Chemical and Isotopic Composition of Sulfide Minerals from the Noho Hydrothermal Field in the Okinawa Trough
Abstract
1. Introduction
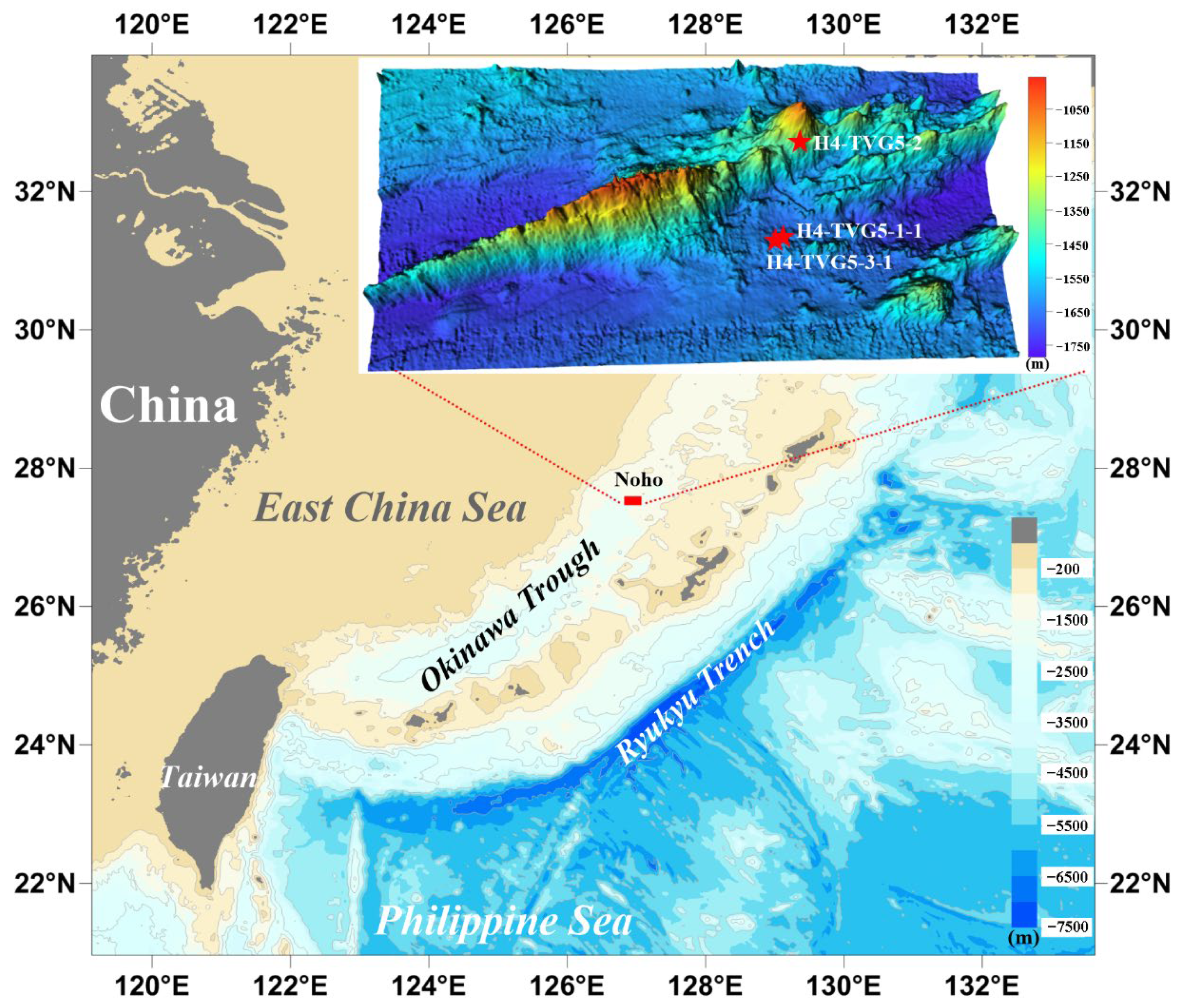
2. Geological Setting
3. Sampling and Methods
3.1. SEM Analysis
3.2. Major Element Analysis
3.3. Trace Element Analysis
3.4. In-Situ S Isotope Analysis
3.5. In-Situ Pb Isotope Analysis
4. Results
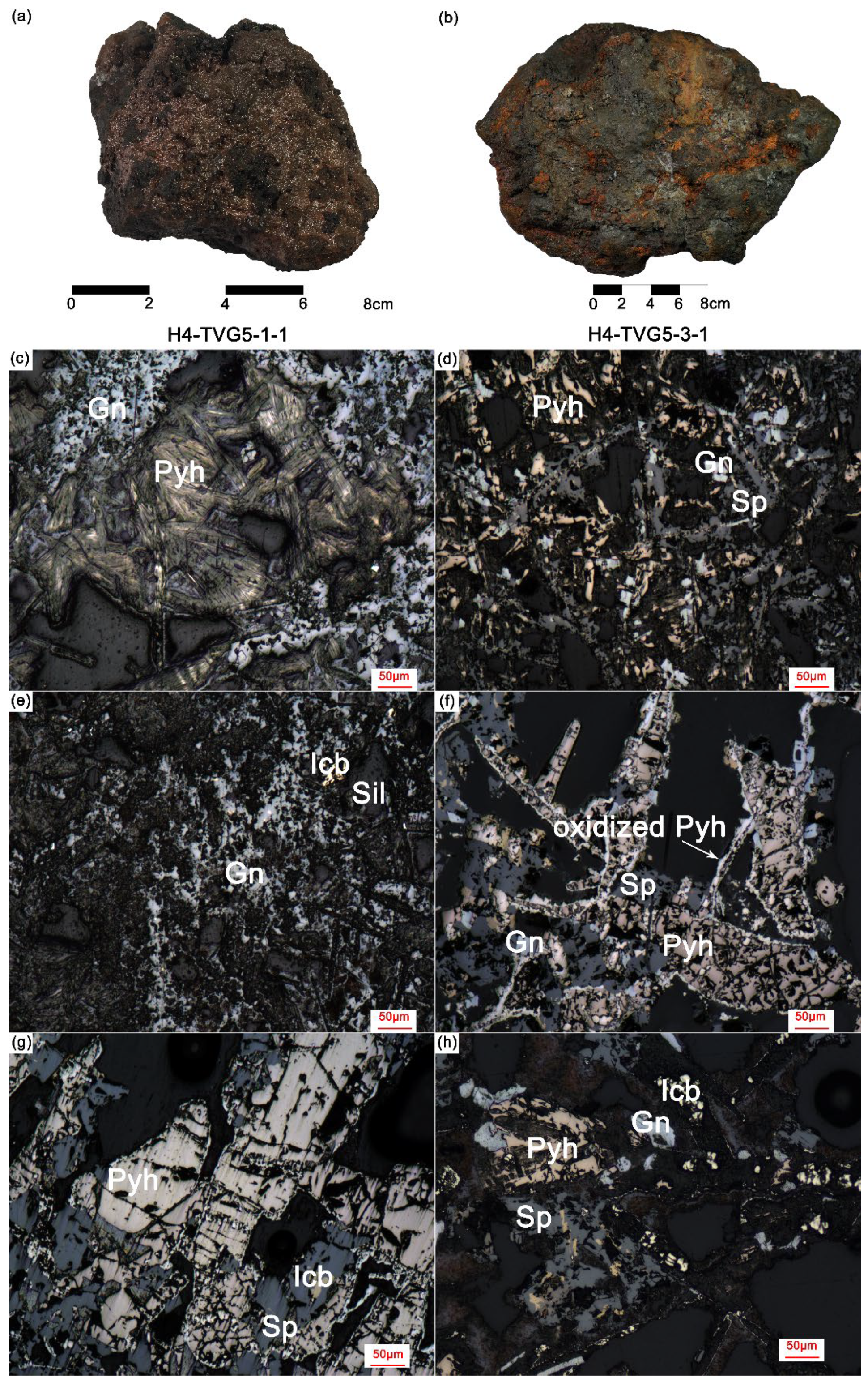
4.1. Mineralogy of Hydrothermal Sulfide Samples
4.1.1. Pyrrhotite
4.1.2. Sphalerite
4.1.3. Galena
4.1.4. Isocubanite and Amorphous Silica
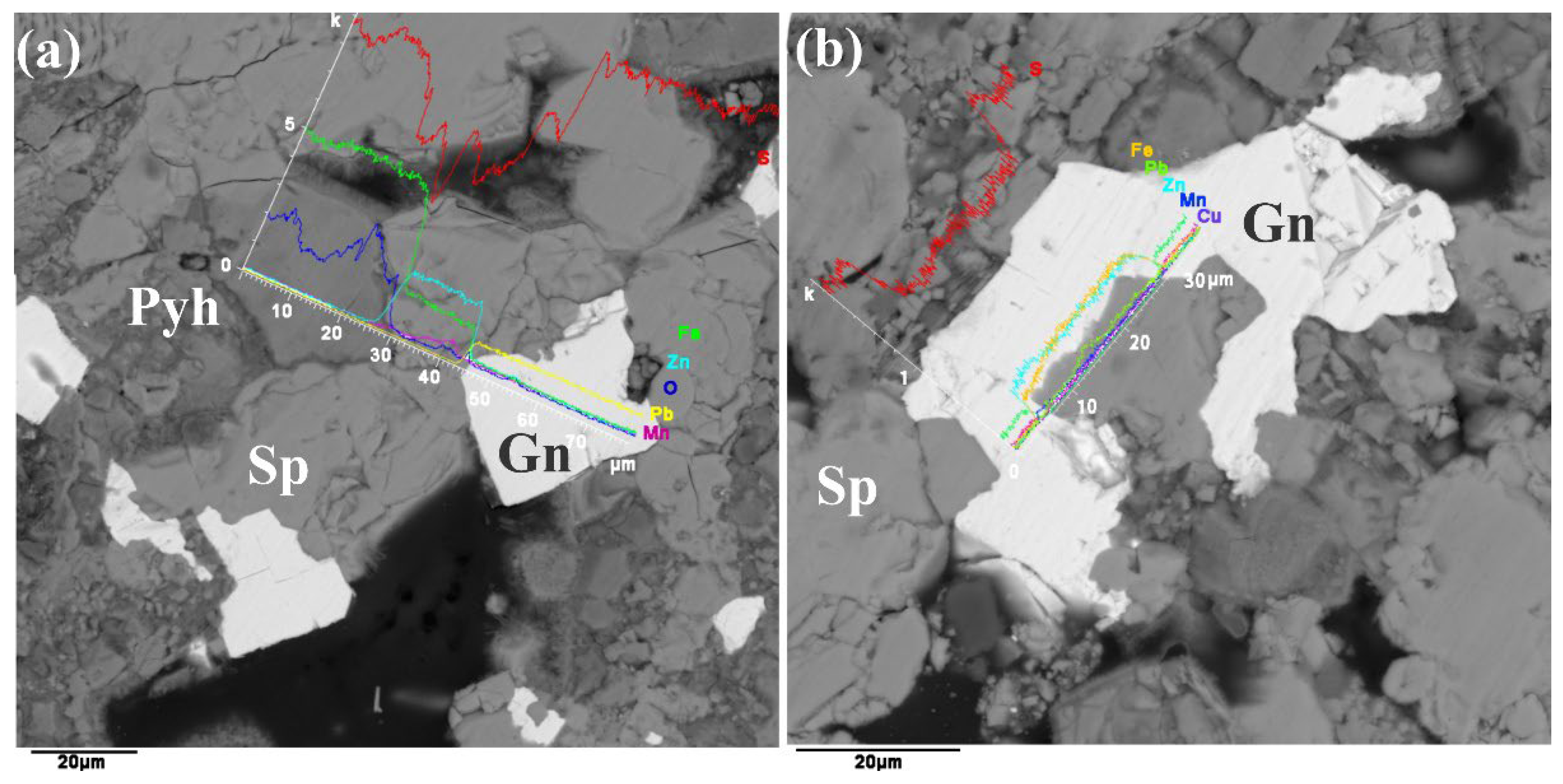
4.2. Major and Trace Element Contents of Sulfide Minerals
4.2.1. Major Element Contents of Sulfide Minerals
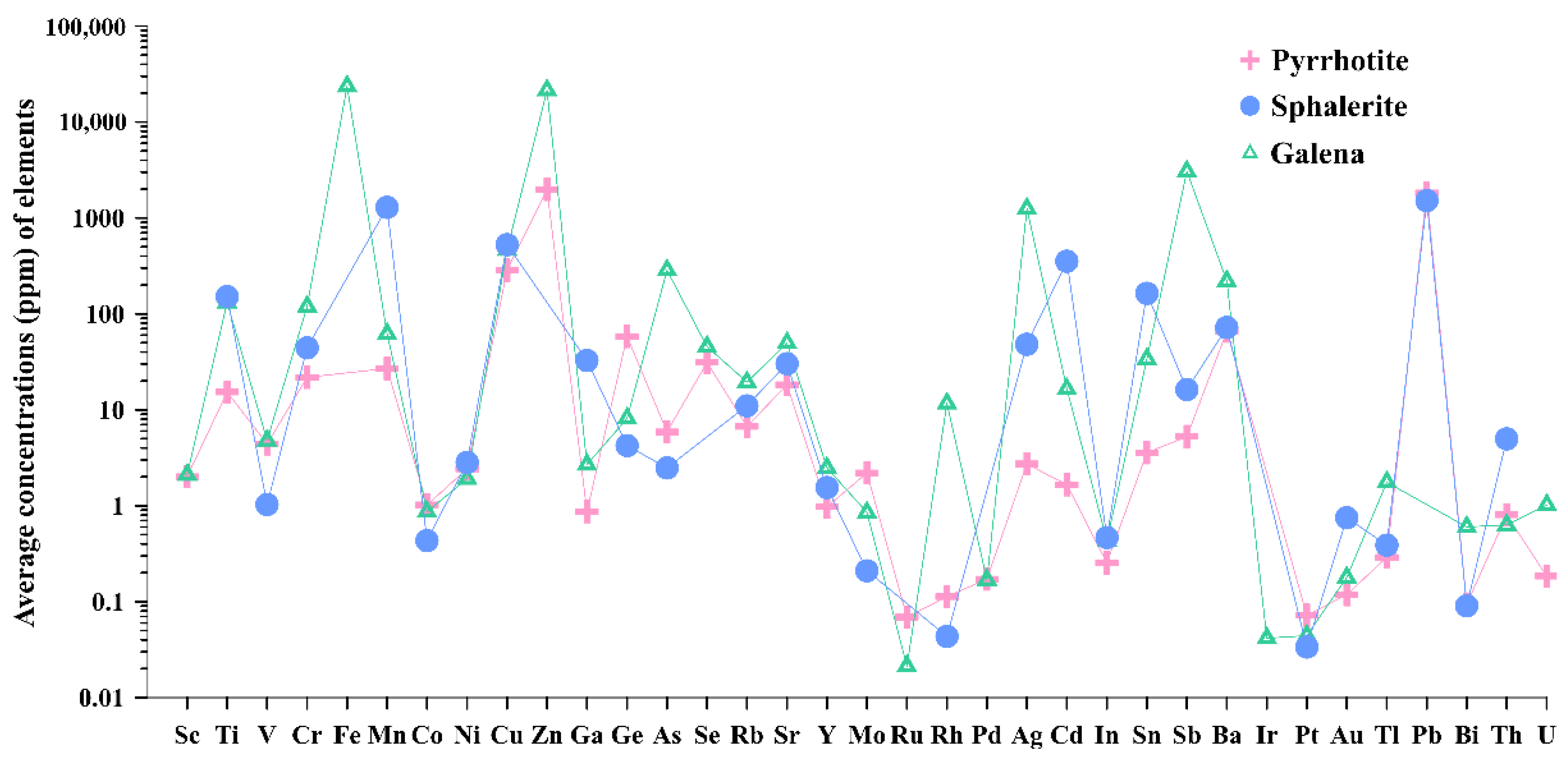
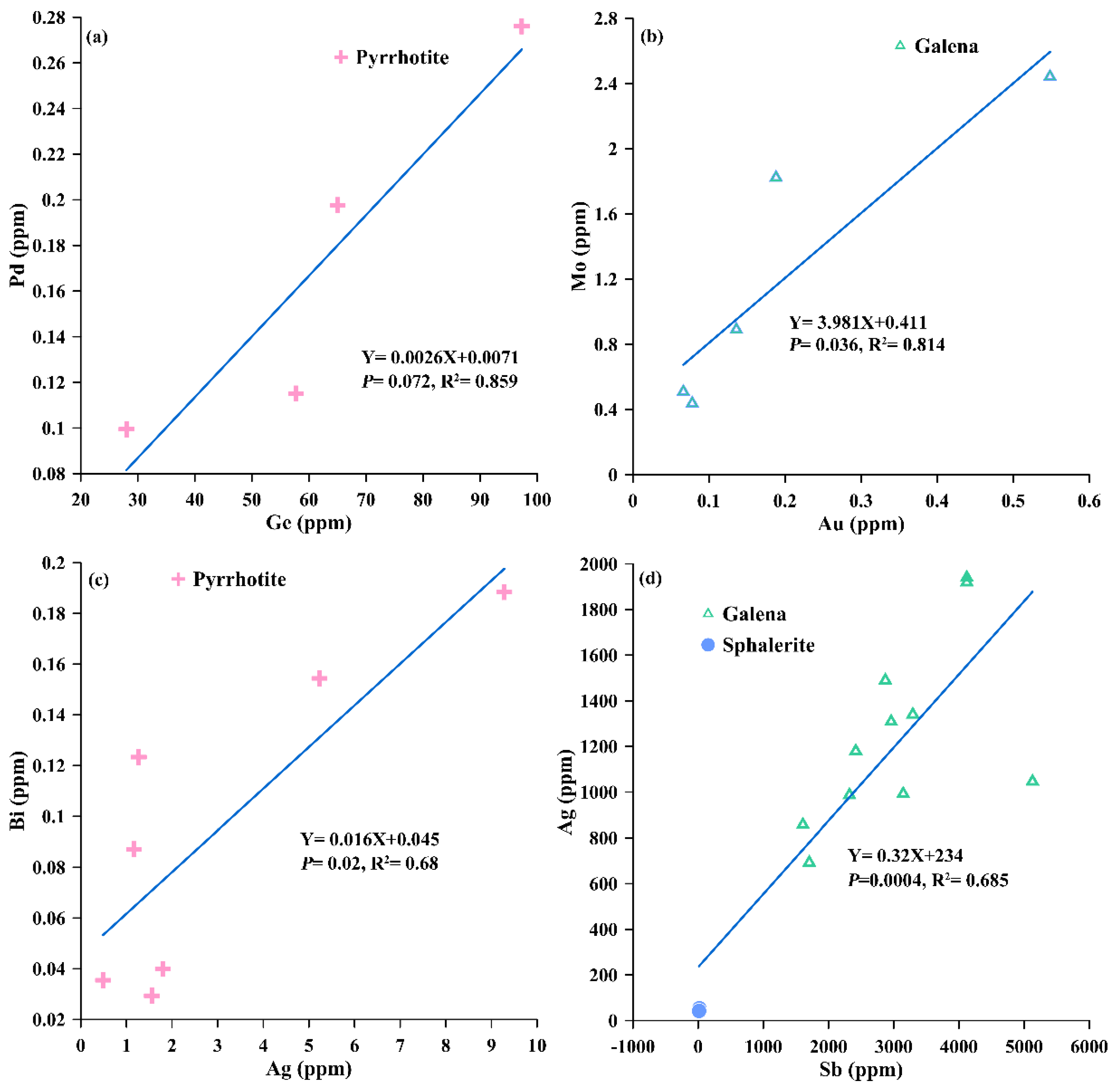
4.2.2. Trace Element Contents of Sulfide Minerals
4.3. Rare Earth Element Compositions of Sulfide Minerals
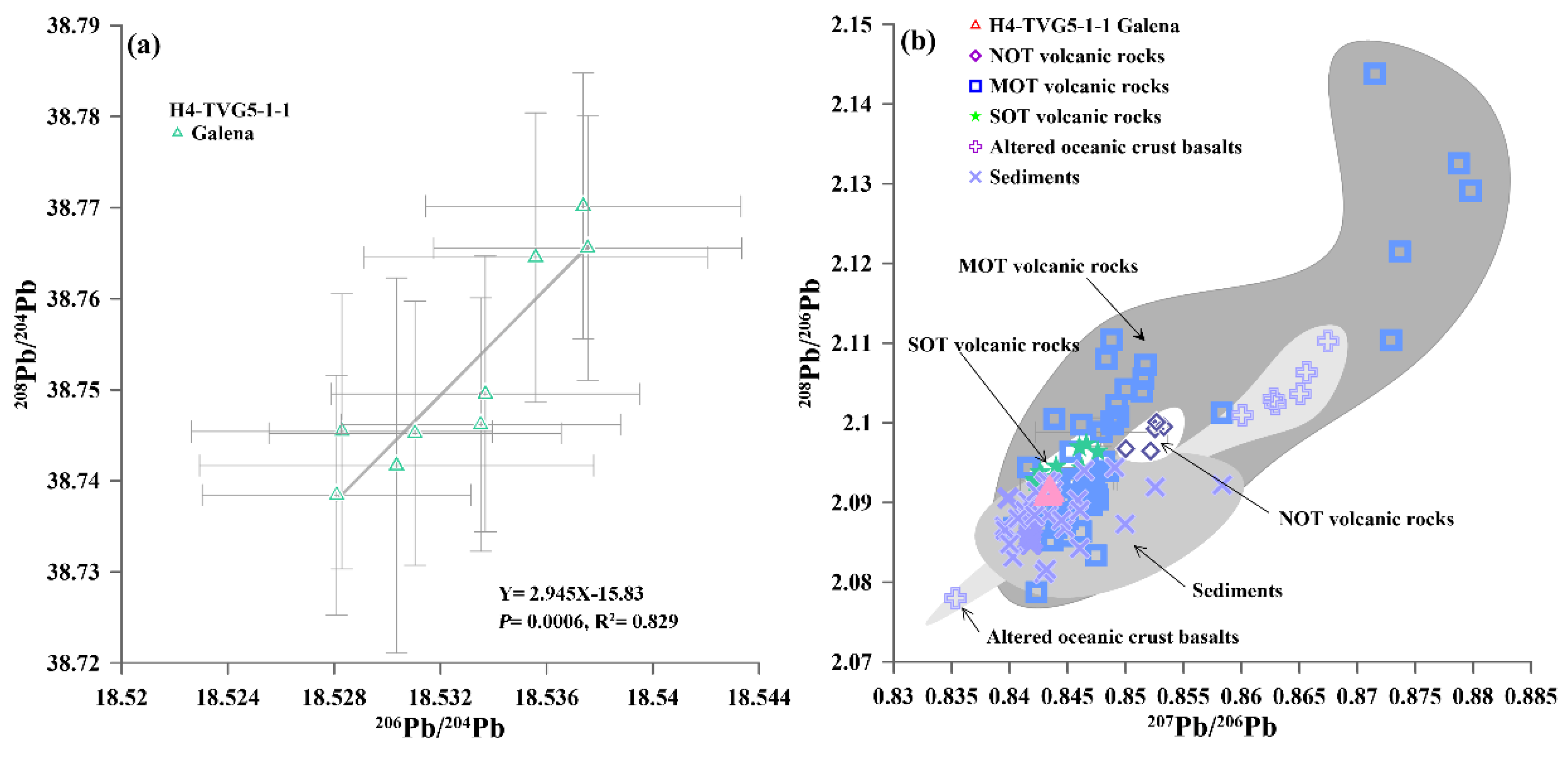
4.4. In-Situ S and Pb Isotopic Compositions of the Sulfide Minerals
5. Discussion
5.1. Seawater–Fluid Mixing and Element Enrichments
5.2. Variable REE Contents and Sources of REEs
5.3. Sources of Sulfur in Sulfide Minerals
5.4. Fluid-Rock Interactions and Source of Lead in Galena
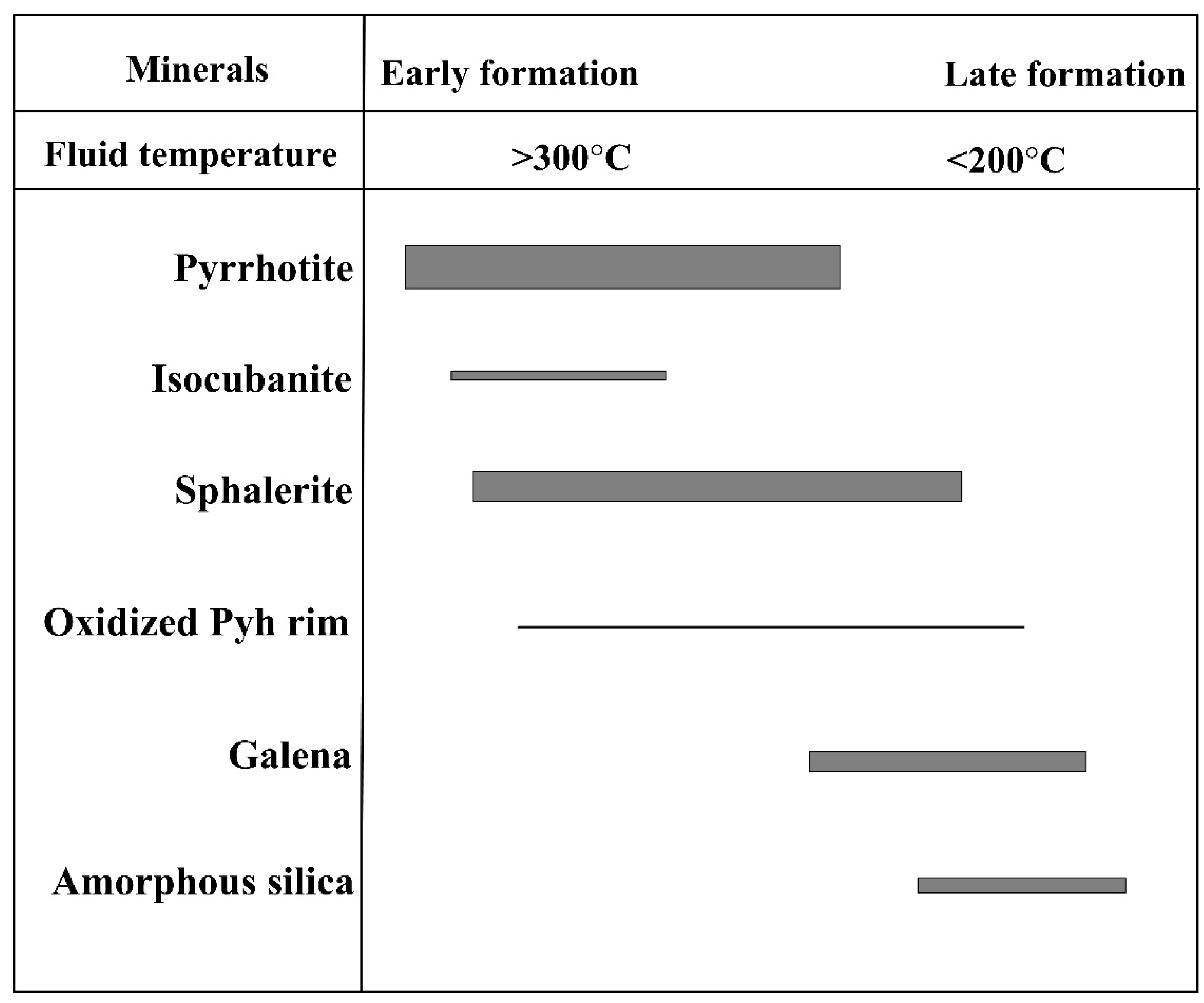
5.5. Hydrothermal Temperature and Redox Conditions during Sulfide Formation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fallon, E.K.; Petersen, S.; Brooker, R.A.; Scott, T.B. Oxidative dissolution of hydrothermal mixed-sulphide ore: An assessment of current knowledge in relation to seafloor massive sulphide mining. Ore Geol. Rev. 2017, 86, 309–337. [Google Scholar] [CrossRef]
- Hawley, J.E.; Nichol, I. Trace elements in pyrite, pyrrhotite and chalcopyrite of different ores. Econ. Geol. 1961, 56, 467–487. [Google Scholar] [CrossRef]
- Arnold, R. Range in composition and structure of 82 natural terrestrial pyrrhotites. Can. Mineral. 1967, 9, 31–50. [Google Scholar]
- Cabri, L.J.; Campbell, J.L.; Laflamme, J.G.; Leigh, R.G.; Maxwell, J.A.; Scott, J.D. Proton-microprobe analysis of trace elements in sulfides from some massive-sulfide deposits. Can. Mineral. 1985, 23, 133–148. [Google Scholar]
- Törmänen, T.O.; Koski, R.A. Gold enrichment and the Bi-Au association in pyrrhotite-rich massive sulfide deposits, Escanaba Trough, Southern Gorda Ridge. Econ. Geol. 2005, 100, 1135–1150. [Google Scholar] [CrossRef]
- Berkenbosch, H.A.; De Ronde, C.E.J.; Gemmell, J.B.; McNeill, A.W.; Goemann, K. Mineralogy and formation of black smoker chimneys from Brothers submarine volcano, Kermadec arc. Econ. Geol. 2012, 107, 1613–1633. [Google Scholar] [CrossRef]
- Tivey, M.; Becker, E.; Beinart, R.; Fisher, C.; Girguis, P.; Langmuir, C.; Michael, P.; Reysenbach, A.-L. Reysenbach. Links from mantle to microbe at the Lau Integrated Study Site: Insights from a back-arc spreading center. Oceanography 2012, 25, 62–77. [Google Scholar] [CrossRef][Green Version]
- Melekestseva, I.Y.; Tret’Yakov, G.A.; Nimis, P.; Yuminov, A.M.; Maslennikov, V.V.; Maslennikova, S.P.; Kotlyarov, V.A.; Beltenev, V.E.; Danyushevsky, L.; Large, R. Barite-rich massive sulfides from the Semenov-1 hydrothermal field (Mid-Atlantic Ridge, 13 30.87′ N): Evidence for phase separation and magmatic input. Mar. Geol. 2014, 349, 37–54. [Google Scholar] [CrossRef]
- Evans, G.N.; Tivey, M.K.; Seewald, J.S.; Wheat, C. Wheat. Influences of the Tonga subduction zone on seafloor massive sulfide deposits along the eastern Lau spreading center and Valu Fa Ridge. Geochim. Cosmochim. Acta 2017, 215, 214–246. [Google Scholar] [CrossRef]
- Fouquet, Y.; Pelleter, E.; Konn, C.; Chazot, G.; Dupré, S.; Alix, A.-S.; Chéron, S.; Donval, J.-P.; Guyader, V.; Etoubleau, J. Volcanic and hydrothermal processes in submarine calderas: The Kulo Lasi example (SW Pacific). Ore Geol. Rev. 2018, 99, 314–343. [Google Scholar] [CrossRef]
- Goodfellow, W.D.; Franklin, J.M. Geology, mineralogy, and chemistry of sediment-hosted clastic massive sulfides in shallow cores, Middle Valley, Northern Juan de Fuca Ridge. Econ. Geol. 1993, 88, 2037–2068. [Google Scholar] [CrossRef]
