Abstract
As one of the youngest back-arc basins, the evolutionary behavior of magmatic volatiles in the Eastern Manus Basin has been poorly studied. Recently, apatite has received widespread attention for its powerful function in recording information on magmatic volatiles. In this paper, by determining the major element compositions and primary volatile abundances (F, Cl, SO3) of apatites in dacite, we analyze the compositions of volatiles before magma eruption in the Eastern Manus Basin, as well as their indications for magmatic oxygen fugacity and petrogenesis, so as to improve the study about the evolution of magmatic volatiles in this region. Experimental data indicate that apatite saturation temperatures range from 935 to 952 °C. All the apatites are magmatic apatites with F contents of 0.87−1.39 wt.%, Cl contents of 1.24−1.70 wt.%, and SO3 ≤ 0.06 wt.%. Analysis reveals that the apatites in this study crystallized from volatile-undersaturated melts, so their chemical compositions can be used as indicators of magmatic compositions. According to calculations, the minimum S concentrations of the host melts range from 2−65 ppm or 8−11 ppm. The crystallization and separation of magnetite caused the reduction state of melts, and the relatively low oxygen fugacity (ΔFMQ = −0.2 ± 0.9) caused low SO3 contents in apatites. In addition, F and Cl contents of the host melt were calculated to be 185−448 ppm and 1059−1588 ppm, corresponding to the H2O contents of 1.4−2.1% and 1.2−1.5% (error ± 30−40%), respectively. The high Cl/F ratio and H2O contents of samples indicate the addition of slab-derived fluids in the mantle source region of the Eastern Manus Basin. High F contents of the melts may be influenced by F-rich sediments, as well as the release of F from lawsonite and phengite decomposition. High Cl appears to originate from the dual influence of subduction-released fluids and Cl-rich seawater-derived components. Further, it is estimated that 14−21% of the total Cl concentrations in melts were added directly from subduction-released fluids, or higher.
1. Introduction
Volatile components of magmas are important for a majority of silicate melts by influencing magma evolution and eruption processes [1]. Despite their comparatively low concentrations, they can influence the thermodynamic stability of minerals, as well as the density and viscosity of melts, thereby playing a primary role in controlling magma storage depth and the crustal-scale structure of sub-volcanic systems [2,3]. Subduction of altered oceanic crust and sediments are involved in global geochemical cycling of volatiles [4], and their migration of H2O-rich slab fluids to mantle wedge is the primary process of realizing volatile circulation in subduction zones [5,6,7,8]. Halogens in volatiles can effectively trace subduction-released fluids, due to their behavior as fluid-mobile elements [9]. The fluid-mobile element Cl can be used as a tracer for slab-derived fluids [10]. Understanding the compositions of volatiles in subduction zone magmas, and the related magmatic processes, is essential for further insight into magma evolution and volatile circulation in subduction zones.
The Manus Basin is a fast spreading back-arc basin between the New Britain and New Ireland arc in the southwestern Pacific, which is bound by the inactive Manus Trench in the north and New Britain Trench in the south (Figure 1) [11]. A series of young volcanoes extend as an en-echelon series in the eastern part of the Manus Basin, which is known as the Eastern Manus Volcanic Zone [11]. Hydrothermal activity is widely developed [11,12,13,14]. Rock types in this region include basalt, basaltic andesite, andesite, dacite, rhyolitic dacite, and rhyolite. Research on the volcanic rocks of the Eastern Manus Basin is important for understanding magmatic processes and related mineralization in the back-arc basins, which are at the early stage of sea-floor spreading. Based on the geochemical characteristics of the whole rock and main rock-forming minerals (e.g., olivine and plagioclase), considerable work has been done on the magma source region and evolution of the Eastern Manus Basin ([11,14,15,16,17,18,19,20]). However, studies concerning the details of the relevant magmatic processes are still lacking, especially regarding the evolutionary behavior of magmatic volatiles. Only Sun et al. [15] have discussed the geochemical behavior of Cl in arc-type magmas in the Eastern Manus Basin through volcanic glasses.

Figure 1.
Regional geologic map of the Manus Basin, western Pacific (base map database from https://www.gebco.net/data_and_products/gridded_bathymetry_data/ (accessed on 28 February 2022); modified from ref. [12,21]).
Apatite is widely distributed in igneous rocks as an accessory mineral, and its molecular formula is commonly expressed as A5(XO4)3Z [22,23,24,25,26]. The A-site can accommodate large cations, such as Ca2+, Sr2+, Pb2+, Ba2+, Mg2+, Mn2+, Fe2+, REE3+, Eu2+, Cd2+, and Na+. The X-site is mainly occupied by PO43+, and can also accommodate other small highly charged cations, such as Si4+, S6+, As5+, and V5+. The Z-site is usually occupied by F− (fluorapatite), Cl− (chlorapatite), and OH− (hydroxyapatite) [22]. Zhang et al. [27] have pointed out that submarine volcanic rocks are susceptible to seawater and hydrothermal alteration, and volatiles in volcanic glasses may undergo diffusion during the cooling process [28]. Apatite exhibits high resistance to weathering, alteration, and diffusion processes [29] and, since it can incorporate volatiles, such as F, Cl, and S into its lattice, it has become one of the most useful minerals in magmatic systems, in that it can provide information about the volatile concentrations in melts [28]. Apatite has important implications for assisting in determining the volatile compositions of magmatic systems, as well as the fugacities and concentrations of related compounds [30,31,32,33], and it can also provide critical geological information on petrogenesis and mineralization [34,35].
Therefore, we chose apatite, which is highly resistant to alteration and diffusion processes, as the object of this study. By analyzing the F, Cl, OH, and SO3 contents of apatites, we have determined the behavior of volatiles and the redox state of magmas before magma eruption in the Eastern Manus Basin. Further, we have estimated the concentrations of magmatic volatiles, so as to improve understanding of the evolutionary behavior of magmatic volatiles and to supplement details of magmatic evolution in the Eastern Manus Basin; thus, deepening the understanding of magmatism in this region, as well as in the trench-arc-basin system.
2. Geological Background
The Manus Basin (3°−4° S, 149°−152° E) is located in the eastern part of the Bismarck Sea, between the Manus Trench and New Britain Trench [12,16,36,37]. Due to the collision of the Ontong Java Plateau, subduction of the Pacific Plate along the Manus Trench almost stopped, and current volcanic activity in New Britain and northwestern Papua New Guinea is primarily the result of northward subduction of the Solomon Sea Plate along the New Britain Trench [16]. Submarine magnetic anomalies indicate that the Manus Basin was formed by asymmetric sea-floor spreading since 3.5 Ma [21]. GPS defined a rotation vector of 10°12′ N, 33°30′ W, 8.75° Ma−1 for the separation of the North Bismarck Sea plate from the South Bismarck Sea plate, which predicts a spreading rate of 115−145 mm·a−1 for the Manus Basin, indicating that it is one of the fastest spreading basins in the world [16,38]. The spreading of the Manus Basin occurs along several rifts and submarine spreading centers offset by the Willaumez, Djaul, and Weitin transforms, which are known as the Western Spreading Rift (WSR), the Extensional Transform Zone (ETZ), the Manus Spreading Center (MSC), the Southern Rift (SR), and the Southeast Rift (SER), respectively (Figure 1), Their spreading patterns are somewhat different [16,36,37].
Located between the Djaul and Weitin transforms, the SER is the easternmost volcanic zone of the Manus Basin with water depths generally below 2000 m [14], consisting of a series of en-echelon neo-volcanic seafloor ridges and domes [20]. Large areas of exposed submarine lavas, and related high sonar reflectivity, indicate that this area has been the site of recent volcanic activity [39]. Since the SER is located within the shortest distance between the old and new trenches, petrochemistry indicates that it is more intensely influenced by subduction components than several other rifts and spreading centers in the Manus Basin (e.g., [11,39]). GPS shows its total spreading rate to be 137−145 mm·a−1, indicating that it is the fastest spreading part of the Manus Basin [38], and is in the early phases of sea-floor spreading [40]. Abundant hydrothermal activity has been developed in this region [13,41].
3. Sample and Analytical Techniques
3.1. Sample and Petrography
The samples analyzed in this paper were obtained from five stations (TVG7 (151°40′21″ E, 3°43′36.6″ S, depth 1692 m), TVG 8(151°40′20.747″ E, 3°43′36.617″ S, depth 1692 m), TVG 9 (151°40′19.873″ E, 3°43′43.341″ S, depth 1713 m), TVG10 (151°40′19.712″ E, 3°43′40.909″ S, depth 1702 m), TVG 12 (151°40′18.096″ E, 3°43′36.946″ S, depth 1683 m)) by using a TV grab during the cruise of the R/V Kexue Hao in 2015, and all the samples are dacite (Figure 1 and Figure 2).

Figure 2.
