Abstract
Penaeus semisulcatus, a commercially valuable aquaculture species, is widely distributed along the southeastern coast of China and throughout the South China Sea. Despite its economic importance, the comprehensive understanding of its germplasm characteristics remains limited. This investigation evaluated nutrient profiles, amino acid composition, lipid constituents, antioxidant capacity, and genomic variability across three farmed aquaculture populations maintained under standardized environmental parameters to discern divergence in nutritive attributes and hereditary characteristics. The results revealed significant interpopulation variation in ash content (p < 0.05), while other proximate components showed comparable levels. Among 17 detected amino acids, glutamic acid was the most abundant (2.07–2.30 g/100 g), while cysteine had the lowest concentration (0.10–0.11 g/100 g). Notably, the PsHK population had a relatively higher amino acid content, indicating superior nutritional value. Fatty acid analysis detected 13 fatty acids, with C18:0 (88.27–108.00 mg/100 g) being the most abundant, and C20:2 (3.40–4.70 mg/100 g) the least. The PsHK population exhibited significantly higher levels of all fatty acids compared to the other two populations (p < 0.05). Antioxidant enzyme activity assays revealed no significant differences in antioxidant capacity across the three populations, indicating a similar overall antioxidant status. Genetic diversity analysis indicated that the PsHK population had the highest genetic diversity, with a relatively pronounced genetic differentiation between the PsHK and PsRP populations. In conclusion, the comparative analysis of these three cultured populations highlights that the PsHK population excels in both nutritional composition and genetic diversity. With its superior nutritional profile and rich genetic background, P. semisulcatus shows great potential for aquaculture development. These findings provide valuable insights for future germplasm improvement and aquaculture optimization efforts, offering a scientific basis for refining breeding strategies and enhancing the nutritional evaluation of P. semisulcatus. Additionally, the comparative analysis of genetic diversity and biochemical composition contributes to a better understanding of population variation, which is essential for the sustainable management and utilization of this species in aquaculture.
1. Introduction
The green tiger shrimp (Penaeus semisulcatus) is a widely distributed species that can be found across an extensive range, stretching from the southwestern Indian Ocean to the Indo-Malayan archipelago. Its habitat spans diverse regions such as the Erythraean Sea, Arabian Gulf, and the western littoral zones of Madagascar, reflecting its adaptability to various coastal environments [1]. This species is considered one of the most significant commercial shrimp in the Indo-Pacific region, with notable economic importance along the eastern Mediterranean coast as well [2]. In China, P. semisulcatus is primarily found along the southeastern coast, particularly in the provinces of Fujian, Taiwan, Guangdong, Guangxi, and Hainan Island. Within Hainan, it is most commonly distributed in the eastern and southern waters, with fewer occurrences along the western and northern coasts. This shrimp is prized for its fast growth, large size, and substantial economic value.
P. semisulcatus, as a highly promising aquaculture species, has limited current research focusing mainly on reproductive biology [3,4], population dynamics [5], and other related areas. In contrast, research on species such as Penaeus monodon [6], Litopenaeus vannamei [7], and Fenneropenaeus chinensis [8] is extensive and in-depth, with several studies on their nutritional aspects. However, studies on P. semisulcatus are relatively limited. Researchers evaluating the nutritional value of male and female shrimp from the Mediterranean (Alexandria) and Indian markets identified 16 amino acids and 15 fatty acids. Their findings showed that the edible muscle fat content of both male and female shrimp from Alexandria was higher than that of the same species from the Indian market. Additionally, the carbohydrate content in the muscle of female shrimp was lower compared to that of males [9]. In terms of genetic diversity, the research on P. semisulcatus is relatively broader. A genetic diversity analysis of populations from the Indo-Pacific region revealed that the species can be divided into two major lineages, with a distinct genetic boundary near Bagan Pasir (BGP) in the Strait of Malacca [1]. Additionally, studies on the genetic structure of populations in the Persian Gulf showed significant genetic differentiation among groups, with some loci deviating from Hardy–Weinberg equilibrium and others exhibiting null alleles, suggesting the presence of genetic bottlenecks or selective pressure within the populations [10]. Furthermore, using the RAPD method to analyze the genetic diversity and differentiation of two wild populations of P. semisulcatus from Xiamen and Shantou coasts in China, the results indicated high genetic diversity and low genetic differentiation, suggesting that these populations may belong to the same group and hold potential for further development [11].
While previous studies have offered initial insights into the germplasm resources and genetic diversity of P. semisulcatus, research in this area remains scarce, especially in China, where region-specific studies are still lacking. To address this gap, the present study selected three representative aquaculture regions in China-Haikou, Raoping, and Sanya—for systematic sample collection. Muscle tissue samples of P. semisulcatus were gathered from these areas to assess their nutritional value, and a comprehensive genetic resource database was established. To assess population-level genomic variability, we conducted molecular profiling of P. semisulcatus across sampled geographic zones. By integrating these analyses, we provide empirical evidence to support science-driven conservation planning, ensuring both ecological sustainability and the continued productivity of local fisheries. The methodological framework developed in this study offers valuable, transferable solutions for the preservation and sustainable management of marine organisms, providing a foundation for similar conservation initiatives in marine aquaculture.
