Soil Fertility and Plant Growth Enhancement Through Compost Treatments Under Varied Irrigation Conditions
Abstract
1. Introduction
- Analyze nutrient retention, focusing on Na+, Cl−, NO3−, and P transport and availability in soil under different irrigation, compost, and soil conditions.
- Assess microbial biomass and activity in response to compost treatments under irrigation, compost, and soil conditions.
- Examine plant growth parameters, including chlorophyll content, biomass, plant height, root length, and ion composition of plant tissues under irrigation, compost, and soil conditions.
2. Materials and Methods
2.1. Experimental Design and Treatment Setup
2.2. Soil Collection and Column Preparation
2.3. Plant Growth Conditions and Irrigation Management
2.4. Plant Growth and Biomass Measurements
2.5. Plant Tissue and Soil Analysis
2.6. Statistical Analysis
3. Results
3.1. Physicochemical Properties of Compost and Soil
3.2. Impact of Compost Treatments on Soil Microbial Biomass, Biological Nutrients, and Protozoa Populations
3.3. Effects of Compost Treatments on Soil NO3−, P, and Organic Matter Content
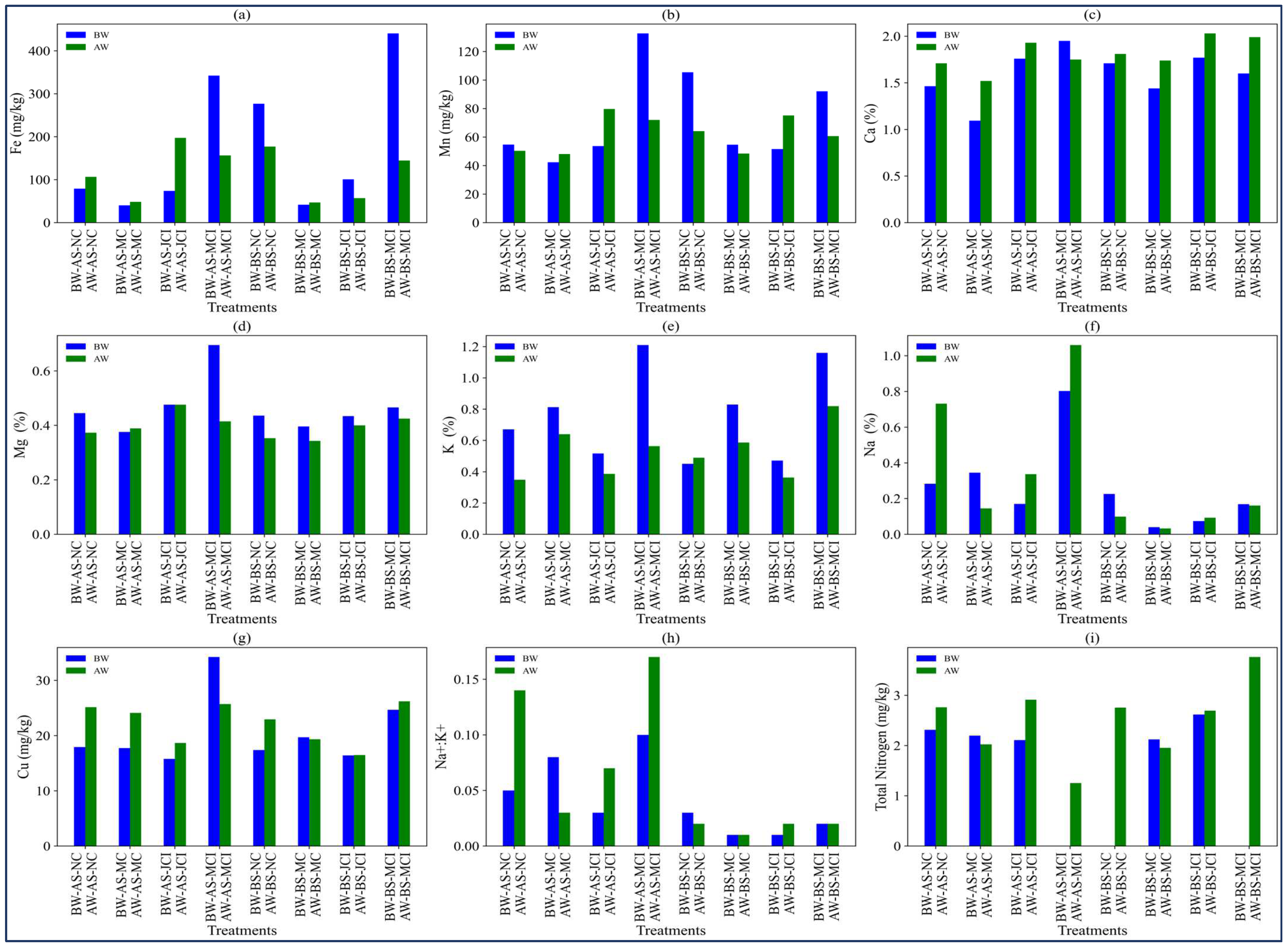
3.4. Impact of Compost Treatments on Soil Cation and Anion Content Under Different Irrigation Regimes

3.5. Principal Component Analysis (PCA) of Soil Ion Dynamics Under Different Treatments
3.6. Nutrient Uptake and Plant Performance Under Soil, Compost, and Irrigation Treatments
3.7. Impact of Irrigation Water, Soil, and Compost Treatments on Plant Growth Parameters
| Treatment | Chlorophyll | Plant Height (cm) | Wet Biomass (g) | Root Length (cm) |
|---|---|---|---|---|
| BW-AS-NC | 36.0 ± 1.0 abcd | 14.7 ± 0.1 def | 4.7 ± 1.2 ef | 15.3 ± 0.6 f |
| BW-AS-MC | 34.3 ± 2.3 bcde | 11.0 ± 2.2 efgh | 4.5 ± 1.3 ef | 15.5 ± 0.4 f |
| BW-AS-JCI | 43.2 ± 1.2 ab | 23.3 ± 4.3 abc | 21.7 ± 2.5 b | 32.3 ± 0.6 c |
| BW-AS-MCI | 28.5 ± 3.9 de | 5.8 ± 0.9 h | 1.9 ± 0.1 f | 10.7 ± 1.8 g |
| BW-BS-NC | 32.8 ± 2.9 cde | 12.9 ± 0.8 defg | 3.7 ± 0.6 ef | 30.5 ± 1.6 cd |
| BW-BS-MC | 37.9 ± 1.1 abc | 17.8 ± 1.9 bcd | 9.3 ± 1.2 cd | 41.2 ± 1.0 b |
| BW-BS-JCI | 42.9 ± 3.9 ab | 23.9 ± 1.1 ab | 29.7 ± 1.5 a | 45.0 ± 0.0 a |
| BW-BS-MCI | 30.5 ± 3.4 cde | 10.2 ± 1.2 fgh | 2.8 ± 1.0 ef | 20.7 ± 0.3 e |
| AW-AS-NC | 26.3 ± 2.5 e | 8.2 ± 0.4 gh | 1.3 ± 0.2 f | 10.5 ± 0.9 g |
| AW-AS-MC | 37.6 ± 2.3 abcd | 17.2 ± 1.3 cde | 9.3 ± 1.2 cd | 16.6 ± 0.9 f |
| AW-AS-JCI | 37.3 ± 2.3 abcd | 25.6 ± 2.4 a | 20.0 ± 3.5 b | 27.9 ± 2.3 d |
| AW-AS-MCI | 30.8 ± 4.2 cde | 8.0 ± 0.7 gh | 2.0 ± 0.5 f | 14.8 ± 1.7 f |
| AW-BS-NC | 36.3 ± 6.4 abcd | 17.6 ± 4.5 cd | 6.7 ± 1.2 de | 45.0 ± 0.0 a |
| AW-BS-MC | 39.1 ± 2.5 abc | 17.6 ± 2.2 cd | 11.3 ± 1.2 c | 42.3 ± 1.2 ab |
