Abstract
Ichnological research on trace fossils from the volcanic islands of Macaronesia (North Atlantic) is reviewed in light of significant advances over the past two decades. These studies contribute to the interpretation of paleoenvironments and enhance our understanding of the biota preserved in Miocene–Holocene shallow marine and non-marine deposits across the Azores, Cape Verde, Canary, Madeira, and Salvagens archipelagos. Trace fossils provide evidence of organisms not always known from body fossils, or whose potential tracemakers are absent from the extant island fauna. They include sedimentary burrows, borings in hard substrates, and traces of plant–insect interactions. Some ichnotaxa are widespread and common (e.g., Bichordites monastiriensis, Dactyloidites ottoi, Macaronichnus segregatis, Ophiomorpha nodosa, Thalassinoides isp.), whereas others are rare. Several new ichnotaxa have also been described from the islands, including Alaichnus kabuverdiensis (cumulative trace of bivalve siphons), Centrichnus dentatus (attachment trace of verrucid barnacles), Diopatrichnus santamariaensis (polychaete tubes armored with shell debris), Ericichnus bromleyi and E. asgaardi (bioerosion grooves of regular echinoids), and Rebuffoichnus guanche (coleopteran pupation chambers). Despite these advances, ichnological research in Macaronesia remains uneven, with many topics still underexplored and significant gaps in the geographic and inventory record.
1. Introduction
Since Darwin’s time, islands isolated from continents by deep seas have been central to understanding the history of life, particularly speciation and the dispersal of biota. Among them, oceanic volcanic islands hold special significance because they arise directly from submarine volcanic activity rather than from continental drift or submergence. Unlike continental fragments, which may already carry biota at the moment of separation, oceanic volcanic islands are initially sterile and must be colonized entirely by incoming organisms [1,2].
Sedimentary rocks are rare or poorly exposed on the emerged surfaces of volcanic islands. For a long time, this led to relatively little attention from geologists and paleontologists, who traditionally focused on sedimentary successions. However, during the past three decades, remarkable progress has been achieved, particularly in the Atlantic archipelagos of Macaronesia, which include the Azores, Madeira, the Salvagens, the Canary Islands, and Cape Verde (Figure 1). Studies have shown that marine deposits on these islands are often fossiliferous. Numerous works have documented body fossils from diverse taxonomic groups in Miocene, Pliocene, and Quaternary deposits, forming a solid basis for palaeoecological and palaeogeographical interpretations [3,4,5,6,7,8].
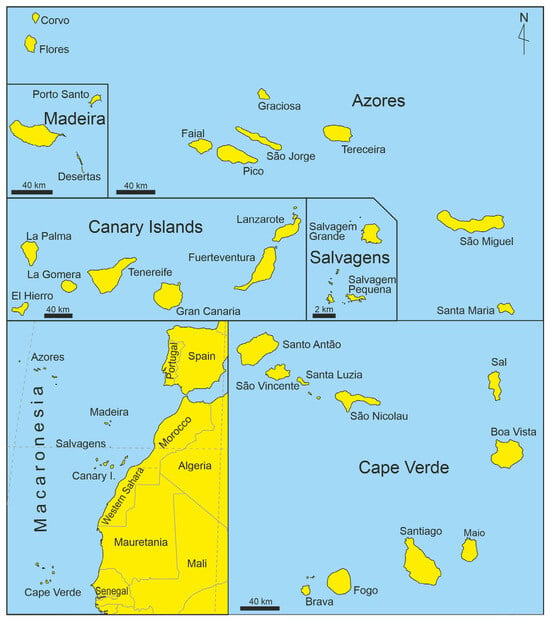
Figure 1.
Simplified maps of Macaronesia.
Complementing the record of body fossils are trace fossils—the preserved evidence of biological activity that modifies the substrate and typically exhibits recurrent morphological patterns. The organisms that produce them are often absent as body fossils, meaning trace fossils provide essential, and sometimes unique, insights into past environments and biotic interactions. They therefore represent a powerful tool for paleoenvironmental reconstruction [9,10,11]. Over the last two decades, the ichnological record from Macaronesia has expanded considerably, including the description of new ichnotaxa. This paper reviews recent advances in the study of trace fossils from these islands and highlights their importance for understanding colonization processes and the ecological history of oceanic volcanic islands.
2. Synopsis of Selected Trace Fossils
The trace fossils preserved in the sedimentary successions of Macaronesia show broadly comparable assemblages across the archipelagos. This chapter outlines the most common and distinctive ichnotaxa. As elsewhere, the ichnological record can be grouped into two main categories: burrows, produced in soft and unconsolidated substrates, and borings, produced in hard substrates such as rocks or skeletal material.
For clarity, the discussion is organized into three parts: marine burrows, marine borings, and trace fossils from continental deposits. The latter include a more diverse range of structures, such as borings, burrows, root traces, and plant-damage traces attributable to insect activity.
2.1. Burrows in Marine Deposits
Bichordites monastiriensis Plaziat & Mahmoudi, 1988 [12] (Figure 2A) is a horizontal, straight to gently winding, complex burrow characterized by meniscate backfill surrounding a central core, typically with a heart-shaped cross section. The upper surface of the core bears a narrow median furrow, whereas the lower surface displays a longitudinal crest. The core, interpreted as the drainage structure of a burrowing irregular echinoid—most likely of the Echinocardium group [12,13,14,15]—is preferentially cemented and thus usually much better preserved than the surrounding meniscate fill.
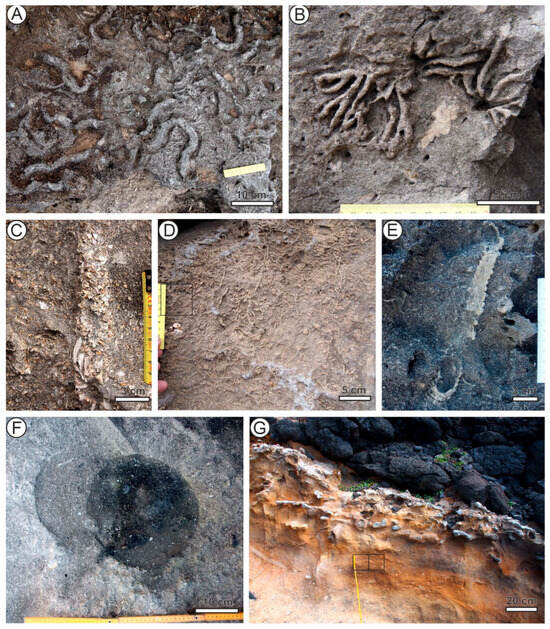
Figure 2.
Trace fossils (fossil burrows) in Pliocene calcarenites–sandstones of Santa Maria, Azores. (A) Bichordites monastiriensis. (B) Dacyloidites ottoi. (C) Diopatrichnus santamariaensis. (D) Macaronichnus segregatis. (E) Ophiomorpha nodosa. (F) Piscichnus waitemata. (G) Thalassinoides isp. (A–F) Malbusca; (G) Castillo.
Dactyloidites ottoi (Geinitz, 1849) in [16] (Figure 2B) forms nearly complete rosette structures composed of outwardly radiating, horizontal to subhorizontal cylindrical elements, occasionally showing spreiten displacement. It is interpreted as a feeding structure [13], most likely produced by polychaete worms [17,18,19].
Diopatrichnus santamariaensis Uchman, Quintino & Rodrigues, 2017, in [20] (Figure 2C) is a vertical to inclined tube, armored predominantly with crushed or uncrushed bivalve shell valves. The valves are arranged concave-upward and oriented mostly perpendicular to the burrow axis. It is attributed to the activity of polychaetes, comparable to the burrowing behavior of extant Owenia [20].
Macaronichnus segregatis Clifton & Thompson, 1978 in [21] (Figure 2D) is a variably oriented, usually straight to winding cylindrical burrow characterized by a light-grained core surrounded by a mantle of darker grains. It is produced by opheliid polychaetes that segregate sand grains, ingesting the lighter fraction—presumably richer in microbial content—while rejecting darker grains [21,22,23,24]. Two ichnosubspecies are recognized: the smaller M. s. segregatis and the larger M. s. degiberti [23].
Ophiomorpha nodosa Lundgren, 1891 in [25] (Figure 2E) is a tubular, rarely branched burrow with walls bearing a distinctly knobby external texture. In some cases, abundant interconnecting branches form a boxwork network. It is interpreted as a dwelling and feeding, or facultative farming, structure produced by decapod crustaceans [9,26,27,28,29,30].
Palaeophycus isp. consists of predominantly horizontal, tubular burrows with smooth walls. It is interpreted as a dwelling and feeding structure, produced by various deposit-feeding or carnivorous invertebrates, most likely polychaetes [31,32].
Piscichnus waitemata Gregory, 1991 in [33] (Figure 2F) is a bowl-shaped depression interpreted as a feeding trace, produced primarily by rays and, in some cases, by mammals hunting invertebrates within the sediment [33].
Thalassinoides isp. (Figure 2G) comprises predominantly horizontal, cylindrical, branched burrows that may form complex networks. It is interpreted as a semi-permanent dwelling and feeding structure, produced mainly by obligatory or facultative deposit-feeding decapod crustaceans [9,34,35].
2.2. Marine Borings
Caulostrepsis ispp. (Figure 3A) are U-shaped galleries, commonly interconnected by a vane and forming pouch-shaped structures. They are borings with a single entrance or embedment structure, wider in the distal portion than at the apertural part and typically display elliptical to dumbbell-shaped cross sections. These structures are produced mainly by spionid polychaetes of the genus Polydora, and less frequently by some eunicid polychaetes [36,37].