Hand specimens of dacite from (a) TVG 7; (b) TVG 8; (c) TVG 9; (d) TVG 10; (e) TVG 12; and transmitted light images of phenocrysts in dacite from (f) and (g) TVG 9; (h) TVG 10 in the Eastern Manus Basin. Abbreviations: Pl—plagioclase, Cpx—clinopyroxene.
The sample hand specimens are black in color and porphyritic, with phenocryst contents <5 vol.%, mainly composed of plagioclase, pyroxene, and minor magnetite. The sample thin sections were observed using a polarized light microscope. The sizes of phenocrysts are 50−600 μm, and they exhibit euhedral to subhedral texture; the matrix is composed of acicular plagioclase micro-crystals, granular pyroxene micro-crystals, and minor granular magnetite micro-crystals with pilotaxitic texture (Figure 2f–h). According to the back-scattering images of the thin sections, apatites exist primarily as micro-crystals in the host dacite, and partly as inclusions in plagioclase, pyroxene, and magnetite phenocrysts, implying the presence of early apatite-saturation in melts (Figure 3a–c) [42]. The apatite micro-crystals in the matrix have a size of ~20 μm, mostly exhibiting equant to sub-equant crystals in euhedral-subhedral texture, and showing uniformly bright surfaces without mineral- or fluid-inclusions (Figure 3d–g), indicating that no hydrothermal replacement has occurred. A very few apatites are highly acicular and large (maximum elongation along the c-axis to more than 300 μm, as in Figure 3h,i), which may precipitate during a rapid quench [24].
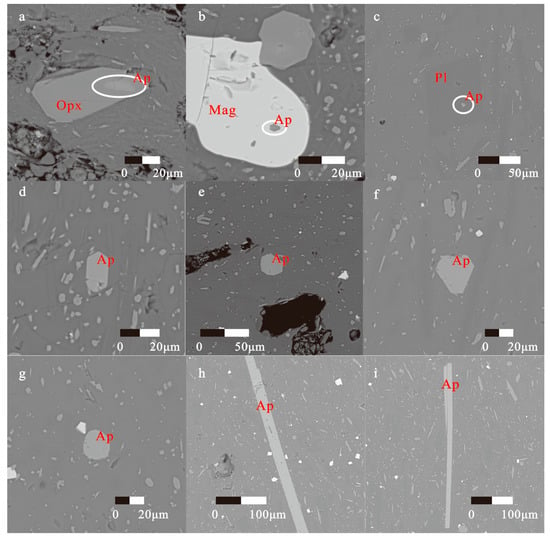
Figure 3.
Back-scattering images of apatites in dacite from the Eastern Manus Basin. (a) Apatite in orthopyroxene; (b) Apatite in magnetite; (c) Apatite in plagioclase; (d–g) Apatite crystals in euhedral-subhedral texture; (h,i) Apatite crystals in highly acicular texture. Abbreviations: Opx—orthopyroxene, Mag—magnetite, Pl—plagioclase, Ap—apatite.
3.2. Analytical Methods
The samples were made into thin sections that met the requirements for onboard testing, then observed and analyzed using a polarized light microscope, scanning electron microscopy (SEM), equipped with an X-ray energy spectrometer, and an electron probe microanalyzer (EPMA).
Preliminary observations were carried out using Axio Scope A1 polarized light microscopy and VEGA3 TESCAN scanning electron microscopy at CAS Key Laboratory of Marine Geology and Environment, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China. The operating conditions of the scanning electron microscope included a voltage of 20 kV, an emission current of 1.4–1.9 nA, and a working distance of 15 mm. Qualitative and semi-quantitative analyses of apatites and other minerals in the thin sections were carried out using the Oxford X-Max20 energy spectrometer fitted to the SEM, which employed an excitation voltage of 20 kV. Quantitative analysis of the mineral compositions was performed using quartz, anorthoclase, and pyrite as specimens. A total of 16 apatite microcrystals in 10 thin sections were finally selected for this study.
Microprobe analyses of apatites were performed using a JOEL JXA-8230 electron microprobe at the State Key Laboratory for Mineral Deposits Research, Nanjing University, Nanjing, China. The operating conditions included an accelerating voltage of 15 kV, a beam current of 20 nA, and, depending on the size of the apatites, a circle size with a diameter of 3 or 5 µm was selected. A total of 33 mineral sites were analyzed for matrix apatite micro-crystals. The standard sample used in this work was the apatite from the American National Bureau of Standards, and the precision of the analysis could reach 0.1%. All test data were corrected by the ZAF (Z, atomic number correction factor; A, X-ray absorption correction factor; F, fluorescence correction factor) program.
4. Results
4.1. Compositions of Apatites
The results of the analysis are shown in Table S1, which includes calculations of OH concentrations of apatites using the method of Ketcham [43], and XF, XCl, and XOH are molar fractions of F, Cl, and OH. It can be seen that the apatite samples all belong to magmatic apatite with high hydroxyl contents (Table S1 in Supplementary Materials, Figure 4 and Figure 5). The dominant components of apatite samples were CaO and P2O5, which exhibited a range of 53.08−54.55% and 40.95−42.55%, respectively. Moreover, the apatite samples exhibited the characteristics of Cl-rich and F-poor with contents of 1.24−1.70% and 0.87–1.39%, respectively, which were between “mantle apatite A” and “mantle apatite B” [44] (Figure 6a). Compared with apatites in dacite from other subduction systems of the western Pacific, our apatites had higher Cl contents, as well as lower F and SO3 contents [42,45] (Figure 6a,b).
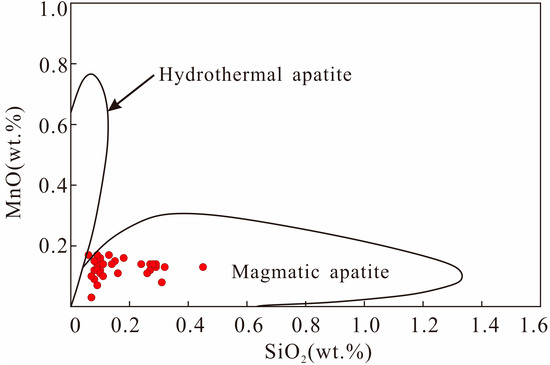
Figure 4.
Plots of MnO versus SiO2 in apatites of dacite ( modified from ref. [46]).
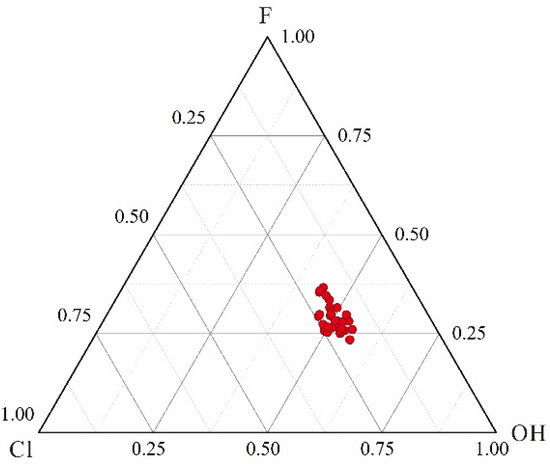
Figure 5.
Volatile contents (molar fraction) of apatites from the dacite.
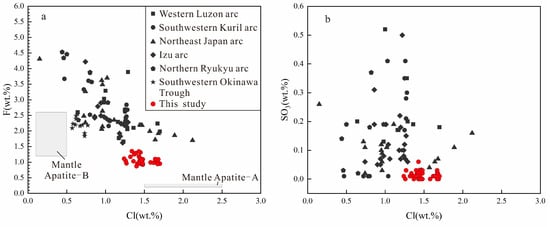
Figure 6.
Plots of (a) F versus Cl in apatites of dacite; (b) SO3 versus Cl in apatites of dacite (data from [42,45] and this study).
4.2. Apatite Saturation Temperatures (AST)
Mostly, the elemental partitioning between apatites and melts varies significantly with temperature [25], so before calculating the individual elemental components of melts, we needed to know the equilibrium temperature of the system apatite-melt. Here we used the apatite saturation temperature () proposed by Harrison and Watson [47] to constrain the equilibrium temperature, where the . Remarkably, there is no reliable method to accurately estimate the instantaneous concentration of melt P2O5 (i.e., ) at the time of apatite crystallization, but given that the samples in this study were all from felsic melts with low crystallization, the whole rock P2O5 concentrations could be approximated here as the P2O5 concentrations of melt. Although there will be some errors, the correction should be small and have little effect on the results [22]. As shown in Table 1, the AST of the apatite samples ranged from 935 to 952 °C.

Table 1.
Apatite saturation temperatures of apatite samples.
