Seasonal Dynamics and Trophic Impact of Mesozooplankton in the Shannon River Estuary System, Ireland
Abstract
1. Introduction
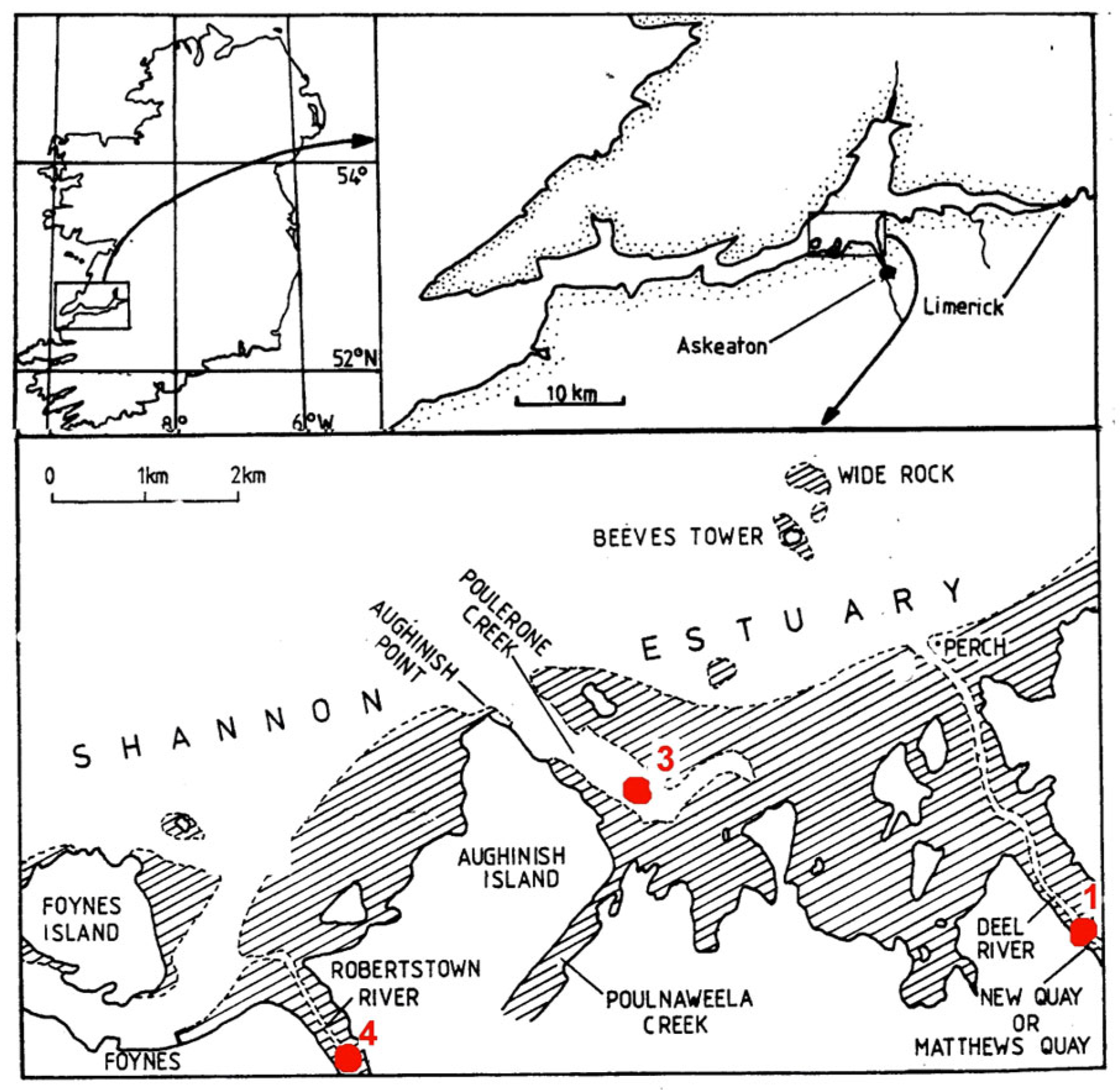
2. Study Area
3. Materials and Methods
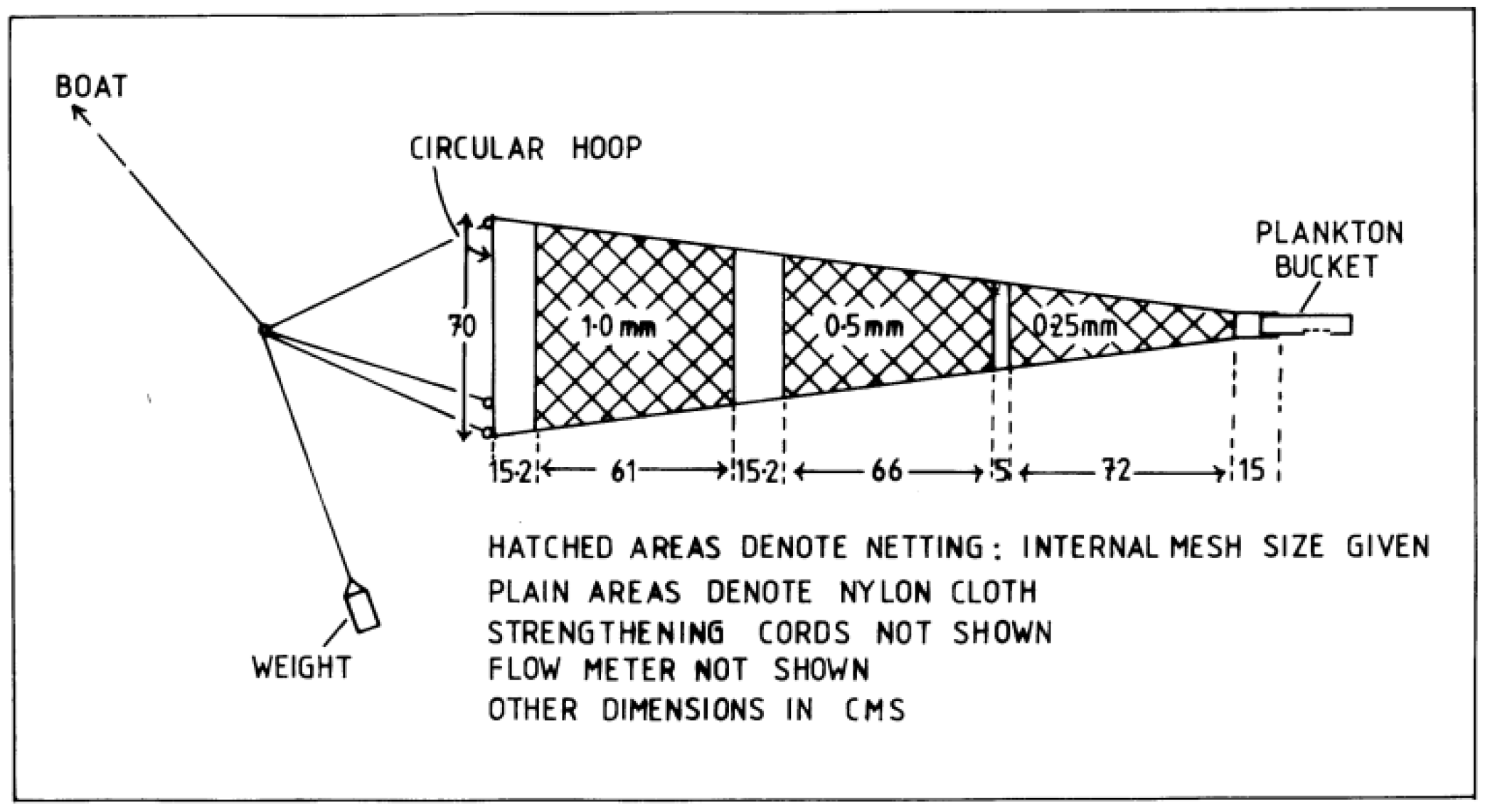
3.1. Sampling, Identification, and Estimation of Abundance
- 210 m3 for organisms > 1.0 mm (e.g., Pleurobrachia pileus, fishes, mysids), sampled by all three meshes.
- 100 m3 for organisms > 0.5 mm (e.g., adult Calanus), sampled by the 500 µm and 250 µm meshes.
- 37 m3 for mesozooplankton > 0.25 mm (e.g., adult copepods, large copepodites, Oikopleura dioica without its house), sampled by the 250 µm mesh only.
