The Grain Protein Content of Polish Cereals Other than Wheat: Can It Be Increased by Combining a Crop Sequence System, Cultivar Selection, and Plant Protection?
Abstract
1. Introduction
2. Materials and Methods
2.1. Site, Soil, Climate
2.2. Experiment Design and Agronomic Management
2.3. Data Collection
2.4. Statistical Analysis
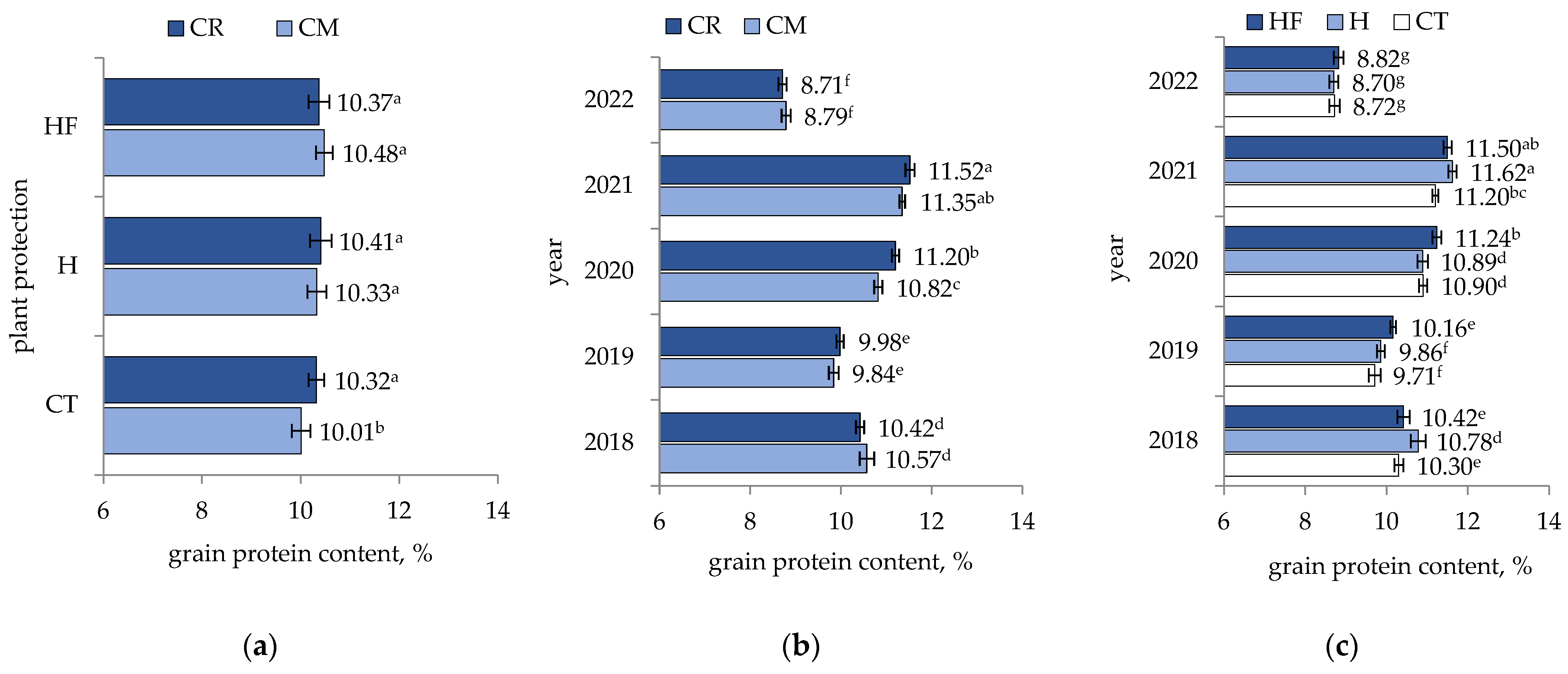
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GPC | Grain protein content |
| CS | Crop sequence system |
| CM | Continuous monocropping |
| CR | Crop rotation |
| Cv | Cultivar |
| PP | Plant protection |
| CT | Control treatment |
| H | Herbicide protection |
| HF | Herbicide and fungicide protection |
| Yr | Year |
| N | Nitrogen |
| COBORU | Centralny Ośrodek Badania Odmian Roślin Uprawnych (Research Center for Cultivar Testing) |
| FYM | Farmyard manure |
References
- FAO. FAOSTAT Database; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022; Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 21 January 2025).
- GUS. Statistical Yearbook of the Republic of Poland 2024; Statistics Poland: Warsaw, Poland, 2024.