5. Discussion
5.1. Behavior of Magmatic Volatiles
When the magmas have reached volatile-saturated conditions, melts will be highly depleted with fluid exsolution, as fluid-mobile elements (e.g., Cl) prefer to enter the fluid phase [48]. Apatites crystallized in such a state could not indicate the compositions of primitive melts well. Hence, before using apatites to extrapolate the compositions of primitive magmas, it was necessary to determine whether volatiles in the melts became saturated before and during the crystallization of apatites. Stock et al. [1] developed a model to determine whether magmatic volatiles have reached saturation by the relative relationship between contents of F, Cl, and OH as well as their ratios in apatites. Combined with this model, as shown in Figure 5 and Figure 7, XF/XCl of the apatite samples exhibited a decreasing trend, while XCl/XOH and XF/XOH exhibited increasing trends, implying that those apatites crystallized under a volatile-undersaturated condition [1]. Therefore, the compositions of apatites in this study could be useful indicators for their host magmas.
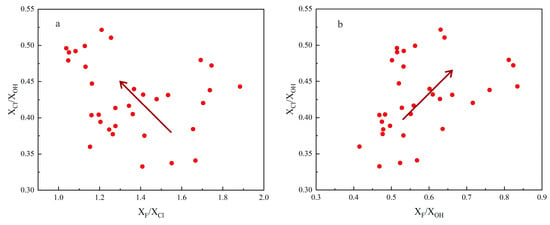
Figure 7.
Plots of (a) XCl/XOH versus XF/XCl in apatites of dacite; (b) XCl/XOH versus XF/XOH in apatites of dacite. The arrow shows the trajectory of apatite compositional evolution.
5.2. Magmatic S Contents and Redox State
5.2.1. Magmatic S Concentrations
The S concentration in apatite is related to that in melts during the crystallization of apatite, so it can trace the S contents of melts [49]. Although there is no formula to accurately calculate the S concentrations of magmas from the SO3 contents of apatites [50], if the chemical exchange between apatites and their host melts can be neglected after their formation, the relative S contents of magmas can be estimated according to two semi-quantitative formulas given by Peng et al. [29] and Parat et al. [28], respectively [49]. The apatite micro-crystals in this study were morphologically intact, small in size, and without zoning, indicating their short contact time with melts after their formation, as well as limited chemical exchange between the two. Thus, we estimated the S contents of melts using the above two semi-quantitative formulae. As shown in Table S2 in Supplementary Materials, the S concentrations of the host melt ranged from 2−65 ppm or 8−11 ppm, which is consistent with the range of S contents in silicate melts produced by partial melting of subduction-related mantle wedge (<4000 ppm [51]). It should be noted that S exists in magmatic systems in five main valence states, including S2−, S1−, S0, S4+, and S6+ [52]. Since S is present mainly as SO42− in apatite lattice, the S contents obtained from our analysis were only related to the concentrations of SO42− (S6+) in the melts, while there may also be considerably reduced sulfur in the melts (more details will be discussed in Section 5.2.2), so the values we obtained here were probably just the minimum S contents of melts [53].
From previous studies, the SO3 content of apatite is a complex function of magmatic temperature, sulfur fugacity and redox state [28,29,54]. According to Peng et al. [29], the partitioning of S between apatites and their host melts is highly dependent on the redox state of the latter. Combining Table 1 and Table S2 in Supplementary Materials, the apatites studied crystallized in narrow ranges of temperature and sulfur fugacity, and their SO3 contents remained essentially stable with magmatic differentiation (Figure 8), which tends to indicate that the magma has a stable redox state during this evolution stage.
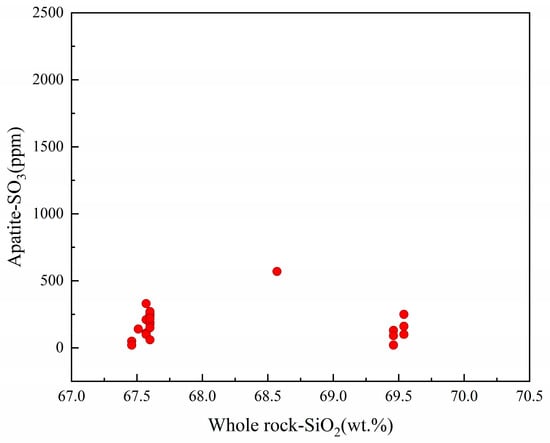
Figure 8.
Correlation between SO3 concentrations in apatites and magmatic differentiation.
5.2.2. Indication for Oxygen Fugacity of Melts
Affected by the magmatic oxygen fugacity, Mn ions may enter the apatite lattice in three valence states: Mn2+, Mn3+ and Mn5+. They enter the apatite lattice through the following displacement reactions [55]:
Mn2+ = Ca2+
Mn3+ + SiO44− = Ca2+ + PO43−
Mn3+ + Na+ = 2Ca2+
MnO43− = PO43−
When the above reactions have occurred, there will be an obvious negative correlation between the ions that undergo displacement. In apatite samples, obviously, no negative correlations are shown between Mn and Ca as well as Mn + Na and 2Ca (Figure 9a,b), while a significant negative correlation exists between Mn + Si and Ca + P (Figure 9c), and a moderate negative correlation between Mn and P (Figure 9d). Therefore, it can be concluded that Mn in our samples entered the apatite lattice mainly as Mn3+, and brought SiO44− into the lattice simultaneously. In addition, a few Mn replaced PO43− as MnO43− in the apatite lattice. It indicates that apatites from this study crystallized in a medium oxygen fugacity condition, and the oxygen fugacity only slightly changed during this process.
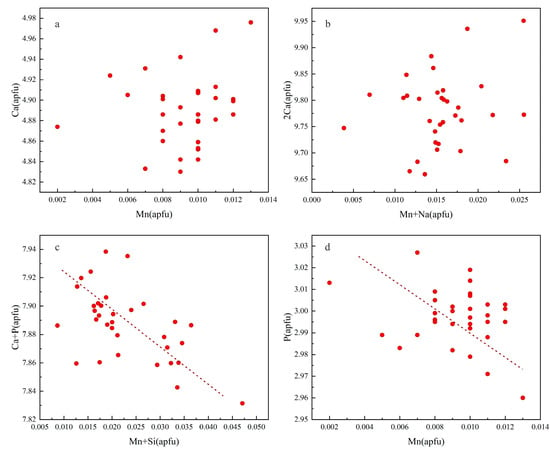
Figure 9.
Covariant relations of cations in apatites from the dacite. (a) Ca versus Mn; (b) 2Ca versus Mn + Na; (c) Ca + P versus Mn + Si; (d) P versus Mn.
The quantitative estimation of the redox state can be achieved by calculating the oxygen fugacity (). The oxygen fugacity is a tool to assess the availability and potential of oxygen to participate in reactions between minerals and fluids. Concentrations of redox-sensitive elements, such as Fe, Mn, Ce, and Eu, in accessory minerals are important for obtaining the redox states of magmas [56]. Miles et al. [56] found that the variation of Mn contents of apatites in intermediate-acid silicate melts is relatively independent, so that the redox states of magmas can be reliably estimated based on the Mn contents of apatites. To further clarify the redox state of magmas and their stabilities, we estimated the absolute and relative oxygen fugacities of the host melts according to MnO contents of apatite samples using the equations given by Miles et al. [56], as well as Myers and Eugster [57]. Since the FMQ oxygen buffer is minimally influenced by pressure [58], the relative oxygen fugacities (ΔFMQ) of melts were calculated here with the oxygen fugacities of the FMQ oxygen buffer as the reference (Table S2 in Supplementary Materials).
The mantle wedge above subduction zones is oxidized relative to the mantle from other tectonic settings [59,60,61], and the water-bearing intermediate silicate magmas in such regions generally have intermediate oxygen fugacity conditions (from general reducing to general oxidizing conditions) [62]. As regards this study, except for one anomalously high value (ΔFMQ = +1.4), all values showed that the host melt was under the reduction condition with a narrow fluctuation range (ΔFMQ = −0.2 ± 0.9, Table S2 in Supplementary Materials). Kleinsasser et al. [62] found experimentally that dacitic melts under redox conditions of ΔFMQ − 0.7~ΔFMQ + 2.08 would remain both sulfide- and sulfate- undersaturated, which is consistent with the observations and calculations of this study. The trace element compositions of dacite in the Eastern Manus Basin distinctly exhibited the characteristics of island arc volcanic rocks, such as enrichment in light rare earth elements (LREE) and large ionic lithophile elements (LILE) [11,14,18]. However, this study indicates that the oxygen fugacity of dacitic magmas in the Eastern Manus Basin is lower than that of island-arc magmas influenced by subduction components (ΔFMQ = +1~+2, [63,64,65]). Two reasons may account for this phenomenon: (1) As shown in previous studies, processes such as crystallization and separation of closed-system, crustal contamination, and water degassing (≤8 wt.%) have just a slight impact on magmatic oxygen fugacity, while sulfur degassing and addition of subduction components have considerable effects on redox states of magmas (e.g., [66,67,68,69]). According to the discussion in 5.1, the host magma has not yet reached fluid saturation, and it is also unlikely to have reached S saturation [70]; the relatively low oxygen fugacities of the dacite samples may be inherited from the mantle source region. However, this is obviously inconsistent with the high oxygen fugacity of the mantle wedge in the subduction zones; (2) Richards [65] found that crystallization and segregation of Fe3+-rich magnetite can result in a reduction of reduced to moderately oxidized magmas, which could be offset by oxidation from degassing of reducing or weakly oxidizing gases (e.g., H2, H2S, etc.), as well as dissolution and migration of Fe2+ in high-temperature saline hydrothermal fluids. The saturation of apatites in the Eastern Manus Basin was concomitant with the saturation of magnetite, and they crystallized from undegassed melts (see the description in Section 3.1, Figure 3b, and previous studies [71] for details). Furthermore, hydrothermal activity is widely developed in the Eastern Manus Basin, but the oxidation process from high-temperature hydrothermal fluids may be slow [65]. When the apatites in melts reached saturation and started crystallizing, the intensity of oxidation from the hydrothermal fluids may not yet have been sufficient to offset the decrease in oxygen fugacity, due to the crystallization and separation of magnetite. The above factors collectively caused the oxygen fugacity reduced from ΔFMQ ≈ +1~+2 in the mantle source region to ΔFMQ = −0.2 ± 0.9 in the host magma recorded by apatites. Apparently, the second explanation is more reasonable. Under such a low oxygen fugacity condition, S existed mainly as low-valence states in melts, which caused the low SO3 contents of apatites in this study.
