Assemblage Distribution of the Larval and Juvenile Myctophid Fish in the Kuroshio Extension Region: Winter 2020
Abstract
1. Introduction
2. Materials and Methods
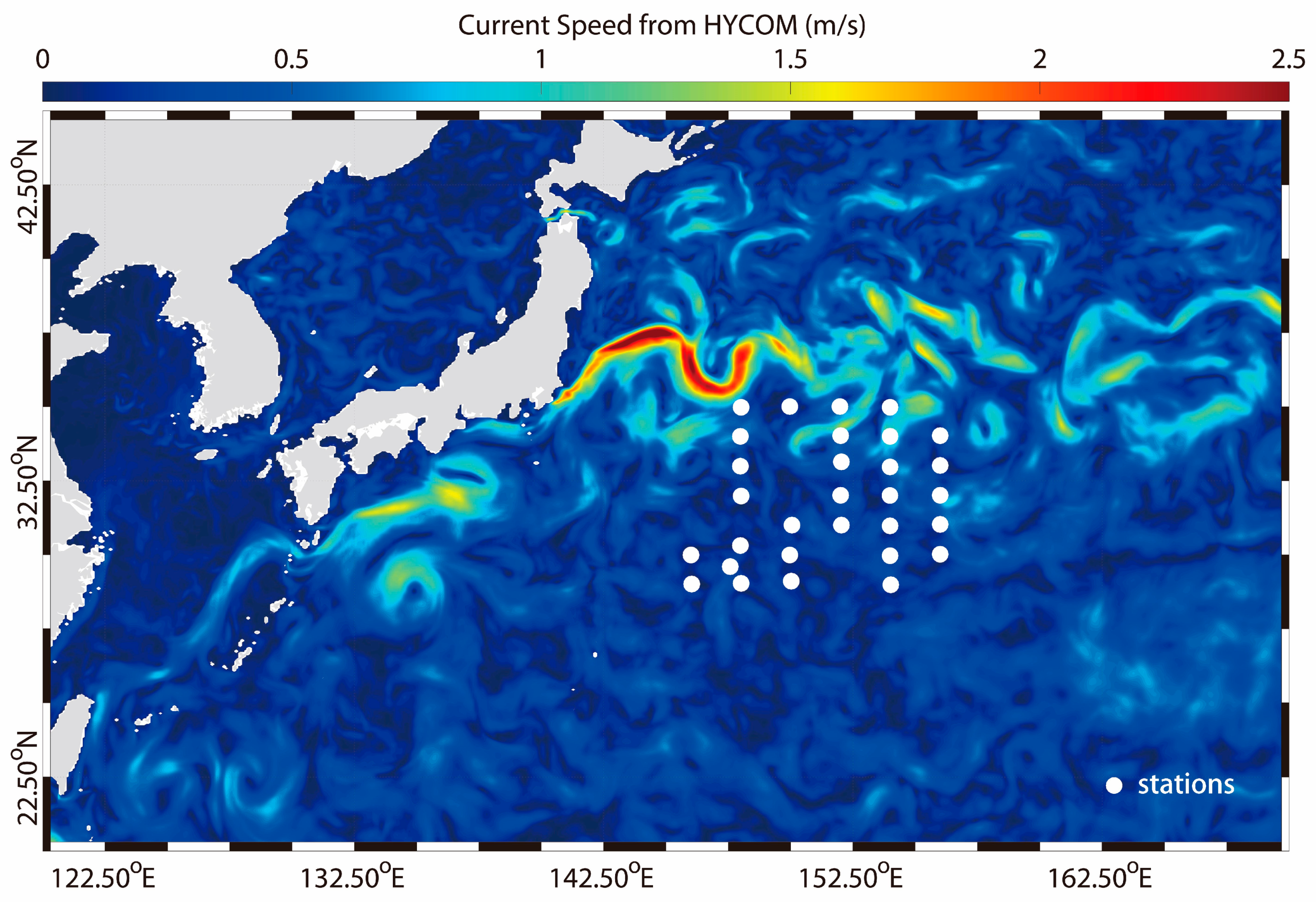
2.1. Sample Collection
2.2. Laboratory Analysis
2.3. Data Analysis
3. Results
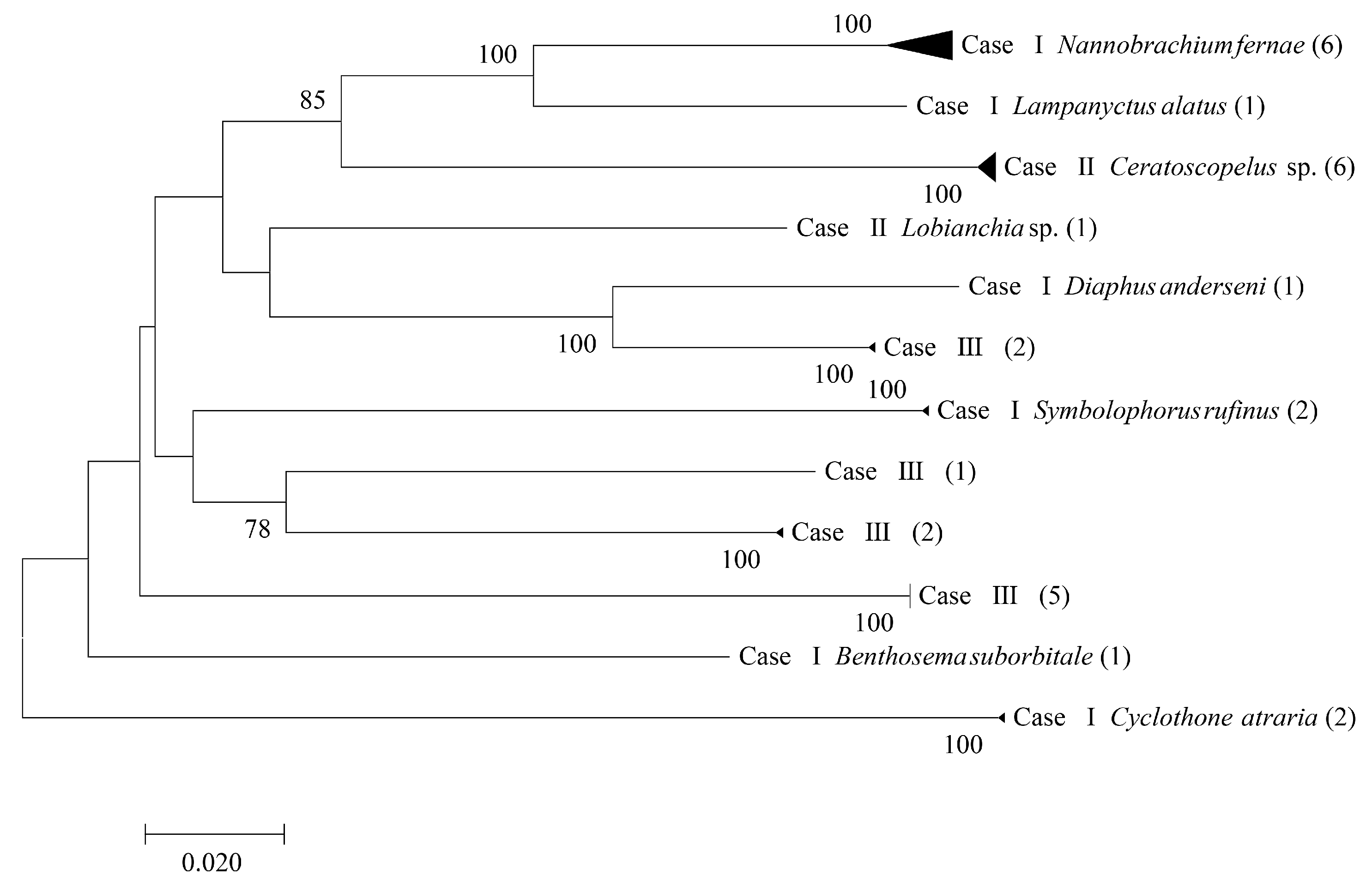
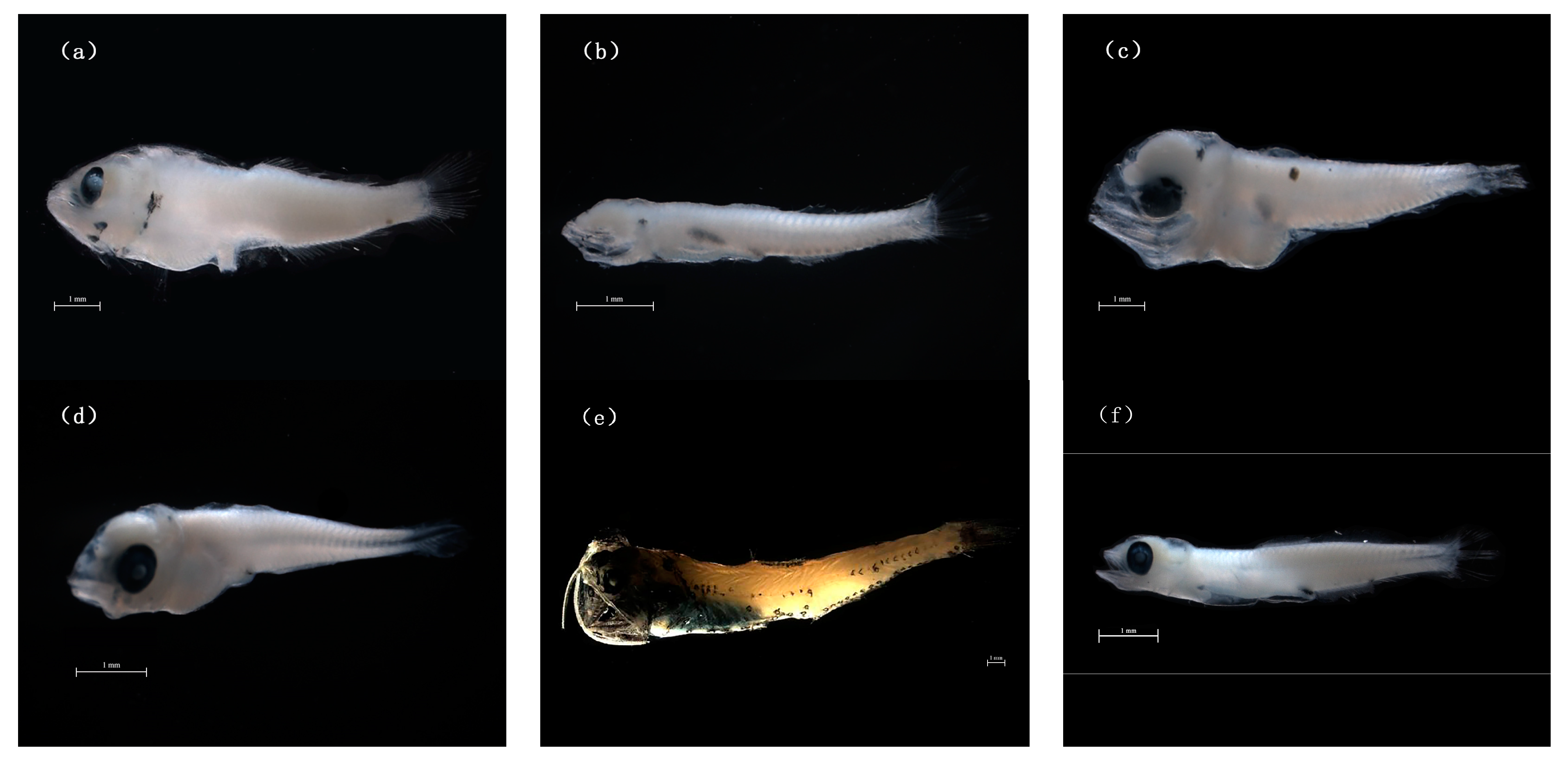