- Sudheesh, C.; Bhat, Z.R.; Aaliya, B.; Sunooj, K.V. Chapter 2—Cereal proteins. In Nutraceuticals and Health Care; Kour, J., Nayik, G.A., Eds.; Academic Press: New York, NY, USA, 2022; pp. 29–60. [Google Scholar]
- Poutanen, K.S.; Kårlund, A.O.; Gómez-Gallego, C.; Johansson, D.P.; Scheers, N.M.; Marklinder, I.M.; Eriksen, A.K.; Silventoinen, P.C.; Nordlund, E.; Sozer, N.; et al. Grains—A major source of sustainable protein for health. Nutr. Rev. 2022, 80, 1648–1663. [Google Scholar] [CrossRef] [PubMed]
- Marinangeli, C.P.F.; Nosworthy, M.G.; Shoveller, A.-K. Cereal proteins in the human diet: Reflecting on their contributions to daily protein intake. J. Cereal Sci. 2024, 117, 103908. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Huang, Y.; Zhao, X.; Dong, Z.; Jin, W.; Huang, W. Amino acid permease 6 regulates grain protein content in maize. Crop J. 2022, 10, 1536–1544. [Google Scholar] [CrossRef]
- Biel, W.; Kazimierska, K.; Bashutska, U. Nutritional value of wheat, triticale, barley and oat grains. Acta Sci. Pol. Zootech. 2020, 19, 19–28. [Google Scholar] [CrossRef]
- Shewry, P.R. Can we increase the use of wheat and other cereals as sources of protein? J. Cereal Sci. 2024, 117, 103899. [Google Scholar] [CrossRef]
- Kartseva, T.; Alqudah, A.M.; Aleksandrov, V.; Alomari, D.Z.; Doneva, D.; Arif, M.A.; Börner, A.; Misheva, S. Nutritional genomic approach for improving grain protein content in wheat. Foods 2023, 12, 1399. [Google Scholar] [CrossRef]
- Humer, E.; Zebeli, Q. Grains in ruminant feeding and potentials to enhance their nutritive and health value by chemical processing. Anim. Feed Sci. Technol. 2017, 226, 133–151. [Google Scholar] [CrossRef]
- Kosová, K.; Prášil, I.T.; Klíma, M.; Nesvadba, Z.; Vítámvás, P.; Ovesná, J. Proteomics of wheat and barley cereals in response to environmental stresses: Current state and future challenges. J. Proteom. 2023, 282, 104923. [Google Scholar] [CrossRef]
- Tomičić, Z.; Pezo, L.; Spasevski, N.; Lazarević, J.; Čabarkapa, I.; Tomičić, R. Diversity of amino acids composition in cereals. Food Feed Res. 2022, 49, 11–22. [Google Scholar] [CrossRef]
- Kowieska, A.; Lubowicki, R.; Jaskowska, I. Chemical composition and nutritional characteristics of several cereal grain. Acta Sci. Pol. Zootech. 2011, 10, 37–50. [Google Scholar]
- Alijošius, S.; Švirmickas, G.J.; Bliznikas, S.; Gružauskas, R.; Šašytė, V.; Racevičiūtė-Stupelienė, A.; Kliševičiūtė, V.; Daukšienė, A. Grain chemical composition of different varieties of winter cereals. Zemdirbyste-Agriculture 2016, 103, 273–280. [Google Scholar] [CrossRef]
- Biel, W.; Jaroszewska, A.; Stankowski, S.; Sobolewska, M.; Kępińska-Pacelik, J. Comparison of yield, chemical composition and farinograph properties of common and ancient wheat grains. Eur. Food Res. Technol. 2021, 247, 1525–1538. [Google Scholar] [CrossRef]
- Nigro, D.; Blanco, E.; Mangini, G.; Laddomada, B.; Sgaramella, N.; Signorile, M.A.; Simeone, R.; Blanco, A. Identifying QTL for grain protein content independent from grain yield-related traits in durum wheat. J. Cereal Sci. 2024, 117, 103894. [Google Scholar] [CrossRef]
- Vishwakarma, M.K.; Mishra, V.K.; Gupta, P.K.; Yadav, P.S.; Kumar, H.; Joshi, A.K. Introgression of the high grain protein gene Gpc-B1 in an elite wheat variety of Indo-Gangetic Plains through marker assisted backcross breeding. Curr. Plant Biol. 2014, 1, 60–67. [Google Scholar] [CrossRef]
- Gupta, P.K.; Balyan, H.S.; Chhuneja, P.; Jaiswal, J.P.; Tamhankar, S.; Mishra, V.K.; Bains, N.S.; Chand, R.; Joshi, A.K.; Kaur, S.; et al. Pyramiding of genes for grain protein content, grain quality, and rust resistance in eleven Indian bread wheat cultivars: A multi-institutional effort. Mol. Breed. 2022, 42, 21. [Google Scholar] [CrossRef]
- Neuweiler, J.E.; Maurer, H.P.; Würschum, T. Genetic architecture of phenotypic indices for simultaneous improvement of protein content and grain yield in triticale (×Triticosecale). Plant Breed. 2021, 140, 232–245. [Google Scholar] [CrossRef]
- Ben Mariem, S.; Soba, D.; Zhou, B.; Loladze, I.; Morales, F.; Aranjuelo, I. Climate change, crop yields, and grain quality of C3 cereals: A meta-analysis of CO2, temperature, and drought effects. Plants 2021, 10, 1052. [Google Scholar] [CrossRef]
- Adhikari, K.; Smith, D.R.; Hajda, C.; Owens, P.R. Can soil health explain grain quality? A case study of a corn field in Texas. Agric. Environ. Lett. 2022, 7, e20078. [Google Scholar] [CrossRef]
