Sediment Nitrate Dissimilatory Reduction Processes along a Salinity Gradient in an Estuarine and Coastal Wetland, China
Abstract
:1. Introduction
2. Materials and Methods
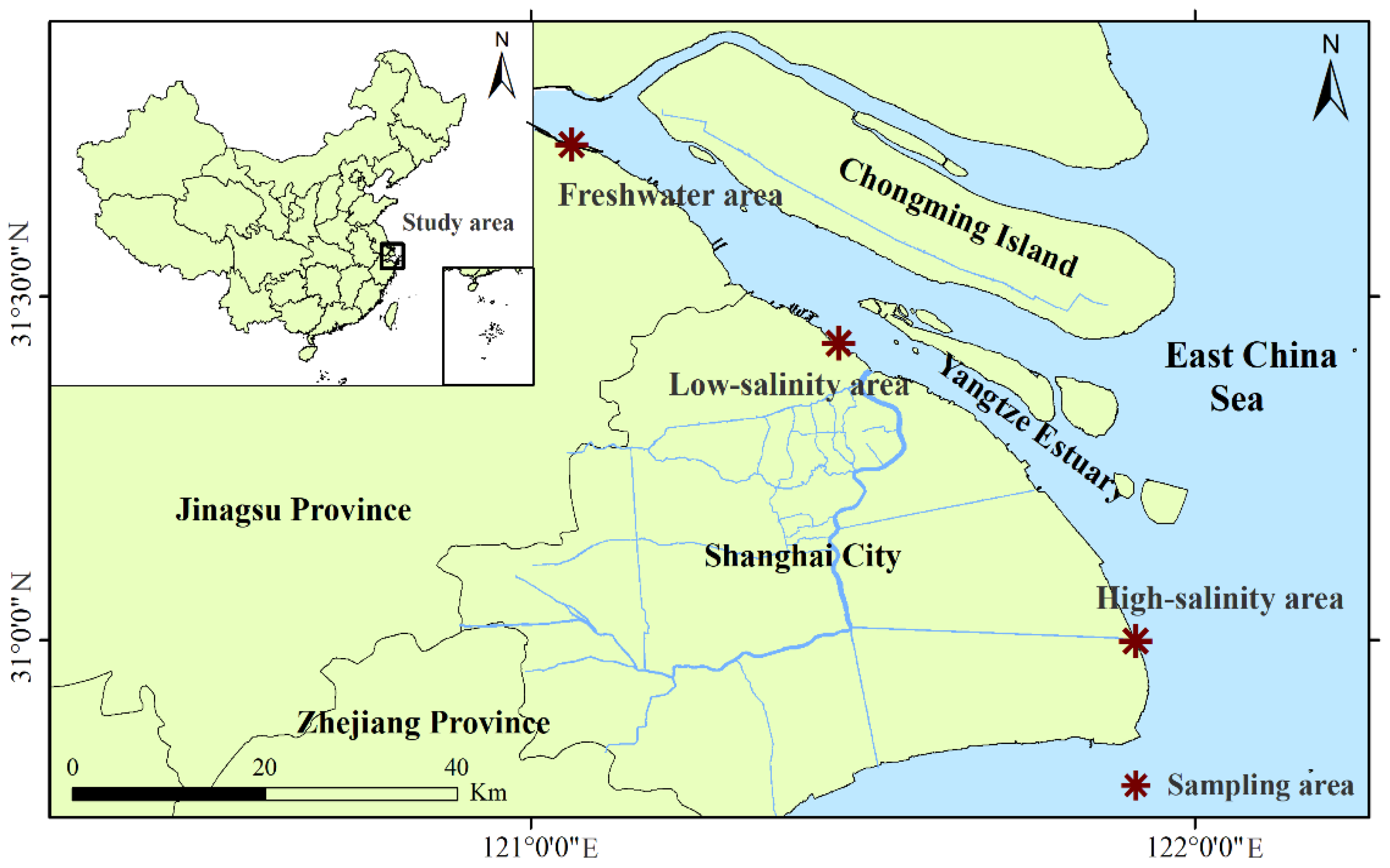
2.1. Study Site and Field Sample Collection
2.2. Sediment Physico-Chemical Properties Analysis
2.3. DNA Extraction and Quantitative PCR (q-PCR) Analysis
2.4. Analyzing Potential NO3− Rates Based on the 15N Tracer Method
2.5. Statistical Analysis
3. Results
3.1. Sediment Physicochemical Parameters
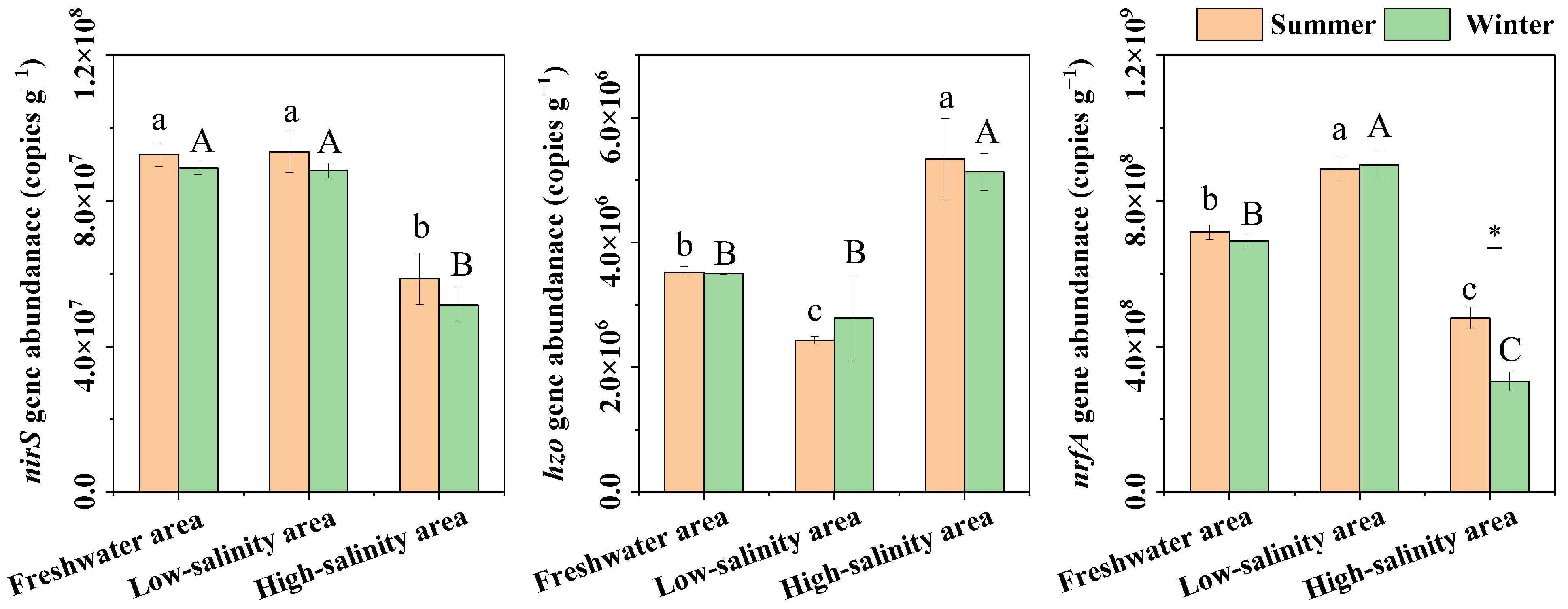
3.2. Sediment nirS, hzo, and nrfA Gene Abundance
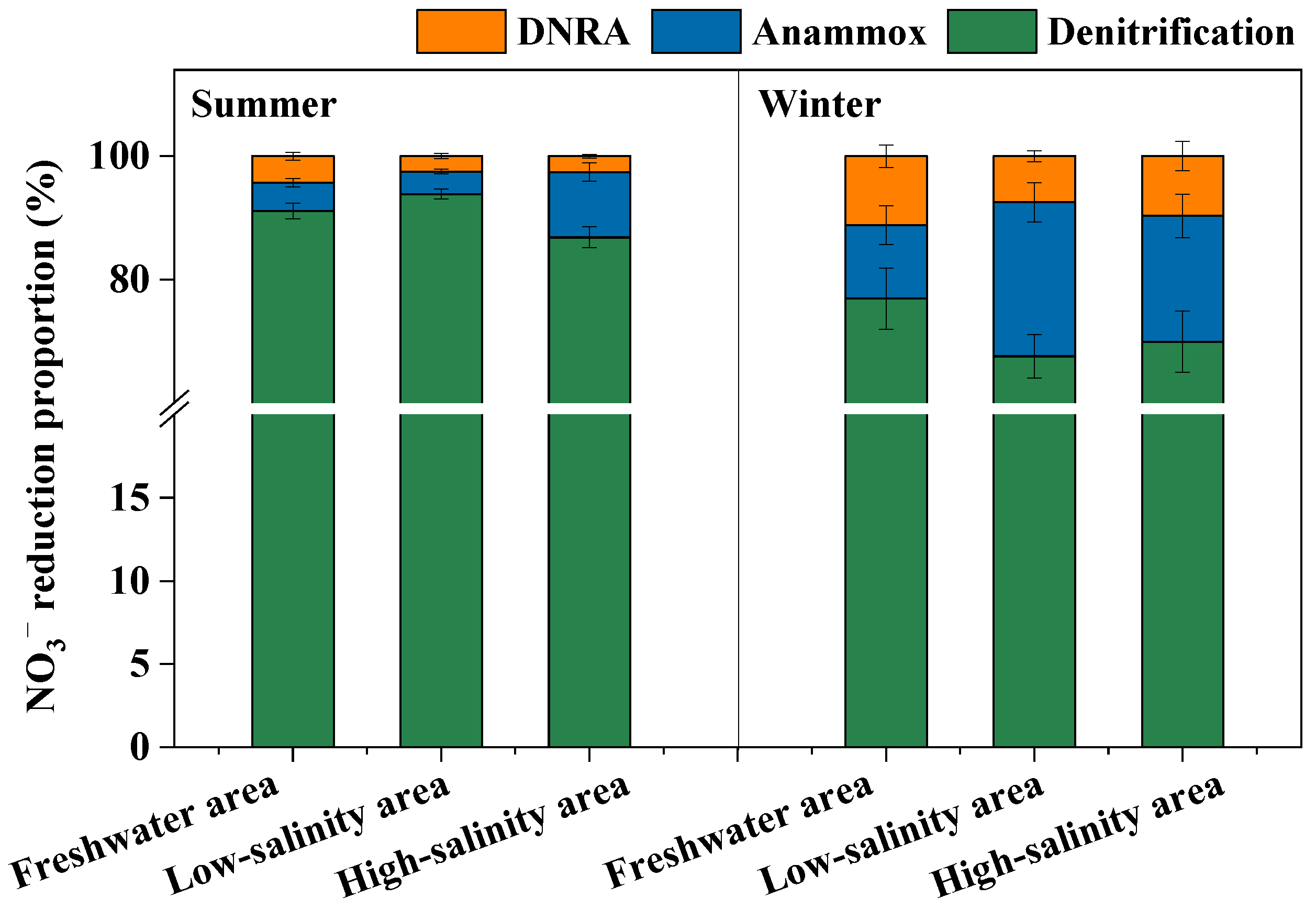
3.3. Sediment Denitrification, Anammox and DNRA Processes
3.4. Sediment Environmental Variables Influencing NO3− Reduction Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.H.; Wang, L.; Xie, X.J.; Huang, L.D.; Wu, Y.H. Effects of invasion of Spartina alterniflora and exogenous N deposition on N2O emissions in a coastal salt marsh. Ecol. Eng. 2013, 58, 77–83. [Google Scholar] [CrossRef]
- Damashek, J.; Francis, C.A. Microbial Nitrogen Cycling in Estuaries: From Genes to Ecosystem Processes. Estuar. Coast. 2018, 41, 626–660. [Google Scholar] [CrossRef]
- Lin, G.M.; Huang, J.; Lu, J.G.; Su, M.; Hu, B.Q.; Lin, X.B. Geochemical and Microbial Insights into Vertical Distributions of Genetic Potential of N-cycling Processes in Deep-sea Sediments. Ecol. Indic. 2021, 125, 107461. [Google Scholar] [CrossRef]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The evolution and future of Earth’s nitrogen cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, F.Y.; Hou, L.J.; Liu, M.; Zheng, Y.L.; Yin, G.Y.; Li, X.F.; Lin, X.B.; Chen, F.; Gao, J.; Jiang, X.F. Dissimilatory nitrate reduction processes and associated contribution to nitrogen removal in sediments of the Yangtze Estuary. J. Geophys. Res. Biogeosci. 2015, 120, 1521–1531. [Google Scholar] [CrossRef]