2. Materials and Methods
2.1. Sample Collection
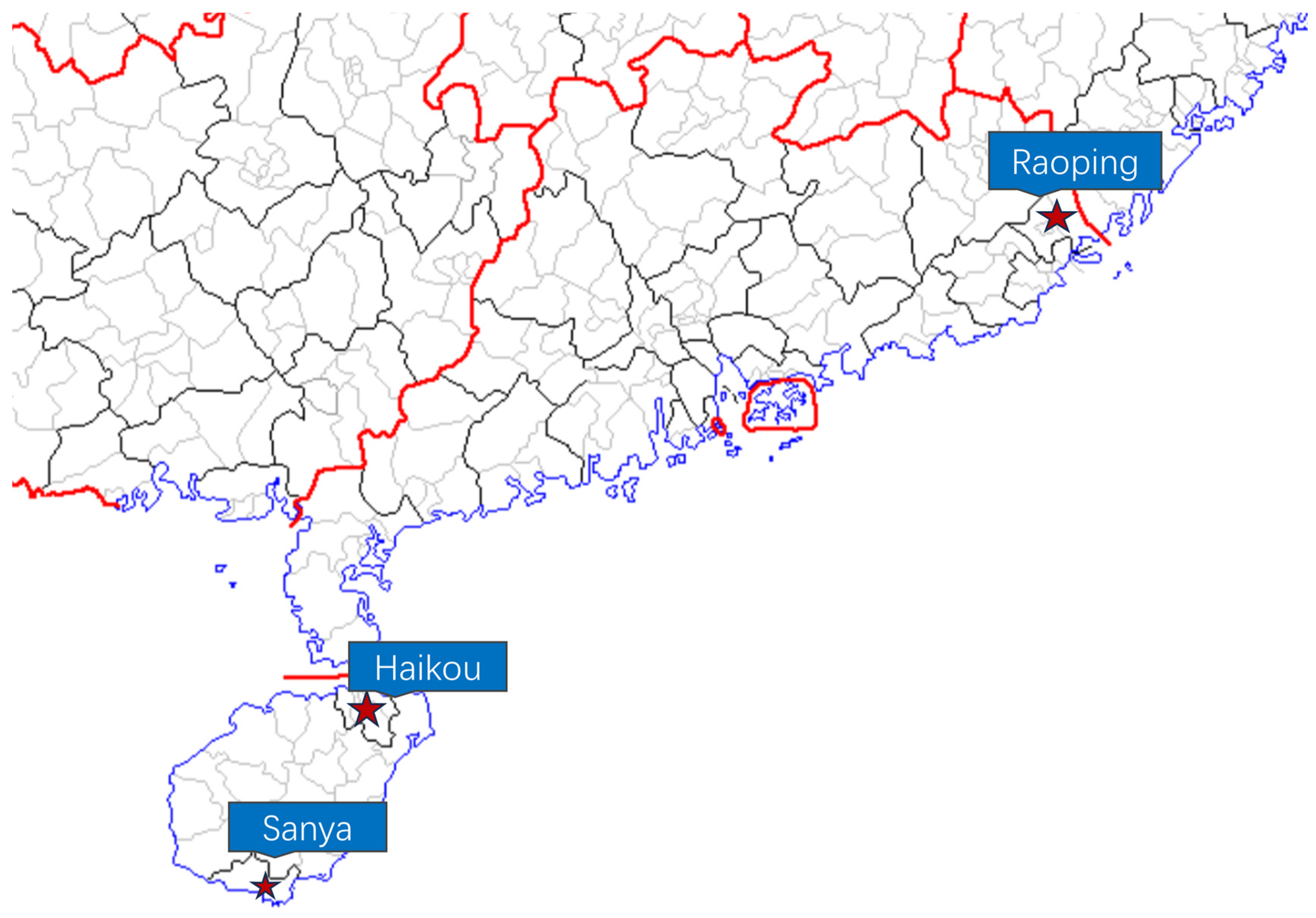
In specific aquaculture regions for P. semisulcatus in China, we randomly selected three representative farming areas: Haikou, Raoping, and Sanya (Table 1, Figure 1). Accurately calibrated instruments, including pH meters, dissolved oxygen sensors, and salinity meters, along with Merck chemical test kits using colorimetric analysis, were employed to measure various aquatic parameters such as salinity, ammonium-N, nitrite levels, pH, dissolved oxygen, and nitrate concentrations. The statistical analysis using ANOVA and t-tests showed significant consistency in these metrics among different breeding groups, further indicating that the aquaculture environments across the surveyed areas are comparable. Notably, recorded water temperatures varied between 27.4 °C and 28.5 °C at measurement points, maintaining stable alkaline conditions with pH levels approximating 8.8. Dissolved oxygen (DO) levels were consistently maintained above 5 mg/L throughout the study. Salinity was carefully monitored using a salinity meter to ensure it remained at 30‰. Additionally, we regularly measured ammonia, nitrite, and nitrate levels to ensure water quality, maintaining ammonia levels below 0.1 mg/L, nitrite below 0.2 mg/L, and nitrate below 50 mg/L at all times. Ninety specimens (approximately 50% males and 50% females) were randomly collected from three aquaculture sites, with thirty specimens selected from each site. These 30 specimens per site were first used to measure body length, weight and genetic diversity. Following this, for the subsequent analyses of amino acid profiling, fatty acid composition and enzyme activity, three individuals were randomly selected from each group for further testing. Muscle tissues (1 g aliquots) were aseptically excised from each organism, transferred to sterile centrifuge tubes, and cryopreserved at −80 °C for subsequent molecular investigations.

Table 1.
Basic information of the sampling sites.
Figure 1.
Map of the survey point. (On the map, red lines mark provincial boundaries, blue represents the coastline, and the circled areas highlight the Special Administrative Regions of Hong Kong and Macao in China.)
2.2. Comprehensive Nutritional Analysis
Moisture quantification was performed in compliance with GB 5009.3-2016 [12] standard protocols, employing precision oven-drying methodology. Pre-weighed samples in calibrated crucibles underwent thermal dehydration at 105 °C for 240 min, followed by vacuum desiccator equilibration and gravimetric measurement through sequential drying cycles until mass stabilization. Ash determination followed GB 5009.4-2016 [13] muffle furnace (Thermo Scientific Thermolyne, Waltham, MA, USA) protocols, while protein content (GB 5009.5-2016 [14]) and lipid fractions (GB/T 5009.6-2016 [15]) were analyzed via micro-Kjeldahl (Kjeltec 8400, Hillerød, Denmark) distillation and automated Soxhlet (LabTech LT-01, Beijing, China) extraction systems, respectively. Carbohydrate composition was established per GB/T 15672-2009 [16] specifications using sulfuric acid–phenol chromogenic reactions with spectrophotometric detection.
2.3. Nutritional and Antioxidant Analysis
Amino acid profiling was conducted per GB 5009.124-2016 [17] specifications through reverse-phase HPLC separation with UV detection (440 nm for proline (Pro) and 570 nm for other amino acids). Lipid characterization was conducted following the GB 5009.168-2016 [18] guidelines, using certified reference materials for the quantitative analysis of fatty acid methyl esters (FAMEs). The preparation of FAMEs involved an acid-catalyzed methylation process, where fatty acids were esterified with methanol in the presence of sulfuric acid as the catalyst, ensuring complete methylation for accurate analysis. Antioxidant biomarkers were evaluated using standardized commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China): Superoxide Dismutase (SOD) activity via WST-8 redox cycling (A001-1-2), Total Antioxidant Capacity (T-AOC) through ferric reducing power assay (A015-1), and Catalase (CAT) enzymatic conversion using ammonium molybdate chromogenic system (A007-1-1).
