Abstract
The experiments were carried out during 2012–2017. There were 5 crops in rotation: Red clover, winter wheat, pea, potato and barley undersown (us) with red clover. There were 5 cropping systems in the experimental setup: 2 conventional systems with chemical plant protection and mineral fertilizers; 3 organic systems which included winter cover crops and farm manure. The aim of the present research was to study the effect of cultivating barley undersown with red clover and the preceding winter cover crop on the soil microbial hydrolytic activity, the change in the content of soil organic carbon (SOC) and total nitrogen (Ntot) compared to the same parameters from the field that was previously under potato cultivation (forecrop of barley in the rotation). The cultivation of barley with red clover (barley (us)) had a positive impact on the soil micro-organisms activity. In organic systems the soil microbial hydrolytic activity increased on average by 19.0%, compared to the conventional systems. By cultivating barley (us) the soil microbial hydrolytic activity had a significant effect on the SOC content only in organic systems where winter cover crops were used. Organic cultivation systems had positive impact on the soil nitrogen content; Ntot in samples taken before sowing the barley (us) was higher by 17.4% and after the cultivation of barley (us) by 14.4% compared to conventional systems, as an average of experimental years. After cultivation of barley (us) with red clover the soil microbial hydrolytic activity had no effect on the soil Ntot content in either cultivation systems.
1. Introduction
The nutrient availability in organic systems depends mainly on the soil fertility [1]. The soil fertility should be maintained through suitable crop rotation, use of cattle manure and green manure [1,2]. According to many studies [3,4] legumes as forecrop have positive effect on the following crop and their cultivation is the most important source of nitrogen [5]. In cropping systems where winter cover crops were used with farm manure the yield and quality parameters of barley increased [6]. By undersowing red clover to barley crop it enabled to decrease the occurrance of weeds [2] and on the areas sown with winter cover crops the content of weed seeds in the soil decreased significantly [7]. The efficiency of cover crops and undersown crops in surpressing the weeds has been studied also by several researchers [8,9,10]. Additionally, undersown crops have positive impact on the physical and chemical parameters of the soil [11,12]. Carter and Kunelius [13] found that barley undersown with clover increased the root mass by 6–11 times compared to cultivating only the barley and also improved the soil structure. Besides physical and chemical parameters of the soil more and more attention is given to biological quality of the soil (incl. microbial and enzymatic activity). The method of hydrolysis of fluorescein diacetate (FDA) as a relatively cheap and reliable method for determination of quantitative parameters of soil microorganisms [14] has been widely applied [15,16,17] and used to study the microbial metabolic activity in soil [18,19,20] showed the positive effect of long-term crop rotations and organic fertilizers on the soil microbial composition and their activity. The microbial activity is significantly more sensitive to changes than the physicochemical parameters of the soil [21,22,23]. It is not yet clear how soil microbial hydrolytic activity influences the content of soil organic carbon (SOC) and total nitrogen (Ntot) in the soil in various conventional and organic cultivation systems. Present research covers these topics based on barley with red clover cultivation.
So far it is not clearly known what effect does the undersowing of red clover and the preceding winter cover crop (also with manure) have on the microbial activity, SOC and Ntot content in the soil. Hence, the aim of our experiment was to investigate the effect of different cropping systems of barley undersown with red clover on the soil microbial hydrolytic activity, SOC and Ntot content in the soil compared to the same parameters in the field after the forecrop (potato) which had been harvested.
2. Materials and Methods
2.1. Experimental Setup
The field experiment was set up at the experimental station of the Estonian University of Life Sciences in Eerika, Tartu, Estonia (58°22′ N, 26°40′ E). The five-field crop rotation (red clover, winter wheat, pea, potato and barley undersown with red clover (barley (us)) experiment with organic and conventional systems was started in 2008. The soil was described as Stagnic Luvisol in the World Reference Base for soil resources [24] classification with 56.5% sand, 34% silt and 9.5% clay, and 20 to 30 cm depth of the ploughing layer [25]. The experiments were established as systematic block design in 4 replications and the size of plots was 60 m2. Data used in present study is based on the 2nd rotation period (2012–2017). Crops under study were: Red clover (Trifolium pratense L., cultivar ‘Varte’) which was undersown into barley (Hordeum vulgare L., cultivar ‘Anni’), which were compared to the field from where previously forecrop (potato) had been harvested. Red clover has been drilled at the same time as barley. Red clover (tetraploid) sowing rate was 7 kg ha−1.