- 100 m3 was arbitrarily assigned as the sampled volume for detritus.
3.2. Statistical Analyses
3.2.1. Diversity
- The Margalef index of richness:
- Pielou’s Evenness index:
3.2.2. Principal Component Analysis (PCA)
3.3. Choice of Environmental Variables
3.4. Calculations of Grazing Rate (Trophic Impact)
3.4.1. General Considerations
3.4.2. Predatory Clearance Rate by Pleurobrachia pileus
3.4.3. Clearance Rates by Eurytemora affinis
3.4.4. Clearance Rates by Acartia spp.
3.4.5. Clearance Rates by Mysids
3.4.6. Clearance Rates by Oikopleura dioica
4. Results
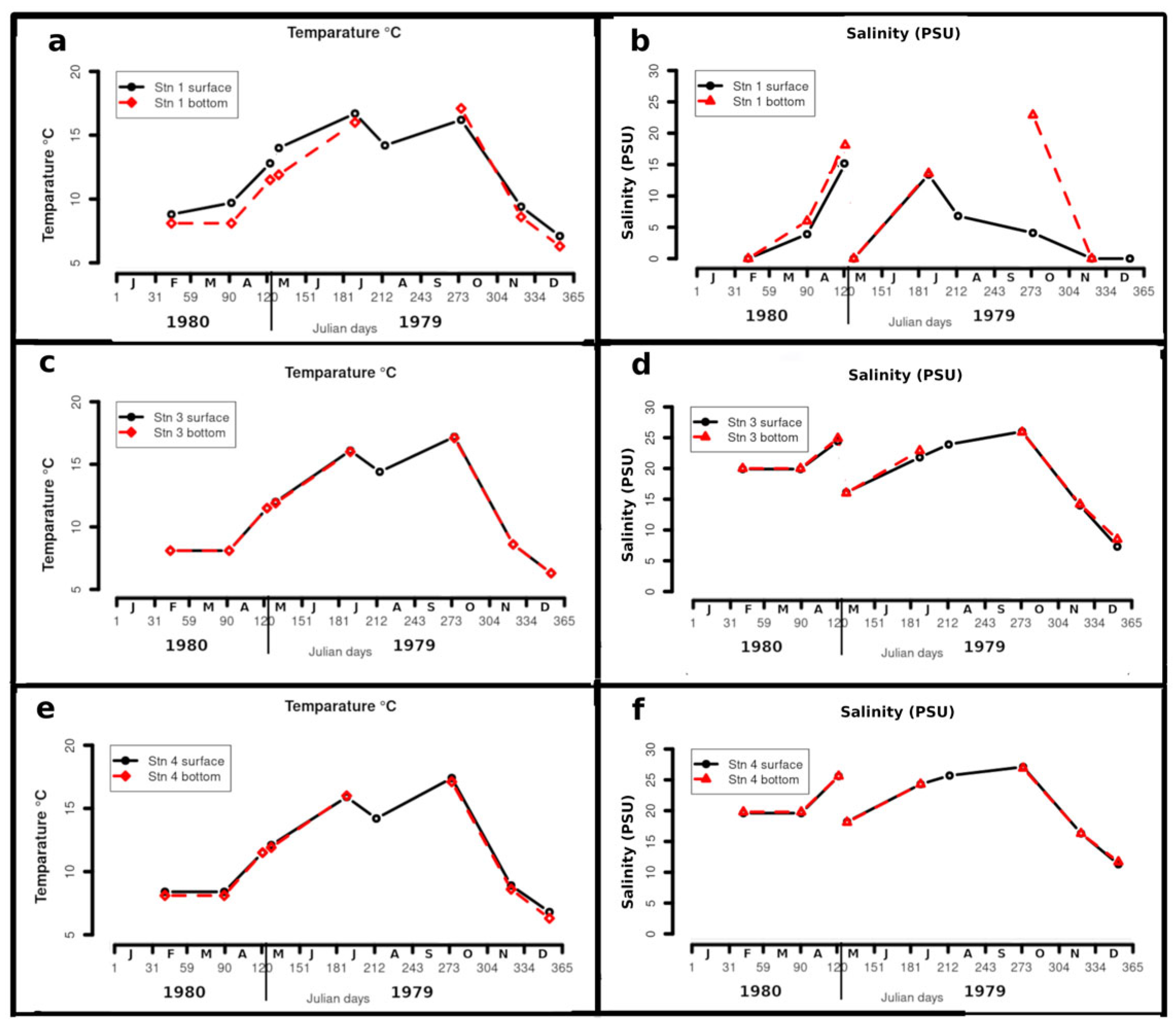
4.1. Temperature and Salinity
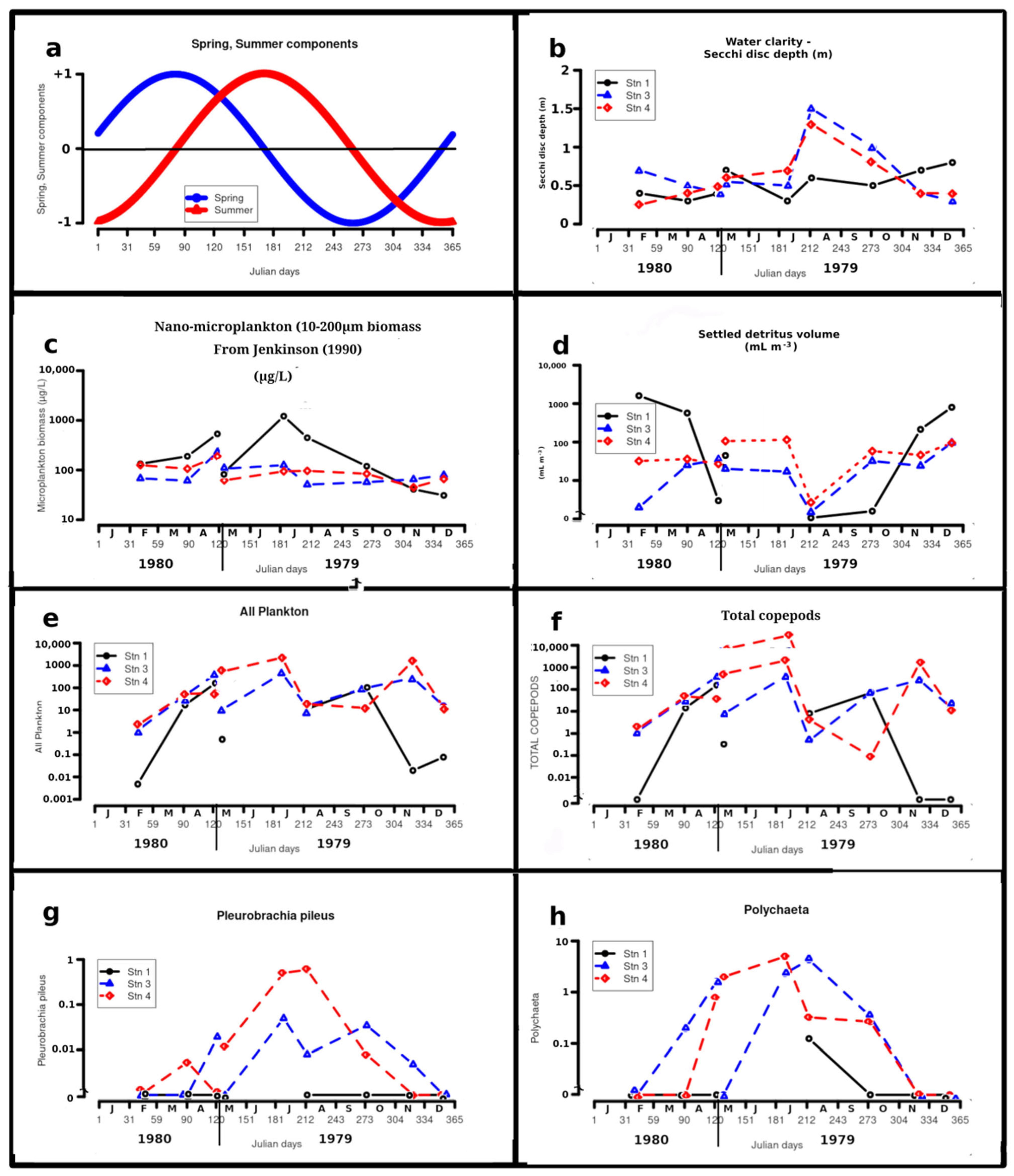
4.2. Celestial Variables
4.3. Secchi Disc Water Clarity and Colour
4.4. Detritus Volume Fraction
4.5. Total Mesozooplankton
4.6. Dominant and Noteworthy Mesozooplankton
4.6.1. Coelenterates
4.6.2. Ctenophores
Pleurobrachia pileus (O.F. Müller) (Figure 4g)
4.6.3. Polychaetes (Figure 4h)
4.6.4. Major Copepods
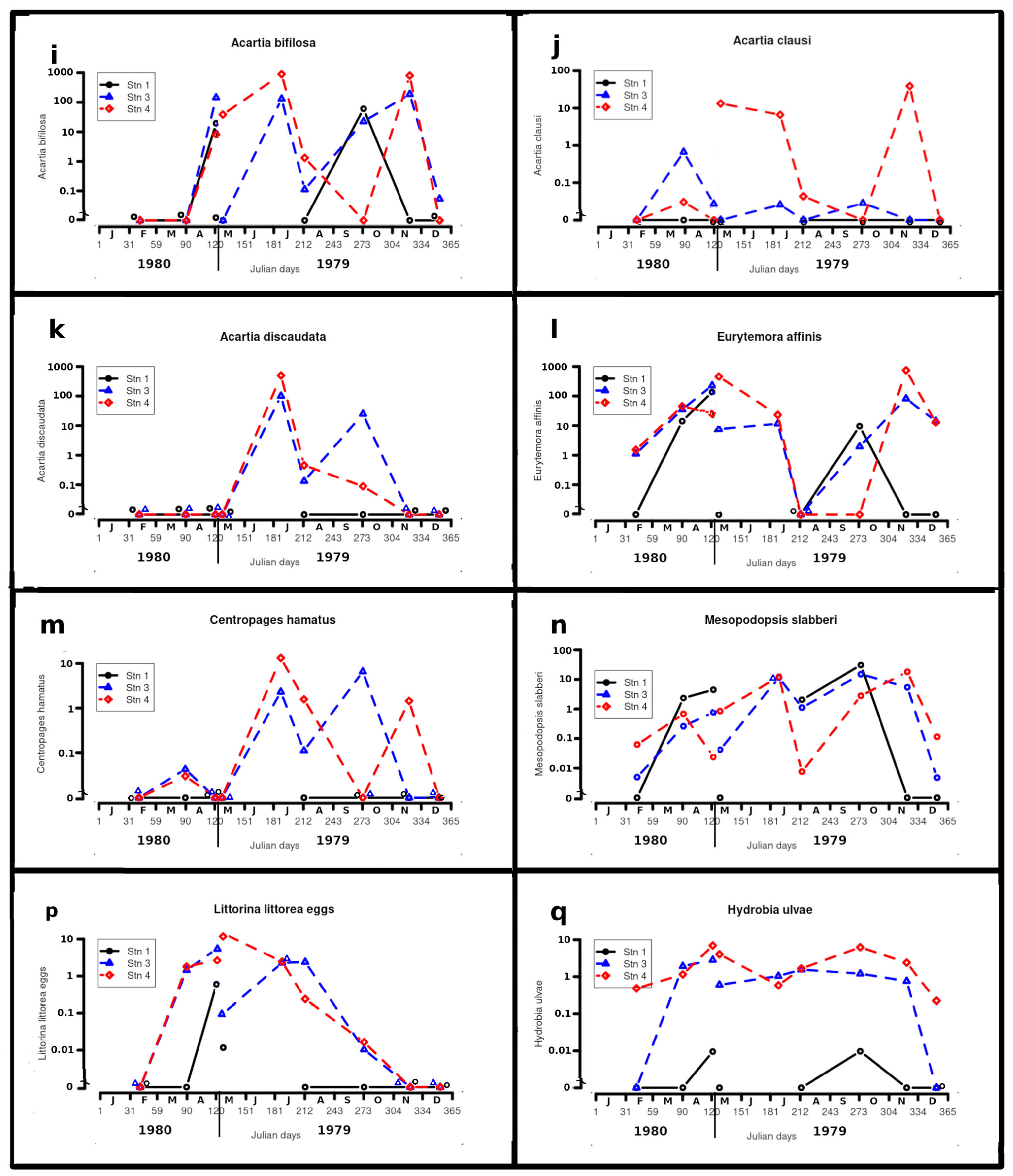
Acartia bifilosa (Giesbrecht) (Figure 4i)
Acartia clausi Giesbrecht (Figure 4j)
Acartia discaudata (Giesbrecht) (Figure 4k)
Eurytemora affinis (Poppe) (Figure 4l)
