3.1. Description of Desmoscolex (Desmoscolex) lanceosetatus sp. nov.
3.1.1. Systematic Accounts
Phylum Nematoda Potts, 1932.
Class Chromadorea Inglis, 1983.
Order Desmoscolecida Filipjev, 1929.
Family Desmoscolecidae Shipley, 1896.
Subfamily Desmoscolecinae Shipley, 1896.
Genus Desmoscolex Claparède, 1863.
Subgenus Desmoscolex (Desmoscolex) Claparède, 1863.
Type species. Desmoscolex (Desmoscolex) minutus Claparède, 1863.
urn:lsid:zoobank.org:act:B0AD85A5-101F-4CA1-B9FD-80B829B00EF6.
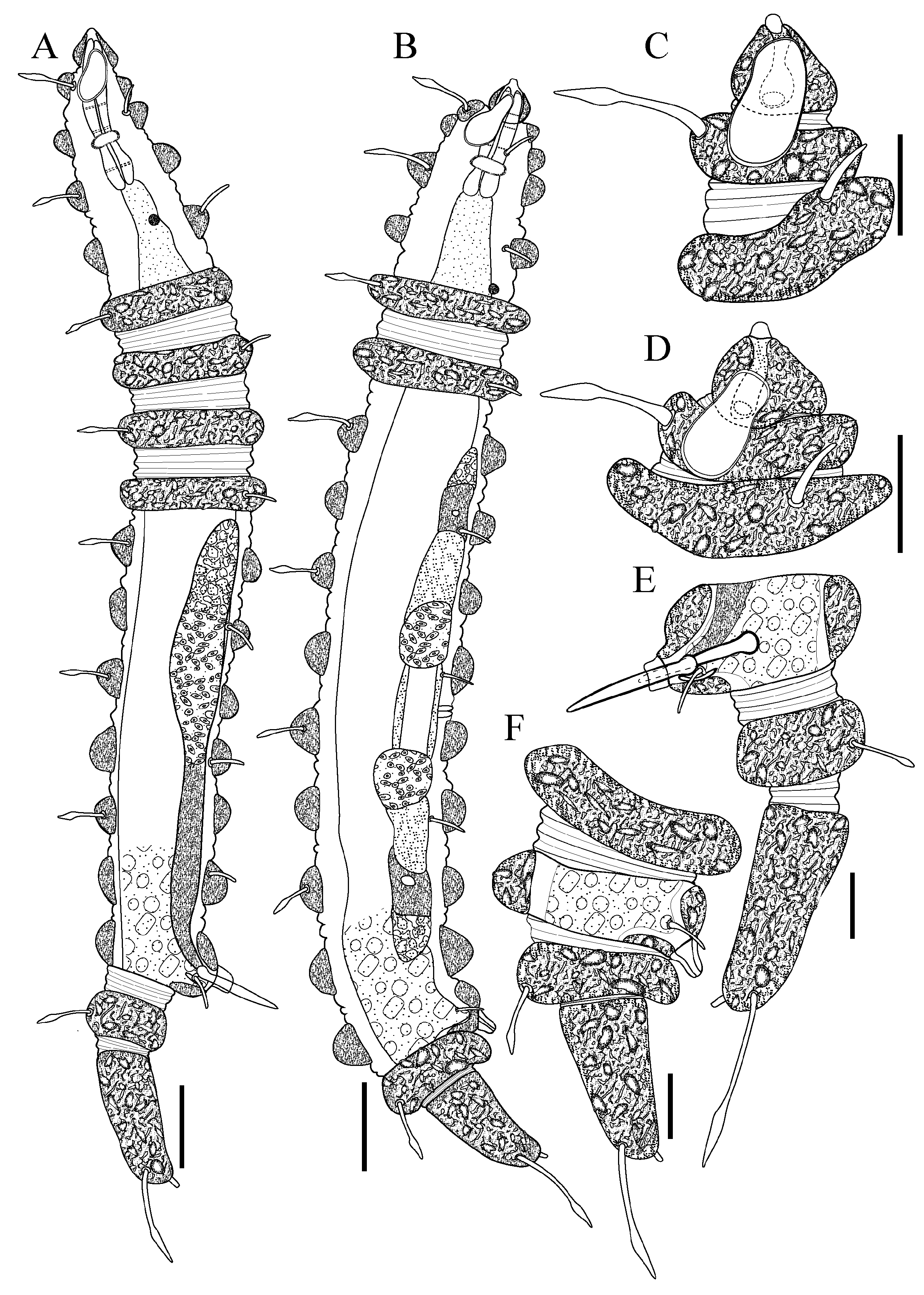
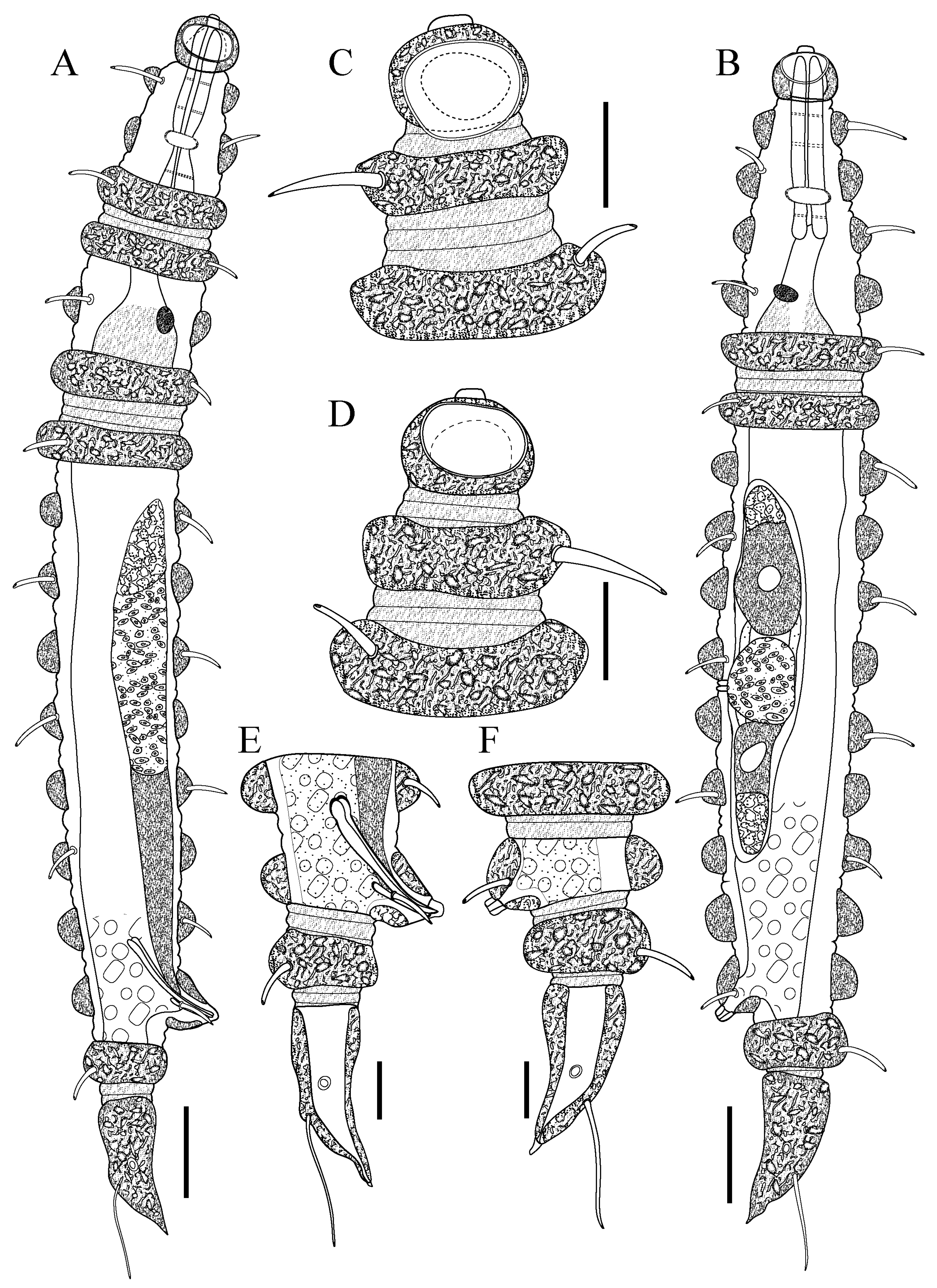
Figure 1.
Desmoscolex (Desmoscolex) lanceosetatus sp. nov.: (A) entire view of the holotype male, lateral view; (B) entire view of the paratype female, lateral view; (C) anterior end of holotype male, surface view; (D) anterior end of paratype female, surface view; (E) posterior end of holotype male, lateral view; (F) posterior end of paratype female, lateral view (scale bars: (A,B) = 20 μm; (C–F) = 10 μm).
Figure 1.
Desmoscolex (Desmoscolex) lanceosetatus sp. nov.: (A) entire view of the holotype male, lateral view; (B) entire view of the paratype female, lateral view; (C) anterior end of holotype male, surface view; (D) anterior end of paratype female, surface view; (E) posterior end of holotype male, lateral view; (F) posterior end of paratype female, lateral view (scale bars: (A,B) = 20 μm; (C–F) = 10 μm).
