Effect of Hydrogen-Containing Fuel on the Mechanical Properties of an Aluminum Alloy ICE Piston
Abstract
1. Introduction
2. Materials and Methods
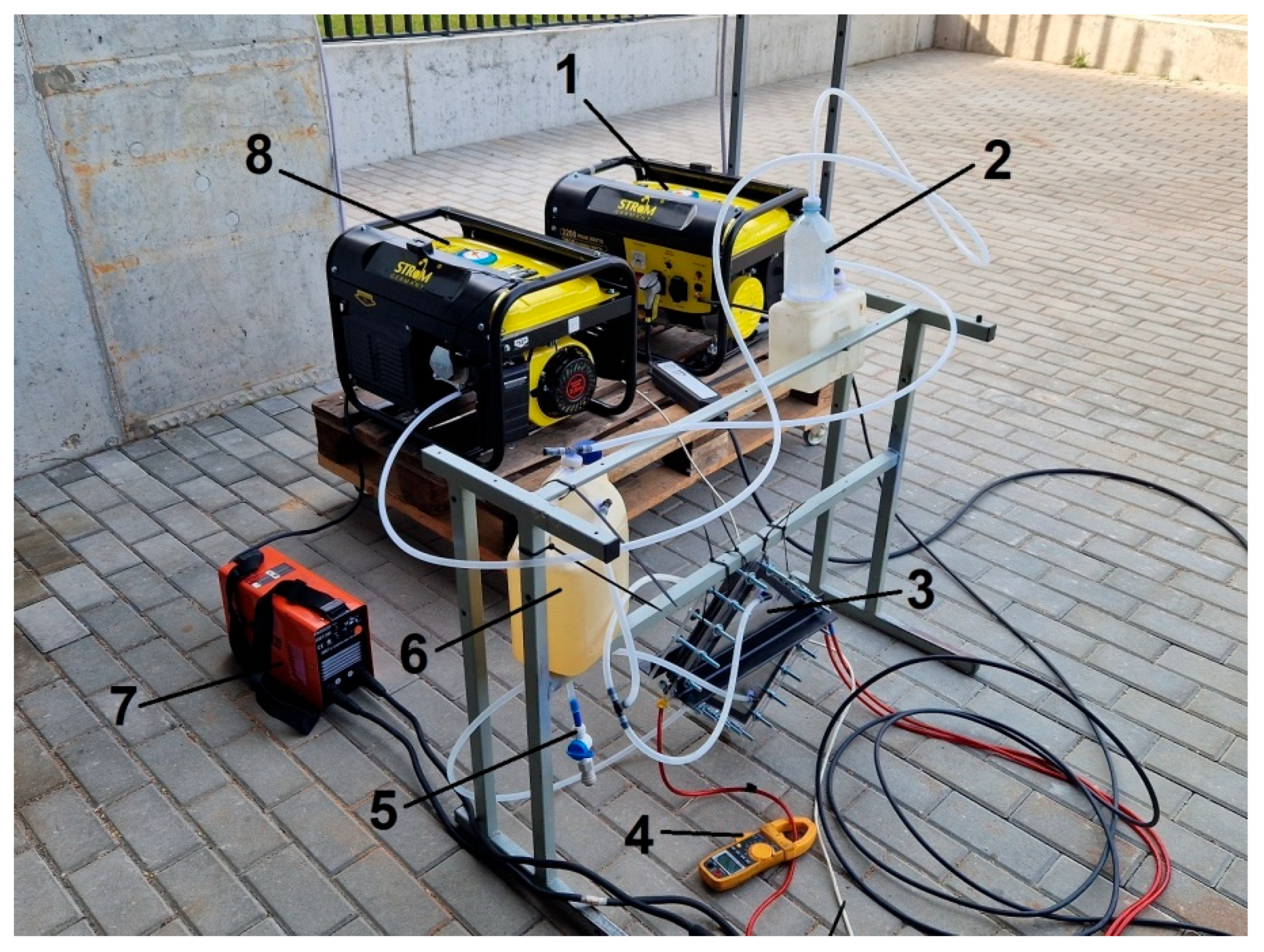
2.1. Experiment Description
- Modulating the electric current. Using a current modulator while maintaining a constant voltage and pulse period, the pulse width of the current is adjusted, thereby changing the current supplied to the gas generator and the amount of HHO gas produced.
- Varying the number of sections of the Brown’s gas generator powered by electric current.
2.2. Evaluation of the Conditions in a Combustion Chamber
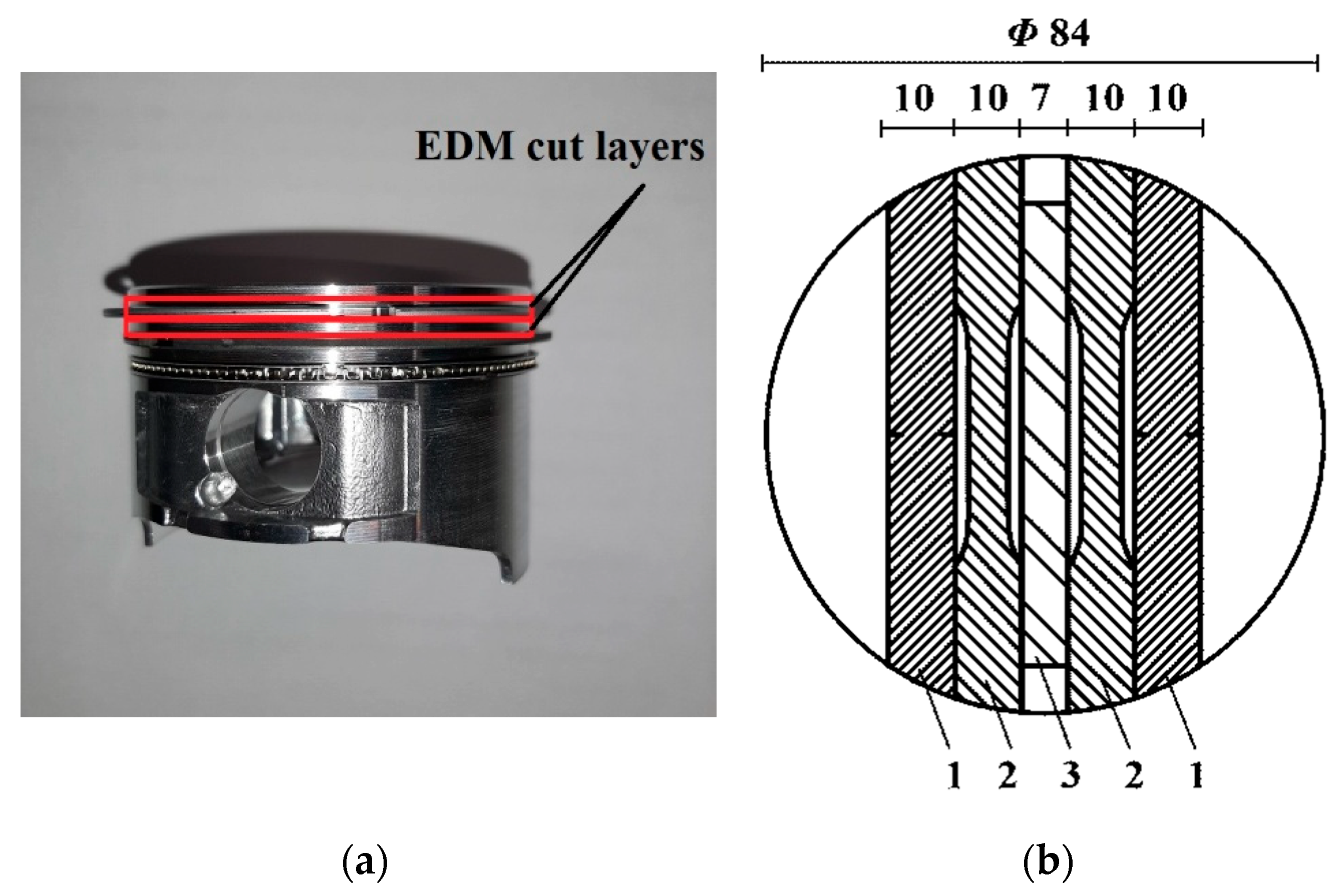
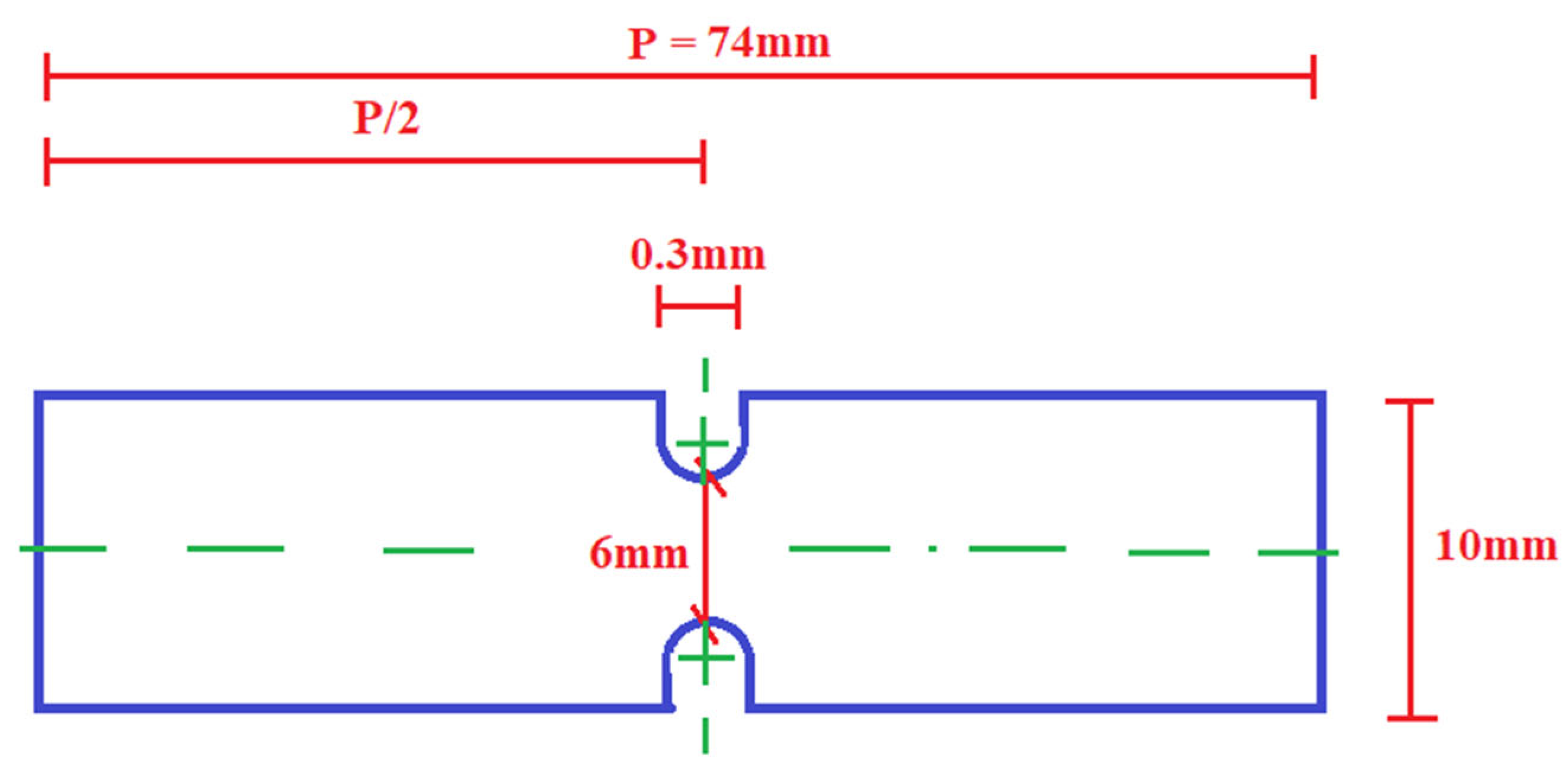
2.3. Testing Methods
3. Results
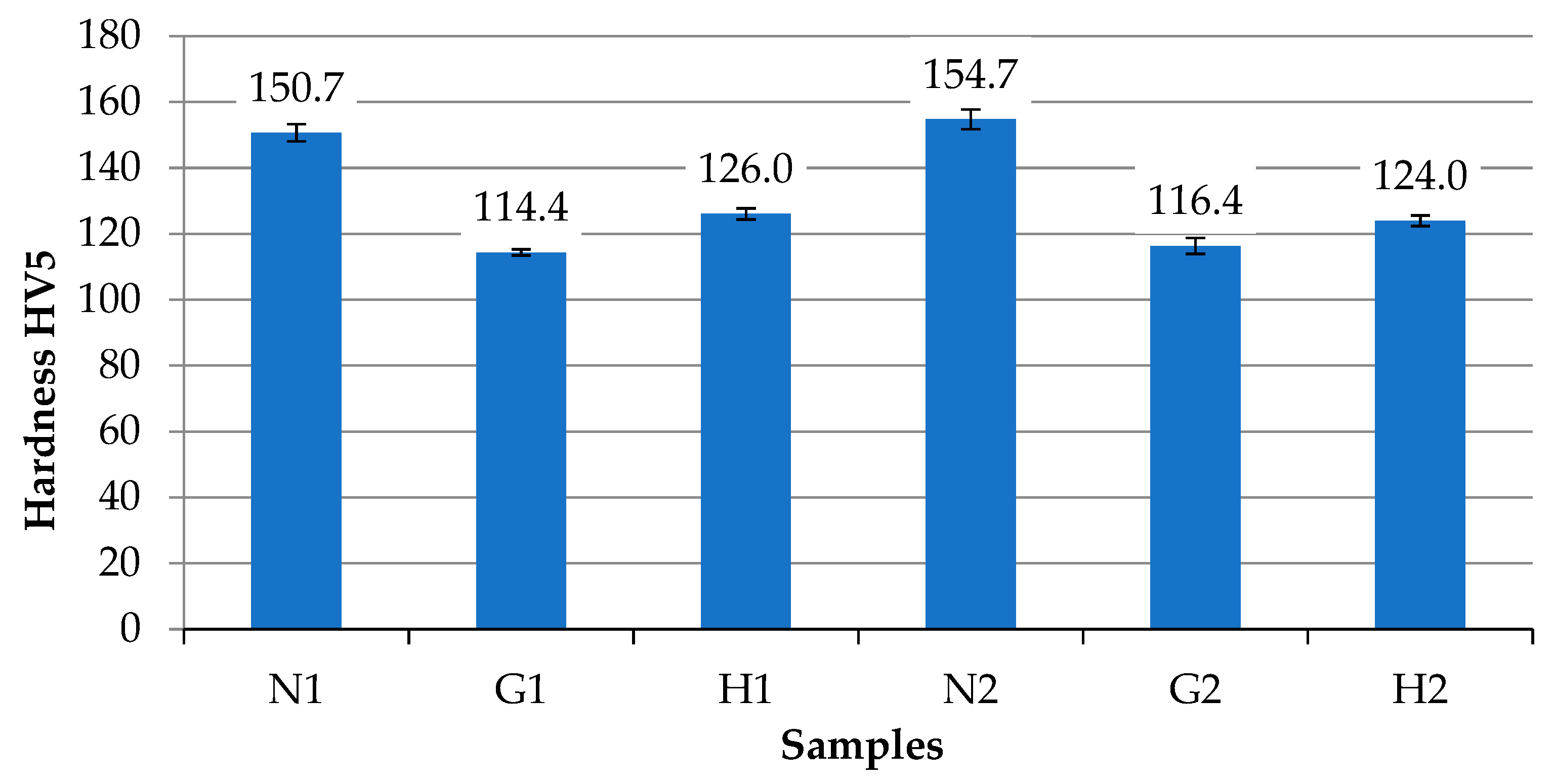
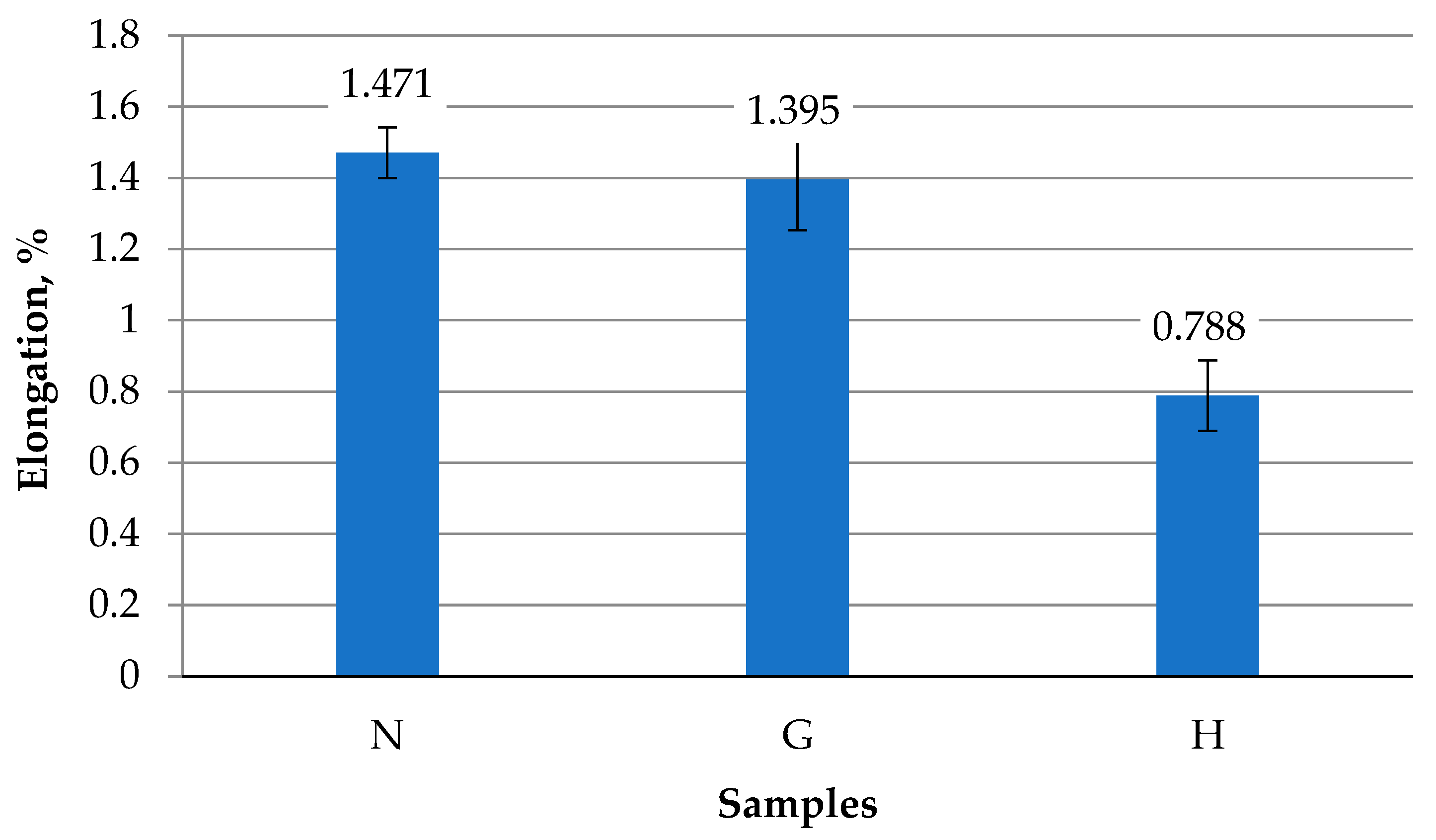
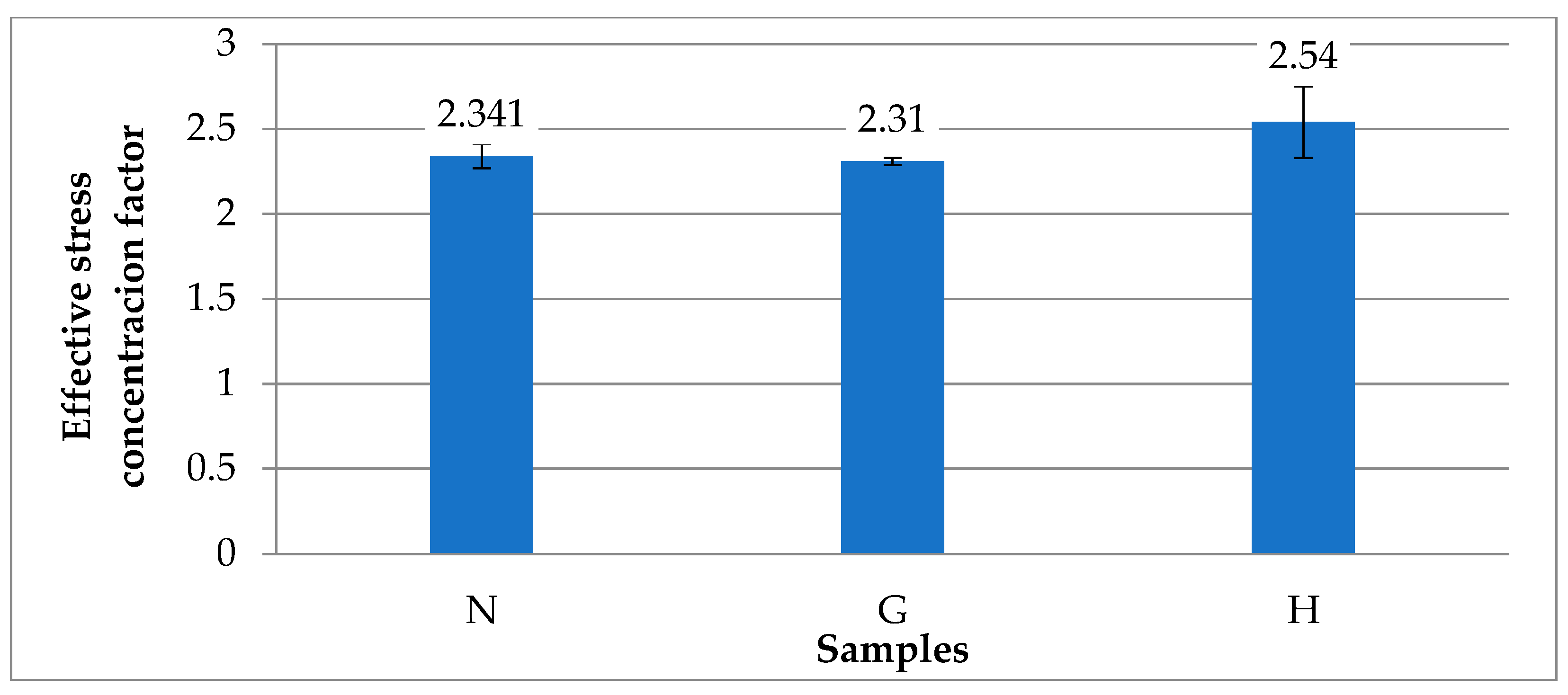
3.1. Analysis of Mechanical Properties
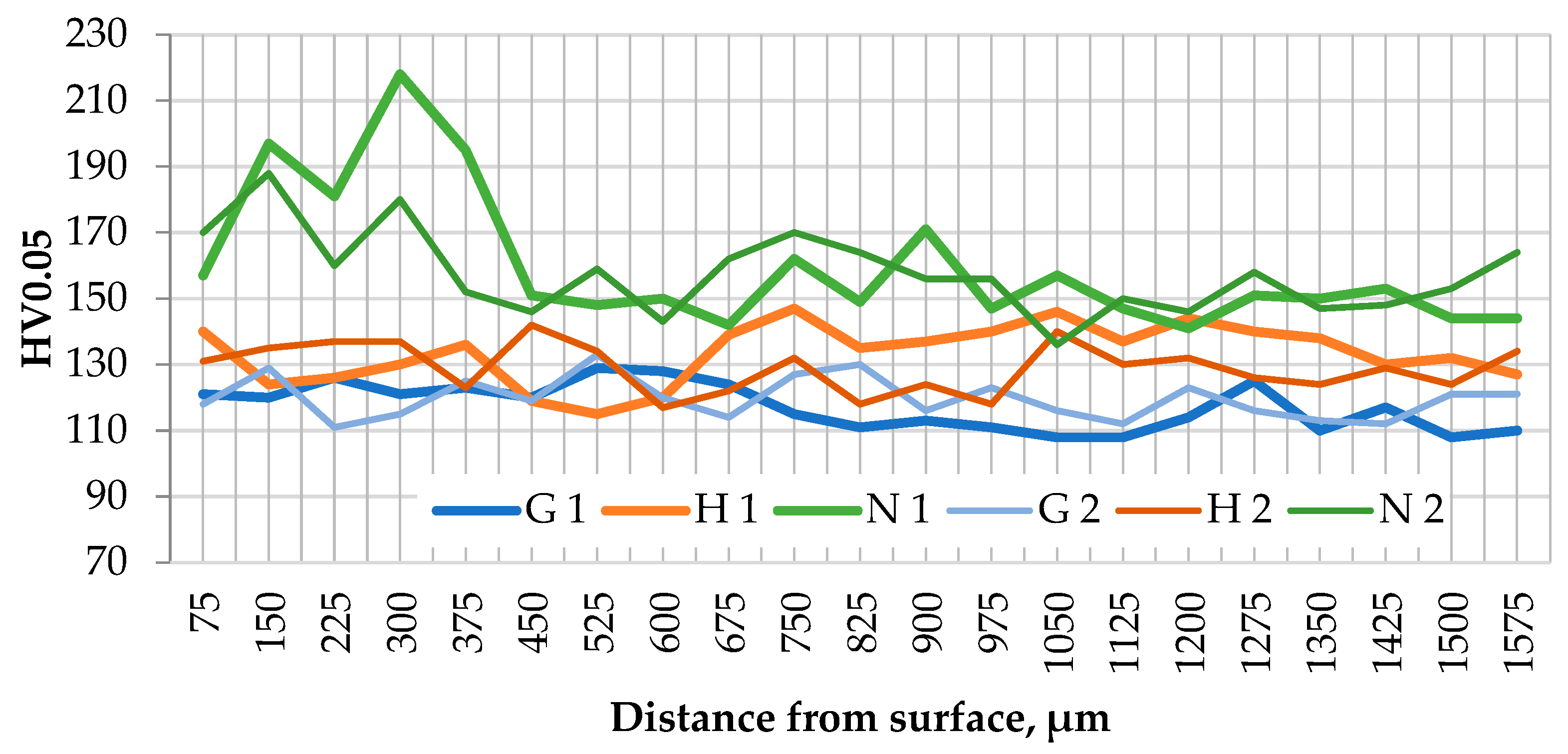
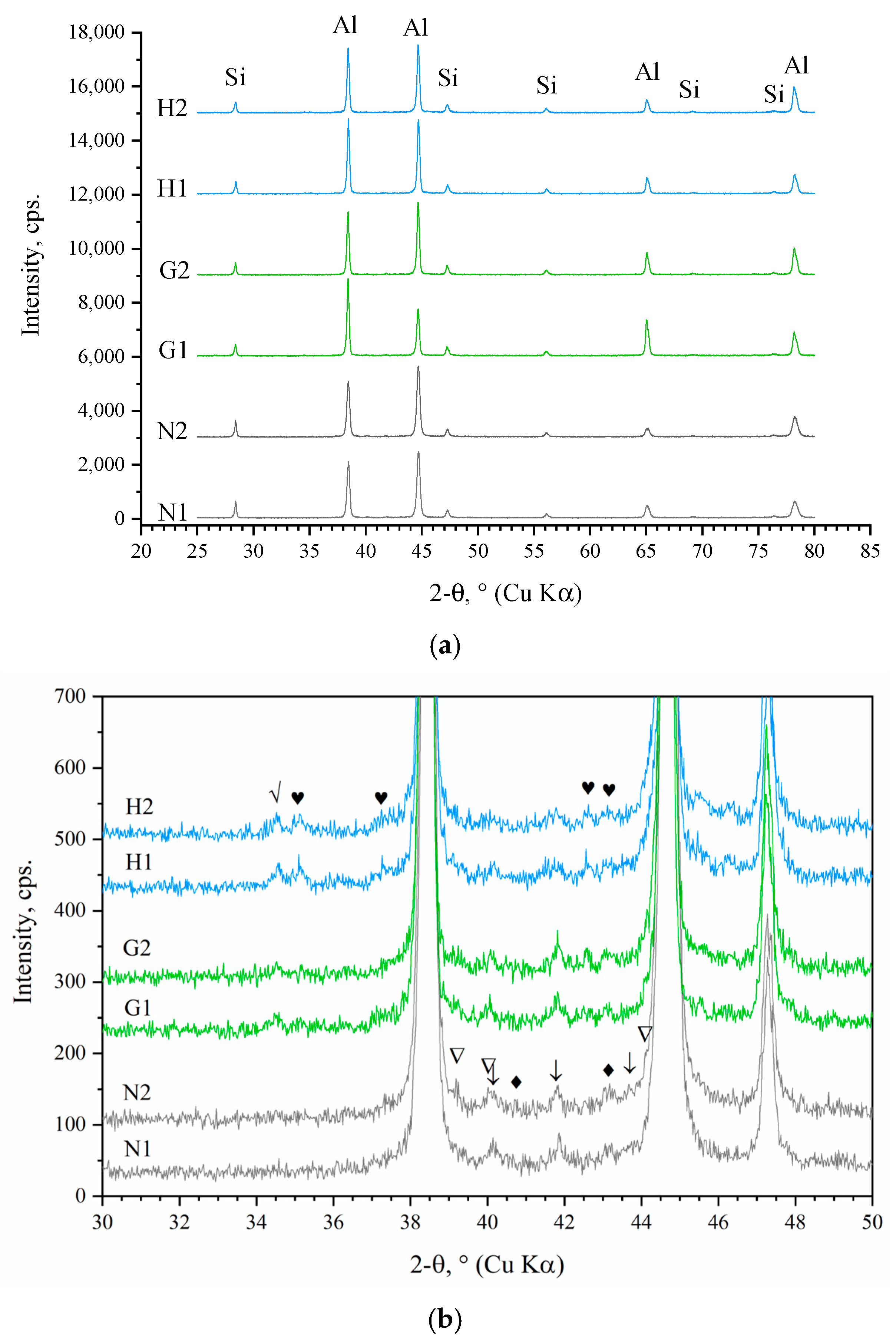
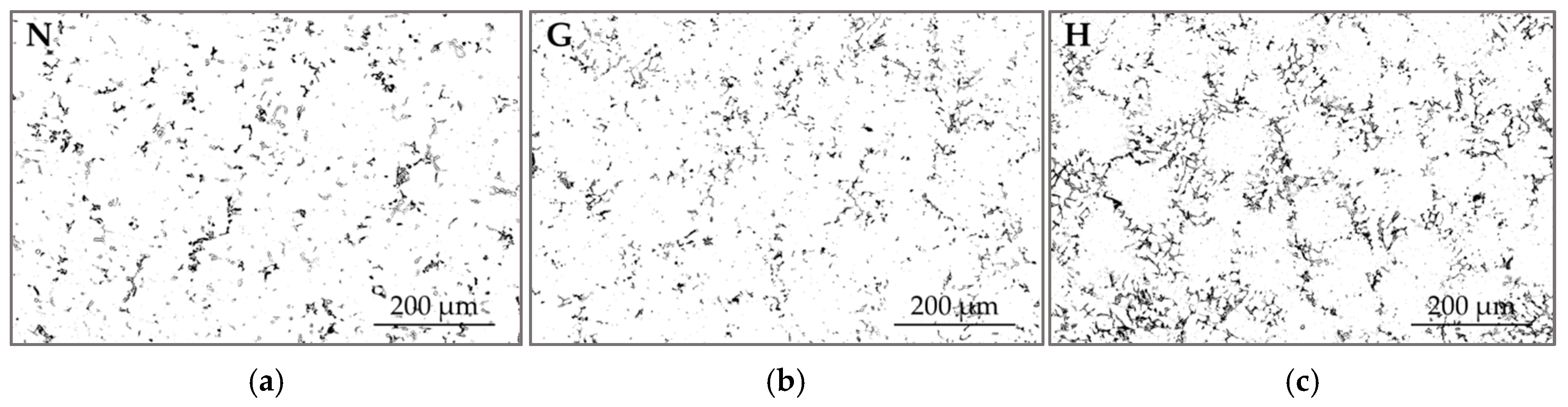
3.2. Microstructural Study and Phase Analysis
4. Conclusions
- -
- The mechanical properties of the internal combustion engine piston alloy are strongly dependent on operating conditions and fuel type. Operation with gasoline alone for 220 h did not significantly affect hardness, tensile strength, stiffness, or ductility, and the alloy’s sensitivity to stress concentrators remains similar to the virgin material. In contrast, operation with a gasoline–oxyhydrogen mixture led to deterioration of mechanical properties: tensile strength decreased by 1.12–1.22 times, elongation at fracture by up to 1.77 times, and maximum bending force by 1.07 times, while maximum deflection at fracture increased by 1.59 times, indicating reduced bending resistance and stiffness.