| AW-BS-JCI | 45.2 ± 2.6 a | 27.4 ± 1.5 a | 30.0 ± 2.0 a | 45.0 ± 0.0 a |
| AW-BS-MCI | 28.5 ± 1.5 de | 9.9 ± 0.5 fgh | 2.9 ± 1.1 ef | 16.2 ± 0.8 f |
| p-value IW | 0.487 | 0.018 | 0.135 | 0.009 |
| S | 0.011 | 0.000 | 0.000 | 0.000 |
| C | 0.000 | 0.000 | 0.000 | 0.000 |
| IW × S | 0.041 | 0.447 | 0.122 | 0.000 |
| IW × C | 0.171 | 0.078 | 0.008 | 0.000 |
| S × C | 0.404 | 0.398 | 0.000 | 0.000 |
| IW × S × C | 0.004 | 0.000 | 0.006 | 0.000 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ingrao, C.; Strippoli, R.; Lagioia, G.; Huisingh, D. Water scarcity in agriculture: An overview of causes, impacts and approaches for reducing the risks. Heliyon 2023, 9, e18507. [Google Scholar] [CrossRef] [PubMed]
- Marchuk, A. Effect of Cations on Structural Stability of Salt-Affected Soils. Ph.D. Thesis, The University of Adelaide, Adelaide, Australia, 2013. [Google Scholar]
- Khondoker, M.; Mandal, S.; Gurav, R.; Hwang, S. Freshwater shortage, salinity increase, and global food production: A need for sustainable irrigation water desalination—A scoping review. Earth 2023, 4, 223–240. [Google Scholar] [CrossRef]
- Adugna, G. A review on impact of compost on soil properties, water use and crop productivity. Acad. Res. J. Agric. Sci. Res. 2016, 4, 93–104. [Google Scholar]
- Gondek, M.; Weindorf, D.C.; Thiel, C.; Kleinheinz, G. Soluble salts in compost and their effects on soil and plants: A review. Compos. Sci. Util. 2020, 28, 59–75. [Google Scholar] [CrossRef]
- Singh, V.K.; Malhi, G.S.; Kaur, M.; Singh, G.; Jatav, H.S. Use of organic soil amendments for improving soil ecosystem health and crop productivity. In Ecosystem Services: Types, Management and Benefits; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2022. [Google Scholar]
- Elshony, M.; Farid, I.M.; Alkamar, F.; Abbas, M.H.; Abbas, H. Ameliorating a sandy soil using biochar and compost amendments and their implications as slow release fertilizers on plant growth. Egypt. J. Soil Sci. 2019, 59, 305–322. [Google Scholar] [CrossRef]
- Li, X.; Zhu, W.; Xu, F.; Du, J.; Tian, X.; Shi, J.; Wei, G. Organic amendments affect soil organic carbon sequestration and fractions in fields with long-term contrasting nitrogen applications. Agric. Ecosyst. Environ. 2021, 322, 107643. [Google Scholar] [CrossRef]
- Ng, J.F.; Ahmed, O.H.; Jalloh, M.B.; Omar, L.; Kwan, Y.M.; Musah, A.A.; Poong, K.H. Soil nutrient retention and pH buffering capacity are enhanced by calciprill and sodium silicate. Agronomy 2022, 12, 219. [Google Scholar] [CrossRef]
- Yüksel, O.; Kavdır, Y. Improvement of soil quality parameters by municipal solid waste compost application in clay-loam soil. Turk. J. Agric.—Food Sci. Technol. 2020, 8, 603–609. [Google Scholar] [CrossRef]