Eurytemora velox (Willieborg)
Temora longicornis (O.F. Müller)
Pseudocalanus elongatus Beck
Centropages hamatus (Lilljeborg) (Figure 4m)
4.6.5. Other Copepods
4.6.6. Cirripedes
4.6.7. Mysids
Gastrosaccus spinifer (Goës)
Mesopodopsis slabberi (Van Beneden) (Figure 4n)
Neomysis integer (Leach)
4.6.8. Isopods
4.6.9. Amphipods
4.6.10. Decapods
Carcinus maenas (L.)
Crangon crangon (L.)
Other Decapods
4.6.11. Molluscs
Hydrobia ulvae (Pennant)—adults (Figure 4q)
Littorina littorea (L.)—egg capsules (Figure 4p)
Other Molluscs
4.6.12. Tunicates
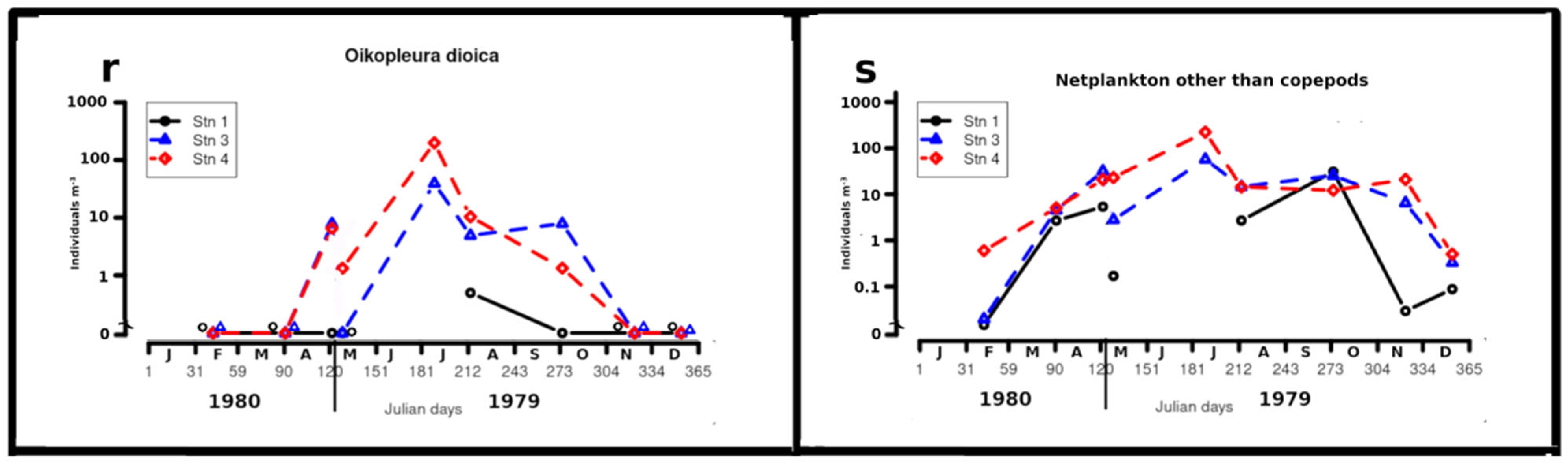
Oikopleura dioica Fol (Figure 4r)
4.6.13. Fishes
- Anguilla anguilla: Elvers (65–75 mm) were found at all stations from December to May, with a maximum of ~25 ind. (~0.7 ind. m−3) at Station 1 in April.
- Gasterosteus aculeatus: One individual from Station 1 in November.
- Gobiidae: Larval gobies occurred at maxima of ~2 ind. m−3 from May to November at all stations.
- Platichthys flesus: Flounder larvae and early juveniles (9–10 mm) occurred, most abundantly at Station 1 (~1 ind. m−3) in April and May.
- Sprattus sprattus: Three sprats (yolk-sac stage to 10 mm) were found at Station 4 in February and May.
