Abstract
Diatoms of the genus Pseudo-nitzschia are producers of the neurotoxin domoic acid that causes serious damage to marine fauna and aquaculture farms. These microalgae are known as the most abundant group of toxic phytoplankton in Peter the Great Bay, Northwestern Sea of Japan, during the bloom season, which, as was previously reported for members of this group in the study region, lasts in the autumn months. Autumnal blooms of toxic diatoms Pseudo-nitzschia spp. were studied in the period from 2017 to 2022 in Ussuri Bay, the largest body of water in Peter the Great Bay, which harbors numerous recreational area and aquaculture farms. As a result, the following changes in the composition of bloom-forming species were recorded: blooms were caused by the Pseudo-nitzschia delicatissima group in the period from 2017 to 2020 and by P. multistriata in 2021–2022. An assumption has been made that one of the factors responsible for blooms of P. multistriata, known as one of the most widespread species in Asian warm-water areas, is an abnormally high water temperature in the autumn of 2021 in the study area.
Keywords:
Pseudo-nitzschia; bloom; domoic acid; temporal changes; warming; Northwestern Sea of Japan 1. Introduction
Several marine diatom species of the genus Pseudo-nitzschia H. Peragallo (1900) can pose serious environmental and economic/health problems due to their ability to produce the neurotoxin domoic acid (DA). This toxin belongs to the class of excitatory amino acids and is known as an agonist of ionotropic glutamate receptors in the mammalian central nervous system [1,2]. As a result of the transfer of DA up the food chain from diatoms to edible mollusks [3,4,5,6], it may have negative health effects, in particular Amnesic Shellfish Poisoning (ASP), on humans consuming edible mollusks contaminated by DA. It has also been reported as a causative agent of numerous poisoning events and mortalities of birds and marine mammals [3,4,5,6]. In addition to the mass poisoning of wild marine animals, DA can cause significant economic damage to aquaculture farms [5,6]. Studies of the temporal dynamics of the Pseudo-nitzschia species associated with environmental or biotic factors or climate change events are carried out in highly productive waters or areas adjacent to aquaculture farms and recreational zones worldwide [7,8,9,10,11]. Such studies are crucial not only for interpreting the temporal patterns of the population dynamics of Pseudo-nitzschia but also for understanding the toxic events and their economic and social effects.
Peter the Great Bay, including Ussuri Bay, is considered one of Russia’s regions with the highest productive waters, where the greatest species diversity and richness have been recorded [12]. The locally high species diversity is explained by the combined influence of the branches of the warm Tsushima and cold Liman currents and by the co-occurrence of arctic–boreal and tropical phytoplankton species [13]. Diatoms of the genus Pseudo-nitzschia are known as the most abundant group of toxic phytoplankton in Peter the Great Bay, reaching 75–98% of the total phytoplankton density in the bloom season, which, as was established earlier, lasts in the autumn months [14]. Although bloom events caused by Pseudo-nitzschia spp. have regularly been reported for the Northwestern Sea of Japan since 1992 until the present time [14,15,16,17,18], data on temporal variations in Pseudo-nitzschia abundance during bloom events in the region are available only for Amur Bay and cover the period from 1992 to 2015 [18]. According to them, the highly toxic species Pseudo-nitzschia multiseries was found in Peter the Great Bay at extremely high concentrations in the 1990s, but after 2002 its concentration decreased sharply, and in 2005–2015, the toxic Pseudo-nitzschia calliantha and P. multistriata dominated the plankton [18]. However, there is a lack of information about the population dynamics of the bloom-forming Pseudo-nitzschia spp. in the Northwestern Sea of Japan after 2015 in the scientific literature. To date, no studies on temporal variations in the composition of the bloom-forming Pseudo-nitzschia species have been carried out in Ussuri Bay, the largest body of water in Peter the Great Bay, Sea of Japan, which has commercial and recreational significance.
The objectives of this study were as follows: (1) assess interannual variations in the composition and density of the bloom-forming Pseudo-nitzschia groups in the surface waters of Ussuri Bay off the city of Vladivostok, Northwestern Sea of Japan, during the period from 2017 to 2022; (2) attempt to identify relationships between temporal variations in species composition of the bloom-forming Pseudo-nitzschia and environmental changes in the region.
2. Materials and Methods
2.1. Study Area, Sampling, and Processing
Peter the Great Bay is the largest embayment (42°17′–42°40′ N, 130°41′–133°02′ E) in the Northwestern Sea of Japan (Figure 1). It is bounded from the open sea by an imaginary line from the Tumen River estuary to Cape Povorotny and includes two large secondary bays, Amur and Ussuri. Ussuri Bay is the largest body of water in Peter the Great Bay, located in its northeastern part and bounded by the Muravyov-Amursky Peninsula and the islands of the Empress Eugenie Archipelago (Russky, Popov, Reyneke, etc.) on the west and by Askold Island on the east. Various facilities of ship repair, building, fuel, and agriculture industries are operated on the shores of Ussuri Bay. These waters are used for coastal fishing and aquaculture activities. In the northern, upper part of the bay, there is also a large recreation zone [19].
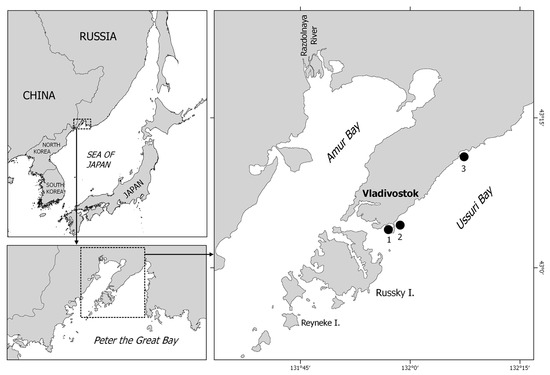
Figure 1.
Location of the study area in the Northwestern Sea of Japan and position of the sampling stations (1–3) in Ussuri Bay.