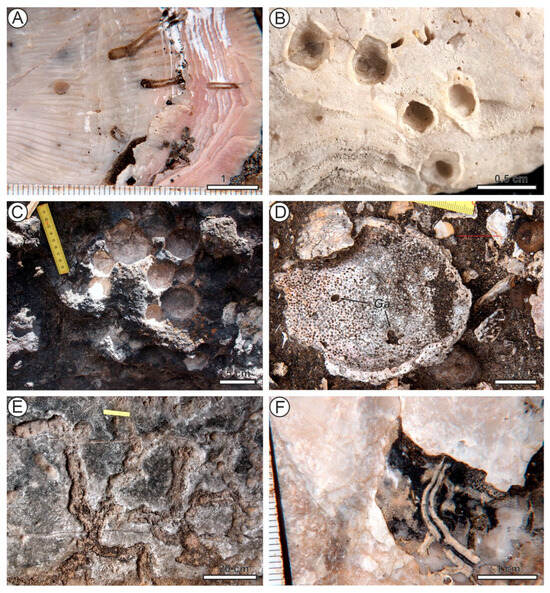
Figure 3.
Trace fossils (borings) from Santa Maria, Azores. (A) Caulostrepsis isp. in Spondylus shell valve, Pliocene, Baía de Nossa Senhora. (B) Centrichnus dentatus in an oyster shell valve, Pliocene, Pdara-que-pica. (C) Circolites kotoucensis in basalt, Pleistocene, Prainha. (D) Entobia isp. and Gastrochaenolits isp. (Ga) in a bivalve shell, Pliocene, Malbusca. (E) Ericichnus bromleyi in calcarenite, Plaistocene, Ichnofossil Cave. (F) Maendropolydora isp., Pliocene, Baía de Nossa Senhora.
Centrichnus dentatus Uchman, Wisshak, Madeira, Melo, Sacchetti, Ávila & Ávila, 2025 in [38] (Figure 3B) is a small, symmetric or asymmetric depression on shells, circular or elliptical in outline, and bounded by a groove and/or a series of pits. It is interpreted as an attachment trace produced by verrucid barnacles [38].
Circolites kotoucensis Mikuláš, 1992 in [39] (Figure 3C) are bowl-shaped structures formed in rocky substrates, including basalts. They are interpreted as traces produced by regular echinoids, and in modern Macaronesia by Paracentrotus lividus [39,40].
Entobia ispp. (Figure 3D) are branched systems of galleries composed of small chambers interconnected by tunnels and opening to the surface. They are produced by clionaid sponges in calcareous hard substrates, most commonly in mollusk shells. Several ichnospecies of Entobia are distinguished based on morphological details [41,42,43].
Ericichnus bromleyi Santos et al., 2015 in [44] (Figure 3E) is an irregularly sinuous groove on the rock surface, which may converge or diverge, and form irregular networks. It is produced by regular echinoids [44].
Gastrochaenolites ispp. (Figure 3D) are flask-shaped cavities in hard substrates, consisting of a chamber and a neck. They are interpreted as borings produced by bivalves [45,46].
Maeandropolydora ispp. (Figure 3F) are long, sinuous, and typically contorted cylindrical galleries in hard calcareous substrates, opening to the surface. The limbs of the galleries may be interconnected. They are interpreted as borings produced by suspension-feeding spionid polychaetes [36,47].
2.3. Trace Fossils in Continental Deposits
Cuniculonomus isp. is a linear, undulating to sinuous leaf mine, of uniform width or with a slight terminal widening [48,49]. It is interpreted as the feeding trace of flies (Agromyzidae) or moths (Gracillariidae) [50].
Palmiraichnus castellanosi (Roselli, 1987) in [51] is an ovoid to subcylindrical structure with a smooth, shiny chamber. It is straight or slightly curved, concave or truncated on one side, and rounded on the other, where a spiral closure separates the main chamber from the ante-chamber. The ante-chamber may be filled with structureless sediment. This trace fossil occurs in palaeosols and is interpreted as the nesting burrow of solitary bees of the family Andrenidae [52,53].
Phagophytichnus ekowskii Van Amerom, 1966 in [54] consists of semicircular excisions along the margins of plant leaves that do not reach the midrib, forming an incomplete oval to elliptical outline. It is interpreted as a feeding trace produced by butterfly caterpillars or locust larvae [49].
Rebuffoichnus guanche Genise et al., 2013 in [53] (Figure 4A) comprises subovoid to subcylindrical structures composed of a chamber and a wall. The outer wall surface is rough and lumpy, whereas the inner surface is smooth or nearly smooth. The chamber is ellipsoid in outline and circular in cross section. The wall may exhibit a rounded opening [55].
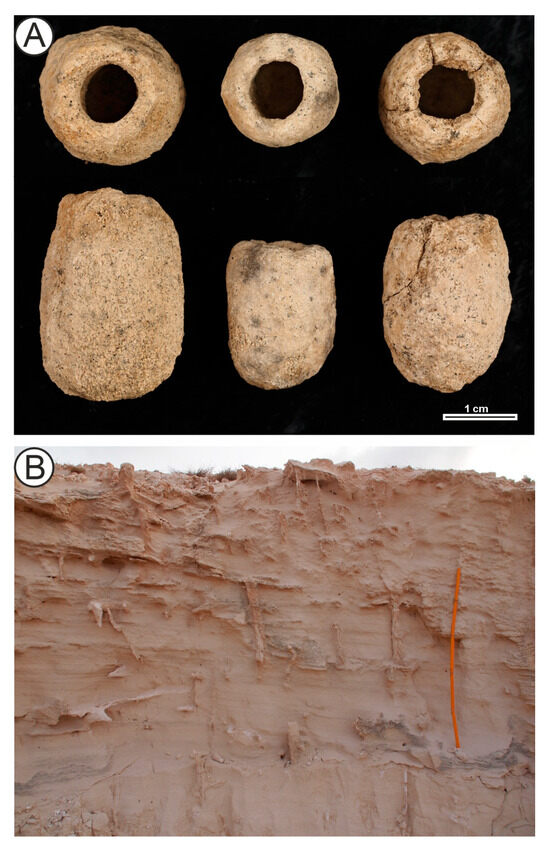
Figure 4.
Some continental trace fossils and biogenic structures. (A) specimens of Rebuffoichnus guanche. Top view in the upper row and side view in the lower row. Pleistocene, El Altillo, Gran Canaria, Canary Islands. (B) Root structures in Quaternary aeolianites, road on side of the road from Deserto de Viana to Ervatão, Boa Vista island, Cape Verde.
Xylonichnus trypetus Genise, 1995 in [55] consists of a boxwork of anastomosing longitudinal, tangential, and radial tunnels, rectangular to circular in cross section, developed at various depths within the xylem. The tunnels may be filled with frass. This trace is interpreted as the work of buprestid beetles or termites [49].
3. Ichnological Investigations in Macaronesia
3.1. Burrows in Marine Deposits
Exposed marine deposits in the Macaronesian islands are almost exclusively restricted to shallow-marine Miocene–Holocene facies, influenced primarily by wave and storm activity. These deposits, typically lacking quartz grains, consist mainly of rocks ranging from calcarenites to lithic sandstones, with variable contributions from volcanic-derived components. Conglomerates also occur, mostly in fan deltas and fossil beach deposits. Characteristic depositional architectures include shoreface-to-backshore sheet-like covers or patches (at outcrop scale) preserved in cliffs and marine terraces, clinoforms prograding into the open ocean, and fan-delta cones. In several cases, sedimentary lithosomes are encapsulated between lava flows. Trace fossils are common and locally abundant in Miocene and Pleistocene calcarenite–sandstone exposures. The most widespread ichnotaxa include Macaronichnus segregatis, Bichordites monastiriensis, Thalassinoides isp., Ophiomorpha nodosa, and Dactyloidites ottoi, with other forms occurring more sporadically.
The first comprehensive ichnological study of marine sediments on volcanic islands was conducted by Mayoral et al. [56], who examined Miocene and Pleistocene deposits of the Cape Verde Archipelago. Some ichnotaxa had already been reported in related papers from the same period [57,58,59,60]. For example, Sinusichnus isp. was documented from São Nicolau Island [60]. Mayoral et al. [56] recognized two principal ichnoassociations: (1) a Miocene Thalassinoides ichnoassociation (restricted to Maio Island) and (2) a Pleistocene Macaronichnus–Dactyloidites ichnoassociation (Santiago, Maio, São Nicolau). Within the latter, mostly horizontal trace fossils such as Dactyloidites ottoi, Dactyloidites isp., Bichordites monastiriensis, Bichordites isp., Cardioichnus isp., Phycodes isp., Teichichnus rectus, aff. Taenidium isp., and Palaeophycus tubularis were assigned to the proximal Cruziana ichnofacies. In contrast, Macaronichnus and vertical burrows including Skolithos, Conichnus conicus, Ophiomorpha nodosa, and Ophiomorpha isp. were referred to the Skolithos ichnofacies. Importantly, based on the geometric relationship between submarine and subaerial lava flows, as well as the stratigraphic distribution of ichnofossil-bearing deposits, Mayoral et al. [56] calibrated the transition from the proximal Cruziana ichnosubfacies to the Skolithos ichnofacies at water depths of approximately 12–18 m.
The Miocene Thalassinoides ichnoassociation comprises Thalassinoides suevicus, Thalassinoides isp., Conichnus conicus, Dactyloidites ottoi, and Macaronichnus segregatis, and belongs to the proximal Cruziana ichnofacies. In addition, a Psilonichnus ichnofacies, represented by a monospecific assemblage of Psilonichnus upsilon (produced by crabs), was recognized in a Pleistocene section of Maio Island, indicating an upper foreshore–backshore setting. At the Nossa Senhora da Luz section (Santiago), protected-bay sediments contain almost exclusively Thalassinoides [61].