3.1. Species Identification and Abundance
3.2. Model Performance
3.3. Results of Model
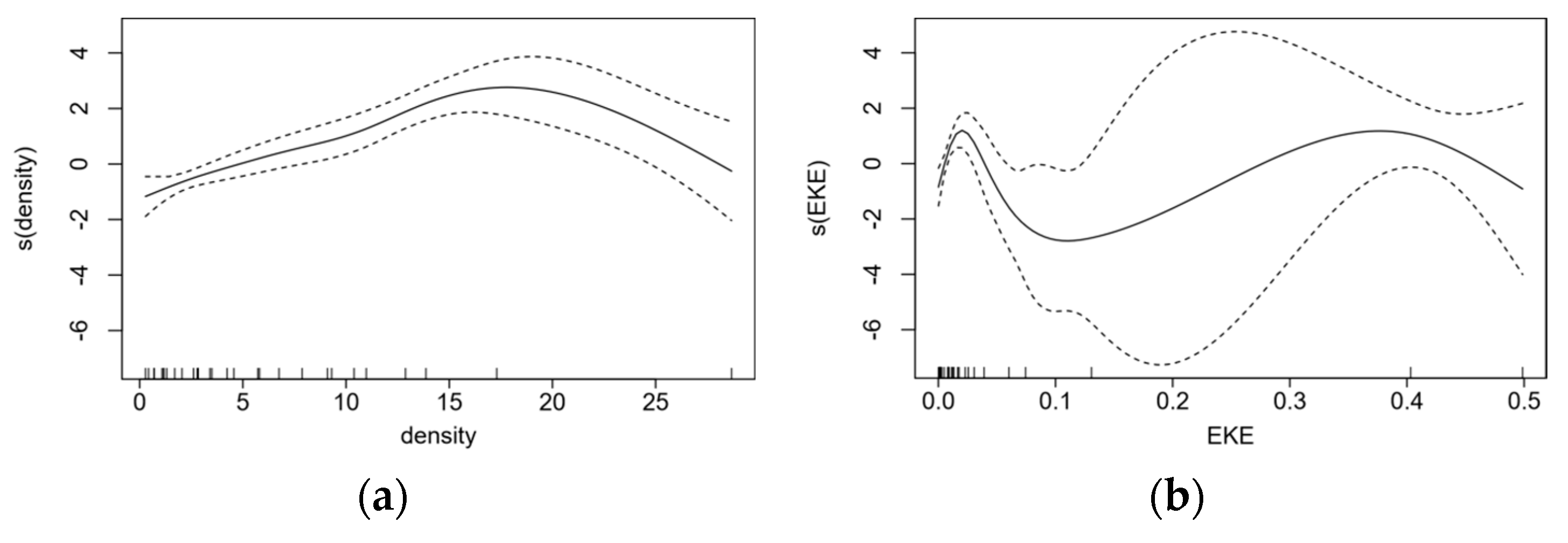
3.4. Relationship between Abundance and Environmental Factors
4. Discussion
4.1. Species Composition and Abundance
4.2. Effect of Biological Factors
4.3. Effect of Physical Factors
5. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Gjoesaeter, J.; Kawaguchi, K. A review of the world resources of mesopelagic fish. FAO Fish. Tech. Pap. 1980, 193, 1–151. [Google Scholar]
- Fricke, R.; Eschmeyer, W.N.; Van der Laan, R. Eschmeyer’s Catalog of Fishes: Genera, Species, References. 2023. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 14 April 2023).