The oceanographic regime of the Peter the Great Bay waters is determined by the following major factors: monsoon climate, daily and annual fluctuations in water temperature associated with the general climatic conditions of the region, free water exchange with the open part of the Sea of Japan; effect of river discharge; wind-induced inflowing and outflowing currents that raise or lower the water level in the bay, which affects the pattern and intensity of horizontal movement of waters; coast orography; and the degree of isolation of secondary coves and bays [20]. The trend towards an increase in temperature in the surface layer of the Sea of Japan and in the upper layer of intermediate waters has been recorded over the past few decades [21,22,23]. Furthermore, the weakening of monsoon winds has been indicated as a direct factor determining the present-day scale of climate changes off the Northwestern Sea of Japan coast [21]. Detailed oceanographic characteristics of the sampling area and an analysis of long-term climatic changes in the region are provided in some publications elsewhere [20,22,24,25].
The study used materials collected at three stations in the southwestern near-shore part of Ussuri Bay off Vladivostok during plankton surveys carried out in the autumn seasons from 2017 to 2022. An earlier study of the dynamics of the Pseudo-nitzschia spp. density in the Northwestern Sea of Japan showed high cell densities in autumn, which can be considered as the typical season of Pseudo-nitzschia bloom events [14]. For this reason, we conducted long-term observations of phytoplankton in the autumn season. Thus, phytoplankton samples were collected from the surface horizon, as a rule, from September to December twice a month in order to estimate the cell density during and after the Pseudo-nitzschia bloom events (Table 1). Samples were collected with a 4-L Niskin bottle. A sample volume of 1 L was concentrated by precipitation [26] and fixed with Utermöhl’s solution [27], an aqueous solution containing iodine, potassium iodine, and sodium acetate.

Table 1.
Dates, locations, and number of samples of the sampling stations 1–3 in Ussuri Bay, Northwestern Sea of Japan. See Figure 1 for the positions of stations.
Species were identified under an Olympus BX-41 LM (Tokyo, Japan) with bright-field optics and a Sigma 300 VP transmission electron microscope (TEM) (Carl Zeiss, Cambridge, UK).
The density of Pseudo-nitzschia cells was estimated using light microscopy (LM) by counting in a 1-mL Sedgewick–Rafter chamber. A minimum of 100 cells of Pseudo-nitzschia were counted to estimate species density with an accuracy of ±20% [28]. Based on the previously conducted monitoring of phytoplankton in coastal waters of the Northwestern Sea of Japan, we assumed a concentration >1 × 105 cells L−1 to be a provisional limit (a limit level), above which any increase in the density of Pseudo-nitzschia species would be considered as a fact of bloom [14].
For TEM observations specimens from the samples with abundant Pseudo-nitzschia species were prepared to examine the valve and gridle-bands structure by acidifying with a 40% H2SO4 solution, followed by rinsing by centrifugation several times in deionized water. A drop of the rinsed specimen was then placed on a Formvar-coated grid and air-dried before examination under TEM. Thus, the 42 samples with a high density of Pseudo-nitzschia spp. (>1 × 104 cells L−1) were examined using TEM. In LM, Pseudo-nitzschia cells were examined for the shape of cells and cell length (Table 2). In TEM, Pseudo-nitzschia cells were examined for cell width, density of valve poroids, valve striae and fibulae, and density of valvocopula striae (Table 2). The TEM studies were carried out at the Far Eastern Center of Electron Microscopy, A.V. Zhirmunsky National Scientific Center of Marine Biology, Far Eastern Branch, Russian Academy of Sciences.

Table 2.
Morphometric and morphological data and identification of Pseudo-nitzschia in Ussuri Bay, Northwestern Sea of Japan.
2.2. Data Analysis
To identify statistically significant differences in the density of Pseudo-nitzschia between different years, we used the nonparametric Kruskal–Wallis test (non-normal data distribution, Shapiro–Wilk test; p < 0.05). In addition, relationships between the Pseudo-nitzschia density in 2017–2022 and sea surface temperature (SST) were assessed using Spearman’s rank correlation analysis of the whole data set. Statistical data processing was performed using the Statistica 7 software (StatSoft Inc., Tulsa, OK, USA).
The similarity of species compositions and densities among samples collected during 2017–2022 was analyzed using the Bray–Curtis similarity coefficient (similarity matrix) based on abundance data for different Pseudo-nitzschia groups [29]. Nonmetric multidimensional scaling (NMDS) ordination was performed for a graphical representation of the complete data set from the sampling period of 2017–2022. As a result of NMDS, three groups (clusters) were distinguished that differed in Pseudo-nitzschia composition and density. Abnormal warming of surface waters has been recorded from Peter the Great Bay in the autumn of 2021. Therefore, we performed a one-way ANOSIM to compare their density between two periods: before the abnormal warming of surface waters (2017–2020) and after the warming (2021–2022). A square-root transformation of the data for ANOSIM was performed. The NMDS and ANOSIM analyses were carried out using the PRIMER 6.1.18 software (Primer-E Ltd., Plymouth, UK) [30,31].
3. Results
3.1. Composition of Pseudo-nitzschia spp. Bloom Events
Based on TEM examination of the samples with a high density of Pseudo-nitzschia spp. (>1 × 104 cells L−1), a total of four species that caused bloom events in the area of our study were identified: P. calliantha Lundholm, Moestrup et Hasle, P. multistriata (Takano) Takano, P. pungens (Grunow ex Cleve) Hasle, and P. hasleana Lundholm. Their identification was confirmed based on electron microscopy and morphometric measurements (Table 2, Figure 2).
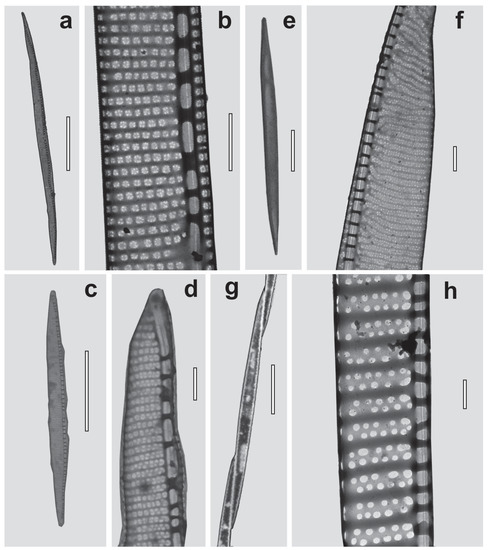
Figure 2.