In conventional system crop rotation mineral fertilizers and chemical pest control were applied (plots with red clover did not receive any mineral fertilizers nor chemical pest control). In organic farming system crop rotations winter cover crops were sown after the harvest of main crops: Ryegrass (sowing rate 25 kg ha−1) (since 2014 ryegrass was replaced with winter oil turnip, 7 kg ha −1) followed winter wheat, winter oilseed rape (7.1 kg ha−1, since 2014 mixture of winter rye and winter oil turnip) followed peas and winter rye (220 kg ha−1) followed potato, in order to keep the green cover in rotation until spring when these were ploughed into the soil. Following description of experimental materials and methods applies specifically to potato as being forecrop and barley (us):
- Conventional system variants:
- Conv 0—no fertilizers, but chemical pest control was used
- Conv II—plots with barley (us) recieved phosphorous (P) 25 kg ha−1 and potassium (K) 95 kg ha−1 and mineral nitrogen (N) 120 kg ha−1 For weed protection in barley crop in both conventional variants MCPA-750 (rate of 1.0 l ha−1) and also fungicides for barley diseases were used.
- Organic system variants (no mineral fertilizers nor chemical pest control was used):
- Org 0—without winter cover crops, without organic fertilizers (control)
- Org CC—with winter cover crops. Winter rye was sown as a winter cover crop after harvesting potato (after precrop) that was ploughed into the soil as green manure in spring.
- Org CC+M—with winter cover crops and cattle manure. Composted cattle manure applied to potato (here as forecrop) at a rate of 20 t ha−1 and cereals (barley (us)) in spring at a rate of 10 t ha−1, that were both ploughed into the soil in spring as green manure.
Biomass of winter cover crops have been reported by Kauer et al. [26]. More detailed trial plan perfomed by Kauer et al. [26] and Sánchez de Cima et al. [27]. In organic systems no mineral fertilizers nor pesticides were used.
2.2. Chemical Analysis
The spectrophotometric determination of soil microbial hydrolytic activity is a simple and fast method for evaluation of the microbial activity in the soil [28]. For determination of soil microbial hydrolytic activity, the samples of 500 g were taken from the depth of 5–10 cm according to ISO 10381-6 [29] method and these were sieved through the 2 mm sieve [30]. The preparation of reagents for following soil microbial hydrolytic activity analysis was performed according to the method described by Adam and Duncan [17]. Once a year in mid-April before starting of field operations, soil samples were taken from the depth of 0–25 cm. Eight samples were taken from each plot to obtain the average for each plot. Air-dried soil samples were sieved through a 2 mm sieve. The content of SOC was measured using the Tjurin method [31], and Ntot content was measured using the Kjeldahl method [30].
2.3. Statistical Analyses
The statistical analysis of collected data was performed with the software Statistica 13 (Quest Software Inc, Aliso Viejo, CA, USA). Full-factorial analysis of variance (ANOVA) was used to test the statistical significance of year, farming system and their interaction effects on soil properties (soil microbial hydrolytical activity, SOC and Ntot). Fisher (LSD) test [32] was applied for pairwise comparisions of the factors. Correlation analysis was used as linear correlation coefficients between variables and the significance of coefficients were taken as p < 0.001, p < 0.01, p < 0.05 or ns (no significant).
3. Results and Discussion
Results of factorial analysis showed that the significant (p < 0.05) effect of the climatic conditions of the experimental years and the combined effect of years and farming systems were noticed only in soil Ntot content (Table 1). The significant effects of the farming systems on all the parameters under investigation were apparent.

Table 1.
Impact of trial factors on soil microbial hydrolytic activity (μg of fluorescein g−1 soil dry weight h−1), soil organic carbon (%) and total nitrogen (%) content.
3.1. Soil Microbial Hydrolytic Activity
The cultivation of barley (us) increased the soil microbial hydrolytic activity of the soil in all farming systems. Soil microbial hydrolytic activity data presented in Table 2 describe the result of the end of the first crop rotation (2008–2012) and the initial status of the new crop rotation (2013–2017) after the cultivation of the forecrop (potato) of the barley (us). The methods used in organic systems during the previous 5 years (absence of mineral fertilizers and chemical plant protection and the application of winter cover crops and farm manure) increased the soil microbial hydrolytic activity in organic systems on average by 19.0%, compared to the conventional systems.