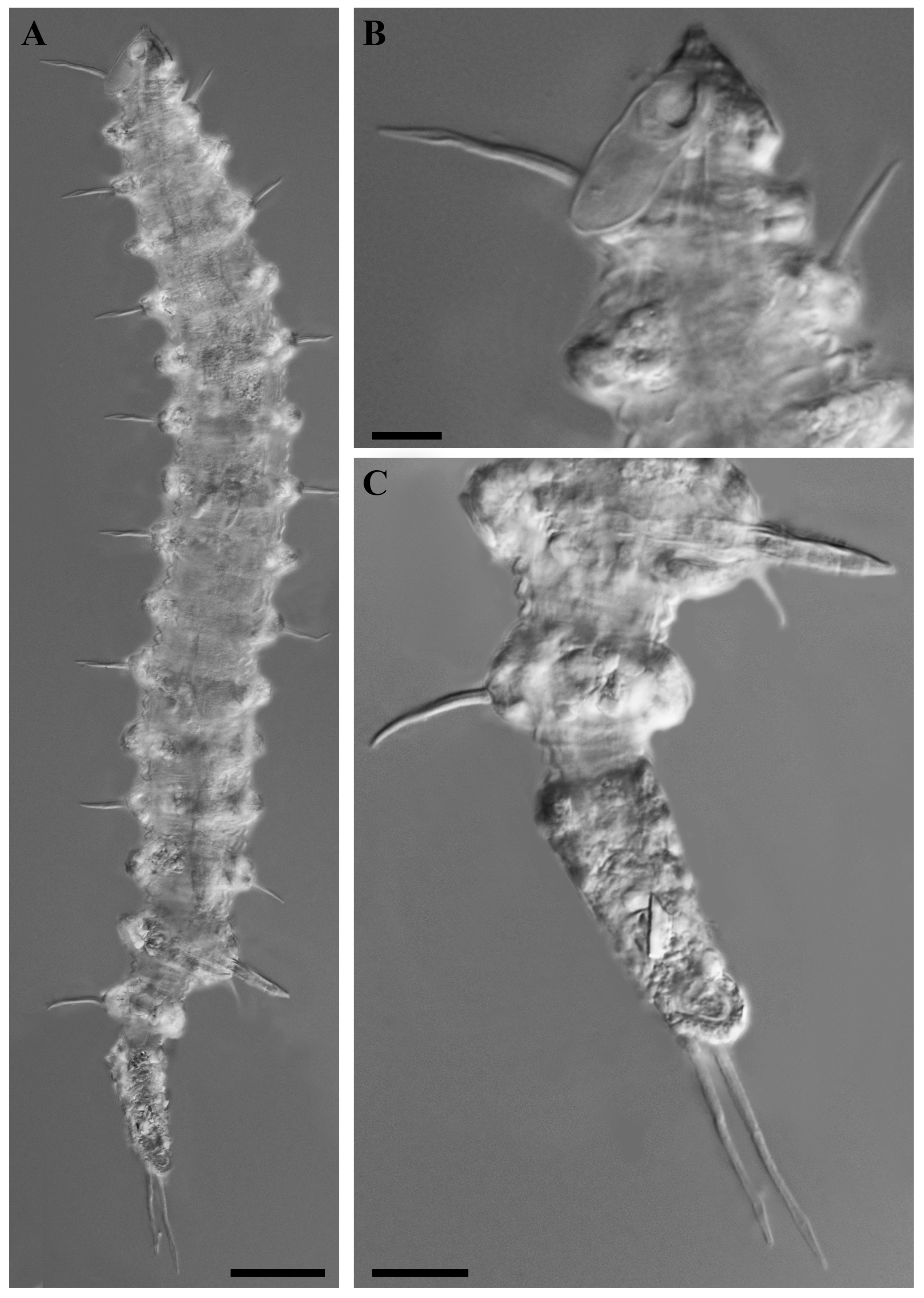
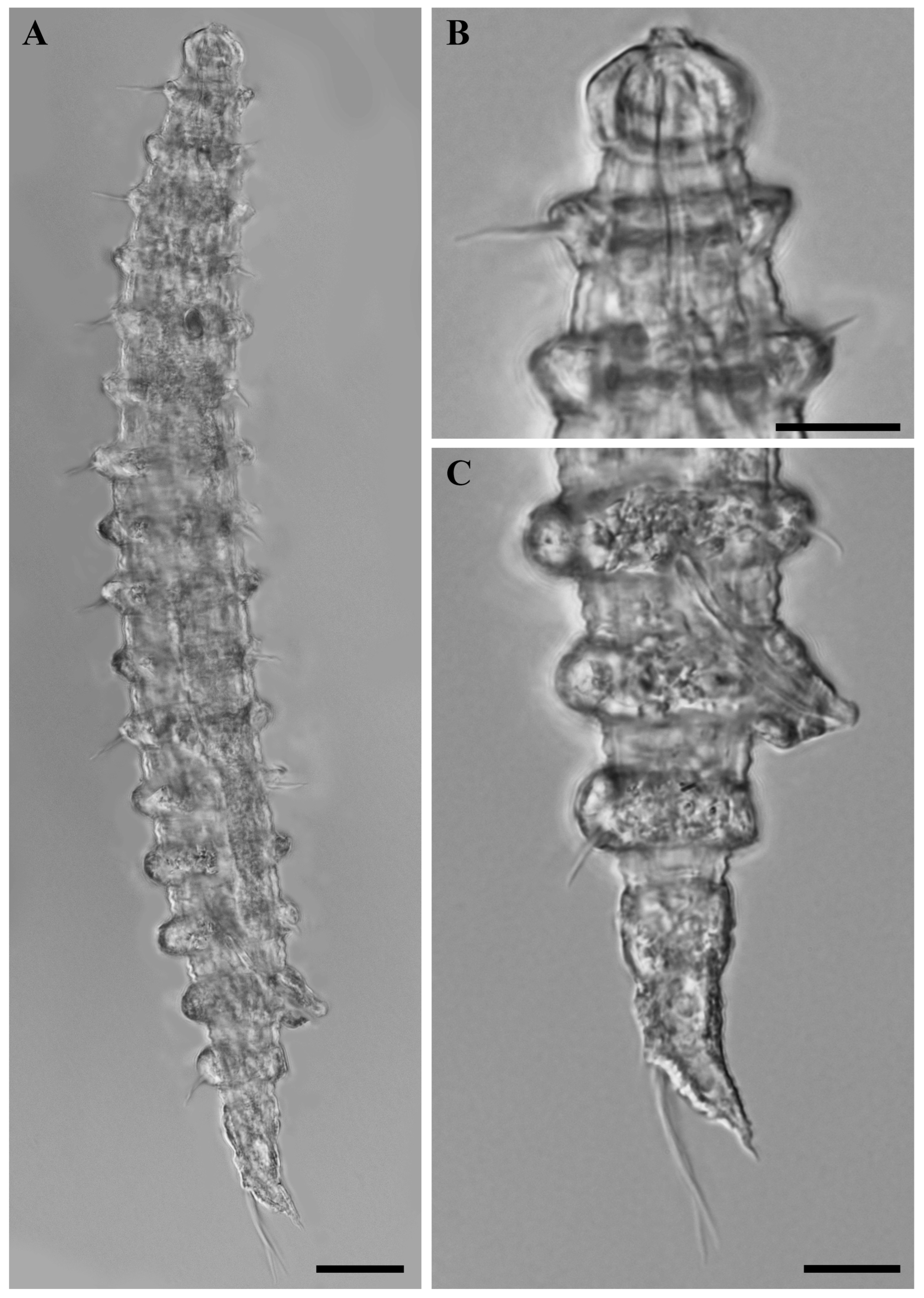
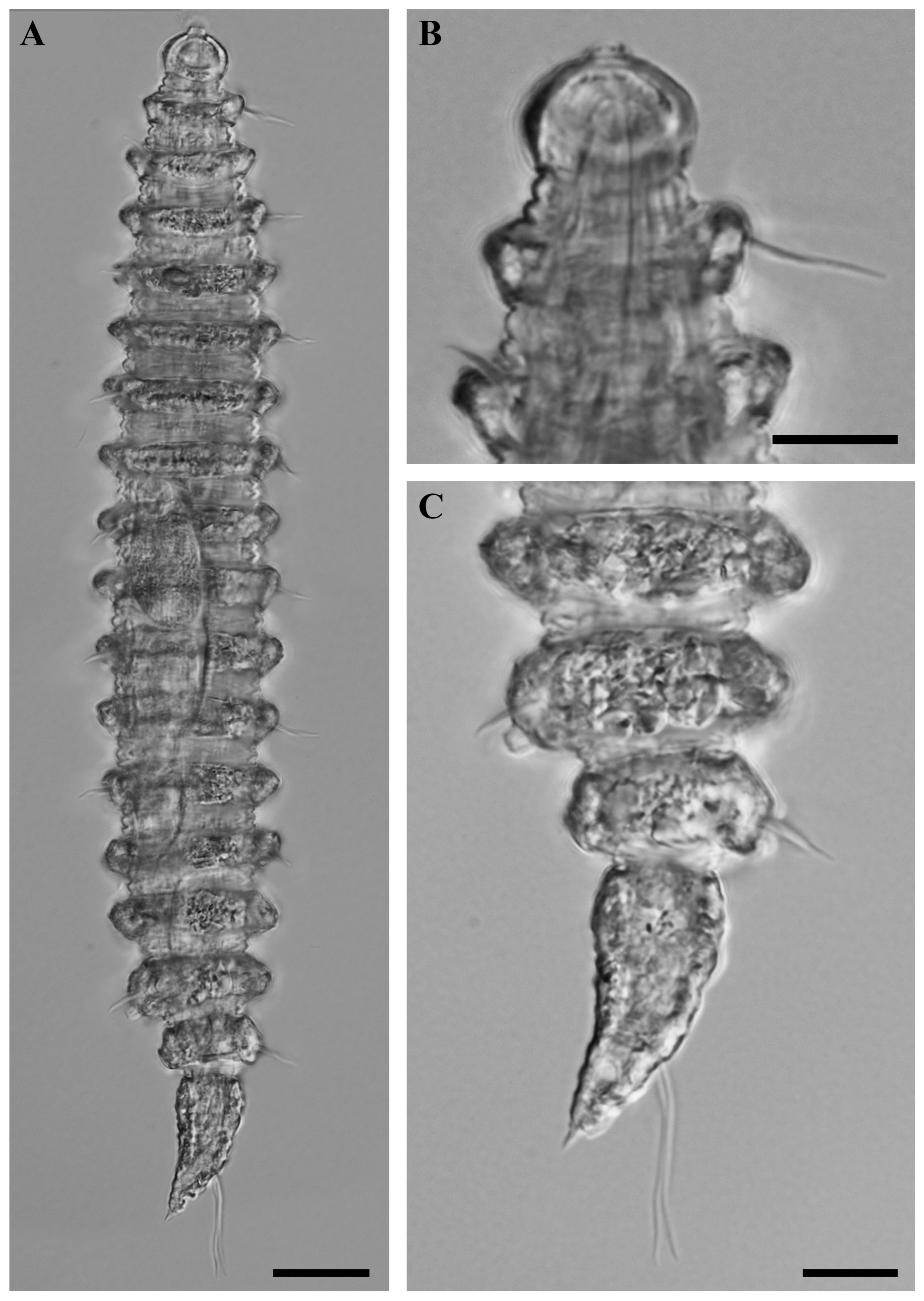
Figure 2.
Desmoscolex (Desmoscolex) lanceosetatus sp. nov., holotype male, DIC micrographs: (A) entire view of holotype male, lateral view; (B) surface view of the anterior body region, lateral view; (C) tail region, lateral view (scale bars: (A) = 20 μm; (B,C) = 10 μm).
Figure 2.
Desmoscolex (Desmoscolex) lanceosetatus sp. nov., holotype male, DIC micrographs: (A) entire view of holotype male, lateral view; (B) surface view of the anterior body region, lateral view; (C) tail region, lateral view (scale bars: (A) = 20 μm; (B,C) = 10 μm).