Transmission electron microscopy images of bloom-forming Pseudo-nitzschia species from Ussuri Bay. Pseudo-nitzschia calliantha: (a)—whole valve; (b)—fragment of the valve showing striae and fibulae. Pseudo-nitzschia hasleana: (c)—valve view; (d)—a part of the valve showing apex. Pseudo-nitzschia multistriata: (e)—a whole valve; (f)—part of the valve. Pseudo-nitzschia pungens: (g)—one cell in a colony; (h)—a part of the valve showing striae and fibulae. Scale bars = 10 μm (a,c,e,g); 1 μm (b,d,f,h).
Based on LM data, the abundance of three Pseudo-nitzschia groups was estimated: (1) Pseudo-nitzschia multistriata (sigmoid cells with a cell width > 3 μm); (2) Pseudo-nitzschia delicatissima group including P. calliantha and P. hasleana (linear-lanceolate cells with a cell width < 2.5 μm); (3) Pseudo-nitzschia seriata group including P. pungens (lanceolate cells with a cell width > 3 μm).
During the five years of the study, seven bloom events caused by diatoms of the genus Pseudo-nitzschia (exceeding a cell concentration level of 105 cells L−1) were registered (Table 3).

Table 3.
Pseudo-nitzschia bloom events and sea surface temperature (SST) in Ussuri Bay the Northwestern Sea of Japan during 2017–2022.
During the study period, bloom events caused by the P. delicatissima group were recorded twice. The most intense bloom of the P. delicatissima group (2.3 × 105 cells L−1, or 97% of the total phytoplankton density), was observed in November 2019 at stn. 3 at an SST of 10 °C (Table 3, Figure 3). The highest density of P. multistriata in the autumn of 2019 reached 1.3 × 103 cells L−1. In early October 2020, the P. delicatissima group (1.6 × 105 cells L−1) caused a bloom event at stn. 2 again at an SST of 16 °C. Along with this group, the P. multistriata (3.1 × 104 cells L−1) and P. seriata group (1 × 103 cells L−1) also reached relatively high densities in the phytoplankton. During this period, the total density of Pseudo-nitzschia spp. constituted 71% of the total phytoplankton density. All bloom events in 2021–2022 were caused by P. multistriata. The most intense bloom event caused by this species (2 × 105 cells L−1, or 77% of the total phytoplankton density) was observed in the first ten days of November 2021 at stn. 2 at an SST of 11 °C. Along with this bloom event, the density of P. multistriata exceeded the limit level of 1 × 105 cells L−1 in four more cases: 1.7 × 105 cells L−1 in the third ten days of October 2021 at stn. 2; 1.1 × 105 cells L−1 in the second ten days of November 2021 at stn. 2; 1.2 × 105 cells L−1 in mid-September 2022 at stn. 1; and 1.7 × 105 cells L−1 in mid-September 2022 at stn. 2 (Table 3).
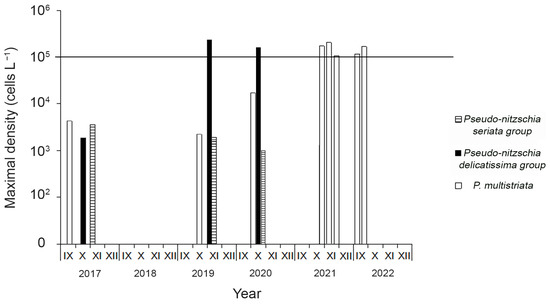
Figure 3.
The dominant Pseudo-nitzschia groups during their bloom events in Ussuri Bay in 2017–2022. The bold line indicates a provisional limit level of cell density above which values are considered as a fact of bloom.
Thus, the highest densities of Pseudo-nitzschia in Ussuri Bay in 2019–2020 and 2021–2022 were determined by blooms of the P. delicatissima group and P. multistriata, respectively (Figure 3). On the other hand, in the autumn seasons of 2017, 2019, and 2020, the P. seriata group was almost permanently present in the phytoplankton at low densities (up to 3.6 × 103 cells L−1).
Cell concentrations of P. multistriata increased in the autumns of 2021 and 2022 compared to the respective data in the previous years, while the highest density values of the P. delicatissima group were recorded in the autumn of 2019, and in 2021–2022, they decreased significantly (Kruskal–Wallis test; p < 0.05) (Figure 4). No statistically significant interannual differences in P. seriata-group density were found (Kruskal–Wallis test; p > 0.05).
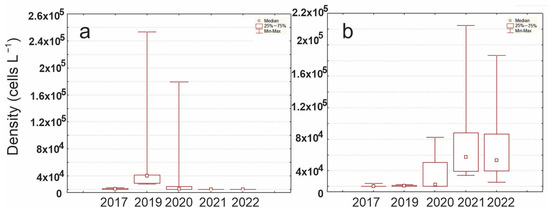
Figure 4.
Interannual variations in P. delicatissima group (a) and P. multistriata (b) densities during 2017–2022.
As the data obtained show, in the autumn seasons of 2021 and 2022, there was a shift in the composition of the dominant groups: blooms of the P. delicatissima group were not observed, and P. multistriata became the causative organism of Pseudo-nitzschia bloom events.
A correlation analysis was performed to test the relationship between the density of the bloom-causing Pseudo-nitzschia groups and SST (Table 4).

Table 4.
Spearman’s coefficient of correlation (r) between the cell density of the bloom-forming Pseudo-nitzschia groups and SST based on data of phytoplankton sampling (n = 91) in Ussuri Bay in the autumn seasons of 2017–2022. The statistically significant result (p < 0.05) is highlighted in bold.
Thus, a moderate positive correlation was recorded between the P. multistriata density and SST (r = 0.43, p < 0.05). No relationship was found between SST and the densities of the P. delicatissima group and P. seriata group (Table 4).