- Bogdanov, Y.A.; Lein, A.Y.; Sagalevich, A.M.; Ul’yanov, A.A.; Dorofeev, S.; Ul’yanova, N.V. Hydrothermal sulfide deposits of the Lucky Strike vent field, Mid-Atlantic Ridge. Geochem. Int. 2006, 44, 403–418. [Google Scholar] [CrossRef]
- Bogdanov, Y.A.; Lein, A.Y.; Maslennikov, V.V.; Li, S.; Ul’Yanov, A.A. Mineralogical-geochemical features of sulfide ores from the Broken Spur hydrothermal vent field. Oceanology 2008, 48, 679–700. [Google Scholar] [CrossRef]
- Mozgova, N.N.; Trubkin, N.V.; Borodaev, Y.S.; Cherkashev, G.A.; Stepanova, T.V.; Semkova, T.A.; Uspenskaya, T.Y. Mineralogy of massive sulfides from the Ashadze hydrothermal field, 13 N, Mid-Atlantic Ridge. Can. Mineral. 2008, 46, 545–567. [Google Scholar] [CrossRef]
- Paradis, S.; Jonasson, I.R.; le Cheminant, G.M.; Watkinsons, D.H. Two zinc-rich chimneys from the Plume Site, southern Juan de Fuca Ridge. Can. Mineral. 1988, 26, 637–654. [Google Scholar]
- Tivey, M.K.; Stakes, D.S.; Cook, T.L.; Hannington, M.D.; Petersen, S. A model for growth of steep-sided vent structures on the Endeavour Segment of the Juan de Fuca Ridge: Results of a petrologic and geochemical study. J. Geophys. Res. Solid Earth 1999, 104, 22859–22883. [Google Scholar] [CrossRef]
- Marques, A.F.A.; Barriga, F.; Chavagnac, V.; Fouquet, Y. Mineralogy, geochemistry, and Nd isotope composition of the Rainbow hydrothermal field, Mid-Atlantic Ridge. Miner. Depos. 2006, 41, 52–67. [Google Scholar] [CrossRef]
- Hannington, M.D.; Jonasson, I.R.; Herzig, P.M.; Petersen, S. Physical and chemical processes of seafloor mineralization at mid-ocean ridges. In Seafloor Hydrothermal Systems: Physical, Chemical, Biological, and Geological Interactions; Washington DC American Geophysical Union Geophysical Monograph Series; American Geophysical Union: Washington, DC, USA, 1995; Volume 91, pp. 115–157. [Google Scholar]
- Monecke, T.; Petersen, S.; Hannington, M.D.; Grant, H.; Samson, I.M. The minor element endowment of modern sea-floor massive sulfides and comparison with deposits hosted in ancient volcanic successions. Rev. Econ. Geol. 2016, 18, 245–306. [Google Scholar]
- Ueno, H. Ore and gangue minerals of sulfide chimneys from the North Knoll, Iheya Ridge, Okinawa Trough, Japan. JAMSTEC Deep-Sea Res. 2003, 22, 49–62. [Google Scholar]
- Zeng, Z.; Ma, Y.; Chen, S.; Selby, D.; Wang, X.; Yin, X. Sulfur and lead isotopic compositions of massive sulfides from deep-sea hydrothermal systems: Implications for ore genesis and fluid circulation. Ore Geol. Rev. 2017, 87, 155–171. [Google Scholar] [CrossRef]
- Tong, X.; Song, S.X.; He, J. Research on Mineral Processing of Marmatite Ore. Metal Mine 2006, 6, 8–12. [Google Scholar]
- Cook, N.J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Shimizu, M.; Danyushevsky, L.; Saini-Eidukat, B.; Melcher, F. Trace and minor elements in sphalerite: A LA-ICPMS study. Geochim. Cosmochim. Acta 2009, 73, 4761–4791. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Guo, J. A DFT study on the effect of lattice impurities on the electronic structures and floatability of sphalerite. Miner. Eng. 2010, 23, 1120–1130. [Google Scholar] [CrossRef]
- Waida, C.; Ueno, H. Ore and gangue minerals of seafloor hydrothermal deposits in the Mariana trough. JAMSTEC Rep. Res. Dev. 2005, 1, 1–12. [Google Scholar]
- Suzuki, R.; Ishibashi, J.-I.; Nakaseama, M.; Konno, U.; Tsunogai, U.; Gena, K.; Chiba, H. Diverse range of mineralization induced by phase separation of hydrothermal fluid: Case study of the Yonaguni Knoll IV Hydrothermal Field in the Okinawa Trough Back-Arc Basin. Resour. Geol. 2008, 58, 267–288. [Google Scholar] [CrossRef]
- Petersen, S.; Monecke, T.; Westhues, A.; Hannington, M.D.; Gemmell, J.B.; Sharpe, R.; Peters, M.; Strauss, H.; Lackschewitz, K.; Augustin, N.; et al. Drilling shallow-water massive sulfides at the Palinuro volcanic complex, Aeolian island arc, Italy. Econ. Geol. 2014, 109, 2129–2158. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Chen, Y.; Guo, J. A DFT study of the effect of natural impurities on the electronic structure of galena. Int. J. Miner. Process. 2011, 98, 132–136. [Google Scholar] [CrossRef]