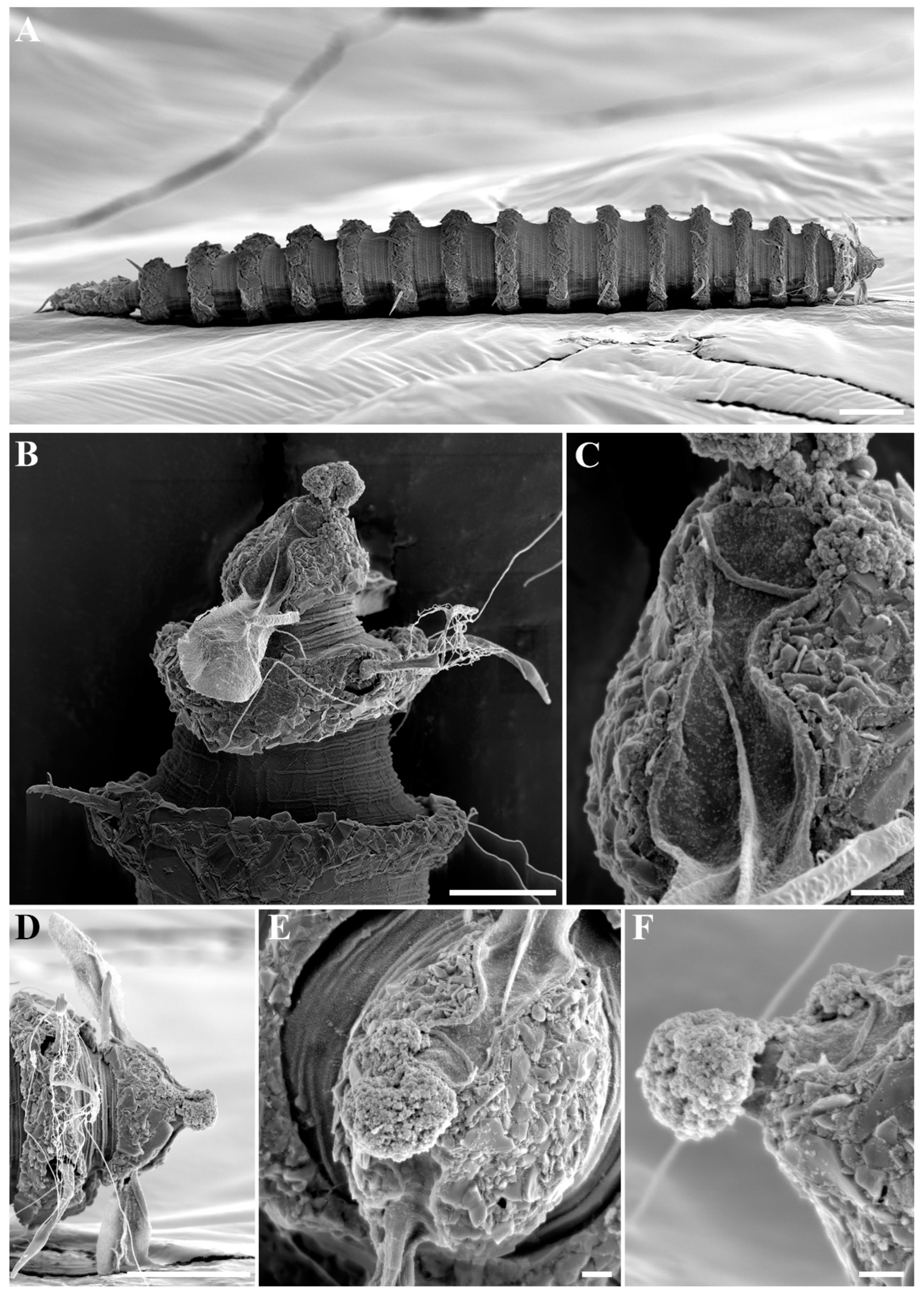
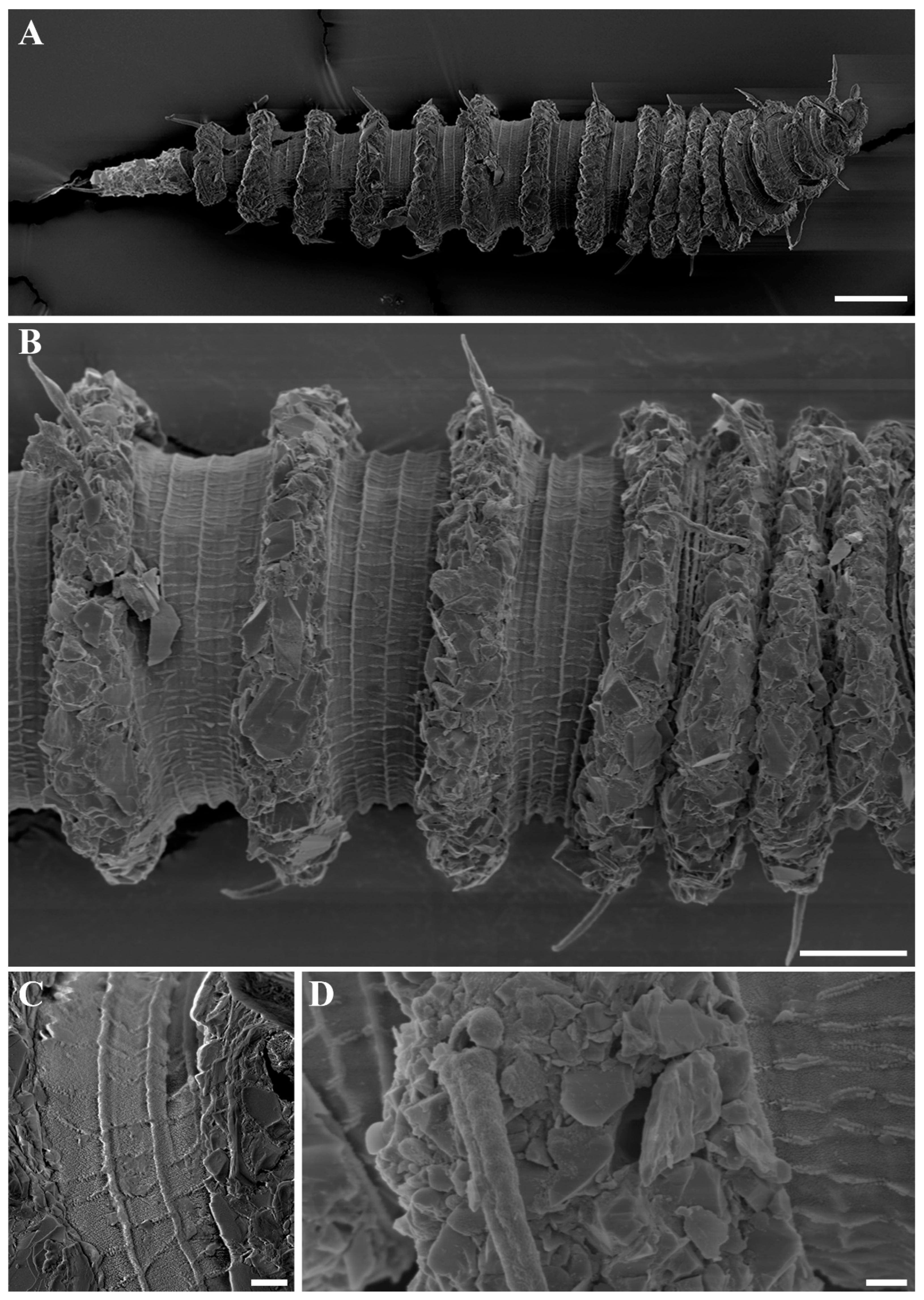
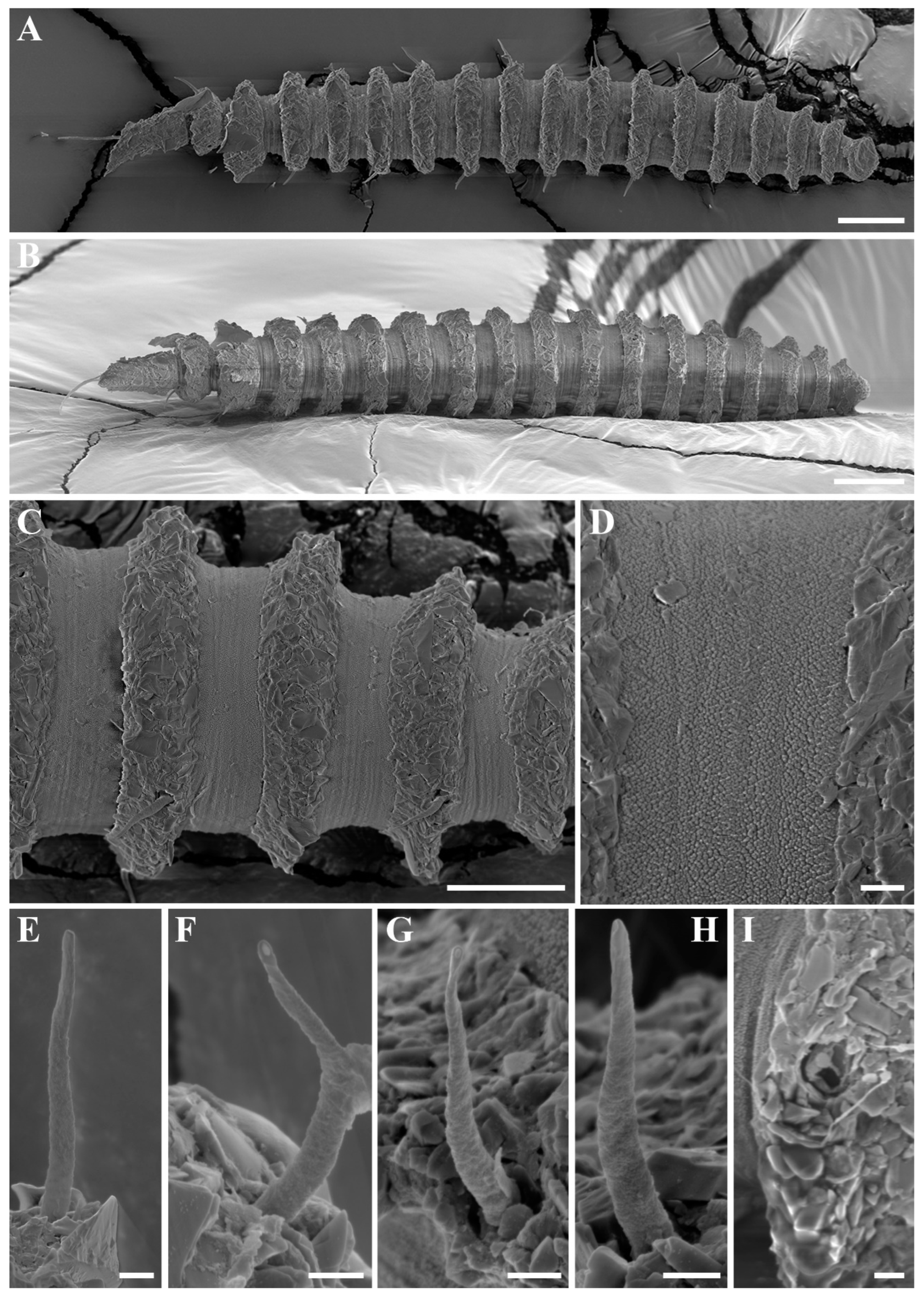
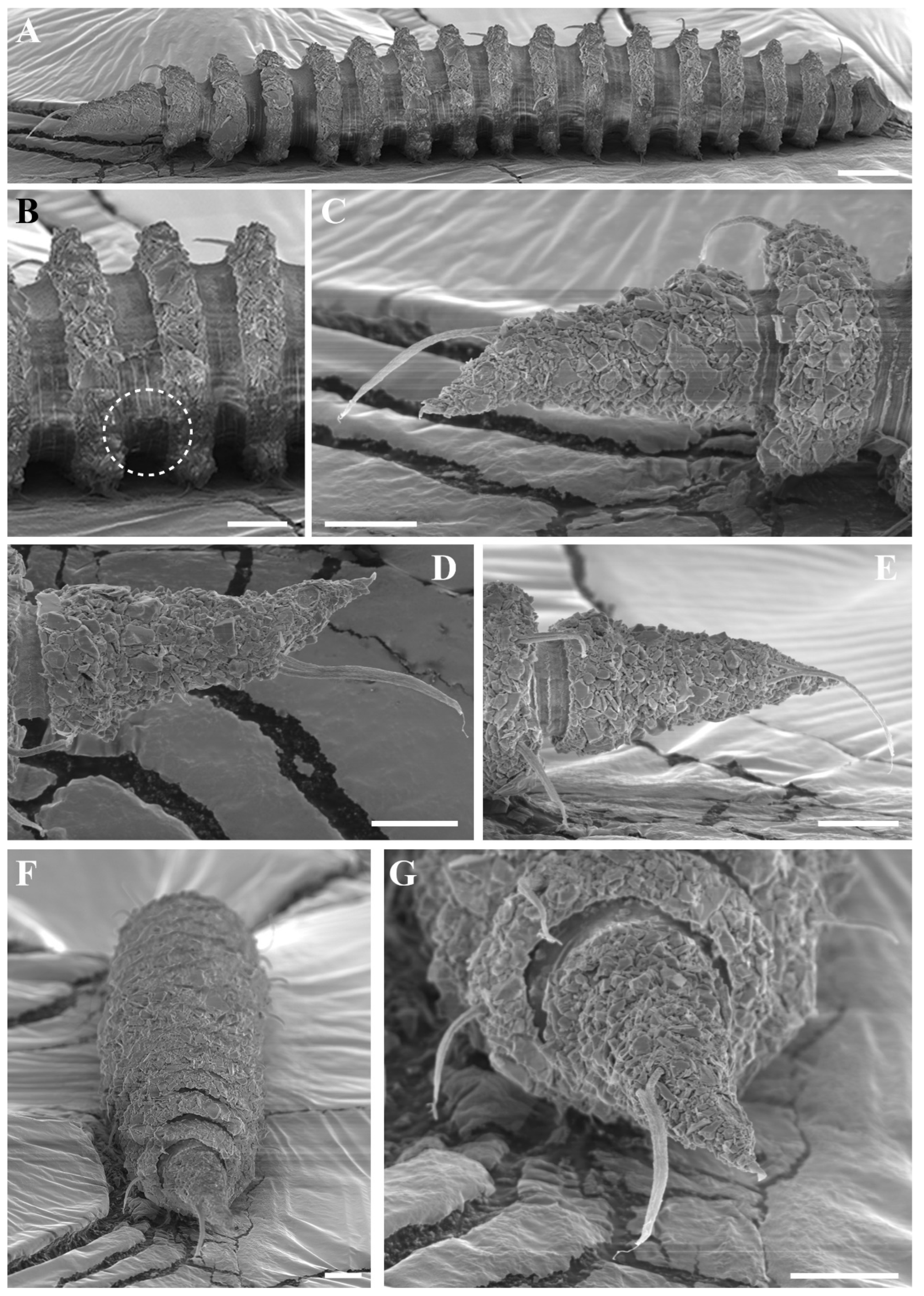
Figure 3.
Desmoscolex (Desmoscolex) lanceosetatus sp. nov., male, SEM micrographs: (A) entire view, lateral view; (B) body from the 12th to the 14th main ring, lateral view; (C) detailed view of the interzone between the 12th and 13th main ring, lateral view; (D) head region, enface view; (E) subdorsal setae of the terminal ring, subdorsal view (scale bars: (A) = 20 μm; (B,D,E) = 10 μm; (C) = 1 μm).
Figure 3.
Desmoscolex (Desmoscolex) lanceosetatus sp. nov., male, SEM micrographs: (A) entire view, lateral view; (B) body from the 12th to the 14th main ring, lateral view; (C) detailed view of the interzone between the 12th and 13th main ring, lateral view; (D) head region, enface view; (E) subdorsal setae of the terminal ring, subdorsal view (scale bars: (A) = 20 μm; (B,D,E) = 10 μm; (C) = 1 μm).
Figure 4.
Desmoscolex (Desmoscolex) lanceosetatus sp. nov., male, SEM micrographs: (A) entire view, dorsal view; (B) anterior end, lateral view; (C) detailed view of the head region, lateral view; (D) anterior end, dorsal view; (E) detailed view of the head region, enface view; (F) detailed view of the labial region covered in particles, ventral view (scale bars: (A) = 20 μm; (B,D) = 10 μm; (C,E,F) = 1 μm).
Figure 4.
Desmoscolex (Desmoscolex) lanceosetatus sp. nov., male, SEM micrographs: (A) entire view, dorsal view; (B) anterior end, lateral view; (C) detailed view of the head region, lateral view; (D) anterior end, dorsal view; (E) detailed view of the head region, enface view; (F) detailed view of the labial region covered in particles, ventral view (scale bars: (A) = 20 μm; (B,D) = 10 μm; (C,E,F) = 1 μm).
