Abstract
Dissimilatory nitrate reduction to ammonium (DNRA) can save N by converting nitrate into ammonium and avoiding nitrate leaching and runoff in saltmarshes. However, little is known about the effects of invasive plants on DNRA in the upper and deeper soil layers in salt marshes. Here, we investigated DNRA rates in the soils of six different depth layers (0–5, 5–10, 10–20, 20–30, 30–50, and 50–100 cm) from the invasive Spartina alterniflora marshland, two native plants Scirpus mariqueter and Phragmites australis marshlands, and bare mudflat on Chongming Island, located in the Yangtze River Estuary, China. Our results show that S. alterniflora significantly increased DNRA rates in both the upper 50 cm soil and deeper 50–100 cm soil layers. With respect to the entire soil profile, the NO3− reduction content calculated from DNRA in S. alterniflora marshland was 502.84 g N m−2 yr−1, increased by 47.10%, 49.42%, and 38.57% compared to bare mudflat, S. mariquete, and P. australis, respectively. Moreover, NO3− reduction content from the 50–100 cm soil layers was almost identical to that in the upper 50 cm of the soil. In the month of May, DNRA is primarily regulated by SO42− and pH in the upper and deeper soil layers, respectively, whereas, in the month of October, soil pH accounted for the most variables of DNRA in both the upper and deeper soil layers. Altogether, these results from a new perspective confirm that S. alterniflora invasion increases soil N pool and may further push its invasion in salt marshes, and the importance of deeper soil in nitrogen cycling cannot be ignored.
1. Introduction
Nitrogen (N) is the main limiting factor for plant growth and net primary productivity in wetlands [1]. However, almost 50% of the N used for production is lost to the environment [2,3]. The nitrification process can convert ammonium (NH4+) into nitrate (NO3−) via soil microbes, which may accelerate the NO3− leaching and nitrous oxide (N2O, a very important long-lived greenhouse gas) emissions [4,5,6]. Compared to NO3−, NH4+ has a positive charge, which is less likely to be lost through gaseous emissions or leaching and is more readily absorbed by plants due to it having a lower energy requirement [7]. Therefore, the rapid conversion of NO3− to NH4+ may play a key role in the transformation of N conversion in ecosystems [8]. Dissimilatory nitrate reduction to ammonium (DNRA) increases the pool of NH4+ and decreases the size of the NO3− pool, which can provide more NH4+ for assimilation and absorption by primary producers [9,10,11,12]. The DNRA is often ignored in the study of soil N pool in salt marshes, but it has been shown to play an important role in grassland, agriculture, and forest, where DNRA accounted for 0~54%, 3.9~25.4%, and 41.6~75% of NO3− reduction, respectively [13,14,15]. Additionally, research on DNRA in salt marshes under different habitat types has not been well studied.
Salt marshes, which are located in the transitional zones between land and sea, can provide unique ecosystem services such as flood mitigation, carbon storage, and wildlife production [16]; however, salt marshes are fragile ecosystems vulnerable to the invasive species Spartina alterniflora (S. alterniflora), which is a species native to the Atlantic and Gulf Coasts of North America [17]. S. alterniflora was intentionally introduced to China in the 1970s for accentuating sediment accumulation and forming land, planted in several locations in the 1980s, extensively spread in the 1990s, and now has widely spread throughout the country’s coastline due to its high ecological adaptability [18,19,20]. Numerous studies have reported that S. alterniflora invasion significantly impacts soil key N cycle by increasing the total N stocks in the tissues and the soil [21], enhancing the ecosystem N pools [22] and altering microbial community structure and soil physicochemical properties [23].
There exists little data about the effects of the invasive species on the DNRA in salt marshes. Few papers were found involving the effects of S. alterniflora invasion on DNRA, which are helpful to understand the importance of exotic plant invasion on nitrogen cycling in salt marshes [24,25,26,27]; however, these studies are limited to upper soil layers between 0 and 50 cm, and no studies have been reported on DNRA in deeper soil layers. Studies in other ecosystems have shown that the role of the N cycle in deeper soil layers cannot be ignored [28,29,30]. Salt marshes are one of the most important blue carbon ecosystems [31], and investigations of carbon stocks in these ecosystems generally consider a fixed soil depth, usually a 0–100 cm layer [32]. Moreover, N leaching also affects deeper layers through tidal and subsurface runoff and the root biomass of S. alterniflora is higher than that of native species in the 0–100 cm soil profile [21,33]. Thus, studies focusing on S. alterniflora invasion on DNRA at the soil profile to a depth of 100 cm are generally considered appropriate for obtaining an overall understanding of the DNRA process in the salt marsh ecosystem.