- Chen, F.; Hou, L.; Liu, M.; Zheng, Y.; Yin, G.; Lin, X.; Li, X.F.; Zong, H.B.; Deng, F.Y.; Gao, J.; et al. Net anthropogenic nitrogen inputs (NANI) into the Yangtze River basin and the relationship with riverine nitrogen export. J. Geophys. Res. Biogeosci. 2016, 121, 451–465. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.J.; Zheng, Y.L.; Jiang, X.F. Anaerobic ammonium oxidation and its contribution to nitrogen removal in China’s coastal wetlands. Sci. Rep. 2015, 5, 15621. [Google Scholar] [CrossRef]
- Wankel, S.D.; Ziebis, W.; Buchwald, C.; Charoenpong, C.; de Beer, D.; Dentinger, J.; Xu, Z.J.; Zengler, K. Evidence for fungal and chemodenitrification based N2O flux from nitrogen impacted coastal sediments. Nat. Commun. 2017, 8, 15595. [Google Scholar] [CrossRef]
- Hardison, A.K.; Algar, C.K.; Giblin, A.E.; Rich, J.J. Influence of organic carbon and nitrate loading on partitioning between dissimilatory nitrate reduction to ammonium (DNRA) and N2 production. Geochim. Cosmochim. Acta 2015, 164, 146–160. [Google Scholar] [CrossRef] [Green Version]
- Plummer, P.; Tobias, C.; Cady, D. Nitrogen reduction pathways in estuarine sediments: Influences of organic carbon and sulfide. J. Geophys. Res. Biogeosci. 2015, 120, 1958–1972. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.F.; Sobey, M.N.; Smith, C.J.; Rusmana, I.; Phillips, W.; Stott, A.; Osborn, A.M.; Nedwell, D.B. Dissimilatory reduction of nitrate to ammonium, not denitrification or anammox, dominates benthic nitrate reduction in tropical estuaries. Limnol. Oceanogr. 2011, 56, 279–291. [Google Scholar] [CrossRef]
- Murphy, A.E.; Anderson, I.C.; Smyth, A.R.; Song, B.; Luckenbach, M.W. Microbial nitrogen processing in hard clam (Mercenaria mercenaria) aquaculture sediments: The relative importance of denitrification and dissimilatory nitrate reduction to ammonium (DNRA). Limnol. Oceanogr. 2016, 61, 1589–1604. [Google Scholar] [CrossRef] [Green Version]
- Gao, D.Z.; Li, X.F.; Lin, X.B.; Wu, D.M.; Jin, B.S.; Huang, Y.P.; Liu, M.; Chen, X. Soil dissimilatory nitrate reduction processes in the Spartina alterniflora invasion chronosequences of a coastal wetland of southeastern China: Dynamics and environmental implications. Plant Soil 2017, 421, 383–399. [Google Scholar] [CrossRef]