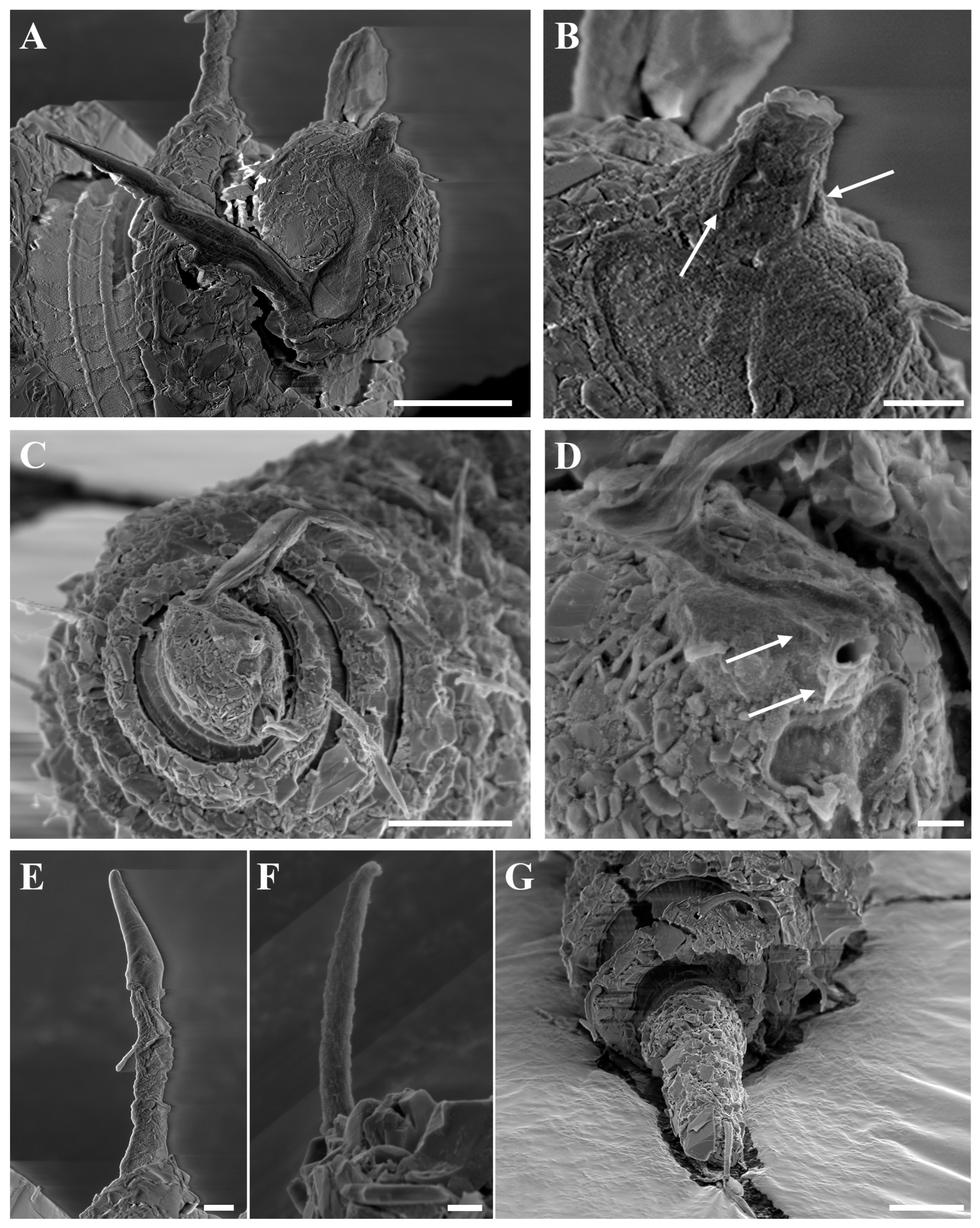
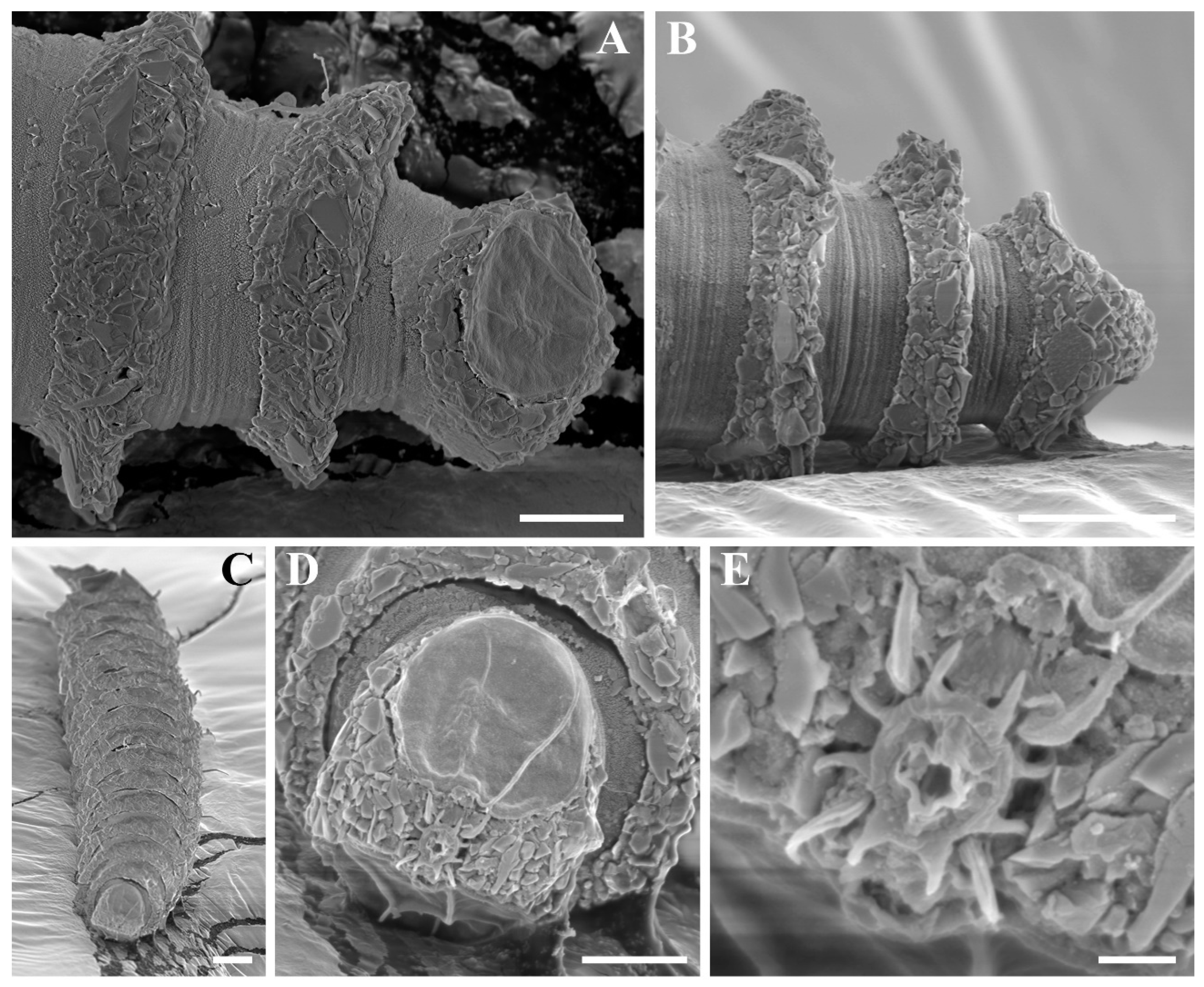
Figure 5.
Desmoscolex (Desmoscolex) lanceosetatus sp. nov., male, SEM micrographs: (A) subdorsal seta of the 7th main ring, lateral view; (B) detailed view of the subdorsal seta of the 7th main ring, lateral view; (C) subventral seta of the 4th main ring, lateral view; (D) detailed view of the subventral seta of the 4th main ring; (E) insertion region of the subdorsal seta on the first main ring; (F) cloacal region with cloacal tube and protruding spicule; (G) terminal main ring, lateral view; (H) detailed view of the insertion region of the terminal subdorsal setae on the terminal main ring (scale bars: (A,C,E,F,H) = 1 μm; (B,D) = 0.1 μm; (G) = 10 μm).
Figure 5.
Desmoscolex (Desmoscolex) lanceosetatus sp. nov., male, SEM micrographs: (A) subdorsal seta of the 7th main ring, lateral view; (B) detailed view of the subdorsal seta of the 7th main ring, lateral view; (C) subventral seta of the 4th main ring, lateral view; (D) detailed view of the subventral seta of the 4th main ring; (E) insertion region of the subdorsal seta on the first main ring; (F) cloacal region with cloacal tube and protruding spicule; (G) terminal main ring, lateral view; (H) detailed view of the insertion region of the terminal subdorsal setae on the terminal main ring (scale bars: (A,C,E,F,H) = 1 μm; (B,D) = 0.1 μm; (G) = 10 μm).
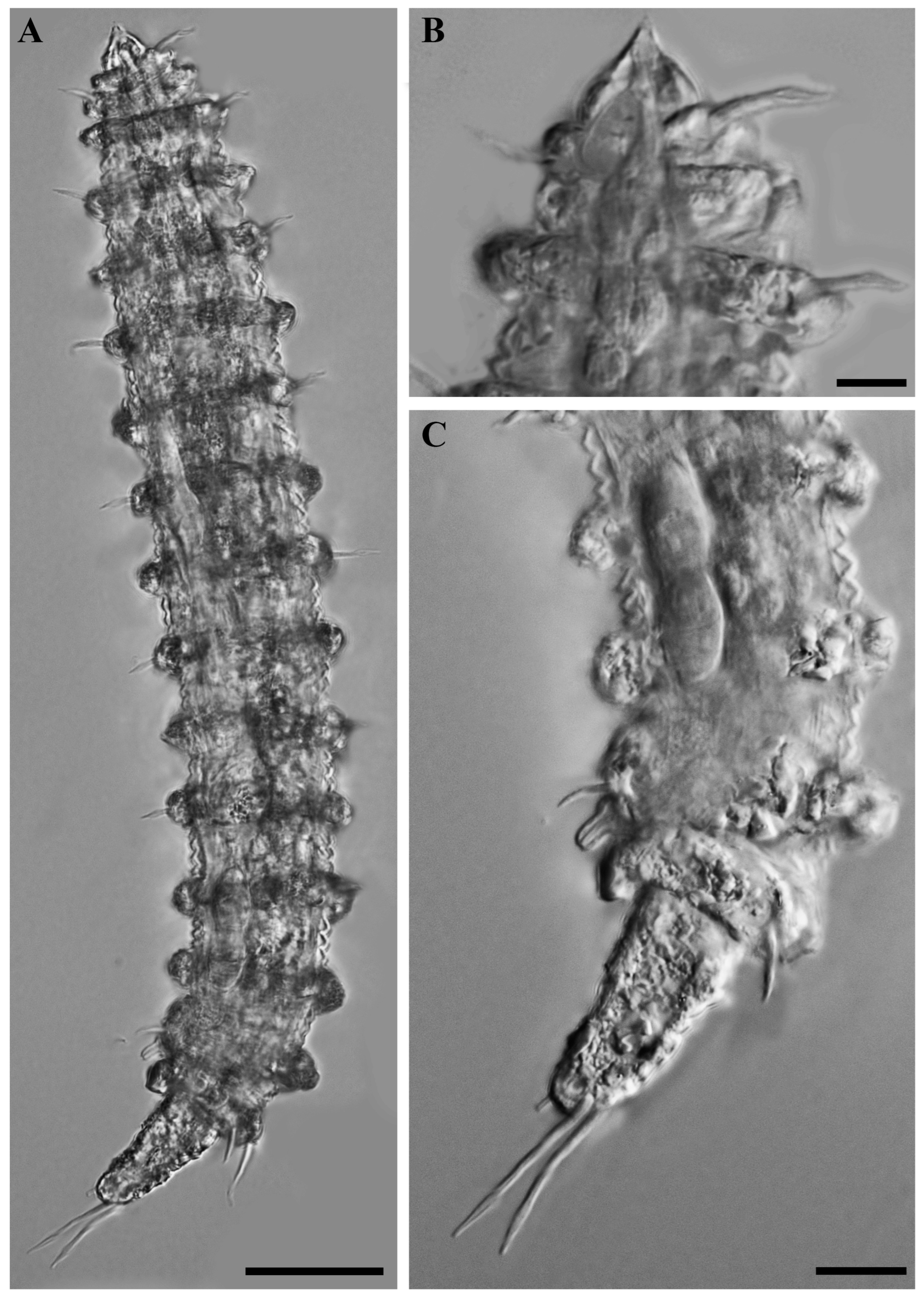
Figure 6.
Desmoscolex (Desmoscolex) lanceosetatus sp. nov., paratype female, DIC micrographs: (A) entire view of paratype female, lateral view; (B) surface view of the anterior body region, lateral view; (C) posterior body region, lateral view (scale bars: (A) = 20 μm; (B,C) = 10 μm).