Table 2.
Soil microbial hydrolytic activity (μg of fluorescein g−1 soil dry weight h−1), organic carbon (%) and total nitrogen content (%) after forecrop and after barley (us) cultivation.
The highest soil microbial hydrolytic activity as an average of the experimental years was recieved after barley (us) in organic system where soil was enriched with cover crops and manure (soil microbial hydrolytic activity, Org CC+M, Table 2). Addition of manure to cultivated winter cover crops (Org CC+M) increased soil microbial hydrolytic activity by 7.1% compared to cultivation of winter cover crops alone (Org CC) in the areas released from the forecrop (potato). Interestingly, by investigating the effect of red clover grown with the barley, the difference between the soil microbial hydrolytic activity of Org CC+M and Org CC after harvesting barley was reduced to 4.5%.
Every year the lowest soil microbial hydrolytic activity value was observed in conventional system Conv 0 which was conformed by Madsen et al. [33] results. The highest rise of soil microbial hydrolytic activity value meanwhile barley growth (us) was observed in conventional system and it did not depend on the fertilization rates. In soils of fertilized (mineral fertilizers) as well as unfertilized the soil microbial hydrolytic activity gain was almost equal (16.1% and 15.4% respectively). A comparison between cultivation systems showed the increase of soil microbial hydrolytic activity after barley undersown with red clover compared to the soil after forecrop was 3.9 (6.7%) and 8.7 (15.8%) in organic and conventional systems, respectively. It can be concluded that as an average of experimental years the soil microbial hydrolytic activity values in conventional systems increased by 4.8 (9.1%) more than organic systems. Higher soil microbial hydrolytic activity values were probably caused by easier availability of mineral N compared to organic N in organic systems and it results in up to 61% higher grain yield of barley (as main crop) in conventional systems [34].
The study previously carried out on the same plot showed that as an average of crop rotation the highest soil microbial hydrolytic activity was measured in organic system where the amount of organic material (plant residues, cover crops and manure) ploughed into the soil was the highest [35]. Moreover, higher biological activity has been observed in the soil with undersown crops because their roots are suitable habitat for bacteria, which produce soil-binding polysaccharides and the soil becomes loose [11]. The physical fractions of the soil which contain various organic compounds, enable to develop the structural and functional properties of the soil organic carbon [12]. For the increase of SOC the significant increase in organic material introduced to soil and enabling its decoposition is needed. It could be carried out directly by taking the amount of organic material needed to the soil and mixing it with soil or indirectly, by enabling the activity of soil organisms. Shallow cultivation methods [27,35,36] showed that winter cover crops and farm manure have significant influence on the enzymatic activity of soil microorganisms. This was also concluded in our study (2012–2017) by comparing the average soil microbial hydrolytic activity and SOC values. As an average of all cultivation systems there was a strong positive correlation between soil microbial hydrolytic activity and SOC values (r = 0.78; p < 0.001), before sowing barley (us) and after its harvest (r = 0.56; p < 0.01). Hence, the undersowing of red clover had a positive impact on the soil microorganisms through increasing their hydrolytic activity. Bayer et al. [37] noted that by spectroscopic methods more labile organic compounds were detected in the plots were yearly organic carbon was added compared to farming systems with lower carbon inputs (based on the mineral fertilizers). High amounts of labile organic carbon indicate that the soil shows good quality only if this fraction is able to provide plant nutrients [38].
3.2. Soil Organic Carbon Content
Methods used in organic cultivation systems during the previous 5 years had positive effect on the SOC. In samples taken after forcrop organic systems, the content of SOC was 11.6% higher than in conventional systems, as average of experimental years (Table 2) although it decreased to 8.6% after the cultivation of barley undersown with red clover. The SOC content after cultivation of red clover did not show any significant changes compared to the soil after forecrop. Large part of carbon is bound by growing red clover and the cut straw of harvested main crop remains on top of the field. Nevertheless, large amount of organic material has not been decomposed by the spring. Paustian et al. [39] noted that changes in SOC content due to management practices are difficult to quantify as they occur slowly. Changes in inputs, which regulate the soil microbial activity and mineralization rates, will ultimately be reflected in the SOC content [40]. Compared to SOC content of the soil after forecrop, after cultivation of barley SOC content decreased by 2.6% in organic systems and increased by 1.4% in conventional systems. Also, the highest increase (5.2% on average) in SOC content occurred in Conv 0.
