Gypsum and Tillage Practices for Combating Soil Salinity and Enhancing Crop Productivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Soil Characteristics
2.2. Determination of Gypsum Amendment
2.3. Soil Sampling
2.4. Physical and Chemical Analyses
2.5. Soil Microbiome Analysis
2.5.1. Mechanical Cell Disruption
2.5.2. DNA Extraction
2.5.3. Sequencing Library Preparation (16S and ITS Regions)
2.6. Statistical Data Analysis
3. Results
3.1. Soil DNA Extraction
3.2. Sequencing Results
3.3. Physical Parameter of Soil
3.4. Microbial Community at Soil Level A
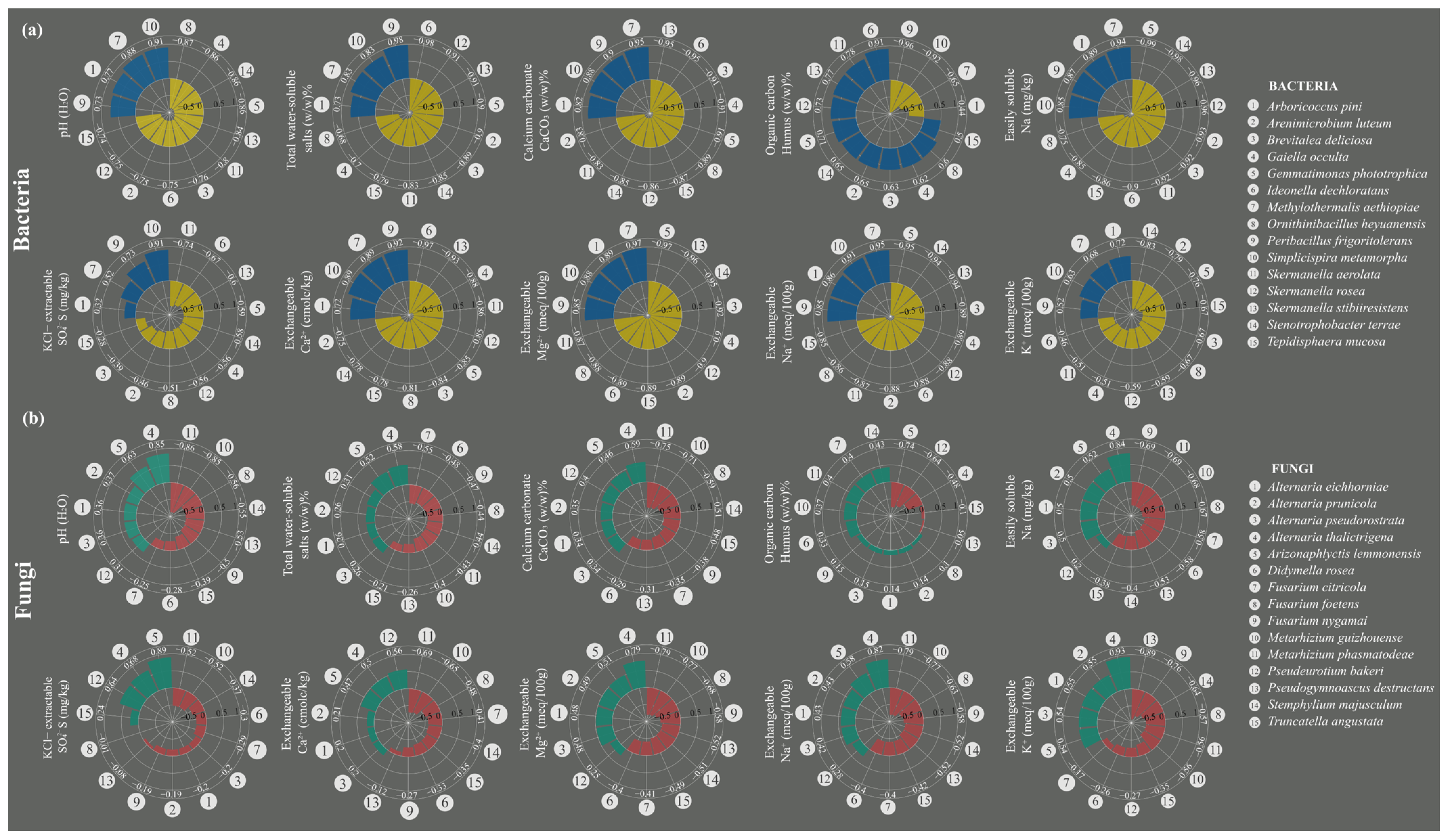
3.5. Examination of Genera Associated with Beneficial Functions for Soil Health
3.6. Chemical Parameters of Soil
3.7. Species Exhibiting Strong Correlations with Changes in Soil Chemistry
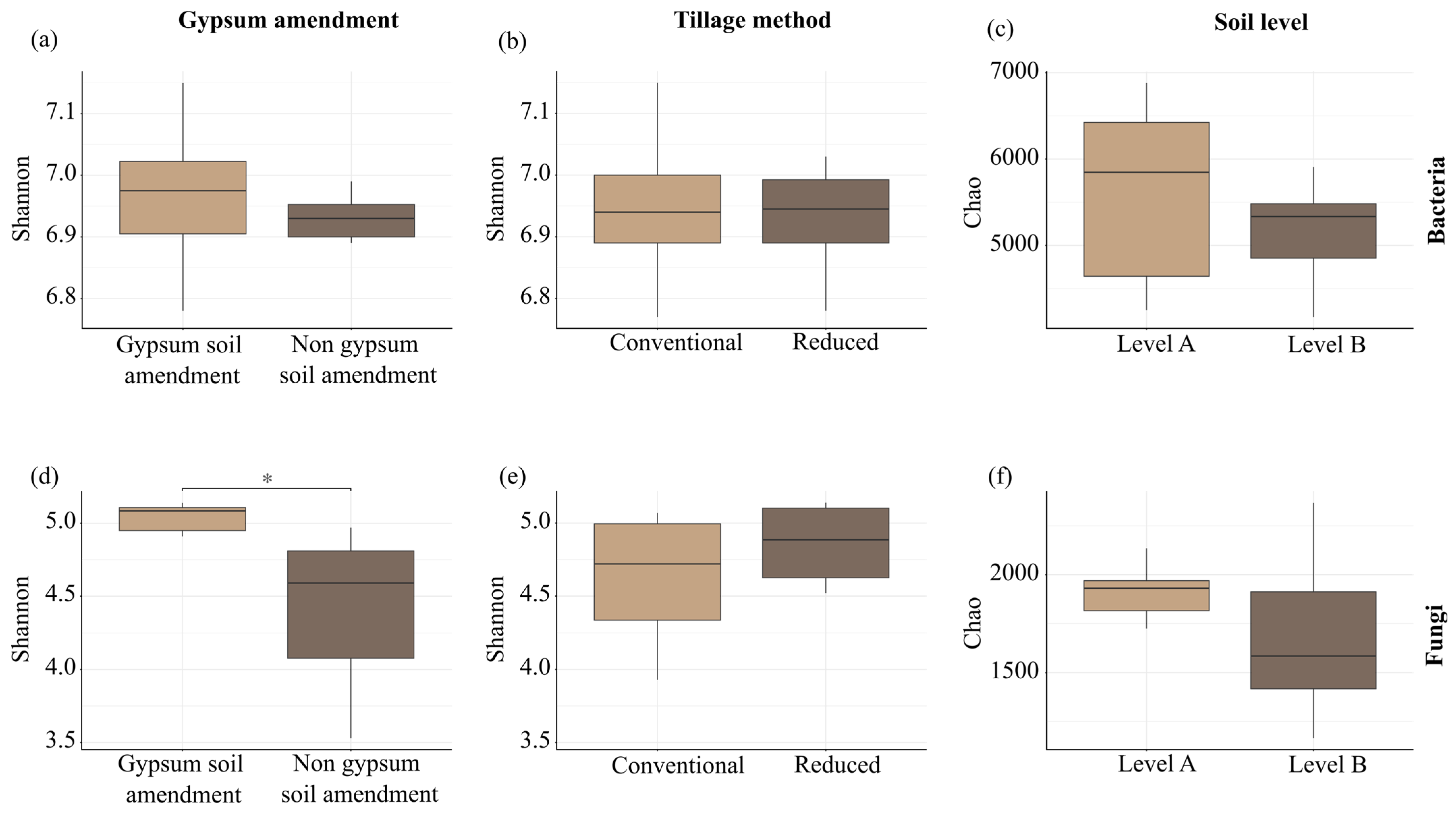
3.8. Overall Diversity of Soil
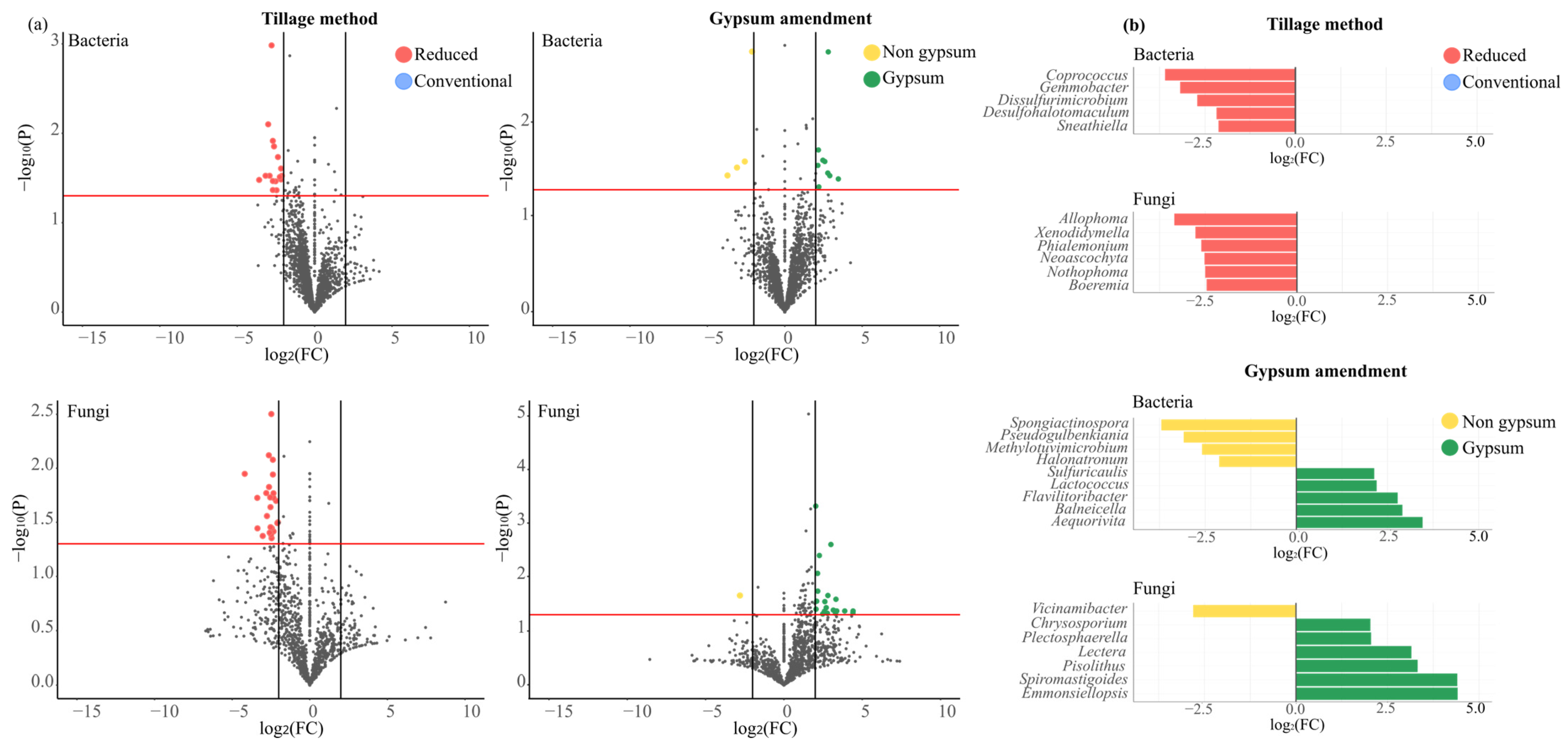
3.9. Microbial Community Dynamics Under Different Tillage Practices and Gypsum Application
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [PubMed]
- FAOSTAT. Food Balances. 2010. Available online: https://www.fao.org/faostat/en/#data/FBS (accessed on 12 December 2024).
- FAO Soils. Where Food Begins: How Can Soils Continue to Sustain the Growing Need for Food Production in the Current Fertilizer Crisis? FAO: Rome, Italy, 2023. [Google Scholar]
- FAO. Global Map of Salt-Affected Soils; FAO: Rome, Italy, 2021. [Google Scholar]
- Singh, A. Soil salinity: A global threat to sustainable development. Soil Use Manag. 2021, 38, 39–67. [Google Scholar]
- FAO. FAO Launches First Major Global Assessment of Salt-Affected Soils in 50 Years; FAO: Rome, Italy, 2024. [Google Scholar]
- Negacz, K.; Malek, Ž.; de Vos, A.; Vellinga, P. Saline soils worldwide: Identifying the most promising areas for saline agriculture. J. Arid Environ. 2022, 203, 104775. [Google Scholar]
- Eswar, D.; Karuppusamy, R.; Chellamuthu, S. Drivers of soil salinity and their correlation with climate change. Curr. Opin. Environ. Sustain. 2021, 50, 310–318. [Google Scholar]
- Yuvaraj, M.; Bose, K.S.C.; Elavarasi, P.; Tawfik, E.; Yuvaraj, M.; Bose, K.S.C.; Elavarasi, P.; Tawfik, E. Soil Salinity and Its Management. In Soil Moisture Importance; IntechOpen: London, UK, 2021; ISBN 978-1-83968-096-0. [Google Scholar]
- Laiskhanov, S.U.; Smanov, Z.M.; Kaimuldinova, K.D.; Myrzaly, N.B.; Ussenov, N.E.; Poshanov, M.N.; Azimkhanov, B.; Smanov, Z.M.; Poshanov, M.N. A Study of the Effects of Soil Salinity on the Growth and Development of Maize (Zea mays L.) by using Sentinel-2 Imagery. OnLine J. Biol. Sci. 2022, 22, 323–332. [Google Scholar] [CrossRef]
- El-Ramady, H.; Prokisch, J.; Mansour, H.; Bayoumi, Y.A.; Shalaby, T.A.; Veres, S.; Brevik, E.C. Review of Crop Response to Soil Salinity Stress: Possible Approaches from Leaching to Nano-Management. Soil Syst. 2024, 8, 11. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innovation 2020, 1, 100017. [Google Scholar]
- Liu, C.; Jiang, X.; Yuan, Z. Plant Responses and Adaptations to Salt Stress: A Review. Horticulturae 2024, 10, 1221. [Google Scholar] [CrossRef]
- Tripathi, S.; Kumari, S.; Chakraborty, A.; Gupta, A.; Chakrabarti, K.; Bandyapadhyay, B.K. Microbial biomass and its activities in salt-affected coastal soils. Biol. Fertil. Soils 2006, 42, 273–277. [Google Scholar]
