The Influence of Summer Cyclonic Circulation in the Southern Gulf of California on Planktonic Copepod Communities
Abstract
1. Introduction
2. Materials and Methods
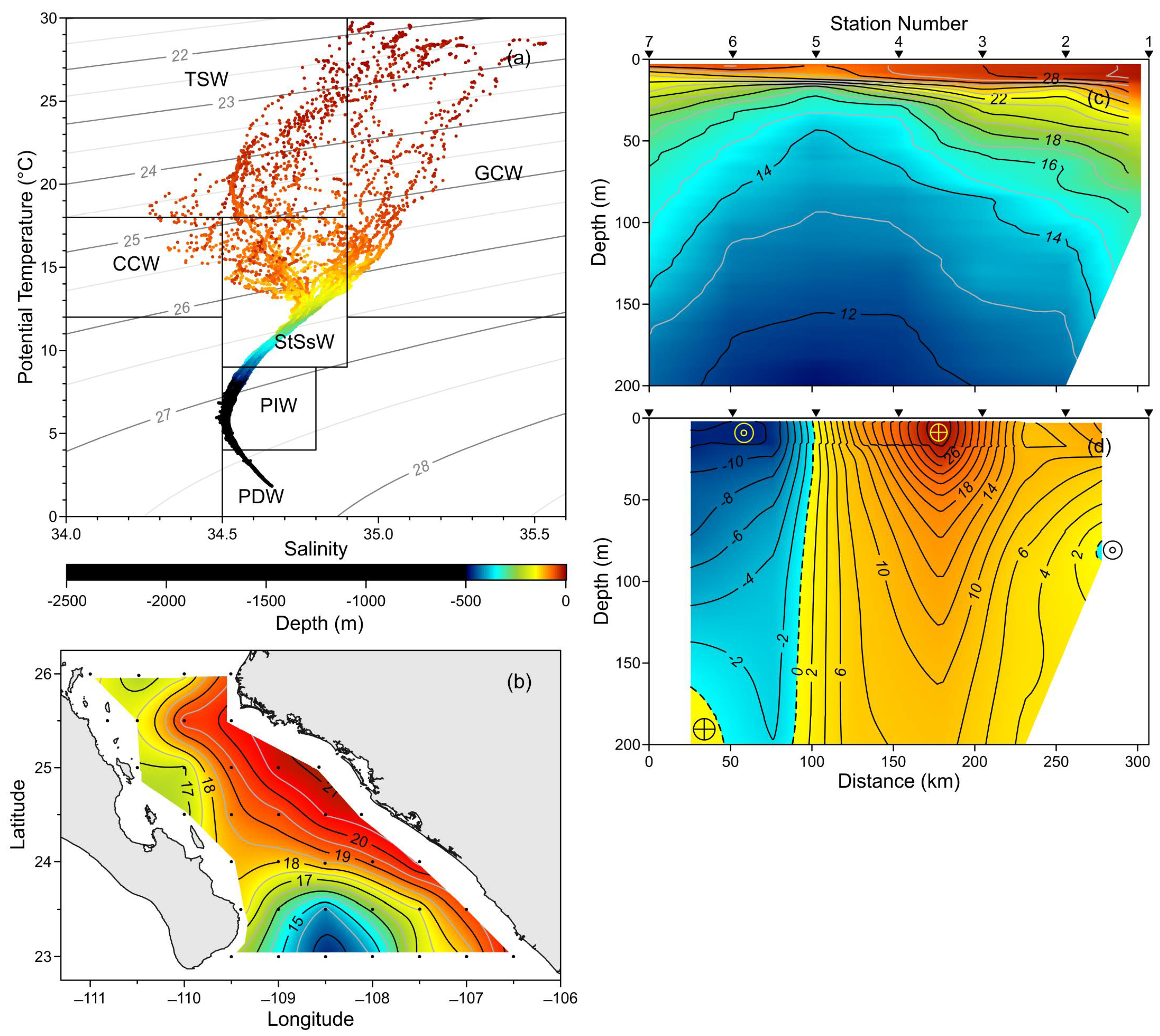
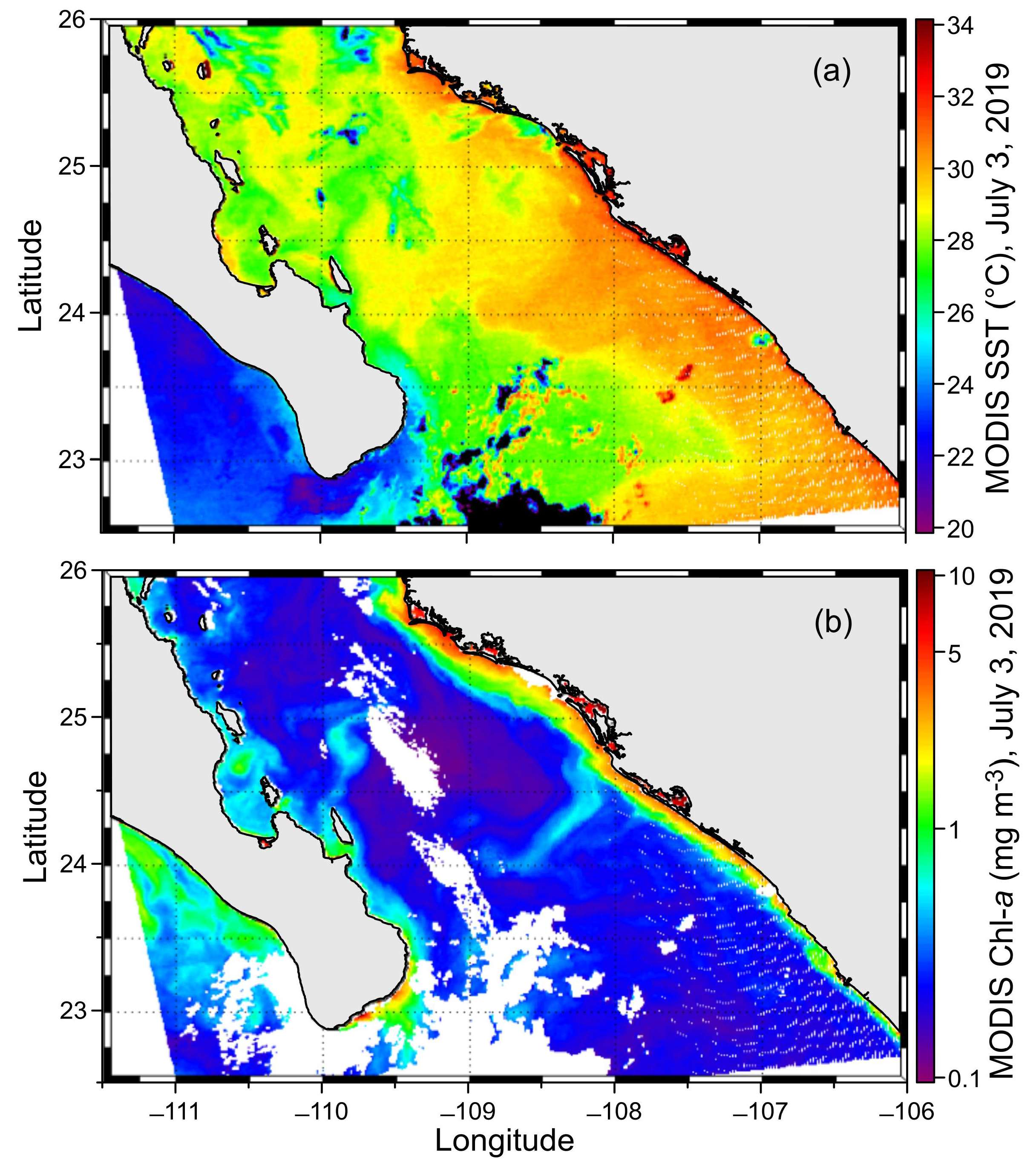
2.1. Study Area
2.2. Sampling
2.3. Data Processing
2.4. Laboratory Analyses
2.5. Statistical Analyses
3. Results
3.1. Hydrography and Circulation Pattern
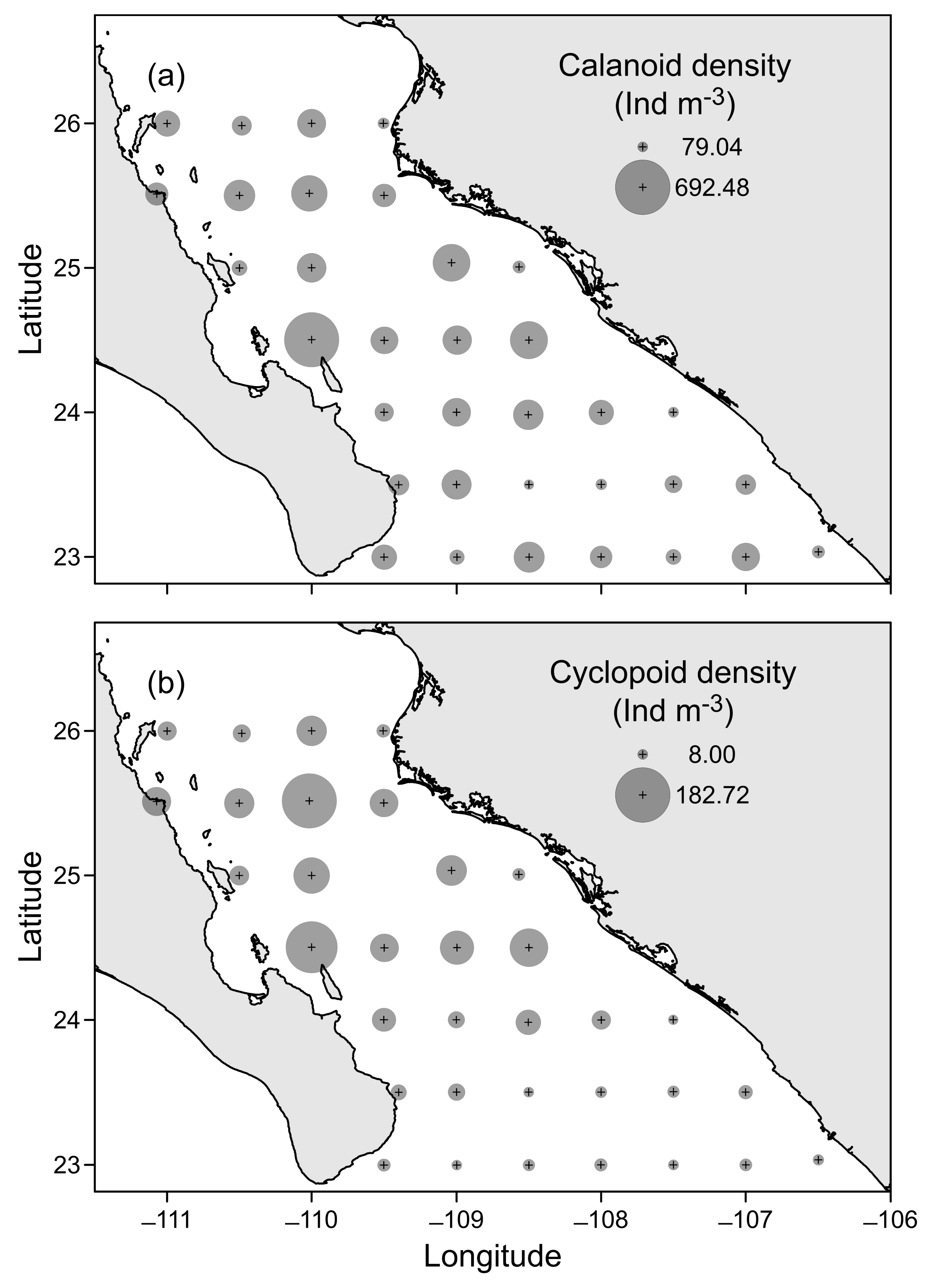
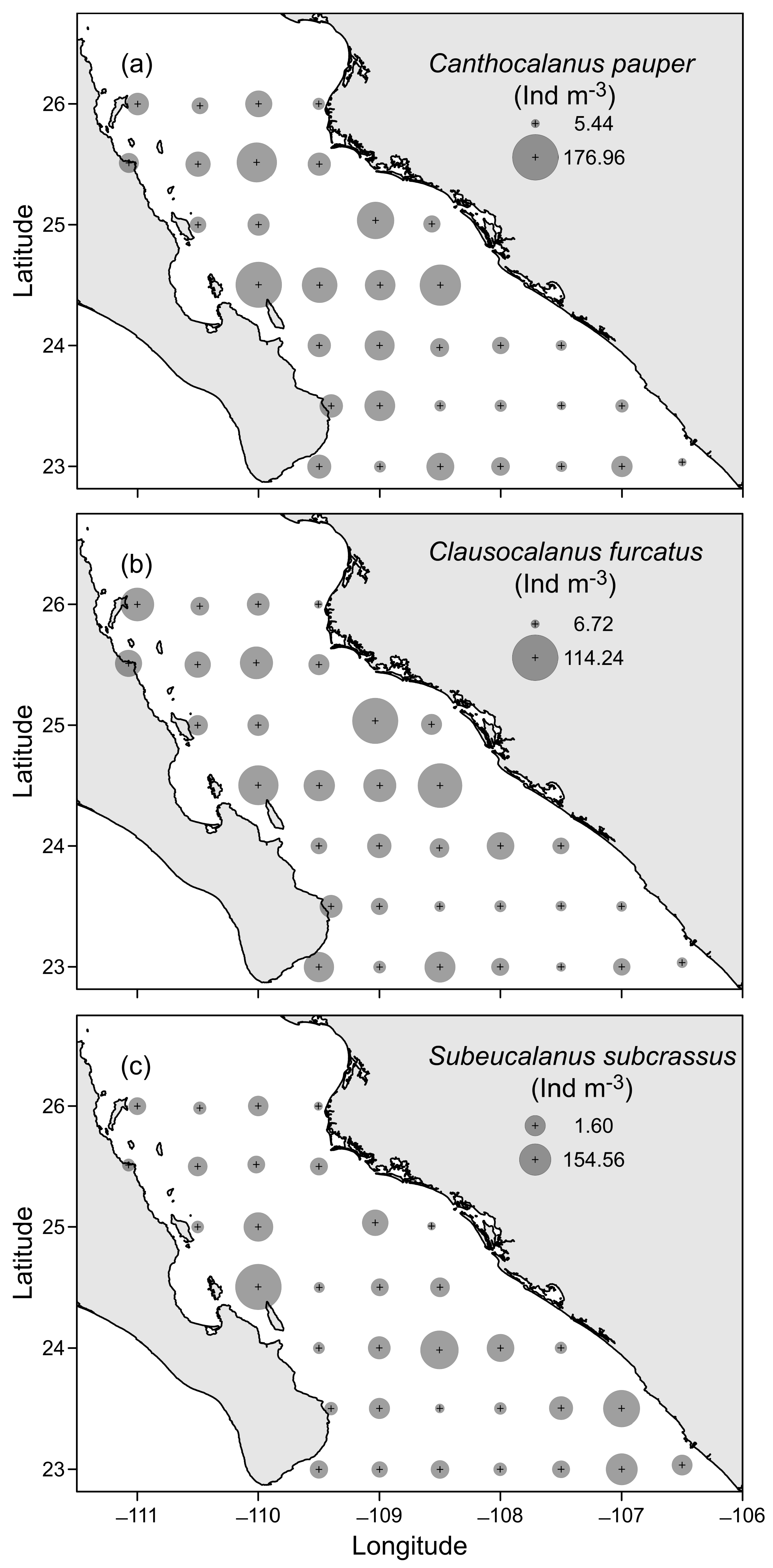
3.2. Copepod Community Structure
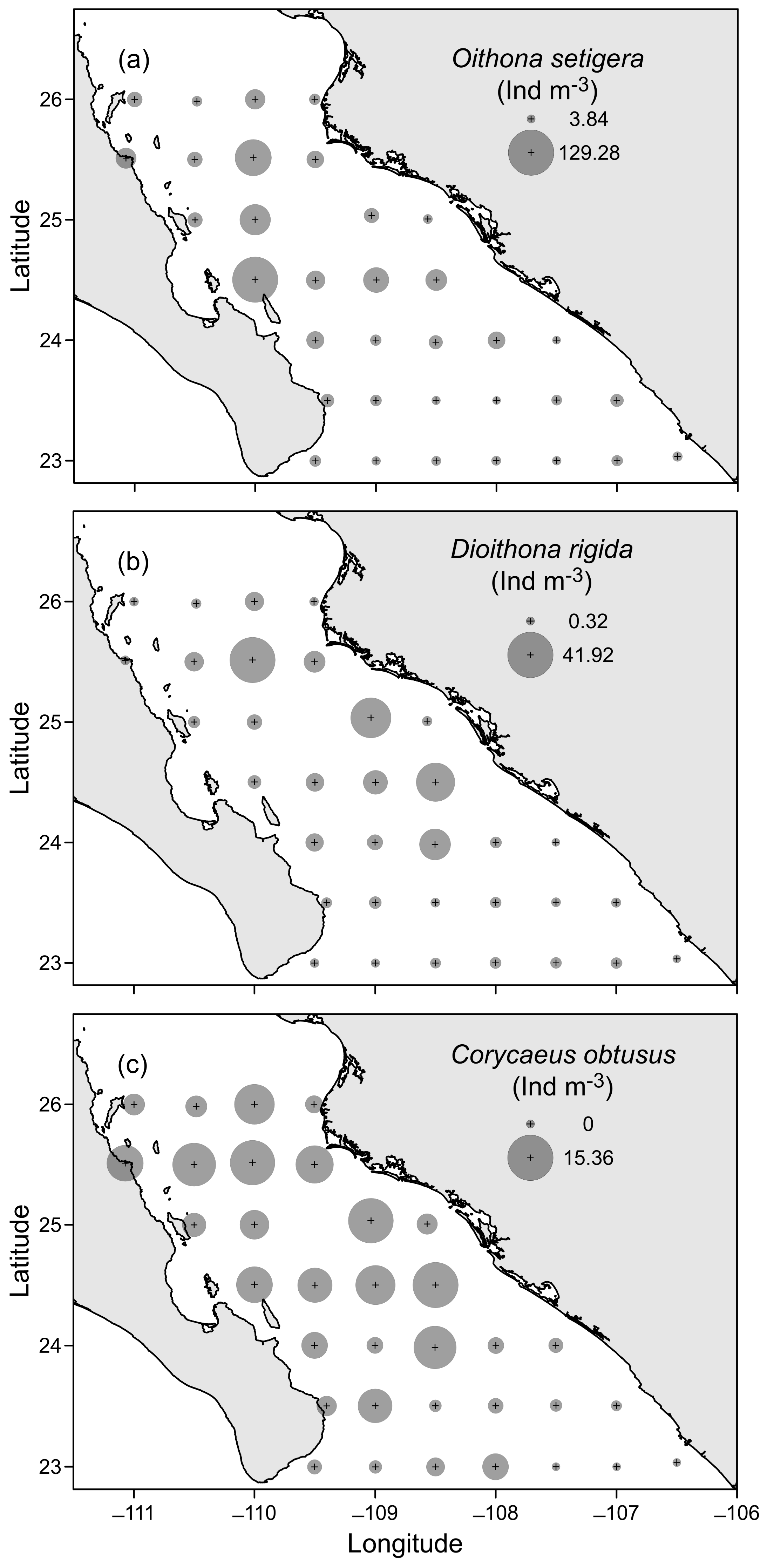
3.3. Horizontal Distribution of Copepods
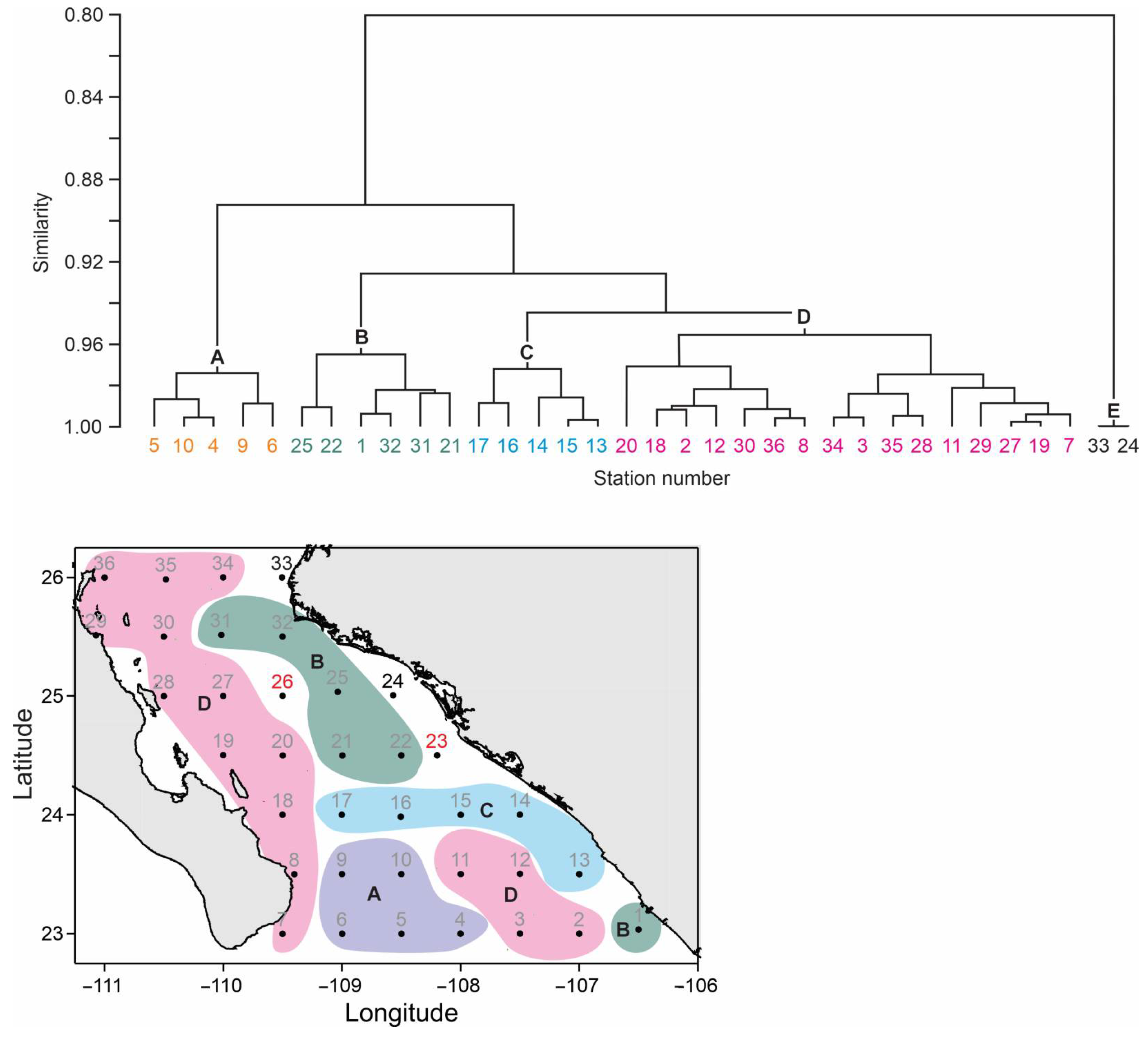
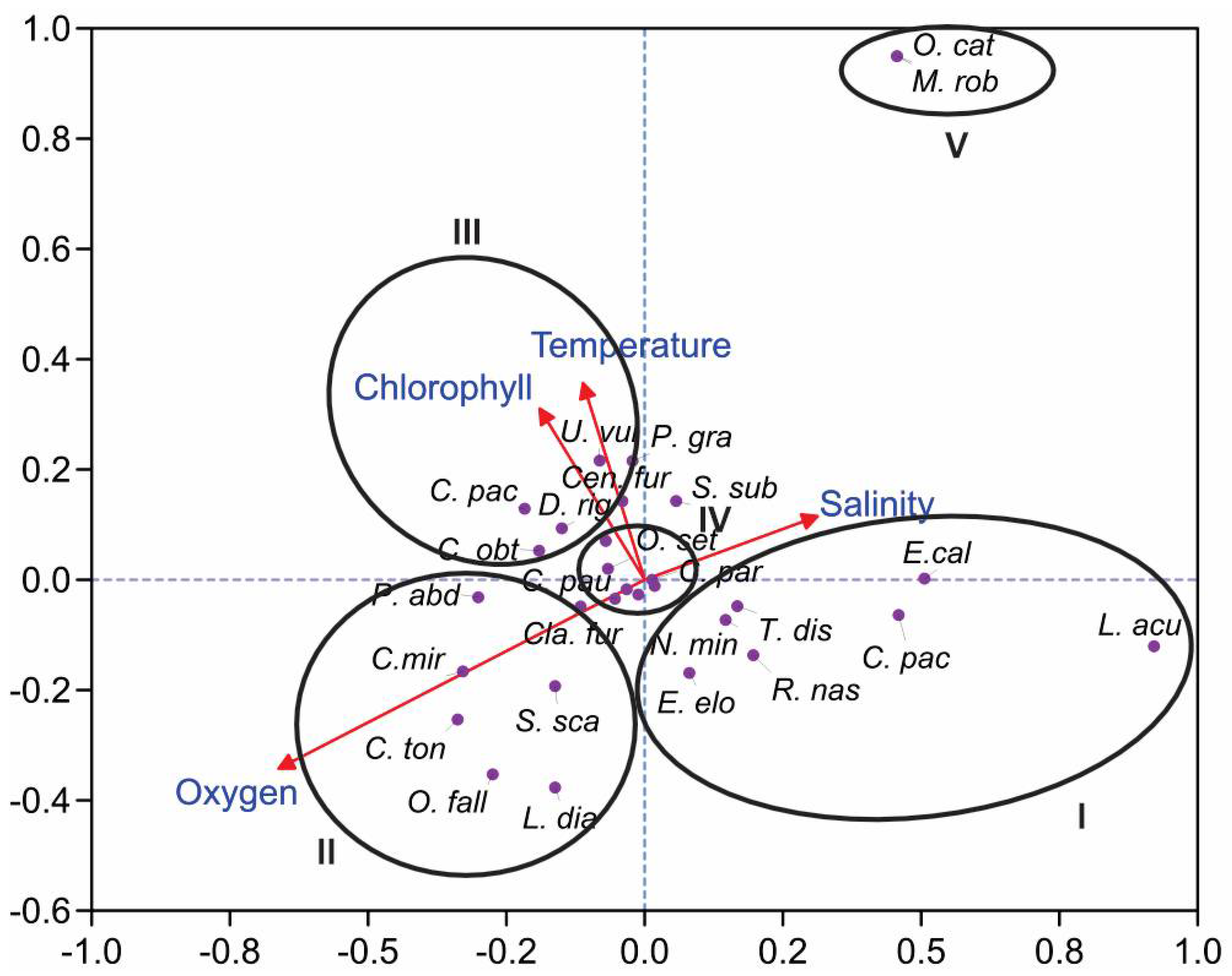
3.4. Statistical Analyses
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lavín, M.F.; Beier, E.; Badan, A. Estructura hidrográfica y circulación del Golfo de California: Escalas estacional e interanual. In Contribuciones a la Oceanografía Física en México; Monografía No. 3; Lavín, M.F., Ed.; Unión Geofísica Mexicana: Ensenada, Mexico, 1997; p. 171. [Google Scholar]
- Lavín, M.F.; Castro, R.; Beier, E.; Godínez, V.M.; Amador, A.; Guest, P. SST, thermohaline structure, and circulation in the southern Gulf of California in June 2004 during the North American Monsoon Experiment. J. Geophys. Res. 2009, 114, C02025. [Google Scholar] [CrossRef]
- Lavín, M.F.; Marinone, S.G. An overview of the physical oceanography of the Gulf of California. In Nonlinear Processes in Geophysical Fluid Dynamics; Velasco-Fuentes, O.U., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 173–204. [Google Scholar]
- Durán-Campos, E.; Salas-de-Léon, D.A.; Coria-Monter, E.; Monreal-Gómez, M.A.; Aldeco-Ramírez, J.; Quiroz-Martínez, B. ENSO effects in the southern gulf of California estimated from satellite data. Cont. Shelf. Res. 2023, 266, 105084. [Google Scholar] [CrossRef]
- Álvarez-Borrego, S. Phytoplankton biomass and production in the Gulf of California: A review. Bot. Mar. 2012, 55, 119–128. [Google Scholar] [CrossRef]