Mayoral et al. [62] described thin pockets of Upper Pleistocene biocalcirudites encapsulated between lava flows on Santiago Island. These contained Thalassinoides, Rhizocorallium commune irregulare, Rhizocorallium jenense versum (a new ichnosubspecies), Alaichnus kabuverdiensis (a new ichnogenus and ichnospecies, interpreted as a cumulative trace of bivalve siphons), and cf. Dactyloidites. Taphonomic analysis allowed interpretation of erosion–sedimentation events, colonization sequences, and estimates of erosion depth during deposition.
In the Canary Islands, ichnological investigations are less numerous. Sánchez-Pinto et al. [63] reported Dactyloidites ottoi from Pliocene deposits of Fuerteventura, also mentioned by Martín González [64] and Martín González & Castillo [65]. Schneider et al. [66] described Skolithos ichnofacies traces in Miocene–Pliocene fan-delta deposits of the Cuevas los Guinchos section, Las Palmas Detritic Formation, Gran Canaria. Mayoral et al. [67] recognized Rosselia socialis, Macaronichnus segregatis, Bichordites monastiriensis, and Ophiomorpha nodosa in the same formation, assigning them to the Skolithos and proximal Cruziana ichnofacies. Uchman et al. [68] reinterpreted these assemblages in terms of sedimentation dynamics within a transgressive–regressive cycle of fan-delta progradation, rather than as indicators of bathymetric change. At the Las Rehoyas section of the same formation, Macaronichnus segregatis, Ophiomorpha nodosa, Rosselia socialis, Skolithos linearis, and indeterminate Thalassinoides have been described, assigned to the Skolithos ichnofacies, and interpreted as evidence of seafloor stabilization within sand-dominated intervals of a conglomeratic fan-delta system [69]. Ophiomorpha nodosa was described from Mio-Pliocene deposits in a few localities of Gran Canaria and Fuertenventura [70,71,72].
By contrast, extensive ichnological studies have been carried out on Santa Maria Island (Azores), well known for its fossiliferous formations. Meireles et al. [73] reported Diplocraterion, Crossopodia, Rhizocorallium, Ophiomorpha, and Thalassinoides from storm-influenced Miocene–Pliocene sandy deposits of the Castello section, though the presence of the first three ichnotaxa is doubtful based on later observations. In the Ichnofossil Cave, Santos et al. [44] documented Arenicolites isp., Bichordites isp., Macaronichnus segregatis, Dactyloidites ottoi, and Ophiomorpha nodosa from Miocene–Pliocene deposits, see also [74]. In the Pedra-que-pica section, associated with an exceptional coquina, assemblages include Macaronichnus segregatis, ?Ophiomorpha isp. (misidentified as Thalassinoides isp. by Kirby et al. [75]), Asterosoma isp., and Bichordites isp. [76,77]. These traces helped refine the palaeoenvironmental interpretation and stratigraphic position of the coquina.
Uchman et al. [78,79] described unusual vertically oriented Macaronichnus segregatis from Miocene–Pliocene calcarenites and sandstones of Santa Maria. These deposits, formed on an erosionally truncated island surface and prograding clinoforms on its southern coast, are interpreted as reflecting vertical migration of trace makers following the mixing zone of fresh and marine waters. This ichnotaxon cooccurs with Piscichnus isp., Diopatrichnus isp., ?Palaeophycus isp., ?Parmaichnus isp., Thalassinoides isp., Ophiomorpha nodosa, ?Psilonichnus isp., Asterosoma isp., and Dactyloidites ottoi, which belong to the Psilonichnus, Skolithos, and Cruziana ichnofacies, confirming shallow-water deposition [80]. A similar assemblage, including ?Scolicia isp. and ?Curvolithus isp., is linked to a major hurricane bed (Figure 5) in the Malbusca section [81]. Diopatrichnus santamariaensis [20], a new ichnospecies from Santa Maria, is a vertical tube armored with shell fragments, interpreted as the trace of owenid polychaetes. Piscichnus waitemata, produced mainly by hunting rays, is abundant in the sediment cover of the truncated island but less common in clinoform deposits on the island’s flanks, probably due to lower temperatures and reduced preservation potential [82]. Subtidal clinoforms encapsulated between lava flows on the eastern margin of the island yielded Thalassinoides, Scolicia isp., Palaeophycus isp., Diopatrichnus santamariaensis, Macaronichnus segregatis, Ophiomorpha isp., and Piscichnus waitemata, which refine interpretations of depositional environments [83]. The presence of crustacean burrows (Ophiomorpha isp., Ophiomorpha nodosa, Thalassinoides isp., ?Psilonichnus isp., ?Parmaichnus isp.) has further clarified the diversity of burrowing decapods in the Pliocene–Pleistocene of Santa Maria [84].

Figure 5.
Major hurricane bed (Pliocene calcareous sandy deposits) in between volcanic and volcanogenic rocks at Malbusca, Santa Maria, Azores.
In contrast, information about fossil burrows from Madeira and the Salvagens is scarce. From middle Miocene calcarenites of the Madeira Archipelago, Thalassinoides was reported from Ilhéu de Cima [40], and Bichordites isp. and Dactyloidites isp. from Ilhéu de Baixo [85].
3.2. Marine Bioerosion Structures
Marine bioerosion structures in Macaronesia occur mostly on rocky substrates—typically calcareous, more rarely volcanic—and on skeletal elements, especially mollusk shells.
In the Cape Verde Archipelago, Santos et al. [86] reported Gastrochaenolites isp. in Pliocene–Pleistocene limestones, corals, and basalt boulders at Tarrafal outcrop and Ponta das Bicudas (Santiago Island). Mayoral et al. [56] described Circolites kotoucensis (echinoid boring, also found in basalt), bivalve borings (Gastrochaenolites torpedo, G. cluniformis, Gastrochaenolites isp.), and sponge borings (Entobia isp.) developed on the Miocene–Pleistocene unconformity of Maio Island and on basaltic paleocliffs at Ponta das Bicudas, Santiago, see also [62]. These assemblages belong to the Entobia ichnofacies. Baarli et al. [61] also documented Gastrochaenolites isp. and C. kotoucensis on Santiago, noting that Gastrochaenolites penetrated through hydrozoan Millepora alcicornis into the underlying basalt. C. kotoucensis has also been recorded from São Nicolau [44,59], Sal, and Santo Antão (in basalts), and Brava (in phonolites) [74,87].
In the Canary Islands, Martín-González et al. [88] reported Entobia, Gastrochaenolites, and probable vermetid borings in Pliocene mollusk shells from Fuerteventura. Verde et al. [89] introduced Santichnus mayorali as a new ichnogenus and ichnospecies representing the attachment trace of vermetid gastropods, identified in Miocene–Pliocene mollusk shells from Fuerteventura and La Graciosa (near Lanzarote). Circolites kotoucensis, Gastrochaenolites torpedo, Gastrochaenolites isp., and Maeandropolydora sulcans, Caulostrepsis taeniola, Entobia ovunamesla, and Entobia isp. were described from Mio-Pliocene deposits of Gran Canaria and Fuerteventura [70], and Entobia from the Pleistocene of Fuerteventura [90].
In the Madeira Archipelago, Santos et al. [91] documented Gastrochaenolites torpedo, G. vivus, G. lapidicus, and Entobia isp. in Miocene algal crusts and corals on a basaltic rocky shore at Ilhéu de Cima near Porto Santo; notably, G. lapidicus also penetrated into basalt. Subsequent work at the same locality revealed G. ornatus, which likewise entered basalt [86]. Additional Gastrochaenolites isp. were found in Miocene corals and rhodoliths [92]. Santos et al. [93] also described Imbutichnus costatum, a bioclaustration structure resulting from overgrowth of a Miocene hermatypic coral on a pyrgomatid barnacle. However, bioclaustration features are no longer regarded as trace fossils [94,95].
In the Azores, records are concentrated on Santa Maria Island, with only rare finds from other islands. Boring traces by regular echinoids—now recognized as Circolites kotoucensis—were first reported from Holocene basalts of São Miguel [96,97]. Ávila et al. [98] described Pleistocene Circolites kotoucensis (Figure 2C) in basalts and Gastrochaenolites produced by Leiosolenus ariestatus in algal limestones at Prainha (Santa Maria). In the Ichnofossil Cave (Santa Maria), Santos et al. [44] distinguished two new ichnospecies of echinoid grazing grooves, Ericichnus bromleyi (Figure 3E) and E. asgaardi, as well as C. kotoucensis. Similar grooves were reported from tuffites in Sal (Cape Verde). Ávila et al. [74,77]) noted Gastrochaenolites isp., Entobia isp., and Caulostrepsis isp. in bivalve shells from the Pliocene coquina of the Pedra-que-pica geosite and the Ponta do Cedro section [83]. Gastrochaenolites and Entobia also occur in a paleocliff below a large storm bed at Malbusca [81] and in shells armoring Diopatrichnus santamariaensis (Figure 2C) [20]. Gastrochaenolites cf. torpedo (produced by Leiosolenus ariestatus) was reported in bioclasts, rhodoliths, and algal encrustations at Ponta do Cedro [83]. This boring, along with Entobia, is also present in rhodoliths of Malbusca [81] and in Pleistocene (MIS 5e) algal buildups [99].