- Rietz, D.N.; Haynes, R.J. Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol. Biochem. 2003, 35, 845–854. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, J.; Chen, R.; Zhong, L.; Lin, X.; Feng, Y. Microbial Community Composition and Activity in Saline Soils of Coastal Agro–Ecosystems. Microorganisms 2022, 10, 835. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Li, E.; Yang, X.; Yang, J. Shifts in Microbial Community Structure and Co-occurrence Network along a Wide Soil Salinity Gradient. Microorganisms 2024, 12, 1268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Shi, Y.; Cui, X.; Yue, P.; Li, K.; Liu, X.; Tripathi, B.M.; Chu, H. Salinity Is a Key Determinant for Soil Microbial Communities in a Desert Ecosystem. mSystems 2019, 4, 10-1128. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, C.; Xue, R.; Wang, L. Effects of salinity on the soil microbial community and soil fertility. J. Integr. Agric. 2019, 18, 1360–1368. [Google Scholar] [CrossRef]
- Wang, X.; Chi, Y.; Song, S. Important soil microbiota’s effects on plants and soils: A comprehensive 30-year systematic literature review. Front. Microbiol. 2024, 15, 1347745. [Google Scholar]
- Bidalia, A.; Vikram, K.; Yamal, G.; Rao, K.S. Effect of Salinity on Soil Nutrients and Plant Health. In Salt Stress, Microbes, and Plant Interactions: Causes and Solution; Akhtar, M.S., Ed.; Springer: Singapore, 2019; Volume 1, pp. 273–297. ISBN 9789811388019. [Google Scholar]
- Athar, T.; Kanwal, N. Significance of soil health and soil life for sustainable food production. Emergent Life Sci. Res. 2022, 08, 1–4. [Google Scholar] [CrossRef]
- Murtaza, G.; Murtaza, B.; Usman, H.; Ghafoor, A. Amelioration of Saline-sodic Soil using Gypsum and Low Quality Water in Following Sorghum-berseem Crop Rotation. Int. J. Agric. Biol. 2013, 15, 640–648. [Google Scholar]
- McKenna, B.A.; Kopittke, P.M.; Macfarlane, D.C.; Dalzell, S.A.; Menzies, N.W. Changes in soil chemistry after the application of gypsum and sulfur and irrigation with coal seam water. Geoderma 2019, 337, 782–791. [Google Scholar] [CrossRef]
- Bello, S.K.; Alayafi, A.H.; AL-Solaimani, S.G.; Abo-Elyousr, K.A.M. Mitigating Soil Salinity Stress with Gypsum and Bio-Organic Amendments: A Review. Agronomy 2021, 11, 1735. [Google Scholar] [CrossRef]
- Liu, R.; Liang, B.; Zhao, H.; Zhao, Y. Impacts of various amendments on the microbial communities and soil organic carbon of coastal saline–alkali soil in the Yellow River Delta. Front. Microbiol. 2023, 14, 1239855. [Google Scholar]
- Saqib, A.; Ahmed, K.; Naseem, A.; Qadir, G.; Nawaz, M.; Khalid, M.; Warraich, I.; Arif, M. Integrated Use of Humic Acid and Gypsum under Saline-Sodic Conditions. Pak. J. Agric. Res. 2020, 33, 684–691. [Google Scholar]
- Bossolani, J.W.; Crusciol, C.A.C.; Merloti, L.F.; Moretti, L.G.; Costa, N.R.; Tsai, S.M.; Kuramae, E.E. Long-term lime and gypsum amendment increase nitrogen fixation and decrease nitrification and denitrification gene abundances in the rhizosphere and soil in a tropical no-till intercropping system. Geoderma 2020, 375, 114476. [Google Scholar]
- López-Lozano, N.E.; Eguiarte, L.E.; Bonilla-Rosso, G.; García-Oliva, F.; Martínez-Piedragil, C.; Rooks, C.; Souza, V. Bacterial Communities and the Nitrogen Cycle in the Gypsum Soils of Cuatro Ciénegas Basin, Coahuila: A Mars Analogue. Astrobiology 2012, 12, 699–709. [Google Scholar] [PubMed]
- Somenahally, A.C.; McLawrence, J.; Chaganti, V.N.; Ganjegunte, G.K.; Obayomi, O.; Brady, J.A. Response of soil microbial Communities, inorganic and organic soil carbon pools in arid saline soils to alternative land use practices. Ecol. Indic. 2023, 150, 110227. [Google Scholar]