2.4. Genetic Diversity
DNA samples that met the quality criteria were sequenced using the MGISEQ series sequencer (MGI Tech Co., Ltd., Shenzhen, China). In this study, a total of 90 individuals were sequenced, generating a total of 2562.36 Gb of data. On average, each sample produced 28.47 Gb of sequencing data, which corresponds to an average sequencing depth of 14.23X. After read alignment, we observed that the average alignment rate to the reference genome was 64.88%, with an average sequencing depth of 50.67X across the aligned reads. The process involved random fragmentation, fragment selection, end repair, adapter ligation, PCR preparation of DNA nanoballs (DNBs), and sequencing on a patterned sequencing chip. The sequencing quality was assessed using Phred scores, and Raw sequencing reads underwent quality control processing via fastp (v0.23.2) with adapter trimming and quality filtering (Q20 > 90%, read length ≥ 150 bp), generating high-fidelity data suitable for downstream genomic analyses. Next, clean data were aligned to the reference genome using BWA (Wellcome Trust Sanger Institute, Cambridge, UK), with re-alignment performed around InDel edge regions. SNP detection was performed using GATK’s HaplotypeCaller (Broad Institute, GATK 4.x, https://gatk.broadinstitute.org/), generating mutation files in VCF format with variant annotations. For multi-sample analysis, gVCF files were first generated for each individual sample and subsequently processed using GATK’s GenotypeGVCFs (Broad Institute, GATK 4.x, https://gatk.broadinstitute.org/) tool for joint genotyping, enabling the identification of population-level genetic variations.
2.5. Data Analysis
Quantitative data processing was executed in SPSS (v16.0) through hierarchical analytical protocols. Primary statistical evaluation employed one-way ANOVA to detect intergroup variations, followed by Duncan’s post-hoc testing when omnibus significance thresholds (α = 0.05) were attained, establishing pairwise differential significance. All values are expressed as x ± s. In our analysis, various indices were used to measure genetic variation, including SNP density = SNP count/genome size (Kb), Ho = het/(het + hom), PIC = 1 − Σpi2 − ΣΣ2pi2pj2, π = n/(n − 1) Σpipjπij, F = (Ho − He)/(n − He).
3. Results
3.1. Analysis of Overall Nutritional Composition
A routine nutritional analysis in the three P. semisulcatus aquaculture populations (Table 2) revealed the following ranges: moisture (74.07–74.57 g/100 g), ash (1.77–2.07 g/100 g), crude fat (0.80–0.83 g/100 g), crude protein (21.77–22.37 g/100 g), and total sugar (29.92–30.92%). Overall, the nutritional composition showed minimal variation among the populations, with no significant differences in moisture, crude protein, crude fat, or total sugar content (p > 0.05). However, ash content varied significantly (p < 0.05), with the PsRP population having the highest value (2.07 g/100 g), significantly differing from PsHK and PsSY (p < 0.05). In contrast, no significant difference was found between PsHK (1.77 g/100 g) and PsSY (1.80 g/100 g) (p > 0.05).

Table 2.
Nutrient profile in the myotomal tissues across three populations of P. semisulcatus.
3.2. Analysis of Amino Acids
Chromatographic quantification of muscle amino acids in triplicate P. semisulcatus stocks (Table 3) resolved 17 proteogenic components, comprising seven essential (EAA), two semi-essential (SEAA), and eight non-essential (NEAA) variants. Multivariate analysis revealed statistical divergences (p < 0.05) in 13 amino acid concentrations across cohorts, whereas Thr, Ser, Cys, and Arg demonstrated conserved concentrations (p > 0.05) irrespective of population origin.

Table 3.
Amino acid composition and levels in the myotomal tissues of the three P. semisulcatus populations.
Among the 17 amino acids, Glu had the highest concentration (2.07–2.30 g/100 g). The PsSY population had the lowest Glu content (2.07 g/100 g), which was significantly lower than that of PsHK (2.30 g/100 g) and PsRP (2.24 g/100 g) (p < 0.05). In contrast, Cys had the lowest concentration (0.10–0.11 g/100 g), with no significant differences among the populations (p > 0.05). Overall, amino acid levels were highest in PsHK, followed by PsRP, with PsSY having the lowest content.
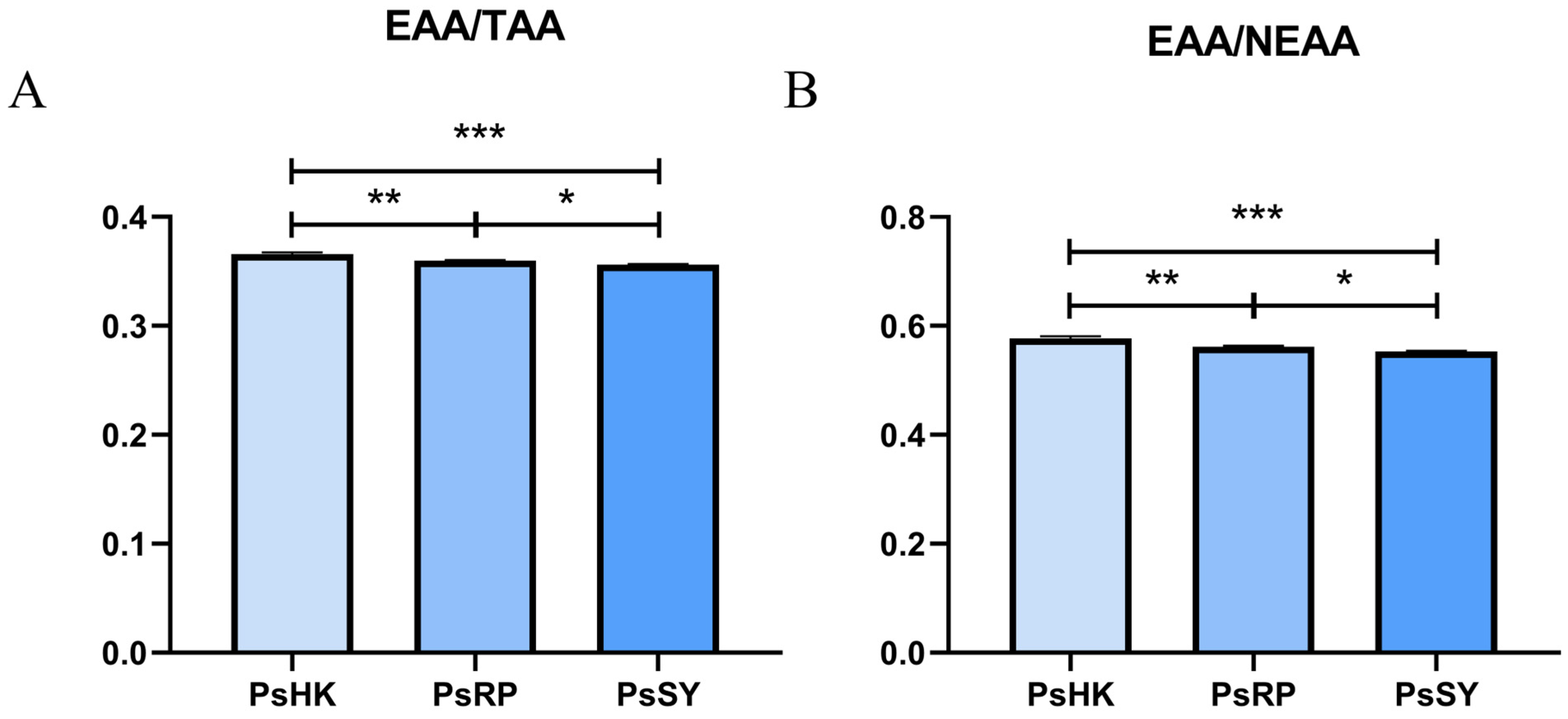
Comparison of essential amino acids (EAA) among the three populations revealed significant differences (p < 0.05). The total EAA content ranged from 4.99 to 5.49 g/100 g, with PsHK exhibiting the highest levels. Additionally, delicious amino acids (DAA) ranged from 5.77 to 6.08 g/100 g, semi-essential amino acids (SEAA) from 1.89 to 11.98 g/100 g, and non-essential amino acids (NEAA) from 9.01 to 9.54 g/100 g. The total amino acid (TAA) content varied between 14.00 and 15.00 g/100 g. Although no significant differences were observed in total amino acid content among the populations (p > 0.05), significant variations were noted in the EAA/TAA and SEAA ratios between groups (p < 0.05) (Figure 2).