Figure 6.
Desmoscolex (Desmoscolex) lanceosetatus sp. nov., paratype female, DIC micrographs: (A) entire view of paratype female, lateral view; (B) surface view of the anterior body region, lateral view; (C) posterior body region, lateral view (scale bars: (A) = 20 μm; (B,C) = 10 μm).
Figure 7.
Desmoscolex (Desmoscolex) lanceosetatus sp. nov., female, SEM micrographs: (A) entire view, sublateral view; (B) body from the 5th to the 11th main ring, sublateral view; (C) detailed view of the interzone between the 1st and 2nd main ring, sublateral view; (D) detailed view of the interzone between the 15th and 17th main ring, sublateral view (scale bars: (A) = 20 μm; (B) = 10 μm; (C,D) = 1 μm).
Figure 7.
Desmoscolex (Desmoscolex) lanceosetatus sp. nov., female, SEM micrographs: (A) entire view, sublateral view; (B) body from the 5th to the 11th main ring, sublateral view; (C) detailed view of the interzone between the 1st and 2nd main ring, sublateral view; (D) detailed view of the interzone between the 15th and 17th main ring, sublateral view (scale bars: (A) = 20 μm; (B) = 10 μm; (C,D) = 1 μm).
Figure 8.
Desmoscolex (Desmoscolex) lanceosetatus sp. nov., female, SEM micrographs: (A) anterior end, sublateral view; (B) detailed view of the labial region with four protrusions surrounding the oral opening indicated by arrows at the base of the rostrum, sublateral view; (C) anterior end, enface view; (D) detailed view of the labial region with four protrusions surrounding the oral opening indicated by arrows at the base of the rostrum, enface view; (E) subdorsal seta of the first main ring; (F) subventral seta of the 8th main ring; (G) posterior end, lateral view (scale bars: (A) = 5 μm; (B,D–F) = 1 μm; (C,G) = 10 μm).