- El-Ramady, H.; Prokisch, J.; Mansour, H.; Bayoumi, Y.A.; Shalaby, T.A.; Veres, S.; Brevik, E.C. Review of crop response to soil salinity stress: Possible approaches from leaching to nano-management. Soil Syst. 2024, 8, 11. [Google Scholar] [CrossRef]
- Duong, T.T.T. Compost Effects on Soil Properties and Plant Growth. Ph.D. Thesis, The University of Adelaide, Adelaide, Australia, 2013. [Google Scholar]
- Gross, A.; Glaser, B. Meta-analysis on how manure application changes soil organic carbon storage. Sci. Rep. 2021, 11, 5516. [Google Scholar] [CrossRef]
- Liu, Y.; Xun, W.; Chen, L.; Xu, Z.; Zhang, N.; Feng, H.; Zhang, Q.; Zhang, R. Rhizosphere microbes enhance plant salt tolerance: Toward crop production in saline soil. Comput. Struct. Biotechnol. J. 2022, 20, 6543–6551. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef]
- El Hayany, B.; El Fels, L.; Kouisni, L.; Yasri, A.; Hafidi, M. An Insight into Role of Microorganisms in Composting and Its Applications in Agriculture. In Microbial BioTechnology for Sustainable Agriculture Volume 1; Springer: Singapore, 2022; pp. 185–203. [Google Scholar]
- Wei, X.; Xie, B.; Wan, C.; Song, R.; Zhong, W.; Xin, S.; Song, K. Enhancing soil health and plant growth through microbial fertilizers: Mechanisms, benefits, and sustainable agricultural practices. Agronomy 2024, 14, 609. [Google Scholar] [CrossRef]
- Sayara, T.; Basheer-Salimia, R.; Hawamde, F.; Sánchez, A. Recycling of organic wastes through composting: Process performance and compost application in agriculture. Agronomy 2020, 10, 1838. [Google Scholar] [CrossRef]
- Xu, D.; Yu, X.; Li, X.; Chen, J.; Li, J. Effects of compost as a soil amendment on bacterial community diversity in saline–alkali soil. Front. Microbiol. 2023, 14, 1253415. [Google Scholar] [CrossRef]
- Wang, D.; Lin, J.Y.; Sayre, J.M.; Schmidt, R.; Fonte, S.J.; Rodrigues, J.L.; Scow, K.M. Compost amendment maintains soil structure and carbon storage by increasing available carbon and microbial biomass in agricultural soil—A six-year field study. Geoderma 2022, 427, 116117. [Google Scholar] [CrossRef]
- Vassilev, N.; Mendes, G.D.O. Soil Fungi in Sustainable Agriculture. Microorganisms 2024, 12, 163. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Hu, J.; Zhang, T.; Wu, X.; Yang, Y. Arbuscular mycorrhizal fungi and glomalin play a crucial role in soil aggregate stability in pb-contaminated soil. Int. J. Environ. Res. Public Health 2022, 19, 5029. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Rengasamy, P.; McDonald, G.K. High concentrations of Na+ and Cl− ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J. Exp. Bot. 2010, 61, 4449–4459. [Google Scholar] [CrossRef]