Relationships between soil SOC content and soil microbial hydrolytic activity value in organic farming system (Figure 1A)—correlation after forecrop between SOC and soil microbial hydrolytic activity was r = 0.32, p > 0.05 and after barley (us) cultivation r = 0.56; p < 0.01 respectively. In conventional cropping system the correlation between SOC and soil microbial hydrolytic activity was r = 0.79; p < 0.001 and r = 0.35, p > 0.05 after forecrop (Figure 1B) and after barley cultivation, respectively. Hence, through barley (us) cultivation the soil microbial hydrolytic activity influenced the content of SOC significantly only in organic farming system.
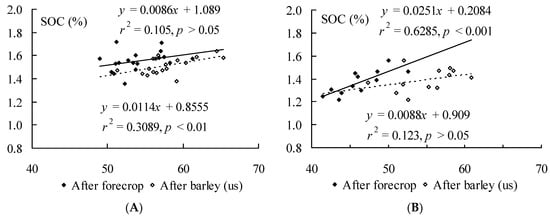
Figure 1.
The relationships between soil hydrolytic activity (μg of fluorescein g−1 soil dry mass h−1) and organic carbon (SOC, %) content in organic (A) and conventional (B) farming system after forecrop and after barley (us) cultivation.
Kauer et al. [26] found that in well developed crop rotations high rates of N fertilizers might be useful for stabilisation of SOC. This could have been the reason why the changes in soil SOC content in barley cultivation (us) showed increasing tendency compared to the forecrop field in conventional farming system while slight decrease was seen in organic farming system.
3.3. Soil Total Nitrogen Content
Undertaken study on organic farming systems had positive effect on the soil nitrogen content. In soil samples taken from plots after forecrop the Ntot content in organic farming systems was 17.4% higher than conventional systems as an average of experimental years (Table 2). The highest content was related to plots after forecrop in 2016 (by 32.4%) and the lowest content was observed at the year (by 8.0%) but after the cultivation of barley undersown with red clover. Results showed that after the cultivation of barley (us), the Ntot content in organic farming system was 14.4% higher than conventional one. As an average of experimental years, the cultivation of barley (us) decreased the soil Ntot content in both farming systems. The soil Ntot content after the barley (us) cultivation was 2.8% and 5.6% lower than after forecrop, in a row, in conventional and organic systems. The cultivation of barley (us) decreased the Ntot content every year due to the decrease of proportion of available nitrogen in the soil. Probably there were increase of its share bound in by the undersown red clover crop from where the nitrogen will be released only for the next year’s crop. In a study by Känkänen and Eriksson [41], undersown red clover did not increase the soil nitrogen content. At the same time, it was found that clover did not compete with the main crop and it was possible for them to bind nitrogen which was potentially beneficial for the next crop. In case of red clover compared to other undersown crops also the yield of the catch crop was higher [42].
The relationships between soil microbial hydrolytic activity and Ntot could be observed only based on the samples taken before the sowing of barley in spring where soil microbial hydrolytic activity correlation (r = 0.37; p > 0.05) with N was apparent in all farming systems. In organic (Figure 2A) conventional system (Figure 2B) the correlation between soil microbial hydrolytic activity and N after forecrop were r < 0.62, p < 0.05 and r = 0.02, p > 0.05, respectively. Therefore, during the cultivation of barley (us) the soil microbial hydrolytic activity did not influence the soil Ntot conent in either farming system.
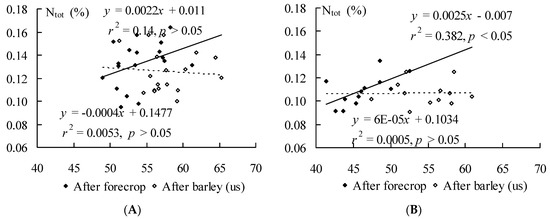
Figure 2.
Relationships between soil hydrolytic activity (μg of fluorescein g−1 soil dry mass h−1) and total nitrogen (Ntot, %) content in organic (A) and conventional (B) farming system after forecrop and after barley (us) cultivation.