- Syngnathus rostellatus: Pipefish (16–100 mm) were encountered, mostly singly, at Stations 1 and 3 from July to October.
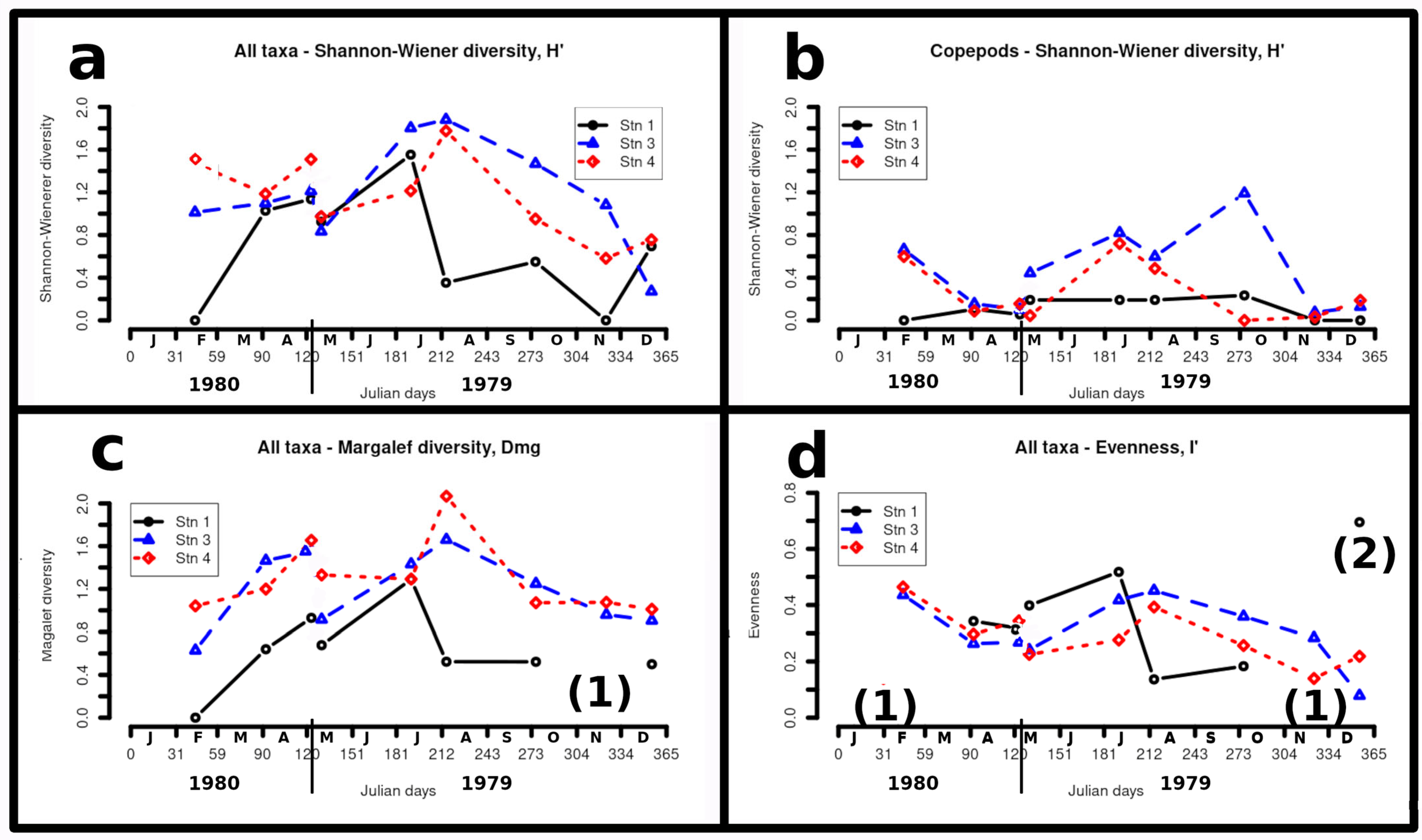
4.7. Mesozooplankton Diversity
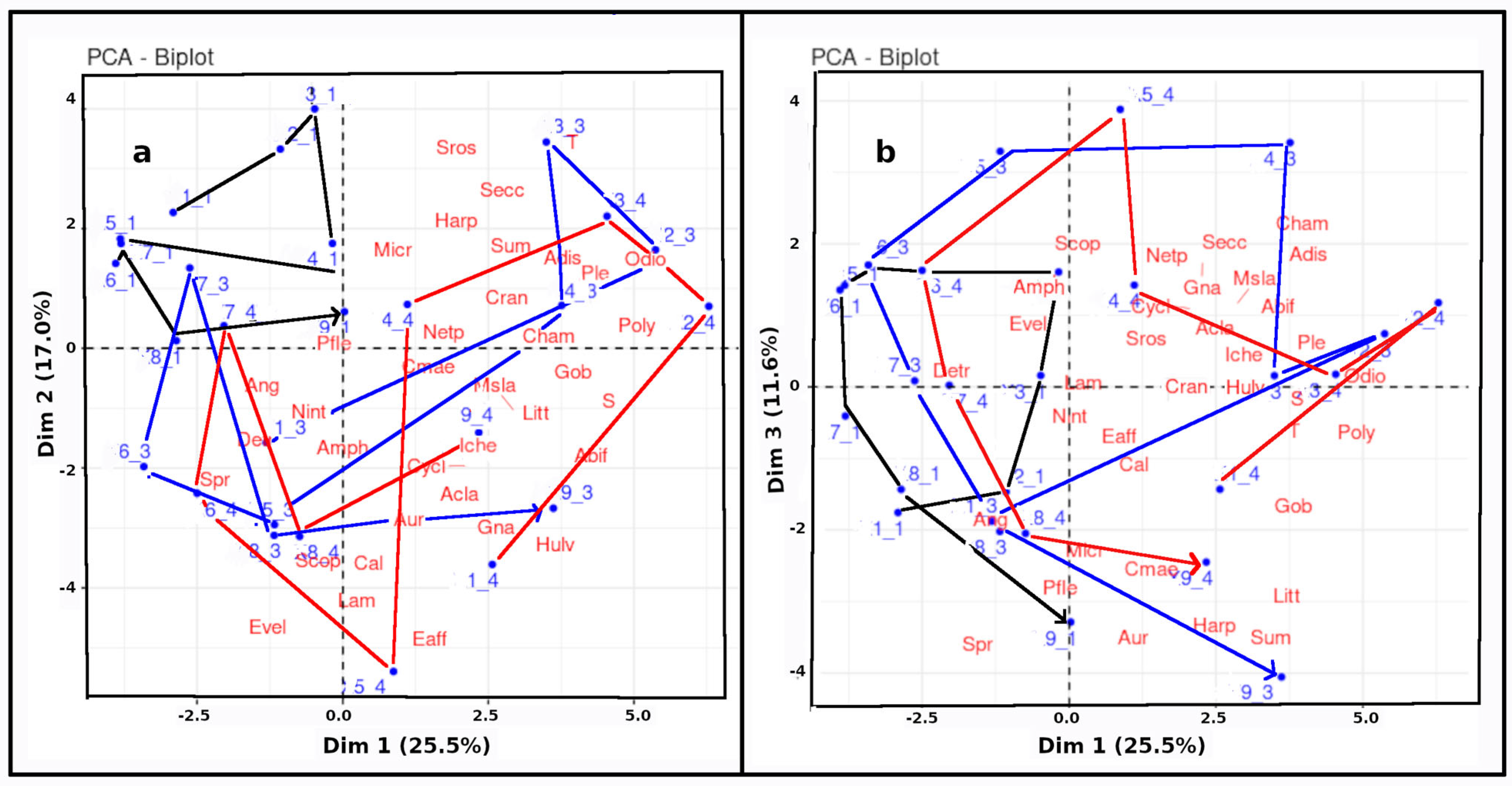
4.8. Principal Component Analysis (PCA) Interpretation
4.9. Grazing Rates (Trophic Impact)
4.9.1. Predatory Grazing Rates by Pleurobrachia pileus
4.9.2. Eurytemora affinis
4.9.3. Grazing Rates by Acartia spp.
4.9.4. Grazing Rates by All Copepods
4.9.5. Grazing Rates by Mysids
4.9.6. Grazing Rates by Oikopleura dioica
4.9.7. Total Filter-Feeding Grazing Rates (Trophic Impact) by the Mesozooplankton
5. Discussion
5.1. Inclusion of Celestial Variables: An Innovation
5.2. Trophic Structuring by the Mesozooplankton
5.3. The Mesozooplankton Community
5.3.1. General Considerations
5.3.2. The Importance of Our Three-Mesh Net
5.3.3. The Mesozooplankton Taxa
The Major Copepods: Eurytemora and Acartia
Position Maintenance by Estuarine Meso Plankton: The Case of Eurytemora affinis
Mesopodopsis slabberi
Oikopleura dioica
Pleurobrachia pileus
Absence of Cladocerans
Hydrobia ulvae
5.4. Diversity
5.5. The Factorial Analysis
5.6. Grazing Impact of Mesozooplankton on the Estuary Ecosystem
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lotze, H.K.; Lenihan, H.; Bourque, B.; Bradbury, R.; Cooke, R.; Kay, M.; Kidwell, S.; Kirby, M.; Peterson, C.; Jackson, J. Depletion, Degradation, and Recovery Potential of Estuaries and Coastal Seas. Science 2006, 312, 1806–1809. [Google Scholar] [CrossRef]
- Jeffrey, D.; Wilson, J.G.; Harris, C.; Tomlinson, D. The application of two simple indices to Irish estuary pollution studies. In Estuarine Management and Quality Assessment; Wilson, J., Halcrow, W., Eds.; Plenum Press: London, UK, 1985; pp. 147–161. [Google Scholar]
- Wilson, J.; Brennan, B. Spatial and temporal variation in sediments and their nutrient concentrations in the unpolluted Shannon estuary, Ireland. Arch. Für Hydrobiol. 1993, 75, 451–486. [Google Scholar]
- Wilson, J.; Brennan, B.; Brennan, M. Horizontal and vertical gradients in sediment nutrients on mudflats in the Shannon estuary, Ireland. Neth. J. Aquat. Ecology. 1993, 27, 173–180. [Google Scholar] [CrossRef]
- Wilson, J.; Brennan, M. Nutrient interchange between the sediment and the overlying water on the intertidal mudflats of the Shannon estuary. Arch. Für Hydrobiologie. 1993, 75, 423–450. [Google Scholar]
- McCarthy, T.K.; Frankiewicz, P.; Cullen, P.; Blaszkowski, M.; O’Connor, W.; Doherty, D. Long-term effects of hydropower installations and associated river regulation on River Shannon eel populations: Mitigation and management. Hydrobiologia 2008, 609, 109–124. [Google Scholar] [CrossRef]
- Anon. and Conservation Through Education. All Gates Closed. The Life and Death of the Atlantic Salmon. 2025. Available online: https://www.youtube.com/watch?v=PcgFo0odp2w (accessed on 2 January 2025).
- Byrne, P. Seasonal Composition of Meroplankton in the Dunkellin Estuary, Galway Bay. In Biology and Environment: Proceedings of the Royal Irish Academy; Royal Irish Academy: Dublin, Ireland, 1995; Volume 95B, pp. 35–48. Available online: http://www.jstor.org/stable/20504491 (accessed on 1 August 2025).
- Ó Céidigh, P. The marine Decapoda of Counties Galway and Clare. In Proceedings of the Royal Irish Academy. Section B: Biological, Geological, and Chemical Science; Royal Irish Academy: Dublin, Ireland, 1961; Volume 62, pp. 151–174. [Google Scholar]
- Fives, J.M. Investigations of the plankton of the west coast of Ireland. II. Planktonic Copepoda raken off County Galway and adjacent areas in plankton surveys during the years 1958 to 1963. In Proceedings of the Royal Irish Academy. Section B: Biological, Geological, and Chemical Science; Royal Irish Academy: Dublin, Ireland, 1968; Volume 67, pp. 233–259. [Google Scholar]