- Tian, Y.; Jiang, W.; Chen, G.; Wang, X.; Li, T. Gypsum and organic materials improved soil quality and crop production in saline-alkali on the loess plateau of China. Front. Environ. Sci. 2024, 12, 1434147. [Google Scholar] [CrossRef]
- Ibáñez, A.; Sombrero, A.; Santiago-Pajón, A.; Santiago-Calvo, Y.; Manzanera, M.C.A.S. Effect of long-term conservation tillage management on microbial diversity under Mediterranean rainfed conditions. Soil Tillage Res. 2024, 236, 105923. [Google Scholar]
- Kurm, V.; Schilder, M.; Haagsma, W.; Bloem, J.; Scholten, O.; Postma, J. Reduced tillage increases soil biological properties but not suppressiveness against Rhizoctonia solani and Streptomyces scabies. Appl. Soil Ecol. 2023, 181, 104646. [Google Scholar]
- 34. Singh, O.; Shahi, U.P.; Dutta, D.; Shivangi; Rajput, V.D.; Singh, A.; Singh, O.; Shahi, U.P.; Dutta, D.; Shivangi; et al. Strategic Tillage for Improved Soil Health and Nutrient Dynamics. In Strategic Tillage and Soil Management—New Perspectives; IntechOpen: London, UK, 2024; ISBN 978-1-83769-223-1. [Google Scholar]
- van Balen, D.; Cuperus, F.; Haagsma, W.; de Haan, J.; van den Berg, W.; Sukkel, W. Crop yield response to long-term reduced tillage in a conventional and organic farming system on a sandy loam soil. Soil Tillage Res. 2023, 225, 105553. [Google Scholar]
- Birkás, M.; Szalai, T.; Gyuricza, C.; Gecse, M.; Bordás, K. Effects of disk tillage on soil condition, crop yield and weed infestation. Plant Soil Environ. 2002, 48, 20–26. [Google Scholar] [CrossRef]
- Finch, H.J.S.; Samuel, A.M.; Lane, G.P.F. Cropping techniques. In Lockhart & Wiseman’s Crop Husbandry Including Grassland; Elsevier: Amsterdam, The Netherlands, 2014; pp. 193–217. ISBN 978-1-78242-371-3. [Google Scholar]
- FAO. Guidelines for Soil Description; FAO: Rome, Italy, 2006. [Google Scholar]
- FAO. Standard Operating Procedure for Soil pH Determination; FAO: Rome, Italy, 2021. [Google Scholar]
- Ferrarezi, R.S.; Lin, X.; Gonzalez Neira, A.C.; Tabay Zambon, F.; Hu, H.; Wang, X.; Huang, J.-H.; Fan, G. Substrate pH Influences the Nutrient Absorption and Rhizosphere Microbiome of Huanglongbing-Affected Grapefruit Plants. Front. Plant Sci. 2022, 13, 856937. [Google Scholar]
- Filep, G. Talajvizsgálat. Egyetem Mezőgazdaságtudományi Kar Talajtani és Mikrobiológiai Tanszék, Debreceni Agrártudományi: Debrecen, Hungary, 1995; p. 81. [Google Scholar]
- Csubák, M. Talajok kémhatása. In Talajtan, Talajökológia; katai, J., Ed.; Az Eszak—Alföld Régiert Kht.: Debrecen, Hungary, 2008; pp. 86–92. [Google Scholar]
- Lukács, A.; Rédly, L. A talajok sótartalmának és sóösszetételének vizsgálata. In Talaj- és Agrokémiai Vizsgálati Módszerkönyv 2; Mezőgazdasági Kiadó: Budapest, Hungary, 1988; pp. 174–210. [Google Scholar]
- Buzás, I. A talajfizikai, vízgazdálkodási és ásványtani vizsgálata. Inda4231 Kiadó. 1933, 1, 37–42. [Google Scholar]
- Hargitai, L. Az ekvivalens humuszkészlet agrokémiai és környezetvédelmi jelentősége. Kertészeti Egy. Közleményei Bp. 1987, 51, 260–267. [Google Scholar]
- Búzás, I. Talaj és Agrokcmiai Vizsgálatok Módszerkonyv; INDA 4231 Kiadó: Budapest, Hungary, 1988. [Google Scholar]
- Wick, R.R.; Judd, L.M.; Holt, K.E. Performance of neural network basecalling tools for Oxford Nanopore sequencing. Genome Biol. 2019, 20, 129. [Google Scholar] [CrossRef]
- Wright, C. epi2me-labs/fastcat. Github. 2025. Available online: https://github.com/epi2me-labs/fastcat (accessed on 17 February 2025).