- Coria-Monter, E.; Monreal-Gómez, M.A.; Salas-de-León, D.A.; Durán-Campos, E. Zooplankton abundance during summer in the Bay of La Paz (southwestern Gulf of California, Mexico). Lat. Am. J. Aquat. Res. 2020, 48, 794–805. [Google Scholar] [CrossRef]
- Brierley, A.S. Plankton. Curr. Biol. 2017, 27, R478–R483. [Google Scholar] [CrossRef] [PubMed]
- Bron, J.E.; Frisch, D.; Goetze, E.; Johnson, S.C.; Lee, C.E.; Wyngaard, G.A. Observing copepods through a genomic lens. Front. Zool. 2011, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F.; Vogt, M.; Righetti, D.; Guilhaumon, F.; Ayata, S.D. Do functional groups of planktonic copepods differ in their ecological niches? J. Biogeogr. 2018, 45, 604–616. [Google Scholar] [CrossRef]
- Jackson, J.M.; Lenz, P.H. Predator-prey interactions in the plankton: Larval fish feeding on evasive copepods. Sci. Rep. 2016, 6, 33585. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Matsuno, K.; Homma, T. Spatial changes in the vertical distribution of calanoid copepods down to great depths in the North Pacific. Zool. Stud. 2015, 54, 13. [Google Scholar] [CrossRef] [PubMed]
- Khodami, S.; Mercado-Salas, N.F.; Tang, D.; Martinez-Arbizu, P. Molecular evidence for the retention of the Thaumatopsyllidae in the order Cyclopoida (Copepoda) and establishment of four suborders and two families within the Cyclopoida. Mol. Phylogenet. Evol. 2019, 138, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Fleminger, A. Geographical distribution and morphological divergence in American coastal-zone planktonic copepods of the genus Labidocera. In Chemistry, Biology and the Estuarine System; Cronin, L.E., Ed.; Academic Press: New York, NY, USA, 1975; pp. 392–419. [Google Scholar]
- Alldredge, A.L.; King, J.M. Effects of moonlight on the vertical migration patterns of demersal zooplankton. J. Exp. Mar. Biol. Ecol. 1980, 44, 133–156. [Google Scholar] [CrossRef]
- Jiménez-Pérez, L.C.; Lara-Lara, J.R. Zooplankton biomass and copepod community structure in the Gulf of California during the 1982-1983 El Niño event. CalCOFI Rep. 1988, 12, 122–128. [Google Scholar]