- Kashyap, S.; Reddy, B.H.R.; Devi, S.; Kurpad, A.V. Potential impact of climate change on dietary grain protein content and its bioavailability-a mini review. Front. Nutr. 2024, 11, 1397219. [Google Scholar] [CrossRef]
- Harasim, E.; Wesołowski, M. Yield and some quality traits of winter wheat (Triticum aestivum L.) grain as influenced by the application of different rates of nitrogen. Acta Agrobot. 2013, 66, 67–72. [Google Scholar] [CrossRef]
- Järvan, M.; Lukme, L.; Tupits, I.; Akk, A. The productivity, quality and bread-making properties of organically and conventionally grown winter rye. Zemdirbyste-Agriculture 2018, 105, 323–330. [Google Scholar] [CrossRef]
- Dang, H.; Sun, R.; She, W.; Hou, S.; Li, X.; Chu, H.; Wang, T.; Huang, T.; Huang, Q.; Siddique, K.H.M.; et al. Updating soil organic carbon for wheat production with high yield and grain protein. Field Crop Res. 2024, 317, 109549. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH-Monograph; Blackwell Wissenschafts-Verlag: Berlin, Germany; Boston, MA, USA, 1997; p. 622. [Google Scholar]
- Kumar, S.; Chatterjee, U.; David Raj, A.; Sooryamol, K.R. Global warming and climate crisis/extreme events. In Climate Crisis: Adaptive Approaches and Sustainability; Chatterjee, U., Shaw, R., Kumar, S., Raj, A.D., Das, S., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 3–18. [Google Scholar]
- Dessart, F.J.; Barreiro-Hurlé, J.; van Bavel, R. Behavioural factors affecting the adoption of sustainable farming practices: A policy-oriented review. Eur. Rev. Agric. Econ. 2019, 46, 417–471. [Google Scholar] [CrossRef]
- Jańczak-Pieniążek, M.; Kaszuba, J. The influence of agrotechnical factors on the yield and quality parameters of winter triticale grain. Agriculture 2024, 14, 2219. [Google Scholar] [CrossRef]
- Lachutta, K.; Jankowski, K.J. The quality of winter wheat grain by different sowing strategies and nitrogen fertilizer rates: A case study in Northeastern Poland. Agriculture 2024, 14, 552. [Google Scholar] [CrossRef]
- Trovato, M.; Funck, D.; Forlani, G.; Okumoto, S.; Amir, R. Editorial: Amino Acids in Plants: Regulation and Functions in Development and Stress Defense. Front. Plant Sci. 2021, 12, 772810. [Google Scholar] [CrossRef]
- Geleta, B.; Atak, M.; Baenziger, P.S.; Nelson, L.A.; Baltenesperger, D.D.; Eskridge, K.M.; Shipman, M.J.; Shelton, D.R. Seeding rate and genotype effect on agronomic performance and end-use quality of winter wheat. Crop Sci. 2002, 42, 827–832. [Google Scholar] [CrossRef]
- Tian, Z.; Yin, Y.; Li, B.; Zhong, K.; Liu, X.; Jiang, D.; Cao, W.; Dai, T. Optimizing planting density and nitrogen application to mitigate yield loss and improve grain quality of late-sown wheat under rice-wheat rotation. J. Integr. Agric. 2024; in press. [Google Scholar] [CrossRef]
- Twizerimana, A.; Niyigaba, E.; Mugenzi, I.; Ngnadong, W.A.; Li, C.; Hao, T.Q.; Shio, B.J.; Hai, J.B. The combined effect of different sowing methods and seed rates on the quality features and yield of winter wheat. Agriculture 2020, 10, 153. [Google Scholar] [CrossRef]
- Chaieb, N.; Rezguia, M.; Ayedb, S.; Bahria, H.; Cheikh, H.; M’hameda, M.R.; Annabia, M. Effects of tillage and crop rotation on yield and quality parameters of durum wheat in Tunisia. J. Anim. Plant Sci. 2020, 44, 7654–7676. [Google Scholar] [CrossRef]
- Wanic, M.; Jastrzębska, M.; Kostrzewska, M.K.; Parzonka, M. Spelt in diversified and spelt-based crop rotations: Grain yield and technological and nutritional quality. Agriculture 2024, 14, 1123. [Google Scholar] [CrossRef]
- Woźniak, A.; Haliniarz, M. Response of winter wheat to 35-year cereal monoculture. Agriculture 2025, 15, 489. [Google Scholar] [CrossRef]
- Noworolnik, K.; Leszczyńska, D. Effect of selected herbicides on yielding and malting quality of spring barley cultivars. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2017, 67, 303–307. [Google Scholar] [CrossRef]
- Malalgoda, M.; Simsek, S. Pesticide residue in grain-based food: Effects on health, grain quality, and chemical properties of biomacromolecules. Cereal Chem. 2021, 98, 8–16. [Google Scholar] [CrossRef]
- Jastrzębska, M.; Kostrzewska, M.K.; Marks, M.; Jastrzębski, W.P.; Treder, K.; Makowski, P. Crop rotation compared with continuous rye cropping for weed biodiversity and rye yield. A case study of a long-term experiment in Poland. Agronomy 2019, 9, 644. [Google Scholar] [CrossRef]
- Wanic, M.; Denert, M.; Treder, K. Effect of forecrops on the yield and quality of common wheat and spelt wheat grain. J. Elem. 2019, 24, 369–383. [Google Scholar] [CrossRef]
