Abstract
Light is one of the most important environmental factors affecting the growth and reproduction of algae. In this study, the effect of various LED colors on the productivity, chlorophyll (Chl-a, Chl-b, and total Chl), protein, and carbohydrate content of Isochrysis zhanjiangensis in indoor culture was investigated. Microalgae monocultures were cultivated under five different colors (red, green, blue, yellow, and white) for twenty-one days. The microalgae cultured under red light exhibited a higher specific growth rate (0.4431 ± 0.0055 µ day−1), and under white light a higher productivity (0.0728 ± 0.0013 g L−1 day−1). The poorest performance was observed under yellow and green lights. Interestingly, green light exhibited the highest levels of chlorophylls (Chl-a, 1.473 ± 0.037 mg L−1; Chl-b, 1.504 ± 0.001 mg L−1; total Chl, 2.827 ± 0.083 mg L−1). The highest protein content was observed under the white light (524.1935 ± 6.5846 mg L−1), whereas the carbohydrate content was remarkably high under the blue light (24.4697 ± 0.0206 mg L−1). This study is important in terms of the selection of light at the appropriate color (wavelength) to increase the content of organic compounds desired to be obtained indoors with the potential for commercially produced cultures.
1. Introduction
The microalgae Isochrysis zhanjiangensis, a marine single-cellular golden-brown flagellated species isolated from Nansan Island of Zhanjiang of Guangdong Province, China [1], is an important species in the aquaculture economy and commonly used in the fodder industry and various mariculture systems [2]. Due to the small size, fast-growing, cellulose-free cell walls, and nutrient-richness, especially in polyunsaturated fatty acid (Omega-3), chlorophylls, and carotenoids, Isochrysis zhanjiangensis has been mass-produced for feeding fish, shrimp, shellfish seedlings, as well as larvae of a variety of aquaculture animals [3,4]. In particular, I. zhanjiangensis is used as a food supply worldwide during broodstock hatchery conditioning. Additionally, it is especially used to culture suspension-feeding larvae and early juvenile bivalve mollusks, because of its nutritional properties that support shell growth and the high survival rates when used as a mono-species diet [5,6].
The growth and propagation of microalgae are affected by several environmental factors, such as temperature, salinity, light, and pH. Light, the main source of energy for algae growth, is one important key factor in regulating its growth and development [7]. Photosynthetic microorganisms do not utilize the whole solar spectrum but only a fraction of it, in particular from 400 to 700 nm. The absorption wavelengths of visible light by algae and plants are mainly concentrated in the blue-violet light region of 400–510 nm and the red-orange light region of 610–720 nm. However, the wavelengths absorbed by microalgae differ according to species [8]. Several investigations have been focused on either the single or combined influence of light quality (meaning the different wavelengths which are absorbed by water to various extents) [9,10,11,12], light quantity (different light intensities) [13,14,15,16,17,18,19], and light periodicity (different photoperiods) [20,21,22,23], thus indicating that illumination is a complex external factor for microalgae cultivation. Moreover, researchers have found that light quality plays an important role in regulating the growth and development, morphology, photosynthesis, and metabolism of algae [24,25,26,27]. For instance, red light is an efficient light quality for the growth of Arthrospira (Spirulina) platensis, while it has a significant inhibitory effect on the chlorophyll content [28]. The cultivation of chlorophytes under a mix of green and blue LEDs may prove optimal for growth, biomass productivity, pigments, proteins, and lipids [29,30,31,32]. Green light enhanced growth rates, protein, and lipid contents in Brachiomonas submarina, and pigment content in Kirchneriella aperta. High- and low-intensity green LEDs enhanced lutein biosynthesis compared to red or blue LEDs in B. submarina and Scenedesmus obliquus [33,34]. High-intensity blue LEDs increased the carotenoid zeaxanthin, and white light was optimal for phycobiliprotein in Rhodella sp. and fucoxanthin content for Stauroneis sp. and Phaeothamnion sp. [33]. Although, the use of any white light sources (fluorescent lamps, RGB LEDs, and white LEDs) for the cultivation of green algae seems to not affect growth. A species-specific response of algae to light intensity has been described in Desmodesmus quadricauda, Parachlorella kessleri, and Chlamydomonas reinhardtii [35]. In P. kessleri cells, the concentration of pigments decreased with increasing light intensity, a response found not only in the genus Chlorella [13,36,37,38], but also in other green algae [39].
Light quality, intensity, and photoperiod also affect the growth, biochemical composition, and physiology of Isochrysis sp. [40,41,42]. Microalgal pigments change with algal variety. Therefore, the influences of different light qualities on the physiological properties of algae, such as growth, photosynthesis, and cellular metabolism, are diverse [43]. The ability of the microalgae to utilize different light qualities is determined by this composition of pigments in their cells, and different pigments absorb different light qualities. The growth and development of microalgae and the generation of metabolites are related to light quality, and the light quality that is most suitable for the growth of one microalgae species may not be suitable for another [33]. Therefore, it is of great significance to explore the optimum light quality for the growth of I. zhangjiangensis. Thus, this study aims to examine the effects of different LED light qualities on the productivity, chlorophyll, protein, and carbohydrate content of I. zhanjiangensis in indoor culture. Our results will aid the optimization of the light conditions for the growth of I. zhanjiangensis. Additionally, the results will provide the basis for the optimization of microalgae propagation in indoor conditions and other systems that require artificial illumination in general.
2. Materials and Methods
2.1. Microalgae Culture Condition
The stock of the microalgae species I. zhanjiangensis was obtained from the Microalgae Laboratory of the College of Oceanography at Hainan University (Haikou, China). For the enrichment of the culture media, the nutrient medium Ningbo 3# was dissolved in filtered and sterilized seawater (29 PSU) with the composition per 1 L of: 100 mg NaNO3, 10 mg NaH2PO4, 2.5 mg FeSO4, 10 mg EDTA-2Na, 0.25 mg MnSO4, 0.5 × 10−3 μg vitamin B12, and 6 μg vitamin B1.
The microalgae were cultured in 5 L flat-bottom glass flasks at 25 ± 1 °C, with the illumination provided by light-emitting diodes (LED, 191.8 μmoles/m2/s) with a 14:10 h light/dark photoperiod. To enhance growth and prevent the algae from settling, continuous aeration was applied using air stones at 20 L/min. The initial culture media inoculation of the microalgae was 100,000 cells/mL, and the illumination was immediately provided using green (495–530 nm), blue (450–480 nm), red (615–650 nm), white (450–465 nm), and yellow (580–595 nm) light-emitting diodes. Each illumination treatment was set separately to avoid any light interference from the neighboring treatments and set in triplicate.