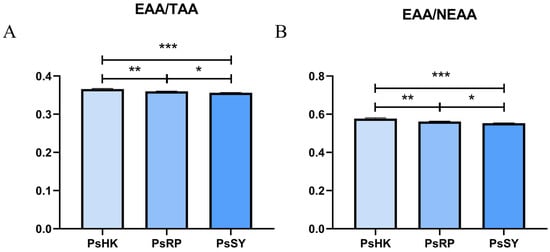
Figure 2.
Amino acid composition and levels in the myotomal tissues of the three P. semisulcatus populations (n = 3). (A): EAA/TAA: essential amino acids/total amino acids. (B): EAA/NEAA: essential amino acids/total non-essential amino acids. Different numbers of symbols between treatments indicate significant differences (* 0.01 < p < 0.05; ** p < 0.01, *** p < 0.001).
3.3. Analysis of Fatty Acids
Fatty acid analysis in the three P. semisulcatus aquaculture populations revealed 13 identified fatty acids (Table 4). C18:0 had the highest concentration (88.27–108.00 mg/100 g), while C20:2 had the lowest (3.40–4.70 mg/100 g). Except for C22:1n9, the PsHK population exhibited the highest levels of all other fatty acids, showing significant differences from the other two populations (p < 0.05). In contrast, PsRP and PsSY displayed varying fatty acid contents, but most differences were not significant (p > 0.05), indicating similar fatty acid compositions.

Table 4.
Muscle fatty acid composition and content of three groups of P. semisulcatus.
When comparing the contents of total fatty acids, saturated fatty acids, monounsaturated fatty acids, and polyunsaturated fatty acids, the PsHK population had the highest content in all these categories, with significant differences from the PsRP and PsSY populations (p < 0.05). However, no significant difference was observed between the PsRP and PsSY populations. Specifically, the saturated fatty acid content in the three populations ranged from 176.70–222.50 mg/100 g, the monounsaturated fatty acid content ranged from 59.10–91.27 mg/100 g, and the total fatty acid content ranged from 403.50–506.70 mg/100 g.
3.4. Analysis of Antioxidant Enzymes
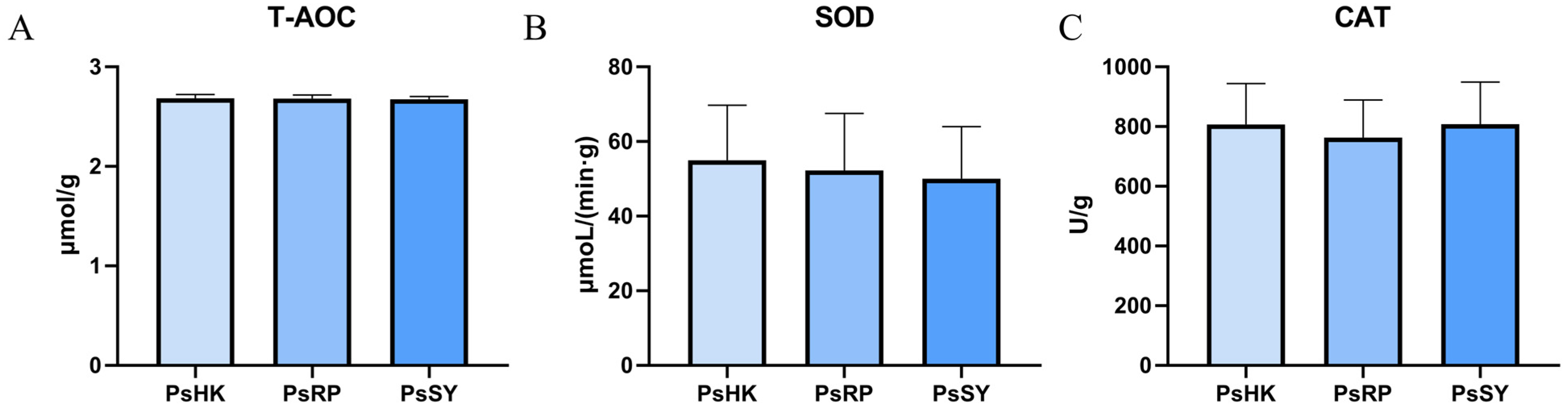
In the assessment of the activity of three antioxidant enzymes (T-AOC, SOD, and CAT) across the three P. semisulcatus populations, the results showed no significant differences among the populations (p > 0.05) (Figure 3). Specifically, the T-AOC activity ranged from 2.67–2.68 μmol/g, SOD activity ranged from 50.07–54.96 μmol/(min·g), and CAT activity ranged from 762.7–807.8 U/g. Among these three enzyme activities, the PsHK population generally exhibited higher enzyme activity, indicating stronger antioxidant capacity. In contrast, the PsSY population showed the lowest levels of T-AOC and SOD activities, suggesting weaker antioxidant capacity. Notably, the PsRP population displayed the lowest CAT activity at 762.7 U/g, which was significantly lower than the other two populations, indicating a relatively reduced ability to scavenge peroxides.
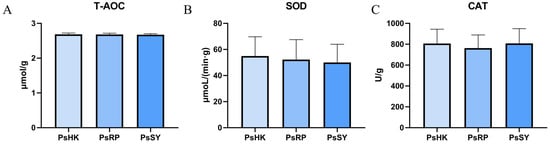
Figure 3.
Physiological and biochemical indicators in the muscle of the three P. semisulcatus groups. (A): The activity of T-AOC. (B): The activity of SOD. (C): The activity of CAT. Mean ± standard deviation (n = 3).