5.3. F, Cl and H2O Contents of Melts
Apatite is one of the rare magmatic minerals containing halogens. For extrusive and hypabyssal rocks, the rapid cooling of magmas allows the preservation of information about magmatic F, Cl, and H2O contents recorded by apatite during its crystallization [72]. Further, apatites crystallized at temperatures above 500 °C are resistant to hydrothermal alteration [73,74], therefore they can be used to deduce the F, Cl, and H2O contents of the host magma.
5.3.1. F, Cl Contents of Melts
Before deducing the volatile contents of melts by using that of apatites, the partition behavior of volatiles between the two phases needs to be known [75]. Since F, Cl, and OH occupy the same position in the apatite lattice, their abundances are controlled by stoichiometric numbers, which prevent the use of Nernst partition coefficients for the estimations of F, Cl, and OH contents in melts [76]. To better represent partitioning behavior between apatites and the host melts, previous works have proposed the exchange coefficient Kd involving two volatile elements (e.g., [75,77,78]), which can be expressed as the molar fraction (i.e., ) of two anions (e.g., A and B) in both apatites and the host melt:
In this paper, we used the regular ternary solution model for apatite established by Li and Hermann [78], combined with the modified Kd expression by Li and Costa [75], to calculate the F, Cl contents of melts as follows:
where T was substituted by AST calculated in 4.2, in K. With the method described above, the F and Cl contents of the host melt were estimated to be about 185−448 ppm and 1059−1588 ppm, respectively (Table S3 in Supplementary Materials), which are consistent with the range of that in subduction-related dacite (0.01−0.15 wt.% for F and 0.01−0.3 wt.% for Cl, [79]).
Magmatic F and Cl contents will differ widely depending on tectonic settings and magma compositions [80]. At concentrations of a few tens to 1000 ppm, they will not affect the fundamental magma properties (e.g., melting behavior and melt rheology) [79]. The Cl content of our dacite samples exhibited relatively high values. Cl at such concentrations may have implications on the composition and timing of exsolved fluids, as well as on the rheology of host magma [81,82,83,84]. From Figure 6a, the F and Cl contents of the apatite samples were similar to the “mantle apatite A”, which is formed by the account of Cl-, CO2- and H2O-rich fluids, indicating that the host melt might have been influenced by exotic Cl-rich components. Moreover, F is highly compatible with melt, while Cl behaves as a fluid-mobile element [79], so the high Cl/F ratio in the samples indicates the addition of slab-derived fluids in the parent magmas [35], which is parallel with the results of previous studies on volcanic rocks in the Eastern Manus Basin (e.g. [16,18,39,85]). Additionally, the non-negative correlation between the Cl/F ratio and SiO2 contents of samples verifies again that the melt remained in a fluid-undersaturated condition (Figure 10).
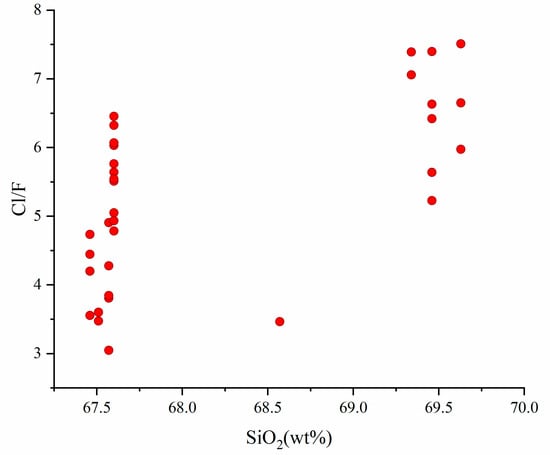
Figure 10.
Plots of Cl/F versus SiO2 in dacitic melts of the Eastern Manus Basin.
5.3.2. Abundance of H2O in Melts
Boyce and Herving [86] found that the variation of F and OH in apatites can be well-matched with their variation in coexisting melts, indicating that OH is a valid indicator for evaluating the H2O content of coexisting melts. Since the water in melts exists as both molecular water (H2Om) and hydroxyl (OH) [87], the calculation of its molar fraction is complicated. Li and Costa [75] developed an online calculation program based on the thermodynamic model for apatite. This program for the determination of H2O concentrations in multicomponent silicate melts is based on a series of thermodynamic formulae. As mentioned above, the exchange coefficients Kd between apatite-melt can be obtained by using AST as well as F, Cl, and OH contents of apatites, and from that, and can be calculated. Furthermore, according to Li and Costa [75], , , and can be expressed by the mass fractions of F, Cl, and H2O (, , and , respectively) in melts as follows:
where W is the molar fraction of dry silicate melts, which was taken here as 33 g/mol [75]; is the molar fraction of total water in melts; is the equilibrium constant for the conversion from H2Om to OH:
According to Liu et al. [88], for dacite, a equals 1.49 and b equals −2634. Concentrations of H2O in the Eastern Manus Basin dacite obtained by this calculation program ranged from 1.4−2.1 wt. % (based on F concentrations in melts, error ±30−40%) and 1.2−1.5 wt.% (based on Cl concentrations in melts, error ±30−40%), respectively (Table S4 in Supplementary Materials). Sun et al. [15] have measured H2O contents of volcanic glasses in the East Manus Basin, and the results ranged from 1.11−1.67 wt.%, which is generally consistent with the range we estimated, indicating that the results obtained here should be reliable.
Previous studies have revealed that the H2O contents of MORB magmas are generally in the range of 100−450 ppm [89], while the primary melts of basalts in subduction zones generally contain higher H2O contents, indicating the influence of water as a fluid phase transferred from the subduction slab to the mantle wedge [90]. The H2O contents of dacitic magmas in the Eastern Manus Basin are obviously higher than those of MORB magmas, again indicating the addition of slab fluids. To be noted, in previous linear regression calculations of Kd, the low F contents in experimental melts caused a larger analysis error than that of Cl [75]. Thus, the H2O contents calculated from Cl concentrations may be closer to the true value, which indicates that the magma chamber of dacitic melts in the Eastern Manus Basin is located about 2 km below the seafloor at a pressure of 0.05 GPa−0.1 GPa [15].
5.4. Sources of F- and Cl-Rich Components
The F and Cl concentrations in melts of the Eastern Manus Basin are significantly higher than those in the primitive mantle (25 ppm F and 17 ppm Cl, [91]). Moreover, since halogens are incompatible with mantle minerals, their concentrations will be further lost in the mantle during its partial melt [92]. Subduction is an important mechanism for the circulation of halogens from surface reservoirs to the mantle [9,92,93]. According to the above discussion, magmas in the Eastern Manus Basin are influenced by the incorporation of subduction components during subduction. Moreover, numerous previous studies have also proven that magmas in this region are obviously influenced by subduction components (mainly by fluids released from the subducting altered oceanic crust, as well as a little sediment melt, e.g. [16,18,39,85] and references therein), yet no significant magmatic mixing and crustal contamination occurred during magma evolution. Hence, the high F and Cl concentrations of our samples may be related to the pollution in the mantle source region.
Seawater has a high Cl, but low F, abundance, which is 19,500 ppm and 1.3 ppm respectively [93]. Therefore, the direct addition of seawater is too low to explain the enrichment of F in samples. In contrast, sediments near subduction zones may be a reasonable source of F; for example, up to 1300 ppm in pelagic clay [94]. The repeated leaching of overlying sediments and assimilation of oceanic crust near the subduction zones by seawater may cause an increase of F contents in the oceanic crust before its subduction [92]. Since F is an incompatible element with fluids [79], the enrichment of F caused by the above process may not be enough. According to Pagé et al. [92], apatite is the mineral phase that most preferentially accommodates F in subduction zones. F may be redistributed to phengite and lawsonite as apatite eventually decomposes at ∼200 km, then lawsonite and phengite decomposition releases F to the deeper upper mantle at ∼280–300 km in cold subduction zones [35,92], which could be another reasonable source of F-rich components. Additionally, the F-rich seafloor sediments could also contribute F efficiently to the mantle source region as melts during subduction.