Substrate, carbon source, oxygen content, and pH are recognized as the key important factors affecting DNRA rates [34]. Previous studies have shown that there are some differences in the terms of the physical and chemical properties between S. alterniflora and native plants. Notably, the root biomass of S. alterniflora in the 0–100 cm soil layer is higher than that of native plants, which benefits S. alterniflora to improve root respiration and transport more oxygen to the soil. In addition, the roots of S. alterniflora can also contribute to the secretion of organic acids that lower soil pH and alter soil carbon and nitrogen pools [22]. Compared with indigenous plants, S. alterniflora also has a stronger siltation-promoting ability, which helps the burial of the surface soil to deeper soil layers. Therefore, we assumed that DNRA rates in S. alterniflora soils were significantly higher than those in other habitat types both at the upper soil layers (0–50 cm) and deeper soil layer (50–100 cm). To test our hypothesis, we conducted a field study to investigate whether differences in DNRA in the 0–100 cm soil profile exist between S. alterniflora and native species in Chongming Island, located in the Yangtze River Estuary, China.
2. Materials and Methods
2.1. Study Site and Experimental Design
The current study was carried out on Chongming Island locates in Yangtze Estuary (31°69′ N, 121°65′ E). The island, with a total area of 1267 km2, is China’s largest estuarine Island [35]. This region has a typical subtropical monsoon climate with annual average precipitation and temperature of approximately 1200 mm and 16 °C, respectively [36]. This area is experiencing regular semidiurnal tides with two different diurnal tidal periods. S. alterniflora invasion on the island are serious, and its rapid growth has outcompeted the local species Phragmites australis and Scirpus mariqueter since its introduction in this area in 2001 [37].
From the water–land margin to salt marsh, the habitat types are bare mudflat, S. mariqueter marshland, S. alterniflora marshland, and P. australis marshland, located at 0, 50, 400, and 500 m from water–land margin, respectively. Four filed plots (10 × 10 m) spaced at an average of 100 m apart in each habitat types were set up in 2018 (Figure 1), and experimental design had been described elsewhere [37].
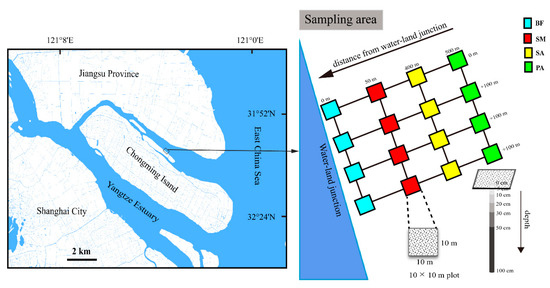
Figure 1.
Location of the study area and sampling sites. SM: S. mariqueter; SA: S. alterniflora; PA: P.australis; BF: bare mudflat.
2.2. Sample Collection
Soil cores (0–5, 5–10, 10–20, 20–30, 30–50, and 50–100 cm in depth) were collected on 5 May 2018 and 25 October 2018 with an Eijkelkamp gouge auger during the diurnal ebb-tide hours, and a total of 96 samples were obtained. To determine the bulk density and soil moisture, vertical soil profiles were collected using standard 100-cm3 containers at the same depths as soil core samples were obtained. These soil sections were then oven-dried at 105 °C for 24 h to measure bulk density and soil moisture. The soil temperature and redox potential (Eh) of each soil layer were recorded immediately. Three 0.5 × 0.5 m quadrats were randomly set at 0.5 m away from the sampling point to measure above-ground biomass. After collection, the soil samples were sealed in sterile plastic bags and then transported to the laboratory and divided into two parts: the first part was stored at 4 °C to measure the DNRA rates, and the second part was air-dried and sieved through a 2 mm filter to analyze soil properties.
2.3. Analysis of Soil Characteristics
Soil salinity and pH were measured with a modular multi-channel benchtop pH meter after soil was mixed with deionized water free CO2 at a ratio of 1:2.5 [38,39]. The concentrations of NH4+, NO3− and NO2− were measured on a continuous flow injection analyzer (SAN plus, Skalar, the Netherlands) after extraction with 2 M KCI solution [40,41,42]. The total carbon (TC) and total nitrogen (TN) were analyzed using CN Elementary Analyser after soil carbonates were removed by acidifing with 1 mol L−1 HCl [43].
2.4. Measurement of the DNRA Rates
Slurry incubations combining 15N isotope-tracing method were conducted to measure DNRA rates [44]. Specifically, soil slurries were made with 25 g of fresh soil and 175 mL of tidal water, and then filled with helium (He) gas for approximately 30 min to achieve anaerobic conditions. After pre-incubation for 48h in 12-mL screw-capped Exetainer vials (Labco) under in situ temperature (23.5 C for May, 25 °C for October) in dark conditions to deplete the residual nitrate, nitrite and oxygen, samples were then spiked with 100 μL of 15NO3− (12.5 mM, 15N at 99%). Immediately after, these samples were injected with ZnCl2 (200 μL 50% w/v) to inhibit microbial activity in time-series incubations at 0, 2, 4, and 8 h, respectively. The Hypobromite method was used to convert 15NH4+ from the DNRA process to 29N2 and 30N2, and then these produced gases were determined by membrane inlet mass spectrometry (MIMS) [45]. Furthermore, 15NH4+ with concentrations of 0, 5, 10, and 20 μmol L−1 were also oxidized by using the hypobromite method, respectively. Thus, a standard curve was established to calculate the concentrations of 15NH4+ from the incubation slurries. The DNRA rates were calculated by the following equation:
where RDNRA corresponds to DNRA rates (nmol 15N g−1h−1), S is the slope of 15NH4+ concentrations versus incubation time (nmol 15N L−1h−1), V denotes the vial volume (L), and W is the soil weight (g).