- Lavaniegos-Espejo, B.E.; Lara-Lara, J.R. Zooplankton of the Gulf of California after the 1982-1983 EI Niño Event: Biomass Distribution and Abundance. Pac. Sci. 1990, 44, 297–310. [Google Scholar]
- Palomares-García, R.J.; Gómez-Gutiérrez, J.; Robinson, C.J. Winter and summer vertical distribution of epipelagic copepods in the Gulf of California. J. Plankton Res. 2013, 35, 1009–1026. [Google Scholar] [CrossRef]
- Beltrán-Castro, J.R.; Hernández-Trujillo, S.; Gómez-Gutiérrez, J.; Trasviña-Castro, A.; González-Rodríguez, E.; Aburto-Oropeza, O. Copepod species assemblage and carbon biomass during two anomalous warm periods of distinct origin during 2014–2015 in the southern Gulf of California. Cont. Shelf Res. 2020, 207, 104215. [Google Scholar] [CrossRef]
- Velázquez-Ornelas, K.E.; Hendrickx, M.E. Latitudinal and seasonal variation in the copepods (Multicrustacea: Copepoda) of the Gulf of California based on the CORTES cruises (1985). Nauplius 2023, 31, e2023027. [Google Scholar] [CrossRef]
- Cruz-Hernández, J.; Sánchez-Velasco, L.; Godínez, V.M.; Beier, E.; Palomares-García, J.R.; Barton, E.D.; Santamaría-del-Ángel, E. Vertical distribution of calanoid copepods in a mature cyclonic eddy in the Gulf of California. Crustaceana 2018, 91, 63–84. [Google Scholar] [CrossRef]
- Cruz-Hernández, J.; Sánchez-Velasco, L.; Beier, E.; Godínez, V.M.; Barton, E.D. Distribution of calanoid copepods across the mesoscale frontal zone of tropical-subtropical convergence off México. Deep Sea Res. 2019, 169–170, 104678. [Google Scholar] [CrossRef]
- Coria-Monter, E.; Monreal-Gómez, M.A.; Salas-de-León, D.A.; Durán-Campos, E. Impact of the “Godzilla El Niño” event of 2015- 2016 on sea surface temperature and chlorophyll-a in the southern Gulf of California, Mexico, as evidenced by satellite and in situ data. Pac. Sci. 2018, 72, 411–422. [Google Scholar] [CrossRef]
- Sherman, H.; Hempel, G. Perspectives on Regional Seas and the Large Marine Ecosystem Approach. In The UNEP Large Marine Ecosystem Report; Sherman, K., Hempel, G., Eds.; United Nations Environment Programme: Nairobi, Kenya, 2009; pp. 3–22. [Google Scholar]