Studies of back-arc magmatism have indicated that slab fluids contributing to magmatism in back-arc basins are Cl-rich, as shown by off-axis seamount lavas of the Lau Basin and submarine basaltic glasses of the Mariana back-arc trough, both of which exhibit Cl-rich characteristics due to subduction [6,10,95]. Although the contribution of slab fluids can be identified with Cl/K2O and Cl/TiO2 to Ba/Nb ratios, the mobility of K differs from that of Cl, and the value of Cl/TiO2 changes during magma evolution, since Cl is more incompatible than Ti [10]. So neither Cl/K2O nor Cl/TiO2 is appropriate for quantifying the contribution of subduction-released fluids to Cl. Sun et al. [15] indicated that the plots of Cl/K2O and Cl/TiO2 versus Ba/Nb can be refined with the plots of Cl/Nb versus Ba/Nb and U/Nb. Cl, Ba, U, and Nb are incompatible elements, where Ba and U are fluid-mobile LILE and the behavior of Cl resembles them, while Nb is one of the most inactive elements during subduction [15,48,96,97]. The ratios of Cl, Ba, U to Nb can maximally offset the partial melting of the mantle, as well as the crystallization and differentiation of magmas, thereby providing a useful indication of the mobility of these elements during subduction. Here, we plot the Cl/Nb of our samples and the collected volcanic rocks of other subduction systems in the western Pacific against Ba/Nb and U/Nb, respectively (Figure 11; Cl in our samples was calculated from apatite components, and the rest of data are shown in Table S4).
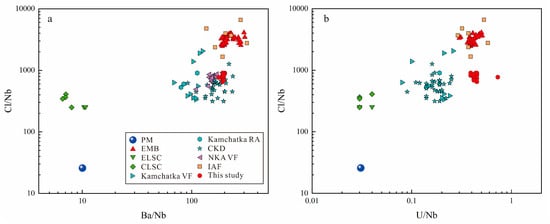
Figure 11.
Plots of (a) Cl/Nb versus Ba/Nb and (b) Cl/Nb versus U/Nb in subduction-related volcanic rocks (data from: PM, Primitive Mantle [91]; EMB, Eastern Manus Basin [15]; ELSC, Eastern Lau Spreading Center [10,98]; CLSC, Central Lau Spreading Center [10,98]; Kamchatka VF, volcanic front of Kamchatka [99]; Kamchatka RA, rear-arc of Kamchatka [99]; CKD, The Central Kamchatka Depression [99]; NKA VF, volcanic front of Northern Kurile Arc [99]; IAF, Izu arc front [93]).
Figure 11 indicates that, compared to the primitive mantle, the mantle source of this study exhibited intense enrichment of Cl, Ba, and U, implying that it has been influenced by Cl-, Ba-, and U-rich components. Such components are most probably the subduction-released fluids [10]. Moreover, the elemental ratios of samples in this study and volcanic rocks from other subduction-related systems did not exhibit a single linear trend with the primitive mantle. At the same Cl/Nb values, volcanic rocks from the Kamchatka, Izu arc, and Lau basin exhibit lower Ba/Nb and U/Nb values relative to those of this study, which appears to indicate the influence of other Cl-rich and Ba-, U-poor components.
Kent et al. [10] indicated that shallow assimilation of Cl-rich seawater derived components during magma transport, storage, or eruption in submarine environments can also result in increased Cl concentrations in magmas, and, compared to subduction-released fluids, such materials are characterized as Ba- and U-poor. Sun et al. [15] also found that the samples were commonly influenced by Cl-rich seawater derived components through the analysis of matrix glasses in several back-arc basins and active spreading ridges, including the Eastern Manus Basin. Compared to the matrix glasses of the Eastern Manus Basin analyzed by Sun et al. [15], samples in this study had similar Ba/Nb and U/Nb values, but lower Cl/Nb values, indicating that samples in this study were relatively poorly influenced by Cl-rich seawater derived components. Kent et al. [10] analyzed matrix glasses of the Eastern Lau Spreading Center and Central Lau Spreading Center, finding that their Cl/Nb ratios are significantly higher than that of the primitive mantle, while the Ba/Nb values are low to the MORB, which indicated the minimal subduction components input, hence they considered that the high Cl/Nb ratios in magmas are the result of assimilation by Cl-rich seawater-derived components. As shown in Table S4, the Cl/Nb values of our dacite samples ranged from 649 to 909, while the contribution of the primitive mantle lay around 26 only [91]. Taking the average of the Cl/Nb values in magmas from the Eastern Lau Spreading Center and Central Lau Spreading Center (about 400 [15]) as the influence from Cl-rich seawater derived components, then approximately 14−21% of Cl in the Eastern Manus Basin magma source region is contributed by subduction-released fluids, and mainly by fluids released from subducting altered oceanic crust. However, as discussed above, the mantle source region of this study has been less influenced by Cl-rich seawater derived components, so the contribution of subduction-released fluids to Cl may be higher.
6. Conclusions
In this study, dacite from the Eastern Manus Basin was selected as the object for study, and apatites in dacite were analyzed by EMPA. The results were as follows:
- (1)
- Apatites in dacite samples crystallized under a volatile-undersaturated condition with temperatures ranging from 935 to 952 °C. Compared with apatites in dacite of other subduction systems in the western Pacific, they exhibited rich in Cl but poor in F and SO3.
- (2)
- The minimum S contents of the host melts have a narrow range of 2−65 ppm and 8−11 ppm. Additionally, the host magma was under weak reduction conditions, and the relatively low oxygen fugacity could be caused by reduction brought from crystallization and separation of Fe3+-rich magnetite, which also caused the low SO3 contents in apatites.
- (3)
- The F and Cl contents of melts in this study were within the range of subduction-related dacite, which is 185−448 ppm and 1059−1588 ppm, respectively, and such relatively high Cl concentrations of melts may have an impact on the compositions and exsolution of magmatic fluids, as well as on magmatic rheology. Further, the H2O contents of melts were deduced to be in the range of 1.4−2.1% and 1.2−1.5% based on the F and Cl contents of melts, respectively, which indicates the location of the magma chamber at a depth of ~2 km. The high Cl/F ratio and H2O contents of samples indicated the addition of slab-derived fluids in the parent magmas.
- (4)
- The high F and Cl values exhibited in melts may come from both pollution in the magma source region, where the F may be influenced by F-rich seafloor sediments, as well as the release of F from lawsonite and phengite decomposition; high Cl may come from slab fluids and shallow assimilation of Cl-rich seawater derived components, where the Cl contributed by slab fluids to the mantle source region may account for 14−21% of the total Cl contents in melts, and possibly even higher.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmse10050698/s1, Tables S1–S4 and Text S1. Table S1: Abundances of major elements (wt.%) in apatites from the dacite; Table S2: Estimations of S concentrations and magmatic redox states from compositions in apatite and whole-rock; Table S3: Calculations of F, Cl and H2O contents in melts from apatite compositions; Table S4: Cl and representative trace element concentrations of volcanic rocks from this study and other subduction related systems (ppm); Text S1 include the formulas used for calculations and the references of Tables S1–S4.
Author Contributions
Methodology, X.D. and Z.C.; software, X.D.; formal analysis, X.D.; validation, X.D.; investigation, X.D.; resources, Z.Z.; data curation, X.D.; writing—original draft preparation, X.D.; writing—review and editing, X.D., Z.C. and Z.Z.; visualization, X.D.; supervision, Z.Z.; project administration, Z.Z.; funding acquisition, Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 91958213), Strategic Priority Research Program (B) of the Chinese Academy of Sciences (Grant No. XDB42020402), National Basic Research Program of China (Grant No. 2013CB429700), and Special Fund for the Taishan Scholar Program of Shandong Province (Grant No. ts201511061).
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are given in the Supporting Information.