2.5. Statistical Analysis
The data in this study were analyzed by using SPSS Statistics version 19.0 (SPSS, Inc., Chicago, IL, USA) and Rstudio software version 3.5.1 (Rstudio, Inc., Boston, MA, USA). Mean and standard error values of each parameter were calculated (Mean ± SD, n = 4). Analysis of two-way ANOVA was carried out to observe the significance of soil depth and habitat type on DNRA rates and NO3− retention content, and analysis of one-way ANOVA using LSD’s test was employed to evaluate the differences of NO3− retention content among habitat types. Post hoc analyses of significant results were conducted using Tukey’s multiple comparison tests, and the significance level was set at p < 0.05. To determine the most concise model, equations with the lowest AIC were used to select the most suitable model through stepwise multiple regression. Stepwise multiple regression analysis was carried out using R software with soil physical and chemical properties as variables and DNRA as the dependent variable.
We consolidated data into annual NO3− reduction content (NR) to obtain a better overview of different habitat types on DNRA. NO3− retention content (NR) from a given habitat type with s soil layers was calculated using the following equation:
where NR (g N m−2 y−1) is NO3− retention content; n and s represent the soil layer and number of soil layers, respectively; and are the DNRA rate (nmol N g−1 h−1) and bulk density (g cm−3) of soil layer n in the month of May, respectively; and are the DNRA rate (nmol N g−1h−1) and bulk density (g cm−3) of soil layer n in the month of October, respectively denotes the thickness of soil layer n (cm); t denotes time (24 × 365 h); and c = represents a unit conversion factor.
3. Results
3.1. Potential Rates of DNRA
In the month of May, DNRA rates were not affected by soil depth or habitat type (Figure 2). No significant differences were found in the DNRA rates between habitat types in either upper soil layers or in the deeper soil layers. The DNRA rates in S. alterniflora marshland were not significantly different from those in the other habitat types in the month of May. In the month of October, the DNRA rates in the S. alterniflora marshland were significantly higher than those in other habitat types in all soil layers. DNRA in the deeper soil layers (50–100 cm) in the month of October were markedly higher than those in the 0–5 cm and 5–10 cm the upper soil layers. The two-month-averaged DNRA in the S. alterniflora stands was significantly higher than that of other habitat types (Figure 3).
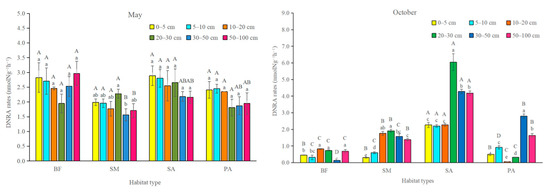
Figure 2.
Potential rates of dissimilatory nitrate reduction to ammonium (DNRA). Bars represent standard errors of the quadruplicate samples. SM: S. mariqueter; SA: S. alterniflora; PA: P. australis; BF: bare mudflat. Bars with different uppercase letters and lowercase letters denote significantly different in habitat types and soil depths, respectively (p < 0.05).
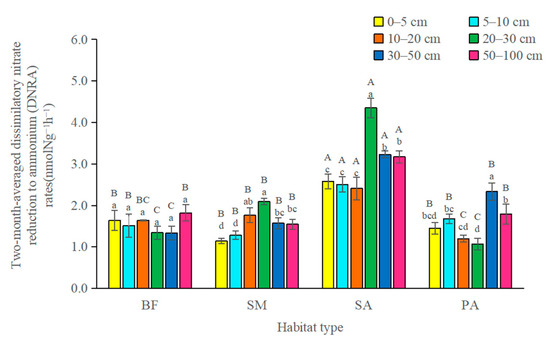
Figure 3.
Two-month-averaged dissimilatory nitrate reduction to ammonium (DNRA) rates. Error bars in the column represent standard errors. SM: S. mariqueter; SA: S. alterniflora; PA: P. australis; BF: bare mudflat. Bars with different uppercase letters and lowercase letters denote significantly different in habitat types and soil depths, respectively (p < 0.05).