3.2. Interannual Changes in Composition of the Bloom-Forming Pseudo-nitzschia
Phytoplankton samples were analyzed to assess interannual variations in the composition of the bloom-forming Pseudo-nitzschia groups. The NMDS ordination plot graphically represents the changes in the composition of Pseudo-nitzschia groups during their bloom events in 2017–2022 (Figure 5a,b). Three clusters (A–C) based on dominant Pseudo-nitzschia-group density were identified (Figure 5a). The overlay of the NMDS plot with the cluster dendrogram similarity lines indicated the respective maximum boundary values for the discrimination of clusters. The maximum similarity between cluster A and cluster B was 12% (Bray–Curtis similarity). Cluster C most distinctly differed from clusters A and B: the relative similarity between cluster C and the group of clusters A and B was 3%. The obtained clusters are determined by densities of P. multistriata, P. delicatissima group, and P. seriata group. Cluster C, formed mainly by P. multistriata-dominated collections of 2021–2022, differed from cluster A, formed predominantly by the P. delicatissima group-dominated collections of 2019, as well as from cluster B, formed predominantly by the 2017 and 2020 collections, where both the P. delicatissima group and the P. seriata group were found (Figure 5a,b). The relatively low density of the P. delicatissima group (5–6 × 104 cells L−1) was found in the 2020 collections included in Cluster C, and the relatively low density of P. multistriata (3.1 × 104 cells L−1) was found in October 2020, when blooms caused by the P. delicatissima group occurred (Cluster A). Five bloom events of P. multistriata with maximum densities (2 × 105 cells L−1) were recorded in 2021–2022, while the densities of the P. delicatissima group and the P. seriata group in the collections of 2017 that formed Cluster B were lower than 2.5 × 104 cells (Figure 5a,b).
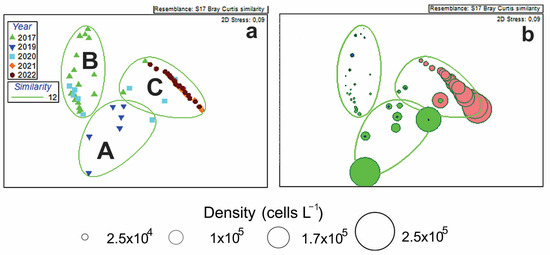
Figure 5.
Nonmetric multidimensional scaling (NMDS) ordination analysis of cell density data of bloom-forming Pseudo-nitzschia groups during 2017–2022 (a) showing three clusters (A–C), and densities of P. multistriata ((b), red circles), P. delicatissima group ((b), green circles), and P. seriata group ((b), blue circles) are identified for clusters.
A one-way ANOSIM was performed to compare the density of Pseudo-nitzschia species between two periods: before the abnormal warming of surface waters (2017–2020) and after the warming (2021–2022). There was a significant difference between the samples collected before and after the warming (Global R: 0.713, significance level: 0.1%). Our analyses supported the conclusion about the interannual changes in the composition of the bloom-forming Pseudo-nitzschia groups: P. delicatissima group, dominated in 2019–2020 (before abnormal warming of surface waters); in 2021–2022 (after the warming), P. multistriata became the dominant species.
4. Discussion
We identified four Pseudo-nitzschia species based on TEM investigation. Their morphological and morphometric characteristics matched those previously reported [32,33,34,35] and were consistent with the data of a morphological study of Pseudo-nitzschia performed recently in Russian waters of the Sea of Japan [36].
Gradual shifts in the composition of dominant species, both on a long-term and short-term scale, where one of the Pseudo-nitzschia species almost completely vanishes and another appears in abundance, are characteristic of phytoplankton communities [37,38,39]. Some authors, in their reviews [4,40], summarize information on the effects of various environmental factors on the bloom formation, growth rate, and domoic acid (DA) production of various Pseudo-nitzschia species and put a significant focus on water temperature and note that the effect of temperature increase on these physiological parameters is species specific [41]. It has been experimentally proven that a water temperature increase only can stimulate the growth and production of DA and affect the competitive dominance of different Pseudo-nitzschia species regardless of other possible triggering environmental factors [41].
In the Sea of Japan, as the monsoon circulation weakened, a pronounced positive trend of average integral water-temperature values and multidirectional trends of decreasing or increasing water salinity in the surface layer of various parts of the area have been revealed in recent decades [21,22]. On the other hand, an abnormal warming of surface waters was recorded from Peter the Great Bay in the summer and autumn of 2021 [42]. In particular, the coastal waters of the Northwestern Sea of Japan were found to be significantly warmed in the second half of the summer and autumn. Thus, the SST in June was, on average, already above the long-term average by 0.5–2.0 °C. In July, SST anomalies in the Sea of Japan reached a record-breaking value of +6.5 °C. Such substantial positive anomalies in these waters were documented for the first time in the 21st century [43]. Furthermore, the surfaces of these water bodies remained very warm, both in August (temperature anomalies reached +4.0 °C) and in the autumn months. In October and November, deviations from the norm remained within a range of +1 to +3 °C [42]. In 2021, P. multistriata bloom events were recorded from Ussuri Bay in the third ten days of October and in the first and the second ten days of November (Table 3, Figure 3). They coincided in time with positive anomalies of water temperature. Thus, in the third ten days of October 2021, the SST water temperatures in Ussuri Bay were in a region of positive anomalies of about 1–2 °C (Figure 6a–c). In November 2021, the SST in this area was still characterized by an abnormal distribution: the surface layer of water was warmer than usual by 1–3 °C throughout the month (Figure 6d–f).
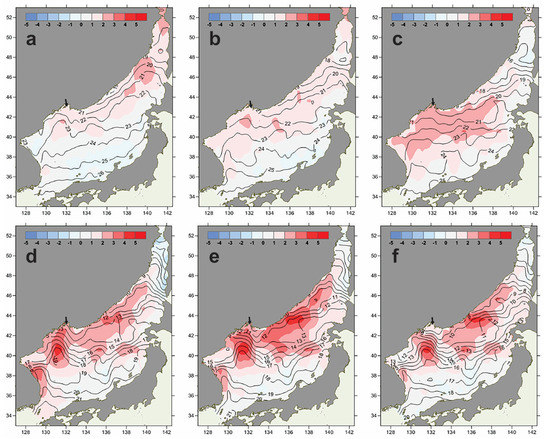
Figure 6.