Acknowledgments
We are grateful for the valuable comments and suggestions from the anonymous reviewers and editors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stock, M.J.; Humphreys, M.C.S.; Smith, V.C.; Isaia, R.; Brooker, R.A.; Pyle, D.M. Tracking volatile behaviour in sub-volcanic plumbing systems using apatite and glass: Insights into pre-eruptive processes at Campi Flegrei, Italy. J. Petrol. 2018, 59, 2463–2491. [Google Scholar] [CrossRef] [Green Version]
- Howarth, G.H.; Pernet-Fisher, J.F.; Bodnar, R.J.; Taylor, L.A. Evidence for the exsolution of cl-rich fluids in martian magmas: Apatite petrogenesis in the enriched lherzolitic shergottite northwest Africa 7755. Geochim. Cosmochim. Acta 2015, 166, 234–248. [Google Scholar] [CrossRef]
- Annen, C.; Blundy, J.D.; Sparks, R.S.J. The genesis of intermediate and silicic magmas in deep crustal hot zones. J. Petrol. 2006, 47, 505–539. [Google Scholar] [CrossRef] [Green Version]
- Wallace, P.J. Volatiles in subduction zone magmas: Concentrations and fluxes based on melt inclusion and volcanic gas data. J. Volcanol. Geotherm. Res. 2005, 140, 217–240. [Google Scholar] [CrossRef]
- Anderson, A. Parental basalts in subduction zones—Implications for continental evolution. J. Geophys. Res. 1982, 87, 7047–7060. [Google Scholar] [CrossRef]
- Stolper, E.; Newman, S. The role of water in the petrogenesis of mariana trough magmas. Earth Planet. Sci. Lett. 1994, 121, 293–325. [Google Scholar] [CrossRef]
- Grove, T.L.; Parman, S.W.; Bowring, S.A.; Price, R.C.; Baker, M.B. The role of an H2O-rich fluid component in the generation of primitive basaltic andesites and andesites from the Mt. Shasta region, N California. Contrib. Mineral. Petrol. 2002, 142, 375–396. [Google Scholar] [CrossRef]
- Manning, C.E. The chemistry of subduction-zone fluids. Earth Planet. Sci. Lett. 2004, 223, 1–16. [Google Scholar] [CrossRef]
- Barnes, J.D.; Manning, C.E.; Scambelluri, M.; Selverstone, J. The behavior of halogens during subduction-zone processes. In The Role of Halogens in Terrestrial and Extraterrestrial Geochemical Processes; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Kent, A.J.R.; Peate, D.W.; Newman, S.; Stolper, E.M.; Pearce, J.A. Chlorine in submarine glasses from the Lau Basin: Seawater contamination and constraints on the composition of slab-derived fluids. Earth Planet. Sci. Lett. 2002, 202, 361–377. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Binns, R.A.; Gemmell, J.B.; Crawford, A.J.; Mernagh, T.P.; Maas, R.; Steele, D. Parental basaltic melts and fluids in eastern Manus backarc Basin: Implications for hydrothermal mineralisation. Earth Planet. Sci. Lett. 2001, 184, 685–702. [Google Scholar] [CrossRef]
- Miller, D.J.; Vanko, D.A.; Paulick, H. Data report: Petrology and geochemistry of fresh, recent dacite lavas at Pual Ridge, Papua New Guinea, from an active, felsic-hosted seafloor hydrothermal system. Proc. Ocean Drill. Program Sci. Results 2007, 193, 1–31. [Google Scholar] [CrossRef]
- Yang, K.; Scott, S.D. Magmatic degassing of volatiles and ore metals into a hydrothermal system on the modern sea floor of the eastern Manus Back-Arc Basin, western Pacific. Econ. Geol. 2002, 97, 1079–1100. [Google Scholar] [CrossRef]
- Beier, C.; Bach, W.; Turner, S.; Niedermeier, D.; Woodhead, J.; Erzinger, J.; Krumm, S. Origin of silicic magmas at spreading centres—An example from the south east Rift, Manus Basin. J. Petrol. 2015, 56, 255–272. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.D.; Binns, R.A.; Fan, A.C.; Kamenetsky, V.S.; Wysoczanski, R.; Wei, G.J.; Hu, Y.H.; Arculus, R.J. Chlorine in submarine volcanic glasses from the eastern Manus Basin. Geochim. Cosmochim. Acta 2007, 71, 1542–1552. [Google Scholar] [CrossRef]
- Martinez, F.; Taylor, B. Controls on back-arc crustal accretion: Insights from the Lau, Manus and Mariana Basins. Geol. Soc. Lond. Spec. Publ. 2003, 219, 19–54. [Google Scholar] [CrossRef]
- Siegburg, M.; Klügel, A.; Rocholl, A.; Bach, W. Magma plumbing and hybrid magma formation at an active back-arc basin volcano: North Su, Eastern Manus Basin. J. Volcanol. Geotherm. Res. 2018, 362, 1–16. [Google Scholar] [CrossRef]
- Yao, M.A.; Zeng, Z.; Chen, S.; Yin, X.; Wang, X. Origin of the volcanic rocks erupted in the eastern Manus Basin: Basaltic andesite-andesite-dacite associations. J. Ocean Univ. China 2017, 16, 389–402. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, L.; Sun, J.; Huang, P.; Yan, L.I.; Gao, Y. Petrogenesis of volcanic rocks from eastern Manus Basin: Indications in mineralogy and geochemistry. J. Oceanol. Limnol. 2021, 39, 21. [Google Scholar] [CrossRef]
- Seewald, J.S.; Reeves, E.P.; Bach, W.; Saccocia, P.J.; Craddock, P.R.; Iii, W.; Sylva, S.P.; Pichler, T.; Rosner, M.; Walsh, E. Submarine venting of magmatic volatiles in the eastern Manus Basin, Papua New Guinea. Geochim. Cosmochim. Acta 2015, 163, 178–199. [Google Scholar] [CrossRef] [Green Version]
- Martinez, F.; Taylor, B. Backarc Spreading, Rifting, and Microplate Rotation, between Transform Faults in the Manus Basin. Mar. Geophys. Res. 1996, 18, 203–224. [Google Scholar] [CrossRef]
- Piccoli, P.M.; Candela, P.A. Apatite in igneous systems. In Phosphates: Geochemical, Geobiological, and Materials Importance; Kohn, M.J., Rakovan, J., Hughes, J.M., Eds.; Mineralogical Society of America: Washington, DC, USA, 2002; Volume 48, pp. 255–292. ISBN 978-0-939950-60-7. [Google Scholar]
- Moore, P.B. Apatite. Its Crystal Chemistry, Mineralogy, Utilization and Geologic and Biologic Occurrences; McConnell, D., Ed.; Applied Mineralogy; Springer: New York, NY, USA, 1973; Volume 5, p. 111. [Google Scholar]
- Wyllie, P.J.; Cox, K.G.; Biggar, G.M. The habit of apatite in synthetic systems and igneous rocks. J. Petrol. 1962, 3, 238–243. [Google Scholar] [CrossRef]
- Webster, J.D.; Piccoli, P.M. Magmatic apatite: A powerful, yet deceptive, mineral. Elements 2015, 11, 177–182. [Google Scholar] [CrossRef]
- Elliott, J.C.; Wilson, R.; Dowker, S.E.P. Apatite structures. Adv. X-ray Anal. 2002, 45, 172–181. [Google Scholar]
- Zhang, Y.; Zeng, Z.; Yin, X.; Qi, H.; Chen, S.; Wang, X.; Shu, Y.; Chen, Z. Petrology and Mineralogy of Pumice from the Iheya North Knoll, Okinawa Trough: Implications for the Differentiation of Crystal-Poor and Volatile-Rich Melts in the Magma Chamber. Geol. J. 2018, 53, 2732–2745. [Google Scholar] [CrossRef]
- Parat, F.; Holtz, F.; Kluegel, A. S-rich apatite-hosted glass inclusions in Xenoliths from La Palma: Constraints on the volatile partitioning in evolved alkaline magmas. Contrib. Mineral. Petrol. 2011, 162, 463–478. [Google Scholar] [CrossRef]
- Peng, G.Y.; Luhr, J.F.; McGee, J.J. Factors controlling sulfur concentrations in volcanic apatite. Am. Miner. 1997, 82, 1210–1224. [Google Scholar] [CrossRef]
- Kusebauch, C.; John, T.; Whitehouse, M.J.; Engvik, A.K. Apatite as probe for the halogen composition of metamorphic fluids (Bamble Sector, SE Norway). Contrib. Mineral. Petrol. 2015, 170, 34. [Google Scholar] [CrossRef]
- Webster, J.D.; Goldoff, B.A.; Flesch, R.N.; Nadeau, P.A.; Silbert, Z.W. Hydroxyl, Cl, and F partitioning between high-silica rhyolitic melts-apatite-fluid(s) at 50–200 MPa and 700–1000 degrees C. Am. Miner. 2017, 102, 61–74. [Google Scholar] [CrossRef]
- Boudreau, A.E.; McCallum, I.S. Investigations of the stillwater complex: Part V. apatites as indicators of evolving fluid composition. Contrib. Mineral. Petrol. 1989, 102, 138–153. [Google Scholar] [CrossRef]
- Kusebauch, C.; John, T.; Whitehouse, M.J.; Klemme, S.; Putnis, A. Distribution of halogens between fluid and apatite during fluid-mediated replacement processes. Geochim. Cosmochim. Acta 2015, 170, 225–246. [Google Scholar] [CrossRef] [Green Version]