- Petersen, S.; Herzig, P.M.; Schwarz-Schampera, U.; Hannington, M.D.; Jonasson, I.R. Hydrothermal precipitates associated with bimodal volcanism in the Central Bransfield Strait, Antarctica. Miner. Depos. 2004, 39, 358–379. [Google Scholar] [CrossRef]
- Lee, C.-S.; Shor, G.G.; Bibee, L.; Lu, R.S.; Hilde, T.W. Okinawa Trough: Origin of a back-arc basin. Mar. Geol. 1980, 35, 219–241. [Google Scholar] [CrossRef]
- Sibuet, J.-C.; Deffontaines, B.; Hsu, S.-K.; Thareau, N.; Le Formal, J.-P.; Liu, C.-S. Okinawa trough backarc basin: Early tectonic and magmatic evolution. J. Geophys. Res. Solid Earth 1998, 103, 30245–30267. [Google Scholar] [CrossRef]
- Kimura, M. Genesis and formation of the Okinawa Trough, Japan. Mem. Geol. Soc. 1990, 34, 77–88. [Google Scholar]
- Shinjo, R.; Kato, Y. Geochemical constraints on the origin of bimodal magmatism at the Okinawa Trough, an incipient back-arc basin. Lithos 2000, 54, 117–137. [Google Scholar] [CrossRef]
- Zeng, Z.-G.; Chen, Z.-X.; Zhang, Y.-X. Zhang. Zircon record of an Archaean crustal fragment and supercontinent amalgamation in quaternary back-arc volcanic rocks. Sci. Rep. 2021, 11, 12367. [Google Scholar] [CrossRef] [PubMed]
- Sibuet, J.-C.; Letouzey, J.; Barbier, F.; Charvet, J.; Foucher, J.-P.; Hilde, T.W.C.; Kimura, M.; Chiao, L.-Y.; Marsset, B.; Muller, C.; et al. Back arc extension in the Okinawa Trough. J. Geophys. Res. Solid Earth 1987, 92, 14041–14063. [Google Scholar] [CrossRef]
- Gena, K.; Chiba, H.; Kase, K.; Nakashima, K.; Ishiyama, D. The Tiger Sulfide Chimney, Yonaguni Knoll IV Hydrothermal Field, Southern Okinawa Trough, Japan: The First Reported Occurrence of Pt-Cu-Fe-Bearing Bismuthinite and Sn-Bearing Chalcopyrite in an Active Seafloor Hydrothermal System. Resour. Geol. 2013, 63, 360–370. [Google Scholar] [CrossRef]
- Shinjo, R.; Chung, S.-L.; Kato, Y.; Kimura, M. Geochemical and Sr-Nd isotopic characteristics of volcanic rocks from the Okinawa Trough and Ryukyu Arc: Implications for the evolution of a young, intracontinental back arc basin. J. Geophys. Res. Solid Earth 1999, 104, 10591–10608. [Google Scholar] [CrossRef]
- Halbach, P.; Hansmann, W.; Köppel, V.; Pracejus, B. Whole-rock and sulfide lead-isotope data from the hydrothermal JADE field in the Okinawa back-arc trough. Miner. Depos. 1997, 32, 70–78. [Google Scholar] [CrossRef]
- Li, X.; Zeng, Z.; Chen, S.; Ma, Y.; Yang, H.; Zhang, Y.; Chen, Z. Geochemical and Sr-Nd-Pb isotopic compositions of volcanic rocks from the Iheya Ridge, the middle Okinawa Trough: Implications for petrogenesis and a mantle source. Acta Oceanolog. Sin. 2018, 37, 73–88. [Google Scholar] [CrossRef]
- Ishibashi, J.-I.; Gamo, T.; Sakai, H.; Nojiri, Y.; Igarashi, G.; Shitashima, K.; Tsubota, H. Geochemical evidence for hydrothermal activity in the Okinawa Trough. Geochem. J. 1988, 22, 107–114. [Google Scholar] [CrossRef]
- Yamano, M.; Uyeda, S.; Foucher, J.-P.; Sibuet, J.-C. Heat flow anomaly in the middle Okinawa Trough. Tectonophysics 1989, 159, 307–318. [Google Scholar] [CrossRef]
- Nakamura, K.; Kawagucci, S.; Kitada, K.; Kumagai, H.; Takai, K.; Okino, K. Water column imaging with multibeam echo-sounding in the mid-Okinawa Trough: Implications for distribution of deep-sea hydrothermal vent sites and the cause of acoustic water column anomaly. Geochem. J. 2015, 49, 579–596. [Google Scholar] [CrossRef]
- Halbach, P.; Nakamura, K.-I.; Wahsner, M.; Lange, J.; Sakai, H.; Käselitz, L.; Hansen, R.-D.; Yamano, M.; Post, J.; Prause, B.; et al. Prause. Probable modern analogue of Kuroko-type massive sulphide deposits in the Okinawa Trough back-arc basin. Nature 1989, 338, 496–499. [Google Scholar] [CrossRef]
- Sakai, H.; Gamo, T.; Kim, E.-S.; Tsutsumi, M.; Tanaka, T.; Ishibashi, J.; Wakita, H.; Yamano, M.; Oomori, T. Venting of carbon dioxide-rich fluid and hydrate formation in mid-Okinawa trough backarc basin. Science 1990, 248, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Gamo, T. Growth mechanism of the hydrothermal mounds at the CLAM Site, Mid Okinawa Trough, inferred from their morphological, mineralogical and chemical characteristics. JAMSTEC Deep Sea Res. 1991, 7, 163–184. [Google Scholar]