- Gawęda, D.; Haliniarz, M. Grain yield and quality of winter wheat depending on previous crop and tillage system. Agriculture 2021, 11, 133. [Google Scholar] [CrossRef]
- Woźniak, A.; Nowak, A.; Gawęda, D. The effect of the three-field crop rotation system and cereal monoculture on grain yield and quality and the economic efficiency of durum wheat production. Pol. J. Environ. Stud. 2021, 30, 5297–5305. [Google Scholar] [CrossRef]
- Jastrzębska, M.; Kostrzewska, M.K.; Marks, M. Is diversified crop rotation an effective non-chemical strategy for protecting triticale yield and weed diversity? Agronomy 2023, 13, 1589. [Google Scholar] [CrossRef]
- Adamiak, E. Struktura Zachwaszczenia i Produktywność Wybranych Agrocenoz Zbóż Ozimych i Jarych w Zależności od Systemu Następstwa Roślin i Ochrony Łanu; Wydawnictwo Uniwersytetu Warmińsko-Mazurskiego: Olsztyn, Poland, 2007; Volume 129, p. 146. [Google Scholar]
- Zawiślak, K.; Kostrzewska, M. Konkurencja pokarmowa chwastów w łanach żyta ozimego uprawianego w płodozmianie iw wieloletniej monokulturze. II. Zawartość i pobranie makroelementów w nadziemnej biomasie żyta ozimego i chwastów. Ann. UMCS Sect. E LV Suppl. 2000, 33, 269–275. [Google Scholar]
- Tariq, M.; Ali, H.; Hussain, N.; Nasim, W.; Mubeen, M.; Ahmad, S.; Hasanuzzaman, M. Fundamentals of Crop rotation in agronomic management. In Agronomic Crops: Volume 1: Production Technologies; Hasanuzzaman, M., Ed.; Springer: Singapore, 2019; pp. 545–559. [Google Scholar]
- Gorooei, A.; Gaiser, T.; Aynehband, A.; Rahnama, A.; Kamali, B. The effect of farming management and crop rotation systems on chlorophyll content, dry matter translocation, and grain quantity and quality of wheat (Triticum aestivum L.) grown in a Semi-Arid region of Iran. Agronomy 2023, 13, 1007. [Google Scholar] [CrossRef]
- Fan, H.; Miao, R.; Guo, C.; Bao, X.; He, W.; Sun, Y.; Zhao, C. Research on the effect of diversified cropping on crop quality: A review. Agriculture 2025, 15, 456. [Google Scholar] [CrossRef]
- Woźniak, A. Yield and chemical composition of spring triticale grain depending on cropping and tillage systems. Int. J. Plant Prod. 2016, 10, 45–52. [Google Scholar] [CrossRef]
- Babulicová, M.; Gavurníková, S. The influence of cereal share in crop rotations on the grain yield and quality of winter wheat. Agriculture 2015, 61, 12–21. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Kijewski, L.; Dubis, B. Milling quality and flour strength of the grain of winter wheat grown in monoculture. Rom. Agric. Res. 2015, 32, 191–200. [Google Scholar]
- Borghi, B.; Giordani, G.; Corbellini, M.; Vaccino, P.; Guermandi, M.; Toderi, G. Influence of crop rotation, manure and fertilizers on bread making quality of wheat (Triticum aestivum L.). Eur. J. Agron. 1995, 4, 37–45. [Google Scholar] [CrossRef]
- Lykogianni, M.; Bempelou, E.; Karamaouna, F.; Aliferis, K.A. Do pesticides promote or hinder sustainability in agriculture? The challenge of sustainable use of pesticides in modern agriculture. Sci. Total Environ. 2021, 795, 148625. [Google Scholar] [CrossRef]
- Hernández Plaza, E.; Bastida, F.; Gibson, D.J.; Barro, F.; Giménez, M.J.; Pallavicini, Y.; Izquierdo, J.; González-Andújar, J.L. Grain quality as influenced by the structural properties of weed communities in Mediterranean wheat crops. Agronomy 2023, 13, 49. [Google Scholar] [CrossRef]
- Cheaib, A.; Killiny, N. Photosynthesis responses to the infection with plant pathogens. Mol. Plant Microbe Interact. 2025, 38, 9–29. [Google Scholar] [CrossRef]
- Kumar, R.; Rathor, V.S. Nature and types of damage by insect pests. J. Entomol. Res. 2020, 44, 639–646. [Google Scholar] [CrossRef]
- Simón, M.R.; Fleitas, M.C.; Castro, A.C.; Schierenbeck, M. How foliar fungal diseases affect nitrogen dynamics, milling, and end-use quality of wheat. Front. Plant Sci. 2020, 11, 569401. [Google Scholar] [CrossRef]
- Gaju, O.; Allard, V.; Martre, P.; Le Gouis, J.; Moreau, D.; Bogard, M.; Hubbart, S.; Foulkes, M.J. Nitrogen partitioning and remobilization in relation to leaf senescence, grain yield and grain nitrogen concentration in wheat cultivars. Field Crop Res. 2014, 155, 213–223. [Google Scholar] [CrossRef]
- Matzen, N.; Ravn Jørgensen, J.; Holst, N.; Nistrup Jørgensen, L. Grain quality in wheat—Impact of disease management. Eur. J. Agron. 2019, 103, 152–164. [Google Scholar] [CrossRef]
- PAN. PAN Pesticide Database; Pesticide Action and Agroecology Network: Berkeley, CA, USA, 2025; Available online: http://www.pesticideinfo.org/ (accessed on 13 March 2025).