- Zafar, T.; Leng, C.-B.; Zhang, X.-C.; Rehman, H.U. Geochemical attributes of magmatic apatite in the kukaazi granite from western Kunlun Orogenic Belt, NW China: Implications for granite petrogenesis and Pb-Zn (-Cu-W) mineralization. J. Geochem. Explor. 2019, 204, 256–269. [Google Scholar] [CrossRef]
- Zafar, T.; Rehman, H.U.; Mahar, M.A.; Alam, M.; Oyebamiji, A.; Rehman, S.U.; Leng, C.-B. A critical review on petrogenetic, metallogenic and geodynamic implications of granitic rocks exposed in north and east China: New insights from apatite geochemistry. J. Geodyn. 2020, 136, 101723. [Google Scholar] [CrossRef]
- Lee, S.-M.; Ruellan, E. Tectonic and magmatic evolution of the Bismarck Sea, Papua New Guinea: Review and new synthesis. Geophys. Monogr. 2006, 166, 263–286. [Google Scholar] [CrossRef]
- Fourre, E.; Jean-Baptiste, P.; Charlou, J.L.; Donval, J.P.; Ishibashi, J.I. Helium isotopic composition of hydrothermal fluids from the Manus Back-Arc Basin, Papua New Guinea. Geochem. J. 2006, 40, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Tregoning, P. Plate kinematics in the western Pacific derived from geodetic observations. J. Geophys. Res. Solid Earth 2002, 107, ECV-7. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-H.; Lee, S.-M.; Kamenov, G.D.; Kwon, S.-T.; Lee, K.-Y. Tracing the origin of subduction components beneath the south east rift in the Manus Basin, Papua New Guinea. Chem. Geol. 2010, 269, 339–349. [Google Scholar] [CrossRef]
- Bach, W.; Roberts, S.; Vanko, D.A.; Binns, R.A.; Yeats, C.J.; Craddock, P.R.; Humphris, S.E. Controls of fluid chemistry and complexation on rare-earth element contents of anhydrite from the pacmanus subseafloor hydrothermal system, Manus Basin, Papua New Guinea. Miner. Depos. 2003, 38, 916–935. [Google Scholar] [CrossRef]
- Yang, K.H.; Scott, S.D. Vigorous exsolution of volatiles in the magma chamber beneath a hydrothermal system on the modern sea floor of the eastern Manus Back-Arc Basin, western Pacific: Evidence from melt inclusions. Econ. Geol. 2005, 100, 1085–1096. [Google Scholar] [CrossRef]
- Chen, Z.; Zeng, Z.; Wang, X.; Yin, X.; Chen, S.; Guo, K.; Lai, Z.; Zhang, Y.; Ma, Y.; Qi, H. U-Th/He dating and chemical compositions of apatite in the dacite from the southwestern Okinawa trough: Implications for petrogenesis. J. Asian Earth Sci. 2018, 161, 1–13. [Google Scholar] [CrossRef]
- Ketcham, R.A. Technical note: Calculation of stoichiometry from EMP data for apatite and other phases with mixing on monovalent anion sites. Am. Miner. 2015, 100, 1620–1623. [Google Scholar] [CrossRef]
- O’Reilly, S.Y.; Griffin, W.L. Apatite in the mantle: Implications for metasomatic processes and high heat production in phanerozoic mantle. Lithos 2000, 53, 217–232. [Google Scholar] [CrossRef]
- Imai, A. Variation of Cl and SO3 contents of microphenocrystic apatite in intermediate to silicic igneous rocks of cenozoic Japanese Island Arcs: Implications for porphyry Cu metallogenesis in the western Pacific Island Arcs. Resour. Geol. 2004, 54, 357–372. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, C.; Jia, F.; Liu, H.; Yan, Q. Apatite and zircon geochemistry in Yao’an alkali-rich porphyry gold deposit, southwest China: Implications for petrogenesis and mineralization. Minerals 2021, 11, 1293. [Google Scholar] [CrossRef]
- Harrison, T.M.; Watson, E.B. The behavior of apatite during crustal anatexis: Equilibrium and kinetic considerations. Geochim. Cosmochim. Acta 1984, 48, 1467–1477. [Google Scholar] [CrossRef]
- Straub, S.M.; Layne, G.D. Decoupling of fluids and fluid-mobile elements during shallow subduction: Evidence from halogen-rich andesite melt inclusions from the Izu arc volcanic front. Geochem. Geophys. Geosyst. 2003, 4, 9003. [Google Scholar] [CrossRef] [Green Version]
- Parat, F.; Holtz, F.; Streck, M.J. Sulfur-bearing magmatic accessory minerals. In Sulfur in Magmas and Melts: Its Importance for Natural and Technical Processes; Behrens, H., Webster, J.D., Eds.; Mineralogical Society of America & Geochemical Society: Chantilly, France, 2011; Volume 73, pp. 285–314. ISBN 978-0-939950-87-4. [Google Scholar]
- Zhu, J.-J.; Richards, J.P.; Rees, C.; Creaser, R.; DuFrane, S.A.; Locock, A.; Petrus, J.A.; Lang, J. Elevated magmatic sulfur and chlorine contents in ore-forming magmas at the red chris porphyry Cu-Au deposit, northern British Columbia, Canada. Econ. Geol. 2018, 113, 1047–1075. [Google Scholar] [CrossRef] [Green Version]
- Johnson, E.R.; Wallace, P.J.; Cashman, K.V.; Delgado Granados, H. Degassing of volatiles (H2O, CO2, S, Cl) during ascent, crystallization, and eruption at mafic monogenetic volcanoes in central Mexico. J. Volcanol. Geotherm. Res. 2010, 197, 225–238. [Google Scholar] [CrossRef]
- Fleet, M.E. Xanes spectroscopy of sulfur in earth materials. Can. Mineral. 2005, 43, 1811–1838. [Google Scholar] [CrossRef] [Green Version]
- Chelle-Michou, C.; Chiaradia, M. Amphibole and apatite insights into the evolution and mass balance of Cl and S in magmas associated with porphyry copper deposits. Contrib. Mineral. Petrol. 2017, 172, 105. [Google Scholar] [CrossRef] [Green Version]
- Richards, J.P.; Lopez, G.P.; Zhu, J.-J.; Creaser, R.A.; Locock, A.J.; Mumin, A.H. Contrasting tectonic settings and sulfur contents of magmas associated with cretaceous porphyry Cu +/− Mo +/− Au and intrusion-related iron oxide Cu-Au deposits in northern chile. Econ. Geol. 2017, 112, 295–318. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.M.; Fleet, M.E. Compositions of the apatite-group minerals: Substitution mechanisms and controlling factors. In Phosphates: Geochemical, Geobiological, and Materials Importance; Kohn, M.J., Rakovan, J., Hughes, J.M., Eds.; Mineralogical Society of America & Geochemical Society: Chantilly, France, 2002; Volume 48, pp. 13–49. ISBN 978-0-939950-60-7. [Google Scholar]
- Miles, A.J.; Graham, C.M.; Hawkesworth, C.J.; Gillespie, M.R.; Hinton, R.W.; Bromiley, G.D. Apatite: A new redox proxy for silicic magmas? Geochim. Cosmochim. Acta 2014, 132, 101–119. [Google Scholar] [CrossRef] [Green Version]
- Myers, J.; Eugster, H. The system Fe-Si-O—Oxygen buffer calibrations to 1500k. Contrib. Mineral. Petrol. 1983, 82, 75–90. [Google Scholar] [CrossRef]
- Kress, V.; Carmichael, I. The compressibility of silicate liquids containing Fe2o3 and the effect of composition, temperature, oxygen fugacity and pressure on their redox states. Contrib. Mineral. Petrol. 1991, 108, 82–92. [Google Scholar] [CrossRef]
- Parkinson, I.J.; Arculus, R.J. The redox state of subduction zones: Insights from arc-peridotites. Chem. Geol. 1999, 160, 409–423. [Google Scholar] [CrossRef]
- Wood, B.; Bryndzia, L.; Johnson, K. Mantle oxidation-state and its relationship to tectonic environment and fluid speciation. Science 1990, 248, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Arculus, R. Aspects of magma genesis in arcs. Lithos 1994, 33, 189–208. [Google Scholar] [CrossRef]
- Kleinsasser, J.M.; Simon, A.C.; Konecke, B.A.; Kleinsasser, M.J.; Beckmann, P.; Holtz, F. Sulfide and sulfate saturation of dacitic melts as a function of oxygen fugacity. Geochim. Cosmochim. Acta 2022, 326, 1–16. [Google Scholar] [CrossRef]
- Kelley, K.A.; Cottrell, E. Water and the oxidation state of subduction zone magmas. Science 2009, 325, 605–607. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Kleinsasser, J.M.; Richards, J.P.; Tapster, S.R.; Jugo, P.J.; Simon, A.C.; Kontak, D.J.; Robb, L.; Bybee, G.M.; Marsh, J.H.; et al. Oxidized sulfur-rich arc magmas formed porphyry Cu deposits by 1.88 Ga. Nat. Commun. 2021, 12, 2189. [Google Scholar] [CrossRef]