Figure 8.
Desmoscolex (Desmoscolex) lanceosetatus sp. nov., female, SEM micrographs: (A) anterior end, sublateral view; (B) detailed view of the labial region with four protrusions surrounding the oral opening indicated by arrows at the base of the rostrum, sublateral view; (C) anterior end, enface view; (D) detailed view of the labial region with four protrusions surrounding the oral opening indicated by arrows at the base of the rostrum, enface view; (E) subdorsal seta of the first main ring; (F) subventral seta of the 8th main ring; (G) posterior end, lateral view (scale bars: (A) = 5 μm; (B,D–F) = 1 μm; (C,G) = 10 μm).
3.1.2. Diagnosis
Desmoscolex (Desmoscolex) lanceosetatus sp. nov. is distinguished by its asymmetrical, dorsally curving head with rounded posterior border anteriorly tapered to a protruding rostrum. The amphidial fovea are large, vesicular structures that nearly cover the entire head laterally and extend to the posterior margin of the first main ring. In females, the somatic setae arrangement deviates from the typical pattern of species with 17 main rings, due to the absence of subventral setae on the 14th main ring, resulting in a sexual dimorphism where males typically exhibit nine subdorsal and eight subventral setae, while females have seven subventral setae. The subdorsal setae have a broad base with a lance-shaped, open tip, and the terminal setae are distinctly elongated and located near the end of the 17th main ring. The terminal ring curves ventrally and ends in a short, uncovered spinneret.
3.1.3. Type Locality
Species was discovered in benthic sediments at a depth of 48 m in the southeastern waters of Jeju Island, Korea (33°00′00.0″ N, 126°30′00.0″ E), on 16 November 2015.
3.1.4. Other Locality
Other specimens were discovered in benthic sediments at a depth of 103 m in the southeastern waters of Jeju Island, Korea (33°00′00.0″ N, 127°00’00.0″ E), on 7 November 2015.
3.1.5. Type Material
The holotype male (MABIK NA00158722) and one paratype female (MABIK NA00158723), mounted in glycerin on HS slides, were preserved in the nematode collection of the Marine Biodiversity Institute of Korea (MABIK), Seochun, Korea. Additionally, one paratype male (KIOST NEM-1-2720) and four paratype females (KIOST NEM-1-2722 to KIOST NEM-1-2725), also mounted in glycerin on HS slides, were deposited in the nematode collection of the specimen conservation room at the Bio-Resources Bank of Marine Nematodes (BRBMN), East Sea Research Institute, Korea Institute of Ocean Science & Technology (KIOST), Korea.
3.1.6. Etymology
The species name Desmoscolex (Desmoscolex) lanceosetatus sp. nov. is derived from the characteristic lance-like shape of the subdorsal somatic setae. The prefix “lanceo-“ means “lance”, and “setatus” refers to the presence of bristles (setae), highlighting this distinctive morphological feature.
3.1.7. Measurements
Holotype male: L = 295, hd = 10, sd1 = 14, sd3 = 11, sd5 = 11, sd7 = 11, sd9 = 11, sd11 = 12, sd13 = 9, sd16 = 13, sd17 = 26, sv2 = 9, sv4 = 9, sv6 = 7, sv8 = 9, sv10 = 9, sv12 = 8, sv14 = 9, sv15 = 9, mbd = 36, (mbd) = 29, oes = 36, spic = 22, gub = 7, abd = 34, t = 61, tmrw = 13, tmr = 34, a = 8.2, b = 8.2, c = 4.8
Paratype male (n = 1): L = 440, hd = 14, sd1 = 19, sd3 = 16, sd5 = 16, sd7 = 16, sd9 = 15, sd11 = 15, sd13 = 14, sd16 = 12, sd17 = 37, sv2 = 11, sv4 = 12, sv6 = 10, sv8 = 11, sv10 = 10, sv12 = 9, sv14 = 11, sv15 = 9, mbd = 59, (mbd) = 50, oes = 56, spic = 39, gub = 11, abd = 52, t = 95, tmrw = 23, tmr = 52, a = 7.4, b = 7.9, c = 4.6
Paratype females (n = 5): L = 250–310, hd = 9–11, sd1 = 11–14, sd3 = 9–11, sd5 = 9–11, sd7 = 9–10, sd9 = 10–11, sd11 = 9–11, sd13 = 7–11, sd16 = 9–12, sd17 = 21–23, sv2 = 6–8, sv4 = 7–8, sv6 = 6–10, sv8 = 6–9, sv10 = 8–9, sv12 = 7–9, sv15 = 7–8, mbd = 38–46, (mbd) = 29–37, oes = 30–35, abd = 30–34, t = 43–49, tmrw = 12–15, tmr = 23–33, a = 6.2–7.1, b= 7.1–10.1, c = 5.4–6.7, V = 52.5–56.5%
3.1.8. Description
For males, the body gradually tapers towards both extremities and is composed of 17 main rings (
Figure 1A,
Figure 2A and
Figure 3A). Each main ring is composed of numerous foreign particles. The width of the main rings in the midsection ranges between 10 µm and 11 µm (
Figure 3B and
Figure 4A). In the anterior part of the body and the tail, the main rings are separated by narrower intermediate zones, which consist of two to three secondary rings. In the rest of the body, the interzone is divided into three to four narrow rings. The secondary rings in these intermediate zones range from 2 µm to 3 µm in width. SEM observations of the interzones reveal numerous tubercle- or granular-like protrusions distributed across the entire surface. The secondary rings of the interzone show multiple transverse ridges crossing them, and these rings are uniformly covered with tubercle- or granular-like protrusions (
Figure 3C).
The head appears asymmetrical, curving dorsally. The posterior part is broad and rounded, covered with concretion particles, and tapers anteriorly towards a protruding rostrum (
Figure 1C,
Figure 2B,
Figure 3D and
Figure 4B,D–E). In the SEM enface view, the labial region features four protrusions surrounding the oral opening (
Figure 8B,D). This feature is visible in females under SEM but cannot be observed in the male specimen due to particle attachment in the labial area (
Figure 4F). However, based on the similarity between the male and female labial regions observed in other SEM images, it is concluded that this feature is common to both sexes. Additionally, the labial region is covered with cephalic tubercles that extend towards the rostrum-shaped oral aperture (
Figure 4C,F). The oral opening is bordered by a crown-like membrane, which is free of cephalic tubercles. Although this feature is not visible in the male specimen due to particle attachment, it is presumed to be present in both sexes, as observed in the female specimen. Notably, there are no cephalic setae, and no traces of them were found in their usual positions. The amphidial groove begins at the rostrum, narrows in the middle, and then widens again as it extends towards the posterior region of the head (
Figure 4C).
The amphidial fovea are large vesicular structures, measuring 19 µm in length and 8 µm in width, nearly covering the entire lateral side of the head region. They narrow at the front and extend towards the distal end, reaching the posterior margin of the first main ring.
The pharynx is narrow and cylindrical, extending toward the anterior region of the body. It is encircled by the nerve ring at the level of the second main ring, with the pharynx–intestinal junction occurring at the third main ring. The intestine, exhibiting a typical structure, extends through the 16th main ring, while the ocelli are positioned between the 3rd and 5th main rings.
The somatic setae follow the typical arrangement for species with 17 main rings:
Subdorsal: 1, 3, 5, 7, 9, 11, 13, 16, 17 = 9
Subventral: 2, 4, 6, 8, 10, 12, 14, 15 = 8
The subdorsal setae have a wider basal shaft with a smooth, swollen, lance-shaped open tip. The broader basal shaft exhibits a distinct pattern of numerous tubercles or wrinkles, which differ from those in the interzone and gradually disappear around two-thirds of the way up, forming the lance-shaped tip (
Figure 5A,B). Unlike the other subdorsal setae, the terminal setae display tubercles or wrinkles only along the first quarter of their length. Beyond this point, the wrinkles persist while the tubercles disappear (
Figure 5H), eventually forming a lance-shaped open tip. The subdorsal setae on the first main ring are longer than those on the subsequent rings, maintaining nearly equal length until the 11th main ring. The subdorsal setae on the 13th and 16th main rings are relatively shorter, while the terminal setae are significantly elongated.
The shorter subventral setae have a typical bristle structure with a naked open tip and are of similar length. Like the subdorsal setae, the subventral setae also exhibit numerous tubercles or wrinkles, visible along up to four-fifths of their length, after which the setae become smooth, forming an open-tipped distal part (
Figure 5C,D). SEM observations show that the insertion points of all body setae are directly embedded into the body (
Figure 5E).
The male reproductive system, typical for the subgenus, consists of a single, outstretched testis, starting with a germinal zone followed by a vesicula seminalis that transitions into a finely granular vas deferens. The spicules are nearly straight, with a slender distal portion that broadens toward the base and terminates in a capitulum. A gubernaculum, positioned parallel to the spicules, accompanies them. The naked cloacal tube, 6 µm long, is protruding from the ventrally enlarged 15th main ring (
Figure 1A,E and
Figure 5F).
The terminal ring tapers slightly towards the tail end. The terminal setae are located near the end of the 17th main ring (
Figure 3E and
Figure 5G). The terminal ring is slightly rounded and bends ventrally, ending in a very short spinneret (
Figure 1E and
Figure 2C). Phasmata are not observed.
Female: The female resembles the male in most aspects. Like the male, the body gradually tapers towards both extremities and is comprised of 17 main rings (
Figure 1B,
Figure 6A and
Figure 7A). The interzones between the main rings have two to three secondary rings, displaying tubercles- or granular-like protrusions across the surface (
Figure 7B–D).
The subdorsal setae have a broad base with a lance-shaped tip marked by tubercles or wrinkles that fade towards the tip (
Figure 8E). The terminal setae differ, showing wrinkles along the first quarter of their length (
Figure 8G). The subventral setae are similar but exhibit tubercles along four-fifths of their length (
Figure 8F). SEM observations reveal that the setae are directly embedded into the body.
The head is asymmetrical, curving dorsally, with a broad, rounded posterior tapering into a protruding rostrum (
Figure 1D,
Figure 6B and
Figure 8A,C). The labial region shows four protrusions surrounding the oral opening, and cephalic tubercles cover the labial region.
The oral opening is surrounded by a crown-like membrane without tubercles (
Figure 8B,D). Notably, there are no cephalic setae, and no traces of them were found in their usual positions. The shape of the amphidial groove and terminal ring is consistent with that observed in the male.