2.2. Measurement of I. Zhanjiangensis Growth
The growth of I. zhanjiangensis cultured in the experiment was measured using cell density and biomass (dry weight) to precisely determine the growth pattern of the microalgae. Microalgae cell density was determined daily by counting the cell number using a hemacytometer under a white-light field microscope and was presented as the number of cells/mL. Cell dry weight was estimated by filtering 20 mL of cultured microalgae using a pre-weighed Whatman GF/C filter (47 mm ∅), washed three times with filtered seawater, and dried to constant weight. Samples were always collected at the same time of the day.
Productivity (Px) and specific growth rate (µ) of I. zhangjiangensis were calculated according to the following Formulae [44]:
where, Xi = initial biomass concentration (g/L), Xm = maximum biomass concentration (g/L), and Tc = cultivation time related to the maximum biomass concentration (days).
Px = (Xm − Xi) (Tc)−1
µ = (lnXm − lnXi) (Tc)−1
2.3. Measurement of Photosynthetic Pigments, Protein, and Carbohydrate Content
Chlorophyll concentration (Chl) content was determined using a modified method from Jeffrey and Humphrey [45]. Briefly, 20 mL samples were withdrawn from the culture flasks and transferred to opaque plastic bottles, kept away from light, and warmed to room temperature, then filtered using Whatman GF/C filters (25 mm ∅). After filtration, the filters containing the microalgae biomass were folded and stored separately at −2 °C. Chl extraction was carried out by grinding the filters in 90% acetone (2–4 mL) in a glass homogenizer on an ice bath under low-light conditions for up to 1 min. After grinding, the Chl extracts were transferred to a graduated and stoppered centrifuge tube and rinsed with 10 mL of acetone 90% (10 mL + dead volume of filter). The extract was then centrifuged for 10 min at 500 g. After completion of centrifugation, the absorbance of the supernatant (OD) was measured at 750, 664, 647, and 630 nm against a 90% acetone blank. The concentrations of Chl-a, Chl-b, and the total Chl were calculated according to the following Equations [45,46]:
where, Cchla, Cchlb, and Ctch1 are the concentrations of Chl-a, Chl-b, and the total Chl (mg/L), and V is the filtered volume (mL).
Cchla = (11.85 E664 − 1.54 E647 − 0.08 E630) × 10/V
Cchlb = (21.03 E647 − 5.43 E664 − 2.66 E630) × 10/V
Ctch1 = (20.21 E647 + 8.02 E664) × 10/V
The protein content of each sample was determined by the Lowry method [47]. Carbohydrates were measured by the phenol sulfuric acid method [48].
2.4. Statistical Analysis
Significant differences (p < 0.05) among variables were first identified using the t-test. Before analysis, data were tested for normality using Kolmogorov–Smirnov’s test and for homogeneity of variance using Cochran’s C test. All statistical analyses were performed using DPS14.5 software (Hangzhou Rui Feng Information Technology Co., Ltd., Hangzhou, China).
3. Results
3.1. Specific Growth Rate and Biomass Productivity
The dry weight, specific growth rate, and productivity of I. zhangjiangensis under different light conditions are shown in Table 1. The highest and lowest accumulated biomass (dry weight) were observed in the microalgae cultured under the white and red lights, with 1.1761 ± 0.0212 and 0.5683 ± 0.0284 g/L, respectively. Significant differences were observed among all the specific growth rate values of I. zhangjiangensis cultured under different illumination conditions. The specific growth rates from the highest to the lowest values were under the red, blue, white, and green lights, with 0.4431 ± 0.0055, 0.4089 ± 0.0029, 0.2948 ± 0.0011, and 0.2466 ± 0.0035 µ/day, respectively.

Table 1.
Growth parameters of I. zhangjiangensis cultured under different colored LEDs.
The highest productivity value, a parameter related to potential large-scale microalgae production systems, was observed under the white light (0.0728 ± 0.0013 g/L/day) and was significant when compared to the values observed under the red and blue lights (0.0620 ± 0.0032 and 0.0617 ± 0.0018 g/L/day, respectively). Low values were observed under the green and yellow lights, with 0.0490 ± 0.0032 and 0.0390 ± 0.0028 g/L/day, respectively.
3.2. Algal Growth
The daily mean growth of the microalgae I. zhangjiangensis cultured under the five different colored LEDs was calculated. The stationary phase was attained after the 11th day from the inoculation in most of the treatments (different light colors), and significant differences were observed when microalgae density values (cells/mL) were compared at their respective stationary phases (Figure 1). The microalgae cultured under white light exhibited a pause in its growing tendency on day 9, but on day 13 resumed exponential cell proliferation until day 18 at the highest value (1100.0 × 104 cells/mL). A similar growth pattern was observed in the microalgae cultured under the blue light when cell proliferation paused on day 9 and resumed on day 14, reaching 502.1 × 104 cells/mL. The microalgae cultured under the green light also exhibited a lag on day 12, but resumed on day 15, reaching maximum cell density, 755.0 × 104 cells/mL, on day 17. The microalgae that were cultured under the red light reached the stationary phase on day 10 but exhibited the lowest cell density, 0.4 × 107 cells/mL, among all treatments.
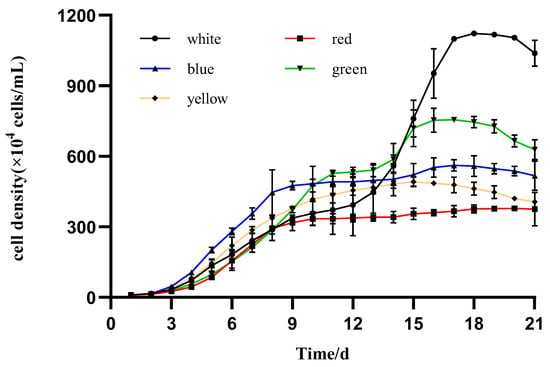
Figure 1.
Daily mean growth of I. zhangjiangensis cultured under different colored LEDs (n = 3, p < 0.05 t-test).