- Kawagucci, S.; Toki, T.; Ishibashi, J.; Takai, K.; Ito, M.; Oomori, T.; Gamo, T. Isotopic variation of molecular hydrogen in 20–375 C hydrothermal fluids as detected by a new analytical method. J. Geophys. Res. Biogeosci. 2010, 115, G03021. [Google Scholar] [CrossRef]
- Ishibashi, J.-I.; Ikegami, F.; Tsuji, T.; Urabe, T. Hydrothermal activity in the Okinawa Trough back-arc basin: Geological background and hydrothermal mineralization. In Subseafloor Biosphere Linked to Hydrothermal Systems: TAIGA Concept; Ishibashi, J.-I., Okino, K., Sunamura, M., Eds.; Springer: Tokyo, Japan, 2015; pp. 337–359. [Google Scholar]
- Chiba, M.; Koizumi, A.; Oshika, J.; Tanahashi, M.; Ueda, S.; Ishikawa, N.; Okamoto, N.; Ishizuka, O.; Shimoda, G. Discovery of Sulfide Mounds in the Okinawa Trough; Abstracts with Programs; The Society of Resource: Tokyo, Japan, 2014; Volume 64, p. 24. [Google Scholar]
- Liu, Y.; Hu, Z.; Gao, S.; Günther, D.; Xu, J.; Gao, C.; Chen, H. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Bao, Z.; Chen, L.; Zong, C.; Yuan, H.; Chen, K.; Dai, M. Development of pressed sulfide powder tablets for in situ sulfur and lead isotope measurement using LA-MC-ICP-MS. Int. J. Mass Spectrom. 2017, 421, 255–262. [Google Scholar] [CrossRef]
- Chen, L.; Chen, K.; Bao, Z.; Liang, P.; Sun, T.; Yuan, H. Preparation of standards for in situ sulfur isotope measurement in sulfides using femtosecond laser ablation MC-ICP-MS. J. Anal. At. Spectrom. 2017, 32, 107–116. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, X.; Chen, L.; Bao, Z.; Chen, K.; Zong, C.; Li, X.-C.; Qiu, J.W. Simultaneous measurement of sulfur and lead isotopes in sulfides using nanosecond laser ablation coupled with two multi-collector inductively coupled plasma mass spectrometers. J. Asian Earth Sci. 2018, 154, 386–396. [Google Scholar] [CrossRef]
- Chen, K.; Yuan, H.; Bao, Z.; Zong, C.; Dai, M. Precise and accurate in situ determination of lead isotope ratios in NIST, USGS, MPI-DING and CGSG glass reference materials using femtosecond laser ablation MC-ICP-MS. Geostand. Geoanal. Res. 2014, 38, 5–21. [Google Scholar]
- Yuan, H.; Yin, C.; Liu, X.; Chen, K.; Bao, Z.; Zong, C.; Dai, M.; Lai, S.; Wang, R.; Jiang, S. High precision in-situ Pb isotopic analysis of sulfide minerals by femtosecond laser ablation multi-collector inductively coupled plasma mass spectrometry. Sci. China Earth Sci. 2015, 58, 1713–1721. [Google Scholar] [CrossRef]
- Belzile, N.; Chen, Y.-W.; Cai, M.; Li, Y. A review on pyrrhotite oxidation. J. Geochem. Explor. 2004, 84, 65–76. [Google Scholar] [CrossRef]
- Evensen, N.; Hamilton, P.; O’Nions, R. Rare-earth abundances in chondritic meteorites. Geochim. Cosmochim. Acta 1978, 42, 1199–1212. [Google Scholar] [CrossRef]
- Hongo, Y.; Obata, H.; Gamo, T.; Nakaseama, M.; Ishibashi, J.; Konno, U.; Saegusa, S.; Ohkubo, S.; Tsunogai, U. Rare Earth Elements in the hydrothermal system at Okinawa Trough back-arc basin. Geochem. J. 2007, 41, 1–15. [Google Scholar] [CrossRef]
- Xu, Z.; Lim, D.; Li, T.; Kim, S.; Jung, H.; Wan, S.; Chang, F.; Cai, M. REEs and Sr-Nd isotope variations in a 20 ky-sediment core from the middle Okinawa Trough, East China Sea: An in-depth provenance analysis of siliciclastic components. Mar. Geol. 2019, 415, 105970. [Google Scholar] [CrossRef]
- Steele, J.H.; Thorpe, S.A.; Turekian, K.K. Marine Chemistry and Geochemistry: A Derivative of Encyclopedia of Ocean Sciences, 2nd ed.; Elsevier Science: London, UK, 2010; pp. 601–602. [Google Scholar]
- Shu, Y.; Nielsen, S.G.; Zeng, Z.; Shinjo, R.; Blusztajn, J.; Wang, X.; Chen, S. Tracing subducted sediment inputs to the Ryukyu arc-Okinawa Trough system: Evidence from thallium isotopes. Geochim. Cosmochim. Acta 2017, 217, 462–491. [Google Scholar] [CrossRef]
- Hannington, M.; Herzig, P.; Scott, S.; Thompson, G.; Rona, P. Comparative mineralogy and geochemistry of gold-bearing sulfide deposits on the mid-ocean ridges. Mar. Geol. 1991, 101, 217–248. [Google Scholar] [CrossRef]
- Kawasumi, S.; Chiba, H. Redox state of seafloor hydrothermal fluids and its effect on sulfide mineralization. Chem. Geol. 2017, 451, 25–37. [Google Scholar] [CrossRef]
- Moss, R.; Scott, S.D. Geochemistry and mineralogy of gold-rich hydrothermal precipitates from the eastern Manus Basin, Papua New Guinea. Can. Mineral. 2001, 39, 957–978. [Google Scholar] [CrossRef]