- Lanfear, R.; Schalamun, M.; Kainer, D.; Wang, W.; Schwessinger, B. MinIONQC: Fast and simple quality control for MinION sequencing data. Bioinformatics 2019, 35, 523–525. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- McMurdie, P.J. joey711/phyloseq. Github. 2025. Available online: https://github.com/joey711/phyloseq (accessed on 17 February 2025).
- Pedersen, T.L. tidyverse/ggplot2. Github. 2025. Available online: https://github.com/tidyverse/ggplot2 (accessed on 17 February 2025).
- Taiyun. taiyun/corrplot. Beijing, China. Github. 2024. Available online: https://github.com/taiyun/corrplot (accessed on 17 February 2025).
- Foster, Z.S.L.; Sharpton, T.J.; Grünwald, N.J. Metacoder: An R package for visualization and manipulation of community taxonomic diversity data. PLoS Comput. Biol. 2017, 13, e1005404. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Slessarev, E.; Kan, M.; McFarlane, K.; Oerter, E.; Pett-Ridge, J.; Nuccio, E.; Berhe, A.A. Active microbial biomass decreases, but microbial growth potential remains similar across soil depth profiles under deeply-vs. shallow-rooted plants. Soil Biol. Biochem. 2021, 162, 108401. [Google Scholar] [CrossRef]
- Rumpel, C.; Kögel-Knabner, I. Deep soil organic matter—A key but poorly understood component of terrestrial C cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Liu, X.; Rezaei Rashti, M.; Van Zwieten, L.; Esfandbod, M.; Rose, M.T.; Chen, C. Microbial carbon functional responses to compaction and moisture stresses in two contrasting Australian soils. Soil Tillage Res. 2023, 234, 105825. [Google Scholar] [CrossRef]
- Tapaça, I.d.P.E.; Obieze, C.C.; Pereira, G.V.d.M.; Fangueiro, D.; Coutinho, J.; Fraga, I.; Partelli, F.L.; Ramalho, J.C.; Marques, I.; Ribeiro-Barros, A.I. Irradiance level and elevation shape the soil microbiome communities of Coffea arabica L. Environ. Microbiome 2024, 19, 75. [Google Scholar] [CrossRef]
- Amon, C.E.R.; Fossou, R.K.; Ebou, A.E.T.; Koua, D.K.; Kouadjo, C.G.; Brou, Y.C.; Voko Bi, D.R.R.; Cowan, D.A.; Zézé, A. The core bacteriobiome of Côte d’Ivoire soils across three vegetation zones. Front. Microbiol. 2023, 14, 1220655. [Google Scholar]
- Gaballah, M.S.; Sobhi, M.; Guo, J.; Philbert, M.; Min, L.; Zheng, Y.; Ghorab, M.A.; Dong, R. Evaluating the removal and impacts of combined tetracycline, oxytetracycline, sulfadiazine, and norfloxacin during swine manure storage process at ambient temperatures. J. Environ. Chem. Eng. 2023, 11, 109765. [Google Scholar]
- Dall’Asta, C.; Cirlini, M.; Falavigna, C. Mycotoxins from Alternaria. In Advances in Molecular Toxicology; Fishbein, J.C., Heilman, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 8, pp. 107–121. [Google Scholar]
- Wu, X.; Cai, W.; Zhu, P.; Peng, Z.; Zheng, T.; Li, D.; Li, J.; Zhou, G.; Zhang, J.; Du, G. Function-driven design of Bacillus kochii and Filobasidium magnum co-culture to improve quality of flue-cured tobacco. Front. Microbiol. 2023, 13, 1024005. [Google Scholar]
- Zeng, Z.-Q.; Zhuang, W.-Y. Three New Species of Fusicolla (Hypocreales) from China. J. Fungi 2023, 9, 572. [Google Scholar] [CrossRef] [PubMed]
- Vandepol, N.; Liber, J.; Yocca, A.; Matlock, J.; Edger, P.; Bonito, G. Linnemannia elongata (Mortierellaceae) stimulates Arabidopsis thaliana aerial growth and responses to auxin, ethylene, and reactive oxygen species. PLoS ONE 2022, 17, e0261908. [Google Scholar]
- Das, K.; Abrol, S.; Verma, R.; Annapragada, H.; Katiyar, N.; Senthilkumar, M. Chapter 8—Pseudomonas. In Beneficial Microbes in Agro-Ecology; Amaresan, N., Senthil Kumar, M., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 133–148. ISBN 978-0-12-823414-3. [Google Scholar]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar]
- Orozco-Mosqueda, M.d.C.; Santoyo, G.; Glick, B.R. Recent Advances in the Bacterial Phytohormone Modulation of Plant Growth. Plants 2023, 12, 606. [Google Scholar] [CrossRef] [PubMed]
- Pino-Hurtado, M.S.; Fernández-Fernández, R.; Torres, C.; Robredo, B. Searching for Antimicrobial-Producing Bacteria from Soils through an Educational Project and Their Evaluation as Potential Biocontrol Agents. Antibiotics 2024, 13, 29. [Google Scholar]
- Millar, B.C.; Nelson, D.; Moore, R.E.; Rao, J.R.; Moore, J.E. Antimicrobial properties of basidiomycota macrofungi to Mycobacterium abscessus isolated from patients with cystic fibrosis. Int. J. Mycobacteriology 2019, 8, 93–97. [Google Scholar]