- Filonov, A.E.; Lavín, M. Internal tides in the Northern Gulf of California. J. Geophys. Res. 2003, 108, 3151. [Google Scholar] [CrossRef]
- Beier, E. A numerical investigation of the annual variability in the Gulf of California. J. Phys. Oceanogr. 1997, 27, 615–632. [Google Scholar] [CrossRef]
- Quiroz-Martínez, B.; Salas-de-León, D.A.; Gil-Zurita, A.; Monreal-Gómez, M.A.; Coria-Monter, E.; Durán-Campos, E. Latitudinal and archipelago effect on the composition, distribution, and abundance of zooplanktonic organisms in the Gulf of California. Oceanologia 2023, 65, 371–385. [Google Scholar] [CrossRef]
- Lluch-Cota, S.E. Coastal upwelling in the eastern Gulf of California. Oceanol. Acta 2000, 23, 731–740. [Google Scholar] [CrossRef]
- Monreal-Gómez, M.A.; Pérez-Cruz, L.; Durán-Campos, E.; Salas-de-León, D.A.; Torres-Martínez, C.M.; Coria-Monter, E. Phytoplankton Communities in the Eastern Tropical Pacific Ocean off Mexico and the Southern Gulf of California During the Strong El Niño of 2023/24. Plants 2025, 14, 1375. [Google Scholar] [CrossRef] [PubMed]
- Pond, S.; Pickard, G.L. Introductory Dynamical Oceanography; Pergamon Press: Oxford, UK, 1983; p. 329. [Google Scholar]
- Baith, K.; Lindsay, R.; Fu, G.; McClain, C.R. SeaDAS, a data analysis system for ocean-color satellite sensors. EoS 2001, 82, 202. [Google Scholar] [CrossRef]
- Rocha-Díaz, F.A.; Monreal-Gómez, M.A.; Coria-Monter, E.; Salas-de-León, D.A.; Durán-Campos, E.; Merino-Ibarra, M. Copepod abundance distribution in relation to a cyclonic eddy in a coastal environment in the southern Gulf of California. Cont. Shelf Res. 2021, 222, 104436. [Google Scholar] [CrossRef]
- Mojica-Ramírez, E.; Monreal-Gómez, M.A.; Hernández-Trujillo, S.; Salas-de-León, D.A. Influence of a cyclonic gyre and a thermohaline front on the population structure of Centropages furcatus in Bay of La Paz, Gulf of California. Cienc. Mar. 2023, 49, 33370. [Google Scholar] [CrossRef]