- -
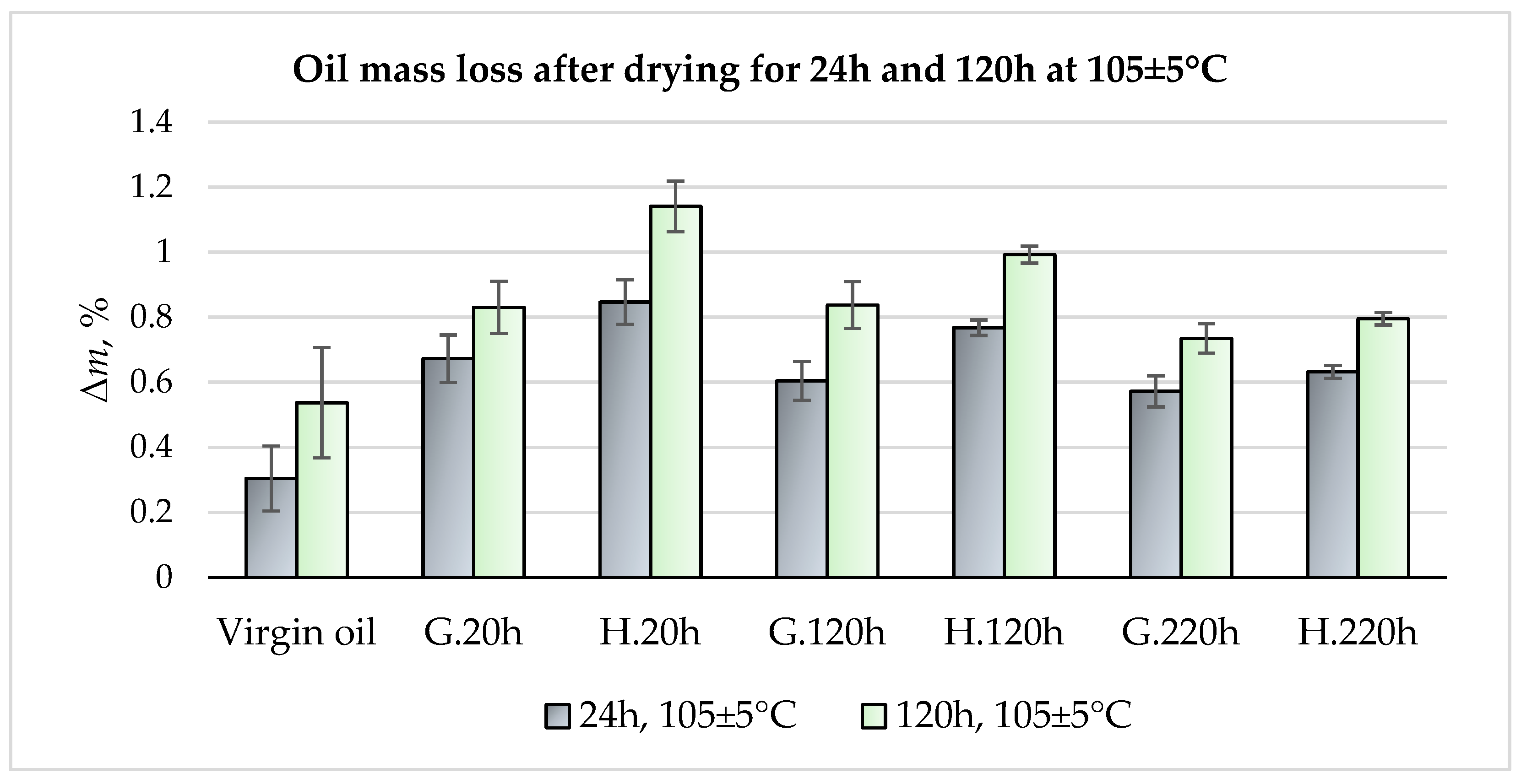
- Exhaust gas monitoring revealed that oxyhydrogen addition lowered the average combustion chamber temperature, but induced local temperature spikes, accelerating operational aging. This was manifested as intermetallic precipitation along α-A grain boundaries and formation of a dual-scale net-like structure, which acted as a brittle matrix, weakening cohesion between aluminum grains and Al–Si islands and restricting overall plastic deformation. The increased temperature and humidity in the chamber due to oxyhydrogen addition also accelerated the degradation of the piston crown surface.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| N | unused piston |
| G | piston operated with gasoline |
| H | piston operated with a mixture of gasoline and oxyhydrogen |
| ICE | internal combustion engine |
| SEM | scanning electron microscopy |
| EDS | energy-dispersive spectroscopy |
References
- Harfoot, M.B.J.; Tittensor, D.P.; Knight, S.; Arnell, A.P.; Blyth, S.; Brooks, S.; Butchart, S.H.M.; Hutton, J.; Jones, M.I.; Kapos, V.; et al. Present and Future Biodiversity Risks from Fossil Fuel Exploitation. Conserv. Lett. 2018, 11, e12448. [Google Scholar] [CrossRef]
- Gattuso, J.-P.; Magnan, A.K.; Bopp, L.; Cheung, W.W.L.; Duarte, C.M.; Hinkel, J.; Mcleod, E.; Micheli, F.; Oschlies, A.; Williamson, P.; et al. Ocean Solutions to Address Climate Change and Its Effects on Marine Ecosystems. Front. Mar. Sci. 2018, 5, 337. [Google Scholar] [CrossRef]