3.2. NO3− Reduction Content
Where the volume of soil for each habitat type was used to compute NO3− reduction content per unit area, the results showed that NO3− retention content in the 0–50 cm layer was almost equivalent to that in the deeper layers (50–100 cm). In the 0–50 cm layer, NO3− retention content in S. alterniflora soil was increased by 50.51%, 46.81%, and 40.45% compared with that of bare mudflat, S. mariqueter, and P. australis, respectively (Figure 4a). In the deeper soil layer, NO3− retention content in S. alterniflora was 47.38%, 52.43%, and 41.42% higher than that in the soils of bare mudflat, S. mariqueter, and P. australis, respectively. NO3− retention content in the whole 0–100 cm soil profile increased in the following order: S. mariqueter (254.32 g N m−2 yr−1) < bare mudflat (281.15 g N m−2 yr−1) < P. australis (308.89 g N m−2 yr−1) < S. alterniflora (502.84 g N m−2 yr−1) (Figure 4b).
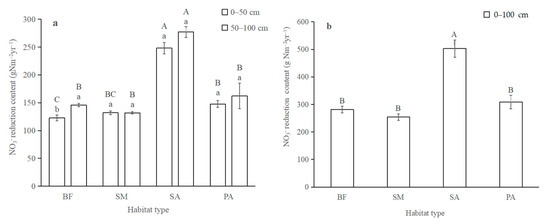
Figure 4.
NO3− retention content between four habitat types: (a) in the upper 50 cm and deeper 50 cm, (b) at the 0–100 cm depth. Error bars in the column represent standard errors. SM: S. mariqueter; SA: S. alterniflora; PA: P. australis; BF: bare mudflat. Bars with different uppercase letters and lowercase letters denote significantly different in habitat types and soil depths, respectively (p < 0.05).
3.3. Influences of Soil Variables on Potential Rates of DNRA
Multiple linear regression analyses to were used to determine which linear combination of independent variables was best conductive to predict DNRA rates (Table 1 and Table S1). In the month of May, SO42−, NO2−, and temperature accounted for the most variables of DNRA in the soil to the depth of 50 cm, and DNRA rates in deeper soil from 50–100 cm were the largest, as explained by pH, bulk density, and NO2−. In the month of October, pH and salinity were best explained by the changes in DNRA rates in the upper soil layers, while in the deeper soil layer, DNRA rates were best accounted for by pH and bulk density.

Table 1.
Multiple linear regression equations for DNRA versus environmental factors measured at 0–50 cm and 50–100 cm depths across the region in two months.
4. Discussion
In this study, we investigated the effect of exotic S. alterniflora invasion on DNRA rates in both the upper and deeper soil layers in the 0–100 cm soil profile. Our results showed that S. alterniflora significantly increased DNRA rates compared to other habitat types (Figure 2 and Figure 3), which is consistent with findings in previous studies [24,45]. The higher NO3− reduction content in S. alterniflora may acerbate the export of more NH4+ into the soil, which in turn causes S. alterniflora to absorb more N to accelerate plant growth and promote further invasion of S. alterniflora [22]. In addition, our results also found that the ability of NO3− to convert to NH4+ under the influence of different habitat types is almost equal to that of upper sediments (Figure 4), indicating that we cannot ignore the importance of deeper soils in the comprehensive evaluation of the conversion of inorganic N pools from DNRA in salt marshes.
In the month of May, in the upper soil layers, SO42− was an important factor influencing the DRNA rates, which is consistent with the observation that reduced sulfides (S2−, SO32−) can provide an electron donor for DNRA [46,47]. SO42− is one of the most abundant ions in seawater, and its morphology and distribution in inter-root sediments can be affected by plant types [48]. Previous studies have demonstrated that SO42− concentration was significantly enhanced after S. alterniflora [49,50]. However, during the early growth stages in the month of May, the sulfide in S. alterniflora tissues in the previous year is decomposed slowly; thus, SO42− concentration in the soil of S. alterniflora marshland was not higher than that of other habitat types during this phase (Figure S1i), which may explain why DNRA rates had no difference between S. alterniflora and other habitat types in the early growth phase in the upper soil layers [51]. In deeper soil layers, we found that pH and bulk density explained the largest proportion of the variance in DNRA (Table 1 and Table S1). DNRA bacteria are susceptible to changes in pH and oxygen contents [52]. It is widely accepted that the pH for DNRA lies between 5 and 9, and a high pH value enhances the DNRA process [34]. In the early growth phase, roots of S. alterniflora and two other native plants used more energy to grow and may not secrete small molecules of organic acids to change soil pH, which may contribute to unchanged DNRA in the 50–100 cm layer between the different habitat types [22].
In the month of October, soil pH explained the largest proportion of the variance of DNRA in both the upper and deeper soil layers (Table 1 and Table S1). During the late growth phase, the biomass of S. alterniflora was significantly higher than S. mariqueter and P. australis (Figure S2), and well-developed root tissue was able to secrete more organic acids to alter soil pH, which was more acidic after S. alterniflora invasion compared to S. mariqueter and P. australis [37], which helped to increase DNRA rates in S. alterniflora marshland in both the surface and deep soil layers. Moreover, salinity was positively correlated with DNRA in the top 0–50 cm (Table 1), which is consistent with the observation that microbes are susceptible to changes in salinity mediating DNRA [53]. The higher salinity in the upper soil layers of S. alterniflora marshland recorded in the present study (Figure S1b), may thus have contributed to enhancing DNRA [54]. S. alterniflora invasion alters soil physical properties (e.g., reducing the soil bulk density) that can affect DNRA activity by regulating oxygen and influencing NO3− leakage content [55], which may have contributed to the DNRA rate in the deeper soil layers.