The amphidial fovea are large vesicular structures extending to the posterior margin of the first main ring. However, there is a difference in the arrangement of the somatic setae.
The somatic setae follow this pattern:
Subdorsal: 1, 3, 5, 7, 9, 11, 13, 16, 17 = 9
Subventral: 2, 4, 6, 8, 10, 12, (-), 15 = 7
This arrangement deviates from the typical pattern observed in species with 17 main rings due to the absence of a pair of subventral setae on the 14th main ring. The reproductive system is didelphic-amphidelphic, with both branches outstretched and containing several immature oocytes. Two spermathecae, each containing small globular sperm, are present anterior and posterior to the vulva. The vulva is positioned between the 10th and 11th main rings (
Figure 1F and
Figure 6C).
3.2. Description of Desmoscolex (Desmoscolex) rotundicephalus sp. nov.
urn:lsid:zoobank.org:act:BCEC5738-8875-4ADE-B87E-7EF349517B86.
Figure 9.
Desmoscolex (Desmoscolex) rotundicephalus sp. nov.: (A) entire view of the holotype male, lateral view; (B) entire view of the paratype female, lateral view; (C) anterior end of holotype male, surface view; (D) anterior end of paratype female, surface view; (E) posterior end of holotype male, lateral view; (F) posterior end of paratype female, lateral view (scale bars: (A,B) = 20 μm; (C–F) = 10 μm).
Figure 9.
Desmoscolex (Desmoscolex) rotundicephalus sp. nov.: (A) entire view of the holotype male, lateral view; (B) entire view of the paratype female, lateral view; (C) anterior end of holotype male, surface view; (D) anterior end of paratype female, surface view; (E) posterior end of holotype male, lateral view; (F) posterior end of paratype female, lateral view (scale bars: (A,B) = 20 μm; (C–F) = 10 μm).
Figure 10.
Desmoscolex (Desmoscolex) rotundicephalus sp. nov., holotype male, DIC micrographs: (A) entire view, lateral view; (B) surface view of the anterior body region, lateral view; (C) posterior body region, lateral view (scale bars: (A) = 20 μm; (B,C) = 10 μm).
Figure 10.
Desmoscolex (Desmoscolex) rotundicephalus sp. nov., holotype male, DIC micrographs: (A) entire view, lateral view; (B) surface view of the anterior body region, lateral view; (C) posterior body region, lateral view (scale bars: (A) = 20 μm; (B,C) = 10 μm).
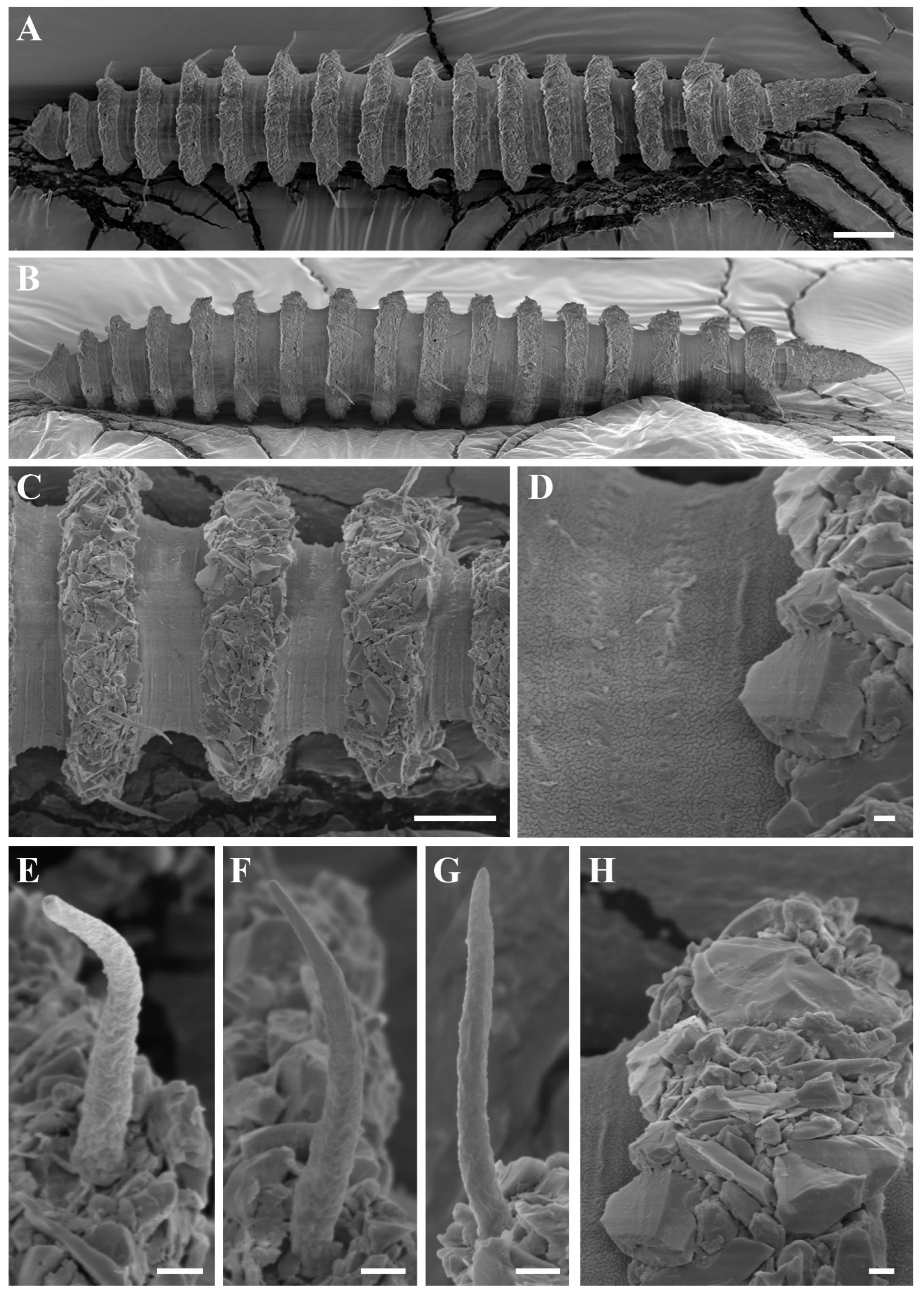
Figure 11.
Desmoscolex (Desmoscolex) rotundicephalus sp. nov., male, SEM micrographs: (A) entire view, lateral view; (B) entire view, ventral view; (C) body from the 1st to the 5th main ring, lateral view; (D) detailed view of the interzone between the 3rd and 4th main ring, lateral view; (E) subdorsal seta of the 11th main ring, lateral view; (F) subdorsal seta of the 13th main ring, lateral view; (G) subventral seta of the 2nd main ring, lateral view; (H) subventral seta of the 10th main ring, lateral view; (I) insertion region of the subdorsal seta on the 1st main ring (scale bars: (A,B) = 20 μm; (C) = 10 μm; (D–I) = 1 μm).
Figure 11.
Desmoscolex (Desmoscolex) rotundicephalus sp. nov., male, SEM micrographs: (A) entire view, lateral view; (B) entire view, ventral view; (C) body from the 1st to the 5th main ring, lateral view; (D) detailed view of the interzone between the 3rd and 4th main ring, lateral view; (E) subdorsal seta of the 11th main ring, lateral view; (F) subdorsal seta of the 13th main ring, lateral view; (G) subventral seta of the 2nd main ring, lateral view; (H) subventral seta of the 10th main ring, lateral view; (I) insertion region of the subdorsal seta on the 1st main ring (scale bars: (A,B) = 20 μm; (C) = 10 μm; (D–I) = 1 μm).
Figure 12.
Desmoscolex (Desmoscolex) rotundicephalus sp. nov., male, SEM micrographs: (A) anterior end, lateral view; (B) anterior end, ventral view; (C) entire view, enface view; (D) anterior end, enface view; (E) detailed view of the labial region, enface view (scale bars: (A,D) = 5 μm; (B,C) = 10 μm; (E) = 1 μm).
Figure 12.
Desmoscolex (Desmoscolex) rotundicephalus sp. nov., male, SEM micrographs: (A) anterior end, lateral view; (B) anterior end, ventral view; (C) entire view, enface view; (D) anterior end, enface view; (E) detailed view of the labial region, enface view (scale bars: (A,D) = 5 μm; (B,C) = 10 μm; (E) = 1 μm).
Figure 13.
Desmoscolex (Desmoscolex) rotundicephalus sp. nov., male, SEM micrographs: (A) body from the 14th to the 17th main ring, lateral view; (B) cloacal region with cloacal tube, lateral view; (C) body from the 14th to the 17th main ring, ventral view; (D) posterior end, enface view, (E) posterior end, lateral view; (F) posterior end with spinneret (scale bars: (A,C–E) = 10 μm; (B,F) = 1 μm).
Figure 13.
Desmoscolex (Desmoscolex) rotundicephalus sp. nov., male, SEM micrographs: (A) body from the 14th to the 17th main ring, lateral view; (B) cloacal region with cloacal tube, lateral view; (C) body from the 14th to the 17th main ring, ventral view; (D) posterior end, enface view, (E) posterior end, lateral view; (F) posterior end with spinneret (scale bars: (A,C–E) = 10 μm; (B,F) = 1 μm).
Figure 14.
Desmoscolex (Desmoscolex) rotundicephalus sp. nov., paratype female, DIC micrographs: (A) entire view, lateral view; (B) surface view of the anterior body region, lateral view; (C), posterior body region, lateral view (scale bars: (A) = 20 μm; (B,C) = 10 μm).
Figure 14.
Desmoscolex (Desmoscolex) rotundicephalus sp. nov., paratype female, DIC micrographs: (A) entire view, lateral view; (B) surface view of the anterior body region, lateral view; (C), posterior body region, lateral view (scale bars: (A) = 20 μm; (B,C) = 10 μm).
Figure 15.
Desmoscolex (Desmoscolex) rotundicephalus sp. nov., female, SEM micrographs: (A) entire view, lateral view; (B) entire view, dorsal view; (C) body from the 12th to the 16th main ring, lateral view; (D) detailed view of the interzone between the 13th and 14th main ring, lateral view; (E) subdorsal seta of the 1st main ring; (F) subdorsal seta of the 5th main ring; (G) subventral seta of the 4th main ring; (H) detailed view of the 14th main ring, lateral view (scale bars: (A,B) = 20 μm; (C) = 10 μm; (D–H) = 1 μm).
Figure 15.