- Campagne, C.S.; Roy, L.-A.; Langridge, J.; Claudet, J.; Mongruel, R.; Beillouin, D.; Thiébaut, É. Existing Evidence on the Impact of Changes in Marine Ecosystem Structure and Functioning on Ecosystem Service Delivery: A Systematic Map. Environ. Evid. 2023, 13, 1–29. [Google Scholar] [CrossRef]
- Roslan, S.B.; Konovessis, D.; Tay, Z.Y. Sustainable Hybrid Marine Power Systems for Power Management Optimisation: A Review. Energies 2022, 15, 29622. [Google Scholar] [CrossRef]
- Radica, G.; Vidović, T.; Šimunović, J.; Jurić, Z. Overview of Hybrid Marine Energy System Configurations and System Component Modelling Approaches. Energies 2025, 18, 1189. [Google Scholar] [CrossRef]
- Foretich, A.; Zaimes, G.G.; Hawkins, T.R.; Newes, E. Challenges and Opportunities for Alternative Fuels in the Maritime Sector. Marit. Transp. Res. 2021, 2, 100033. [Google Scholar] [CrossRef]
- Verhelst, S.; Wallner, T. Hydrogen-Fueled Internal Combustion Engines. Prog. Energy Combust. Sci. 2009, 35, 490–527. [Google Scholar] [CrossRef]
- Ihsan Shahid, M.; Rao, A.; Farhan, M.; Liu, Y.; Ahmad Salam, H.; Chen, T.; Ma, F. Hydrogen Production Techniques and Use of Hydrogen in Internal Combustion Engine: A Comprehensive Review. Fuel 2024, 378, 132769. [Google Scholar] [CrossRef]
- Shadidi, B.; Najafi, G.; Yusaf, T. A Review of Hydrogen as a Fuel in Internal Combustion Engines. Energies 2021, 14, 6209. [Google Scholar] [CrossRef]
- Turner, J.W.G. Future Technological Directions for Hydrogen Internal Combustion Engines in Transport Applications. Appl. Energy Combust. Sci. 2025, 21, 100302. [Google Scholar] [CrossRef]
- Goyal, H.; Jones, P.; Bajwa, A.; Parsons, D.; Akehurst, S.; Davy, M.H.; Leach, F.C.; Esposito, S. Design Trends and Challenges in Hydrogen Direct Injection (H2DI) Internal Combustion Engines—A Review. Int. J. Hydrogen Energy 2024, 86, 1179–1194. [Google Scholar] [CrossRef]