- Scott, S.D.; Kissin, S.A. Sphalerite composition in the Zn-Fe-S system below 300 degrees C. Econ. Geol. 1973, 68, 475–479. [Google Scholar] [CrossRef]
- Yang, B.; Liu, J.; Shi, X.; Zhang, H.; Wang, X.; Wu, Y.; Fang, X. Mineralogy and sulfur isotope characteristics of metalliferous sediments from the Tangyin hydrothermal field in the southern Okinawa Trough. Ore Geol. Rev. 2020, 120, 103464. [Google Scholar] [CrossRef]
- Keith, M.; Häckel, F.; Haase, K.M.; Schwarz-Schampera, U.; Klemd, R. Trace element systematics of pyrite from submarine hydrothermal vents. Ore Geol. Rev. 2016, 72, 728–745. [Google Scholar] [CrossRef]
- Pašava, J.; Vymazalová, A.; Petersen, S.; Herzig, P. PGE distribution in massive sulfides from the PACMANUS hydrothermal field, eastern Manus basin, Papua New Guinea: Implications for PGE enrichment in some ancient volcanogenic massive sulfide deposits. Miner. Depos. 2004, 39, 784–792. [Google Scholar] [CrossRef]
- Pašava, J.; Vymazalová, A.; Petersen, S. PGE fractionation in seafloor hydrothermal systems: Examples from mafic-and ultramafic-hosted hydrothermal fields at the slow-spreading Mid-Atlantic Ridge. Miner. Depos. 2007, 42, 423–431. [Google Scholar] [CrossRef]
- Gamo, T. Wide variation of chemical characteristics of submarine hydrothermal fluids due to secondary modification processes after high temperature water-rock interaction: A review. In Biogeochemical Processes and Ocean Flux in the Western Pacific; Sakai, H., Nozaki, Y., Eds.; Terra Scientific Publishing Company (TERRAPUB): Tokyo, Japan, 1995; pp. 425–451. [Google Scholar]
- Chen, L.-M.; Song, X.-Y.; Danyushevsky, L.; Wang, Y.-S.; Tian, Y.-L.; Xiao, J.-F. A laser ablation ICP-MS study of platinum-group and chalcophile elements in base metal sulfide minerals of the Jinchuan Ni–Cu sulfide deposit, NW China. Ore Geol. Rev. 2015, 65, 955–967. [Google Scholar] [CrossRef]
- Farrow, C.E.; Watkinson, D.H. Diversity of precious-metal mineralization in footwall Cu-Ni-PGE deposits, Sudbury, Ontario; implications for hydrothermal models of formation. Can. Mineral. 1997, 35, 817–839. [Google Scholar]
- Liu, Y.; Brenan, J. Partitioning of platinum-group elements (PGE) and chalcogens (Se, Te, As, Sb, Bi) between monosulfide-solid solution (MSS), intermediate solid solution (ISS) and sulfide liquid at controlled fO2–fS2 conditions. Geochim. Cosmochim. Acta 2015, 159, 139–161. [Google Scholar] [CrossRef]
- Binns, R.A.; Scott, S.D. Actively forming polymetallic sulfide deposits associated with felsic volcanic rocks in the eastern Manus back-arc basin, Papua New Guinea. Econ. Geol. 1993, 88, 2226–2236. [Google Scholar] [CrossRef]
- Wohlgemuth-Ueberwasser, C.C.; Viljoen, F.; Petersen, S.; Vorster, C. Distribution and solubility limits of trace elements in hydrothermal black smoker sulfides: An in-situ LA-ICP-MS study. Geochim. Cosmochim. Acta 2015, 159, 16–41. [Google Scholar] [CrossRef]
- Tivey, M.; Humphris, S.E.; Thompson, G.; Hannington, M.D.; Rona, P.A. Deducing patterns of fluid flow and mixing within the TAG active hydrothermal mound using mineralogical and geochemical data. J. Geophys. Res. Solid Earth 1995, 100, 12527–12555. [Google Scholar] [CrossRef]
- Alt, J.C. The chemistry and sulfur isotope composition of massive sulfide and associated deposits on Green Seamount, eastern Pacific. Econ. Geol. 1988, 83, 1026–1033. [Google Scholar] [CrossRef]
- Mills, R.; Elderfield, H. Rare earth element geochemistry of hydrothermal deposits from the active TAG Mound, 26 N Mid-Atlantic Ridge. Geochim. Cosmochim. Acta 1995, 59, 3511–3524. [Google Scholar] [CrossRef]
- Ishibashi, J.-I.; Urabe, T. Hydrothermal activity related to arc-backarc magmatism in the western Pacific. In Backarc Basins; Springer: New York, NY, USA, 1995; pp. 451–495. [Google Scholar]
- Barrett, T.J.; Jarvis, I.; Jarvis, K.E. Rare earth element geochemistry of massive sulfides-sulfates and gossans on the Southern Explorer Ridge. Geology 1990, 18, 583–586. [Google Scholar] [CrossRef]
- Gillis, K.M.; Smith, A.D.; Ludden, J.N. Trace element and Sr isotopic contents of hydrothermal clays and sulfides from the Snakepit hydrothermal field: ODP site 649. Proc. ODP Sci. Res. 1990, 106, 315–319. [Google Scholar]