4. Conclusions
It was concluded that the cultivation of barley with red clover increased the soil microbial hydrolytic activity in all cultivation systems, as an average of experimental years. In organic systems the soil microbial hydrolytic activity increased on average by 19.0%, compared to the conventional systems. The highest soil microbial hydrolytic activity was in organic systems where winter cover crops were used. The organic cultivation methods had a positive effect on the SOC content. There were no significant changes in SOC content after the cultivation of red clover, compared to the soil after forecrop. When compared to the SOC content after forecrop of barley (us) decreasing tendency in organic systems and increasing trend in conventional systems was observed after the cultivation of barley (us). A comparison between SOC content after the forecrop and after the cultivation of barley (us) showed a decreasing trend in organic systems and slightly increase in conventional systems.
By cultivation of barley (us) the soil microbial hydrolytic activity had a significant effect on the SOC content only in organic system. Organic cultivation systems had positive impact on the Ntot content. The soil Ntot in samples taken after forecrop was higher by 17.4% and after the cultivation of barley (us) by 14.4% compared to conventional system, as an average of experimental years. The cultivation of barley (us) decreased the Ntot of the soil in both cropping systems. After the cultivation of barley (us) the soil microbial hydrolytic activity had no effect on the Ntot content in either cultivation systems.
Author Contributions
Conceptualization, J.K.; Methodology, J.K., V.E., L.T., A.L.; Project administration, E.L., A.L., K.E.-S.; Writing, J.K., V.E.; Review & Editing, J.K., V.E., L.T., E.M., Data curation, M.A., V.E., L.T.; Funding Acquisition, A.L.
Funding
The study has been supported by ERA-NET Core organic project FertilCrop, by Estonian University of Life Sciences projects 8-2/T13001PKTM, P180273PKTT, P170062PKTM and by Institutional Research Project IUT36-2.
Acknowledgments
The technical assistance of Rõhu experimental station from the Estonian University of Life Sciences is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rasmussen, I.A.; Askegaard, M.; Olesen, J.E. The Danish organic crop rotation experiment for cereal production 1997–2004. In Long-Term Field Experiments in Organic Farming; Raupp, J., Pekrun, C., Oltmanns, M., Köpke, U., Eds.; ISOFAR Scientific Series; Verlag Dr. Köster: Berlin, Germany, 2006; pp. 117–134. [Google Scholar]
- Barberi, P.; Aendekerk, R.; Antichi, D.; Armengot, L.; Berner, A.; Bigongiali, F.; Blanco-Moreno, J.M.; Carlesi, S.; Celette, F.; Chamorro, L.; et al. Reduced tillage and cover crops in organic arable systems preserve weed diversity without jeopardising crop yield. In Proceedings of the 4th ISOFAR Scientific Conference ‘Building Organic Bridges’, at the Organic World Congress 2014, Istanbul, Turkey, 13–15 October 2014. [Google Scholar]
- Böhm, H. Effect of a white clover underseed in oil seed rape on yield of the following crop wheat. In Zwischen Tradition und Globalisierung: Beiträge zur 9. Wissenschaftstagung Ökologischer Landbau: Band 1; Zikeli, S., Claupein, W., Eds.; Universität Hohenheim: Stuttgart, Germany, 2007; pp. 153–156. [Google Scholar]
- Loes, A.K.; Henriksen, T.M.; Eltun, R. Repeated Undersowing of Clover in Stockless Organic Grain Production. 2006. Available online: http://orgprints.org/8222/01/gronngjabstract_odense_190406.doc (accessed on 10 March 2019).
- Fuchs, R.; Rehm, A.; Salzeder, G.; Wiesinger, K. Effect of undersowing winter wheat with legumes on the yield and quality of subsequent winter triticale crops. In Proceedings of the 16th IFOAM Organic World Congress, Modena, Italy, 16–20 June 2008; Available online: http://orgprints.org/12544/ (accessed on 23 February 2019).