- Rimkus, A.; Žaglinskis, J. Study of the Combustion Characteristics of a Compression Ignition Engine Fueled with a Biogas–Hydrogen Mixture and Biodiesel. J. Mar. Sci. Eng. 2024, 12, 2192. [Google Scholar] [CrossRef]
- Włodarczyk, P.P.; Włodarczyk, B. Deterioration of Property of Aluminum Alloys (EN AW-1050A, EN AW-5754 and EN AW-6060) by Absorbed Hydrogen. Appl. Sci. 2022, 12, 1392. [Google Scholar] [CrossRef]
- Papantoniou, I.G.; Karmiris-Obratański, P.; Leszczyńska-Madej, B.; Manolakos, D.E. Investigating the Impact of Friction Stir Processing on the Hydrogen Embrittlement in AA6082-T6 Heat-Treatable Aluminum Alloy. Met. Mater. Int. 2024, 30, 2668–2684. [Google Scholar] [CrossRef]
- Shin, D.-H.; Kim, S.-J. Effects of Hard Anodizing and Plasma Ion-Nitriding on Al Alloy for Hydrogen Embrittlement Portection. Corros. Sci. Technol. 2023, 22, 221–231. [Google Scholar] [CrossRef]
- Havazadeh, M.; Raeissi, K. The Effect of Hydrogen on Mechanical Properties of 7075-T6 Al at Elevated Temperatures for High-Duty Compressor Applications. J. Mater. Eng. Perform. 2025, 1–9. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Zhang, C.; Peng, W.; Ma, K.; Oleksandr, M. Effects of hydrogen on the dynamic mechanical properties and microstructure of 7055 and 7A52 aluminum alloys. Mater. Charact. 2023, 203, 113151. [Google Scholar] [CrossRef]
- Gong, C.; Tu, H.; Wu, C.; Wang, J.; Su, X. Study on Microstructure and Mechanical Properties of Hypereutectic Al–18Si Alloy Modified with Al–3B. Materials 2018, 11, 456. [Google Scholar] [CrossRef]