- Collins, M.A.; Xavier, J.C.; Johnston, N.M.; North, A.W.; Enderlein, P.; Tarling, G.A.; Waluda, C.M.; Hawker, E.J.; Cunningham, N.J. Patterns in the distribution of myctophid fish in the northern Scotia Sea ecosystem. Polar. Biol. 2008, 31, 837–851. [Google Scholar] [CrossRef]
- Javadzadeh, N.; Valinassab, T.; Fatemi, M.R. Morphometric features of mesopelagic lanternfish, Diaphus garmani, from the Oman Sea, Iran: First record. World Appl. Sci. J. 2012, 17, 489–493. [Google Scholar]
- Kosenoka, N.S.; Chuchukalo, V.I.; Savinykh, V.F. The Characteristics of Feeding of Diaphus theta (Myctophidae) in the Northwestern Part of the Pacific Ocean in the Summer–Autumn Period. J. Ichthyol. 2006, 46, 606–612. [Google Scholar] [CrossRef]
- Smith, A.D.M.; Brown, C.J.; Bulman, C.M.; Fulton, E.A.; Johnson, P.; Kaplan, I.C.; Lozano-Montes, H.; Mackinson, S.; Marzloff, M.; Shannon, L.J.; et al. Impacts of fishing low–trophic level species on marine ecosystems. Science 2011, 333, 1147–1150. [Google Scholar] [CrossRef]
- Bulman, C.M.; He, X.; Koslow, J.A. Trophic ecology of the mid-slope demersal fish community off southern Tasmania, Australia. Mar. Freshwater. Res. 2002, 53, 59–72. [Google Scholar] [CrossRef]
- Sassa, C.; Kawaguchi, K.; Hirota, Y.; Ishida, M. Distribution patterns of larval myctophid fish assemblages in the subtropical–tropical waters of the western North Pacific. Fish. Oceanogr. 2004, 13, 267–282. [Google Scholar] [CrossRef]
- Sundby, S.; Kristiansen, T. The principles of buoyancy in marine fish eggs and their vertical distributions across the world oceans. PLoS ONE 2015, 10, e0138821. [Google Scholar] [CrossRef] [PubMed]
- Conley, W.J.; Gartner, J.V. Growth among larvae of lanternfishes (Teleostei: Myctophidae) from the Eastern Gulf of Mexico. Bull. Mar. Sci. 2009, 84, 123–135. [Google Scholar]
- Chambers, R.C.; Trippel, E.A. Early Life History and Recruitment in Fish Populations; Springer: Berlin/Heidelberg, Germany, 1997; pp. 515–549. [Google Scholar]
- Fielder, D.S.; Bardsley, W.J.; Allan, G.L.; Pankhurst, P.M. The effects of salinity and temperature on growth and survival of Australian snapper, Pagrus auratus larvae. Aquaculture 2005, 250, 201–214. [Google Scholar] [CrossRef]
- Hou, G.; Chen, Y.Y.; Wang, S.J.; Chen, W.T.; Zhang, H. Formalin-fixed fish larvae could be effectively identified by DNA barcodes: A case study on thousands of specimens in South China Sea. Front. Mar. Sci. 2021, 8, 634575. [Google Scholar] [CrossRef]
- Del Favero, J.M.; Katsuragawa, M.; Zani-Teixeira, M.; Turner, J.T. Spawning areas of engraulis anchoita in the southeastern brazilian bight during late-spring and early summer. Prog. Oceanogr. 2017, 153, 37–49. [Google Scholar] [CrossRef]
- Klimova, T.N.; Subbotin, A.A.; Vdodovich, I.V.; Zagorodnyaya, Y.A.; Podrezova, P.S.; Garbazei, O.A. Distribution of ichthyoplankton in relation to specifics of hydrological regime off the crimean coast (the black sea) in the spring–summer season 2017. J. Ichthyol. 2021, 61, 259–269. [Google Scholar] [CrossRef]