3.3. Photosynthetic Pigment Production
Significant differences were observed among the maximum Chl-a values at the stationary phase of the different treatments (Figure 2). The maximum Chl-a value, 1.473 ± 0.037 mg/L, was observed after day 18 of culture under green light. Microalgae cultivated under white light exhibited the second highest value, 1.073 ± 0.108 mg/L, on the 21st day. The red light initially exhibited low levels, but after the 9th day of cultivation, its growth accelerated and exhibited the third Chl-a value, 0.873 ± 0.007 mg/L, on the 11th day. The Chl-a values under yellow and blue light were 0.693 ± 0.008 and 0.675 ± 0.002 mg/L, respectively, and were the lowest levels of Chl-a concentration among all the treatments.
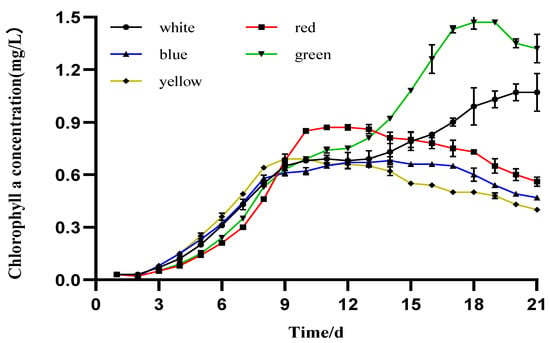
Figure 2.
Daily mean values of chlorophyll-a in I. zhangjiangensis cultured under different colored LEDs (n = 3, p < 0.05 t-test).
The Chl-b levels in the five different colored LEDs are shown in Figure 3, and significance was observed at the maximum levels attained among treatments. The maximum Chl-b level, 1.504 ± 0.001 mg/L, was observed on the 21st day of culture under the green light. The white light evoked the second highest value (0.918 ± 0.001 mg/L), occurring the same day as the green light. The orange, red, and blue lights evoked lower Chl-b levels, with values of 0.360 ± 0.001, 0.275± 0.009, and 0.232 ± 0.003 mg/L, respectively.
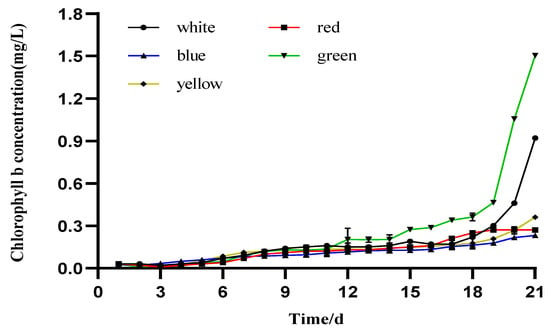
Figure 3.
Daily mean values of chlorophyll-b in I. zhangjiangensis cultured under different colored LEDs (n = 3, p < 0.05 t-test).
The total Chl concentrations of the microalgae I. zhangjiangensis cultured under the five different colored LEDs are shown in Figure 4. In the case of the green and white lights, the highest levels were observed on day 21, with values of 2.827 ± 0.083 and 2.238 ± 0.083 mg/L, respectively. Under the red, yellow, and blue lights, the highest mean total values were observed within the 11th and 13th days of cultivation, with corresponding values of 1.095 ± 0.002, 1.000 ± 0.002, and 0.872 ± 0.049 mg/L, respectively.
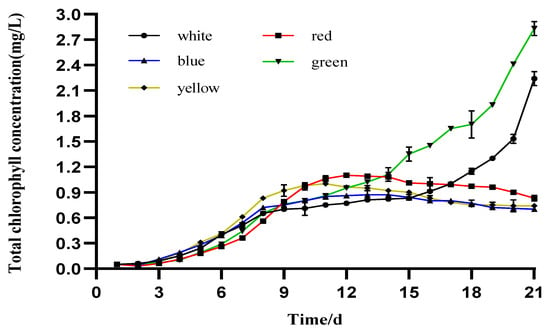
Figure 4.
Daily mean values of total chlorophyll in I. zhangjiangensis cultured under different colored LEDs (n = 3, p < 0.05 t-test).
3.4. Protein and Soluble Carbohydrates’ Production
Significant differences were observed in the total protein content of I. zhangjiangensis grown under different light colors (Table 2). A particularly high content (524.1935 ± 6.5846 mg/L) was observed in the microalgae cultured under the white light. No significance was observed between the blue and yellow lights, in which both lights exhibited lower content than in the white light. The microalgae cultivated under green and red lights observed the lowest values.

Table 2.
Protein and carbohydrate content in I. zhangjiangensis cultured under different colored LEDs.
The mean values of the carbohydrate content were also significant among all light color qualities (Table 2). The highest mean value was 24.4697 ± 0.0206 mg/L, observed in the microalgae cultured under blue light. The green, red, white, and yellow lights exhibited significant mean values within them, varying from 7.8455 ± 0.1247 to 9.3687 ± 0.0206 mg/L.
4. Discussion
4.1. Specific Growth Rate and Biomass Productivity of I. zhangjiangensis
The present study examined the effect of different colored LED illumination on the productivity, Chl, protein, and carbohydrate content in the indoor culture of microalgae I. zhangjiangensis. The highest specific growth rate was observed under the red light, 1.5-fold higher when compared to the white light. Whereas the highest biomass observed under the white light was 2.0-fold higher than that under the red light (Table 1). This observation seems contradictory; however, it is possible that the size of the cells in the microalgae cultured under white light was larger than under the other light colors. Unfortunately, cell size data were not collected at any stage during the experimental period to fully support this idea. Nevertheless, it has been reported that light quality regulates the cell size of the microalgae [49]. For instance, the cell sizes of the green microalgae C. reinhardtii grown under blue light were 1.3 and 1.6 times larger than under white and red light sources, respectively, due to a delay in cellular division processes [50]. Additionally, the light intensity has resulted in cell enlargement by a factor of 2.5 in these species, compared to 1.9 in D. quadricauda, and only 1.3 in P. kessleri. The smaller increase in cell size in P. kessleri was compensated for by a 13.6-fold daily increase in cell number under optimal conditions, as compared with a 9.7-fold increase in C. reinhardtii and D. quadricauda [35]. Another study using different strains of Chlorella sp. revealed that the light spectrum had a significant influence on microalgae cell size. The largest and smallest cells were observed under blue and red lights, respectively [51]. Such an increase in cell size is a specific response of organisms that divide by multiple fission and thus can respond to better growth conditions beyond a simple increase in the cell number. The larger cell size is a mechanism that supports better growth in the next cell cycle, possibly leading to better productivity [35]. Nevertheless, it should be emphasized that although the value of the specific growth rate is important, the most relevant parameter related to potential large-scale microalgae production systems is biomass productivity, which was highest when I. zhangjiangensis was cultivated under white light. It has been proven that multi-chromatic white light, whether provided by a fluorescent lamp or by LED, is more advantageous than monochromatic light for promoting the growth of Isochrysis sp. [42,52,53], which can also be supported by our results. A similar observation has already been reported in the productivity of Nannochloropsis sp. cultured under different colored lights, where pink and white lights exhibited higher biomass productivity [54]. These results are probably related to the differences in energy provided by light and captured by the photosynthetic apparatus of the photosynthetic microorganisms [55]. Particularly, between 380 and 750 nm, the energy content is sufficient to produce chemical changes in the absorbing molecules, as happens throughout the photosynthetic pathways prevailing in the microalgae [56].