- Xia, F.; Dong, X.; Wang, J.; Duan, H.; Ma, Z.; Liang, M. Microstructural Evolution and Tensile Properties of Al-Si Piston Alloys during Long-Term Thermal Exposure. Metals 2024, 14, 535. [Google Scholar] [CrossRef]
- Lin, B.; Li, H.; Xu, R.; Zhao, Y.; Xiao, H.; Tang, Z.; Li, S. Thermal exposure of Al-Si-Cu-Mn-Fe alloys and its contribution to high temperature mechanical properties. J. Mater. Res. Technol. 2020, 9, 1856–1865. [Google Scholar] [CrossRef]
- Liu, Y.; Bian, Z.; Chen, Z.; Wang, M.; Chen, D.; Wang, H. Effect of Mn on the elevated temperature mechanical properties of Al-La alloys. Mater. Character. 2019, 155, 109821. [Google Scholar] [CrossRef]
- Chen, F.; Liu, C.; Zuo, L.; Wu, Z.; He, Y.; Dong, K.; Li, G.; He, W. Effect of Thermal Exposure on Mechanical Properties of Al-Si-Cu-Ni-Mg Aluminum Alloy. Crystals 2023, 13, 236. [Google Scholar] [CrossRef]
- Zhang, M.; Tian, Y.; Zheng, X.; Zhang, Y.; Chen, L.; Wang, J. Research Progress on Multi-Component Alloying and Heat Treatment of High Strength and Toughness Al–Si–Cu–Mg Cast Aluminum Alloys. Materials 2023, 16, 1065. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, Z.-X.; Guo, K.-F. Effect of Equivalence Ratio on H2/Air Combustion Characteristics of Micro Gas Turbine. J. Eng. Therm. Energy Power 2022, 37, 72–78. [Google Scholar] [CrossRef]
- Wang, S.; Ji, C.; Zhang, B.; Zhou, X. Analysis on combustion of a hydrogen-blended gasoline engine at high loads and lean conditions. Energy Procedia 2014, 61, 323–326. [Google Scholar] [CrossRef]
- Romański, L. Wodór Nośnikiem Energii; Wydawnictwo Uniwersytetu Przyrodniczego: Wrocław, Poland, 2007; ISBN 978-83-60574-12-6. [Google Scholar]
- Henkensmeier, D.; Cho, W.-C.; Jannasch, P.; Stojadinovic, J.; Li, Q.; Aili, D.; Jensen, J.O. Separators and Membranes for Advanced Alkaline Water Electrolysis. Chem. Rev. 2024, 124, 6393–6443. [Google Scholar] [CrossRef]