Average ten-day water temperatures (isolines) and its anomalies (isofields) in Ussuri Bay (indicated by arrow), the Northwestern Sea of Japan, ten-day periods of October 2021: 1—(a), 2—(b), 3—(c) [44], and ten-day periods of November 2021: 1—(d), 2—(e), 3—(f) [45]. Reproduced with permission from the Far Eastern Regional Hydrometeorological Research Institute (FERHRI) from the FERHRI official website (Perunova, T.A. Thermal conditions of the Far Eastern Seas. The Sea of Japan. October 2021. Monthly Hydrometeorological Bulletin of FERHRI 2021. Mezentseva, L.I., Ed. Available online: http://www.ferhri.ru/images/stories/FERHRI/Bulletins/Bul_2021/10/2021.10_ch2_hydroterm.pdf (accessed on 17 February 2023) and Perunova, T.A. Thermal conditions of the Far Eastern Seas. The Sea of Japan. November 2021. Monthly Hydrometeorological Bulletin of FERHRI. 2021. Mezentseva, L.I., Ed. Available online: http://www.ferhri.ru/images/stories/FERHRI/Bulletins/Bul_2021/11/2021.11_ch2_hydroterm.pdf (accessed on 17 February 2023).
Along with positive temperature anomalies, the oceanographic conditions in Peter the Great Bay in the summer of 2021 were characterized by other distinctive features: the high salinity values in the upper layer to a depth of 45 m as an indicator of waters of subtropical origin and the lack of signs of the cold Liman Current which is a characteristic feature of all known current patterns in this region [25].
A positive correlation between the density of P. multistriata and the water temperature has been confirmed in the present study (Table 4). Although a statistically reliable positive correlation is not direct proof of a causal relationship, the correlation we found suggests that increasing water temperature may be one of the environmental factors responsible for the formation of P. multistriata blooms in the autumn seasons of 2021 in Ussuri Bay.
P. multistriata is found in tropical and temperate waters [46]. This species has been mentioned among the most widespread species in Asian warm-water areas, including the South China Sea [47]. It has also been recorded from the southern waters of the Pacific Ocean, including Tokyo Bay in Japan [48], off southern China [49], and in New Zealand [50].
Abnormal warming of the waters of Peter the Great Bay in the summer and autumn of 2021 is considered to be the result of the simultaneous influence of several factors contributing to the increase in SST, such as a long period of cloudless weather, the absence of storms stirring the water column, and increased advection of warm and salty subtropical waters into the northern part of the Northwestern Sea of Japan, including Peter the Great Bay. The common reason for the influence of all these factors was the unusual arrangement of the main centers of atmospheric action in the Asia–Pacific region, primarily the increased activity of the Hawaiian anticyclone, whose western spur reached the marginal seas of East Asia, and the weakness of the Okhotsk anticyclone. As a result, there was an early transition from the first phase of the summer monsoon to the second and a powerful removal of tropical air masses to the north, which contributed to the penetration of subtropical waters to the shores of Primorye. This situation is consistent with the current warming trend in global climate change [51]. The increase in SST was accompanied by an increase in salinity and an increase in the number of tropical fish and invertebrate species in the coastal waters of Primorye as indicators of waters of subtropical origin, and a decrease in dissolved-oxygen concentrations and concentrations of nutrients due to the weakening of the vertical mixing of waters [51]. All these data are consistent with our assumption that an abnormally high water temperature in the autumn of 2021 in the surface waters off the coast of Vladivostok may be one of the environmental factors responsible for blooms of P. multistriata, known as a diatom with warm-temperature-water affinity.
Thus, the results of our present study indicate that warming could be one of the factors potentially responsible for the increase in the density of P. multistriata, which is known as a diatom with warm-temperature-water affinity in Ussuri Bay. This event is consistent with the data of other studies that report a relationship between positive water-temperature anomalies and this diatom genus [7,41,52,53]. In particular, the abnormal warming of water induced the change of the dominant Pseudo-nitzschia species and favored the increase in the densities of P. australis and P. delicatissima off the California coast, USA [7,41]. In addition, the effects of other environmental factors and biotic interactions (between diatom species and bacteria and zooplankton) should be addressed. For example, water salinity variations, eutrophication, and silicate and phosphate limitation are often considered as the factors that significantly affect Pseudo-nitzschia growth, competitive ability, and intracellular DA concentration [4,52].
An earlier long-term study of phytoplankton in Peter the Great Bay revealed that the autumn period can be considered the typical season of Pseudo-nitzschia bloom events in this area [14]. Furthermore, it was earlier found that the concentration of chlorophyll a is maximal in the near-surface layer and decreases with depth in the northern part of Peter the Great Bay off the coast of Vladivostok, occupied by estuarine waters [54]. Therefore, our study has been conducted in autumn on the surface horizon. Since this study was limited in time (autumn) and space (surface waters), it allowed us to identify only the short-period (interannual) changes in the composition of the bloom-forming Pseudo-nitzschia on the surface water horizon. Unfortunately, these limitations of our study do not allow us to assess the features of the seasonal and spatial dynamics of Pseudo-nitzschia in the water column and to reveal the mechanisms of the formation of bloom events caused by toxic diatoms. In order to address these objectives, in the future, year-round comprehensive studies of phytoplankton and hydrological and hydrochemical factors in the whole water column are needed.
Thus, a gradual interannual shift in specific dominance occurred after 2020: the density of the P. delicatissima group decreased, and the density of the moderately warm-water P. multistriata increased. The abnormal water warming in the area of our study could contribute to the short-period (interannual) changes in the composition of the bloom-forming Pseudo-nitzschia that we observed. Further studies of the effects of short-term warming phenomena on phytoplankton communities in the Northwestern Sea of Japan are required to predict toxic microalgae blooms.
P. multistriata was previously indicated as a source of DA accumulation in bivalves from the Northwestern Sea of Japan [14]. As the data shows, the potential risk of accumulation of DA, an amnesic neurotoxin associated with numerous blooms of the toxic diatom P. multistriata, by mollusks in Ussuri Bay in the autumn seasons of 2017–2022 remained high. Therefore, these data should be considered when organizing the toxic phytoplankton monitoring and controlling the level of phycotoxins in mollusks from waters near aquaculture farms operated in the Northwestern Sea of Japan.