- Talgre, L.; Tein, B.; Eremeev, V.; Matt, D.; Reintam, E.; Sanches de Cima, D.; Luik, A. In crop rotation green manures as winter cover crops enhance ecosystem services of farming. In Organic Farming Systems as a Driver for Change; NJF Report; Nordic Association for Agricultural Science: Bredsten, Denmark, 2013; pp. 57–58. [Google Scholar]
- Kuht, J.; Eremeev, V.; Talgre, L.; Madsen, H.; Toom, M.; Mäeorg, E.; Luik, A. Soil weed seed bank and factors influencing the number of weeds at the end of conversion period to organic production. Agron. Res. 2016, 14, 1372–1379. [Google Scholar]
- Liebman, M.; Dyck, E. Crop rotation and intercropping strategies for weed management. Ecol. Appl. 1993, 3, 92–122. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Watanabe, Y. Growth and yield of sweet corn with legume living mulches. Jpn. J. Crop. Sci. 2002, 71, 36–42. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.C. Allelopathy for weed control in agricultural systems. Crop Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Russell, E.W. Soil structure: Its maintenance and improvement. Eur. J. Soil Sci. 1971, 22, 137–150. [Google Scholar] [CrossRef]
- Christensen, B.T. Matching measurable soil organic matter fractions with conceptual pools in simulation models of carbon turnover: Revision of model structure. In Evaluation of Soil Organic Matter Models; Powlson, D.S., Smith, P., Smith, J.U., Eds.; Springer: Berlin, Germany, 1996; pp. 143–159. [Google Scholar]
- Carter, M.R.; Kunelius, H.T. Effect of undersowing barley with annual ryegrasses or red clover on soil structure in a barley-soybean rotation. Agric. Ecosyst. Environ. 1993, 43, 245–254. [Google Scholar] [CrossRef]
- Fontvieille, D.A.; Outaguerouine, A.; Thevenot, D.R. Fluorescein diacetate hydrolysis as a measure of microbial activity in aquatic systems: Application to activated sludges. Environ. Technol. 1992, 13, 531–540. [Google Scholar] [CrossRef]
- Söderström, B.E. Vital staining of fungi in pure culture and in soil with fluorescein diacetate. Soil Biol. Biochem. 1977, 9, 59–63. [Google Scholar] [CrossRef]
- Söderström, B.E. Some problems in assessing the fluorescein diacetate-active fungal biomass in the soil. Soil Biol. Biochem. 1979, 11, 147–148. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef]
- Swisher, R.; Carroll, G.C. Fluorescein diacetate hydrolysis as an estimator of microbial biomass on coniferous needle surfaces. Microb. Ecol. 1980, 6, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Liu, H.; Han, J.; Sun, J.; Wu, X.; Yao, J. Monitoring Soil Microbial Activities in Different Cropping Systems Using Combined Methods. Pedosphere 2017, 27, 138–146. [Google Scholar] [CrossRef]
- Edesi, L.; Järvan, M.; Noormets, M.; Lauringson, E.; Adamson, A.; Akk, E. The importance of solid cattle manure application on soil microorganisms in organic and conventional cultivation. Acta Agric. Scand. Sect. B Soil Plant Sci. 2012, 62, 1–12. [Google Scholar] [CrossRef]
- Tejada, M.; Gonzalez, J.L.; Garcia-Martinez, A.M.; Parrado, J. Effects of different green manures on soil biological properties and maize yield. Bioresour. Technol. 2008, 99, 1758–1767. [Google Scholar] [CrossRef] [PubMed]
- Oldare, M.; Pell, M.; Svensson, K. Changes in soil chemical and microbiological properties, during 4 years of application of various organic residues. Waste Manag. 2008, 28, 1246–1253. [Google Scholar]
- Oldare, M.; Arthurson, V.; Pell, M.; Svensson, K.; Nehrenheim, E.; Abubaker, J. Land application of organic waste—Effects on the soil ecosystem. Appl. Energy 2011, 88, 2210–2218. [Google Scholar]
- Deckers, J.A.; Nachtergale, F.O.; Spaargarn, O.C. World Reference Base for Soil Resources: Introduction, 1st ed.; Acco: Leuven, Belgium, 1998; p. 165. [Google Scholar]
- Reintam, E.; Köster, T. The role of chemical indicators to correlate some Estonian soils with WRB and soil taxonomy criteria. Geoderma 2006, 136, 199–209. [Google Scholar] [CrossRef]