- Hodzic, N.; Kazagic, A.; Kadic, K. Qualitative Analysis of the Structure of NOx Emissions during Combustion of Pulverized Coal and Biomass and Staged Air Supply Conditions in Furnace. In New Technologies, Development and Application VI; Lecture Notes in Networks and Systems; Springer: Cham, Switzerland, 2023; Volume 707. [Google Scholar] [CrossRef]
- Studzinski, W.M.; Liiva, P.M.; Choate, P.J.; Brezinsky, K. A Computational and Experimental Study of Combustion Chamber Deposit Effects on NOx Emissions; SAE Technical Paper; SAE International: Warrendale, PA, USA, 1993. [Google Scholar] [CrossRef]
- Ma, T.; Feng, W.; Sun, X.; Jing, G. Numerical Study of NOx Formation Mechanism in Ammonia-Hydrogen Compound Fuel Marine Engines under Varying Conditions. Int. J. Hydrogen Energy 2024, 19, 1422–1434. [Google Scholar] [CrossRef]
- Pan, P.; Wen, Y.; Su, Z.; Li, Z. Effects of Temperature, Reducing Agent Concentration and Water Vapor on NO Emission of Hydrogen Fuel Engines Using NOx Storage and Reduction Technology. J. Xi’an Jiaotong Univ. 2023, 57, 82–90. [Google Scholar] [CrossRef]
- Shudo, T.; Oka, H. Thermophysical Properties of Working Fluid and Heat Transfer in a Hydrogen Combustion Engine. Trans. Jpn. Soc. Mech. Eng. B 2005, 71, 730–736. [Google Scholar] [CrossRef][Green Version]
- Shudo, T.; Nabetani, S. Analysis of Degree of Constant Volume and Cooling Loss in a Hydrogen Fuelled SI Engine; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2001. [Google Scholar] [CrossRef]
- Shudo, T.; Nabetani, S.; Nakajima, Y. Analysis of the Degree of Constant Volume and Cooling Loss in a Spark Ignition Engine Fuelled with Hydrogen. Int. J. Engine Res. 2001, 2, 81–92. [Google Scholar] [CrossRef]