- Cao, W.; Yang, J.; Li, Y.; Liu, B.; Wang, F.; Chang, C. Dissimilatory nitrate reduction to ammonium conserves nitrogen in anthropogenically affected subtropical mangrove sediments in Southeast China. Mar. Pollut. Bull. 2016, 110, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.B.; Liu, M.; Hou, L.J.; Gao, D.Z.; Li, X.F.; Lu, K.J.; Gao, J. Nitrogen Losses in Sediments of the East China Sea: Spatiotemporal Variations, Controlling Factors, and Environmental Implications. J. Geophys. Res. Biogeosci. 2017, 122, 2699–2715. [Google Scholar] [CrossRef]
- Huang, F.J.; Lin, X.B.; Hu, W.F.; Zeng, F.; He, L.; Yin, K.D. Nitrogen Cycling Processes in Sediments of the Pearl River Estuary: Spatial Variations, Controlling Factors, and Environmental Implications. Catena 2021, 206, 105545. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Ge, L.; Huang, Y.F.; Liu, Y.Q.; Wang, S.Y. Coupled relationships among anammox, denitrification, and dissimilatory nitrate reduction to ammonium along salinity gradients in a Chinese estuarine wetland. J. Environ. Sci. 2021, 106, 39–46. [Google Scholar] [CrossRef]
- Gao, J.; Hou, L.J.; Zheng, Y.L.; Liu, M.; Yin, G.Y.; Li, X.F.; Lin, X.B.; Yu, C.D.; Wang, R.; Jiang, X.F.; et al. nirS-Encoding denitrifier community composition, distribution, and abundance along the coastal wetlands of China. Appl. Microbiol. Biot. 2016, 100, 8573–8582. [Google Scholar] [CrossRef]
- An, Z.R.; Gao, D.Z.; Chen, F.Y.; Wu, L.; Zhou, J.; Zhang, Z.X.; Dong, H.P.; Yin, G.Y.; Han, P.; Liang, X.; et al. Crab bioturbation alters nitrogen cycling and promotes nitrous oxide emission in intertidal wetlands: Influence and microbial mechanism. Sci. Total Environ. 2021, 797, 149176. [Google Scholar] [CrossRef]
- Lin, X.B.; Zheng, P.F.; Zou, S.B.; Sun, F.F.; Zhang, X.L.; Gong, J. Seagrass (Zostera marina) Promotes Nitrification Potential and Selects Specific Ammonia Oxidizers in Coastal Sediments. J. Soil Sediment 2021, 21, 3259–3273. [Google Scholar] [CrossRef]