4.2. The Effect of Different LED Colors on the Pigment Content of I. zhangjiangensis
Microalgae, similar to plants, capture light energy (light-harvesting antennas) and produce electrons in the reaction center of the photosystems. For efficient photosynthesis, preserving an excitation balance between the two photosystems (PSI and PSII) is of prime importance. To serve this purpose, microalgae possess specific light-harvesting antennas to expand the available light wavelength. Certain groups of algae contain accessory pigments that help in efficiently harvesting light for photosynthesis [54]. Green algae, in particular, possess a chlorophyll–protein complex which is comprised of Chl-a and b and carotenoids for carrying out the photosynthesis [57]. Chl molecules absorb light energy and transfer this energy to the photochemical reaction centers presented in algae, cyanobacteria, and higher plants by PSI and PSII, where charge separation occurs. Upon illumination, two electrons are extracted from water, mostly (O2 is evolved), and transferred through a chain of electron carriers to produce one molecule of NADPH2 (nicotinamide adenine dinucleotide hydrogen). Simultaneously, protons are transported from an external space (stroma) into the intra-thylakoid space (lumen), forming a pH gradient. According to Mitchel’s chemiosmotic hypothesis, the gradient drives ATP synthesis, which is catalyzed by the protein complex called ATPase or ATP synthase—a reaction called photophosphorylation [58]. In the present investigation, for I. zhangjiangensis, the highest Chl content was attained under green light, while blue light resulted in the lowest Chl content, indicating that different light qualities can evoke different levels of photosynthetic pigments. Green light has promoted the production of Chl in Chlorella vulgaris [46], and also allowed improvement of S. platensis growth [10,59]. A different result has been reported for I. galbana. The highest contents of Chl-a, Chl-c, and Car were obtained under white light, while blue light resulted in the lowest pigment contents [53]. The same report described the highest light absorption in cells cultivated under blue light, but the photochemical reaction was lowered. Cells cultivated under blue and red lights were, respectively, restricted by downregulated photosynthetic efficiency and sufficient light absorption. Meanwhile, green light showed an increase in photosynthetic efficiency, associated with a light absorption close to that for cells exposed to white light, suggesting that green light promotes the photosynthesis of I. galbana by balancing the light absorption and utilization [53]. Light with a shorter wavelength, for example, blue light, has a higher probability to cause growth photo-inhibition by striking the light-harvesting complex of cells at its peak electrical energy due to its high energy [60].
Studies have shown that infrared light can cause cell damage [61], while in some multicellular algae, blue light can significantly increase the content of algal photosynthetic pigments, increasing photosynthetic efficiency and ultimately, the growth rate [62,63]. The content of photosynthetic pigments in I. zhangjiangensis is the highest under green light, which is conducive to the accumulation of its biomass, while the content of photosynthetic pigments is the lowest under blue light. A previous study reported a contrasting result: low-intensity blue light reduced the pigment content of Chaetoceros gracilis but increased it in I. galbana [64]. The effect of light qualities on the high-value pigments has also been reported in five microalgae strains from three distinct lineages [33]. In the Rhodophyte Rhodella sp., the Chl-a levels obtained under red and white LEDs were higher than those reached under green and blue illumination for medium and high intensity. Similarly, the diatom Stauroneis sp. also returned a higher Chl-a content under medium white light intensities. Contrariwise, in the chlorophyte K. aperta and B. submarina, the Chl content was significantly higher under blue and green lights at high and medium intensities, returning two-fold higher Chl-a compared to red and white LEDs. The cultivation of Phaeothamnion sp. under high-intensity blue LEDs also induced a significant increase in Chl-a. In general, responses of each strain to different colored LEDs were generally species-specific. These results indicate that the growth performance of different microalgae under different light qualities is not consistent, which may be due to the different compositions of the pigment system of different microalgae, resulting in different requirements for light quality in photosynthesis. It is also known that the content of the photosynthetic pigments increases as light intensity decreases [65]. In the case of P. kessleri, the concentrations of Chl-a and b and carotenoids decreased with increasing light intensity. Cultures of C. reinhardtii and D. quadricauda maintained similar levels of photosynthetic pigments at low light intensities, but their concentration increased with the time of cultivation at the highest light intensity [35]. This increase in the photosynthetic pigments is attributed to the need of the microorganisms to improve their photosynthetic efficiency and capture as much energy as possible from light [28]. Considering this, a conclusion can be drawn that when Chl content is high under a specific light color, it does not necessarily imply that this illumination provides adequate amounts of energy for biomass synthesis, and this may be the case of I. zhangjiangensis cultured under green light. In addition, light-harvesting ability and energy-transfer processes also differed between green algae species. This has been demonstrated by observing the delayed fluorescence spectra in C. reinhardtii and C. variabilis cells grown under different light qualities. Both types of green algae primarily modified the associations between light-harvesting chlorophyll protein complexes (LHCs) and photosystems (PSII and PSI) [57].