- Srivastava, V.; Sarkar, A.; Singh, S.; Singh, P.; de Araujo, A.S.F.; Singh, R.P. Agroecological responses of heavy metal pollution with special emphasis on soil health and plant performances. Front. Environ. Sci. 2017, 5, 64. [Google Scholar] [CrossRef]
- Otlewska, A.; Migliore, M.; Dybka-Stępień, K.; Manfredini, A.; Struszczyk-Świta, K.; Napoli, R.; Białkowska, A.; Canfora, L.; Pinzari, F. When salt meddles between plant, soil, and microorganisms. Front. Plant Sci. 2020, 11, 553087. [Google Scholar]
- Brar, B.; Bala, K.; Saharan, B.S.; Sadh, P.K.; Duhan, J.S. Bio-boosting agriculture: Harnessing the potential of fungi-bacteria-plant synergies for crop improvement. Discov. Plants 2024, 1, 21. [Google Scholar] [CrossRef]
- Wright, J.; Kenner, S.; Lingwall, B. Utilization of compost as a soil amendment to increase soil health and to improve crop yields. Open J. Soil Sci. 2022, 12, 216–224. [Google Scholar] [CrossRef]
- Nehela, Y.; Mazrou, Y.S.; Alshaal, T.; Rady, A.M.; El-Sherif, A.M.; Omara, A.E.-D.; El-Monem, A.M.A.; Hafez, E.M. The integrated amendment of sodic-saline soils using biochar and plant growth-promoting rhizobacteria enhances maize (Zea mays L.) resilience to water salinity. Plants 2021, 10, 1960. [Google Scholar] [CrossRef]
- El-Ghamry, A.M.; El-Sherpiny, M.A.; Alkharpotly, A.-E.A.; Ghazi, D.A.; Helmy, A.A.; Siddiqui, M.H.; Pessarakli, M.; Hossain, M.A.; Elghareeb, E.M. The synergistic effects of organic composts and microelements co-application in enhancing potato productivity in saline soils. Heliyon 2024, 10, e32694. [Google Scholar] [CrossRef]
- Leogrande, R.; Vitti, C. Use of organic amendments to reclaim saline and sodic soils: A review. Arid. Land Res. Manag. 2019, 33, 1–21. [Google Scholar] [CrossRef]
- Abbas, A.; Naveed, M.; Khan, K.S.; Ashraf, M.; Siddiqui, M.H.; Abbas, N.; Mustafa, A.; Ali, L. The efficacy of organic amendments on maize productivity, soil properties and active fractions of soil carbon in organic-matter deficient soil. Span. J. Soil Sci. 2024, 14, 12814. [Google Scholar] [CrossRef]
- Farooqi, Z.U.R.; Qadir, A.A.; Khalid, S.; Murtaza, G.; Ashraf, M.N.; Javed, W.; Waqas, M.A.; Xu, M. Greenhouse gas emissions, carbon stocks and wheat productivity following biochar, compost and vermicompost amendments: Comparison of non-saline and salt-affected soils. Sci. Rep. 2024, 14, 7752. [Google Scholar]
- Ferdous, J.; Mannan, M.A.; Haque, M.M.; A Mamun, M.A.; Alam, M.S. Chlorophyll content, water relation traits and mineral ions accumulation in soybean as influenced by organic amendments under salinity stress. Aust. J. Crop Sci. 2018, 12, 1806–1812. [Google Scholar] [CrossRef]