- Richards, J.P. The oxidation state, and sulfur and Cu contents of arc magmas: Implications for metallogeny. Lithos 2015, 233, 27–45. [Google Scholar] [CrossRef]
- Crabtree, S.M.; Lange, R.A. An evaluation of the effect of degassing on the oxidation state of hydrous andesite and dacite magmas: A comparison of pre- and post-eruptive Fe2+ concentrations. Contrib. Mineral. Petrol. 2012, 163, 209–224. [Google Scholar] [CrossRef]
- Waters, L.E.; Lange, R.A. No effect of H2O degassing on the oxidation state of magmatic liquids. Earth Planet. Sci. Lett. 2016, 447, 48–59. [Google Scholar] [CrossRef] [Green Version]
- de Hoog, J.C.M.; Hattori, K.H.; Hoblitt, R.P. Oxidized sulfur-rich mafic magma at Mount Pinatubo, Philippines. Contrib. Mineral. Petrol. 2004, 146, 750–761. [Google Scholar] [CrossRef]
- Grocke, S.B.; Cottrell, E.; de Silva, S.; Kelley, K.A. The role of crustal and eruptive processes versus source variations in controlling the oxidation state of iron in central andean magmas. Earth Planet. Sci. Lett. 2016, 440, 92–104. [Google Scholar] [CrossRef] [Green Version]
- Jenner, F.E.; Hauri, E.H.; Bullock, E.S.; Koenig, S.; Arculus, R.J.; Mavrogenes, J.A.; Mikkelson, N.; Goddard, C. The competing effects of sulfide saturation versus degassing on the behavior of the chalcophile elements during the differentiation of hydrous melts. Geochem. Geophys. Geosyst. 2015, 16, 1490–1507. [Google Scholar] [CrossRef] [Green Version]
- Jenner, F.E.; O’Neill, H.S.C.; Arculus, R.J.; Mavrogenes, J.A. The magnetite crisis in the evolution of arc-related magmas and the initial concentration of Au, Ag and Cu. J. Petrol. 2010, 51, 2445–2464. [Google Scholar] [CrossRef] [Green Version]
- Webster, J.D.; Tappen, C.M.; Mandeville, C.W. Partitioning behavior of chlorine and fluorine in the system apatite-melt-fluid. II: Felsic silicate systems at 200 MPa. Geochim. Cosmochim. Acta 2009, 73, 559–581. [Google Scholar] [CrossRef]
- Brenan, J. Partitioning of fluorine and chlorine between apatite and aqueous fluids at high-pressure and temperature—Implications for the F and Cl content of high P-T fluids. Earth Planet. Sci. Lett. 1993, 117, 251–263. [Google Scholar] [CrossRef]
- Tacker, R.; Stormer, J. A Thermodynamic model for apatite solid-solutions, applicable to high-temperature geologic problems. Am. Miner. 1989, 74, 877–888. [Google Scholar]
- Li, W.; Costa, F. A thermodynamic model for F-Cl-OH partitioning between silicate melts and apatite including non-ideal mixing with application to constraining melt volatile budgets. Geochim. Cosmochim. Acta 2020, 269, 203–222. [Google Scholar] [CrossRef]
- Boyce, J.W.; Tomlinson, S.M.; McCubbin, F.M.; Greenwood, J.P.; Treiman, A.H. The lunar apatite paradox. Science 2014, 344, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hermann, J. Apatite as an indicator of fluid salinity: An experimental study of chlorine and fluorine partitioning in subducted sediments. Geochim. Cosmochim. Acta 2015, 166, 267–297. [Google Scholar] [CrossRef]
- Li, H.; Hermann, J. Chlorine and fluorine partitioning between apatite and sediment melt at 2.5 GPa, 800 degrees C: A new experimentally derived thermodynamic model. Am. Miner. 2017, 102, 580–594. [Google Scholar] [CrossRef]
- Aiuppa, A.; Baker, D.R.; Webster, J.D. Halogens in volcanic systems. Chem. Geol. 2009, 263, 1–18. [Google Scholar] [CrossRef]
- Wallace, P.J.; Plank, T.; Edmonds, M.; Hauri, E.H. Volatiles in magmas. In The Encyclopedia of Volcanoes; Academic Press: Cambridge, MA, USA, 2015; pp. 163–183. [Google Scholar] [CrossRef]
- Zimova, M.; Webb, S.L. The combined effects of chlorine and fluorine on the viscosity of aluminosilicate melts. Geochim. Cosmochim. Acta 2007, 71, 1553–1562. [Google Scholar] [CrossRef]
- Zimova, M.; Webb, S. The effect of chlorine on the viscosity of Na2O-Fe2O3-Al2O3-SiO2 melts. Am. Miner. 2006, 91, 344–352. [Google Scholar] [CrossRef]
- Webster, J.D. The exsolution of magmatic hydrosaline chloride liquids. Chem. Geol. 2004, 210, 33–48. [Google Scholar] [CrossRef]
- Signorelli, S.; Carroll, M.R. Experimental constraints on the origin of chlorine emissions at the soufriere hills volcano, montserrat. Bull. Volcanol. 2001, 62, 431–440. [Google Scholar] [CrossRef]
- Kamenov, G.D.; Perfit, M.R.; Mueller, P.A.; Jonasson, I.R. Controls on magmatism in an island arc environment: Study of lavas and sub-arc xenoliths from the Tabar-Lihir-Tanga-Feni island chain, Papua New Guinea. Contrib. Mineral. Petrol. 2008, 155, 635–656. [Google Scholar] [CrossRef]
- Boyce, J.W.; Hervig, R.L. Apatite as a monitor of late-stage magmatic processes at volcan irazA(o), Costa Rica. Contrib. Mineral. Petrol. 2009, 157, 135–145. [Google Scholar] [CrossRef]
- Zhang, Y.X. H2O in rhyolitic glasses and melts: Measurement, speciation, solubility, and diffusion. Rev. Geophys. 1999, 37, 493–516. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Behrens, H.; Zhang, Y.X. The speciation of dissolved H2O in dacitic melt. Am. Miner. 2004, 89, 277–284. [Google Scholar] [CrossRef]
- Danyushevsky, L.V.; Eggins, S.M.; Falloon, T.J.; Christie, D.M. H2O abundance in depleted to moderately enriched mid-ocean ridge magmas; part I: Incompatible behaviour, implications for mantle storage, and origin of regional variations. J. Petrol. 2000, 41, 1329–1364. [Google Scholar] [CrossRef] [Green Version]
- Sobolev, A.V.; Chaussidon, M. H2O concentrations in primary melts from supra-subduction zones and mid-ocean ridges: Implications for H2O storage and recycling in the mantle. Earth Planet. Sci. Lett. 1996, 137, 45–55. [Google Scholar] [CrossRef]
- Mcdonough, W.; Sun, S. The composition of the earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Page, L.; Hattori, K.; de Hoog, J.C.M.; Okay, A.I. Halogen (F, Cl, Br, I) behaviour in subducting slabs: A study of lawsonite blueschists in western Turkey. Earth Planet. Sci. Lett. 2016, 442, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Straub, S.M.; Layne, G.D. The systematics of chlorine, fluorine, and water in izu arc front volcanic rocks: Implications for volatile recycling in subduction zones. Geochim. Cosmochim. Acta 2003, 67, 4179–4203. [Google Scholar] [CrossRef]
- Li, Y.H. A brief discussion on the mean oceanic residence time of elements. Geochim. Cosmochim. Acta 1982, 46, 2671–2675. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Crawford, A.J.; Eggins, S.; Muhe, R. Phenocryst and melt inclusion chemistry of near-axis seamounts, valu fa ridge, Lau Basin: Insight into mantle wedge melting and the addition of subduction components. Earth Planet. Sci. Lett. 1997, 151, 205–223. [Google Scholar] [CrossRef]
- Mc, A.; Jag, B. Geochemical and geodynamical constraints on subduction zone magmatism. Earth Planet. Sci. Lett. 1991, 102, 358–374. [Google Scholar] [CrossRef]
- Pearce, J.A.; Peate, D.W. Tectonic implications of the composition of volcanic arc magmas. Ann. Rev. Earth Planet. Sci. 1995, 23, 251–285. [Google Scholar] [CrossRef]
- Sun, W.D.; Bennett, V.C.; Eggins, S.M.; Arculus, R.J.; Perfit, M.R. Rhenium systematics in submarine MORB and back-arc basin glasses: Laser ablation ICP-MS results. Chem. Geol. 2003, 196, 259–281. [Google Scholar] [CrossRef]
- Portnyagin, M.; Hoernle, K.; Plechov, P.; Mironov, N.; Khubunaya, S. Constraints on mantle melting and composition and nature of slab components in volcanic arcs from volatiles (H2O, S, Cl, F) and trace elements in melt inclusions from the kamchatka arc. Earth Planet. Sci. Lett. 2007, 255, 53–69. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).