4.3. The Effect of Different LED Colors on the Protein and Carbohydrate Content of I. Zhangjiangensis
The effect of light intensity on microalgal cultures has been extensively studied, and it has been demonstrated that light intensity controls not only the growth rate, but also the lipid storage [17,18,20,21,25,41], structural distribution [66], cellular composition (such as proteins and essential fatty acids) [8], and pigment synthesis [67]. While most research has been focused on the effects of light intensity, it has been shown that light quality also plays a key role in algal metabolism and the effects of light wavelengths on growth are species-specific because of the differences in metabolic pathways, pigmentation, and photoreceptors between species [8]. Moreover, the biochemical composition is a pivotal factor in determining the nutritional value of microalgae. In this investigation, the protein and carbohydrate contents of I. zhangjiangensis were influenced by the different LED colors used in the indoor cultures (Table 2). The maximum protein content was measured in the microalgae cultured under white light, followed by the yellow and blue lights, and the lowest level in the red light. Similar results have been reported in microalgal conglomerates of Chlorella variabilis and Scenedesmus obliquus when the protein content of the microalgal consortia was highest under a cool-white light [68]. Contrasting results have been observed when blue light fluorescent tubes were closely related to protein enhancement in Isocrhysis sp. cultured in a bioreactor [42]. However, cell concentration and productivity did not change substantially upon changing the light spectrum during steady-state growth. In addition, experiments conducted with Tisochrysis lutea (previously named I. aff. galbana) under white, blue, green, and red fluorescent lamps in batch cultures revealed that the growth rate and cell density were highest with white light, followed by blue light. Meanwhile, cells under green light had a greater dry weight during exponential growth in comparison with the other light colors, and this monochromatic light also increased the eicosapentaenoic acid and protein contents [69]. In axenic cultures of Dunaliella tertiolecta and Thalassiosira rotula, blue light also allows higher photosynthetic carbon incorporation into protein than white light [70]. It can be inferred that the optimal light color for the cultivation of Isochrysis varies depending on the algal strains and light sources used.
In this study, carbohydrate content was higher in the blue light and the lowest content was in the yellow light. These results concur with the one reported for A. platensis, where the highest carbohydrate content was also measured under blue light [28]. However, there are conflicting results on the influence of blue light on microalgae carbohydrate content: In T. lutea, it did not change [69], but it decreased in Isochrysis sp. [42]. Blue light also enhanced dark respiration, as previously reported for Scenedesmus obliquus [71], Rhodomonas salina [72], or D. tertiolecta and T. rotula [70], confirming a higher rate of carbohydrate degradation under blue light. The evidence presented in this study confirms that light quality can affect the biochemical composition of microalgae cells of I. zhangjiangensis when they are cultured under different light colors.
5. Conclusions
The productivity, Chl (a, b, and total), protein, and carbohydrate content of I. zhangjiangensis can be regulated by different light wavelengths. White light increased the productivity and protein content, and red light increased the specific growth rate. Pigment content was higher under the green light but possibly does not provide adequate amounts of energy for biomass synthesis. The blue light highly promoted carbohydrate content, suggesting that the light influence is a complex phenomenon that is still far from being completely understood. This study is important in terms of the selection of light at the appropriate wavelength to increase the number of metabolites desired in indoor production levels, with the possibility of adaptation to other culture systems that require artificial illumination.
6. Ethics Statement
The experiment complied with the regulations and guidelines established by the Animal Care and Use Committee of Hainan University.
Author Contributions
B.L., investigation, data curation, visualization, formal analysis, writing—original draft; Z.L., investigation, conceptualization, formal analysis; Y.C., investigation, methodology; S.L., investigation, methodology; J.M., investigation, methodology; Z.G., funding acquisition; A.W., project administration; F.Y., formal analysis; X.Z., conceptualization, methodology, formal analysis, writing—original draft, writing—review and editing; H.E.V., methodology, formal analysis, writing—original draft, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Key Research and Development Project of Hainan Province (Grant No. ZDYF2021XDNY277), the Natural Science Foundation for Young Scholars of Hainan Province (Grant No. 320QN207), the Starting Research Fund from Hainan University (Grant No. KYQD-ZR 20061), and the Academician Innovation Center of Hainan Province, China.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hu, H.; Lu, S.; Liu, R. A New Species of Isochrysis (Isochrysidales)-I. Zhanjiangensis and Its Observation on the Fine Structure. Acta Oceanol. Sin. 2007, 29, 111–119. [Google Scholar]
- Phatarpekar, P.V.; Sreepada, R.A.; Pednekar, C.; Achuthankutty, C.T. A Comparative Study on Growth Performance and Biochemical Composition of Mixed Culture of Isochrysis galbana and Chaetoceros calcitrans with Monocultures. Aquaculture 2000, 181, 141–155. [Google Scholar] [CrossRef]