- Kauer, K.; Tein, B.; Sanches de Cima, D.; Talgre, T.; Eremeev, V.; Loit, E.; Luik, A. Soil carbon dynamics estimation and dependence on farming system in a temperate climate. Soil Till. Res. 2015, 154, 53–63. [Google Scholar] [CrossRef]
- Sánchez de Cima, D.; Tein, B.; Eremeev, V.; Luik, A.; Kauer, K.; Reintam, E.; Kahu, G. Winter cover crop effects on soil structural stability and microbiological activity in organic farming. Biol. Agric. Hortic. 2016, 32, 170–181. [Google Scholar] [CrossRef]
- Schnürer, J.; Rosswall, T. Fluorescein Diacetate Hydrolysis as a Measure of Total Microbial Activity in Soil and Litter. Appl. Environ. Microbiol. 1982, 43, 1256–1261. [Google Scholar]
- ISO 10381-6. Soil Quality—Sampling. Guidance on the Collection, Handling and Storage of Soil for the Assessment of Aerobic Microbial Processes in Laboratory; International Organization for Standardization: Geneva, Switzerland, 1993. [Google Scholar]
- van Reeuwijk, L.P. Procedures for Soil Analysis, 6th ed.; Tech. Pap. 9; ISRIC: Wageningen, The Netherlands, 2002; p. 119. [Google Scholar]
- Soil Survey Laboratory Staff. Soil Survey Laboratory Methods Manual; Soil Survey Investigations Report No. 42, Version 3.0; National Soil Survey Center: Lincoln, NE, USA, 1996. [Google Scholar]
- Statsoft. Statistica 7.0; Copyright 1984–2005; StatSoft Inc.: Tulka, OK, USA, 2005; 716p. [Google Scholar]
- Madsen, H.; Talgre, L.; Eremeev, V.; Sànches De Cima, D.; Luik, A. The effect of farming system on soil microbial hydrolytical activity; Programe and Abstarcts: Long-term Agroecosystem Sustainability: Links between Carbon Sequestration in Soils, Food Security and Climate Change. In Proceedings of the International Scientific Conference AgroEco2016, Akademija, Kaunas, Lithuania, 4–6 October 2016; pp. 42–45. [Google Scholar]
- Alaru, M.; Talgre, L.; Luik, A.; Tein, B.; Eremeev, V.; Loit, E. Barley undersown with red clover in organic and conventional systems: Nitrogen aftereffect on legume growth. Zemdirbyste-Agriculture 2017, 104, 131–138. [Google Scholar] [CrossRef]
- Madsen, H.; Talgre, L.; Eremeev, V.; Luik, A. Pestitsiidid suruvad alla mulla mikroobide hüdrolüütilist aktiivsust (Pesticides decreased soil microbial hydrolytic activity). Estonian Plant Prot. 2016, 95, 79–82. (In Estonian) [Google Scholar]
- Post, W.M.; Kwon, K.C. Soil Carbon Sequestration and Land-Use Change: Processes and Potential. Glob. Chang. Biol. 2000, 6, 317–328. [Google Scholar] [CrossRef]
- Bayer, C.; Mielniczuk, J.; Martin-Neto, L.; Ernani, P.R. Stocks and humification degree of organic matter fractions as affected by notillage on a subtropical soil. Plant Soil. 2002, 238, 133–140. [Google Scholar] [CrossRef]
- Whitbread, A.M.; Lefroy, R.D.B.; Blair, G.J. A survey of the impact of cropping on soil physical and chemical properties in north-western New South Wales. Aust. J. Soil Res. 1998, 36, 669–681. [Google Scholar] [CrossRef]
- Paustian, K.; Levine, E.; Post, W.M.; Ryzhova, I.M. The use of models to integrate information and understanding of soil C at the regional scale. Geoderma 1997, 79, 227–260. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Janzen, H.H. Storage of soil carbon in the light fraction and macroorganic matter. In Structure and Organic Matter Storage in Agriculture Soils; Carter, M.R., Steward, B.A., Eds.; CRC Press (Lewis Publishers): Bocaraton, FL, USA, 1996; pp. 167–190. [Google Scholar]
- Känkänen, H.; Eriksson, C. Effects of undersown crops on soil mineral N and grain yield of spring barley. Eur. J. Agron. 2007, 27, 25–34. [Google Scholar] [CrossRef]
- Känkänen, H.; Eriksson, C.; Räkköläionen, M. Effect of annually repeated undersowing on cereal grain yields. Agric. Food Sci. 2008, 10, 197–208. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).