- Fives, J.M. Investigations of the Plankton of the West Coast of Ireland: IV. Larval and Post-Larval Stages of Fishes Taken from the Plankton of the West Coast in Surveys during the Years 1958–1966. In Proceedings of the Royal Irish Academy. Section B: Biological, Geological, and Chemical Science; Royal Irish Academy: Dublin, Ireland, 1970; Volume 70, pp. 15–93. Available online: http://www.jstor.org/stable/20494950 (accessed on 1 August 2025).
- Fives, J.M. Investigations of the Plankton of the West Coast of Ireland: V. Chaetognatha Recorded from the Inshore Plankton off Co. Galway. In Proceedings of the Royal Irish Academy. Section B: Biological, Geological, and Chemical Science; Royal Irish Academy: Dublin, Ireland, 1971; Volume 71, pp. 119–138. Available online: http://www.jstor.org/stable/20518893 (accessed on 1 August 2025).
- O’Brien, F.I. The relationship between temperatur, salinity and Chaetognatha of the Galway Bay area of the west coast of Ireland. In Proceedings of the Royal Irish Academy. Section B: Biological, Geological, and Chemical Science; Royal Irish Academy: Dublin, Ireland, 1977; Volume 77, pp. 245–252. [Google Scholar]
- Boyd, R.J.; Ó Céidigh, P.; Wilkinson, A. Investigations of the Plankton of the West Coast of Ireland: VI. Pelagic Cnidaria of the Galway Bay Area 1956–1972, with a Revision of Previous Records for These Species in Irish Inshore Waters. In Proceedings of the Royal Irish Academy. Section B: Biological, Geological, and Chemical Science; Royal Irish Academy: Dublin, Ireland, 1973; Volume 73, pp. 383–403. Available online: http://www.jstor.org/stable/20518927 (accessed on 1 August 2025).
- Hensey, M. Zooplankton and oceanographic conditions in the Shannon Estuary, Ireland. Master’s Thesis, Department of Oceanography, National University of Ireland, Galway, Ireland, 1980. [Google Scholar]
- Mackas, D.L.; Beaugrand, G. Comparisons of zooplankton time series. J. Mar. Syst. 2010, 79, 286–304. [Google Scholar] [CrossRef]
- Currie, R.; Wyatt, T.; O’Brien, D. Determoinistic signals in fish catches, wine harvests and sea-level, and further experiments. Int. J. Climatol. 1993, 13, 665–687. [Google Scholar] [CrossRef]
- O’Sullivan, G. The intertidal fauna of Aughinish Island, Shannon, Co. Limerick. Ir. Nat. J. 1983, 21, 62–69. [Google Scholar]
- O’Sullivan, G. Seasonal changes in the intertidal fish and crustacean populations of Aughinish Island and the Shannon Estuary. In Irish Fishery Investigations, Series B; Stationery Office: Dublin, Ireland, 1984; Volume 28, pp. 1–15. [Google Scholar]
- Beaulieu, S. The Road to a New Bauxite–Mine and Refinery Optimisation. In TRAVAUX 48, Proceedings of the 37 th International ICSOBA Conference and XXV Conference «Aluminium of Siberia», Krasnoyarsk, Russia, 16–20 September 2019; ICSOBA: Saint-Colomban, QC, Canada, 2019; pp. 137–139. Available online: https://icsoba.org/proceedings/37th-conference-and-exibition-icsoba-2019/?doc=17 (accessed on 1 August 2025).
- Jenkinson, I.R. The microplankton biomass and diversity of the upper Shannon estuary (Ireland) and two tributary estuaries. Br. Phycol. J. 1985, 20, 187. [Google Scholar]
- Jenkinson, I.R. Estuarine plankton of Co Limerick. I. A recurrent bloom summer bloom of the dinoflagellate Glenodinium foliaceum Stein confined to the Deel estuary, with data on microplankton biomass. Ir. Nat. J. 1990, 23, 173–180. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 1 August 2025).
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- The Shannon Airport Group. Traffic Figures. 2024. Available online: https://www.snnairportgroup.ie/2025/shannon-airport-passenger-numbers-climb-7-to-over-104m-in-first-half-of-2025/ (accessed on 8 August 2025).
- Rusal Aughinish Alumina. 2025. Available online: https://rusal.ru/en/about/geography/aughinish-alumina/ (accessed on 2 October 2025).