- Huang, F.J.; Lin, X.B.; Yin, K.D. Effects of Marine Produced Organic Matter on the Potential Estuarine Capacity of NOx− Removal. Sci. Total Environ. 2022, 812, 151471. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, S.C.; Franklin, R.B.; Berrier, D.J. Saltwater intrusion into tidal freshwater marshes alters the biogeochemical processing of organic carbon. Biogeosciences 2013, 10, 8171–8183. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.M.; Hu, M.J.; Ren, H.C.; Li, J.B.; Tong, C.; Musenze, R.S. Seasonal variations of nitrous oxide fluxes and soil denitrification rates in subtropical freshwater and brackish tidal marshes of the Min River estuary. Sci. Total Environ. 2018, 616, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.Y.; Hou, L.J.; Liu, M.; Li, X.F.; Zheng, Y.L.; Gao, J.; Lin, X.B. DNRA in intertidal sediments of the Yangtze Estuary. J. Geophys. Res. Biogeosci. 2017, 122, 1988–1998. [Google Scholar] [CrossRef]
- Hou, L.J.; Wang, R.; Yin, G.Y.; Liu, M.; Zheng, Y.L. Nitrogen fixation in the intertidal sediments of the Yangtze Estuary: Occurrence and environmental implications. J. Geophys. Res. Biogeosci. 2018, 123, 936–944. [Google Scholar] [CrossRef]
- Wang, D.Q.; Chen, Z.L.; Wang, J.; Xu, S.Y.; Yang, H.X.; Chen, H.; Yang, L.Y.; Hu, L.Z. Summer-time denitrification and nitrous oxide exchange in the intertidal zone of the Yangtze Estuary. Estuar. Coast. Shelf Sci. 2007, 73, 43–53. [Google Scholar] [CrossRef]
- Wei, H.C.; Lin, X.B. Shifts in the relative abundance and potential rates of sediment ammonia-oxidizing archaea and bacteria along environmental gradients of an urban river-estuary-adjacent sea continuum. Sci. Total Environ. 2021, 771, 144824. [Google Scholar] [CrossRef]
- Zhang, W.L.; Zeng, C.S.; Tong, C.; Zhai, S.J.; Lin, X.; Gao, D.Z. Spatial distribution of phosphorus speciation in marsh sediments along a hydrologic gradient in a subtropical estuarine wetland, China. Estuar. Coast. Shelf Sci. 2015, 154, 30–38. [Google Scholar] [CrossRef]