5. Conclusions
This study reported the effects of invasive S. alterniflora on DNRA rates in salt marshes in the 0–100 cm soil layer. Our results indicated that S. alterniflora invasion can significantly increase the DNRA rates, and NO3− reduction content in the entire 0–100 cm soil profile. This suggests that increasing soil N pool content, except through litter decomposition and promotion of soil mineralization [25,51], S. alterniflora can also increase DNRA rates to increase inorganic soil N content, which indirectly further promotes S. alterniflora invasion. In addition, the NO3− reduction content via DNRA in the 50–100 cm soil layer was almost identical to that of upper soil layers, which indicates that assessing DNRA capacity in salt marshes and deeper soils should be considered in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jmse10050655/s1, Figure S1: Influence of habitat type, soil depth and month on soil properties (mean ± SE) (a: Water; b: Salinity; c: pH; d: Eh; e: Bulk density; f: Temperature; g: TC; h: TN; i: SO42−; j: NH4+; k: NO3−; l: NO2−). M = May, O = October. BF bare mudflat; SM S. mariqueter; SA S. alterniflora; PA P.australis; Figure S2: Aboveground biomass among different habitat types. Different letters indicate significant differences (p < 0.05) among different habitat types. SM, S. mariqueter; SA, S. alterniflora; PA, P. australis. Table S1: The interpretation rate for each environmental variable obtained from the results of redundancy analysis (RDA) at 0–50 and 50–100 cm soil depths in the two months.
Author Contributions
Conceptualization: J.W., M.N. and N.L.; Sampling: N.L.; Formal analysis and Investigation: N.L.; Writing—review and editing: N.L., J.W., M.N. and M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the NSFC funding (32030067) and the Shanghai Science and Technology Committee (20DZ1204702).
Acknowledgments
We thank Z.K. Liu for the assistance with field work. We also thank two anonymous referees for their valuable comments to the manuscript and their constructive suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fock, H.O. Changes in the Seasonal Cycles of Inorganic Nutrients in the Coastal Zone of the Southeastern North Sea from 1960 to 1997: Effects of Eutrophication and Sensitivity to Meteoclimatic Factors. Mar. Pollut. Bull. 2003, 46, 1434–1449. [Google Scholar] [CrossRef]
- Fang, Y.; Gundersen, P.; Mo, J.; Zhu, W. Nitrogen Leaching in Response to Increased Nitrogen Inputs in Subtropical Monsoon Forests in Southern China. For. Ecol. Manag. 2009, 257, 332–342. [Google Scholar] [CrossRef]
- Lin, X.B.; Liu, M.; Hou, L.J.; Gao, D.Z.; Li, X.F.; Lu, K.J.; Gao, J. Nitrogen Losses in Sediments of the East China Sea: Spatiotemporal Variations, Controlling Factors, and Environmental Implications. J. Geophys. Res. Biogeosci. 2017, 122, 2699–2715. [Google Scholar] [CrossRef]
- Jiang, Y.; Yin, G.; Li, Y.; Hou, L.; Liu, M.; Chen, C.; Zheng, D.; Wu, H.; Gao, D.; Zheng, Y.; et al. Saltwater Incursion Regulates N2O Emission Pathways and Potential Nitrification and Denitrification in Intertidal Wetland. Biol. Fertil. Soils 2022, 12, 234–245. [Google Scholar] [CrossRef]
- Lin, X.B.; Zheng, P.F.; Zou, S.B.; Sun, F.F.; Zhang, X.L.; Gong, J. Seagrass (Zostera marina) Promotes Nitrification Potential and Selects Specific Ammonia Oxidizers in Coastal Sediments. J. Soils Sediments 2021, 21, 3259–3273. [Google Scholar] [CrossRef]
- Wei, H.C.; Lin, X.B. Shifts in the Relative Abundance and Potential Rates of Sediment Ammonia-oxidizing Archaea and Bacteria along Environmental Gradients of an Urban River-estuary-adjacent Sea Continuum. Sci. Total Environ. 2021, 771, 144824. [Google Scholar] [CrossRef]
- Cheng, Y.; Elrys, A.S.; Merwad, A.R.M.A.; Zhang, H.; Chen, Z.; Zhang, J.; Cai, Z.C.; Müller, C. Global Patterns and Drivers of Soil Dissimilatory Nitrate Reduction to Ammonium. Environ. Sci. Technol. 2022, 56, 3791–3800. [Google Scholar] [CrossRef]