A new ichnospecies, Centrichnus dentatus (Figure 3B), was described from the Pedra-que-pica coquina [38]. Produced by the barnacle Verruca spengleri in oyster shells, it co-occurs with Talpina isp., Entobia isp., Maeandropolydora isp., and Gastrochaenolites isp. The assemblage was assigned to the Gnathichnus ichnofacies, reflecting early colonization of hard substrates, often within months.
Dávid et al. [100] recorded 22 ichnotaxa of bioerosion structures in Lower Pliocene deposits of the Baía de Nossa Senhora section (Santa Maria). The assemblage is dominated by well-preserved Entobia, but also includes Gastrochaenolites, Caulostrepsis, Maeandropolydora, Trypanites (sipunculid worms), Talpina (phoronid worms), and Iramena (ctenostome bryozoans). These borings, belonging to the Entobia ichnofacies, closely resemble assemblages from the Paratethys, Mediterranean, and eastern Atlantic regions—consistent with the general Neogene shallow-water faunal affinities of the Azores.
Modern bioerosion has also been studied. Wisshak et al. [101] conducted in situ experiments on the submarine slope of Faial Island, spanning intertidal to bathyal depths. They identified 43 ichnotaxa, including microborings produced by cyanobacteria, chlorophytes, fungi, and other chemotrophs; attachment scars of diatoms, foraminifera, bryozoans, cirripeds, see also [38], gastropods, bivalves, and brachiopods; grazing traces from gastropod radulae and echinoids; and several macroborings (Entobia isp., Caulostrepsis taeniola, Maeandropolydora decipiens, Talpina ramosa).
3.3. Trace Fossils in Continental Environments
Insect nests have been reported from the Canary Islands for over a century, primarily in Pleistocene and Holocene sandy dunes, sandy silts associated with palaeosols, and calcretes of Lanzarote and Fuerteventura [53,102,103,104,105,106,107,108,109,110,111,112]. These structures were initially attributed to Celliforma and interpreted as nests of solitary bees. Genise [113], however, suggested alternative interpretations as coleopteran pupal chambers or lepidopteran cocoons.
Further studies by Meco et al. [114,115] described mass occurrences of these traces in the Pliocene, Pleistocene, and Holocene of Fuerteventura, interpreting them as evidence of episodic locust invasions. Later analyses of material from the island recognized bee nests attributed to Palmiraichnus or to an undescribed ichnogenus of the Celliformidae, as well as coleopteran pupal cells (Rebuffoichnus), both produced under semiarid paleoclimatic conditions during wetter intervals [116]. Rebuffoichnus isp. was subsequently documented in association with megarhizoliths in Pleistocene aeolianites of Gran Canaria [117]. Genise et al. [53] introduced the new ichnospecies Rebuffoichnus guanche, interpreted as pupation chambers of coleopterans (Curculionidae or Scarabaeidae). Moreover, R. casamiquelai Roselli, 1987 [51] is recognized in Fuerteventura and Lanzarote [118]. Palmiraichnus castellanosi has also been reported from the Pleistocene and Holocene of Fuerteventura, Lanzarote, and Gran Canaria [52]. Some Rebuffoichnus guanche specimens show holes or pits in their walls, which belong to the ichnogenus Tombownichnus Mikúlaš & Genise, 2003 [119], with two ichnospecies T. plenus, T. parabolicus produced by some parasitic insects [118].
The distribution of insect nests across the archipelago is uneven. Genise et al. [53] noted abundant occurrences in the eastern islands (Fuerteventura and Lanzarote) but only sporadic records in the western islands (Tenerife, La Palma, La Gomera, El Hierro), where suitable deposits are scarce. Comparable fossils from the more remote Macaronesian archipelagos remain undocumented.
A more recent ichnological development in Macaronesia concerns traces of plant–insect interactions. Pokorný & Borges [49] reported Phagophytichnus ekowskii (marginal feeding traces on leaves), Cuniculonomus isp. (leaf-mining traces; see also [120]), and Xylonichnus trypetus (wood borings) from Quaternary plant collections in the Azores (São Miguel, Terceira, Faial). These findings have since been critically reviewed by Gois-Marques et al. [121]. These traces belong to the Palaeoscolytus ichnofacies.
Borings produced by insects in bones have been examined experimentally in Gran Canaria [122]. However, no evidence of insect borings in fossil bones has yet been reported from Macaronesia.
Plant root structures occupy a conceptual “grey zone” between trace fossils, plant body fossils, and diagenetic features (Figure 4B). Root-induced deformations can represent genuine bioturbation, but differentiating them from abiotic structures is often challenging [94,95]. This issue has been addressed in studies of Pleistocene root structures in Gran Canaria and Tenerife [117,123,124,125]. Similar interpretative difficulties have been noted for rhizobial nodules from the Pleistocene of Madeira [126].
4. Concluding Remarks
The review demonstrates that ichnological research in Macaronesia has made substantial progress over the past two decades, establishing the archipelago as the best-ichnologically studied among volcanic islands. Many islands have been investigated for the first time, often through initiatives led by the University of the Azores. Consequently, the known list of ichnotaxa has expanded considerably, including numerous new ichnogenera and ichnospecies, and understanding of the distribution of trace fossils and their palaeoenvironmental significance has improved markedly. These studies also show that a variety of burrowing and boring animals can colonize newly emerged volcanic islands, even when their extant representatives are not present locally.
Nevertheless, important gaps remain. Microborings in the fossil record of Macaronesia have not yet been studied. Continental ichnology is largely restricted to insect nests in the Canary Islands [52,53] and plant–insect interaction traces [49]. Neoichnological research—such as studies of bioerosion traces [101] and ray-fish burrows [82]—has begun, but much more remains to be done. Fossil burrows are entirely unknown from the Madeira and Salvagens archipelagos. It is also unclear whether the widespread occurrence of the ray-fish hunting burrow Piscichnus waitemata in Santa Maria extends to other islands. In general, geographic trends in trace fossil distribution are poorly understood, and several islands remain completely unexplored ichnologically.
These gaps highlight the need for continued ichnological studies, integrated with other palaeobiological and sedimentological research, to better understand the colonization history and palaeoecology of biota on Macaronesia’s volcanic islands.
Funding
The fieldwork of the author on Santa Maria Island, Azores (2014–2019, 2021, 2013), was supported by the International Workshop Palaeontology in Atlantic Islands. Fieldwork in the Canary Islands (2023) was supported by the PAI2023-CAN International Workshop, sponsored by the Regional Directorate for Science and Technology, Azores Government, through the project Base de Dados da PaleoBiodiversidade da Macaronésia (M1.1.A/INFRAEST CIENT/A/001/2021), within the funding program PRO-SCIENTIA: Eixo 1—Valorizar, Ação 1.1—Capacitar as entidades do SCTA e valorizar as suas atividades, and by the Museums and Centers Autonomous Organism of the Cabildo of Tenerife. Fieldwork in Cape Verde (2024) was supported by FEDER funds through the Operational Program for Competitiveness Factors—COMPETE, and by national funds through FCT (UIDB/50027/2020; POCI-01–0145-FEDER-006821; UIDB/00153/2020; LA/P/0048/2020), as well as through the Regional Government of the Azores (M1.1.a/005/Funcionamento-C-/2016, CIBIO-A; M1.1.A/INFRAEST CIENT/A/001/2021; M3.3.B/ORG.R.C./005/2021). This work also received support from FEDER funds (85%) and the Regional Government of the Azores (15%) through project M1.1.A/INFRAEST CIENT/A/001/2021—Base de Dados da PaleoBiodiversidade da Macaronésia. Additional support was provided by a grant from the Faculty of Geography and Geology under the Strategic Programme Excellence Initiative at Jagiellonian University.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Ramalho, R.S.; Quartau, R.; Trenhaile, A.S.; Mitchell, N.C.; Woodroffe, C.D.; Ávila, S.P. Coastal evolution on volcanic oceanic islands: A complex interplay between volcanism, erosion, sedimentation, sea level change and biogenic production. Earth-Sci. Rev. 2013, 127, 140–170. [Google Scholar] [CrossRef]