- Boltovskoy, D. South Atlantic Zooplankton; Publicaciones Especiales del INIDEP: Mar del Plata, Argentina, 1999; p. 1705. [Google Scholar]
- Tanaka, O. The pelagic copepods of the Izu region middle Japan Systematic Account X. Publ. Seto Mar. Biol. Lab. 1964, 12, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, O.; Omori, M. Additional report on Calanoida Copepods from the Izu region—Part 6. Publ. Seto Mar. Biol. Lab. 1992, 35, 253–271. [Google Scholar] [CrossRef] [PubMed]
- Palomares-García, J.R.; Suárez-Morales, E.; Hernández-Trujillo, S. Catálogo de los Copépodos (Crustacea) Pelágicos del Pacífico Mexicano; CICIMAR/ECOSUR: La Paz, México, 1998; p. 352. [Google Scholar]
- Kramer, D.; Kalin, M.J.; Stevens, E.G.; Thrailkill, J.R.; Zweifel, J.R. Collecting and Processing Data on Fish Eggs and Larvae in the California Current; NOAA Technical Report NMFS 370; NMFS: Seattle, WA, USA, 1972; pp. 1–38. [Google Scholar]
- Field, J.G.; Clarke, K.R.; Warwick, R.M. A practical strategy for analyzing multispecies distribution patterns. Mar. Ecol. Progr. Ser. 1982, 8, 37–52. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 2012; p. 1006. [Google Scholar]
- Ripa, P.; Marinone, S. Seasonal variability of temperature, salinity, velocity, vorticity, and sea level in the central Gulf of California, as inferred from historical data. Quart. J. Roy. Meteor. Soc. 1989, 115, 887–914. [Google Scholar] [CrossRef]
- Ripa, P. Seasonal circulation in the Gulf of California. Ann. Geophys. 1990, 8, 559–564. [Google Scholar]
- Castro, R.; Lavín, M.F.; Ripa, P. Seasonal heat balance in the Gulf of California. J. Geophys. Res. 1994, 99, 3249–3261. [Google Scholar] [CrossRef]
- Ripa, P. Toward a physical explanation of the seasonal dynamics and thermodynamics of the Gulf of California. J. Phys. Oceanogr. 1997, 27, 597–614. [Google Scholar] [CrossRef]
- Siokou-Frangou, I. Zooplankton annual cycle in a Mediterranean coastal area. J. Plank. Res. 1996, 18, 203–223. [Google Scholar] [CrossRef]