- Ottersen, G.; Planque, B.; Belgrano, A.; Post, E.; Reid, P.C.; Stenseth, N.C. Ecological effects of the North Atlantic Oscillation. Oecologia 2001, 128, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; John, M.S.; Lund, T.; Madsen, K.P. Identifying the effect of frontal regimes on condition in larval and juvenile sand lance (Ammodytes sp.): Utilisation of food web specific tracer lipids. Gastroenterological. Endosc. 1998, 50, 1344–1353. [Google Scholar]
- Townsend, D.W.; Pettigrew, N.R. The role of frontal currents in larval fish transport on Georges Bank. Deep-Sea Res. II 1996, 43, 1773–1792. [Google Scholar] [CrossRef]
- Turner, J.T.; Tester, P.A.; Conley, W.J. Zooplankton feeding ecology: Predation by the marine cyclopoid copepod Corycaeus amazonicus F. Dahl upon natural prey. J. Exp. Mar. Biol. Ecol. 1984, 84, 191–202. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Gonzalez-Nuevo, G.; Gonzalez-Pola, C.; Cabal, J. The ichthyoplankton assemblage and the environmental variables off the NW and N Iberian Peninsula coasts, in early spring. Cont. Shelf. Res. 2009, 29, 1145–1156. [Google Scholar] [CrossRef]
- Purcell, J.E. Predation on zooplankton by large jellyfish, Aurelia labiata, Cyanea capillata and Aequorea aequorea, in Prince William Sound, Alaska. Mar. Ecol. Prog. Ser. 2003, 246, 137–152. [Google Scholar] [CrossRef]
- Nagai, T.; Clayton, S. Nutrient interleaving below the mixed layer of the Kuroshio Extension Front. Ocean Dynam. 2017, 67, 1027–1046. [Google Scholar] [CrossRef]
- Vivier, F.; Kelly, K.A.; Thompson, L.A. Heat budget in the Kuroshio Extension region: 1993–99. J. Phys. Oceanogr. 2002, 32, 3436–3454. [Google Scholar] [CrossRef]
- Akihiro, S. Efficiency of water-column light utilization in the subarctic northwestern Pacific. Limnol. Oceanogr. 2000, 45, 982–987. [Google Scholar] [CrossRef]
- Isada, T.; Kuwata, A.; Saito, H.; Ono, T.; Ishii, M.; Yoshikawa-Inoue, H.; Suzuki, K. Photosynthetic features and primary productivity of phytoplankton in the Oyashio and Kuroshio–Oyashio transition regions of the northwest Pacific. J. Plankton Res. 2009, 31, 1009–1025. [Google Scholar] [CrossRef]
- Wang, T.; Chai, F.; Xing, X.; Ning, J.; Jiang, W.; Riser, S.C. Influence of multi-scale dynamics on the vertical nitrate distribution around the Kuroshio Extension: An investigation based on BGC-Argo and satellite data. Prog. Oceanogr. 2021, 193, 102543. [Google Scholar] [CrossRef]
- Chen, X.J.; Cao, J.; Chen, Y.; Liu, B.L.; Tian, S.Q. Effect of the Kuroshio on the spatial distribution of the red flying squid Ommastrephes bartramii in the Northwest Pacific Ocean. Bull. Mar. Sci. 2011, 88, 63–71. [Google Scholar] [CrossRef]
- Watanabe, Y. Latitudinal variation in the recruitment dynamics of small pelagic fishes in the western North Pacific. J. Sea Res. 2007, 58, 46–58. [Google Scholar] [CrossRef]
- Yasuda, I.; Watanabe, T. Chlorophyll a variation in the Kuroshio extension revealed with a mixed-layer tracking float: Implication on the long-term change of pacific saury (Cololabis saira). Fish. Oceanogr. 2007, 16, 482–488. [Google Scholar] [CrossRef]
- Ebisawa, Y.; Kinoshita, T. Relationship of surface water temperature in Boso-Sanriku area and recruitment per spawning biomass of the Japanese sardine. Bull. Ibaraki Prefect. Fish. Exp. Stn. 1998, 36, 49–55. [Google Scholar]