- Johnson, M.E.; Baarli, B.G.; Cachão, M.; Mayoral, E.; Ramalho, R.S.; Santos, A.; da Silva, C.M. On the rise and fall of oceanic islands: Towards a global theory following the pioneering studies of Charles Darwin and James Dwight Dana. Earth-Sci. Rev. 2018, 180, 17–36. [Google Scholar] [CrossRef]
- Ávila, S.P.; Madeira, P.; Zazo, C.; Kroh, A.; Kirby, M.; da Silva, C.M.; Cachão, M.; Martins, A.d.F. Palaeoecology of the Pleistocene (MIS 5.5) outcrops of Santa Maria Island (Azores) in a complex oceanic tectonic setting. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 274, 18–31. [Google Scholar] [CrossRef]
- Ávila, S.P.; Ramalho, R.; Vullo, R. Systematics, palaeoecology and palaeobiogeography of the Neogene fossil sharks from the Azores (Northeast Atlantic). Ann. Paleontol. 2012, 98, 167–189. [Google Scholar] [CrossRef]
- Ávila, S.P.; Azevedo, J.M.; Madeira, P.; Cordeiro, R.; Melo, C.S.; Baptista, L.; Torres, P.; Johnson, M.E.; Vullo, R. Pliocene and late Pleistocene actinopterygian fishes from Santa Maria Island, Azores (NE Atlantic Ocean): Palaeoecological and palaeobiogeographical implications. Geol. Mag. 2020, 157, 1526–1542. [Google Scholar] [CrossRef]
- Freitas, R.; Romeiras, M.; Silva, L.; Cordeiro, R.; Madeira, P.; González, J.A.; Wirtz, P.; Falcón, J.M.; Brito, A.; Floeter, S.R.; et al. Restructuring of the ‘Macaronesia’biogeographic unit: A marine multi-taxon biogeographical approach. Sci. Rep. 2019, 9, 15792. [Google Scholar] [CrossRef]
- Florencio, M.; Patiño, J.; Nogué, S.; Traveset, A.; Borges, P.A.; Schaefer, H.; Amorim, I.R.; Arnedo, M.; Ávila, S.P.; Cardoso, P.; et al. Macaronesia as a fruitful arena for ecology, evolution, and conservation biology. Front. Ecol. Evol. 2021, 9, 718169. [Google Scholar] [CrossRef]
- Ávila, S.P.; Paris, R.; Ramalho, R.S.; Melo, C.S.; Martín-González, E.; Rolán, E.; Madeira, P.; Ávila, G.C.; Porteiro, J.M.; Medeiros, A.M.; et al. Megatsunami deposits and range expansion of cold-temperate marine species towards the tropics in glacial times. Front. Biogeogr. 2025, 18, e138319. [Google Scholar] [CrossRef]
- Bromley, R.G. Trace Fossils: Biology, Taphonomy and Applications; Chapman and Hall: London, UK, 1996; 361p. [Google Scholar]
- Buatois, L.A.; Mángano, M.G. Ichnology: Organism–Substrate Interactions in Space and Time; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Knaust, D.; Bromley, R.G. (Eds.) Trace Fossils as Indicators of Sedimentary Environments; Developments in Sedimentology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 64. [Google Scholar]
- Plaziat, J.-C.; Mahmoudi, M. Trace fossils attributed to burrowing echinoids: A revision including new ichnogenus and ichnospecies. Geobios 1988, 21, 209–233. [Google Scholar] [CrossRef]
- Uchman, A. Taxonomy and palaeoecology of flysch trace fossils: The Marnoso-arenacea Formation and associated facies (Miocene, Northern Apennines, Italy). Beringeria 1995, 15, 3–115. [Google Scholar]
- Uchman, A.; Krenmayr, H.G. Trace fossils from Lower Miocene (Ottnangian) molasse deposits of Upper Austria. Paläontol. Z. 1995, 69, 503–524. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Uchman, A. Bichordites and Bichordites–Rosselia ichnoassemblages from the Lower Pleistocene Tursi Sandstone (southern Italy). In Sediment–Organism Interactions: A Multifaceted Ichnology; Bromley, R.G., Buatois, L.A., Mángano, M.G., Genise, J.F., Melchor, R.N., Eds.; SEPM (Society for Sedimentary Geology) Special Publication 88; SEPM Society for Sedimentary Geology: Tulsa, OK, USA, 2007; pp. 213–221. [Google Scholar]
- Geinitz, H.B. Das Quadersandsteingebirge oder Kreidegebirge in Deutschland; Graz & Gerlach: Freiberg, Germany, 1849–1850; 292p. [Google Scholar]
- Fürsich, F.T.; Bromley, R.G. Behavioural interpretation of a rosetted spreite trace fossil: Dactyloidites ottoi (Geinitz). Lethaia 1985, 18, 199–207. [Google Scholar] [CrossRef]
- de Gibert, J.M.; Martinell, J.; Domènech, R. The rosetted feeding trace fossil Dactyloidites ottoi (Geinitz) from the Miocene of Catalonia. Geobios 1995, 28, 769–776. [Google Scholar] [CrossRef]
- Curran, H.A.; Glumac, B. Dactyloidites ottoi (Geinitz, 1849) in Bahamian Pleistocene carbonates: A shallowest-marine indicator. In The Ichnology of Shallow-Marine and Transitional Environments; Cónsole-Gonella, C., de Valais, S., Díaz-Martínez, I., Citton, P., Verde, M., McIlroy, D., Eds.; Special Publications; Geological Society: London, UK, 2023. [Google Scholar] [CrossRef]
- Uchman, A.; Quintino, V.; Rodrigues, A.M.; Johnson, M.E.; Melo, C.; Cordeiro, R.; Ramalho, R.S.; Ávila, S.P. The trace fossil Diopatrichnus santamariensis isp. nov. —A shell-armored tube from Pliocene sediments of Santa Maria Island, Azores (NE Atlantic Ocean). Geobios 2017, 50, 459–469. [Google Scholar] [CrossRef]
- Clifton, H.E.; Thompson, J.K. Macaronichnus segregatis: A feeding structure of shallow marine polychaetes. J. Sediment. Petrol. 1978, 48, 1293–1302. [Google Scholar] [CrossRef]
- Nara, M.; Seike, K. Macaronichnus segregatis-like traces found in the modern foreshore sediments of the Kujukurihama Coast, Japan. J. Geol. Soc. Jpn. 2004, 110, 545–551. [Google Scholar] [CrossRef]
- Nara, M.; Seike, K. Palaeoecology of Macaronichnus segregatis degiberti: Reconstructing the infaunal lives of the travisiid polychaetes. Palaeogeogr. Palaeoclimat. Palaeoecol. 2019, 516, 284–294. [Google Scholar] [CrossRef]
- Seike, K.; Yanagishima, S.; Nara, M.; Sasaki, T. Large Macaronichnus in modern shoreface sediments: Identification of the producer, the mode of formation, and palaeoenvironmental implications. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 311, 224–229. [Google Scholar] [CrossRef]
- Lundgren, B. Studier öfver fossilförande lösa block. Geol. Föreningen I Stockh. Förhandlinger 1891, 13, 111–121. [Google Scholar] [CrossRef]
- Knaust, D. Atlas of Trace Fossils in Well Core: Appearance, Taxonomy and Interpretation; Springer: Cham, Switzerland, 2017; 271p. [Google Scholar]
- Frey, R.W.; Howard, J.D.; Pryor, W.A. Ophiomorpha: Its morphologic, taxonomic, and environmental significance. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1978, 23, 199–229. [Google Scholar] [CrossRef]
- Bromley, R.G. Trace Fossils. Biology and Taphonomy; Special Topics in Palaeontology Series; Unwin Hyman: London, UK; Boston, MA, USA; Sydney, Australia; Wellington, New Zealand, 1990; 280p. [Google Scholar]
- Schlirf, M. Upper Jurassic trace fossils from the Boulonnais (northern France). Geol. Palaeontol. 2000, 34, 145–213. [Google Scholar]
- Chrząstek, A. Palaeoenvironmental interpretation of the Late Cretaceous Idzików Conglomerate Member (SW Poland, Sudetes, Idzików Quarry) based on analysis of trace fossils. Ann. Soc. Geol. Pol. 2020, 90, 149–194. [Google Scholar] [CrossRef]
- Pemberton, S.G.; Frey, R.W. Trace fossil nomenclature and the Planolites–Palaeophycus dilemma. J. Paleontol. 1982, 56, 843–881. [Google Scholar]
- Keighley, D.G.; Pickerill, R.K. The ichnotaxa Palaeophycus and Planolites: Historical perspectives and recommendations. Ichnos 1995, 3, 301–309. [Google Scholar] [CrossRef]
- Gregory, M.R. New trace fossils from the Miocene of Northland, New Zealand: Roschachichnus amoeba and Piscichnus waitemata. Ichnos 1991, 1, 195–205. [Google Scholar] [CrossRef]
- Frey, R.W.; Curran, A.H.; Pemberton, G.S. Trace-making activities of crabs and their environmental significance: The ichnogenus Psilonichnus. J. Paleontol. 1984, 58, 333–350. [Google Scholar]