- Younkins, M.; Wooldridge, M.S.; Boyer, B.A. Port Injection of Water into a Di Hydrogen Engine; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2015. [Google Scholar] [CrossRef]
- Choi, G.H.; Han, S.B. Performance and Exhaust Emissions Test Results from a Liquid Propane Injected Engine with Hydrogen Enrichment. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2004, 218, 1135–1140. [Google Scholar] [CrossRef]
- Huang, Q.; Miao, H.-Y.; Huang, Z.-H.; Jiang, D.-M.; Zeng, K. Effects of CO2 from EGR on Laminar Combustion Characteristics of Hydrogen. J. Combust. Sci. Technol. 2009, 15, 361–367. [Google Scholar]
- Morovatiyan, M.; Shahsavan, M.; Baghirzade, M.; Mack, J.H. Effect of Hydrogen and Carbon Monoxide Addition to Methane on Laminar Burning Velocity. In Proceedings of the Internal Combustion Engine Division Fall Technical Conference, Chicago, IL, USA, 20–23 October 2019; ASME: Chicago, IL, USA, 2019; p. V001T02A006. [Google Scholar] [CrossRef]
- Du, Y.; Yu, X.; Liu, L.; Li, R.; Zuo, X.; Sun, Y. Effect of Addition of Hydrogen and Exhaust Gas Recirculation on Characteristics of Hydrogen Gasoline Engine. Int. J. Hydrogen Energy 2017, 42, 8288–8298. [Google Scholar] [CrossRef]
- Piqueras, P.; Morena, J.D.l.; Sanchis, E.J.; Pitarch, R. Impact of Exhaust Gas Recirculation on Gaseous Emissions of Turbocharged Spark-Ignition Engines. Appl. Sci. 2020, 10, 7634. [Google Scholar] [CrossRef]
- ISO 6892-1:2019(En); Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature. ISO: Geneva, Switzerland. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:6892:-1:ed-3:v1:en (accessed on 25 September 2025).