- Sassa, C.; Kawaguchi, K.; Taki, K. Larval mesopelagic fish assemblages in the Kuroshio-Oyashio transition region of the western North Pacific. Mar. Biol. 2007, 150, 1403–1415. [Google Scholar] [CrossRef]
- Sassa, C.; Kawaguchi, K.; Hirota, Y.; Ishida, M. Distribution depth of the transforming stage larvae of myctophid fishes in the subtropical–tropical waters of the western North Pacific. Deep-Sea Res. Part I 2007, 54, 2181–2193. [Google Scholar] [CrossRef]
- Collet, A.; Durand, J.D.; Desmarais, E.; Cerqueira, F.; Valade, P.; Ponton, D. DNA barcoding post-larvae can improve the knowledge about fish biodiversity: An example from La Reunion, SW Indian Ocean. Mitochondrial DNA A 2018, 29, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Burrows, M.; Browning, J.; Bønnelycke, E.M.; Zhang, Y.J.; Hu, C.M.; Armenteros, M.; Murawski, S.; Peebles, E.; Breitbart, M. DNA barcoding of fish eggs collected off northwestern Cuba and across the Florida Straits demonstrates egg transport by mesoscale eddies. Fish. Oceanogr. 2020, 29, 340–348. [Google Scholar]
- Batta-Lona, P.G.; Galindo-Sánchez, C.E.; Arteaga, M.C.; Robles-Flores, J.; Jiménez-Rosenberg, S.P.A. DNA barcoding and morphological taxonomy: Identification of lanternfish (Myctophidae) larvae in the Gulf of Mexico. Mitochondrial DNA A 2019, 30, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, A.M.; Cuttitta, A.; Sardella, A.; Musco, M.; Maggio, T.; Patti, B.; Mazzola, S.; Ferrito, V. DNA barcoding and COI sequence variation in Mediterranean lanternfishes larvae. Hydrobiologia 2015, 749, 155–167. [Google Scholar] [CrossRef]
- Ayala, D.; Riemann, L.; Munk, P. Species composition and diversity of fish larvae in the subtropical convergence zone of the sargasso sea from morphology and DNA barcoding. Fish. Oceanogr. 2016, 25, 85–104. [Google Scholar] [CrossRef]
- Hastie, T.J. Generalized additive models. In Statistical Models in S; Routledge: Abingdon, UK, 2017; pp. 249–307. [Google Scholar]
- Hou, G.; Wang, J.; Chen, Z.; Zhou, J.; Huang, W.; Zhang, H. Molecular and morphological identification and seasonal distribution of eggs of four Decapterus fish species in the northern South China Sea: A key to conservation of spawning ground. Front. Mar. Sci. 2020, 7, 970. [Google Scholar] [CrossRef]
- Hubert, N.; Espiau, B.; Meyer, C.; Planes, S. Identifying the ichthyoplankton of a coral reef using DNA barcodes. Mol. Ecol. Resour. 2015, 15, 57–67. [Google Scholar] [CrossRef]
- Li, L.F.; Zhong, J.S.; Jiao, Z.; Rao, Y.Y.; Yang, C.H.; Liu, H. Vertical distribution and changes during day and night of Coilia nasus larvae and juveniles depending on flood and ebb tide in southern branch of Yangtze River estuary. J. Shanghai Ocean Univ. 2020, 29, 74–82. [Google Scholar]
- Munk, P.; Cardinale, M.; Casini, M.; Rudolphi, A. The community structure of over-wintering larval and small juvenile fish in a large estuary. Estuar. Coast. Shelf Sci. 2014, 139, 27–39. [Google Scholar] [CrossRef]
- Long, X.; Wan, R.; Li, Z.; Ren, Y.P.; Song, P.B.; Tian, Y.J.; Xu, B.D.; Xue, Y. Spatio-temporal distribution of Konosirus punctatus spawning and nursing ground in the South Yellow Sea. Acta Oceanol. Sin. 2021, 40, 133–144. [Google Scholar] [CrossRef]