- Lin, X.B.; Hou, L.J.; Liu, M.; Li, X.F.; Zheng, Y.L.; Yin, G.Y.; Gao, J.; Jiang, X.F. Nitrogen mineralization and immobilization in sediments of the East China Sea: Spatiotemporal variations and environmental implications. J. Geophys. Res. Biogeosci. 2016, 121, 2842–2855. [Google Scholar] [CrossRef]
- Liu, R.; Lin, X.B.; Wang, G.Q.; Liu, X. Natural N-bearing Nanoparticles in Sediments of a Shallow Bay of the South China: A New N Form in N-cycling. Ecol. Indic. 2021, 122, 107281. [Google Scholar] [CrossRef]
- Luo, M.; Zeng, C.S.; Tong, C.; Huang, J.F.; Yu, Q.; Guo, Y.B.; Wang, S.H. Abundance and speciation of iron across a subtropical tidal marsh of the Min River Estuary in the East China Sea. Appl. Geochem. 2014, 45, 1–13. [Google Scholar] [CrossRef]
- Cline, J.D. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 1969, 14, 454–458. [Google Scholar] [CrossRef]
- Wei, H.C.; Gao, D.Z.; Liu, Y.; Lin, X.B. Sediment nitrate reduction processes in response to environmental gradients along an urban river-estuary-sea continuum. Sci. Total Environ. 2020, 718, 137185. [Google Scholar] [CrossRef] [PubMed]
- Throback, I.N.; Enwall, K.; Jarvis, A.; Hallin, S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef]
- Fu, L.L.; Chen, Y.Y.; Li, S.Q.; He, H.; Mi, T.Z.; Zhen, Y.; Yu, Z.G. Shifts in the anammox bacterial community structure and abundance in sediments from the Changjiang Estuary and its adjacent area. Syst. Appl. Microbiol. 2019, 42, 383–396. [Google Scholar] [CrossRef]
- Yin, G.Y.; Hou, L.J.; Liu, M.; Liu, Z.F.; Gardner, W.S. A Novel Membrane Inlet Mass Spectrometer Method to Measure (NH4+)-N15 for Isotope-Enrichment Experiments in Aquatic Ecosystems. Environ. Sci. Technol. 2014, 48, 9555–9562. [Google Scholar] [CrossRef]
- Li, N.; Li, B.; Nie, M.; Wu, J. Effects of exotic Spartina alterniflora on saltmarsh nitrogen removal in the Yangtze River Estuary, China. J. Clean. Prod. 2020, 271, 122557. [Google Scholar] [CrossRef]