- Chen, Y.-S.; Huang, C.; Liu, P.-Y.; Yen, H.-W.; Niu, R.; Burr, P.; Moore, K.L.; Martínez-Pañeda, E.; Atrens, A.; Cairney, J.M. Hydrogen Trapping and Embrittlement in Metals—A Review. Int. J. Hydrogen Energy 2025, 136, 789–821. [Google Scholar] [CrossRef]
- Campari, A.; Ustolin, F.; Alvaro, A.; Paltrinieri, N. A Review on Hydrogen Embrittlement and Risk-Based Inspection of Hydrogen Technologies. Int. J. Hydrogen Energy 2023, 48, 35316–35346. [Google Scholar] [CrossRef]
- Luo, X.; Bian, B.; Zhang, K.; Tian, D.; Pan, M.; Chen, X.; Zhao, H. Investigation of Hydrogen Embrittlement in 12Cr2Mo1R(H) Steel. J. Mater. Res. 2018, 33, 3501–3511. [Google Scholar] [CrossRef]
- Scully, J.R.; Young, G.A.; Smith, S.W. Hydrogen Embrittlement of Aluminum and Aluminum-Based Alloys. In Gaseous Hydrogen Embrittlement of Materials in Energy Technologies; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Umeda, H.; Itoh, G.; Kato, Y. Hydrogen Absorption Behavior in Al-Mg Alloys Exposed to an SO2 Atmosphere During Subsequent Annealing. J. Jpn. Inst. Light Met. 2007, 57, 203–209. [Google Scholar] [CrossRef][Green Version]
- Lasa, L.; Rodriguez-Ibabe, J.M. Evolution of the Main Intermetallic Phases in Al-Si-Cu-Mg Casting Alloys during Solution Treatment. J. Mater. Sci. 2004, 39, 1343–1355. [Google Scholar] [CrossRef]
- Zhu, X.; Dong, X.; Blake, P.; Ji, S. Improvement in As-Cast Strength of High Pressure Die-Cast Al–Si–Cu–Mg Alloys by Synergistic Effect of Q-Al5Cu2Mg8Si6 and θ-Al2Cu Phases. Mater. Sci. Eng. A 2021, 802, 140612. [Google Scholar] [CrossRef]
- Cáceres, C.H.; Djurdjevic, M.B.; Stockwell, T.J.; Sokolowski, J.H. The Effect of Cu Content on the Level of Microporosity in Al-Si-Cu-Mg Casting Alloys. Scripta Mater. 1999, 40, 631–637. [Google Scholar] [CrossRef]
- Tupaj, M.; Orłowicz, A.W.; Mróz, M.; Trytek, A.; Dolata, A.J.; Dziedzic, A. A Study on Material Properties of Intermetallic Phases in a Multicomponent Hypereutectic Al-Si Alloy with the Use of Nanoindentation Testing. Materials 2020, 13, 5612. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Samuel, E.; Zedan, Y.; Samuel, A.M.; Doty, H.W.; Samuel, F.H. Intermetallics Formation during Solidification of Al-Si-Cu-Mg Cast Alloys. Materials 2022, 15, 1335. [Google Scholar] [CrossRef]
- Bogdanof, T. The Effect of Microstructural Features, Defects and Surface Quality on the Fatigue Performance in Al-Si-Mg Cast Alloys. Ph.D. Thesis, School of Engineering, Jönköping University, Jönköping, Sweden, 2023. Available online: https://www.diva-portal.org/smash/get/diva2:1813756/FULLTEXT01.pdf (accessed on 20 August 2025).
- Zhao, H.; De Geuser, F.; Kwiatkowski da Silva, A.; Szczepaniak, A.; Gault, B.; Ponge, D.; Raabe, D. Segregation Assisted Grain Boundary Precipitation in a Model Al-Zn-Mg-Cu Alloy. Acta Mater. 2018, 156, 318–329. [Google Scholar] [CrossRef]
- Foley, D.L.; Leff, A.C.; Lang, A.C.; Taheri, M.L. Evolution of β-Phase Precipitates in an Aluminum-Magnesium Alloy at the Nanoscale. Acta Mater. 2020, 185, 279–286. [Google Scholar] [CrossRef]
- Xia, F.; Li, J.-P.; Guo, Y.-C.; Yang, Z. Microstructure Evolution and Mechanical Properties of an Al-Si-Cu-Mg-Ni Aluminium Alloy after Thermal Exposure. Mater. Sci. Forum 2013, 765, 486–490. [Google Scholar] [CrossRef]
- An, M.; Wang, C.; Li, F.; Yan, R. Effects of Fe and Ni Additions and Thermal Exposure on the Microstructure and Mechanical Properties of Hypereutectic Al–Si–Cu–Mg Alloy. Int. J. Metalcast. 2025, 1–14. [Google Scholar] [CrossRef]
- Nagesh Kumar, R.; Prabhu, T.R.; Siddaraju, C. Effect of Thermal Exposure on Mechanical Properties Hypoeutectic Aerospace Grade Aluminium-Silicon Alloy. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2016. [Google Scholar] [CrossRef]
- Fadl, M.; Khalifa, W.; El-Hadad, S. Effect of Soaking Treatment on the Microstructure and Wear Behavior of the Ultrasonic Melt-Treated B390 Hypereutectic Al-Si Alloy. TMS Light. Met. 2016, 2016, 821–825. [Google Scholar] [CrossRef]
- Lee, J.C.; Lee, S.; Lee, D.Y.; Kim, N.J. On the Embrittlement of a Rapidly Solidified Al-Fe-V-Si Alloy After High-Temperature Exposure. Metall. Trans. A 1991, 22, 853–858. [Google Scholar] [CrossRef]








| Fuel | Exhaust Gas Parameter | ||||
|---|---|---|---|---|---|
| CO, % | CO2, % | HC, ppm | NOx, ppm | Temperature, °C | |
| Gasoline (G) | 2.23 ± 0.093 | 7.72 ± 0.042 | 211 ± 1.94 | 153 ± 2.57 | 235 ± 3.15 |
| Mixture of gasoline and oxyhydrogen gas (H) | 2.52 ± 0.080 | 6.93 ± 0.048 | 204 ± 1.18 | 337 ± 6.85 | 214 ± 3.86 |
| Element | Virgin Oil | G (100 h) | H (100 h) |
|---|---|---|---|
| C | 8.11% | 7.58% | 8.60% |
| O | 91.9% | 92.4% | 91.4% |
| Mg | 1.16 ppm | 0.912 ppm | 1.68 ppm |
| Al | - | 0.150 ppm | 0.719 ppm |
| Si | - | 2.43 ppm | 3.71 ppm |
| P | 1.52 ppm | 1.39 ppm | 1.44 ppm |
| S | 3.95 ppm | 5.49 ppm | 9.60 ppm |
| Cl | - | 0.436 ppm | 0.252 ppm |
| Ca | 4.05 ppm | 3.92 ppm | 3.90 ppm |
| Fe | - | 0.0813 ppm | 0.195 ppm |
| Zn | 1.67 ppm | 1.44 ppm | 1.38 ppm |
| Mo | 0.113 ppm | 0.0924 ppm | 0.0833 ppm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škamat, J.; Černašėjus, O.; Pukalskas, S.; Černašėjienė, R. Effect of Hydrogen-Containing Fuel on the Mechanical Properties of an Aluminum Alloy ICE Piston. J. Mar. Sci. Eng. 2025, 13, 1889. https://doi.org/10.3390/jmse13101889
Škamat J, Černašėjus O, Pukalskas S, Černašėjienė R. Effect of Hydrogen-Containing Fuel on the Mechanical Properties of an Aluminum Alloy ICE Piston. Journal of Marine Science and Engineering. 2025; 13(10):1889. https://doi.org/10.3390/jmse13101889
Chicago/Turabian StyleŠkamat, Jelena, Olegas Černašėjus, Saugirdas Pukalskas, and Raimonda Černašėjienė. 2025. "Effect of Hydrogen-Containing Fuel on the Mechanical Properties of an Aluminum Alloy ICE Piston" Journal of Marine Science and Engineering 13, no. 10: 1889. https://doi.org/10.3390/jmse13101889
APA StyleŠkamat, J., Černašėjus, O., Pukalskas, S., & Černašėjienė, R. (2025). Effect of Hydrogen-Containing Fuel on the Mechanical Properties of an Aluminum Alloy ICE Piston. Journal of Marine Science and Engineering, 13(10), 1889. https://doi.org/10.3390/jmse13101889