- Sassa, C.; Kawaguchi, K. Larval feeding habits of Diaphus theta, Protomyctophum thompsoni, and Tarletonbeania taylori (Pisces: Myctophidae) in the transition region of the western North Pacific. Mar. Ecol. Prog. Ser. 2005, 298, 261–276. [Google Scholar] [CrossRef]
- Rodriguez-Grana, L.; Castro, L.; Loureiro, M.; Gonzalez, H.E.; Calliari, D. Feeding ecology of dominant larval myctophids in an upwelling area of the humboldt current. Mar. Ecol. Prog. 2005, 290, 119–134. [Google Scholar] [CrossRef]
- Jia, F.; Wu, L.; Qiu, B. Seasonal Modulation of Eddy Kinetic Energy and Its Formation Mechanism in the Southeast Indian Ocean. J. Phys. Oceanogr. 2011, 41, 657–665. [Google Scholar] [CrossRef]
- Shono, H. Application of the tweedie distribution to zero-catch data in cpue analysis. Fish. Res. 2008, 93, 154–162. [Google Scholar] [CrossRef]
- Watanabe, H.; Kawaguchi, K. Decadal change in abundance of surface migratory myctophid fishes in the Kuroshio region from 1957 to 1994. Fish. Oceanogr. 2003, 12, 100–111. [Google Scholar] [CrossRef]
- Sassa, C.; Takahashi, M. Comparative larval growth and mortality of mesopelagic fishes and their predatory impact on zooplankton in the Kuroshio region. Deep-Sea Res. Part I 2018, 131, 121–132. [Google Scholar] [CrossRef]
- Flores, E.A.; Castro, L.R.; Narváez, D.A.; Lillo, S.; Balbon, F.; Osorio-Zúñiga, F. Inter-annual and seasonal variations in the outer and inner sea spawning zones of southern hake, Merluccius australis, inferred from early life stages distributions in Chilean Patagonia. Prog. Oceanogr. 2019, 171, 93–107. [Google Scholar] [CrossRef]
- Moku, M.; Tsuda, A.; Kawaguchi, K. Spawning season and migration of the myctophid fish Diaphus theta in the western North Pacific. Ichthyol. Res. 2003, 50, 0052–0058. [Google Scholar] [CrossRef]
- Sassa, C.; Kawaguchi, K.; Oozeki, Y.; Sugisaki, H. Distribution patterns of larval myctophid fishes in the transition region of the western North Pacific. Mar. Biol. 2004, 144, 417–428. [Google Scholar] [CrossRef]
- Sassa, C.; Hirota, Y. Seasonal occurrence of mesopelagic fish larvae on the onshore side of the Kuroshio off southern Japan. Deep-Sea Res. Part I 2013, 81, 49–61. [Google Scholar] [CrossRef]
- Landaeta, M.F.; Bustos, C.A.; Contreras, J.E.; Salas-Berríos, F.; Palacios-Fuentes, P.; Alvarado-Niño, M.; Letelier, J.; Balbontín, F. Larval fish feeding ecology, growth and mortality from two basins with contrasting environmental conditions of an inner sea of northern Patagonia, Chile. Mar. Environ. Res. 2015, 106, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Ojaveer, H.; Lankov, A.; Teder, M.; Simm, M.; Klais, R. Feeding patterns of dominating small pelagic fish in the Gulf of Riga, Baltic Sea. Hydrobiologia 2017, 792, 331–344. [Google Scholar] [CrossRef]
- Bernal, A.; Olivar, M.P.; Beckley, L.E. Dietary composition of myctophid larvae off Western Australia. Deep-Sea Res. Part II 2020, 179, 104841. [Google Scholar] [CrossRef]
- Bernal, A.; Castro, L.R.; Soto, S.; Cubillos, L.A. Ichthyoplankton distribution and feeding habits of fish larvae at the inshore zone of northern Patagonia, Chile. Mar. Biodivers. 2020, 50, 56. [Google Scholar] [CrossRef]
- Contreras-Catala, F.; Sanchez-Velasco, L.; Lavín, M.F.; Victor, M.G. Three-dimensional distribution of larval fish assemblages in an anticyclonic eddy in a semi-enclosed sea (Gulf of California). J. Plankton Res. 2012, 34, 548–562. [Google Scholar] [CrossRef]