- Cornils, A.; Niehoff, B.; Richter, C.; Al-Najjar, T.; Schnack-Schiel, B. Seasonal abundance and reproduction of clausocalanid copepods in the northern Gulf of Aqaba (Red Sea). J. Plank. Res. 2007, 29, 57–70. [Google Scholar] [CrossRef]
- Mazzocchi, M.G.; Paffenhöfer, G.A. Swimming and feeding behaviour of the planktonic copepod Clausocalanus furcatus. J. Plankton Res. 1999, 21, 1501–1518. [Google Scholar] [CrossRef]
- Belkin, N.; Guy-Haim, T.; Rubin-Blum, M.; Lazar, A.; Sisma-Ventura, G.; Kiko, R.; Morov, A.R.; Ozer, T.; Gertman, I.; Herut, B.; et al. Influence of cyclonic and anticyclonic eddies on plankton in the southeastern Mediterranean Sea during late summertime. Ocean Sci. 2022, 18, 693–715. [Google Scholar] [CrossRef]
- Gaona-Hernández, A.; Suárez-Morales, E.; Linacre, L.; Compaire, J.C.; Lara-Lara, J.R.; Herzka, S.Z. Seasonal variability drives differences in the structure of the calanoid copepod community in two contrasting regions of the Gulf of Mexico. J. Plank. Res. 2024, 46, 158–173. [Google Scholar] [CrossRef]
- Huntley, M.E.; Lopez, M.D.G. Temperature-dependent production on marine copepods: A global synthesis. Am. Nat. 1992, 140, 201–242. [Google Scholar] [CrossRef] [PubMed]
- Horne, C.R.; Hirst, A.G.; Atkinson, D.; Neves, A.; Kiørboe, T. A global synthesis of seasonal temperature–size responses in copepods. Global Ecol. Biogeogr. 2016, 25, 988–999. [Google Scholar] [CrossRef]
- Ashlock, L.; García-Reyes, M.; Gentemann, C.; Batten, S.; Sydeman, W. Temperature and Patterns of Occurrence and Abundance of Key Copepod Taxa in the Northeast Pacific. Front. Mar. Sci. 2021, 8, 670795. [Google Scholar] [CrossRef]
- Molinero, J.C.; Ibanez, F.; Souissi, S.; Chifflet, M.; Nival, P. Phenological changes in the Northwestern Mediterranean copepods Centropages typicus and Temora stylifera linked to climate forcing. Oecologia 2005, 145, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Hooff, R.C.; Peterson, W. Copepod biodiversity as an indicator of changes in ocean and climate conditions of the northern California current ecosystem. Limnol. Oceanogr. 