- Zeng, Z.; Ma, Y.; Yin, X.; Selby, D.; Kong, F.; Chen, S. Factors affecting the rare earth element compositions in massive sulfides from deep-sea hydrothermal systems. Geochem. Geophys. Geosyst. 2015, 16, 2679–2693. [Google Scholar] [CrossRef]
- Schade, J.; Cornell, D.H.; Theart, H.F.J. Rare earth element and isotopic evidence for the genesis of the Prieska massive sulfide deposit, South Africa. Econ. Geol. 1989, 84, 49–63. [Google Scholar] [CrossRef]
- Sverjensky, D.A. Europium redox equilibria in aqueous solution. Earth Planet. Sci. Lett. 1984, 67, 70–78. [Google Scholar] [CrossRef]
- Wood, S.A.; Williams-Jones, A.E. The aqueous geochemistry of the rare-earth elements and yttrium 4. Monazite solubility and REE mobility in exhalative massive sulfide-depositing environments. Chem. Geol. 1994, 115, 47–60. [Google Scholar] [CrossRef]
- Haas, J.R.; Shock, E.L.; Sassani, D.C. Rare earth elements in hydrothermal systems: Estimates of standard partial molal thermodynamic properties of aqueous complexes of the rare earth elements at high pressures and temperatures. Geochim. Cosmochim. Acta 1995, 59, 4329–4350. [Google Scholar] [CrossRef]
- Allen, D.E.; Seyfried, W., Jr. REE controls in ultramafic hosted MOR hydrothermal systems: An experimental study at elevated temperature and pressure. Geochim. Cosmochim. Acta 2005, 69, 675–683. [Google Scholar] [CrossRef]
- De Baar, H.J.; Brewer, P.G.; Bacon, M.P. Anomalies in rare earth distributions in seawater: Gd and Tb. Geochim. Cosmochim. Acta 1985, 49, 1961–1969. [Google Scholar] [CrossRef]
- Michard, A.; Albarede, F. The REE content of some hydrothermal fluids. Chem. Geol. 1986, 55, 51–60. [Google Scholar] [CrossRef]
- Rimskaya-Korsakova, M.N.; Dubinin, A.V. Rare earth elements in sulfides of submarine hydrothermal vents of the Atlantic Ocean. Dokl. Earth Sci. 2003, 389, 432–436. [Google Scholar]
- Bau, M.; Dulski, P. Comparing yttrium and rare earths in hydrothermal fluids from the Mid-Atlantic Ridge: Implications for Y and REE behaviour during near-vent mixing and for the Y/Ho ratio of Proterozoic seawater. Chem. Geol. 1999, 155, 77–90. [Google Scholar] [CrossRef]
- Langmuir, C.; Humphris, S.; Fornari, D.; Van Dover, C.; Von Damm, K.; Tivey, M.; Colodner, D.; Charlou, J.-L.; Desonie, D.; Wilson, C.; et al. Hydrothermal vents near a mantle hot spot: The Lucky Strike vent field at 37 N on the Mid-Atlantic Ridge. Earth Planet. Sci. Lett. 1997, 148, 69–91. [Google Scholar] [CrossRef]
- Rye, R.O.; Ohmoto, H. Sulfur and carbon isotopes and ore genesis: A review. Econ. Geol. 1974, 69, 826–842. [Google Scholar] [CrossRef]
- Ohomoto, H.; Rye, R.O. Isotopes of sulfur and carbon. In Geochemistry of Hydrothermal Ore Deposits, 2nd ed.; Barnes, H.L., Ed.; J Wiley and Sons: New York, NY, USA, 1979; pp. 509–567. [Google Scholar]
- Sakai, H.; Marais, D.; Ueda, A.; Moore, J. Concentrations and isotope ratios of carbon, nitrogen and sulfur in ocean-floor basalts. Geochim. Cosmochim. Acta 1984, 48, 2433–2441. [Google Scholar] [CrossRef]
- Alt, J.C.; Anderson, T.F.; Bonnell, L. The geochemistry of sulfur in a 1.3 km section of hydrothermally altered oceanic crust, DSDP Hole 504B. Geochim. Cosmochim. Acta 1989, 53, 1011–1023. [Google Scholar] [CrossRef]
- Shanks, W.C.; Böhlke, J.K.; Seal, R.R. Stable isotopes in mid-ocean ridge hydrothermal systems: Interactions between fluids, minerals, and organisms. Geophys. Monogr. Am. Geophys. Union 1995, 91, 194. [Google Scholar]
- Alt, J.C.; Shanks, W.C., III. Serpentinization of abyssal peridotites from the MARK area, Mid-Atlantic Ridge: Sulfur geochemistry and reaction modeling. Geochim. Cosmochim. Acta 2003, 67, 641–653. [Google Scholar] [CrossRef]
- Ueda, A.; Sakai, H. Sulfur isotope study of Quaternary volcanic rocks from the Japanese Islands Arc. Geochim. Cosmochim. Acta 1984, 48, 1837–1848. [Google Scholar] [CrossRef]
- Woodhead, J.D.; Harmon, R.S.; Fraser, D. O, S, Sr, and Pb isotope variations in volcanic rocks from the Northern Mariana Islands: Implications for crustal recycling in intra-oceanic arcs. Earth Planet. Sci. Lett. 1987, 83, 39–52. [Google Scholar] [CrossRef]