- Mackay, J.E.; Bernhardt, L.T.; Smith, R.G.; Ernakovich, J.G. Tillage and pesticide seed treatments have distinct effects on soil microbial diversity and function. Soil Biol. Biochem. 2023, 176, 108860. [Google Scholar]
- Chen, J.; Song, D.; Liu, D.; Sun, J.; Wang, X.; Zhou, W.; Liang, G. Soil Aggregation Shaped the Distribution and Interaction of Bacterial-Fungal Community Based on a 38-Year Fertilization Experiment in China. Front. Microbiol. 2022, 13, 824681. [Google Scholar] [CrossRef] [PubMed]
- Armalytė, J.; Skerniškytė, J.; Bakienė, E.; Krasauskas, R.; Šiugždinienė, R.; Kareivienė, V.; Kerzienė, S.; Klimienė, I.; Sužiedėlienė, E.; Ružauskas, M. Microbial Diversity and Antimicrobial Resistance Profile in Microbiota From Soils of Conventional and Organic Farming Systems. Front. Microbiol. 2019, 10, 892. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.M.H.; Vilas-Boas, Â.; Sousa, C.A.; Soares, H.M.V.M.; Soares, E.V. Comparison of five bacterial strains producing siderophores with ability to chelate iron under alkaline conditions. AMB Express 2019, 9, 78. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Bacterial Siderophores: Classification, Biosynthesis, Perspectives of Use in Agriculture. Plants 2022, 11, 3065. [Google Scholar] [CrossRef]
- Holinsworth, B.; Martin, J.D. Siderophore production by marine-derived fungi. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2009, 22, 625–632. [Google Scholar] [CrossRef]
- Zhou, C.; Guo, J.; Zhu, L.; Xiao, X.; Xie, Y.; Zhu, J.; Ma, Z.; Wang, J. Paenibacillus polymyxa BFKC01 enhances plant iron absorption via improved root systems and activated iron acquisition mechanisms. Plant Physiol. Biochem. PPB 2016, 105, 162–173. [Google Scholar] [CrossRef]
- Soares, F.L.; Melo, I.S.; Dias, A.C.F.; Andreote, F.D. Cellulolytic bacteria from soils in harsh environments. World J. Microbiol. Biotechnol. 2012, 28, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Kumar, V.; Kumar, S.; Singh, D. Diverse culturable bacterial communities with cellulolytic potential revealed from pristine habitat in Indian trans-Himalaya. Can. J. Microbiol. 2018, 64, 798–808. [Google Scholar] [CrossRef]
- Gunathilake, K.M.D.; Ratnayake, R.R.; Kulasooriya, S.A.; Karunaratne, D.N. Evaluation of cellulose degrading efficiency of some fungi and bacteria and their biofilms. J. Natl. Sci. Found. Sri Lanka 2013, 41, 155. [Google Scholar] [CrossRef]
- Rabha, J.; Devi, S.P.; Das, S.; Roy, N.; Jha, D.K. Chapter 3—Microbial conversion of biomass to value-added chemicals. In Value-Addition in Agri-food Industry Waste Through Enzyme Technology; Kuddus, M., Ramteke, P., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 37–64. ISBN 978-0-323-89928-4. [Google Scholar]
- Trujillo-Cabrera, Y.; Ponce-Mendoza, A.; Vásquez-Murrieta, M.S.; Rivera-Orduña, F.N.; Wang, E.T. Diverse cellulolytic bacteria isolated from the high humus, alkaline-saline chinampa soils. Ann. Microbiol. 2013, 63, 779–792. [Google Scholar] [CrossRef]
- Srinivasan, R.; Prabhu, G.; Prasad, M.; Mishra, M.; Chaudhary, M.; Srivastava, R. Chapter 32—Penicillium. In Beneficial Microbes in Agro-Ecology; Amaresan, N., Senthil Kumar, M., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 651–667. ISBN 978-0-12-823414-3. [Google Scholar]
- Pimenta, R.S.; Monteiro dos Santos Baliza, D.D.; Moreira da Silva, J.F. Chapter 30—Aspergillus. In Beneficial Microbes in Agro-Ecology; Amaresan, N., Senthil Kumar, M., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 611–624. ISBN 978-0-12-823414-3. [Google Scholar]
- Stephanopoulos, G.N.; Aristidou, A.A.; Nielsen, J. Chapter 6—Examples of Pathway Manipulations: Metabolic Engineering in Practice. In Metabolic Engineering; Stephanopoulos, G.N., Aristidou, A.A., Nielsen, J., Eds.; Academic Press: San Diego, CA, USA, 1998; pp. 203–283. ISBN 978-0-12-666260-3. [Google Scholar]
- Hardy, A.; Kever, L.; Frunzke, J. Antiphage small molecules produced by bacteria—Beyond protein-mediated defenses. Trends Microbiol. 2023, 31, 92–106. [Google Scholar]
- Gemechu, G.W. Antibiotics Production from Fungi. Adv. Biotechnol. Microbiol. 2024, 18, 001–004. [Google Scholar] [CrossRef]