2006, 51, 2607–2620. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Sivakumar, K.; Baskar, G. Seasonal dynamics of body size in calanoid copepods (Calanoida: Copepoda) from the stressed tropical coast of India, Chennai, Bay of Bengal. Aquat. Ecol. 2024, 58, 363–373. [Google Scholar] [CrossRef]
- Wang, L.; Du, F.; Wang, X.; Li, Y.; Ning, J. Distribution and role of the genus Oithona (Copepoda: Cyclopoida) in the South China Sea. Oceanologia 2017, 59, 300–310. [Google Scholar] [CrossRef]

| Species | ||
|---|---|---|
| Calanoida | Abbreviation | Density (ind m−3) |
| Acartia (Acanthacartia) tonsa Dana, 1849–1852 | A. ton | 98.24 |
| Calanus pacificus Brodsky, 1948 | C. pac | 197.44 |
| Candacia parafalcifera Brodsky, 1950 | C. par | 78.08 |
| Canthocalanus pauper (Giesbrecht, 1888) | C. pau | 2316.80 |
| Centropages furcatus (Dana, 1849–1852) | Cen. fur | 332.80 |
| Clausocalanus furcatus Brady, 1883 | Cla. fur | 1593.60 |
| Eucalanus californicus Johnson M.W., 1938 | E. cal | 65.92 |
| Eucalanus elongatus (Dana, 1849–1852) | E. elo | 261.12 |
| Labidocera acuta (Dana, 1849–1852) | L. acu | 2.24 |
| Labidocera diandra Fleminger, 1967 | L. dia | 12.80 |
| Nannocalanus minor (Claus, 1863) | N. min | 865.60 |
| Odontacartia lilljeborgii Giesbrecht, 1889 | O. lil | 389.12 |
| Pleuromamma abdominalis (Lubbock, 1856) | P. abd | 64.32 |
| Pleuromamma gracilis Claus, 1863 | P. gra | 512.00 |
| Pseudochirella pacifica (Brodsky, 1950) | P. pac | 71.04 |
| Rhincalanus nasutus Giesbrecht, 1888 | R. nas | 432.64 |
| Scolecithrix danae (Lubbock, 1856) | S. dan | 121.60 |
| Subeucalanus subcrassus (Giesbrecht, 1888) | S. sub | 1584.64 |
| Temora discaudata Giesbrecht, 1889 | T. dis | 129.92 |
| Undinula vulgaris (Dana, 1849–1852) | U. vul | 33.28 |
| Cyclopoida | ||
| Copilia mirabilis Dana, 1849–1852 | C. mir | 49.92 |
| Corycaeus obtusus Dana, 1849–1852 | C. obt | 139.52 |
| Dioithona rigida (Giesbrecht, 1896) | D. rig | 290.56 |
| Macrocyclops albidus (Jurine, 1820) | M. alb | 7.68 |