5. Conclusions
As a result of a five-year study of phytoplankton in 2017–2022, we have recorded the interannual changes in the composition of the toxic bloom-forming Pseudo-nitzschia groups. After 2020, the density of the P. delicatissima group decreased, and the density of P. multistriata, known as one of the most widespread species in Asian warm-water areas, increased. Therefore, we assume that the abnormal water-temperature increase in Peter the Great Bay in the autumn of 2021 could be one of the factors responsible for the change of the dominant Pseudo-nitzschia. Furthermore, due to the relatively high intracellular DA level, as was reported earlier for cultures of P. multistriata from our study area, there is a risk of accumulation of amnesic shellfish toxin in bivalves from Ussuri Bay.
Author Contributions
I.V.S. provided phytoplankton analysis, proposed conceptualization, performed formal analysis and visualization, and wrote the manuscript. A.A.Z. provided phytoplankton analysis, and performed formal analysis and visualization. All authors have read and agreed to the published version of the manuscript.
Funding
Analysis of interannual variations in the composition and density of the bloom-forming Pseudo-nitzschia groups was carried out within the framework of the state assignment of A.V. Zhirmunsky National Scientific Center of Marine Biology, FEB RAS. The study of relationships between temporal variations in species composition of the bloom-forming Pseudo-nitzschia and environmental changes in the region was supported by the Federal Service for Hydrometeorology and Environmental Monitoring of the Russian Federation (Agreement No. 169-15-2023-002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pulido, O.M. Domoic acid toxicologic pathology: A review. Mar. Drugs 2008, 6, 180–219. [Google Scholar] [CrossRef]
- Bates, S.; Bird, C.; de Freitas, A.; Foxall, R.; Gilgan, M.; Hanic, L.; Johnson, G.; McCulloch, A.; Odense, P.; Pocklington, R.; et al. Pennate diatom Nitzschia pungens as the primary source of domoic acid, a toxin in shellfish from eastern Prince Edward Island, Canada. Can. J. Fish. Aquat. Sci. 1989, 46, 1203–1215. [Google Scholar] [CrossRef]
- Lefebvre, K.; Bargu, S.; Kieckhefer, T.; Silver, M. From sanddabs to blue whales: The pervasiveness of domoic acid. Toxicon 2002, 40, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Lelong, A.; Hégaret, H.; Soudant, P.; Bates, S.S. Pseudo-nitzschia (Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: Revisiting previous paradigms. Phycologia 2012, 51, 168–216. [Google Scholar] [CrossRef]
- Trainer, V.L.; Bates, S.S.; Lundholm, N.; Thessen, A.E.; Cochlan, W.P.; Adams, N.G.; Trick, C.G. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef]
- Bates, S.S.; Hubbard, K.A.; Lundholm, N.; Montresor, M.; Leaw, C.P. Pseudo-nitzschia, Nitzschia, and domoic acid: New research since 2011. Harmful Algae 2018, 79, 3–43. [Google Scholar] [CrossRef]
- McCabe, R.; Hickey, B.; Kudela, R.; Lefebvre, K.; Adams, N.; Bill, B.; Gulland, F.; Thomson, R.; Cochlan, W.; Trainer, V. An unprecedented coastwide toxic algal bloom linked to anomalous ocean conditions. Geophys. Res. Lett. 2016, 43, 10366–10376. [Google Scholar] [CrossRef]
- Cabrini, M.; Fornasaro, D.; Cossarini, G.; Lipizer, M.; Virgilio, D. Phytoplankton temporal changes in a coastal northern Adriatic site during the last 25 years. Estuar. Coast. Shelf Sci. 2012, 115, 113–124. [Google Scholar] [CrossRef]
- Bresnan, E.; Cook, K.B.; Hughes, S.L.; Hay, S.J.; Smith, K.; Walsham, P.; Webster, L. Seasonality of the plankton community at an east and west coast monitoring site in Scottish waters. J. Sea Res. 2015, 105, 16–29. [Google Scholar] [CrossRef]
- Louw, D.C.; Doucette, G.J.; Voges, E. Annual patterns, distribution and long-term trends of Pseudo-nitzschia species in the northern Benguela upwelling system. J. Plankton Res. 2017, 39, 35–47. [Google Scholar] [CrossRef]
- Roche, K.M.; Sterling, A.R.; Rynearson, T.A.; Bertin, M.J.; Jenkins, B.D. A decade of time series sampling reveals thermal variation and shifts in Pseudo-nitzschia species composition that contribute to harmful algal blooms in an Eastern US estuary. Front. Mar. Sci. 2022, 9, 1126. [Google Scholar] [CrossRef]
- Adrianov, A.V.; Kussakin, O.G. A Check-List of Biota of the Peter the Great Bay, the Sea of Japan; Dalnauka: Vladivostok, Russia, 1998; p. 349. (In Russian) [Google Scholar]
- Semina, G.I. Phytoplankton of the Pacific Ocean; Academy of Sciences of the USSR. Soviet National Committee for the International Biological Programme; House Nauka: Moscow, Russia, 1974; p. 239. (In Russian) [Google Scholar]
- Stonik, I.V.; Orlova, T.Y.; Chikalovets, I.V.; Aizdaicher, N.A.; Aleksanin, A.I.; Kachur, V.A.; Morozova, T.V. Pseudo-nitzschia species (Bacillariophyceae) and the domoic acid concentration in Pseudo-nitzschia cultures and bivalves from the northwestern Sea of Japan, Russia. Nova Hedwig. 2019, 108, 73–93. [Google Scholar] [CrossRef]
- Orlova, T.Y.; Zhukova, N.V.; Stonik, I.V. Bloom-forming Diatom Pseudo-nitzschia pungens in Amurskii Bay (the Sea of Japan): Morphology, ecology and biochemistry. In Harmful and Toxic Algal Blooms; Yasumoto, T., Oshima, Y., Fukuyo, Y., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 1996; pp. 147–150. [Google Scholar]