3.5. Genetic Diversity Analysis
The genetic diversity analysis of three cultured P. semisulcatus populations revealed that the average SNP density ranged from 1.541–2.109 (Table 5). Nucleotide diversity (π) values were similar among populations, ranging from approximately 3.37 × 10−4 to 4.50 × 10−4. The inbreeding coefficient (FHOM) ranged from 6.20% to 9.21%, with the PsRP population showing the lowest value, indicating the least risk of inbreeding. The average polymorphic information content (PIC) ranged from 0.094 to 0.129, indicating the genetic diversity of the markers employed. The observed heterozygosity (Ho) had an average value between 0.118 and 0.130, reflecting the frequency of heterozygous individuals within the populations.

Table 5.
Statistics of genetic diversity parameters of three populations of P. semisulcatus.
4. Discussion
4.1. Comparative Analysis of Fundamental Nutritional Components in P. semisulcatus Populations
Water is a fundamental component for all living organisms, and for aquatic species, the regulation of water balance—between intake and loss—is primarily governed by osmotic processes. Aquatic organisms regulate their internal osmotic pressure to ensure equilibrium with the surrounding environment and maintain proper water balance [19]. Additionally, minerals in the ash content are important components that make up the skeleton and hard shells of aquatic organisms [20] and are also essential trace elements for maintaining physiological functions [21]. Protein is the material basis of life and an important component of all living organisms, playing a crucial role in biological activities [22]. Crude protein is an estimate of the nitrogen content in protein, mainly providing energy, forming the body, and regulating physiological functions [23]. Crude fat is the energy storage form in organisms [24] and also a part of cell membranes. In aquatic organisms, crude fat helps maintain body temperature and protects internal organs [25]. Total sugars are one of the main energy sources in organisms, providing rapid energy for aquatic organisms to support their activity and growth [26,27]. Therefore, in P. semisulcatus, these routine nutrients are essential components of their bodily functions and primary energy sources.
In the analysis of the routine nutritional components of P. semisulcatus, it was found that the crude fat content ranged from 0.80 to 0.83 g/100 g, which is lower than that of L. vannamei (0.8–1.1%) [28], Marsupenaeus japonicus (2.09 g/100 g) [29], and Metapenaeus ensis (0.83–1.03 g/100 g) [30]. Meanwhile, the crude protein content of P. semisulcatus ranged from 21.77–22.37 g/100 g, which is higher than that of Fenneropenaeus merguiensis (20.83–21.97 g/100 g) [31], M. japonicus (14.60 g/100 g), Procambarus clarkii (12.33 g/100 g), and P. monodon (13.29 g/100 g) [32]. Compared to P. chinensis (1.69 g/100 g) and L. vannamei (1.52 g/100 g) [33], the ash content of P. semisulcatus (1.77–2.07 g/100 g) is relatively higher, with significant differences among the populations (p < 0.05). Overall, P. semisulcatus is a protein-rich, low-fat food. Among the three populations of P. semisulcatus, the PsRP population had relatively low crude fat content but higher ash content, which may give it an advantage in terms of routine nutritional components.
4.2. Comparative Analysis of Amino Acid Profiles in P. semisulcatus Populations
Amino acids in aquatic organisms serve several critical functions. They are fundamental building blocks of proteins and act as precursors to various essential biomolecules, including enzymes, hormones, and antibodies. These molecules are crucial for growth and development, and they play vital roles in osmoregulation, hypoxia tolerance, shell formation, metabolic processes, antioxidant defense, and immune response [34,35,36]. Amino acids assist aquatic organisms in regulating their internal osmotic pressure, adapting to environments with fluctuating salinity, and enhancing their ability to survive under hypoxic conditions [37,38]. Additionally, they are involved in synthesizing important biomolecules, such as hormones and neurotransmitters, and play a role in antioxidant and immune responses, thus enhancing the overall health and survival ability of aquatic organisms [34]. Furthermore, certain amino acids can serve as an energy source, providing support during stress responses or periods of hunger [39,40]. The balance and metabolism of amino acids have significant effects on the growth, development, and reproduction of aquatic organisms.
In this study, EAA content in P. semisulcatus ranged from 4.99 to 5.49 g/100 g, which is lower than that of P. monodon (5.44–6.41 g/100 g) [6]. Among these, glutamic acid had the highest content, while cysteine had the lowest, a trend similar to that seen in M. ensis [30]. The total amino acid content in P. semisulcatus ranged from 14.00–15.00 g/100 g, slightly lower than that in P. monodon (15.68–18.63 g/100 g) [6]. According to the ideal model proposed by FAO/WHO, the EAA/TAA ratio for high-quality proteins should be around 40%, and P. semisulcatus’s EAA/TAA ratio generally meets this requirement, indicating a good amino acid balance. However, the overall amino acid level in P. semisulcatus is relatively low, which may suggest nutritional deficiencies or environmental stress. Among the three populations of P. semisulcatus, the PsHK population exhibited relatively higher amino acid levels, demonstrating an advantage in terms of amino acid content.
4.3. Comparative Analysis of Fatty Acid Profiles Across P. semisulcatus Populations
Fatty acids in aquatic organisms fulfill several essential functions. They are key components of cell membranes, where they influence membrane fluidity and functionality. Additionally, fatty acids serve as a primary energy source. Through β-oxidation in the mitochondria, fatty acids release energy that is critical for the growth, development, and reproduction of aquatic organisms [41]. Particularly, polyunsaturated fatty acids (PUFAs) regulate inflammatory responses, promote cell proliferation and differentiation, and are critical for the growth, development, reproductive health, and maintenance of the nervous system in aquatic organisms [42,43,44]. Monounsaturated fatty acids (MUFAs) have a positive effect on promoting blood circulation, regulating blood glucose and lipid metabolism, and effectively lowering cholesterol levels [45]. Additionally, certain fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have positive effects on the immune system of aquatic organisms, enhancing disease resistance and improving survival rates [46]. These fatty acids are converted in aquatic organisms through a series of dehydrogenation and elongation reactions, playing a key role in maintaining the integrity and function of cell membranes [47].
In this study, P. semisulcatus had the highest content of C18:0 and the lowest content of C20:2, a trend similar to that observed in M. ensis [30]. All samples showed a higher content of polyunsaturated fatty acids (ΣPUFA) compared to monounsaturated fatty acids (ΣMUFA). The saturated fatty acid content ranged from 176.70–222.50 mg/100 g, slightly higher than that of P. monodon (125.49–221.79 mg/100 g) [6]. The monounsaturated fatty acid content ranged from 59.10–91.27 mg/100 g, slightly lower than that in M. ensis (75.1–104.2 mg/100 g) [30] but higher than in L. vannamei (14.79–17.67 mg/100 g) [28] and F. merguiensis (18.85 mg/100 g) [33]. Moreover, the DHA + EPA content in this study ranged from 103.70–123.20 mg/100 g, higher than that of F. merguiensis (90.20–131.4 mg/100 g) [31] and Penaeus japonicus (11.58 mg/100 g) [32]. Overall, P. semisulcatus has relatively high levels of fatty acids and shows similar trends to other shrimp species, indicating a higher nutritional value and relatively balanced fatty acid levels. Among the three populations of P. semisulcatus, the PsHK population exhibited the highest levels of all fatty acids, suggesting that PsHK has a relatively higher nutritional value in terms of fatty acid content.