- Maie, N.; Boyer, J.N.; Yang, C.; Jaffe, R. Spatial, geomorphological, and seasonal variability of CDOM in estuaries of the Florida Coastal Everglades. Hydrobiologia 2006, 569, 135–150. [Google Scholar] [CrossRef]
- O’Connor, B.L.; Hondzo, M. Enhancement and inhihition of denitrification by fluid-flow and dissolved oxygen flux to stream sediments. Environ. Sci. Technol. 2008, 42, 119–125. [Google Scholar] [CrossRef]
- Shan, J.; Zhao, X.; Sheng, R.; Xia, Y.Q.; Ti, C.P.; Quan, X.F.; Wang, S.W.; Wei, W.X.; Yan, X.Y. Dissimilatory nitrate reduction processes in typical Chinese Paddy soils: Rates, relative contributions, and influencing factors. Environ. Sci. Technol. 2016, 50, 9972–9980. [Google Scholar] [CrossRef]
- Egli, K.; Fanger, U.; Alvarez, P.J.; Siegrist, H.; van der Meer, J.R.; Zehnder, A.J. Enrichment and characterization of an anammox bacterium from a rotating biological contactor treating ammonium-rich leachate. Arch. Microbiol. 2001, 175, 198–207. [Google Scholar] [CrossRef]
- Canion, A.; Overholt, W.A.; Kostka, J.E.; Huettel, M.; Lavik, G.; Kuypers, M.M. Temperature response of denitrification and anaerobic ammonium oxidation rates and microbial community structure in Arctic fjord sediments. Environ. Microbiol. 2014, 16, 3331–3344. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Dong, L.F.; Wilson, J.; Stott, A.; Osborn, A.M.; Nedwell, D. Seasonal variation in denitrification and dissimilatory nitrate reduction to ammonia process rates and corresponding key functional genes along an estuarine nitrate gradient. Front. Microbiol. 2015, 6, 542. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.H.; Shi, Y.L.; Groffman, P.M.; Schlesinger, W.H.; Zhu, Y.G. Centennial-scale analysis of the creation and fate of reactive nitrogen in China (1910–2010). Proc. Natl. Acad. Sci. USA 2013, 110, 2052–2057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, G.M.; Lin, X.B. Bait input altered microbial community structure and increased greenhouse gases production in coastal wetland sediment. Water Res. 2022, 218, 118520. [Google Scholar] [CrossRef]