- Vélez, J.A.; Watson, W.; Arntz, W.; Wolff, M.; Schnack-Schiel, S.B. Larval fish assemblages in Independencia Bay, Pisco, Peru: Temporal and spatial relationships. Mar. Biol. 2005, 147, 77–91. [Google Scholar] [CrossRef]
- Sánchez-Hernández, J.; Gabler, H.M.; Amundsen, P.A. Prey diversity as a driver of resource partitioning between river-dwelling fish species. Ecol. Evol. 2017, 7, 2058–2068. [Google Scholar] [CrossRef]
- Lobel, P.S.; Robinson, A.R. Larval fishes and zooplankton in a cyclonic eddy in Hawaiian waters. J. Plankton Res. 1988, 10, 1209–1223. [Google Scholar] [CrossRef]
- Wu, C.Y.; Wang, X.G.; Zhong, J.S.; Ju, J.L.; Li, C.H. Spatial Patterns of Larval and Juvenile Fish Assemblages in an Eddy Area in the Western South China Sea. Front. Mar. Sci. 2022, 9, 832817. [Google Scholar] [CrossRef]
- Shi, Z.H.; Xie, M.M.; Peng, S.M.; Zhang, C.J.; Gao, Q.X. Effects of temperature stress on activities of digestive enzymes and serum biochemical indices of Pampus argenteus juveniles. Prog. Fish. Sci. 2016, 37, 30–36. [Google Scholar]
- Fu, C.; Cao, Z.D.; Fu, S.J. The influence of temperature and starvation on resting metabolic rate and spontaneous movement of juvenile Cyprinus carpio. Chin. J. Zool. 2012, 47, 85–90. [Google Scholar]
- Nonaka, M.; Xie, S.P. Covariations of sea surface temperature and wind over the Kuroshio and its extension: Evidence for ocean-to-atmosphere feedback. J. Clim. 2003, 16, 1404–1413. [Google Scholar] [CrossRef]

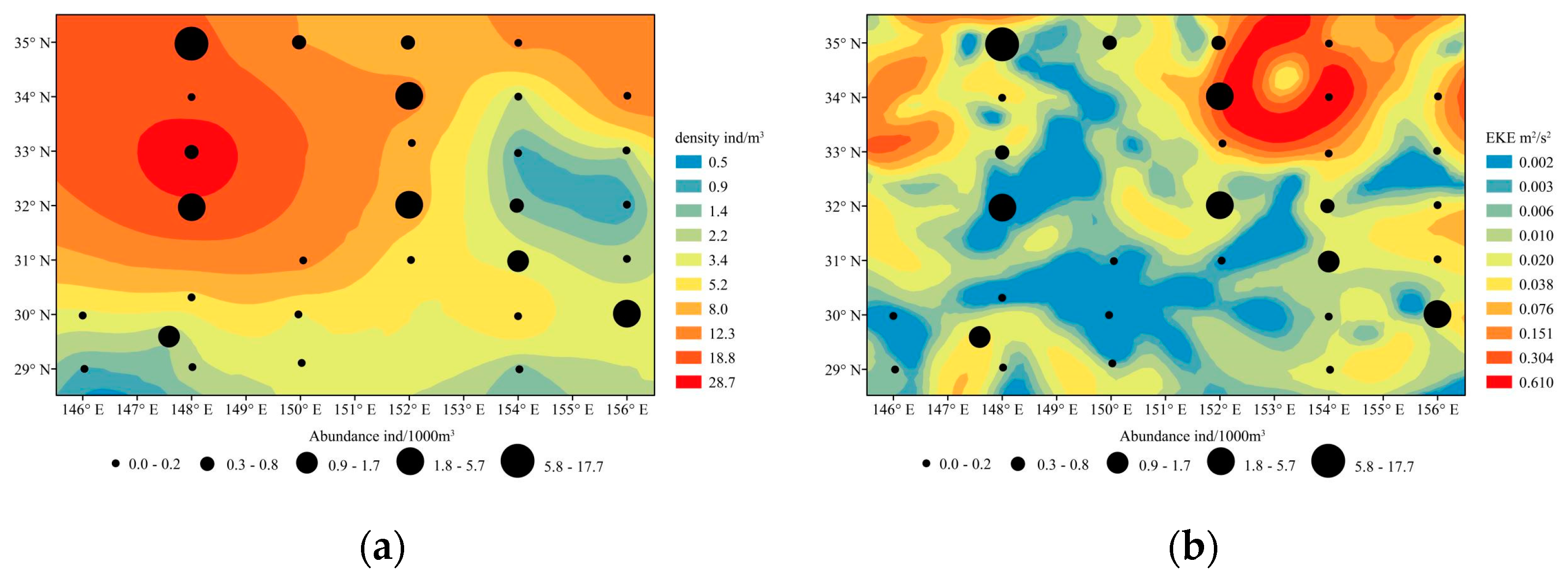
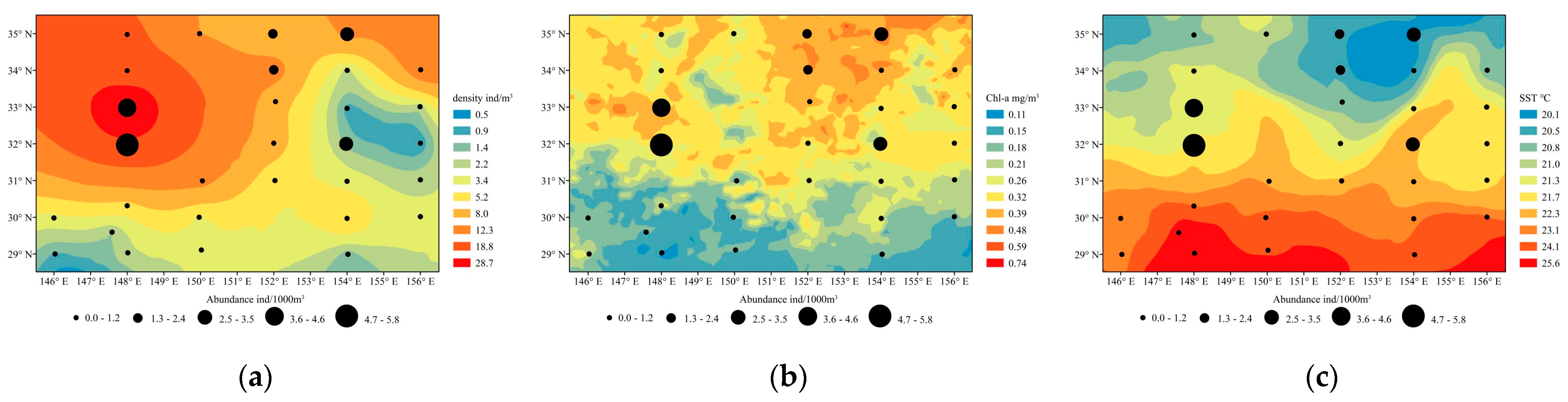
| Explanatory Variable | SST | SLA | SSS | Chl−a | EKE | Density |
|---|---|---|---|---|---|---|
| VIF | 2.20 | 1.15 | 1.11 | 1.96 | 1.30 | 1.16 |
| Model | Explanatory Variable | AIC Value | Cumulative Deviance Explained | Deviance Explanation of Each Factor | p |
|---|---|---|---|---|---|
| Horizontal-GAM | +density | 99.42 | 45.1% | 45.1% | <0.001 *** |
| +EKE | 80.69 | 69.4% | 24.3% | 0.005 ** | |
| Vertical- GAM | +density | 60.54 | 22.1% | 22.1% | <0.001 *** |
| +SST | 38.75 | 72.4% | 50.3% | <0.001 *** | |
| +Chl−a | 32.48 | 81.2% | 8.8% | 0.04 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Liu, B.; Cao, Y. Assemblage Distribution of the Larval and Juvenile Myctophid Fish in the Kuroshio Extension Region: Winter 2020. J. Mar. Sci. Eng. 2023, 11, 898. https://doi.org/10.3390/jmse11050898
Xu H, Liu B, Cao Y. Assemblage Distribution of the Larval and Juvenile Myctophid Fish in the Kuroshio Extension Region: Winter 2020. Journal of Marine Science and Engineering. 2023; 11(5):898. https://doi.org/10.3390/jmse11050898
Chicago/Turabian StyleXu, Hao, Bilin Liu, and Yangming Cao. 2023. "Assemblage Distribution of the Larval and Juvenile Myctophid Fish in the Kuroshio Extension Region: Winter 2020" Journal of Marine Science and Engineering 11, no. 5: 898. https://doi.org/10.3390/jmse11050898
APA StyleXu, H., Liu, B., & Cao, Y. (2023). Assemblage Distribution of the Larval and Juvenile Myctophid Fish in the Kuroshio Extension Region: Winter 2020. Journal of Marine Science and Engineering, 11(5), 898. https://doi.org/10.3390/jmse11050898