- Pandey, C.B.; Kumar, U.; Kaviraj, M.; Minick, K.J.; Mishra, A.; Singh, J.S. DNRA: A Short-Circuit in Biological N-Cycling to Conserve Nitrogen in Terrestrial Ecosystems. Sci. Total Environ. 2020, 738, 139710. [Google Scholar] [CrossRef]
- Huang, F.J.; Lin, X.B.; Hu, W.F.; Zeng, F.; He, L.; Yin, K.D. Nitrogen Cycling Processes in Sediments of the Pearl River Estuary: Spatial Variations, Controlling Factors, and Environmental Implications. Catena 2021, 206, 105545. [Google Scholar] [CrossRef]
- Huang, F.J.; Lin, X.B.; Yin, K.D. Effects of Marine Produced Organic Matter on the Potential Estuarine Capacity of NOx− Removal. Sci. Total Environ. 2022, 812, 151471. [Google Scholar] [CrossRef]
- Wei, H.C.; Gao, D.Z.; Liu, Y.; Lin, X.B. Sediment nitrate reduction processes in response to environmental gradients along an urban river-estuary-sea continuum. Sci. Total Environ. 2020, 718, 137185. [Google Scholar] [CrossRef] [PubMed]
- Minick, K.J.; Pandey, C.B.; Fox, T.R.; Subedi, S. Dissimilatory Nitrate Reduction to Ammonium and N2O Flux: Effect of Soil Redox Potential and N Fertilization in Loblolly Pine Forests. Biol. Fertil. Soils. 2016, 52, 601–614. [Google Scholar] [CrossRef]
- Rütting, T.; Clough, T.J.; Müller, C.; Lieffering, M.; Newton, P.C.D. Ten Years of Elevated Atmospheric Carbon Dioxide Alters Soil Nitrogen Transformations in a Shee-Grazed Pasture. Glob. Chang. Biol. 2010, 16, 2530–2542. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, H.; Shi, W. Dissimilatory Nitrate Reduction to Ammonium in an Anaerobic Agricultural Soil as Affected by Glucose and Free Sulfide. Eur. J. Soil Biol. 2013, 58, 98–104. [Google Scholar] [CrossRef]
- Huygens, D.; Rütting, T.; Boeckx, P.; van Cléemput, O.; Godoy, R.; Müller, C. Soil Nitrogen Conservation Mechanisms in a Pristine South Chilean Nothofagus Forest Ecosystem. Soil Biol. Biochem. 2007, 39, 2448–2458. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Bernal, B.; Nahlik, A.M.; Mander, Ü.; Zhang, L.; Anderson, C.; Jørgensen, S.E.; Brix, H. Wetlands, Carbon, and Climate Change. Landsc. Ecol. 2012, 28, 583–597. [Google Scholar] [CrossRef]
- Xia, L.; Geng, Q.; An, S. Rapid Genetic Divergence of an Invasive Species, Spartina alterniflora, in China. Front. Genet. 2020, 11, 284. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Wu, J.; Pennings, S.C. Contrasting Latitudinal Clines of Nematode Diversity in Spartina Alterniflora Salt Marshes between Native and Introduced Ranges. Divers. Distrib. 2020, 26, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Meng, L.; Zhang, Y.; Weng, Q.; Morris, J.T. Tidal and Meteorological Influences on the Growth of Invasive Spartina alterniflora: Evidence from UAV Remote Sensing. Remote Sens. 2019, 11, 1208. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.; Lin, X. Bait Input Altered Microbial Community Structure and Increased Greenhouse Gases Production in Coastal Wetland Sediment. Water Res. 2022, 218, 118520. [Google Scholar] [CrossRef]
- Liao, C.; Luo, Y.; Jiang, L.; Zhou, X.; Wu, X.; Fang, C.; Chen, J.; Li, B. Invasion of Spartina Alterniflora Enhanced Ecosystem Carbon and Nitrogen Stocks in the Yangtze Estuary, China. Ecosystems 2007, 10, 1351–1361. [Google Scholar] [CrossRef]
- Peng, R.; Fang, C.; Li, B.; Chen, J.K. Spartina Alterniflora Invasion Increases Soil Inorganic Nitrogen Pools through Interactions with Tidal Subsidies in the Yangtze Estuary, China. Oecologia 2010, 165, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Xie, X.; Huang, L.; Wu, Y. Effects of Invasion of Spartina alterniflora and Exogenous N Deposition on N2O Emissions in a Coastal Salt Marsh. Ecol. Eng. 2013, 58, 77–83. [Google Scholar] [CrossRef]
- Gao, D.; Li, X.; Lin, X.; Wu, D.; Jin, B.; Huang, Y.; Liu, M.; Chen, X. Soil Dissimilatory Nitrate Reduction Processes in the Spartina alterniflora Invasion Chronosequences of a Coastal Wetland of Southeastern China: Dynamics and Environmental Implications. Plant Soil 2017, 421, 383–399. [Google Scholar] [CrossRef]