- Giannetti, A.; Monaco, P.; Caracuel, J.E.; Soria, J.; Yébenes, A. Functional morphology and ethology of decapod crustaceans gathered by Thalassinoides branched burrows in Mesozoic shallow-water environments. Mem. Soc. Ital. Sci. Nat. Mus. Civ. Stor. Nat. Milano 2007, 35, 48–52. [Google Scholar]
- Bromley, R.G.; D’Alessandro, A.S. Bioerosion in the Pleistocene of southern Italy: Ichnogenera Caulostrepsis and Maeandropolydora. Riv. Ital. Paleontol. Stratigr. 2020, 89, 283–309. [Google Scholar]
- Gaaloul, N.; Uchman, A.; Ben Ali, S.; Janiszewska, K.; Stolarski, J.; Kołodziej, B.; Riahi, S. In vivo and post-mortem bioerosion traces in solitary corals from the upper Pliocene deposits of Tunisia. Acta Palaeontol. Pol. 2023, 68, 659–681. [Google Scholar] [CrossRef]
- Uchman, A.; Wisshak, M.; Madeira, P.; Melo, C.S.; Sacchetti, C.; Ávila, G.C.; Ávila, S.P. A new attachment trace of a verrucid barnacle on Pliocene bivalve shells, Santa Maria Island, Azores (Central Atlantic). Acta Palaeontol. Pol. 2025, 70, 143–157. [Google Scholar] [CrossRef]
- Mikuláš, R. Early Cretaceous borings from Štramberk (Czechoslovakia). Čas. Mineral. Geol. 1992, 37, 297–312. [Google Scholar]
- Johnson, M.E.; da Silva, C.M.; Santos, A.; Baarli, B.G.; Cachão, M.; Mayoral, E.J.; Rebelo, A.C.; Ledesma-Vázquez, J. Rhodolith transport and immobilization on a volcanically active rocky shore: Middle Miocene at Cabeço das Laranjas on Ilhéu de Cima (Madeira Archipelago, Portugal). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 300, 113–127. [Google Scholar] [CrossRef]
- Bromley, R.G. Borings as trace fossils and Entobia cretacea Portlock, as an example. Geol. J. 1970, 3, 49–90. [Google Scholar]
- Bromley, R.G.; D’Alessandro, A. Ichnological study of shallow marine endolithic sponges from the Italian coast. Riv. Ital. Paleontol. Strat. 1989, 95, 279–340. [Google Scholar]
- Garilli, V.; Dávid, Á.; Dominici, S. Natural casts of Entobia from the late Caenozoic of Sicily. Riv. Ital. Paleontol. Strat. 2022, 128, 211–228. [Google Scholar] [CrossRef]
- Santos, A.; Mayoral, E.; Dumont, C.P.; da Silva, C.M.; Ávila, S.P.; Baarli, B.G.; Cachão, M.; Johnson, M.E.; Ramalho, R.S. Role of environmental change in rock-boring echinoid trace fossils. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 432, 1–14. [Google Scholar] [CrossRef]
- Kelly, S.R.A.; Bromley, R.G. Ichnological nomenclature of clavate borings. Palaeontology 1984, 27, 793–807. [Google Scholar]
- Donovan, S.K. Club-shaped borings: Gastrochaenolites. Geol. Today 2025, 41, 61–64. [Google Scholar] [CrossRef]
- de Gibert, J.M.; Domènech, R.; Martinell, J. Bioerosion in shell beds from the Pliocene Roussillon Basin, France: Implications for the (macro) bioerosion ichnofacies model. Acta Palaeont. Pol. 2007, 52, 783–798. [Google Scholar]
- Straus, A. Gallen, Minen und andere Fraßspuren im Pliokän von Willershauen am Harz. Verh. Bot. Ver. Prov. Brandenbg. 1977, 113, 43–80. [Google Scholar]
- Pokorný, R.; Borges, P.A. Uma descoberta interessante de folhas fósseis com vestígios de interações planta-animal nas colecções do Museu Vulcanoespeleológico “Os Montanheiros”. Pingo Lava 2022, 44, 76–78. [Google Scholar]
- Robledo, J.M.; Sarzetti, L.C.; Anzótegui, L.M. New records and ichnospecies of linear leaf mines from the Late Miocene–Pliocene from Argentina and the establishment of leaf-mining ichnotaxobases. Riv. Ital. Paleontol. Stratigr. 2016, 122, 55–70. [Google Scholar] [CrossRef]
- Roselli, F.L. Paleoicnología: Nidos de insectos fósiles de la cubertura Mesozoica del Uruguay. Publ. Mus. Municip. Nueva Palmira 1987, 1, 1–56. [Google Scholar]
- La Roche, F.; Genise, J.F.; Castillo, C.; Quesada, M.L.; García-Gotera, C.M.; De la Nuez, J. Fossil bee cells from the Canary Islands: Ichnotaxonomy, palaeobiology and palaeoenvironments of Palmiraichnus castellanosi. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 409, 249–264. [Google Scholar] [CrossRef]
- Genise, J.F.; Alonso-Zarza, A.M.; Verde, M.; Meléndez, A. Insect trace fossils in aeolian deposits and calcretes from the Canary Islands: Their ichnotaxonomy, producers, and palaeoenvironmental significance. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 377, 110–124. [Google Scholar] [CrossRef]
- Van Amerom, H.W.J. Phagophytichnus ekowskii nov. ichnogen. & nov. ichnosp., eine Missbildung infolge von Insektenfrass, aus dem Spanischen Stephanien (Provinz Leon). Leidse Geol. Meded. 1966, 38, 181–184. [Google Scholar]
- Genise, J.F. Upper Cretaceous trace fossils in permineralized plant remains from Patagonia, Argentina. Ichnos 1995, 3, 287–299. [Google Scholar] [CrossRef]
- Mayoral, E.; Ledesma-Vázquez, J.; Baarli, B.G.; Santos, A.; Ramalho, R.; Cachão, M.; da Silva, C.M.; Johnson, M.E. Ichnology in oceanic islands: Case studies from the Cape Verde Archipelago. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 381, 47–66. [Google Scholar] [CrossRef]
- Johnson, M.E.; Baarli, B.G.; Cachão, M.; da Silva, C.M.; Ledesma-Vázquez, J.; Mayoral, E.J.; Ramalho, R.S.; Santos, A. Rhodoliths, uniformitarianism, and Darwin: Pleistocene and Recent carbonate deposits in the Cape Verde and Canary archipelagos. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 329, 83–100. [Google Scholar] [CrossRef]
- Johnson, M.E.; Baarli, B.G.; da Silva, C.M.; Cachão, M.; Ramalho, R.S.; Ledesma-Vázquez, J.; Mayoral, E.J.; Santos, A. Coastal dunes with high content of rhodolith (coralline red algae) bioclasts: Pleistocene formations on Maio and São Nicolau in the Cape Verde Archipelago. Aeolian Res. 2013, 8, 1–9. [Google Scholar] [CrossRef]
- Johnson, M.E.; Ramalho, R.S.; Baarli, B.G.; Cachão, M.; da Silva, C.M.; Mayoral, E.J.; Santos, A. Miocene–Pliocene rocky shores on São Nicolau (Cape Verde Islands): Contrasting windward and leeward biofacies on a volcanically active oceanic island. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 395, 131–143. [Google Scholar] [CrossRef]
- Baarli, B.G.; Santos, A.G.; Mayoral, E.J.; Ledesma-Vázquez, J.; Johnson, M.E.; da Silva, C.M.; Cachão, M. What Darwin did not see: Pleistocene fossil assemblages on a high-energy coast at Ponta das Bicudas, Santiago, Cape Verde Islands. Geol. Mag. 2013, 150, 183–189. [Google Scholar] [CrossRef]
- Melo, M.; Madeira, J.; Ramalho, R.S.; Rebelo, A.C.; Rasser, M.; González, E.; Uchman, A.; Madeira, P.; Rolán, E.; Silva, L.; et al. Last Interglacial fossiliferous sequences from Santiago Island (Cabo Verde Archipelago): The palaeoecology of the Nossa Senhora da Luz section, a rare example of a protected bay in volcanic oceanic islands. In Proceedings of the EGU General Assembly 2020, EGU2020-10423, Online, 4–8 May 2020. [Google Scholar] [CrossRef]
- Mayoral, E.; Santos, A.; Vintaned, J.G.; Ledesma-Vázquez, J.; Baarli, B.G.; Cachão, M.; da Silva, C.M.; Johnson, M.E. Upper Pleistocene trace fossils from Ponta das Bicudas, Santiago, Cape Verde Islands: Systematics, taphonomy and palaeoenvironmental evolution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 498, 83–98. [Google Scholar] [CrossRef]
- Sanchez-Pinto, L.; García-Talavera, F.; López Rondón, J.; Martín Oval, M. Sobre la presencia del icnofósil Dactyloidites ottoi (Geinitz, 1849) en sedimentos neógenos de la costa occidental de Fuerteventura (Islas Canarias). In Homenaje al Profesor Dr. Wolfredo Wildpret de la Torre; Beltrán Tejera, E., Afonso-Carrillo, J., García Gallo, A., Rodríguez Delgado, O., Eds.; Instituto de Estudios Canarios: La Laguna, Spain, 2009; Monografía LXXVIII; pp. 229–244. [Google Scholar]
- Martín González, E. Paleontología de Canarias: Los yacimientos marinos fósiles. Macaronesia 2009, 11, 70–87. [Google Scholar]
- Martín-González, E.; Castillo, C. Los yacimientos marinos del Neógeno de Lanzarote y Fuerteventura (Islas Canarias). Correlaciones con el sur de la Península Ibérica y otros archipiélagos de la Macaronesia. In III Congreso Ibérico de Paleontología; Universidad De Zaragoza: Lisboa, Portugal, 2010; Volume 1, 5p. [Google Scholar]
- Schneider, J.L.; Torrado, F.J.P.; Torrente, D.G.; Wassmer, P.; Santana, M.D.C.C.; Carracedo, J.C. Sedimentary signatures of the entrance of coarse-grained volcaniclastic flows into the sea: The example of the breccia units of the Las Palmas Detritic Formation (Mio–Pliocene, Gran Canaria, Eastern Atlantic, Spain). J. Volcanol. Geotherm. Res. 2004, 138, 295–323. [Google Scholar] [CrossRef]