- Mottl, M.J.; Holland, H.D.; Corr, R.F. Chemical exchange during hydrothermal alteration of basalt by seawater—II. Experimental results for Fe, Mn, and sulfur species. Geochim. Cosmochim. Acta 1979, 43, 869–884. [Google Scholar] [CrossRef]
- Shanks, W.; Jeffrey, N. Sulfur isotope studies of hydrothermal anhydrite and pyrite, Deep Sea Drilling Project leg 64, Guaymas Basin, Gulf of California. Init. Rep. Deep Sea Drill. Proj. 1982, 64, 1137–1142. [Google Scholar]
- Rees, C.; Jenkins, W.; Monster, J. The sulphur isotopic composition of ocean water sulphate. Geochim. Cosmochim. Acta 1978, 42, 377–381. [Google Scholar] [CrossRef]
- Michard, G.; Albarede, F.; Michard, A.; Minster, J.-F.; Charlou, J.-L.; Tan, N. Chemistry of solutions from the 13 N East Pacific Rise hydrothermal site. Earth Planet. Sci. Lett. 1984, 67, 297–307. [Google Scholar] [CrossRef]
- Butterfield, D.; Massoth, G.J.; McDuff, R.E.; Lupton, J.E.; Lilley, M.D. Geochemistry of hydrothermal fluids from Axial Seamount hydrothermal emissions study vent field, Juan de Fuca Ridge: Subseafloor boiling and subsequent fluid-rock interaction. J. Geophys. Res. Solid Earth 1990, 95, 12895–12921. [Google Scholar] [CrossRef]
- Shanks, W.C., III. Stable isotopes in seafloor hydrothermal systems: Vent fluids, hydrothermal deposits, hydrothermal alteration, and microbial processes. Rev. Mineral. Geochem. 2001, 43, 469–525. [Google Scholar] [CrossRef]
- Fouquet, Y.; Knott, R.; Cambon, P.; Fallick, A.; Rickard, D.; Desbruyères, D. Formation of large sulfide mineral deposits along fast spreading ridges. Example from off-axial deposits at 12 43′ N on the East Pacific Rise. Earth Planet. Sci. Lett. 1996, 144, 147–162. [Google Scholar] [CrossRef]
- Gamo, T.; Sakai, H.; Kim, E.-S.; Shitashima, K.; Ishibashi, J.-I. High alkalinity due to sulfate reduction in the CLAM hydrothermal field, Okinawa Trough. Earth Planet. Sci. Lett. 1991, 107, 328–338. [Google Scholar] [CrossRef]
- Nakagawa, S.; Takai, K.; Inagaki, F.; Chiba, H.; Ishibashi, J.-I.; Kataoka, S.; Hirayama, H.; Nunoura, T.; Horikoshi, K.; Sako, Y. Variability in microbial community and venting chemistry in a sediment-hosted backarc hydrothermal system: Impacts of subseafloor phase-separation. FEMS Microbiol. Ecol. 2005, 54, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Kawagucci, S.; Chiba, H.; Ishibashi, J.-I.; Yamanaka, T.; Toki, T.; Muramatsu, Y.; Ueno, Y.; Makabe, A.; Inoue, K.; Yoshida, N.; et al. Hydrothermal fluid geochemistry at the Iheya North field in the mid-Okinawa Trough: Implication for origin of methane in subseafloor fluid circulation systems. Geochem. J. 2011, 45, 109–124. [Google Scholar] [CrossRef]
- Kawagucci, S. Fluid geochemistry of high-temperature hydrothermal fields in the Okinawa Trough. In Subseafloor Biosphere Linked to Hydrothermal Systems: TAIGA Concept; Ishibashi, J.-I., Okino, K., Sunamura, M., Eds.; Springer: Tokyo, Japan, 2015; pp. 387–403. [Google Scholar]
- Takai, K.; Nakagawa, S.; Nunoura, T. Comparative investigation of microbial communities associated with hydrothermal activities in the Okinawa trough. In Subseafloor Biosphere Linked to Hydrothermal Systems: TAIGA Concept; Ishibashi, J.-I., Okino, K., Sunamura, M., Eds.; Springer: Tokyo, Japan, 2015; pp. 421–435. [Google Scholar]
- Lüders, V.; Pracejus, B.; Halbach, P. Fluid inclusion and sulfur isotope studies in probable modern analogue Kuroko-type ores from the JADE hydrothermal field (Central Okinawa Trough, Japan). Chem. Geol. 2001, 173, 45–58. [Google Scholar] [CrossRef]
- Alt, J.C.; Shanks, W.C.; Bach, W.; Paulick, H.; Garrido, C.J.; Beaudoin, G. Hydrothermal alteration and microbial sulfate reduction in peridotite and gabbro exposed by detachment faulting at the Mid-Atlantic Ridge, 15 20′ N (ODP Leg 209): A sulfur and oxygen isotope study. Geochem. Geophys. Geosyst. 2007, 8, Q08002. [Google Scholar] [CrossRef]
- Delacour, A.; Früh-Green, G.L.; Bernasconi, S.M.; Kelley, D.S. Sulfur in peridotites and gabbros at Lost City (30 N, MAR): Implications for hydrothermal alteration and microbial activity during serpentinization. Geochim. Cosmochim. Acta 2008, 72, 5090–5110. [Google Scholar] [CrossRef]