- Stonik, I.V.; Orlova, T.Y.; Lundholm, N. Diversity of Pseudo-nitzschia H. Peragallo from the western North Pacific. Diatom Res. 2011, 26, 121–134. [Google Scholar] [CrossRef]
- Stonik, I.V.; Orlova, T.Y.; Shevchenko, O.G. Morphology and ecology of the species of the genus Pseudo-nitzschia (Bacillariophyta) from Peter the Great Bay, Sea of Japan. Russ. J. Mar. Biol. 2001, 27, 362–366. [Google Scholar] [CrossRef]
- Stonik, I.V. Long-term variations in species composition of bloom-forming toxic Pseudo-nitzschia diatoms in the North-western Sea of Japan during 1992–2015. J. Mar. Sci. Eng. 2021, 9, 568. [Google Scholar] [CrossRef]
- Lukyanova, O.N.; Cherkashin, S.A.; Nigmatulina, L.V.; Chernyaev, A.P.; Veideman, E.L.; Ireykina, S.A.; Pryazhevskaya, T.S. Integral chemical-ecological assessment of the state of Ussuri Bay (the Sea of Japan). Water Resour. 2009, 36, 586–593, (In Russian with English abstract). [Google Scholar] [CrossRef]
- Luchin, V.A.; Tikhomirova, E.A.; Kruts, A.A. Oceanographic regime of Peter the Great Bay (Japan sea). Izvestya TINRO 2005, 140, 130–169, (In Russian with English abstract). [Google Scholar]
- Zuenko, Y.I. Monitoring of oceanographic conditions in Peter the Great Bay and adjacent waters of the centennial standard section along 132° E. In Proceedings of the Russian Scientific Conference with International Participation, Dedicated to the 20th Anniversary of the UNESCO “Marine Ecology” International Chair of FEFU, Vladivostok, Russia, 8–10 November 2018; Khristophorova, N.K., Tsygankov, V.Y., Eds.; Publishing House of the Far Eastern Federal University: Vladivostok, Russia, 2018; pp. 51–53, (In Russian with English abstract). [Google Scholar]
- Rostov, I.D.; Rudykh, N.I.; Rostov, V.I.; Vorontsov, A.A. Tendencies of climatic and anthropogenic changes of the marine environments in the coastal areas of Russia in the Japan Sea for the last decades. Izvestya TINRO 2016, 186, 163–181, (In Russian with English abstract). [Google Scholar] [CrossRef]
- Krovnin, A.S.; Zuenko, Y.I.; Figurkin, A.L.; Khen, G.V.; Kivva, K.K.; Novikov, Y.V.; Tepnin, O.B. Oceanographic conditions within the main area of walleye pollock. Tr. VNIRO 2022, 189, 16–44, (In Russian with English abstract). [Google Scholar] [CrossRef]
- Khen, G.V.; Ustinova, E.I.; Sorokin, Y.D.; Matyushenko, L.Y. Long-term changes of the thermal characteristics in the Japan Sea surface water and Peter the Great Bay and their relationship with large-scale climatic processes. Tr. VNIRO 2020, 180, 72–87. Available online: https://agris.fao.org/agris-search/search.do?recordID=R22020800661 (accessed on 24 March 2023). (In Russian with English abstract). [CrossRef]
- Far Eastern Regional Hydrometeorological Research Institute (FERHRI) Official Website; Danchenkov, M.A. Oceanographic Conditions of Peter the Great Bay According to Expedition Observations in 2021–2022 (Analytical review). 2022. Available online: http://www.ferhri.ru/images/stories/FERHRI/science/zalivPV/Obzor2022/oceanography_22.pdf (accessed on 6 March 2023).
- Sukhanova, I.N. Phytoplankton concentrating in the sample. In Modern methods for Quantifying the Distribution of Marine Plankton; House Nauka: Moscow, Russia, 1983; pp. 97–105. (In Russian) [Google Scholar]
- Utermöhl, H. Zur vervollkommnung der quantitativen phytoplankton-methodik: Mit 1 Tabelle und 15 abbildungen im Text und auf 1 Tafel. Int. Ver. Für Theor. Und Angew. Limnol. Mitt. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Andersen, P.; Throndsen, J. Estimating cell numbers. In Manual on Harmful Marine Microalgae; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; UNESCO Publishing: Paris, France, 2003; pp. 99–129. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E Ltd.: Plymouth, UK, 2006; p. 192. Available online: http://www.ap.smu.ca/~dclarke/home/documents/byDAC/mhd_primer.pdf (accessed on 4 May 2023).
- Clarke, K.R.; Gorley, R.N.; Somerfield, P.J.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; PRIMER-E: Plymouth, UK, 2014; p. 262. [Google Scholar]
- Hasle, G.R.; Lange, C.B.; Syvertsen, E.E. A review of Pseudo-nitzschia, with special reference to the Skagerrak, North Atlantic, and adjacent waters. Helgoländer Meeresunters. 1996, 50, 131–175. [Google Scholar] [CrossRef]
- Lundholm, N.; Moestrup, Ø.; Hasle, G.R.; Hoef-Emden, K. A study of the Pseudo-nitzschia pseudodelicatissima/cuspidata complex (Bacillariophyceae): What is P. pseudodelicatissima? J. Phycol. 2003, 39, 797–813. [Google Scholar] [CrossRef]
- Lundholm, N.; Bates, S.S.; Baugh, K.A.; Bill, B.D.; Connell, L.B.; Léger, C.; Trainer, V.L. Cryptic and pseudo-cryptic diversity in diatoms—With descriptions of Pseudo-nitzschia hasleana sp. nov. and P. fryxelliana sp. nov. J. Phycol. 2012, 48, 436–454. [Google Scholar] [CrossRef]
- Moschandreou, K.K.; Baxevanis, A.D.; Katikou, P.; Papaefthimiou, D.; Nikolaidis, G.; Abatzopoulos, T.J. Inter-and intra-specific diversity of Pseudo-nitzschia (Bacillariophyceae) in the northeastern Mediterranean. Eur. J. Phycol. 2012, 47, 321–339. [Google Scholar] [CrossRef]