- Pergner, I.; Lippert, C. On the effects that motivate pesticide use in perspective of designing a cropping system without pesticides but with mineral fertilizer—A review. Agron. Sustain. Dev. 2023, 43, 24. [Google Scholar] [CrossRef]
- Cao, S.; Liu, B.; Wang, D.; Rasheed, A.; Xie, L.; Xia, X.; He, Z. Orchestrating seed storage protein and starch accumulation toward overcoming yield–quality trade-off in cereal crops. J. Integr. Plant Biol. 2024, 66, 468–483. [Google Scholar] [CrossRef] [PubMed]
- GUS. Agriculture in 2023; Statistics Poland: Warsaw, Poland, 2024.
- Hutorowicz, H.; Grabowski, J.; Olba-Zięty, E. Frequency of occurrence of dry spells and droughts in two mesoregions of Masurian Lakeland. Acta Agrophys. 2008, 12, 663–673. [Google Scholar]
- Jastrzębska, M.; Kostrzewska, M.K.; Marks, M. Over 50 years of a field experiment on cropping systems in Bałcyny, Poland: Assessing pesticide residues in soil and crops from the perspective of their field application history. Eur. J. Agron. 2024, 159, 127270. [Google Scholar] [CrossRef]
- Kostrzewska, M.K.; Jastrzębska, M. Exploiting the yield potential of spring barley in poland: The roles of crop rotation, cultivar, and plant protection. Agriculture 2024, 14, 1355. [Google Scholar] [CrossRef]
- StatSoft, I. TIBCO Software Inc. Statistica (Data Analysis Software System), Version 13.3; TIBCO Software Inc.: Palo Alto, CA, USA, 2017.
- COBORU. Post-Registration Variety Testing System and Variety Recommendation; Centralny Ośrodek Badania Odmian Roślin Uprawnych: Słupia Wielka, Poland, 2024. Available online: https://www.coboru.gov.pl/pdo (accessed on 10 December 2024).
- An, H.-Y.; Han, J.-J.; He, Q.-N.; Zhu, Y.-L.; Wu, P.; Wang, Y.-C.; Gao, Z.-Q.; Du, T.-Q.; Xue, J.-F. Influence of nitrogen application rate on wheat grain protein content and composition in China: A meta-analysis. Agronomy 2024, 14, 1164. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Z.; Xu, J.; Huang, C.; Dang, H.; Mu, W.; Zhang, L.; Hou, S.; Huang, N.; Li, C.; et al. Nutrient requirements determined by grain yield and protein content to optimize N, P, and K fertilizer management in China. Sci. Total Environ. 2024, 946, 174187. [Google Scholar] [CrossRef]
- Mi, X.; Bai, N.; Liu, Y.; He, G.; Wang, Z. Exploring nitrogen management methods for depressing the decline of wheat grain protein in plastic film mulch via 15N-labelling technique. Soil Tillage Res. 2023, 228, 105632. [Google Scholar] [CrossRef]
- Pielech-Przybylska, K.; Balcerek, M.; Ługowoj, S.; Królak, K.; Dziekońska-Kubczak, U.; Kuta, A.; Rozbicki, J.; Studnicki, M. Effects of rye cultivars and management intensity on volatile profile of rye-based spirit distillates. J. Cereal Sci. 2022, 108, 103552. [Google Scholar] [CrossRef]
- Stępniewska, S.; Cacak-Pietrzak, G.; Fraś, A.; Jończyk, K.; Studnicki, M.; Wiśniewska, M.; Gzowska, M.; Salamon, A. Effect of genotype and environment on yield and technological and nutrition traits on winter rye grain from organic production. Agriculture 2024, 14, 2249. [Google Scholar] [CrossRef]
- Fraś, A.; Gołębiewska, K.; Gołębiewski, D.; Mańkowski, D.R.; Boros, D.; Szecówka, P. Variability in the chemical composition of triticale grain, flour and bread. J. Cereal Sci. 2016, 71, 66–72. [Google Scholar] [CrossRef]
- Kociuba, W.; Kramek, A. Variability of yield traits and disease resistance in winter triticale genetic resources accessions. Acta Agrobot. 2014, 67, 67–76. [Google Scholar] [CrossRef]
- Kramek, A.; Kociuba, W. Wykorzystanie materiałów kolekcyjnych pszenżyta ozimego jako genetycznego źródła zawartości białka w ziarnie. Agron. Sci. 2020, 75, 81–90. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Boros, D.; Zych, J. Nutritional value of selected mixtures of spring cereals with legumes. Biuletyn IHAR 2020, 289, 51–62. [Google Scholar] [CrossRef]