4.4. Comparative Analysis of Antioxidant Enzyme Activity Across P. semisulcatus Populations
Antioxidant enzymes in aquatic animals, such as T-AOC, SOD, and CAT, play crucial roles in the organism’s defense system against oxidative stress. These enzymes work together to protect cells from damage caused by reactive oxygen species (ROS) [48,49]. T-AOC is a key indicator of antioxidant capacity, helping to neutralize free radicals and reactive oxygen species (ROS) in the body to prevent oxidative damage [50]. SOD primarily converts superoxide anion radicals into hydrogen peroxide and oxygen, thereby reducing the accumulation of free radicals and protecting cells from oxidative stress [48]. CAT further catalyzes the breakdown of hydrogen peroxide into water and oxygen, preventing its conversion into more harmful hydroxyl radicals in the presence of metal ions [51]. It is a key defense against the accumulation of hydrogen peroxide in cells. These antioxidant enzymes not only protect aquatic organisms from oxidative stress but also help maintain the redox balance within the body under environmental stress conditions, mitigate oxidative damage, and enhance adaptability and immune function.
In this study, the total antioxidant capacity (T-AOC) activity in P. semisulcatus from three different regions in China ranged from 2.67–2.68 μmol/g, higher than that of P. clarkii (2.57 U/mg) [52]. The SOD activity ranged from 50.07 to 54.96 μmol/(min·g), which is higher than that of P. monodon (39.65–47.29 U/g) [6] and F. merguiensis (46.85–53.98 U/g) [31]. Higher levels of T-AOC, SOD, and CAT indicate stronger antioxidant and immune capabilities. P. semisulcatus exhibits higher antioxidant enzyme activity compared to other shrimp species, suggesting it has stronger antioxidant and immune capabilities, enabling it to better resist oxidative stress. Among the three populations of P. semisulcatus, the PsHK group displayed the highest antioxidant enzyme activity, indicating that it has a stronger antioxidant capacity within these farming populations.
4.5. Genetic Diversity Comparison Among P. semisulcatus Populations
Genetic diversity is the basis of biological variation and a cornerstone for species’ adaptation, survival, and evolutionary potential in complex and ever-changing environments [53]. Increased genetic diversity helps mitigate the detrimental effects of inbreeding, reduces the impact of genetic drift, and promotes the overall health and vitality of populations. Genetic diversity also underpins selective breeding in aquaculture, enabling the improvement of economically important traits like growth rate, meat quality, and disease resistance, thus enhancing farming efficiency. SNP density indicates the frequency of SNP loci in the genome, with higher density typically linked to increased genetic variation [54]. Nucleotide polymorphism (π) measures sequence variation within specific genomic regions and serves as a key parameter for evaluating intraspecific genetic diversity [55]. In the genetic diversity analysis of P. semisulcatus, the PsHK population showed higher values in various genetic parameters compared to the other two populations. The analytical results indicate that all three investigated P. semisulcatus populations exhibit comparatively limited genetic variability. Notably, the PsHK population displayed marginally elevated genetic diversity levels within this constrained variability framework.
5. Conclusions
This study systematically evaluated the nutritional composition, antioxidant properties, and genetic diversity of three cultured populations of P. semisulcatus. The results indicated that the PsHK population had relatively higher amino acid and fatty acid contents, reflecting superior nutritional value. Antioxidant enzyme activity showed no significant differences between populations, indicating similar antioxidant capacities. Genetic analysis revealed that the PsHK population exhibited the highest genetic diversity and greater genetic differentiation from the PsRP population. Overall, P. semisulcatus exhibited aquaculture traits of low protein and high fat, with good nutritional quality, making it suitable for broader aquaculture promotion. Among the three populations, PsHK stood out in both nutritional composition and genetic background, providing valuable insights for future germplasm conservation, genetic improvement, and industrial development.
Author Contributions
F.Z. and T.L. conceived the project and supervised the work. Y.L., Z.J. and J.C. performed the bioinformatics analysis and prepared the manuscript, the table and figures. Y.L. and J.C. conducted the experiment. L.Y., S.J., Q.Y., J.H., Y.D. and J.S. collected the samples and performed sequencing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key R&D Program of China (2022YFD2401900); Central Public-interest Scientific Institution Basal Research Fund, CAFS (2023TD34); China Agriculture Research System (CARS-48); Guangdong Basic and Applied Basic Research Foundation (2023A1515012410); Hainan Provincial Natural Science Foundation of China (323MS127); Earmarked fund for HNARS (HNARS-10-ZJ01); Provincial Rural Revitalization Strategy Special Fund Seed Industry Revitalization Project (2024-SPY-00-005); Hainan Seed Industry Laboratory-Joint Unveiling of the Project (B24YQ0010); Central Public-interest Scientific Institution Basal Research Fund, South China Sea Fisheries Research Institute, CAFS (No. 2024RC06); Research on breeding technology of candidate species for Guangdong modern marine ranching (2024-MRB-00-001).