- Johnson, D. Johnson Su Bioreactor. March 2016. Available online: https://www.youtube.com/watch?v=DxUGk161Ly8 (accessed on 11 November 2022).
- Baath, G.S.; Shukla, M.K.; Bosland, P.W.; Walker, S.J.; Saini, R.K.; Shaw, R. Water use and yield responses of chile pepper cultivars irrigated with brackish groundwater and reverse osmosis concentrate. Horticulturae 2020, 6, 27. [Google Scholar] [CrossRef]
- Fort, D.J.; Mathis, M.B.; Walker, R.; Tuominen, L.K.; Hansel, M.; Hall, S.; Richards, R.; Grattan, S.; Anderson, K. Toxicity of sulfate and chloride to early life stages of wild rice (Zizania palustris). Environ. Toxicol. Chem. 2014, 33, 2802–2809. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture, N.R.C.S. Chapter 2—Irrigation water requirements. In National Engineering Handbook; U.S. Department of Agriculture: Washington, DC, USA, 1993. [Google Scholar]
- Hagage, M.; Abdulaziz, A.M.; Elbeih, S.F.; Hewaidy, A.G.A. Monitoring soil salinization and waterlogging in the northeastern Nile Delta linked to shallow saline groundwater and irrigation water quality. Sci. Rep. 2024, 14, 27838. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Coskun, D.; Schulze, L.M.; Wong, J.R.; Britto, D.T. Sodium as nutrient and toxicant. Plant Soil 2013, 369, 1–23. [Google Scholar]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Brown, R.W.; Rhymes, J.M.; Jones, D.L. Saltwater intrusion induces shifts in soil microbial diversity and carbon use efficiency in a coastal grassland ecosystem. Soil Biol. Biochem. 2022, 170, 108700. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, T.; Zhang, B.; Liu, Z.; Cheng, Y.; Feng, H. Cation composition of saline water affects soil structure by altering the formation of macropores and cracks in illite soils. Soil Tillage Res. 2024, 239, 106052. [Google Scholar] [CrossRef]
- Tomaz, A.; Palma, P.; Fialho, S.; Lima, A.; Alvarenga, P.; Potes, M.; Costa, M.J.; Salgado, R. Risk assessment of irrigation-related soil salinization and sodification in mediterranean areas. Water 2020, 12, 3569. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L.Q. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, B.; Achterberg, E.P.; Yuan, H.; Song, J.; Duan, L.; Li, X. Rapid cycling of bacterial particulate organic matter in the upper layer of the western pacific warm pool. Geophys. Res. Lett. 2023, 50, e2023GL102896. [Google Scholar] [CrossRef]
- Stout, J.D. The role of protozoa in nutrient cycling and energy flow. In Advances in Microbial Ecology; Springer: Boston, MA, USA, 1980; pp. 1–50. [Google Scholar]