- Kosewska, O.; Nietupski, M.; Koronkiewicz, S.; Przemieniecki, S.W. The chemical grain composition of wheat and barley affects the development of the lesser grain borer (Rhyzopertha dominica F.) and the rice weevil (Sitophilus oryzae L.). J. Plant Prot. Res. 2024, 64, 275–287. [Google Scholar] [CrossRef]
- Noworolnik, K. Plonowanie i jakość ziarna odmian jęczmienia jarego w zależności od dawki azotu. Fragm. Agron. 2013, 30, 123–131. [Google Scholar]
- Noworolnik, K. Plonowanie nowych odmian jęczmienia jarego w zależności od dawki azotu. Pol. J. Agron. 2016, 27, 100–105. [Google Scholar] [CrossRef]
- Panasiewicz, K.; Koziara, W.; Sulewska, H.; Chrzanowski, R. Chemical composition and nutritive value of different oat forms as influence by sprinkling irrigation and nitrogen fertilization. Rom. Agric. Res. 2017, 34, 157–164. [Google Scholar]
- Łabanowska, M.; Kurdziel, M.; Filek, M.; Wesełucha-Birczyńska, A. The impact of biochemical composition and nature of paramagnetic species in grains on stress tolerance of oat cultivars. J. Plant Physiol. 2016, 199, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Drygaś, B.; Depciuch, J.; Zaguła, G.; Puchalski, C. Ascophyllum nodosum application and pre-sowing stimulation with low-frequency magnetic field as factors influencing oat grains (Avena sativa L.) composition. Agronomy 2020, 10, 1164. [Google Scholar] [CrossRef]
- Kordulasińska, I.; Bulińska-Radomska, Z. Ocena cech morfologicznych, użytkowych i składu chemicznego obiektów owsa zgromadzonych w Krajowym Centrum Roślinnych Zasobów Genowych w Radzikowie. Pol. J. Agron. 2016, 16, 3–12. [Google Scholar] [CrossRef]
- Woźniak, A. Production efficiency of different crop rotations and tillage systems. Span. J. Agric. Res. 2021, 19, e0907. [Google Scholar] [CrossRef]
- Gaj, R.; Gorski, D.; Wielgusz, K.; Kukawka, R.; Spychalski, M.; Borowski, J. Nitrogen management impact on winter triticale grain yield and nitrogen use efficiency. J. Elem. 2023, 28, 561–593. [Google Scholar] [CrossRef]
- Amato, G.; Ruisi, P.; Frenda, A.S.; Di Miceli, G.; Saia, S.; Plaia, A.; Giambalvo, D. Long-term tillage and crop sequence effects on wheat grain yield and quality. Agron. J. 2013, 105, 1317–1327. [Google Scholar] [CrossRef]
- Agegnehu, G.; Lakew, B.; Nelson, P.N. Cropping sequence and nitrogen fertilizer effects on the productivity and quality of malting barley and soil fertility in the Ethiopian highlands. Arch. Agron. Soil Sci. 2014, 60, 1261–1275. [Google Scholar] [CrossRef]
- Fossati, D.; Fossati, A.; Feil, B. Relationship between grain yield and grain nitrogen concentration in winter triticale. Euphytica 1993, 71, 115–123. [Google Scholar] [CrossRef]
- Barmeier, G.; Hu, Y.; Schmidhalter, U. Partitioning and translocation of dry matter and nitrogen during grain filling in spring barley varieties and their roles in determining malting quality. Front. Plant Sci. 2021, 12, 722871. [Google Scholar] [CrossRef]
- Yan, W.; Frégeau-Reid, J.; Pageau, D.; Martin, R. Genotype-by-environment interaction and trait associations in two genetic populations of oat. Crop Sci. 2016, 56, 1136–1145. [Google Scholar] [CrossRef]
- Łysoń, E.; Biel, W. Assessing chemical composition of grain of some selected rye (Secale cereale L.) cultivars from organic and conventional crops. Food Sci. Technol. Qual. 2016, 3, 91–101. [Google Scholar] [CrossRef]
- Jaśkiewicz, B.; Panasiewicz, K. Składniki pokarmowe w ziarnie pszenżyta ozimego w zależności od zmianowania i technologii produkcji. Agron. Sci. 2020, 75, 95–104. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Fraś, A.; Dmoch, A. Variability of selected quantitative traits in new spring barley genotypes. Plant Breed. Seed Sci. 2021, 82, 9–30. [Google Scholar] [CrossRef]
- Kaszuba, J.; Woś, H.; Shchipak, G.V. Bread making quality parameters of some Ukrainian and Polish triticale cultivars. Euphytica 2024, 220, 15. [Google Scholar] [CrossRef]
- COBORU. Lista Opisowa Odmian Roślin Rolniczych 2019. Zbożowe; Centralny Ośrodek Badania Odmian Roślin Uprawnych: Słupia Wielka, Poland, 2019.