- Sakai, H. Isotopic properties of sulfur compounds in hydrothermal processes. Geochem. J. 1968, 2, 29–49. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J. Calculation of sulfur isotope fractionation in sulfides. Geochim. Cosmochim. Acta 2006, 70, 1789–1795. [Google Scholar] [CrossRef]
- Vidal, P.; Clauer, N. Pb and Sr isotopic systematics of some basalts and sulfides from the East Pacific Rise at 21 N (project RITA). Earth Planet. Sci. Lett. 1981, 55, 237–246. [Google Scholar] [CrossRef]
- Chen, J. U, Th, and Pb isotopes in hot springs on the Juan de Fuca Ridge. J. Geophys. Res. Solid Earth 1987, 92, 11411–11415. [Google Scholar] [CrossRef]
- Hegner, E.; Tatsumoto, M. Pb, Sr, and Nd isotopes in basalts and sulfides from the Juan de Fuca Ridge. J. Geophys. Res. Solid Earth 1987, 92, 11380–11386. [Google Scholar] [CrossRef]
- Hinkley, T.K.; Tatsumoto, M. Metals and isotopes in Juan de Fuca Ridge hydrothermal fluids and their associated solid materials. J. Geophys. Res. Solid Earth 1987, 92, 11400–11410. [Google Scholar] [CrossRef]
- Fouquet, Y.; Marcoux, E. Lead isotope systematics in Pacific hydrothermal sulfide deposits. J. Geophys. Res. Solid Earth 1995, 100, 6025–6040. [Google Scholar] [CrossRef]
- Charlou, J.; Donval, J.; Fouquet, Y.; Jean-Baptiste, P.; Holm, N. Geochemistry of high H2 and CH4 vent fluids issuing from ultramafic rocks at the Rainbow hydrothermal field (36 14′ N, MAR). Chem. Geol. 2002, 191, 345–359. [Google Scholar] [CrossRef]
- Yao, H.-Q.; Zhou, H.-Y.; Peng, X.-T.; Bao, S.-X.; Wu, Z.-J.; Li, J.-T.; Sun, Z.-L.; Chen, Z.-Q.; Li, J.-W.; Chen, G.-Q. Metal sources of black smoker chimneys, Endeavour Segment, Juan de Fuca Ridge: Pb isotope constraints. Appl. Geochem. 2009, 24, 1971–1977. [Google Scholar] [CrossRef]
- Allègre, C.J.; Hamelin, B.; Dupré, B. Statistical analysis of isotopic ratios in MORB: The mantle blob cluster model and the convective regime of the mantle. Earth Planet. Sci. Lett. 1984, 71, 71–84. [Google Scholar] [CrossRef]
- Hamelin, B.; Dupré, B.; Allègre, C.J. Lead-strontium isotopic variations along the East Pacific Rise and the Mid-Atlantic Ridge: A comparative study. Earth Planet. Sci. Lett. 1984, 67, 340–350. [Google Scholar] [CrossRef]
- Andrieu, A.; Honnorez, J.; Lancelot, J. Lead isotope compositions of the TAG mineralization, Mid-Atlantic Ridge, 26 08′ N. In Ocean Drilling Program; Scientific Results; Elsevier Science: Amsterdam, The Netherlands, 1998; pp. 101–109. [Google Scholar]
- Thompson, G.; Humphris, S.E.; Schroeder, B.; Sulanowska, M.; Rona, P.A. Active vents and massive sulfides at 26 degrees N (TAG) and 23 degrees N (Snakepit) on the Mid-Atlantic Ridge. Can. Mineral. 1988, 26, 697–711. [Google Scholar]
- Davis, A.S.; Clague, D.A.; Zierenberg, R.; Wheat, C.; Cousens, B.L. Sulfide formation related to changes in the hydrothermal system on Loihi Seamount, Hawai’i, following the seismic event in 1996. Can. Mineral. 2003, 41, 457–472. [Google Scholar] [CrossRef]
- Murowchick, J.B. Marcasite inversion and the petrographic determination of pyrite ancestry. Econ. Geol. 1992, 87, 1141–1152. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Z.; Chen, Z.; Qi, H.; Zhu, B. Chemical and Isotopic Composition of Sulfide Minerals from the Noho Hydrothermal Field in the Okinawa Trough. J. Mar. Sci. Eng. 2022, 10, 678. https://doi.org/10.3390/jmse10050678
Zeng Z, Chen Z, Qi H, Zhu B. Chemical and Isotopic Composition of Sulfide Minerals from the Noho Hydrothermal Field in the Okinawa Trough. Journal of Marine Science and Engineering. 2022; 10(5):678. https://doi.org/10.3390/jmse10050678
Chicago/Turabian StyleZeng, Zhigang, Zuxing Chen, Haiyan Qi, and Bowen Zhu. 2022. "Chemical and Isotopic Composition of Sulfide Minerals from the Noho Hydrothermal Field in the Okinawa Trough" Journal of Marine Science and Engineering 10, no. 5: 678. https://doi.org/10.3390/jmse10050678
APA StyleZeng, Z., Chen, Z., Qi, H., & Zhu, B. (2022). Chemical and Isotopic Composition of Sulfide Minerals from the Noho Hydrothermal Field in the Okinawa Trough. Journal of Marine Science and Engineering, 10(5), 678. https://doi.org/10.3390/jmse10050678