- Li, X.; Gao, D.; Hou, L.; Liu, M. Soil Substrates Rather than Gene Abundance Dominate DNRA Capacity in the Spartina alterniflora Ecotones of Estuarine and Intertidal Wetlands. Plant Soil 2018, 436, 123–140. [Google Scholar] [CrossRef]
- Gao, G.; Li, P.; Zhong, J.; Shen, Z.; Chen, J.; Li, Y.; Isabwe, A.; Zhu, X.; Ding, Q.; Zhang, S.; et al. Spartina alterniflora Invasion Alters Soil Bacterial Communities and Enhances Soil N2O Emissions by Stimulating Soil Denitrification in Mangrove Wetland. Sci. Total Environ. 2019, 653, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Ledford, T.C.; Mortazavi, B.; Tatariw, C.; Mason, O.U. Elevated Nutrient Inputs to Marshes Differentially Impact Carbon and Nitrogen Cycling in Two Northern Gulf of Mexico Saltmarsh Plants. Biogeochemistry 2020, 149, 1–16. [Google Scholar] [CrossRef]
- Hill, A.R.C.; Vidon, P.G.; Langat, J.K. Denitrification Potential in Relation to Lithology in Five Headwater Riparian Zones. J. Environ. Qual. 2004, 33, 911–919. [Google Scholar] [CrossRef]
- Jahangir, M.M.R.; Khalil, M.I.; Johnston, P.; Cárdenas, L.M.; Hatch, D.J.; Butler, M.R.; Barrett, M.; O’Flaherty, V.; Richards, K.G. Denitrification Potential in Subsoils: A Mechanism to Reduce Nitrate Leaching to Groundwater. Agric. Ecosyst. Environ. 2012, 147, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Bai, R.; Xi, D.; He, J.; Hu, H.; Fang, Y.; Zhang, L. Activity, Abundance and Community Structure of Anammox Bacteria along Depth Profiles in Three Different Paddy Soils. Soil Biol. Biochem. 2015, 91, 212–221. [Google Scholar] [CrossRef] [Green Version]
- Kirwan, M.L.; Mudd, S.M. Response of Salt-marsh Carbon Accumulation to Climate Change. Nature. 2012, 489, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, B.; Zhong, Y.; Chen, J. Local Competitive Effects of Introduced Spartina alterniflora on Scirpus mariqueter at Dongtan of Chongming Island, the Yangtze River Estuary and Their Potential Ecological Consequences. Hydrobiologia 2004, 528, 99–106. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The Vertical Distribution of Soil Organic Carbon and Its Relation to Climate and Vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Yang, S.; Wu, S.J.; Cai, Y.J.; Zhou, W.Z.; Zhu, T.B.; Wang, Y.; Huang, P. The Synergetic and Competitive Mechanism and the Dominant Factors of Dissimilatory Nitrate Reduction Processes: A Review. Acta Ecol. Sin. 2016, 36, 113–142. [Google Scholar]
- Jiang, Y.; Yin, G.; Hou, L.; Liu, M.; Gao, D.; Zhang, Z.; Zheng, Y.; Han, P. Variations of Dissimilatory Nitrate Reduction Processes along Reclamation Chronosequences in Chongming Island, China. Soil Tillage Res. 2021, 206, 104815. [Google Scholar] [CrossRef]
- Wu, D.; Deng, L.; Sun, Y.; Wang, R.; Zhang, L.; Wang, R.; Song, Y.; Gao, Z.; Haider, H.; Wang, Y.; et al. Climate Warming, but Not Spartina Alterniflora Invasion, Enhances Wetland Soil HONO and NOx Emissions. Sci. Total Environ. 2022, 823, 153710. [Google Scholar] [CrossRef]
- Li, N.; Li, B.; Nie, M.; Wu, J. Effects of Exotic Spartina Alterniflora on Saltmarsh Nitrogen Removal in the Yangtze River Estuary, China. J. Clean. Prod. 2020, 271, 122557. [Google Scholar] [CrossRef]
- Lin, G.M.; Huang, J.; Lu, J.G.; Su, M.; Hu, B.Q.; Lin, X.B. Geochemical and Microbial Insights into Vertical Distributions of Genetic Potential of N-cycling Processes in Deep-sea Sediments. Ecol. Indic. 2021, 125, 107461. [Google Scholar] [CrossRef]
- Liu, R.; Lin, X.B.; Wang, G.Q.; Liu, X. Natural N-bearing Nanoparticles in Sediments of a Shallow Bay of the South China: A New N Form in N-cycling. Ecol. Indic. 2021, 122, 107281. [Google Scholar] [CrossRef]
- Shan, J.; Zhao, X.; Sheng, R.; Xia, Y.; Ti, C.; Quan, X.; Wang, S.; Wei, W.; Yan, X. Dissimilatory Nitrate Reduction Processes in Typical Chinese Paddy Soils: Rates, Relative Contributions, and Influencing Factors. Environ. Sci. Technol. 2016, 50, 9972–9980. [Google Scholar] [CrossRef]