- Sturite, I.; Kronberga, A.; Strazdina, V.; Kokare, A.; Aassveen, M.; Bergjord Olsen, A.K.; Sterna, V.; Straumite, E. Adaptability of hull-less barley varieties to different cropping systems and climatic conditions. Acta Agric. Scand.—B Soil Plant Sci. 2019, 69, 1–11. [Google Scholar] [CrossRef]
- Yanev, M.; Neshev, N.; Mitkov, A.; Manilov, T.; Dimitrova, M.; Moskova, C. Tank mixture of plant protection products with biostimulant in winter rye (Secale cereale L.). Sci. Papers Ser. A Agron. 2023, 66, 426–431. [Google Scholar]
- Brzozowska, I.; Brzozowski, J. Zawartość makroelementów w ziarnie pszenżyta ozimego odmiany Grenado, w zależności od metody pielęgnacji i poziomu nawożenia azotem. Zesz. Probl. Post. Nauk Rol. 2016, 585, 35–44. [Google Scholar]
- Kraska, P.; Pałys, E. Plonowanie i skład chemiczny ziarna pszenżyta ozimego uprawianego w monokulturze w warunkach stosowania zróżnicowanych dawek herbicydów. Agron. Sci. 2008, 63, 1–7. [Google Scholar] [CrossRef]
- Turkington, T.K.; O’Donovan, J.T.; Edney, M.J.; Juskiw, P.E.; McKenzie, R.H.; Harker, K.N.; Clayton, G.W.; Xi, K.; Lafond, G.P.; Irvine, R.B. Effect of crop residue, nitrogen rate and fungicide application on malting barley productivity, quality, and foliar disease severity. Can. J. Plant Sci. 2012, 92, 577–588. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; McMillan, T.; Tittlemier, S.A.; O’Donovan, J.; Tidemann, B.D. Effects of different timing and rate of glyphosate application on the residue level, grain quality, and processing performance of two Canadian malting barley varieties. Cereal Chem. 2024, 101, 1305–1315. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Lozowicka, B.; Kaczynski, P.; Konecki, R. Multifactorial wheat response under Fusarium culmorum, herbicidal, fungicidal and biostimulator treatments on the biochemical and mycotoxins status of wheat. J. Saudi Soc. Agric. Sci. 2021, 20, 443–453. [Google Scholar] [CrossRef]
- Ruske, R.E.; Gooding, M.J.; Jones, S.A. The effects of triazole and strobilurin fungicide programmes on nitrogen uptake, partitioning, remobilization and grain N accumulation in winter wheat cultivars. J. Agric. Sci. 2003, 140, 395–407. [Google Scholar] [CrossRef]
- Landolfi, V.; Visioli, G.; Blandino, M. Effect of nitrogen fertilization and fungicide application at heading on the gluten protein composition and rheological quality of wheat. Agronomy 2021, 11, 1687. [Google Scholar] [CrossRef]
- Singh, A.P.; Lal, M.; Pal, A.K.; Singh, A.P. Effect of FYM, potassium and zinc on yield, quality and uptake of nutrients in forage oat in alluvial soil. Ann. Plant Soil Res. 2016, 18, 338–341. [Google Scholar]
- Goswami, R.K.; Pandey, M. Effect of integrated use of nutrients and FYM on yield, quality and uptake of nutrients by barley (Hordeum valgare). Ann. Plant Soil Res. 2018, 20, 422–427. [Google Scholar]
- Pandey, M.; Singh, O. Productivity, nutrients uptake and quality of forage oat (Avena sativa) and residual soil fertility as influenced by nitrogen and FYM. Ann. Plant Soil Res. 2021, 23, 42–47. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, J.P. Integrated effect of copper and farmyard manure on yield, quality and uptake of nutrients in wheat. Ann. Plant Soil Res. 2013, 15, 156–159. [Google Scholar]
- Kadam, P.V.; Jat, S.L.; Parihar, C.M.; Singh, A.K.; Mahala, D.M.; Kumar, A.; Radheshyam; Naragund, R.; Singh, R. Effect of residue and nitrogen management in maize (Zea mays) on mustard (Brassica juncea) productivity and profitability under conservation agriculture. Indian J. Agric. Sci. 2022, 92, 637–642. [Google Scholar] [CrossRef]
- Lindsey, L.E.; Steinke, K.; Warncke, D.D.; Everman, W.J. Nitrogen release from weed residue. Weed Sci. 2013, 61, 334–340. [Google Scholar] [CrossRef]
- Rauber, R.B.; Demaría, M.R.; Arroyo, D.N.; Poggio, S.L. Crop type and management are key filtering factors of functional traits in the weed communities of regions with contrasting soils and climates. Appl. Veg. Sci. 2021, 24, e12622. [Google Scholar] [CrossRef]
- Wang, J.; Wei, X.; Kamran, M.; Chen, T.; White, J.F.; Li, C. Quality and nutrition of oat seed as influenced by seed-borne fungal pathogens during storage. J. Plant Dis. Prot. 2022, 129, 243–252. [Google Scholar] [CrossRef]



| Source of Variation | Winter Rye | Winter Triticale | Spring Barley | Spring Oats |
|---|---|---|---|---|
| Crop sequence system (CS) | 6.82 * | 7.31 ** | 0.0 | 2.8 |