- Wonham, M.; Carlton, J. Trends in marine biological invasions at local and regional scales: The Northeast Pacific Ocean as a model system. Biol. Invasions 2005, 7, 369–392. [Google Scholar] [CrossRef]
- Department of Oceanography. Data Report No. 4, 1977-1978; Department of Oceanography, University College: Galway, Ireland, 1979. [Google Scholar]
- Department of Oceanography. Data Report No. 5, 1979-1980; Department of Oceanography, University College: Galway, Ireland, 1981. [Google Scholar]
- McMahon, T.; Raine, R.; Fast, T.; Kies, L.; Patching, J. Plankton biomass, light attenuation and md mixing in the Shannon estuary, Ireland. J. Mar. Biol. Assoc. UK 1992, 72, 709–720. [Google Scholar] [CrossRef]
- Grandremy, N.; Bourriau, P.; Daché, E.; Danielou, M.-M.; Doray, M.; Dupuy, C.; Forest, B.; Jalabert, L.; Huret, M.; Le Mestre, S.; et al. Metazoan zooplankton in the Bay of Biscay: A 16-year record of individual sizes and abundances obtained using the ZooScan and ZooCAM imaging systems. Earth Syst. Sci. Data 2024, 16, 1265–1282. [Google Scholar] [CrossRef]
- Smith, P.E.; Counts, R.C.; Clutter, R.I. Changes in Filtering Efficiency of Plankton Nets Due to Clogging Under Tow. ICES J. Mar. Sci. 1968, 32, 232–248. [Google Scholar] [CrossRef]
- Shannon, C.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1963. [Google Scholar]
- Abdullah Al, M.; Gao, Y.; Xu, G.; Wang, Z.; Xu, H.; Warren, A. Variations in the community structure of biofilm-dwelling protozoa at different depths in coastal waters of the Yellow Sea, northern China. J. Mar. Biol. Assoc. UK 2019, 99, 43–50. [Google Scholar] [CrossRef]
- Rajkumar, M.; Ashok Prabu, V.; Rahman, M.M.; Jenkinson, I.R. Community structure of microzooplanktonin a tropical estuary (Uppanar) and amangrove (Pichavaram) from the south-east coast of India. J. Mar. Biol. Assoc. India 2022, 64, 13–28. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2025. Available online: https://cran.r-project.org/doc/manuals/r-release/fullrefman.pdf (accessed on 1 August 2025).
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology. Package. 2022. Available online: https://www.researchgate.net/publication/313502495_Vegan_Community_Ecology_Package (accessed on 1 August 2025).
- Longobardi, L.; Dubroca, L.; Margiotta, F.; Sarno, D.; Zingone, A. Photoperiod-driven rhythms reveal multi-decadal stability of phytoplankton communities in a highly fluctuating coastal environment. Sci. Rep. 2022, 12, 3908. [Google Scholar] [CrossRef]
- Häfkar, N.S.; Meyer, B.; Last, K.S.; Pond, D.W.; Hüppe, L.; Teschke, M. Circadian Clock Involvement in Zooplankton Diel Vertical Migration. Curr. Biol. 2017, 27, 2194–2201. [Google Scholar] [CrossRef]
- Båmstedt, U. Trophodynamics of Pleurobrachia pileus (Ctenophora, Cydippida) and ctenophore summer occurrence off the Norwegian north-west coast. Sarsia 1998, 83, 169–181. [Google Scholar] [CrossRef]
- Hardy, A. The Open Sea: The World of Plankton ; Collins: London, UK, 1956. [Google Scholar]
- Purcell, J.; Sturdevant, M.; Galt, C. A review of appendicularians as prey of invertebrate and fish predators. In Response of Marine Ecosystems to Global Change: Ecological Ipact of Appendicularians; Gorsky, G., Youngbluth, M., Deibel, D., Eds.; Editions Scientifiques: Paris, France, 2005; pp. 259–435. [Google Scholar]
- Yip, S.Y. A preliminary study on the planktonic ctenophora of the west coast of Ireland with special reference to Pleurobrachia pileus from Galway Bay. In Proceedings of the Royal Irish Academy. Section B: Biological, Geological, and Chemical Science; Royal Irish Academy: Dublin, Ireland, 1981; Volume 81, pp. 89–109. [Google Scholar]
- Williams, R. Zooplankton of the Bristol Channel and Severn Estuary. Mar. Pollut. Bull. 1984, 15, 66–70. [Google Scholar] [CrossRef]
- Lee, C.E. Global phylogeography of a cryptic copepod species complex and reproductive isolation between genetically proximate “populations”. Evolution 2000, 54, 2014–2027. [Google Scholar] [CrossRef] [PubMed]
- Tackx, M.; Pauw, N.D.; Mieghem, R.V.; Azémar, F.; Hannouti, A.; Damme, S.; Fiers, F.; Daro, N.; Meire, P. Zooplankton in the Schelde estuary, Belgium and The Netherlands. Spatial and temporal patterns. J. Plankton Res. 2004, 26, 133–141. [Google Scholar] [CrossRef]
- David, V.; Sautour, B.; Galois, R.; Chardy, P. The paradox high zooplankton biomass-low vegetal particulate organic matter in high turbidity zones: What way for energy transfer? J. Exp. Mar. Biol. Ecol. 2006, 333, 202–218. [Google Scholar] [CrossRef]
- Poulet, S.A. Comparison between five co-existing species of marine copepods feeding on naturally occurring particulate matter. Limnol. Oceanogr. 1978, 23, 126–143. [Google Scholar] [CrossRef]
- Mullin, M.; Sloan, P.; Eppley, R. Relationship between carbon content, cell volume, and area in phytoplankton. Limnol. Oceanogr. 1966, 11, 307–311. [Google Scholar] [CrossRef]
- Castel, J. Aspects de l’étude écologique du plancton de l’estuaire de la Gironde. Oceanis 1981, 6, 353–577. [Google Scholar]
- Heinle, D.; Flemer, D. Carbon requirements of a population of the estuarine copepod, Eurytemora affinis. Mar. Biol. 1975, 31, 235–247. [Google Scholar] [CrossRef]
- Richman, S.; Heinle, D.; Huff, R. Grazing by adult estuarine copepods of the Chesapeake Bay. Mar. Biol. 1977, 42, 69–84. [Google Scholar] [CrossRef]
- Roman, N.R. Feeding of the copepod Acartia tonsa on the diatom Nitzschia closterium and brown alga (Fucus vesiculosus) detritus. Mar. Biol. 1977, 42, 149–155. [Google Scholar] [CrossRef]
- Kiørboe, T.; Møhlenberg, F.; Hamburger, K. Bioenergetics of the planktonic copepod Acartia tonsa: Relation between feeding, egg production, and composition of specific dynamic action. Mar. Ecol. Prog. Ser. 1985, 26, 85–97. [Google Scholar] [CrossRef]
- Webb, P.; Perissinotto, R.; Wooldridge, T. Feeding of Mesopodopsis slabberi (Crustacea, Mysidaceae) on naturally occurring phytoplankton. Mar. Ecol. Prog. Ser. 1987, 38, 115–123. [Google Scholar] [CrossRef]
- David, V. Dynamique spatio-temporelle du zooplancton dans l’estuaire de la Gironde et implications au sein du réseau trophique planctonique. Ph.D. Thesis, Université de Bordeaux I, Bordeaux, France, 2006. [Google Scholar]
- King, K.R. The population biology of the larvacean Oikopleura dioica in enclosed water columns. In Marine Mesocosms; Grice, O.D., Reeve, M.R., Eds.; Springer: New York, NY, USA, 1982; pp. 341–352. [Google Scholar]
- Alldredge, A. The impact of appendicularian grazing on natural food concentrations in situ. Limnol. Oceanogr. 1981, 26, 247–257. [Google Scholar] [CrossRef]
- Acuňa, J.; Kiefer, M. Functional response of the appendicularian Oikopleura dioica. Limnol. Oceanogr. 2000, 45, 608–618. [Google Scholar] [CrossRef]