- Demain, A.L. Importance of microbial natural products and the need to revitalize their discovery. J. Ind. Microbiol. Biotechnol. 2014, 41, 185–201. [Google Scholar] [CrossRef]
- Roberts, J.M. Antibiotic Substances Produced by Species of Cephalosporium, with a Description of a New Species. Mycologia 1952, 44, 292–306. [Google Scholar] [CrossRef]
- Ngo, M.T.; Van Nguyen, M.; Han, J.W.; Kim, B.; Kim, Y.K.; Park, M.S.; Kim, H.; Choi, G.J. Biocontrol Potential of Aspergillus Species Producing Antimicrobial Metabolites. Front. Microbiol. 2021, 12, 804333. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Song, C.; Li, Z.; Kuipers, O.P. Antimicrobial activity screening of rhizosphere soil bacteria from tomato and genome-based analysis of their antimicrobial biosynthetic potential. BMC Genomics 2021, 22, 29. [Google Scholar] [CrossRef]
- Schütze, E.; Klose, M.; Merten, D.; Nietzsche, S.; Senftleben, D.; Roth, M.; Kothe, E. Growth of streptomycetes in soil and their impact on bioremediation. J. Hazard. Mater. 2014, 267, 128–135. [Google Scholar] [CrossRef]
- Dell’ Anno, F.; Rastelli, E.; Sansone, C.; Brunet, C.; Ianora, A.; Dell’ Anno, A. Bacteria, Fungi and Microalgae for the Bioremediation of Marine Sediments Contaminated by Petroleum Hydrocarbons in the Omics Era. Microorganisms 2021, 9, 1695. [Google Scholar] [CrossRef]
- Perpetuini, G.; Nzobouh Fossi, P.A.; Kwak, S.; Namessi, O.; Petchkongkaew, A.; Tofalo, R.; Waché, Y. Pesticides in Foods: Towards Bioremediation Biocatalysts? Catalysts 2023, 13, 1055. [Google Scholar] [CrossRef]
- Kundu, D.; Hazra, C.; Chaudhari, A. Bioremediation Potential of Rhodococcus pyridinivorans NT2 in Nitrotoluene-Contaminated Soils: The Effectiveness of Natural Attenuation, Biostimulation and Bioaugmentation Approaches. Soil Sediment Contam. Int. J. 2016, 25, 637–651. [Google Scholar] [CrossRef]
- Bolton, S.L.; Brannen, P.M.; Glenn, A.E. A Novel Population of Fusarium fujikuroi Isolated from Southeastern U.S. Winegrapes Reveals the Need to Re-Evaluate the Species’ Fumonisin Production. Toxins 2016, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- de Souza, R.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar]
- Wani, F.; Ahmad, D.; Ali, T.; Mushtaq, A. Role of Microorganisms in Nutrient Mobilization and Soil Health—A Review. J. Pure Appl. Microbiol. 2015, 9, 1401–1410. [Google Scholar]
- Misra, V.; Ansari, M.I. Role of Trichoderma in Agriculture and Disease Management. In Plant Growth-Promoting Microbes for Sustainable Biotic and Abiotic Stress Management; Mohamed, H.I., El-Beltagi, H.E.-D.S., Abd-Elsalam, K.A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 425–440. ISBN 978-3-030-66587-6. [Google Scholar]
- Vio, S.A.; García, S.S.; Casajus, V.; Arango, J.S.; Galar, M.L.; Bernabeu, P.R.; Luna, M.F. Chapter 15—Paraburkholderia. In Beneficial Microbes in Agro-Ecology; Amaresan, N., Senthil Kumar, M., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 271–311. ISBN 978-0-12-823414-3. [Google Scholar]
- Tsukanova, K.A.; Chebotar, V.K.; Meyer, J.J.M.; Bibikova, T.N. Effect of plant growth-promoting Rhizobacteria on plant hormone homeostasis. S. Afr. J. Bot. 2017, 113, 91–102. [Google Scholar]
- Ercole, T.G.; Kava, V.M.; Aluizio, R.; Pauletti, V.; Hungria, M.; Galli-Terasawa, L.V. Co-inoculation of Bacillus velezensis and Stenotrophomonas maltophilia strains improves growth and salinity tolerance in maize (Zea mays L.). Rhizosphere 2023, 27, 100752. [Google Scholar]
- Hossain, M.M.; Sultana, F.; Hossain, M.M.; Sultana, F. Application and Mechanisms of Plant Growth Promoting Fungi (PGPF) for Phytostimulation. In Organic Agriculture; IntechOpen: London, UK, 2020; ISBN 978-1-78984-669-0. [Google Scholar]
- Li, G.; Wei, M.; Wei, G.; Chen, Z.; Shao, Z. Efficient heterotrophic nitrification by a novel bacterium Sneathiella aquimaris 216LB-ZA1-12T isolated from aquaculture seawater. Ecotoxicol. Environ. Saf. 2023, 266, 115588. [Google Scholar] [CrossRef]
- Rahmeh, R.; Akbar, A.; Kumar, V.; Al-Mansour, H.; Kishk, M.; Ahmed, N.; Al-Shamali, M.; Boota, A.; Al-Ballam, Z.; Shajan, A.; et al. Insights into Bacterial Community Involved in Bioremediation of Aged Oil-Contaminated Soil in Arid Environment. Evol. Bioinforma. 2021, 17, 11769343211016887. [Google Scholar]
- Murindangabo, Y.T.; Kopecký, M.; Perná, K.; Nguyen, T.G.; Konvalina, P.; Kavková, M. Prominent use of lactic acid bacteria in soil-plant systems. Appl. Soil Ecol. 2023, 189, 104955. [Google Scholar]