- Cao, J.-Y.; Kong, Z.-Y.; Ye, M.-W.; Ling, T.; Chen, K.; Xu, J.-L.; Zhou, C.-X.; Liao, K.; Zhang, L.; Yan, X.-J. Comprehensive Comparable Study of Metabolomic and Transcriptomic Profiling of Isochrysis galbana Exposed to High Temperature, an Important Diet Microalgal Species. Aquaculture 2020, 521, 735034. [Google Scholar] [CrossRef]
- Zhu, C.; Han, D.; Li, Y.; Zhai, X.; Chi, Z.; Zhao, Y.; Cai, H. Cultivation of Aquaculture Feed Isochrysis zhangjiangensis in Low-Cost Wave Driven Floating Photobioreactor without Aeration Device. Bioresour. Technol. 2019, 293, 122018. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xu, J.; Zong, C.; Zhu, S.; Ye, M.; Zhou, C.; Chen, H.; Yan, X. Effect of High Temperature on the Lipid Composition of Isochrysis galbana Parke in Logarithmic Phase. Aquac. Int. 2017, 25, 327–339. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Zang, Z.; Li, J.; Wang, A.; Shi, Y.; Zhang, X.; Gu, Z.; Zheng, X.; Vasquez, H.E. Effect of Fresh and Spray-Dried Microalgal Diets on the Growth, Digestive Enzymatic Activity, and Gut Microbiota of Juvenile Winged Pearl Oyster Pteria penguin. Aquac. Rep. 2022, 25, 101251. [Google Scholar] [CrossRef]
- Takada, J.; Murase, N.; Abe, M.; Noda, M.; Suda, Y. Growth and Photosynthesis of Ulva prolifera under Different Light Quality from Light Emitting Diodes. Aquac. Sci. 2011, 59, 101–107. [Google Scholar]
- Kwan, P.P.; Banerjee, S.; Shariff, M.; Yusoff, F.M. Influence of Light on Biomass and Lipid Production in Microalgae Cultivation. Aquac. Res. 2021, 52, 1337–1347. [Google Scholar] [CrossRef]
- Li, Y.; Li, R.; Yi, X. Effects of Light Quality on Growth Rates and Pigments of Chaetoceros gracilis (Bacillariophyceae). J. Ocean. Limnol. 2020, 38, 795–801. [Google Scholar] [CrossRef]
- Ravelonandro, P.H.; Ratianarivo, D.H.; Joannis-Cassan, C.; Isambert, A.; Raherimandimby, M. Influence of Light Quality and Intensity in the Cultivation of Spirulina platensis from Toliara (Madagascar) in a Closed System. J. Chem. Technol. Biotechnol. 2008, 83, 842–848. [Google Scholar] [CrossRef]
- Shu, C.-H.; Tsai, C.-C.; Liao, W.-H.; Chen, K.-Y.; Huang, H.-C. Effects of Light Quality on the Accumulation of Oil in a Mixed Culture of Chlorella sp. and Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 2012, 87, 601–607. [Google Scholar] [CrossRef]
- You, T.; Barnett, S.M. Effect of Light Quality on Production of Extracellular Polysaccharides and Growth Rate of Porphyridium cruentum. Biochem. Eng. J. 2004, 19, 251–258. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Effect of Light Intensity and Quality on Growth Rate and Composition of Chlorella vulgaris. Plants 2019, 9, 31. [Google Scholar] [CrossRef]
- Pang, N.; Fu, X.; Fernandez, J.S.M.; Chen, S. Multilevel Heuristic LED Regime for Stimulating Lipid and Bioproducts Biosynthesis in Haematococcus pluvialis under Mixotrophic Conditions. Bioresour. Technol. 2019, 288, 121525. [Google Scholar] [CrossRef] [PubMed]
- Hallenbeck, P.C.; Grogger, M.; Mraz, M.; Veverka, D. The Use of Design of Experiments and Response Surface Methodology to Optimize Biomass and Lipid Production by the Oleaginous Marine Green Alga, Nannochloropsis gaditana in Response to Light Intensity, Inoculum Size and CO2. Bioresour. Technol. 2015, 184, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Solovchenko, A.E.; Khozin-Goldberg, I.; Didi-Cohen, S.; Cohen, Z.; Merzlyak, M.N. Effects of Light Intensity and Nitrogen Starvation on Growth, Total Fatty Acids and Arachidonic Acid in the Green Microalga Parietochloris incisa. J. Appl. Phycol. 2008, 20, 245–251. [Google Scholar] [CrossRef]
- Takeshita, T.; Ota, S.; Yamazaki, T.; Hirata, A.; Zachleder, V.; Kawano, S. Starch and Lipid Accumulation in Eight Strains of Six Chlorella Species under Comparatively High Light Intensity and Aeration Culture Conditions. Bioresour. Technol. 2014, 158, 127–134. [Google Scholar] [CrossRef]
- Yeesang, C.; Cheirsilp, B. Effect of Nitrogen, Salt, and Iron Content in the Growth Medium and Light Intensity on Lipid Production by Microalgae Isolated from Freshwater Sources in Thailand. Bioresour. Technol. 2011, 102, 3034–3040. [Google Scholar] [CrossRef]
- Mandotra, S.K.; Kumar, P.; Suseela, M.R.; Nayaka, S.; Ramteke, P.W. Evaluation of Fatty Acid Profile and Biodiesel Properties of Microalga Scenedesmus abundans under the Influence of Phosphorus, PH and Light Intensities. Bioresour. Technol. 2016, 201, 222–229. [Google Scholar] [CrossRef]
- Sirisuk, P.; Ra, C.-H.; Jeong, G.-T.; Kim, S.-K. Effects of Wavelength Mixing Ratio and Photoperiod on Microalgal Biomass and Lipid Production in a Two-Phase Culture System Using LED Illumination. Bioresour. Technol. 2018, 253, 175–181. [Google Scholar] [CrossRef]
- Babuskin, S.; Radhakrishnan, K.; Babu, P.A.S.; Sivarajan, M.; Sukumar, M. Effect of Photoperiod, Light Intensity and Carbon Sources on Biomass and Lipid Productivities of Isochrysis galbana. Biotechnol. Lett. 2014, 36, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Krzemińska, I.; Pawlik-Skowrońska, B.; Trzcińska, M.; Tys, J. Influence of Photoperiods on the Growth Rate and Biomass Productivity of Green Microalgae. Bioprocess Biosyst. Eng. 2014, 37, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ding, W.; Zhou, X.; Jin, W.; Han, W.; Chi, K.; Chen, Y.; Zhao, Z.; He, Z.; Jiang, G. Sub-Pilot Scale Cultivation of Tetradesmus dimorphus in Wastewater for Biomass Production and Nutrients Removal: Effects of Photoperiod, CO2 Concentration and Aeration Intensity. J. Water Process Eng. 2022, 49, 103003. [Google Scholar] [CrossRef]
- De Mooij, T.; de Vries, G.; Latsos, C.; Wijffels, R.H.; Janssen, M. Impact of Light Color on Photobioreactor Productivity. Algal Res. 2016, 15, 32–42. [Google Scholar] [CrossRef]