Institutional Review Board Statement
The animal study was reviewed and approved by the Animal Care and Use Committee of the South China Sea Fisheries Research Institute.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Halim, S.A.A.A.; Othman, A.S.; Akib, N.A.M.; Jamaludin, N.-A.; Esa, Y.; Nor, S.A.M. Mitochondrial Markers Identify a Genetic Boundary of the Green Tiger Prawn (Penaeus semisulcatus) in the Indo-Pacific Ocean. Zool. Stud. 2021, 60, 8. [Google Scholar] [CrossRef]
- Kumlu, M.; Eroldogan, O.T.; Aktas, M. Effects of Temperature and Salinity on Larval Growth, Survival and Development of Penaeus semisulcatus. Aquaculture 2000, 188, 167–173. [Google Scholar] [CrossRef]
- Rajkumar, M.; Pillai, S.L.; Rameshkumar, P.; Saravanan, R.; Thirumalaiselvan, S.; Jose, J.; Sobhana, K.S.; George, M.R. Reproductive Biology of Green Tiger Shrimp Penaeus semisulcatus De Haan, 1844 in Palk Bay, Southeast Coast of India. Reg. Stud. Mar. Sci. 2023, 66, 103161. [Google Scholar]
- Niamaimandi, N.; Aziz, A.; Siti Khalijah, D.; Che Roos, S.; Kiabi, B. Reproductive Biology of the Green Tiger Prawn (Penaeus semisulcatus) in Coastal Waters of Bushehr, Persian Gulf. ICES J. Mar. Sci. 2008, 65, 1593–1599. [Google Scholar]
- Alrashada, Y.N.; Tharwat, A.; Boqursain, A. Population Dynamics of the Green Tiger Prawn, Penaeus semisulcatus de Haan, 1844 in the Saudi Coast of the Arabian Gulf. Indian J. Anim. Res. 2019, 1–6. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Jiang, S.; Huang, J.; Jiang, S.; Yang, Q.; Yang, L.; Shi, J.; Zhou, F. A Comprehensive Study on Nutritional Quality, Physiological Enzyme Activity and Genetic Diversity in Six Populations of Penaeus Monodon. Aquacult. Int. 2024, 32, 10141–10157. [Google Scholar] [CrossRef]
- Li, Y.; Cao, S.; Jiang, S.; Huang, J.; Yang, Q.; Jiang, S.; Yang, L.; Zhou, F. Comparative Study of Nutritional Composition, Physiological Indicators, and Genetic Diversity in Litopenaeus vannamei from Different Aquaculture Populations. Biology 2024, 13, 722. [Google Scholar] [CrossRef]
- Meng, X.; Fu, Q.; Luan, S.; Luo, K.; Sui, J.; Kong, J. Genome Survey and High-Resolution Genetic Map Provide Valuable Genetic Resources for Fenneropenaeus chinensis. Sci. Rep. 2021, 11, 7533. [Google Scholar]
- El-Gendy, A.M.; El-Feky, F.; Mahmoud, N.H.; Elsebakhy, G.S. Evaluation of Nutritional Quality of Green Tiger Prawn, Penaeus semisulcatus from Land Fisheries (Alexandria) and Market (India). Egypt. J. Hosp. Med. 2018, 70, 924–934. [Google Scholar] [CrossRef]
- Tamadoni Jahromi, S.; Sofman Othman, A.; Rosazlina, R.; Pourmozaffar, S.; Gozari, M. Population Genetics of Penaeus semisulcatus from Persian Gulf and Oman Sea Using Newly Developed DNA Microsatellite Markers. Iran. J. Fish. Sci. 2021, 20, 157–178. [Google Scholar] [CrossRef]
- Shuhua, T.A.N.; Guizhong, W.; Qiongwu, L.I.N. Genetic Diversity of Two Wild Populations of Penaeus semisulcatus Revealed by RAPD Technique. Acta Ecol. Sin. 2006, 26, 3907–3911. [Google Scholar]
- GB 5009.3-2016; Determination of Moisture in Food. National Standards of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.4-2016; National Food Safety Standard—Determination of Ash in Food. National Standards of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.5-2016; National Standards for Food Safety—Determination of Proteins in Foods. National Standards of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.6-2016; National Food Safety Standard—Determination of Crude Fat in Food. National Standards of the People’s Republic of China: Beijing, China, 2016.
- GB/T 15672-2009; Determination of Total Sugar Content in Edible Fungi. National Standards of the People’s Republic of China: Beijing, China, 2009.
- GB 5009.124-2016; National Food Safety Standard—Determination of Amino Acids in Foods by Hydrochloric Acid Hydrolysis. National Standards of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.168-2016; National Food Safety Standard—Determination of Fatty Acids in Foods by Gas Chromatography. National Standards of the People’s Republic of China: Beijing, China, 2016.
- Jin, X.; Wang, X.; Tse, W.K.F.; Shi, Y. Homeostasis and Physiological Regulation in the Aquatic Animal during Osmotic Stress. Front. Physiol. 2022, 13, 977185. [Google Scholar] [CrossRef]
- Boyd, C.E.; Lawrence, J.M. The Mineral Composition of Several Fresh-Water Algae. 1967. Available online: https://seafwa.org/sites/default/files/journal-articles/BOYD-413.pdf (accessed on 1 March 2025).
- Lall, S.P.; Kaushik, S.J. Nutrition and Metabolism of Minerals in Fish. Animals 2021, 11, 2711. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z. Foundations for the Study of Structure and Function of Proteins. In Basics of Bioinformatics; Jiang, R., Zhang, X., Zhang, M.Q., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 303–336. ISBN 978-3-642-38950-4. [Google Scholar]
- Alhotan, R.A.; Pesti, G.M.; Billard, L. The Linear Relationship between True Protein and Nitrogen Contents of Feed and Food Ingredients: Calculating True Protein from a New Conversion Factor. Cogent Food Agric. 2024, 10, 2428821. [Google Scholar] [CrossRef]
- Ahmed, I.; Jan, K.; Fatma, S.; Dawood, M.A.O. Muscle Proximate Composition of Various Food Fish Species and Their Nutritional Significance: A Review. Anim. Physiol. Nutr. 2022, 106, 690–719. [Google Scholar] [CrossRef] [PubMed]
- Kainz, M.; Brett, M.T.; Arts, M.T. (Eds.) Lipids in Aquatic Ecosystems; Springer: New York, NY, USA, 2009; ISBN 978-0-387-88607-7. [Google Scholar]
- dos Santos Carvalho, C.; Fernandes, M.N. Effect of Copper on Liver Key Enzymes of Anaerobic Glucose Metabolism from Freshwater Tropical Fish Prochilodus lineatus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 437–442. [Google Scholar] [CrossRef]
- Dos Santos Carvalho, C.; Fernandes, M.N. Effects of Copper Toxicity at Different pH and Temperatures on the in Vitro Enzyme Activity in Blood and Liver of Fish, Prochilodus lineatus. Mol. Biol. Rep. 2019, 46, 4933–4942. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Li, H.; Jiang, X.; Ji, L.; Liu, T.; Sun, Y. Chemical and Quality Evaluation of Pacific White Shrimp Litopenaeus vannamei: Influence of Strains on Flesh Nutrition. Food Sci. Nutr. 2021, 9, 5352–5360. [Google Scholar] [CrossRef]