- Li, N.; Li, B.; Nie, M.; Wu, J.H.; Zhao, J.Y. Contrasting effects of aboveground litters of native Phragmites australis and invasive Spartina alterniflora on nitrification and denitrification. Sci. Total Environ. 2021, 764, 144283. [Google Scholar] [CrossRef]

| Target Genes | Primers | Sequence (5′-3′) | PCR Conditions | Reference |
|---|---|---|---|---|
| nirS | cd3aF | GTSAACGTSAAGGARACSGG | 50 °C for 2 min, 95 °C for 10 min, 45 × [95 °C for 30 s, 58 °C for 40 s, 72 °C for 1 min] | [34] |
| R3cd | GASTTCGGRTGSGTCTTGA | |||
| hzo | hzo5F | AGTATGGGTATGTCHAATG | 50 °C for 2 min, 95 °C for 10 min, 45 × [94 °C for 50 s, 54 °C for 1 min, 72 °C for 40 s] | [35] |
| hzo5R | CATCWGTCCATACCAAA | |||
| nrfA | nrfA-2F | CACGACAGCAAGACTGCCG | 50 °C for 2 min, 95 °C for 10 min, 40 × [95 °C for 30 s, 60 °C for 1 min, 72 °C for 1 min] | [24] |
| nrfA-2R | CCGGCACTTTCGAGCCC |
| Summer | Winter | |||||
|---|---|---|---|---|---|---|
| Freshwater Area | Low-Salinity Area | High-Salinity Area | Freshwater Area | Low-Salinity Area | High-Salinity Area | |
| WC (%) | 56.76 ± 2.78 a | 56.54 ± 3.38 a | 53.80 ± 4.23 a | 54.26 ± 2.94 A | 55.08 ± 2.46 A | 50.56 ± 2.85 A |
| pH | 7.25 ± 0.22 c | 7.95 ± 0.14 b | 8.25 ± 0.13 a | 7.21 ± 0.04 C | 7.82 ± 0.13 B | 8.21 ± 0.11 A |
| Salinity | 0.13 ± 0.02 c | 0.28 ± 0.04 b | 5.48 ± 0.50 a | 0.13 ± 0.02 C | 0.28 ± 0.02 B | 6.41 ± 0.39 A |
| TOC (g C kg−1) | 15.15 ± 1.12 b | 18.73 ± 1.84 a | 12.53 ± 1.73 b | 13.70 ± 0.56 B | 18.35 ± 2.57 A | 11.70 ± 0.35 C |
| TN (g N kg−1) | 1.14 ± 0.01 b | 2.22 ± 0.14 a | 0.98 ± 0.13 c | 1.19 ± 0.16 B | 2.11 ± 0.20 A | 1.10 ± 0.21 B |
| NH4+ (mg N kg−1) | 50.39 ± 5.65 b | 86.75 ± 9.00 a | 36.11 ± 4.35 c | 42.39 ± 5.40 B | 78.98 ± 5.28 A | 36.12 ± 4.90 B |
| NOx− (μg N kg−1) | 8.49 ± 2.31 b | 13.75 ± 1.42 a | 7.54 ± 0.95 b | 8.00 ± 1.71 B | 13.89 ± 1.34 A | 7.32 ± 0.89 B |
| Fe2+ (g Fe kg−1) | 1.37 ± 0.32 b | 2.07 ± 0.34 a | 1.24 ± 0.13 b | 1.27 ± 0.10 B | 1.81 ± 0.22 A | 1.19 ± 0.19 B |