- von Vaupel-Klein, J.; Weber, R. Distribution of Eurytemora affinis (Copepoda: Calanoida) in relation to salinity: Field and laboratory observations. Neth. J. Sea Res. 1975, 9, 297–310. [Google Scholar] [CrossRef]
- Devreker, D.; Souissi, S.; Molinero, J.C.; Nkubito, F. Trade-offs of the copepod Eurytemora affinis in mega-tidal estuaries. Insights from high frequency sampling in the Seine Estuary. J. Plankton Res. 2008, 30, 1329–1342. [Google Scholar] [CrossRef]
- Hemmingsen, W.; MacKenzie, K.; Sagerup, K.; Remen, M.; Bloch-Hansen, K.; Dagbjartarson Imsland, A.K. Caligus elongatus and other sea lice of the genus Caligus as parasites of farmed salmonids: A review. Aquaculture 2020, 522, 735160. [Google Scholar] [CrossRef]
- Tattersall, W.; Tattersall, O. The British Mysidacea; The Ray Society: London, UK, 1951. [Google Scholar]
- Naylor, E. British Marine Isopods; Linnean Society and Academic Press: London, UK, 1972. [Google Scholar]
- Graham, A. British Prosobranch and Other Operculate Gastropod Molluscs; Academic Press: London, UK, 1971. [Google Scholar]
- Gibson, R.; Hextall, B.; Rogers, A. Photographic Guide to the Sea and Shore Life of Britain and North-west Europe; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Little, C.; Nix, W. The burrowing and floating behaviour of the gastropod Hydrobia ulvae Estuarine Coastal and Marine. Science 1976, 4, 537–544. [Google Scholar] [CrossRef]
- Wiebe, P.H.; Harris, R.; Gislason, A.; Margonski, P.; Skjoldal, H.R.; Benfield, M.; Hay, S.; O’Brien, T.; Valdés, L. The ICES Working Group on Zooplankton Ecology: Accomplishments of the first 25 years. Prog. Oceanogr. 2015, 141, 179–201. [Google Scholar] [CrossRef]
- Clutter, R.; Anraku, M. Avoidance of samplers. In Zooplankton Sampling; UNESCO: Paris, France, 1968; pp. 57–76. [Google Scholar]
- øller, L.F.; Canon, J.M.; Tiselius, P. Bioenergetics and growth in the ctenophore Pleurobrachia pileus. Hydrobiologia 2010, 645, 167–178. [Google Scholar] [CrossRef]
- Peitsch, A.; Köpcke, B.; Bernát, N. Long-term investigation of the distribution of Eurytemora affinis (Calanoida; Copepoda) in the Elbe Estuary. Limnologica 2000, 30, 175–182. [Google Scholar] [CrossRef]
- Favier, J.-B.; Winkler, G. Coexistence, distribution patterns and habitat utilization of the sibling species complex Eurytemora affinis in the St Lawrence estuarine transition zone. J. Plankton Res. 2014, 26, 1247–1261. [Google Scholar] [CrossRef]
- Troedson, C.; Frischer, M.E.; Nejstgaard, J.C.; Thompson, E.M. Molecular quantification of differential ingestion and particle trapping rates by the appendicularian Oikopleura dioica as a function of prey size and shape. Limnol. Oceanogr. 2007, 52, 416–427. [Google Scholar] [CrossRef]
- Raven, J.A.; Beardall, J. Evolution of Phytoplankton in Relation to Their Physiological Traits. J. Mar. Sci. Eng. 2022, 10, 194. [Google Scholar] [CrossRef]
- Skjoldal, H.R.; Aarflot, J.M.; Knutsen, T.; Wiebe, P.H. Comparison of WP-2 and MOCNESS plankton samplers for measuring zooplankton biomass in the Barents Sea ecosystem. J. Plankton Res. 2024, 46, 654–672. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, S.; Uyé, S.; Onbé, T. Calanoid copepod eggs in sea-bottom muds. II. Seasonal cycles of abundance in the populations of several species of copepods and their eggs in the Inland Sea of Japan. Mar. Biol. 1975, 31, 373–384. [Google Scholar] [CrossRef]
- Soltanpour-Gargari, A.; Wellershaus, S. Very low salinity stretches in estuaries—The main habitat of Eurytemora affinis, a plankton copepod. Meeresforschung 1997, 31, 199–208. [Google Scholar]
- Castel, J.; Courties, C.; Poli, J. Dynamique du copépode Eurytemora hirundoides dans l’estuaire de la Gironde: Effet de la température. In Oceanologica Acta, Special Issue; Gauthier-Villars: Paris, France, 1983; pp. 57–61. [Google Scholar]
- Collins, N.; Williams, R. Zooplankton of the Bristol Channel and Severn Estuary. The distribution of four copepods in relation to salinity. Mar. Biol. 1981, 64, 273–283. [Google Scholar] [CrossRef]
- Rees, C. The plankton in the upper reaches of the Bristol Channel. J. Mar. Biol. Assoc. UK 1939, 23, 397–5425. [Google Scholar] [CrossRef]
- Chervin, M. Assimilation of particulate organic carbon by estuarine and coastal copepods. Mar. Biol. 1978, 49, 265–275. [Google Scholar] [CrossRef]
- Heinle, D.R. Population dynamics of exploited cultures of calanoid copepods. Helgoländer Wissenshaftliche Meeresunters 1970, 20, 360–372. [Google Scholar] [CrossRef]
- Katona, S.K. Growth characteristics of the copepods Eurytemora affinis and Eurytemora herdmanni in laboratory culture. Helgoländer Wissenschalftliche Meeresunters 1970, 20, 373–384. [Google Scholar] [CrossRef]
- Berk, S.; Brownlee, D.; Heinle, D.; Kling, H.; Colwell, R. Ciliates as a food source for marine plankton copepods. Microb. Ecol. 1977, 4, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Heinle, D.; Harris, R.; Ustach, J.; Flemer, D. Detritus as food for estuarine copepods. Mar. Biol. 1977, 40, 341–353. [Google Scholar] [CrossRef]
- Poli, J.; Castel, J. Cycle biologique en laboratoire d’un copépode planctonique de l’estuaire de la Gironde: Eurytemora hirundoides (Nordquist, 1888). Vie Milieu Life Environ. 1983, 33, 79–86. [Google Scholar]
- Bradford-Greve, J.M. Copepoda: Calanoida: Acartiidae: Acartia, Paracartia, Pteriacartia. In ICES Identification Leaflets for Plankton No. 181; Lindley, J., Ed.; International Council for the Exploration of the Sea: Copenhagen, Denmark, 1999; pp. 1–19. [Google Scholar]
- Marques, S.C.; Azeiteiro, U.M.; Marques, J.C.; Neto, J.M.; Pardal, M.A. Zooplankton and ichthyoplankton communities in a temperate estuary: Spatial and temporal patterns. J. Plankton Res. 2006, 28, 297–312. [Google Scholar] [CrossRef]
- Le Fèvre-Lehoëroff, G. Distribution et variations saisonnières du plancton en ‘rivière de Morlaix Comptes-rendus hebdomadaires des Séances de l’Académie des Sciences de Paris. Ann. De L’institut Océanographique Monaco Nouv. Série 1972, 275, 1681–1684. [Google Scholar]
- Le Fèvre-Lehoërff, G. Variabilité de l’indice de diversité spécifique de copépodes dans l’estuaire à marée (Baie de Morlaix): Sa signification écologique. Ann. De L’institut Océanographique Monaco Nouv. Série 1974, 50, 59–66. [Google Scholar]
- Remerie, T.; Bourgois, T.; Peelaers, D.; Vierstraete, A.; Vanfleteren, J.; Vanreusel, A. Phylogeographic patterns of the mysid Mesopodopsis slabberi (Crustacea, Mysida) in Western Europe: Evidence for high molecular diversity and cryptic speciation. Mar. Biol. 2006, 149, 465–481. [Google Scholar] [CrossRef]