| Cultivar (Cv) | 157.77 *** | 58.72 *** | 14.2 *** | 8.8 ** |
| Plant protection (PP) | 10.62 *** | 1.55 | 0.1 | 8.1 *** |
| Year (Yr) | 18.81 *** | 24.84 *** | 202.5 *** | 289.5 *** |
| CS × Cv | 10.29 ** | 2.78 | 0.4 | 3.7 |
| CS × PP | 7.78 *** | 0.97 | 6.0 ** | 5.1 ** |
| Cv × PP | 0.80 | 3.87 * | 2.3 | 0.1 |
| CS × Yr | 14.71 *** | 0.48 | 7.3 *** | 2.9 * |
| Cv × Yr | 1.56 | 6.37 *** | 2.3 | 2.4 |
| PP × Yr | 2.51 * | 2.88 ** | 1.6 | 2.5 * |
| CS × Cv × PP | 16.62 *** | 0.45 | 3.8 * | 2.7 |
| CS × Cv × Yr | 1.44 | 0.90 | 0.3 | 0.7 |
| CS × PP × Yr | 0.92 | 2.58 * | 1.1 | 1.9 |
| Cv × PP × Yr | 1.06 | 0.48 | 3.2 ** | 0.5 |
| CS × Cv × PP × Yr | 1.17 | 2.13 * | 1.0 | 1.3 |
| Source of Variation | Winter Rye | Winter Triticale | Spring Barley | Spring Oats |
|---|---|---|---|---|
| Crop sequence system (CS) | ||||
| Continuous monocropping (CM) | 8.32 ± 0.06 a 3 | 9.46 ± 0.09 b | 9.91 ± 0.11 a | 10.28 ± 0.11 a |
| Crop rotation (CR) | 8.17 ± 0.08 b | 9.68 ± 0.08 a | 9.90 ± 0.09 a | 10.37 ± 0.10 a |
| Cultivar (Cv) 1 | ||||
| A | 7.89 ± 0.06 b | 9.88 ± 0.08 a | 9.79 ± 0.09 b | 10.24 ± 0.11 b |
| B | 8.60 ± 0.06 a | 9.26 ± 0.08 b | 10.02 ± 0.11 a | 10.40 ± 0.11 a |
| Plant protection (PP) | ||||
| CT 2 | 8.07 ± 0.08 b | 9.61 ± 0.13 a | 9.90 ± 0.13 a | 10.17 ± 0.12 b |
| H | 8.37 ± 0.10 a | 9.63 ± 0.09 a | 9.90 ± 0.12 a | 10.37 ± 0.14 a |
| HF | 8.29 ± 0.07 a | 9.47 ± 0.09 a | 9.92 ± 0.12 a | 10.43 ± 0.13 a |
| Year (Yr) | ||||
| 2018 | 8.42 ± 0.09 a | 9.49 ± 0.09 b | 8.97 ± 0.09 c | 10.50 ± 0.09 c |
| 2019 | 7.99 ± 0.12 b | 9.71 ± 0.16 ab | 9.73 ± 0.08 b | 9.91 ± 0.07 d |
| 2020 | 8.51 ± 0.12 a | 10.06 ± 0.09 a | 10.78 ± 0.10 a | 11.01 ± 0.07 b |
| 2021 | 7.92 ± 0.09 b | 9.76 ± 0.13 ab | 10.98 ± 0.06 a | 11.44 ± 0.06 a |
| 2022 | 8.39 ± 0.10 a | 8.84 ± 0.10 c | 9.06 ± 0.06 c | 8.75 ± 0.07 e |
| Cereal Species | Cultivar | Grain Protein Content, % | Year | Reference |
|---|---|---|---|---|
| Winter rye | KWS Binntto | 6.9–9.4 | 2018–2022 | this study |
| KWS Binntto | 12.2–12.7 | 2019 | [73] | |
| Dańkowskie Diament | 7.3–10.2 | 2018–2022 | this study | |
| Dańkowskie Diament | 13.5–13.8 | 2019 | [73] | |
| different | 12–15 1 | no data | [8] | |
| different | 8.6–11.4 | 2019–2020 | [74] | |
| different | 11.1–14.3 | 2019 | [73] | |
| Winter triticale | Trapero | 7.8–12.2 | 2018–2022 | this study |
| Trapero | 15.2 | 2013 | [75] | |
| Borowik | 7.5–11.3 | 2018–2022 | this study | |
| different | 5.9–19.3 | 1982–2010 | [76] | |
| different | 7.9–19.7 | 2011–2019 | [77] | |
| different | 11.8–15.2 | 2013 | [75] | |
| Spring barley | Radek | 7.6–11.6 | 2018–2022 | this study |
| Radek | 9.7 | 2017 | [78] | |
| Radek | 10.6 | no data | [79] | |
| Skald | 8.1–11.9 | 2018–2022 | this study | |
| Skald | 10.5–13.0 | 2009–2010 | [80] | |
| different | 8–15 1 | no data | [8] | |
| different | 10.3–12.6 | no data | [79] | |
| different | 9.8–13.9 | 2011–2013 | [81] | |
| Spring oats | Elegant | 8.0–11.8 | 2018–2022 | this study |
| Bingo | 8.3–12.3 | 2018–2022 | this study | |
| Bingo | 11.4 | 2010–2011 | [82] | |
| Bingo | 10.7 | no data | [83] | |
| Bingo | 10.4–13.1 | 2016–2018 | [84] | |
| different | 12–24 1 | no data | [8] | |
| different | 9.4–16.7 | 2009–2011 | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostrzewska, M.K.; Jastrzębska, M. The Grain Protein Content of Polish Cereals Other than Wheat: Can It Be Increased by Combining a Crop Sequence System, Cultivar Selection, and Plant Protection? Agriculture 2025, 15, 1016. https://doi.org/10.3390/agriculture15091016
Kostrzewska MK, Jastrzębska M. The Grain Protein Content of Polish Cereals Other than Wheat: Can It Be Increased by Combining a Crop Sequence System, Cultivar Selection, and Plant Protection? Agriculture. 2025; 15(9):1016. https://doi.org/10.3390/agriculture15091016
Chicago/Turabian StyleKostrzewska, Marta K., and Magdalena Jastrzębska. 2025. "The Grain Protein Content of Polish Cereals Other than Wheat: Can It Be Increased by Combining a Crop Sequence System, Cultivar Selection, and Plant Protection?" Agriculture 15, no. 9: 1016. https://doi.org/10.3390/agriculture15091016
APA StyleKostrzewska, M. K., & Jastrzębska, M. (2025). The Grain Protein Content of Polish Cereals Other than Wheat: Can It Be Increased by Combining a Crop Sequence System, Cultivar Selection, and Plant Protection? Agriculture, 15(9), 1016. https://doi.org/10.3390/agriculture15091016






