; Dejen, E. Cryopreservation of sperm of Labeobarbus brevicephalus (Pisces: Cyprinidae) from Lake Tana (Ethiopia). Aquaculture 2022, 548, 737697. [Google Scholar] [CrossRef]
- Dhanasekar, K.; Selvakumar, N.; Munuswamy, N. Cryopreservation of sperm in cobia, Rachycentron canadum (Linnaeus, 1766). Aquaculture 2022, 557, 738313. [Google Scholar] [CrossRef]
- Handayani, L.; Maulida, S.; Rahayu, S.; Razi, N.M.; Kocabas, M.; Kocabas, F.K.; Wilkes, M.; Siti-Azizah, M.N.; Eriani, K.; Fadli, N.; et al. Effect of cryoprotectant and concentration on the sperm quality of walking catfish, Clarias batrachus, post-cryopreservation. CryoLetters 2024, 45, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Hai, E.; Li, B.; Zhang, J.; Zhang, J. Sperm freezing damage: The role of regulated cell death. Cell Death Discov. 2024, 10, 239. [Google Scholar] [CrossRef]
- Ding, X.; Tian, Y.; Qiu, Y.; Duan, P.; Wang, X.; Li, Z.; Li, L.; Liu, Y.; Wang, L. Effects of long-term cryopreservation on the transcriptomes of giant grouper sperm. Genes 2024, 15, 523. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhu, K.; Liu, J.; Guo, H.; Liu, B.; Zhang, N.; Xian, L.; Sun, J.; Zhang, D. Cryopreservation of goldlined seabream Rhabdosargus sarba (Forsskål, 1775) sperm: CASA observation and enzyme activity evaluation. Aquaculture 2024, 582, 740494. [Google Scholar] [CrossRef]
- Balamurugan, R.; Karthik, S.; Arul, V. Effect of cryopreservation on motility, DNA integrity and gene expression in grey mullet, Mugil cephalus sperm. Cryobiology 2024, 114, 104848. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hu, E.; Tiersch, T.; Carmichael, C.; Matthews, J.; Varga, Z.M. Temporal and concentration effects of methanol on cryopreservation of zebrafish (Danio rerio) sperm. Zebrafish 2020, 17, 233–242. [Google Scholar] [CrossRef]
- Zilli, L.; Bianchi, A.; Sabbagh, M.; Pecoraro, L.; Schiavone, R.; Vilella, S. Development of sea bream (Sparus aurata) semen vitrification protocols. Theriogenology 2018, 110, 103–109. [Google Scholar] [CrossRef]
- Kása, E.; Lujić, J.; Marinović, Z.; Kollár, T.; Bernáth, G.; Bokor, Z.; Urbányi, B.; Lefler, K.K.; Jesenšek, D.; Horváth, Á. Development of sperm vitrification protocols for two endangered salmonid species: The Adriatic grayling, Thymallus thymallus, and the marble trout, Salmo marmoratus. Fish Physiol. Biochem. 2018, 44, 1499–1507. [Google Scholar] [CrossRef]
- Varela, J.A.; Jardim, R.D.; Streit, D.J.; Cardoso, T.F.; Silva, E.F.; Lucia, T.J.; Figuelredo, M.; Corcini, C.D. Trehalose in extenders for cryopreservation of tambaqui (Colossoma macropomum) sperm. CryoLetters 2022, 43, 264–268. [Google Scholar] [CrossRef]
- Pandey, D.; Ryu, Y.; Matsubara, T. Features of sperm motility and circadian rhythm in Japanese anchovy (Engraulis japonicus). Fish. Aquac. J. 2017, 8, 203. [Google Scholar] [CrossRef]
- Cosson, J.; Groison, A.L.; Suquet, M.; Fauvel, C.; Dreanno, C.; Billard, R. Studying sperm motility in marine fish: An overview on the state of the art. J. Appl. Ichthyol. 2008, 24, 460–486. [Google Scholar] [CrossRef]
- França, T.S.; González-López, W.A.; Sanchez, M.P.; Ferrão, L.; Fernández-García, F.; Borges, L.P.; Belenguer, A.; Holorea, P.G.; Calduch-Giner, J.C.; Felip, A.; et al. Successful cryopreservation in biodegradable containers of sperm from aquaculture Mediterranean fishes. Theriogenology 2024, 216, 53–61. [Google Scholar] [CrossRef]
- Salinas-Flores, L.; Paniagua-Chavez, C.G.; Jenkins, J.A.; Tiersch, T.R. Cryopreservation of sperm of red abalone (Haliotis rufescens). J. Shellfish Res. 2005, 24, 415–420. [Google Scholar] [CrossRef]
- Ferrão, L.; Morini, M.; González-Lopéz, W.A.; Galledo, V.; Felip, A.; Pérez, L.; Asturiano, J.F. Effects of cold seawater pre-treatments on induction of early sexual maturation and sperm production in European eel (Anguilla anguilla). Fish Physiol. Biochem. 2024, 50, 2489–2503. [Google Scholar] [CrossRef] [PubMed]
- Shaliutina-Loginova, A.; Loginov, D.S. Oxidative stress and DNA fragmentation in frozen/thawed common carp (Cyprinus carpio) sperm with and without supplemental proteins. Anim. Reprod. Sci. 2023, 251, 107213. [Google Scholar] [CrossRef]
- Stanger, S.J.; Law, E.A.; Jamsai, D.; O’Bryan, M.K.; Nixon, B.; McLaughlin, E.A.; Aitken, R.J.; Roman, S.D. A novel germ cell protein, SPIF (sperm PKA interacting factor), is essential for the formation of a PKA/TCP11 complex that undergoes conformational and phosphorylation changes upon capacitation. FASEB J. 2016, 30, 2777–2791. [Google Scholar] [CrossRef]
- Xin, M.; Siddique, M.A.M.; Dzyuba, B.; Cuevas-Uribe, R.; Shaliutina-Kolešová, A.; Linhart, O. Progress and challenges of fish sperm vitrification: A mini review. Theriogenology 2017, 98, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, X.; Liu, X.; Zhou, Y.; Zheng, J.; Zheng, X. Sperm cryopreservation of Amphioctopus fangsiao (Mollusca: Cephalopoda). Aquaculture 2025, 598, 742026. [Google Scholar] [CrossRef]
- Kiriyakit, A.; Gallardo, W.G.; Bart, A.N. Successful hybridization of groupers (Epinephelus coioides × Epinephelus lanceolatus) using cryopreserved sperm. Aquaculture 2011, 320, 106–112. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).