- Foissner, W. An update of ‘basic light and scanning electron microscopic methods for taxonomic studies of ciliated protozoa’. Int. J. Syst. Evol. Microbiol. 2014, 64, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; He, J.; Zhou, Z.; Xia, L.; Hu, Y.; Zhang, Y.; Zhang, Y.; Luo, Y.; Chu, H.; Liu, W.; et al. Organic amendments enhance soil microbial diversity, microbial functionality and crop yields: A meta-analysis. Sci. Total. Environ. 2022, 829, 154627. [Google Scholar] [CrossRef] [PubMed]
- Beevers, L.; Hageman, R.H. Nitrate and nitrite reduction. In Amino Acids and Derivatives; Elsevier: Amsterdam, The Netherlands, 1980; pp. 115–168. [Google Scholar]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef]
- Chaganti, V.N.; Culman, S.W. Historical perspective of soil balancing theory and identifying knowledge gaps: A review. Crop. Forage Turfgrass Manag. 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Omara, A.E.-D.; Hafez, E.M.; Osman, H.S.; Rashwan, E.; El-Said, M.A.A.; Alharbi, K.; El-Moneim, D.A.; Gowayed, S.M. Collaborative impact of compost and beneficial rhizobacteria on soil properties, physiological attributes, and productivity of wheat subjected to deficit irrigation in salt affected soil. Plants 2022, 11, 877. [Google Scholar] [CrossRef]
- Ayyaswamy, G. A Super-Ion Called Magnesium: The Unsung Hero of Vitality and Well-Being; Notion Press: Chennai, India, 2023. [Google Scholar]
- Beecher, H. Effect of saline water on rice yields and soil properties in the Murrumbidgee Valley. Aust. J. Exp. Agric. 1991, 31, 819–823. [Google Scholar] [CrossRef]
- Rezapour, S.; Nouri, A.; Asadzadeh, F.; Barin, M.; Erpul, G.; Jagadamma, S.; Qin, R. Combining chemical and organic treatments enhances remediation performance and soil health in saline-sodic soils. Commun. Earth Environ. 2023, 4, 285. [Google Scholar] [CrossRef]
- Dhaliwal, S.; Naresh, R.; Mandal, A.; Singh, R.; Dhaliwal, M. Dynamics and transformations of micronutrients in agricultural soils as influenced by organic matter build-up: A review. Environ. Sustain. Indic. 2019, 1, 100007. [Google Scholar] [CrossRef]
- Khoshru, B.; Mitra, D.; Nosratabad, A.F.; Reyhanitabar, A.; Mandal, L.; Farda, B.; Djebaili, R.; Pellegrini, M.; Guerra-Sierra, B.E.; Senapati, A.; et al. Enhancing manganese availability for plants through microbial potential: A sustainable approach for improving soil health and food security. Bacteria 2023, 2, 129–141. [Google Scholar] [CrossRef]