- Gieskes, W. Ecology of the cladocera of the North Atlantic and the North Sea, 1960–1967. Neth. J. Sea Res. 1971, 5, 342–376. [Google Scholar] [CrossRef]
- Helenius, L.K.; Leskinen, E.; Lehtonen, H.; Nurminen, L. Spatial patterns of littoral zooplankton assemblages along a salinity gradient in a brackish sea: A functional diversity perspective. Estuar. Coast. Shelf Sci. 2017, 198, 400–412. [Google Scholar] [CrossRef]
- Meireles, S.; Queiroga, H. Use of artificial collectors shows semilunar rhythm of planktonic dispersal in juvenile Hydrobia ulvae (Gastropoda: Prosobranchia). J. Mar. Biol. Assoc. UK 2004, 84, 761–766. [Google Scholar] [CrossRef]
| Abbreviation | Variable | Transform | Abbreviation | Variable | Transform |
|---|---|---|---|---|---|
| Acla | Acartia clausi | Log(n + 1) | Abif | Acartia bifilosa | Log(n + 1) |
| Amph | Amphipods | Log(n + 1) | Adis | Acartia discaudata | Log(n + 1) |
| Aur | Aurelia aurita | Log(n + 1) | Ang | Anguilla anguilla—elvers | Log(n + 1) |
| Cham | Centropages hamatus | Log(n + 1) | Cal | Calanus helgolandicus and C. sp. | Log(n + 1) |
| Cran | Crangon crangon | Log(n + 1) | Cmae | Carcinus maenas larvae | Log(n + 1) |
| Detr | Detritus volume | Log(volume) | Cycl | Freshwater cyclopoid copepods | Log(n + 1) |
| Evel | Eurytemora velox | Log(n + 1) | Eaff | Eurytemora affinis | Log(n + 1) |
| Gob | Gobiid larvae | Log(n + 1) | Gna | Gnathiid isopods | Log(n + 1) |
| Hulv | Hydrobia ulvae adults | Log(n + 1) | Harp | Harpacticoid copepods | Log(n + 1) |
| Lam | Lamellibranch larvae | Log(n + 1) | Iche | Idotea chelipes | Log(n + 1) |
| Micr | Biovolume of nano-microplankton 10–200 µm (from Jenkinson, 1990) [15]) | Log(volume) | Litt | Littorina littorea egg capsules (2–3 eggs) | Log(n + 1) |
| Netp | Total netplankton concentration | Log(n + 1) | Msla | Mesopodopsis slabberi | Log(n + 1) |
| Odio | Oikopleura dioica | Log(n + 1) | Nint | Neomysis integer | Log(n + 1) |
| Ple | Pleurobrachia pileus | Log(n + 1) | Pfle | Platychthys flesus | Log(n + 1) |
| S | Salinity (mean of surface and bottom) | No transform | Poly | sPolychaete larvae | Log(n + 1) |
| Secc | Secchi disc depth (m)—Water clarity | No transform | Scop | Small copepods, Paracalanus parvus and Pseudocalanus elongatus | Log(n + 1) |
| ros | Sygnathus rostratus | Log(n + 1) | Spr | Spring equinox component (Autumn component is the negative of this) | None |
| T | Water temperature (mean of surface and bottom) | No transform | Sum | Summer solstice component (Winter component is the negative of this) | None |
| Cruise | Station | ||
|---|---|---|---|
| 1 | 3 | 4 | |
| May | 0.00 | 0.00 | 0.07 |
| July | 0.00 | 0.30 | 3.02 |
| August | 0.00 | 0.05 | 3.54 |
| October | 0.00 | 0.21 | 0.05 |
| November | 0.00 | 0.03 | 0.00 |
| December | 0.00 | 0.00 | 0.00 |
| February | 0.00 | 0.00 | 0.00 |
| April | 0.00 | 0.00 | 0.03 |
| May | 0.00 | 0.11 | 0.00 |
| MEAN | 0.00 | 0.078 | 0.75 |
| Cruise | Station | ||
|---|---|---|---|
| 1 | 3 | 4 | |
| May | 0 | 0.03 | 0.84 |
| July | P | 0.03 | 0.07 |
| August | 0 | 0 | 0 |
| October | 0.01 | 0.01 | 0 |
| November | 0 | 0.29 | 2.9 |
| December | 0 | 0.11 | 0.03 |
| February | 0 | 0 | 0 |
| April | 0.05 | 0.09 | 0.18 |
| May | 0.31 | 0.41 | 0.07 |
| MEAN | 0.05 | 0.11 | 0.45 |
| Cruise | Station | ||
|---|---|---|---|
| 1 | 3 | 4 | |
| May | 0 | 0 | 0.08 |
| July | 0 | 1 | 5.5 |
| August | 0.01 | 0 | 0.01 |
| October | 0.2 | 0.17 | 0 |
| November | 0 | 0.62 | 0.38 |
| December | 0 | 0 | 0 |
| February | 0 | 0 | 0 |
| April | 0 | 0 | 0 |
| May | 0.06 | 0.38 | 0.02 |
| MEAN | 0.03 | 0.24 | 0.67 |
| Cruise | Station | ||
|---|---|---|---|
| 1 | 3 | 4 | |
| May | 0 | 0.03 | 0.92 |
| July | P | 1.03 | 5.57 |
| August | 0.01 | 0 | 0.01 |
| October | 0.21 | 0.18 | 0 |
| November | 0 | 0.91 | 3.28 |
| December | 0 | 0.11 | 0.03 |
| February | 0 | 0 | 0 |
| April | 0.05 | 0.09 | 0.18 |
| May | 0.37 | 0.79 | 0.09 |
| MEAN | 0.08 | 0.35 | 1.12 |
| Cruise | Station | ||
|---|---|---|---|
| 1 | 3 | 4 | |
| May total | 0.00 | 0.95 | 15.13 |
| Adults | 0.00 | 0.95 | 14.42 |
| Juveniles | 0.00 | 0.00 | 0.71 |
| July total | P | 111.84 | 148.25 |
| Adults | P | 7.63 | 66.34 |
| Juveniles | P | 104.22 | 81.90 |
| August total | 19.82 | 10.48 | 0.07 |
| Adults | 0.98 | 0.15 | 0.00 |
| Juveniles | 18.84 | 10.34 | 0.07 |
| October total | 491.29 | 140.87 | 26.36 |
| Adults | 404.57 | 0.74 | 0.00 |
| Juveniles | 86.72 | 140.12 | 26.36 |
| November | 0.00 | 50.93 | 169.38 |
| Adults | 0.00 | 0.00 | 0.00 |
| Juveniles | 0.00 | 50.93 | 169.38 |
| December | 0.00 | 0.04 | 1.07 |
| Adults | 0.00 | 0.00 | 0.00 |
| Juveniles | 0.00 | 0.04 | 1.07 |
| February | 0.00 | 0.05 | 0.59 |
| Adults | 0.00 | 0.00 | 0.00 |
| Juveniles | 0.00 | 0.05 | 0.59 |
| April | 26.26 | 2.56 | 6.34 |
| Adults | 8.36 | 0.21 | 0.00 |
| Juveniles | 17.90 | 2.35 | 6.34 |
| May | 82.50 | 12.84 | 0.31 |
| Adults | 80.41 | 11.23 | 0.18 |
| Juveniles | 2.09 | 1.60 | 0.13 |
| MEAN | 77.48 | 36.73 | 40.83 |
| Adults | 61.79 | 2.32 | 8.99 |
| Juveniles | 15.69 | 34.41 | 31.84 |
| Cruise | Station | ||
|---|---|---|---|
| 1 | 3 | 4 | |
| May | 0 | 0 | 0.12 |
| July | P | 4.3 | 22 |
| August | 0.11 | 0.55 | 1.1 |
| October | 0 | 0 | 0 |
| November | 0 | 0 | 0 |
| December | 0 | 0 | 0 |
| February | 0 | 0 | 0 |
| April | 0 | 0 | 0 |
| May | 0 | 0.21 | 0.80 |
| MEAN | 0.01 | 0.56 | 2.70 |
| Cruise | Station | ||
|---|---|---|---|
| 1 | 3 | 4 | |
| May | 0 | 0.95 | 16 |
| July | P | 134 | 175 |
| August | 20 | 11 | 1.2 |
| October | 491 | 141 | 26 |
| November | 0 | 52 | 173 |
| December | 0 | 0.15 | 1.1 |
| February | 0 | 0.052 | 1.1 |
| April | 26 | 2.7 | 6.5 |
| May | 83 | 14 | 14 |
| MEAN | 77.5 | 39.5 | 46.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jenkinson, I.R.; Ryan, T.H. Seasonal Dynamics and Trophic Impact of Mesozooplankton in the Shannon River Estuary System, Ireland. J. Mar. Sci. Eng. 2025, 13, 1966. https://doi.org/10.3390/jmse13101966
Jenkinson IR, Ryan TH. Seasonal Dynamics and Trophic Impact of Mesozooplankton in the Shannon River Estuary System, Ireland. Journal of Marine Science and Engineering. 2025; 13(10):1966. https://doi.org/10.3390/jmse13101966
Chicago/Turabian StyleJenkinson, Ian R., and Tom H. Ryan. 2025. "Seasonal Dynamics and Trophic Impact of Mesozooplankton in the Shannon River Estuary System, Ireland" Journal of Marine Science and Engineering 13, no. 10: 1966. https://doi.org/10.3390/jmse13101966
APA StyleJenkinson, I. R., & Ryan, T. H. (2025). Seasonal Dynamics and Trophic Impact of Mesozooplankton in the Shannon River Estuary System, Ireland. Journal of Marine Science and Engineering, 13(10), 1966. https://doi.org/10.3390/jmse13101966