- Xu, L.; Yan, B. Nutritional Component Analysis and Quality Evaluation of Penaeus Japonicus. Food Sci. 2011, 32, 297–301. [Google Scholar]
- Li, Y.; Chen, J.; Jiang, S.; Yang, Q.; Yang, L.; Huang, J.; Shi, J.; Zhang, Y.; Lu, Z.; Zhou, F. A Comprehensive Assessment of Nutritional Value, Antioxidant Potential, and Genetic Diversity in Metapenaeus ensis from Three Different Populations. Biology 2024, 13, 838. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Cao, S.; Jiang, Z.; Jiang, S.; Yang, Q.; Yang, L.; Huang, J.; Shi, J.; Ma, Z. A Comprehensive Assessment of the Nutritional Value, Antioxidant Potential, and Genetic Diversity of Fenneropenaeus merguiensis from Three Different Regions in China. Biology 2024, 13, 1002. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Q.; Zhang, D.; Wei, S.; Sun, Q.; Xia, Q.; Shi, W.; Ji, H.; Liu, S. Comparison of the Proximate Composition and Nutritional Profile of Byproducts and Edible Parts of Five Species of Shrimp. Foods 2021, 10, 2603. [Google Scholar] [CrossRef]
- Wang, J. Comparision of Nutritional Composition in Muscle of Penaeus chinensis, Penaeus vannamei Boone and Penaeus japonicuss Bate. Food Sci. Technol. 2013, 38, 145–150. [Google Scholar]
- Li, P.; Yin, Y.-L.; Li, D.; Kim, S.W.; Wu, G. Amino Acids and Immune Function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar]
- Huang, M.; Gao, Q.; Yang, X.; Jiang, W.; Hao, L.; Yu, Y.; Tian, Y. Free Amino Acids in Response to Salinity Changes in Fishes: Relationships to Osmoregulation. Fish Physiol. Biochem. 2023, 49, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Functional Amino Acids in Nutrition and Health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef]
- Niu, J.; Hu, X.L.; Ip, J.C.; Ma, K.Y.; Tang, Y.; Wang, Y.; Qin, J.; Qiu, J.-W.; Chan, T.F.; Chu, K.H. Multi-Omic Approach Provides Insights into Osmoregulation and Osmoconformation of the Crab Scylla paramamosain. Sci. Rep. 2020, 10, 21771. [Google Scholar] [PubMed]
- Huo, D.; Zhang, L.; Yang, H.; Sun, L. Adaptation to Hypoxic Stress Involves Amino Acid Metabolism: A Case in Sea Cucumber. Environ. Pollut. 2023, 330, 121766. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.; Hildebrandt, T.M. The Role of Amino Acid Metabolism During Abiotic Stress Release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, H.; Zou, Z.; Yao, H.; Lin, Z.; Dong, Y. Effects of Medium-and Long-Term High-Salinity Environments on Free Amino Acid Content and Related Genes of Sinonovacula constricta. Front. Mar. Sci. 2024, 11, 1368952. [Google Scholar] [CrossRef]
- Houten, S.M.; Violante, S.; Ventura, F.V.; Wanders, R.J.A. The Biochemistry and Physiology of Mitochondrial Fatty Acid β-Oxidation and Its Genetic Disorders. Annu. Rev. Physiol. 2016, 78, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X.; Weylandt, K.H. Modulation of Inflammatory Cytokines by Omega-3 Fatty Acids. In Lipids in Health and Disease; Quinn, P.J., Wang, X., Eds.; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2008; Volume 49, pp. 133–143. ISBN 978-1-4020-8830-8. [Google Scholar]
- Fritsche, K.L. The Science of Fatty Acids and Inflammation. Adv. Nutr. 2015, 6, 293S–301S. [Google Scholar] [PubMed]
- Rashid, M.A.; Haque, M.; Akbar, M. Role of Polyunsaturated Fatty Acids and Their Metabolites on Stem Cell Proliferation and Differentiation. In The Benefits of Natural Products for Neurodegenerative Diseases; Essa, M.M., Akbar, M., Guillemin, G., Eds.; Advances in Neurobiology; Springer International Publishing: Cham, Switzerland, 2016; Volume 12, pp. 367–380. ISBN 978-3-319-28381-4. [Google Scholar]
- Cao, X.; Xia, J.; Zhou, Y.; Wang, Y.; Xia, H.; Wang, S.; Liao, W.; Sun, G. The Effect of Mufa-Rich Food on Lipid Profile: A Meta-Analysis of Randomized and Controlled-Feeding Trials. Foods 2022, 11, 1982. [Google Scholar] [CrossRef]
- Calder, P.C. N-3 Fatty Acids, Inflammation and Immunity: New Mechanisms to Explain Old Actions. Proc. Nutr. Soc. 2013, 72, 326–336. [Google Scholar] [PubMed]
- Lee-Okada, H.-C.; Xue, C.; Yokomizo, T. Recent Advances on the Physiological and Pathophysiological Roles of Polyunsaturated Fatty Acids and Their Biosynthetic Pathway. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2025, 1870, 159564. [Google Scholar]
- Zhang, L.; Zhao, M.; Wang, Y. A Review on Superoxide Dismutases of Hydrobios. 2012, pp. 800–804. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20123388727 (accessed on 1 March 2025).
- Attia, H.G.; El-Morshedy, S.M.; Nagy, A.M.; Ibrahim, A.M.; Aleraky, M.; Abdelrahman, S.S.; Osman, S.M.; Alasmari, S.M.; El Raey, M.A.; Abdelhameed, M.F. Citrus Clementine Peel Essential Oil Ameliorates Potassium Dichromate-Induced Lung Injury: Insights into the PI3K/AKT Pathway. Metabolites 2024, 14, 68. [Google Scholar] [CrossRef]
- Silvestrini, A.; Meucci, E.; Ricerca, B.M.; Mancini, A. Total Antioxidant Capacity: Biochemical Aspects and Clinical Significance. Int. J. Mol. Sci. 2023, 24, 10978. [Google Scholar] [CrossRef]
- Carmo De Carvalho E Martins, M.D.; Martins; Da Silva Santos Oliveira, A.S.; Da Silva, L.A.A.; Primo, M.G.S.; De Carvalho Lira, V.B. Biological Indicators of Oxidative Stress [Malondialdehyde, Catalase, Glutathione Peroxidase, and Superoxide Dismutase] and Their Application in Nutrition. In Biomarkers in Nutrition; Patel, V.B., Preedy, V.R., Eds.; Biomarkers in Disease: Methods, Discoveries and Applications; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–25. ISBN 978-3-030-81304-8. [Google Scholar]
- Tan, S.H.; Deng, X.Y.; Jiang, W.M.; He, F.L. Effects of High Level Chromium on Antioxidant Enzyme System in Gill and Hepatopancreas of Procambarus Clarkii. J. Agro-Environ. Sci. 2007, 23, 24–34. [Google Scholar]
- O’Connell, M.; Wright, J.M. Microsatellite DNA in Fishes. Rev. Fish Biol. Fish. 1997, 7, 331–363. [Google Scholar] [CrossRef]
- Matukumalli, L.K.; Lawley, C.T.; Schnabel, R.D.; Taylor, J.F.; Allan, M.F.; Heaton, M.P.; O’Connell, J.; Moore, S.S.; Smith, T.P.; Sonstegard, T.S. Development and Characterization of a High Density SNP Genotyping Assay for Cattle. PLoS ONE 2009, 4, e5350. [Google Scholar]
- Luo, W.; Luo, C.; Wang, M.; Guo, L.; Chen, X.; Li, Z.; Zheng, M.; Folaniyi, B.S.; Luo, W.; Shu, D. Genome Diversity of Chinese Indigenous Chicken and the Selective Signatures in Chinese Gamecock Chicken. Sci. Rep. 2020, 10, 14532. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).