| Monocorycaeus robustus (Giesbrecht, 1891) | M. rob | 0.64 |
| Oithona fallax Farran, 1913 | O. fall | 70.08 |
| Oithona setigera Dana, 1853–1855 | O. set | 963.44 |
| Oncaea venusta Philippi, 1843 | O. ven | 235.52 |
| Onychocorycaeus catus (Dahl F., 1894) | O. cat | 0.32 |
| Sapphirina scarlata Giesbrecht, 1891 | S. sca | 30.72 |
| Species | Average Dissimilarity | Percentage of Contribution | Cumulative Percentage |
|---|---|---|---|
| C. pauper | 13.21 | 30.85 | 30.85 |
| S. subcrassus | 10.74 | 25.1 | 55.95 |
| C. furcatus | 8.56 | 20.01 | 75.96 |
| O. setigera | 6.16 | 14.39 | 90.35 |
| D. rigida | 2.56 | 5.98 | 96.33 |
| O. venusta | 1.56 | 3.66 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha-Díaz, F.A.; Monreal-Gómez, M.A.; Coria-Monter, E.; Salas-de-León, D.A.; Durán-Campos, E.; Cházaro-Olvera, S. The Influence of Summer Cyclonic Circulation in the Southern Gulf of California on Planktonic Copepod Communities. J. Mar. Sci. Eng. 2025, 13, 1394. https://doi.org/10.3390/jmse13081394
Rocha-Díaz FA, Monreal-Gómez MA, Coria-Monter E, Salas-de-León DA, Durán-Campos E, Cházaro-Olvera S. The Influence of Summer Cyclonic Circulation in the Southern Gulf of California on Planktonic Copepod Communities. Journal of Marine Science and Engineering. 2025; 13(8):1394. https://doi.org/10.3390/jmse13081394
Chicago/Turabian StyleRocha-Díaz, Franco Antonio, María Adela Monreal-Gómez, Erik Coria-Monter, David Alberto Salas-de-León, Elizabeth Durán-Campos, and Sergio Cházaro-Olvera. 2025. "The Influence of Summer Cyclonic Circulation in the Southern Gulf of California on Planktonic Copepod Communities" Journal of Marine Science and Engineering 13, no. 8: 1394. https://doi.org/10.3390/jmse13081394
APA StyleRocha-Díaz, F. A., Monreal-Gómez, M. A., Coria-Monter, E., Salas-de-León, D. A., Durán-Campos, E., & Cházaro-Olvera, S. (2025). The Influence of Summer Cyclonic Circulation in the Southern Gulf of California on Planktonic Copepod Communities. Journal of Marine Science and Engineering, 13(8), 1394. https://doi.org/10.3390/jmse13081394