- Rugnini, L.; Rossi, C.; Antonaroli, S.; Rakaj, A.; Bruno, L. The Influence of Light and Nutrient Starvation on Morphology, Biomass and Lipid Content in Seven Strains of Green Microalgae as a Source of Biodiesel. Microorganisms 2020, 8, 1254. [Google Scholar] [CrossRef]
- Jeon, Y.-C.; Cho, C.-W.; Yun, Y.-S. Measurement of Microalgal Photosynthetic Activity Depending on Light Intensity and Quality. Biochem. Eng. J. 2005, 27, 127–131. [Google Scholar] [CrossRef]
- Elisabeth, B.; Rayen, F.; Behnam, T. Microalgae Culture Quality Indicators: A Review. Crit. Rev. Biotechnol. 2021, 41, 457–473. [Google Scholar] [CrossRef]
- Markou, G. Effect of Various Colors of Light-Emitting Diodes (LEDs) on the Biomass Composition of Arthrospira platensis Cultivated in Semi-Continuous Mode. Appl. Biochem. Biotechnol. 2014, 172, 2758–2768. [Google Scholar] [CrossRef]
- Atta, M.; Idris, A.; Bukhari, A.; Wahidin, S. Intensity of Blue LED Light: A Potential Stimulus for Biomass and Lipid Content in Fresh Water Microalgae Chlorella vulgaris. Bioresour. Technol. 2013, 148, 373–378. [Google Scholar] [CrossRef]
- Hultberg, M.; Jönsson, H.L.; Bergstrand, K.-J.; Carlsson, A.S. Impact of Light Quality on Biomass Production and Fatty Acid Content in the Microalga Chlorella vulgaris. Bioresour. Technol. 2014, 159, 465–467. [Google Scholar] [CrossRef]
- Teo, C.L.; Atta, M.; Bukhari, A.; Taisir, M.; Yusuf, A.M.; Idris, A. Enhancing Growth and Lipid Production of Marine Microalgae for Biodiesel Production via the Use of Different LED Wavelengths. Bioresour. Technol. 2014, 162, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Ra, C.-H.; Kang, C.-H.; Jung, J.-H.; Jeong, G.-T.; Kim, S.-K. Effects of Light-Emitting Diodes (LEDs) on the Accumulation of Lipid Content Using a Two-Phase Culture Process with Three Microalgae. Bioresour. Technol. 2016, 212, 254–261. [Google Scholar] [CrossRef] [PubMed]
- McGee, D.; Archer, L.; Fleming, G.T.A.; Gillespie, E.; Touzet, N. Influence of Spectral Intensity and Quality of Led Lighting on Photoacclimation, Carbon Allocation and High-Value Pigments in Microalgae. Photosynth. Res. 2020, 143, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-H.; Chan, M.-C.; Liu, C.-C.; Chen, C.-Y.; Lee, W.-L.; Lee, D.-J.; Chang, J.-S. Enhancing Lutein Productivity of an Indigenous Microalga Scenedesmus obliquus Fsp-3 Using Light-Related Strategies. Bioresour. Technol. 2014, 152, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Bialevich, V.; Zachleder, V.; Bišová, K. The Effect of Variable Light Source and Light Intensity on the Growth of Three Algal Species. Cells 2022, 11, 1293. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Fanesi, A.; Martin, T.; Lopes, F. Biomass Production and Physiology of Chlorella vulgaris during the Early Stages of Immobilized State Are Affected by Light Intensity and Inoculum Cell Density. Algal Res. 2021, 59, 102453. [Google Scholar] [CrossRef]
- He, Q.; Yang, H.; Wu, L.; Hu, C. Effect of Light Intensity on Physiological Changes, Carbon Allocation and Neutral Lipid Accumulation in Oleaginous Microalgae. Bioresour. Technol. 2015, 191, 219–228. [Google Scholar] [CrossRef]
- Beale, S.I.; Appleman, D. Chlorophyll Synthesis in Chlorella: Regulation by Degree of Light Limitation of Growth. Plant Physiol. 1971, 47, 230–235. [Google Scholar] [CrossRef][Green Version]
- Da Silva Ferreira, V.; Sant’Anna, C. Impact of Culture Conditions on the Chlorophyll Content of Microalgae for Biotechnological Applications. World J. Microbiol. Biotechnol. 2017, 33, 20. [Google Scholar] [CrossRef]
- Yoshioka, M.; Yago, T.; Yoshie-Stark, Y.; Arakawa, H.; Morinaga, T. Effect of High Frequency of Intermittent Light on the Growth and Fatty Acid Profile of Isochrysis galbana. Aquaculture 2012, 338–341, 111–117. [Google Scholar] [CrossRef]
- Che, C.A.; Kim, S.H.; Hong, H.J.; Kityo, M.K.; Sunwoo, I.Y.; Jeong, G.-T.; Kim, S.-K. Optimization of Light Intensity and Photoperiod for Isochrysis galbana Culture to Improve the Biomass and Lipid Production Using 14-L Photobioreactors with Mixed Light Emitting Diodes (LEDs) Wavelength under Two-Phase Culture System. Bioresour. Technol. 2019, 285, 121323. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, J.; Bougaran, G.; Jauffrais, T.; Lefebvre, S.; Rouxel, C.; Saint-Jean, B.; Lukomska, E.; Robert, R.; Cadoret, J.P. Effects of Blue Light on the Biochemical Composition and Photosynthetic Activity of Isochrysis sp. (T-Iso). J. Appl. Phycol. 2013, 25, 109–119. [Google Scholar] [CrossRef]
- Fernandes, B.D.; Dragone, G.M.; Teixeira, J.A.; Vicente, A.A. Light Regime Characterization in an Airlift Photobioreactor for Production of Microalgae with High Starch Content. Appl. Biochem. Biotechnol. 2010, 161, 218–226. [Google Scholar] [CrossRef]
- Sukumaran, P.; Nulit, R.; Halimoon, N.; Simoh, S.; Omar, H.; Ismail, A. Formulation of Cost-Effective Medium Using Urea as a Nitrogen Source for Arthrospira platensis Cultivation under Real Environment. ARRB 2018, 22, 1–12. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Humphrey, G.F. New Spectrophotometric Equations for Determining Chlorophylls a, b, C1 and C2 in Higher Plants, Algae and Natural Phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Willoughby, N. Luminescent Photobioreactor Design for Improved Algal Growth and Photosynthetic Pigment Production through Spectral Conversion of Light. Bioresour. Technol. 2013, 142, 147–153. [Google Scholar] [CrossRef]
- Lowry, O.H. Protein Measurement with the Folin Phenol Reagent. J. biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Somani, B.L.; Khanade, J.; Sinha, R. A Modified Anthrone-Sulfuric Acid Method for the Determination of Fructose in the Presence of Certain Proteins. Anal. Biochem. 1987, 167, 327–330. [Google Scholar] [CrossRef]