- Stonik, I.V.; Isaeva, M.P.; Aizdaicher, N.A.; Balakirev, E.S.; Ayala, F.J. Morphological and genetic identification of Pseudo-nitzschia H. Peragallo, 1900 (Bacillariophyta) from the Sea of Japan. Russ. J. Mar. Biol. 2018, 44, 192–201. [Google Scholar] [CrossRef]
- Hasle, G.R. Are most of the domoic acid-producing species of the diatom genus Pseudo-nitzschia cosmopolites? Harmful Algae 2002, 1, 137–146. [Google Scholar] [CrossRef]
- Lundholm, N.; Clarke, A.; Ellegaard, M. A 100-year record of changing Pseudo-nitzschia species in a sill-fjord in Denmark related to nitrogen loading and temperature. Harmful Algae 2010, 9, 449–457. [Google Scholar] [CrossRef]
- Bowers, H.A.; Ryan, J.P.; Hayashi, K.; Woods, A.L.; Marin III, R.; Smith, G.J.; Hubbard, K.A.; Douchette, G.F.; Mikukski, C.M.; Gellene, A.G.; et al. Diversity and toxicity of Pseudo-nitzschia species in Monterey Bay: Perspectives from targeted and adaptive sampling. Harmful Algae 2018, 78, 129–141. [Google Scholar] [CrossRef]
- Bates, S.S.; Garrison, D.L.; Horner, R.A. Bloom dynamics and physiology of domoic-acid-producing Pseudo-nitzschia species. In Physiological Ecology of Harmful Algal Blooms; Anderson, D.M., Cembella, A.D., Hallegraeff, G.M., Eds.; Springer: Heidelberg, Germany, 1998; pp. 267–292. [Google Scholar]
- Zhu, Z.; Qu, P.; Fu, F.; Tennenbaum, N.; Tatters, A.O.; Hutchins, D.A. Understanding the blob bloom: Warming increases toxicity and abundance of the harmful bloom diatom Pseudo-nitzschia in California coastal waters. Harmful Algae 2017, 67, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Far Eastern Regional Hydrometeorological Research Institute (FERHRI) Official Website; Mezentseva, L.I.; Gonchukov, L.V. Unusually Hot Summer of 2021 in Primorsky Krai and in the Waters of the Far Eastern Seas. Vladivostok, Russia. 2022. Available online: http://www.ferhri.ru/images/stories/FERHRI/science/Articles/heat_2021.pdf (accessed on 6 March 2023).
- Kulikova, I.A.; Kruglova, E.N.; Sumerova, K.A.; Khan, V.M. The forecasts and observations of heat waves and blocking anticyclones in the summer of 2021. Hydrometeorol. Res. Forecast. 2022, 1, 7–21. [Google Scholar] [CrossRef]
- Far Eastern Regional Hydrometeorological Research Institute (FERHRI) Official Website; Perunova, T.A. Thermal Conditions of the Far Eastern Seas. The Sea of Japan. October 2021. Monthly Hydrometeorological Bulletin of FERHRI. Mezentseva, L.I., Ed.; Vladivostok, Russia. 2021. Available online: http://www.ferhri.ru/images/stories/FERHRI/Bulletins/Bul_2021/10/2021.10_ch2_hydroterm.pdf (accessed on 17 February 2023).
- Far Eastern Regional Hydrometeorological Research Institute (FERHRI) Official Website; Perunova, T.A. Thermal Conditions of the Far Eastern Seas. The Sea of Japan. November 2021. Monthly Hydrometeorological Bulletin of FERHRI. Mezentseva, L.I., Ed.; Vladivostok, Russia. 2021. Available online: http://www.ferhri.ru/images/stories/FERHRI/Bulletins/Bul_2021/11/2021.11_ch2_hydroterm.pdf (accessed on 17 February 2023).
- Thessen, A.E. Taxonomy and Ecophysiology of Pseudo-nitzschia in the Chesapeake Bay. PhD Thesis, University of Maryland, College Park, MD, USA, 2007; p. 231. Available online: https://drum.lib.umd.edu/handle/1903/7707 (accessed on 4 March 2023).
- Lü, S.; Li, Y.; Lundholm, N.; Ma, Y.; Ho, K.C. Diversity, taxonomy and biogeographical distribution of the genus Pseudo-nitzschia (Bacillariophyceae) in Guangdong coastal waters, South China Sea. Nova Hedwig. 2012, 95, 123. [Google Scholar] [CrossRef]
- Takano, H. Marine diatom Nitzschia multistriata sp. nov. common at inlets of southern Japan. Diatom 1993, 8, 39–41. [Google Scholar]
- Qi, Y.; Wang, J.; Zheng, L. The Taxonomy and Bloom Ecology of Pseudo-nitzschia on the Coasts of China. In Proceedings of the IOC-WESTPAC Third International Scientific Symposium, Bali, Indonesia, 22–26 November 1994; pp. 88–95. [Google Scholar]
- Rhodes, L.; Jiang, W.; Knight, B.; Adamson, J.; Smith, K.; Langi, V.; Edgar, M. The genus Pseudo-nitzschia (Bacillariophyceae) in New Zealand: Analysis of the last decade’s monitoring data. N. Z. J. Mar. Freshw. Res. 2013, 47, 490–503. [Google Scholar] [CrossRef]
- Zuenko, Y.I.; Nikitin, A.A.; Figurkin, A.L.; Matveev, V.I. Hot Summer of 2021 in Primorye: Maricultural Aspects. In Proceedings of the Abstracts of the All-Russian Conference (in Commemoration of the Academician Oleg, G. Kussakin), Vladivostok, Russia, 20–23 September 2022; Serkov, V.M., Ed.; NSCMB FEB RAS: Vladivostok, Russia, 2022; pp. 145–146. (In Russian). [Google Scholar]
- Bates, S.S.; Lundholm, N.; Hubbard, K.A.; Montresor, M.; Leaw, C.P. Toxic and harmful marine diatoms. In Diatoms: Fundamentals & Applications. Volume 1 in the Series: Diatoms: Biology & Applications; Gordon, R., Seckbach, J., Eds.; Wiley-Scrivener: Beverly, MA, USA, 2019; pp. 389–434. [Google Scholar] [CrossRef]
- McKibben, S.M.; Peterson, W.; Wood, A.M.; Trainer, V.L.; Hunter, M.; White, A.E. Climatic regulation of the neurotoxin domoic acid. Proc. Natl. Acad. Sci. USA 2017, 114, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Zharova, A.D.; Zuenko, Y.I. Seasonal changes of vertical profiles of chlorophyll a in the Amur Bay (Sea of Japan). Izvestya TINRO 2018, 193, 183–189, (In Russian with English abstract). [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).