- Xie, K.; Cakmak, I.; Wang, S.; Zhang, F.; Guo, S. Synergistic and antagonistic interactions between potassium and magnesium in higher plants. Crop. J. 2021, 9, 249–256. [Google Scholar] [CrossRef]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40 (Suppl. S1), 326–345. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Pandita, S.; Sidhu, G.P.S.; Sharma, A.; Khanna, K.; Kaur, P.; Bali, A.S.; Setia, R. Copper bioavailability, uptake, toxicity and tolerance in plants: A comprehensive review. Chemosphere 2021, 262, 127810. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Liu, Y.; Gu, D.; Zhan, X.; Li, J.; Zhou, K.; Zhang, P.; Zou, Y. Molecular mechanisms of plant responses to copper: From deficiency to excess. Int. J. Mol. Sci. 2024, 25, 6993. [Google Scholar] [CrossRef]
- Mohammadshirazi, F.; McLaughlin, R.A.; Heitman, J.L.; Brown, V.K. A multi-year study of tillage and amendment effects on compacted soils. J. Environ. Manag. 2017, 203, 533–541. [Google Scholar] [CrossRef]


| Water (IW) | Brackish Water—BW (2958 ± 51 µS/cm) | Agricultural Water—AW (796 ± 7 µS/cm) | ||||||||||||||
| Soil (S) | Agricultural Soil (AS) | BGNDRF Soil (BS) | Agricultural Soil (AS) | BGNDRF Soil (BS) | ||||||||||||
| Compost (C) | NC | MC | JCI | MCI | NC | MC | JCI | MCI | NC | MC | JCI | MCI | NC | MC | JCI | MCI |
| Treatment | BW-AS-NC | BW-AS-MC | BW-AS-JCI | BW-AS-MCI | BW-BS-NC | BW-BS-MC | BW-BS-JCI | BW-BS-MCI | AW-AS-NC | AW-AS-MC | AW-AS-JCI | AW-AS-MCI | AW-BS-NC | AW-BS-MC | AW-BS-JCI | AW-BS-MCI |
| Parameters | Compost Type | AS | BS | |||||
|---|---|---|---|---|---|---|---|---|
| JC | MC | 0–15 cm | 15–30 cm | 30–45 cm | 0–15 cm | 15–30 cm | 30–45 cm | |
| pH (1:1) | 7.7 | 8.4 | 8.1 | 8.2 | 8.1 | 7.9 | 7.9 | 8.0 |
| Soluble salts (mS/cm) (1:1) | 0.36 | 0.79 | 0.31 | 0.16 | 0.25 | 1.30 | 1.25 | 1.30 |
| Organic matter (%) | 41.5 | 24.5 | 3.2 | 3.1 | 3.0 | 2.6 | 2.3 | 2.6 |
| NO3− (mg/kg) | 82.5 | 0.7 | 23.2 | 25.2 | 36.9 | 9.6 | 4.2 | 1.2 |
| P (mg/kg) | 193.1 | 36.5 | 24.4 | 23.3 | 21.7 | 6.6 | 4.6 | 3.0 |
| K (mg/kg) | 163 | 1451 | 734 | 679 | 687 | 713 | 577 | 531 |
| Na+ (mg/kg) | 107 | 830 | 108 | 97 | 97 | 140 | 213 | 219 |
| Cl− (mg/kg) | 44.2 | 919.7 | 38.3 | 25.7 | 21.3 | 114.2 | 60.2 | 44.4 |
| Ca2+ (mg/kg) | 4417 | 2832 | 5762 | 5648 | 5568 | 11,550 | 17,140 | 17,960 |
| Mg2+ (mg/kg) | 420 | 274 | 412 | 397 | 403 | 214 | 222 | 267 |
| Cu (mg/kg) | 0.16 | 1.07 | 1.29 | 0.90 | 0.75 | 0.72 | 0.68 | 0.66 |
| Fe (mg/kg) | 4.7 | 41.9 | 5.9 | 3.7 | 4.0 | 2.3 | 2.0 | 2.4 |
| Mn (mg/kg) | 1.7 | 4.4 | 18.9 | 4.0 | 2.4 | 6.5 | 5.5 | 2.6 |
| SO42− (mg/kg) | 116 | 344 | 52 | 47 | 49 | 3442 | 4657 | 3949 |
| CEC/Sum of Cations (meq/100 g) | 26.5 | 23.8 | 34.6 | 33.7 | 33.4 | 62.0 | 90.0 | 94.3 |
| Total Fungi (mg/kg) | Total Bacteria (mg/kg) | Total F: B | Biological Carbon (g/m2) | Biological Nitrogen (g/m2) | Biological Carbon: Biological Nitrogen | Aerobic Fungi (mg/kg) | Aerobic Bacteria (mg/kg) | Aerobic Fungi: Aerobic Bacteria | Flagellates MPN/g | Amoebae MPN/g | Ciliates MPN/g | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW-AS-NC | 12.3 | 141.3 | 0.1 | 18.93 | 3.53 | 5.4 | 8.5 | 3.9 | 2.2 | 6206.6 | 6206.6 | 0.0 |