- Mayoral, E.; Santos, A.; Galindo, I.; Martín-González, E.; Mangas, J. Contenido icnológico del Miembro medio de la Formación Detrítica Las Palmas (Mio–Plioceno) en el yacimiento de Cuevas del Guincho, Gran Canaria. In Libro de Resúmenes, XXXV Jornadas de la SEP; Martínez-Navarro, B., Ed.; Universidad de Málaga: Málaga, Spain, 2019; pp. 183–185. [Google Scholar]
- Uchman, A.; Martín-González, E.; Madeira, J.; Madeira, P.; Hipólito, A.; Ávila, S.P. Ichnofabrics controlled by transgressive-regressive regimes in marine Neogene deposits from Gran Canaria, Canary Islands, Spain. In XVIII International Ichnofabric Workshop, New Trends in Ichnofabrics Analysis; Dorador, J., Rodríguez-Tovar, F.J., Eds.; Universidad de Granada: Granada, Spain, 2025; pp. 93–94. [Google Scholar]
- Rebelo, C.; Uchman, A.; Johnson, M.E.; Melo, C.S.; Vegas, J.; Galindo, I.; Mayoral, E.J.; Santos, A.; González-Rodríguez, A.; Afonso-Carrillo, J.; et al. Rhodoliths and trace fossils record stabilization of a fan-delta system: An example from the Mio–Pliocene deposits of Gran Canaria (Canary Islands, Spain). J. Paleogeogr. 2025, 14, 100266. [Google Scholar] [CrossRef]
- Betancort Lozano, J.F. Fósiles marinos del Neógeno de Canarias (Colección de la ULPGC): Dos neotipos, catálogo y nuevas aportaciones (sistemática, paleoecología y paleoclimatología). Doctoral Dissertation, Universidat del Las Palmas de Gran Canaria, Las Palmas de Gran Canaria, Spain, 2012; 377p. [Google Scholar]
- Tuya, F.; Betancort, J.F.; Haroun, R.; Espino, F.; Lomoschitz, A.; Meco, J. Seagrass paleo-biogeography: Fossil records reveal the presence of Halodule cf. in the Canary Islands (eastern Atlantic). Aquatic Bot. 2017, 143, 1–7. [Google Scholar] [CrossRef]
- Lozano, J.F.B. El patrimonio paleontológico de Gran Canaria: Ventanas abiertas a la historia del Atlántico Norte. In Actas XV Semana Científica Telesforo Bravo; Lozano, J.F.B., Ed.; Instituto de Estudios Hispánicos de Canarias: Puerto de la Cruz, Spain, 2020; pp. 41–59. [Google Scholar]
- Meireles, R.P.; Quartau, R.; Ramalho, R.S.; Rebelo, A.C.; Madeira, J.; Zanon, V.; Ávila, S.P. Depositional processes on oceanic island shelves: Evidence from storm-generated Neogene deposits from the mid-North Atlantic. Sedimentology 2013, 60, 1769–1785. [Google Scholar] [CrossRef]
- Ávila, S.P.; Ramalho, R.S.; da Silva, C.M.; Uchman, A.; Berning, B.; Johnson, M.E.; Melo, C.S.; Madeira, P.; Rebelo, A.C.; Câmara, R.; et al. Os Fósseis de Santa Maria (Açores). 3. O Trilho Marítimo da Rota dos Fósseis; OVGA—Observatório Vulcanológico e Geotérmico dos Açores: Lagoa, Portugal, 2022; 120p, ISBN 978-989-8164-27-8. [Google Scholar]
- Kirby, M.X.; Jones, D.S.; Ávila, S.P. Neogene shallow-marine paleoenvironments and preliminary strontium isotope chronostratigraphy of Santa Maria Island, Azores. In Proceedings of the 1st Atlantic Islands Neogene; Ávila, S.P., de Frias Martins, A.M., Eds.; Açoreana Suplemento; University of Florida: Gainesville, FL, USA, 2007; (Suppl. 5), pp. 112–125. [Google Scholar]
- Ávila, S.P.; Ramalho, R.S.; Habermann, J.M.; Quartau, R.; Kroh, A.; Berning, B.; Johnson, M.E.; Kirby, M.X.; Zanon, V.; Titschack, J.; et al. Palaeoecology, taphonomy, and preservation of a lower Pliocene shell bed (coquina) from a volcanic oceanic island (Santa Maria Island, Azores, NE Atlantic Ocean). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 430, 57–73. [Google Scholar] [CrossRef]
- Ávila, S.P.; Ramalho, R.S.; da Silva, C.M.; Johnson, M.E.; Uchman, A.; Berning, B.; Quartau, R.; Madeira, P.; Melo, C.S.; Rebelo, A.C.; et al. Os fósseis de Santa Maria (Açores). 2. Pedra-Que-Pica: Uma História Com 5 Milhões de Anos; OVGA—Observatório Vulcanológico e Geotérmico dos Açores: Ponta Delgada, Portugal, 2022; 160p. [Google Scholar]
- Uchman, A.; Johnson, M.E.; Rebelo, A.C.; Melo, C.; Cordeiro, R.; Ramalho, R.S.; Ávila, S.P. Vertically-oriented trace fossil Macaronichnus segregatis from Neogene of Santa Maria Island (Azores, NE Atlantic). In Proceedings of the 13th International Ichnofabric Workshop, Ichnofabric Studies Linking Past, Present, and Future, Kochi, Japan, 14–21 May 2015; Nara, M., Ed.; Abstract Book. p. 27. [Google Scholar]
- Uchman, A.; Johnson, M.E.; Rebelo, A.C.; Melo, C.; Cordeiro, R.; Ramalho, R.S.; Ávila, S.P. Vertically-oriented trace fossil Macaronichnus segregatis from Neogene of Santa Maria Island (Azores, NE Atlantic) records a specific palaeohydrological regime on a small oceanic island. Geobios 2016, 49, 229–241. [Google Scholar] [CrossRef]
- Rebelo, A.C.; Rasser, M.W.; Kroh, A.; Johnson, M.E.; Melo, C.; Ramalho, R.S.; Uchman, A.; Zanon, V.; Silva, L.; Neto, A.I.; et al. Rocking around a volcanic island shelf: Pliocene rhodolith beds from Malbusca, Santa Maria Island (Azores, NE Atlantic). Facies 2016, 62, 1–22. [Google Scholar] [CrossRef]
- Johnson, M.E.; Uchman, A.; Costa, P.J.M.; Ramalho, R.S.; Ávila, S.P. Intense hurricane transports sand onshore: Example from the Pliocene Malbusca section on Santa Maria Island (Azores, Portugal). Mar. Geol. 2017, 385, 244–249. [Google Scholar] [CrossRef]
- Uchman, A.; Torres, T.; Johnson, M.E.; Berning, B.; Ramalho, R.S.; Rebelo, A.C.; Melo, C.S.; Baptista, L.; Madeira, P.; Cordeiro, R.; et al. Feeding traces of recent ray fish and abundant occurrences of the trace fossil Piscichnus waitemata from the Pliocene of Santa Maria Island, Azores (Northeast Atlantic). Palaios 2018, 33, 361–375. [Google Scholar] [CrossRef]
- Uchman, A.; Johnson, M.E.; Ramalho, R.S.; Quartau, R.; Berning, B.; Hipólito, A.; Melo, C.; Rebelo, A.C.; Cordeiro, R.; Ávila, S.P. Neogene marine sediments and biota encapsulated between lava flows on Santa Maria Island (Azores, NE Atlantic): An interplay between sedimentary, erosional, and volcanic processes. Sedimentology 2020, 67, 3595–3618. [Google Scholar] [CrossRef]
- Hyžný, M.; Melo, C.S.; Ramalho, R.S.; Cordeiro, R.; Madeira, P.; Baptista, L.; Rebelo, A.C.; Gómez, C.; Torres, P.; Uchman, A.; et al. Pliocene and late Pleistocene (MIS 5e) decapod crustaceans from Santa Maria Island (Azores Archipelago: NE Atlantic): Systematics, palaeoecology and palaeobiogeography. J. Quat. Sci. 2021, 36, 91–109. [Google Scholar] [CrossRef]
- Baarli, B.G.; Cachão, M.; da Silva, C.M.; Johnson, M.E.; Mayoral, E.J.; Santos, A. A Middle Miocene carbonate embankment on an active volcanic slope: Ilhéu de Baixo, Madeira Archipelago, Eastern Atlantic. Geol. J. 2014, 49, 90–106. [Google Scholar] [CrossRef]
- Santos, A.; Mayoral, E.; Johnson, M.E.; Baarli, B.G.; Cachão, M.; da Silva, C.M.; Ledesma-Vázquez, J. Extreme habitat adaptation by boring bivalves on volcanically active paleoshores from North Atlantic Macaronesia. Facies 2012, 58, 325–338. [Google Scholar] [CrossRef]
- Ramalho, R.; Helffrich, G.; Schmidt, D.N.; Vance, D. Tracers of uplift and subsidence in the Cape Verde Archipelago. J. Geol. Soc. Lond. 2010, 167, 519–538. [Google Scholar] [CrossRef]
- Martín-González, E.; Castillo, C.; Castillo Ruiz, C.; Gutiérrez González, M.; Aguirre, J. Estudio paleoambiental de los depósitos litorales someros del Plioceno inferior de Fuerteventura (Islas Canarias). Rev. Esp. Paleontol. 2001, 16, 47–57. [Google Scholar] [CrossRef]
- Verde, M.; Castillo, C.; Martín-González, E.; Cruzado-Caballero, P.; Mayoral, E.; Santos, A. A new Miocene–Pliocene ichnotaxon for vermetid anchoring bioerosion structures. Front. Earth Sci. 2022, 10, 906493. [Google Scholar] [CrossRef]
- González Delgado, J.Á.; Zazo, C.; Goy, J.L.; Civis, J.; Dabrio, C.J.; Cabero, A.; Bardaji, T.; Silva, P.G. Paleontología de las terrazas marinas del valle de Jorós (Fuerteventura, España). Geo-Temas 2008, 10, 1265–1268. [Google Scholar]
- Santos, A.; Mayoral, E.J.; da Silva, C.M.; Cachão, M.; Johnson, M.E.; Baarli, B.G. Miocene intertidal zonation on a volcanically active shoreline: Porto Santo in the Madeira Archipelago, Portugal. Lethaia 2011, 44, 26–32. [Google Scholar] [CrossRef]