- Koc, C.; Anderson, G.A.; Kommareddy, A. Use of Red and Blue Light-Emitting Diodes (LED) and Fluorescent Lamps to Grow Microalgae in a Photobioreactor. Isr. J. Aquac.-Bamidgeh 2013, 65, 20661. [Google Scholar] [CrossRef]
- Oldenhof, H.; Zachleder, V.; Ende, H. Blue Light Delays Commitment to Cell Division in Chlamydomonas Reinhardtii. Plant Biology. 2004, 6, 689–695. [Google Scholar] [CrossRef]
- Izadpanah, M.; Gheshlaghi, R.; Mahdavi, M.A.; Elkamel, A. Effect of Light Spectrum on Isolation of Microalgae from Urban Wastewater and Growth Characteristics of Subsequent Cultivation of the Isolated Species. Algal Res. 2018, 29, 154–158. [Google Scholar] [CrossRef]
- Gómez-Loredo, A.; Benavides, J.; Rito-Palomares, M. Growth Kinetics and Fucoxanthin Production of Phaeodactylum tricornutum and Isochrysis galbana Cultures at Different Light and Agitation Conditions. J. Appl. Phycol. 2016, 28, 849–860. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J. Analysis of Light Absorption and Photosynthetic Activity by Isochrysis galbana under Different Light Qualities. Aquac. Res. 2020, 51, 2893–2902. [Google Scholar] [CrossRef]
- Vadiveloo, A.; Moheimani, N.R.; Cosgrove, J.J.; Bahri, P.A.; Parlevliet, D. Effect of Different Light Spectra on the Growth and Productivity of Acclimated Nannochloropsis sp. (Eustigmatophyceae). Algal Res. 2015, 8, 121–127. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light Requirements in Microalgal Photobioreactors: An Overview of Biophotonic Aspects. Appl. Microbiol. Biotechnol. 2011, 89, 1275–1288. [Google Scholar] [CrossRef]
- Kommareddy, A.; Anderson, G. Study of Light as a Parameter in the Growth of Algae in a Photo-Bio Reactor (PBR). In Proceedings of the 2003 ASAE Annual Meeting, Las Vegas, NV, USA, 27–30 July 2003. [Google Scholar] [CrossRef]
- Ueno, Y.; Aikawa, S.; Kondo, A.; Akimoto, S. Adaptation of Light-Harvesting Functions of Unicellular Green Algae to Different Light Qualities. Photosynth. Res. 2019, 139, 145–154. [Google Scholar] [CrossRef]
- Masojídek, J.; Torzillo, G.; Koblížek, M. Handbook of Microalgal Culture: Applied Phycology and Biotechnolog, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; ISBN 9781118567166. [Google Scholar]
- Wang, C.-Y.; Fu, C.-C.; Liu, Y.-C. Effects of Using Light-Emitting Diodes on the Cultivation of Spirulina platensis. Biochem. Eng. J. 2007, 37, 21–25. [Google Scholar] [CrossRef]
- Das, P.; Lei, W.; Aziz, S.S.; Obbard, J.P. Enhanced Algae Growth in Both Phototrophic and Mixotrophic Culture under Blue Light. Bioresour. Technol. 2011, 102, 3883–3887. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, A.; Lütz, C. Algae and UV Irradiation: Effects on Ultrastructure and Related Metabolic Functions. Micron 2006, 37, 190–207. [Google Scholar] [CrossRef]
- Kuwano, K.; Abe, N.; Nishi, Y.; Seno, H.; Nishihara, G.N.; Iima, M.; Zachleder, V. Growth and Cell Cycle of Ulva Compressa (Ulvophyceae) under LED Illumination. J. Phycol. 2014, 50, 744–752. [Google Scholar] [CrossRef]
- Tsekos, I.; Niell, F.X.; Aguilera, J.; López-Figueroa, F.; Delivopoulos, S.G. Ultrastructure of the Vegetative Gametophytic Cells of Porphyra Leucosticta (Rhodophyta) Grown in Red, Blue and Green Light. Phycol. Res. 2002, 50, 251–264. [Google Scholar] [CrossRef]
- Gorai, T.; Katayama, T.; Obata, M.; Murata, A.; Taguchi, S. Low Blue Light Enhances Growth Rate, Light Absorption, and Photosynthetic Characteristics of Four Marine Phytoplankton Species. J. Exp. Mar. Biol. Ecol. 2014, 459, 87–95. [Google Scholar] [CrossRef]
- Danesi, E.D.G.; Rangel-Yagui, C.O.; Carvalho, J.C.M.; Sato, S. Effect of Reducing the Light Intensity on the Growth and Production of Chlorophyll by Spirulina platensis. Biomass Bioenergy 2004, 26, 329–335. [Google Scholar] [CrossRef]
- George, B.; Pancha, I.; Desai, C.; Chokshi, K.; Paliwal, C.; Ghosh, T.; Mishra, S. Effects of Different Media Composition, Light Intensity and Photoperiod on Morphology and Physiology of Freshwater Microalgae Ankistrodesmus falcatus-A Potential Strain for Bio-Fuel Production. Bioresour. Technol. 2014, 171, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Guðmundsson, Ó.; Paglia, G.; Herjólfsson, G.; Andrésson, Ó.S.; Palsson, B.Ø.; Brynjólfsson, S. Enhancement of Carotenoid Biosynthesis in the Green Microalga Dunaliella salina with Light-Emitting Diodes and Adaptive Laboratory Evolution. Appl. Microbiol. Biotechnol. 2013, 97, 2395–2403. [Google Scholar] [CrossRef]
- Gatamaneni Loganathan, B.; Orsat, V.; Lefsrud, M.; Wu, B.S. A Comprehensive Study on the Effect of Light Quality Imparted by Light-Emitting Diodes (LEDs) on the Physiological and Biochemical Properties of the Microalgal Consortia of Chlorella variabilis and Scenedesmus obliquus Cultivated in Dairy Wastewater. Bioprocess Biosyst. Eng. 2020, 43, 1445–1455. [Google Scholar] [CrossRef]
- Del Pilar Sánchez-Saavedra, M.; Maeda-Martínez, A.N.; Acosta-Galindo, S. Effect of Different Light Spectra on the Growth and Biochemical Composition of Tisochrysis lutea. J. Appl. Phycol. 2016, 28, 839–847. [Google Scholar] [CrossRef]
- Rivkin, R. Influence of Irradiance and Spectral Quality on the Carbon Metabolism of Phytoplankton I. Photosynthesis, Chemical Composition and Growth. Mar. Ecol. Prog. Ser. 1989, 55, 291–304. [Google Scholar] [CrossRef]
- Brinkmann, G.; Senger, H. The Development of Structure and Function in Chloroplasts of Greening Mutants of Scenedesmus IV. Blue Light-Dependent Carbohydrate and Protein Metabolism. Plant Cell Physiol. 1978, 19, 1427–1437. [Google Scholar] [CrossRef]
- Hammer, A.; Schumann, R.; Schubert, H. Light and Temperature Acclimation of Rhodomonas salina (Cryptophyceae): Photosynthetic Performance. Aquat. Microb. Ecol. 2002, 29, 287–296. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).