- Chaudhary, S.; Sindhu, S.S.; Dhanker, R.; Kumari, A. Microbes-mediated sulphur cycling in soil: Impact on soil fertility, crop production and environmental sustainability. Microbiol. Res. 2023, 271, 127340. [Google Scholar] [CrossRef]
- Badalíková, B. Influence of Soil Tillage on Soil Compaction. In Soil Engineering; Dedousis, A.P., Bartzanas, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 19–30. ISBN 978-3-642-03681-1. [Google Scholar]
- Hamza, M.; Anderson, W. Response of soil properties and grain yield to deep ripping and gypsum application in a compacted loamy sand soil contrasted with a sandy clay loam in Western Australia. Crop Pasture Sci. 2003, 54, 273–282. [Google Scholar]
- Peralta Ogorek, L.L.; Gao, Y.; Farrar, E.; Pandey, B.K. Soil compaction sensing mechanisms and root responses. Trends Plant Sci. 2024; ahead of print. [Google Scholar]
- Jin, K.; White, P.J.; Whalley, W.R.; Shen, J.; Shi, L. Shaping an Optimal Soil by Root–Soil Interaction. Trends Plant Sci. 2017, 22, 823–829. [Google Scholar] [CrossRef]
- Bengough, A.G. Water Dynamics of the Root Zone: Rhizosphere Biophysics and Its Control on Soil Hydrology. Vadose Zone J. 2012, 11, vzj2011.0111. [Google Scholar] [CrossRef]
- Munkholm, L.J.; Schjønning, P.; Jørgensen, M.H.; Thorup-Kristensen, K. Mitigation of subsoil recompaction by light traffic and on-land ploughing: II. Root and yield response. Soil Tillage Res. 2005, 80, 159–170. [Google Scholar]
- Sojnóczki, I.; Nagy, J.; Illés, Á.; Kecskés, I.; Bojtor, C. Comparative Analysis of Drought Effects on the Soil Moisture Level and Penetration Resistance in Conventional and Non-Conventional Tillage Systems in Maize Production. Agriculture 2024, 14, 1000. [Google Scholar] [CrossRef]
- Pingel, M.; Reineke, A.; Leyer, I. Disentangling the mixed effects of soil management on microbial diversity and soil functions: A case study in vineyards. Sci. Rep. 2023, 13, 3568. [Google Scholar]
- Casazza, G.; Lumini, E.; Ercole, E.; Dovana, F.; Guerrina, M.; Arnulfo, A.; Minuto, L.; Fusconi, A.; Mucciarelli, M. The abundance and diversity of arbuscular mycorrhizal fungi are linked to the soil chemistry of screes and to slope in the Alpic paleo-endemic Berardia subacaulis. PLoS ONE 2017, 12, e0171866. [Google Scholar]
- Wilkes, T.I. The influence of a soil amendment on the abundance and interaction of arbuscular mycorrhizal fungi with arable soils and host winter wheat. Access Microbiol. 2024, 6, 000581.v5. [Google Scholar] [CrossRef]
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere Bacteria in Plant Growth Promotion, Biocontrol, and Bioremediation of Contaminated Sites: A Comprehensive Review of Effects and Mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef]
- Franzluebbers, A.J. Soil depth distribution of particulate organic carbon and nitrogen in the southeastern United States. Agric. Environ. Lett. 2024, 9, e70005. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Van Goethem, M.W.; Pierneef, R.; Bezuidt, O.K.I.; Van De Peer, Y.; Cowan, D.A.; Makhalanyane, T.P. A reservoir of ‘historical’ antibiotic resistance genes in remote pristine Antarctic soils. Microbiome 2018, 6, 40. [Google Scholar]
- Abbas, F.; Siddique, T.; Fan, R.; Azeem, M. Role of Gypsum in Conserving Soil Moisture Macronutrients Uptake and Improving Wheat Yield in the Rainfed Area. Water 2023, 15, 1011. [Google Scholar] [CrossRef]
- Chaudhary, A.; Chaudhary, P.; Upadhyay, A.; Kumar, A.; Singh, A. Effect of Gypsum on Plant Growth Promoting Rhizobacteria. Environ. Ecol. 2021, 39, 1248–1256. [Google Scholar]
- Rashmi, I.; Meena, B.P.; Rajendiran, S.; Jayaraman, S.; Joshy, C.G.; Ali, S.; Mina, B.L.; Kumar, K.; Kumar, A.; Kumawat, A.; et al. Can gypsum and organic amendments achieve sustainability, productivity and maintain soil health under soybean-mustard cropping in sodic soils of western India. Soil Tillage Res. 2024, 240, 106075. [Google Scholar]
- Costa, J.L.; Aparicio, V.C.; Sallesses, L.F.; Frolla, F.D. Effect of tillage and application of gypsum In a No-Till field under supplementary irrigation with sodium bicarbonate waters. Agric. Water Manag. 2016, 177, 291–297. [Google Scholar]