| Fe3+ (g Fe kg−1) | 1.28 ± 0.51 a | 0.93 ± 0.17 a | 1.08 ± 0.15 a | 1.18 ± 0.13 A | 1.02 ± 0.08 A | 1.04 ± 0.13 A |
| Fe2+/Fe3+ | 1.12 ± 0.18 b | 2.23 ± 0.24 a | 1.17 ± 0.28 b | 1.08 ± 0.10 B | 1.77 ± 0.18 A | 1.15 ± 0.11 B |
| Sulfide (mg S kg−1) | 3.72 ± 0.44 b | 48.84 ± 7.86 a | 2.80 ± 0.22 c | 3.55 ± 0.63 B | 53.75 ± 11.41 A | 2.49 ± 0.84 B |
| nirS | hzo | nrfA | WC | pH | Salinity | TOC | |
|---|---|---|---|---|---|---|---|
| Denitrification | 0.45 | −0.49 * | 0.50 * | 0.42 | 0.07 | −0.18 | 0.53 * |
| Anammox | 0.10 | −0.27 | 0.46 | 0.05 | 0.37 | −0.63 ** | 0.53 * |
| DNRA | 0.79 ** | −0.85 ** | 0.78 ** | 0.43 | −0.34 | 0.10 | 0.77 ** |
| TN | NH4+ | NOx− | Fe2+ | Fe3+ | Fe2+/Fe3+ | Sulfide | |
| Denitrification | 0.53 * | 0.60 * | 0.48 * | 0.64 ** | −0.17 | 0.69 ** | 0.45 |
| Anammox | 0.66 ** | 0.61 ** | 0.64 ** | 0.56 * | −0.21 | 0.55 * | 0.76 ** |
| DNRA | 0.72 ** | 0.81 ** | 0.68 ** | 0.75 ** | 0.07 | 0.62 ** | 0.62 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Liu, C.; Li, M.; Lin, X. Sediment Nitrate Dissimilatory Reduction Processes along a Salinity Gradient in an Estuarine and Coastal Wetland, China. J. Mar. Sci. Eng. 2022, 10, 761. https://doi.org/10.3390/jmse10060761
Gao L, Liu C, Li M, Lin X. Sediment Nitrate Dissimilatory Reduction Processes along a Salinity Gradient in an Estuarine and Coastal Wetland, China. Journal of Marine Science and Engineering. 2022; 10(6):761. https://doi.org/10.3390/jmse10060761
Chicago/Turabian StyleGao, Lin, Chen Liu, Mingcong Li, and Xianbiao Lin. 2022. "Sediment Nitrate Dissimilatory Reduction Processes along a Salinity Gradient in an Estuarine and Coastal Wetland, China" Journal of Marine Science and Engineering 10, no. 6: 761. https://doi.org/10.3390/jmse10060761
APA StyleGao, L., Liu, C., Li, M., & Lin, X. (2022). Sediment Nitrate Dissimilatory Reduction Processes along a Salinity Gradient in an Estuarine and Coastal Wetland, China. Journal of Marine Science and Engineering, 10(6), 761. https://doi.org/10.3390/jmse10060761