- Santos, A.; Mayoral, E.; Johnson, M.E.; Baarli, B.G.; da Silva, C.M.; Cachão, M.; Ledesma-Vázquez, J. Basalt mounds and adjacent depressions attract contrasting biofacies on a volcanically active Middle Miocene coastline (Porto Santo, Madeira Archipelago, Portugal). Facies 2012, 58, 573–585. [Google Scholar] [CrossRef]
- Santos, A.; Mayoral, E.; Baarli, B.G.; da Silva, C.M.; Cachão, M.; Johnson, M.E. Symbiotic association of a pyrgomatid barnacle with a coral from a volcanic Middle Miocene shoreline (Porto Santo, Madeira Archipelago, Portugal). Palaeontology 2012, 55, 173–182. [Google Scholar] [CrossRef]
- Bertling, M.; Braddy, S.; Bromley, R.G.; Demathieu, G.D.; Genise, J.F.; Mikuláš, R.; Nielsen, J.-K.; Nielsen, K.S.S.; Rindsberg, A.K.; Schlirf, M.; et al. Names for trace fossils: A uniform approach. Lethaia 2006, 39, 265–286. [Google Scholar] [CrossRef]
- Bertling, M.; Buatois, L.A.; Knaust, D.; Laing, B.; Mángano, M.G.; Meyer, N.; Mikuláš, R.; Minter, N.J.; Neumann, C.; Rindsberg, A.K.; et al. Names for trace fossils 2.0: Theory and practice in ichnotaxonomy. Lethaia 2022, 55, 1–19. [Google Scholar] [CrossRef]
- John, G. Über bohrende Seeigel. In Inaugural Dissertation zur Erlangung des Doctorgrades der Philosophischen Facultät der Universität Leipzig; Adolph Mehnert: Leipzig, Germany, 1888; 46p. [Google Scholar]
- Otter, G.W. Rojazidoring echinoids. Biol. Rev. 1932, 7, 89–107. [Google Scholar] [CrossRef]
- Ávila, S.P.; Rebelo, A.; Medeiros, A.; Melo, C.; Gomes, C.; Bagaço, L.; Madeira, P.; Borges, P.A.; Monteiro, P.; Cordeiro, R.; et al. Os Fósseis de Santa Maria (Açores). 1. A Jazida da Prainha; Marine PalaeoBiogeography Working Group: Ponta Delgada, Portugal, 2010; 105p. [Google Scholar]
- Rebelo, A.C.; Rasser, M.W.; Ramalho, R.S.; Johnson, M.E.; Melo, C.S.; Uchman, A.; Quartau, R.; Berning, B.; Neto, A.I.; Mendes, A.R.; et al. Pleistocene coralline algal buildups on a mid-ocean rocky shore—insights into the MIS 5e record of the Azores. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 579, 110598. [Google Scholar] [CrossRef]
- Dávid, Á.; Uchman, A.; Ramalho, R.S.; Madeira, J.; Melo, C.S.; Madeira, P.; Rebelo, A.C.; Berning, B.; Johnson, M.E.; Ávila, S.P. Diverse bioerosion structures in lower Pliocene deposits from a volcanic oceanic island: Baía de Nossa Senhora section, Santa Maria Island, Azores (central North Atlantic). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 569, 110284. [Google Scholar] [CrossRef]
- Wisshak, M.; Tribollet, A.; Golubic, S.; Jakobsen, J.; Freiwald, A. Temperate bioerosion: Ichnodiversity and biodiversity from intertidal to bathyal depths (Azores). Geobiology 2011, 9, 492–520. [Google Scholar] [CrossRef]
- Hernández-Pacheco, E. Exploración geológica de Lanzarote y de las Isletas Canarias. Bol. R. Soc. Esp. Hist. Nat. 1907, 7, 339–348. [Google Scholar]
- Hernández-Pacheco, E. Estudio geológico de Lanzarote y de las Islas Canarias. Mem. Real Soc. Esp. Hist. Nat. 1909, 6, 107–342. [Google Scholar]
- Aranda Millán, F. Sobre Moluscos de Lanzarote (Canarias). Bol. Real Soc. Esp. Hist. Nat. 1909, 9, 112–114. [Google Scholar]
- Ellis, W.N.; Ellis-Adam, A.C. Fossil brood cells of solitary bees on Fuerteventura and Lanzarote, Canary Islands (Hymenoptera: Apoidea). Entomol. Ber. 1993, 53, 161–173. [Google Scholar]
- Bravo, T. Geografía General de las Islas Canarias 1; Editorial Goya: Santa Cruz de Tenerife, Spain, 1954; 410p. [Google Scholar]
- Machado, A. Introduction to a faunal study of the Canary Islands laurisilva, with special reference to ground beetles. In Biogeography and Ecology in the Canary Islands; Kunkel, G., Ed.; W. Junk: The Hague, The Netherlands, 1976; pp. 347–411. [Google Scholar]
- Báez, M.; Bacallado, J.J. Los fósiles de Canarias. In Fauna (marina y terrestre) del Archipiélago Canario; Bacallado, J.J., Ed.; Edirca: Las Palmas de Gran Canaria, Spain, 1984; pp. 343–347. [Google Scholar]
- Rothe, P. Kanarische Inseln. Lanzarote, Fuerteventura, Gran Canaria, Tenerife, Gomera, La Palma, Hierro. Samml. Geol. Führer 1986, 81, 1–226. [Google Scholar]
- Edwards, N.; Meco, J. Morphology and palaeoenvironment of brood cells of Quaternary ground-nesting solitary bees (Hymenoptera, Apidae) from Fuerteventura, Canary Islands, Spain. Proc. Geol. Assoc. 2000, 111, 173–183. [Google Scholar] [CrossRef]
- Alonso-Zarza, A.M.; Silva, P.G. Quaternary laminar calcretes with bee nests: Evidences of small-scale climatic fluctuations, Eastern Canary Islands, Spain. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2002, 178, 119–135. [Google Scholar] [CrossRef]
- Von Suchodoletz, H.; Kühn, P.; Hambach, U.; Dietz, M.; Zöller, L.; Faust, D. Loesslike and palaeosol sediments from Lanzarote (Canary Islands / Spain)—indicators of palaeoenvironmental change during the Late Quaternary. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 278, 71–87. [Google Scholar] [CrossRef]
- Genise, J.F. The ichnofamily Celliformidae for Celliforma and allied ichnogenera. Ichnos 2000, 7, 267–284. [Google Scholar] [CrossRef]
- Meco, J.; Petit-Maire, N.; Ballester, J.; Betancort, J.F.; Ramos, A.J.G. The Acridian plagues, a new Holocene and Pleistocene palaeoclimatic indicator. Glob. Planet. Change 2010, 72, 318–320. [Google Scholar] [CrossRef]
- Meco, J.; Muhs, D.R.; Fontugne, M.; Ramos, A.J.G.; Lomoschitz, A.; Patterson, D. Late Pliocene and Quaternary Eurasian locust infestations in the Canary Archipelago. Lethaia 2011, 44, 440–454. [Google Scholar] [CrossRef]
- Genise, J.F.; Edwards, N. Ichnotaxonomy, origin, and paleoenvironment of Quaternary insect cells from Fuerteventura, Canary Islands, Spain. J. Kans. Entomol. Soc. 2003, 76, 320–327. [Google Scholar]
- Alonso-Zarza, A.M.; Genise, J.F.; Cabrera, M.C.; Mangas-Viñuela, J.; Martín-Pérez, A.; Valdeolmillos-Rodríguez, A.; Dorado-Valiño, M. Megarhizoliths in Pleistocene aeolian deposits from Gran Canaria (Spain): Ichnological and palaeoenvironmental significance. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 265, 39–51. [Google Scholar] [CrossRef]
- Genise, J.F. Ichnoentomology: Insect Traces in Soils and Paleosols; Topics in Geobiology; Springer: Berlin/Heidelberg, Germany, 2017; Volume 37, 695p. [Google Scholar]
- Mikúlaš, R.; Genise, J.F. Traces within traces: Holes, pits and galleries in walls and filling of insect trace fossils in paleosols. Geol. Acta 2003, 1, 339–348. [Google Scholar] [CrossRef]
- Pokorný, R.; Borges, P.A. Plant–insect interactions in the Quaternary fossil record of the Azores Archipelago (Portugal). J. Quat. Sci. 2023, 38, 597–607. [Google Scholar] [CrossRef]
- Gois-Marques, C.A.; Madeira, J.; de Sequeira, M.M. Comment on: Plant–insect interactions in the Quaternary fossil record of the Azores Archipelago (Portugal). Pokorný and Borges (2023). J. Quat. Sci. 2024, 39, 340–344. [Google Scholar] [CrossRef]
- Henríquez-Valido, P.; Brito-Mayor, A. Taphonomy on the beach: Experimental approach to bone modifications made by insects on an island (Gran Canaria, Canary Island, Spain). Archaeol. Anthropol. Sci. 2024, 16, 192. [Google Scholar] [CrossRef]
- Alonso-Zarza, A.M.; Genise, J.; Valdeolmillos Rodríguez, A.; Cabrera Santana, M.D.C.; Mangas, J.; Martín-Pérez, A.; Dorado-Valiño, M. Los megarrizolitos de las eolianitas de Tufia (Gran Canaria): Procesos de formación, ichnología y paleoambiente. Geotemas 2008, 10, 1377–1380. [Google Scholar]
- Coello Bravo, J.J. Escrito en la arena: Tubos y diques sedimentarios en paleodepósitos costeros de El Médano (Granadilla de Abona, Tenerife). Macaronesia 2012, 14, 124–135. [Google Scholar]
- Alonso-Zarza, A.M.; Casillas Ruiz, R. El Médano megarhizoliths field, Tenerife: Origin and paleoenvironmental significance. Geogaceta 2019, 65, 27–30. [Google Scholar]
- Pokorný, R. Rhizobial root nodules in aeolian sandstones on Madeira (Piedade Beds, Pleistocene) and their significance for palaeoenvironmental and “originator” hypotheses. Rev. Palaeobot. Palynol. 2022, 306, 104740. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).