Desmoscolex (Desmoscolex) rotundicephalus sp. nov., female, SEM micrographs: (A) entire view, lateral view; (B) entire view, dorsal view; (C) body from the 12th to the 16th main ring, lateral view; (D) detailed view of the interzone between the 13th and 14th main ring, lateral view; (E) subdorsal seta of the 1st main ring; (F) subdorsal seta of the 5th main ring; (G) subventral seta of the 4th main ring; (H) detailed view of the 14th main ring, lateral view (scale bars: (A,B) = 20 μm; (C) = 10 μm; (D–H) = 1 μm).
Figure 16.
Desmoscolex (Desmoscolex) rotundicephalus sp. nov., female, SEM micrographs: (A) anterior end, lateral view; (B) anterior end, dorsal view; (C) anterior end, enface view; (D) detailed view of the labial region, enface view (scale bars: (A) = 5 μm; (B,C) = 10 μm; (D) = 1 μm).
Figure 16.
Desmoscolex (Desmoscolex) rotundicephalus sp. nov., female, SEM micrographs: (A) anterior end, lateral view; (B) anterior end, dorsal view; (C) anterior end, enface view; (D) detailed view of the labial region, enface view (scale bars: (A) = 5 μm; (B,C) = 10 μm; (D) = 1 μm).
Figure 17.
Desmoscolex (Desmoscolex) rotundicephalus sp. nov., female, SEM micrographs: (A) entire view, subventral view; (B) body from the 9th to the 12th main ring, highlighting the vulva region with its position marked by a dashed circle, subventral view; (C) posterior end, subventral view; (D) posterior end, lateral view, (E) posterior end, dorsal view; (F) entire view, enface view; (G) posterior end, enface view (scale bars: (A) = 20 μm; (B–G) = 10 μm).
Figure 17.
Desmoscolex (Desmoscolex) rotundicephalus sp. nov., female, SEM micrographs: (A) entire view, subventral view; (B) body from the 9th to the 12th main ring, highlighting the vulva region with its position marked by a dashed circle, subventral view; (C) posterior end, subventral view; (D) posterior end, lateral view, (E) posterior end, dorsal view; (F) entire view, enface view; (G) posterior end, enface view (scale bars: (A) = 20 μm; (B–G) = 10 μm).
3.2.1. Diagnosis
Desmoscolex (Desmoscolex) rotundicephalus sp. nov. is characterized by its globular head with a prominent labial region, featuring an oral ring with eight distinct projections and four elongated setae-like structures arranged submedially behind the oral ring, adding a unique morphological trait to the labial region. The amphidial fovea are oval, nearly covering the entire lateral sides of the head and featuring a central amphidial pore. The subdorsal setae taper towards the tip, ending in an open tip, while the terminal setae are distinctly elongated and located near the two-thirds of the 17th main ring. The terminal ring curves ventrally and ends in a short, uncovered spinneret, with small, rounded phasmata. In females, the somatic setae arrangement deviates from the typical 17 main ring species pattern due to the absence of subventral setae on the 14th main ring.
3.2.2. Type Locality
Species found in benthic sediments at a depth of 103 m in the southeastern waters of Jeju Island, Korea (33°00′00.0″ N, 127°00′00.0″ E), on 7 November 2015.
3.2.3. Type Material
The holotype male (MABIK NA00158724) and one paratype female (MABIK NA00158725), mounted in glycerin on HS slides, are preserved in the nematode collection of the Marine Biodiversity Institute of Korea (MABIK), Seochun, Korea. Additionally, three paratype females (KIOST NEM-1-2728 to KIOST NEM-1-2730), also mounted in glycerin on HS slides, have been deposited in the nematode collection at the specimen conservation room of the Bio-Resources Bank of Marine Nematodes (BRBMN), East Sea Research Institute, Korea Institute of Ocean Science & Technology (KIOST), Korea.
3.2.4. Etymology
The species name Desmoscolex (Desmoscolex) rotundicephalus sp. nov. is derived from the distinctive round shape of the head. The prefix ‘rotundi-’ means ‘round,’ and the suffix ‘-cephalus’ refers to the head, emphasizing this characteristic feature of the species.
3.2.5. Measurements
Holotype male: L = 275, hd = 14, sd1 = 11, sd3 = 8, sd5 = 8, sd7 = 7, sd9 = 8, sd11 = 8, sd13 = 6, sd16 = 7, sd17 = 21, sv2 = 7, sv4 = 6, sv6 = 6, sv8 = 6, sv10 = 7, sv12 = 7, sv14 = 7, sv15 = 7, mbd = 35, (mbd) = 26, oes = 38, spic = 30, gub = 7, abd = 32, t = 54, tmrw = 12, tmr = 30, a = 7.8, b =7.27, c =5.0
Paratype females (n = 4): L = 255–330, hd = 13–16, sd1 = 12–13, sd3 = 10–11, sd5 = 9–10, sd7 = 9–10, sd9 = 8–10, sd11 = 9–10, sd13 = 8–9, sd16 = 10–12, sd17 = 20–24, sv2 = 7–8, sv4 = 8–9, sv6 = 7–9, sv8 = 8, sv10 = 8–9, sv12 = 8–9, sv15 = 8–10, mbd = 39–52, (mbd) = 32–42, oes = 33–39, abd = 30–36, t = 52–62, tmrw = 12–15, tmr = 33–39, a = 6.3–7.8, b = 6.9–9.9, c = 4.6–5.3, V = 53.4–56.1%
3.2.6. Description
In males, the body gradually tapers towards both extremities and is composed of 17 main rings (
Figure 9A,
Figure 10A,
Figure 11A,B and
Figure 12C). Each main ring is composed of numerous large foreign particles. The width of the main rings in the midsection of the body ranges from 9 µm to 11 µm. In the anterior part of the body and the tail, the main rings are separated by narrower intermediate zones consisting of two secondary rings. In the rest of the body, the interzone is divided into three secondary rings. The secondary rings forming the interzones range from 1 µm to 2 µm in width. SEM observations reveal numerous tubercle- or granular-like protrusions distributed across the entire interzone surface. Additionally, faint junctions between the secondary rings are visible throughout the area (
Figure 11C,D).
When observed from a lateral view, the head appears globular, while in the dorsal or ventral view, it has a rounded triangular shape with blunt edges, except at the amphid regions (
Figure 9C,
Figure 10B and
Figure 12A,B). The head features a narrow, protruding labial region. The oral opening is encircled by an oral ring bearing eight projections, accompanied by four longer setae-like structures positioned submedially just behind the ring. When the labial region is not swollen, as depicted in
Figure 12E, these projections appear distinctly separate, giving the impression of individual structures. No labial papillae or tubercle-like projections were observed in this externally protruding labial area. Notably, there were no cephalic setae, and no traces of them were found in their usual positions. The oval amphidial fovea nearly cover the entire head laterally, with the circular amphidial pore located centrally.
The pharynx is narrow and cylindrical, extending toward the anterior region of the body. It is encircled by the nerve ring at the level of the second main ring, with the pharynx–intestinal junction situated at the third main ring. The intestine, exhibiting a typical structure, extends through the 16th main ring. The ocelli are positioned between the fourth and fifth main rings.
The somatic setae follow the typical arrangement for species with 17 main rings:
Subdorsal: 1, 3, 5, 7, 9, 11, 13, 16, 17 = 9.
Subventral: 2, 4, 6, 8, 10, 12, 14, 15 = 8.
The subdorsal setae taper towards the tip and have an open terminal end. Faint wrinkles are present along most of the subdorsal setae, except for the distal part, which leads to a smooth open tip (
Figure 11E,F). Similarly, the terminal setae show wrinkles along most of their length, ending in a naked open tip. The subdorsal setae on the first main ring are longer than those on the subsequent rings, maintaining nearly equal length until the 16th ring, except on the 13th ring, where the subdorsal setae are relatively shorter. However, the terminal setae are distinctly elongated (
Figure 13C).
The shorter subventral setae exhibit a fine structure and are similar in length. Like the subdorsal setae, wrinkles are present along their length, except for the distal part, which is naked and ends in an open tip (
Figure 11G,H). SEM observations reveal that the insertion points of all body setae are directly embedded into the body (
Figure 11I).
The male reproductive system, characteristic of the subgenus, consists of a single, outstretched testis, starting with a germinal zone followed by a vesicula seminalis that transitions into a finely granular vas deferens. The spicules are slightly bent, with a slender distal portion that widens proximally and terminates in a capitulum. A gubernaculum, positioned parallel to the spicules, accompanies them (
Figure 9E and
Figure 10C). The cloacal tube protrudes from the ventrally enlarged 15th main ring (
Figure 13A). This papilla, devoid of concretion particles, exhibits numerous tubercle- or granular-like protrusions (
Figure 13B)
The terminal ring tapers towards the tail. The subdorsal setae of the terminal ring are inserted at approximately two-thirds of the 17th main ring. Beyond this point, the ring curves ventrally and terminates in a short, uncovered spinneret (
Figure 13D–F). Small, rounded phasmata are located midway along the terminal ring.
The female is similar to the male in most respects. Like the male, the body gradually tapers towards both extremities and is composed of 17 main rings. The main rings are composed of large concretion particles (
Figure 9B,
Figure 14A and
Figure 15A,B). SEM observations reveal numerous tubercle-like protrusions across the interzone (
Figure 15C,D).
The setae taper towards an open tip, with faint wrinkles along most of their length (
Figure 15E–G). The terminal setae are distinctly elongated (
Figure 17A,F,G). The head appears globular in the lateral view but has a rounded triangular shape in the dorsal or ventral views. The oral ring appears more swollen and is viewed at a slightly oblique angle. In the SEM image of the swollen labial region (
Figure 16D), the eight projections and four longer setae-like structures blend together, forming a unified, crown-like arrangement (
Figure 9D,
Figure 14B and
Figure 16A–D). Notably, there are no cephalic setae, and no traces of them were found in their usual positions. The amphidial fovea are oval, nearly cover the entire head laterally, with a central amphidial pore.
The terminal ring bends ventrally and terminates in a short, uncovered spinneret with small, rounded phasmata (
Figure 9F,
Figure 14C and
Figure 17C–E).
There is a difference in the arrangement of the somatic setae. In a paratype female, the somatic setae are arranged as follows:
Subdorsal: 1, 3, 5, 7, 9, 11, 13, 16, 17 = 9.
Subventral: 2, 4, 6, 8, 10, 12, (-), 15 = 7.
In all paratype females, the arrangement deviates from the usual 17 main ring species pattern due to the absence of a pair of subventral setae on the 14th main ring (
Figure 15H).
The reproductive system is didelphic-amphidelphic, with both branches outstretched, each containing several immature oocytes. The two spermathecae, although not always distinctly differentiated, contain small, globular sperm. While the exact location of the vulva is difficult to determine under light microscopy, SEM observations revealed that the vulva is positioned between the 10th and 11th main rings (
Figure 17B).