| BW-AS-MC | 301.0 | 399.0 | 0.8 | 86.23 | 11.07 | 7.8 | 22.8 | 10.8 | 2.1 | 8453.8 | 2240.5 | 2240.5 |
| BW-AS-JCI | 120.8 | 152.0 | 0.8 | 33.61 | 4.24 | 7.9 | 16.2 | 6.6 | 2.4 | 7951.0 | 2107.3 | 0.0 |
| BW-AS-MCI | 13.4 | 154.4 | 0.1 | 20.68 | 3.86 | 5.4 | 20.9 | 4.7 | 4.4 | 780.4 | 2132.7 | 0.0 |
| BW-BS-NC | 11.7 | 134.9 | 0.1 | 18.05 | 3.37 | 5.4 | 18.2 | 5.0 | 3.6 | 0.0 | 200.8 | 0.0 |
| BW-BS-MC | 14.7 | 148.9 | 0.1 | 20.16 | 3.73 | 5.4 | 30.2 | 4.9 | 6.2 | 0.0 | 222.1 | 0.0 |
| BW-BS-JCI | 180.1 | 306.7 | 0.6 | 59.98 | 8.30 | 7.2 | 3.6 | 7.0 | 0.5 | 0.0 | 213.5 | 213.5 |
| BW-BS-MCI | 134.3 | 285.5 | 0.5 | 51.72 | 7.58 | 6.8 | 26.5 | 7.7 | 3.4 | 0.0 | 765.5 | 19,017.6 |
| AW-AS-NC | 12.1 | 139.6 | 0.1 | 18.69 | 3.49 | 5.4 | 22.0 | 4.8 | 4.6 | 7658.4 | 227.6 | 742.7 |
| AW-AS-MC | 173.9 | 309.1 | 0.6 | 59.51 | 8.33 | 7.1 | 20.7 | 9.6 | 2.2 | 741.2 | 2025.7 | 0.0 |
| AW-AS-JCI | 48.4 | 253.9 | 0.2 | 37.24 | 6.45 | 5.8 | 15.1 | 6.9 | 2.2 | 728.3 | 728.3 | 0.0 |
| AW-AS-MCI | 10.0 | 132.2 | 0.1 | 17.51 | 3.29 | 5.3 | 27.0 | 6.5 | 4.2 | 17,466.9 | 7249.8 | 0.0 |
| AW-BS-NC | 195.9 | 319.7 | 0.6 | 63.52 | 8.68 | 7.3 | 17.4 | 3.5 | 5.0 | 0.0 | 0.0 | 0.0 |
| AW-BS-MC | 10.5 | 139.1 | 0.1 | 18.44 | 3.47 | 5.3 | 25.8 | 2.1 | 12.6 | 0.0 | 226.8 | 0.0 |
| AW-BS-JCI | 204.8 | 320.3 | 0.6 | 64.69 | 8.74 | 7.4 | 5.4 | 5.7 | 1.0 | 0.0 | 211.4 | 211.4 |
| AW-BS-MCI | 89.2 | 229.3 | 0.4 | 39.23 | 6.01 | 6.5 | 33.1 | 7.9 | 4.2 | 0.0 | 733.2 | 7560.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suvendran, S.; Acevedo, M.F.; Smithers, B.; Walker, S.J.; Xu, P. Soil Fertility and Plant Growth Enhancement Through Compost Treatments Under Varied Irrigation Conditions. Agriculture 2025, 15, 734. https://doi.org/10.3390/agriculture15070734
Suvendran S, Acevedo MF, Smithers B, Walker SJ, Xu P. Soil Fertility and Plant Growth Enhancement Through Compost Treatments Under Varied Irrigation Conditions. Agriculture. 2025; 15(7):734. https://doi.org/10.3390/agriculture15070734
Chicago/Turabian StyleSuvendran, Subanky, Miguel F. Acevedo, Breana Smithers, Stephanie J. Walker, and Pei Xu. 2025. "Soil Fertility and Plant Growth Enhancement Through Compost Treatments Under Varied Irrigation Conditions" Agriculture 15, no. 7: 734. https://doi.org/10.3390/agriculture15070734
APA StyleSuvendran, S., Acevedo, M. F., Smithers, B., Walker, S. J., & Xu, P. (2025). Soil Fertility and Plant Growth Enhancement Through Compost Treatments Under Varied Irrigation Conditions. Agriculture, 15(7), 734. https://doi.org/10.3390/agriculture15070734