- Lin, X.B.; Hou, L.J.; Liu, M.; Li, X.F.; Zheng, Y.L.; Yin, G.Y.; Gao, J.; Jiang, X.F. Nitrogen Mineralization and Immobilization in Sediments of the East China Sea: Spatiotemporal Variations and Environmental Implications. J. Geophys. Res. Biogeosci. 2016, 121, 2842–2855. [Google Scholar] [CrossRef]
- Lin, X.B.; Hou, L.J.; Liu, M.; Li, X.F.; Yin, G.Y.; Zheng, Y.L.; Deng, F.Y. Gross Nitrogen Mineralization in Surface Sediments of the Yangtze Estuary. PLoS ONE 2016, 11, e0151930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, G.; Zhou, L.M.; Zhao, X.; Jia, Q.; Xie, Y.; Zhou, G. Vertical Distribution of Soil Organic Carbon and Total Nitrogen in Reed Wetland. J. Appl. Ecol. 2006, 17, 384–389. [Google Scholar]
- Bu, C.; Wang, Y.; Ge, C.; Ahmad, H.A.; Gao, B.; Ni, S.Q. Dissimilatory Nitrate Reduction to Ammonium in the Yellow River Estuary: Rates, Abundance, and Community Diversity. Sci. Rep. 2017, 7, 6830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, G.; Hou, L.; Liu, M.; Liu, Z.; Gardner, W.S. A Novel Membrane Inlet Mass Spectrometer Method to Measure 15NH4+ for Isotope-Enrichment Experiments in Aquatic Ecosystems. Environ. Sci. Technol. 2014, 48, 9555–9562. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Gardner, W.S. Dissimilatory Nitrate Reduction to Ammonium (DNRA) as A Nitrogen Link, versus Denitrification as A Sink in A Shallow Estuary (Laguna Madre/Baffin Bay, Texas). Mar. Ecol. Prog. Ser. 2002, 237, 41–50. [Google Scholar] [CrossRef]
- Li, X.B.; Hou, L.; Liu, M.; Zheng, Y.; Yin, G.; Lin, X.; Cheng, L.; Li, Y.; Hu, X. Evidence of Nitrogen Loss from Anaerobic Ammonium Oxidation Coupled with Ferric Iron Reduction in an Intertidal Wetland. Environ. Sci. Technol. 2015, 49, 11560–11568. [Google Scholar] [CrossRef]
- Leonard, E.N.; Mattson, V.R.; Benoit, D.A.; Hoke, R.A.; Ankley, G.T. Seasonal Variation of Acid Volatile Sulfide Concentration in Sediment Cores from Three Northeastern Minnesota Lakes. Hydrobiologia 2004, 271, 87–95. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhang, H.Y.; He, C.; Liu, C.; Liang, X.; Chen, X. Spatiotemporal Variability in Soil Sulfur Storage Is Changed by Exotic Spartina Alterniflora in the Jiuduansha Wetland, China. Ecol. Eng. 2019, 133, 160–166. [Google Scholar] [CrossRef]
- Zeng, A.; Hu, W.; Zeng, C.; Sun, Z.; Gao, D. Litter Decomposition and Nutrient Dynamics of Native Species (Cyperus malaccensis) and Alien Invasive Species (Spartina alterniflora) in a Typical Subtropical Estuary (Min River) in China. Estuaries Coast 2020, 43, 1873–1883. [Google Scholar] [CrossRef]
- Zhu, M.J.; He, P.D.Y.; Zhu, Y.; Huang, P.M.; Zhang, M.Y. Biogeochemical Sulfur Cycling Coupling with Dissimilatory Nitrate Reduction Processes in Freshwater Sediments. Environ. Rev. 2017, 26, 12–132. [Google Scholar] [CrossRef]
- Simek, M.V.; Cooper, J. The Influence of Soil pH on Denitrification: Progress towards the Understanding of This Interaction over the Last 50 years. Eur. J. Soil Sci. 2002, 53, 45–354. [Google Scholar] [CrossRef]
- Zhou, Z.; Ge, L.; Huang, Y.; Liu, Y.; Wang, S. Coupled Relationships among Anammox, Denitrification, and Dissimilatory Nitrate Reduction to Ammonium along Salinity Gradients in a Chinese Estuarine Wetland. J. Environ. Sci. 2021, 106, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Risgaard-Petersen, N.; Meyer, R.L.; Schmid, M.C.; Jetten, M.S.M.; Enrich-Prast, A.; Rysgaard, S.; Revsbech, N.P. Anaerobic Ammonium Oxidation in an Estuarine Sediment. Aquat. Microb. Ecol. 2004, 36, 293–304. [Google Scholar] [CrossRef] [Green Version]
- Wollersheim, R.; Trolldenier, G.; Beringer, H. Effect of Bulk Density and Soil Water Tension on Denitrification in the Rhizosphere of Spring Wheat (Triticum vulgare). Biol. Fertil. Soils. 2004, 5, 181–187. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).