- Khan, M.Z.; Azom, M.G.; Sultan, M.T.; Mandal, S.; Islam, M.A.; Khatun, R.; Billah, S.M.; Ali, A.H.M.Z. Amelioration of Saline Soil by the Application of Gypsum, Calcium Chloride, Rice Husk and Cow Dung. J. Agric. Chem. Environ. 2019, 8, 78–91. [Google Scholar]
- Green, H.; Larsen, P.; Koci, J.; Edwards, W.; Nelson, P.N. Long-term effects of gypsum on the chemistry of sodic soils. Soil Tillage Res. 2023, 233, 105780. [Google Scholar]
- Toma, M.; Sumner, M.E.; Weeks, G.; Saigusa, M. Long-term Effects of Gypsum on Crop Yield and Subsoil Chemical Properties. Soil Sci. Soc. Am. J. 1999, 63, 891–895. [Google Scholar]
- Sharma, M.K.; Kumar, M. Sulphate contamination in groundwater and its remediation: An overview. Environ. Monit. Assess. 2020, 192, 74. [Google Scholar]
- Jalali, M.; Rowell, D.L. Potassium leaching in undisturbed soil cores following surface applications of gypsum. Environ. Geol. 2009, 57, 41–48. [Google Scholar]
- Dubrovina, T.A.; Losev, A.A.; Karpukhin, M.M.; Vorobeichik, E.L.; Dovletyarova, E.A.; Brykov, V.A.; Brykova, R.A.; Ginocchio, R.; Yáñez, C.; Neaman, A. Gypsum soil amendment in metal-polluted soils—An added environmental hazard. Chemosphere 2021, 281, 130889. [Google Scholar]
- Choi, Y.; Ganzorig, M.; Lee, K. Analysis of the Genomes and Adaptive Traits of Skermanella cutis sp. nov., a Human Skin Isolate, and the Type Strains Skermanella rosea and Skermanella mucosa. Microorganisms 2025, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Shivaramu, S.; Tomasch, J.; Kopejtka, K.; Nupur; Saini, M.K.; Bokhari, S.N.H.; Küpper, H.; Koblížek, M. The Influence of Calcium on the Growth, Morphology and Gene Regulation in Gemmatimonas phototrophica. Microorganisms 2023, 11, 27. [Google Scholar]
- Chen, Y.; Yin, S.; Shao, Y.; Zhang, K. Soil bacteria are more sensitive than fungi in response to nitrogen and phosphorus enrichment. Front. Microbiol. 2022, 13, 999385. [Google Scholar]
- Jumpponen, A.; Jones, K.L. Tallgrass prairie soil fungal communities are resilient to climate change. Fungal Ecol. 2014, 10, 44–57. [Google Scholar]
- Wan, Q.; Li, L.; Liu, B.; Zhang, Z.; Liu, Y.; Xie, M. Different and unified responses of soil bacterial and fungal community composition and predicted functional potential to 3 years’ drought stress in a semiarid alpine grassland. Front. Microbiol. 2023, 14, 1104944. [Google Scholar]
- Jaeger, A.C.H.; Hartmann, M.; Six, J.; Solly, E.F. Contrasting sensitivity of soil bacterial and fungal community composition to one year of water limitation in Scots pine mesocosms. FEMS Microbiol. Ecol. 2023, 99, fiad051. [Google Scholar]
- D’Hose, T.; Molendijk, L.; Van Vooren, L.; van den Berg, W.; Hoek, H.; Runia, W.; van Evert, F.; ten Berge, H.; Spiegel, H.; Sandèn, T.; et al. Responses of soil biota to non-inversion tillage and organic amendments: An analysis on European multiyear field experiments. Pedobiologia 2018, 66, 18–28. [Google Scholar]
- Pelosi, C.; Pey, B.; Hedde, M.; Caro, G.; Capowiez, Y.; Guernion, M.; Peigné, J.; Piron, D.; Bertrand, M.; Cluzeau, D. Reducing tillage in cultivated fields increases earthworm functional diversity. Appl. Soil Ecol. 2014, 83, 79–87. [Google Scholar]
- Zhang, L.; Ge, A.-H.; Tóth, T.; Yang, F.; Wang, Z.; An, F. Enrichment of keystone fungal taxa after flue gas desulphurization gypsum application drives reclamation of the saline-sodic soil. Land Degrad. Dev. 2023, 34, 2276–2287. [Google Scholar]
- Lu, G.; Feng, Z.; Xu, Y.; Jin, Y.; Zhang, G.; Hu, J.; Yu, T.; Wang, M.; Liu, M.; Yang, H.; et al. Impact of Phosphogypsum Application on Fungal Community Structure and Soil Health in Saline–Alkali-Affected Paddy Fields. Agronomy 2023, 13, 2726. [Google Scholar] [CrossRef]
- Zhao, H.; Zheng, W.; Zhang, S.; Gao, W.; Fan, Y. Soil microbial community variation with time and soil depth in Eurasian Steppe (Inner Mongolia, China). Ann. Microbiol. 2021, 71, 21. [Google Scholar]
- Fernandez, M.R.; Abdellatif, L.; Lokuruge, P.; Schellenberg, M.P.; Lupwayi, N.Z. Root disease and fungal populations in organic crops under different tillage–cropping systems. Crop Sci. 2022, 62, 1288–1304. [Google Scholar]
- Jenkinson, P.; Parry, D. Isolation of Fusarium Species from Common Broad-Leaved Weeds and Their Pathogenicity to Winter-Wheat. Mycol. Res. 1994, 98, 776–780. [Google Scholar]

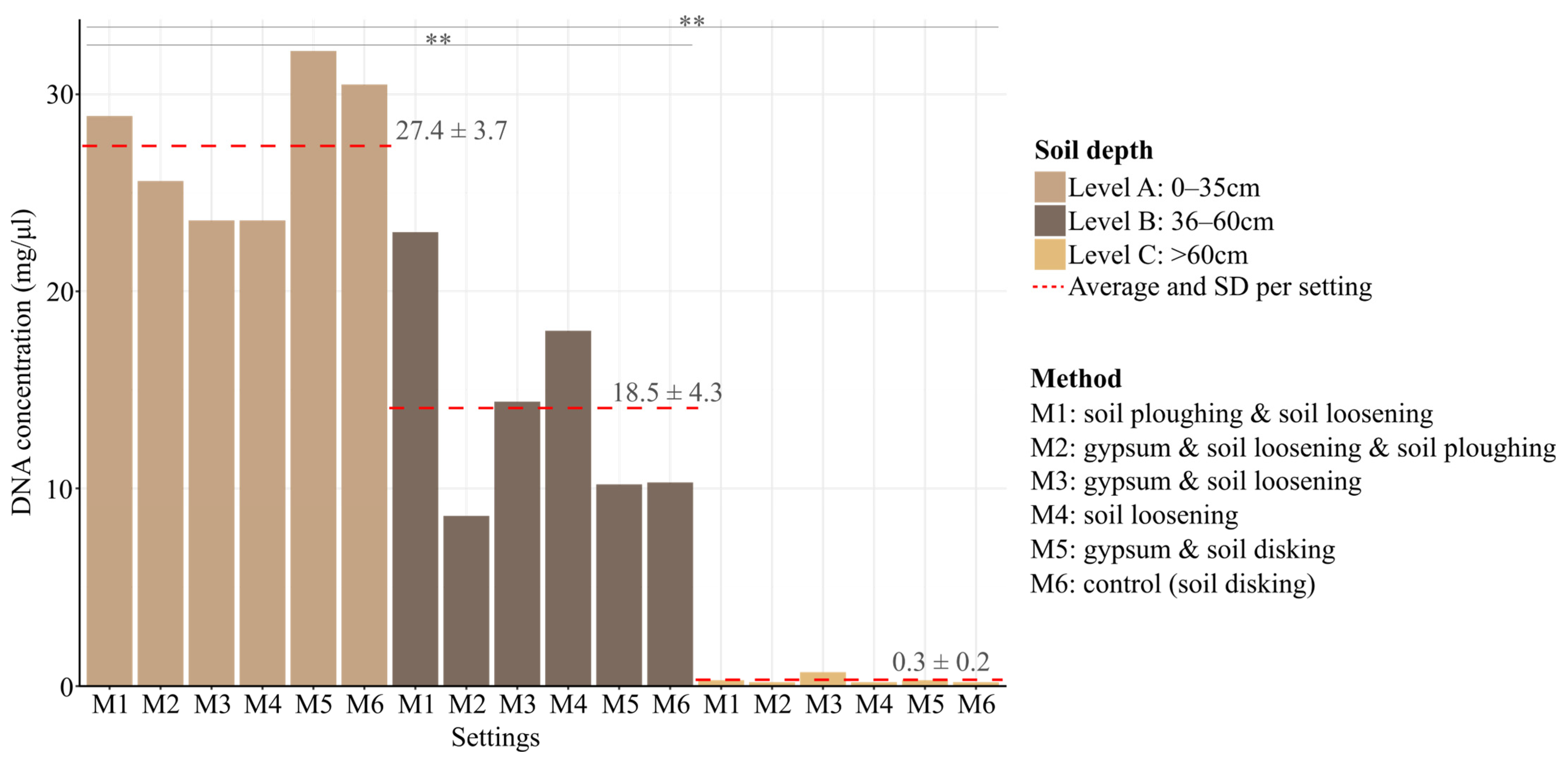
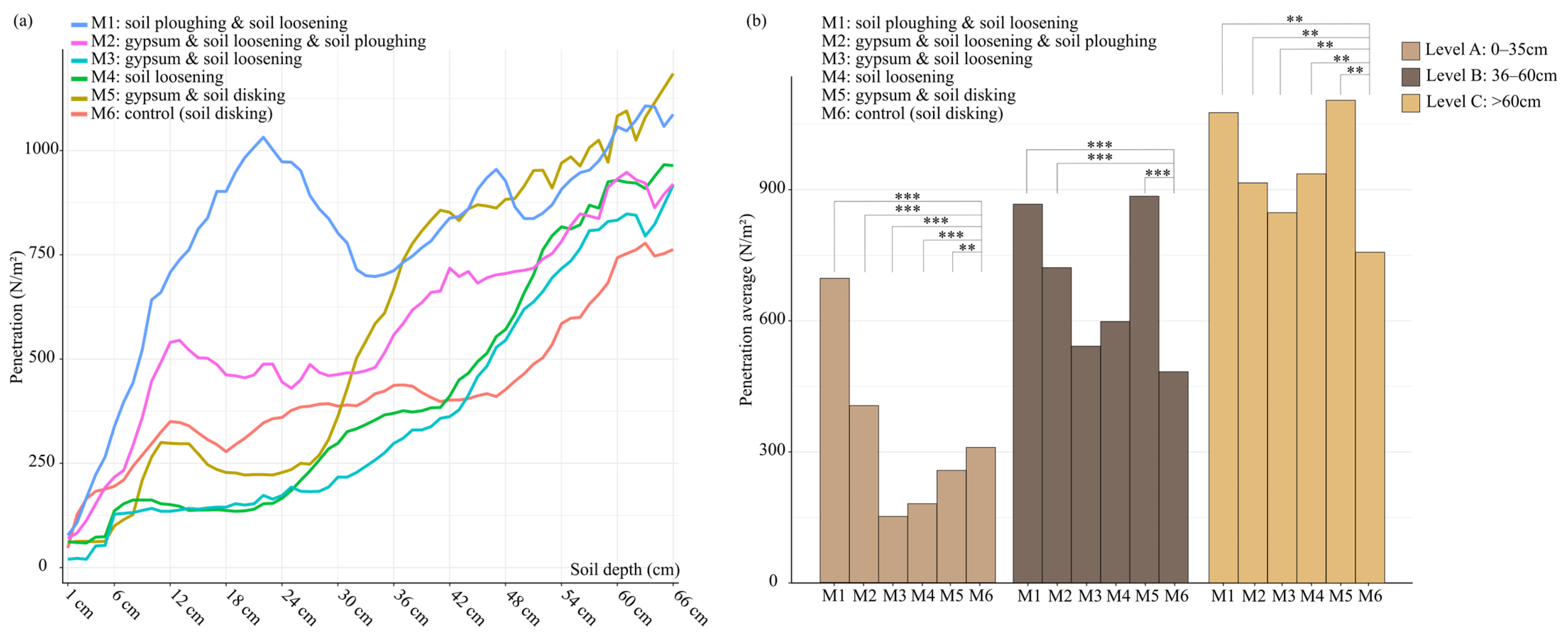
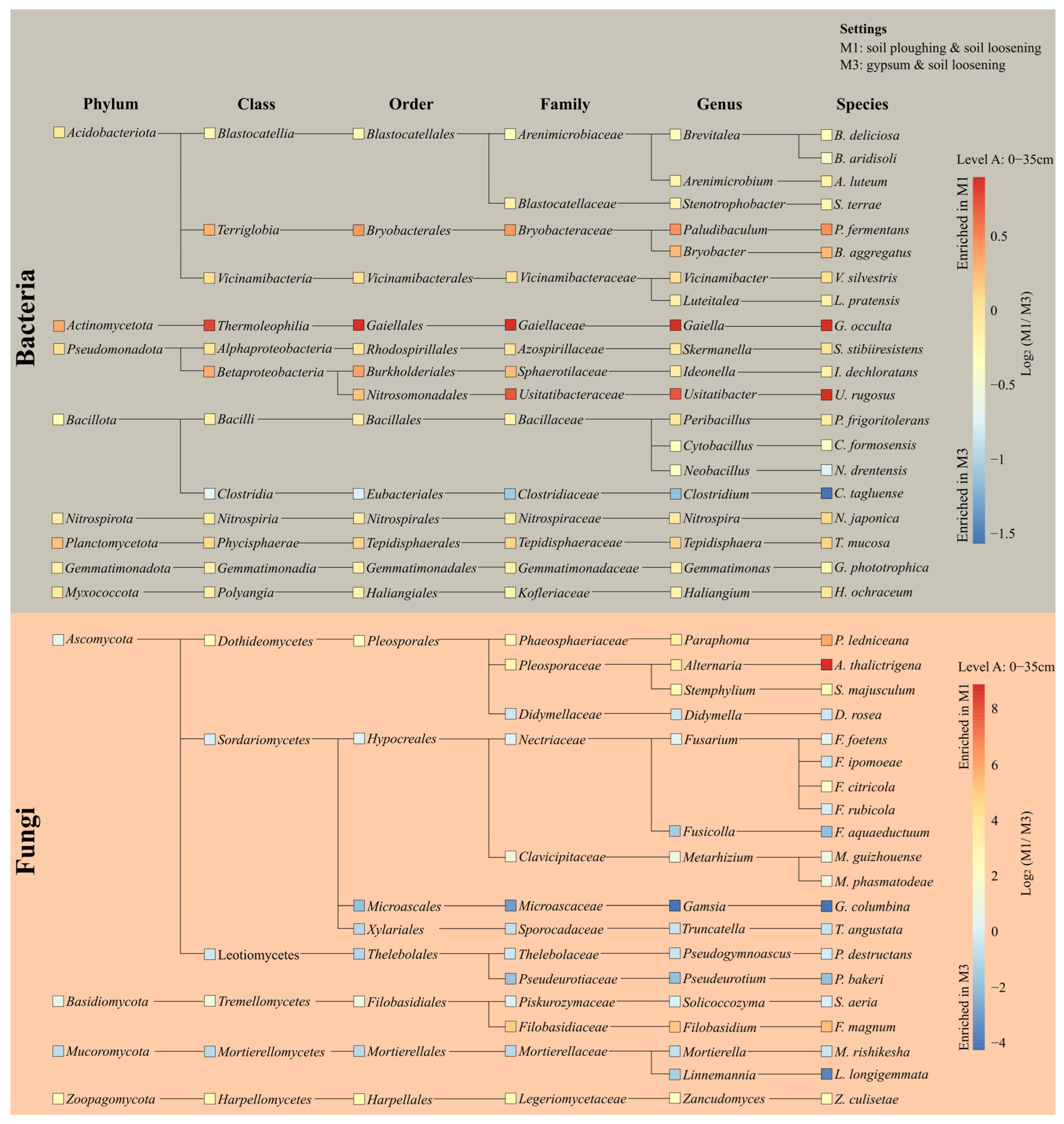

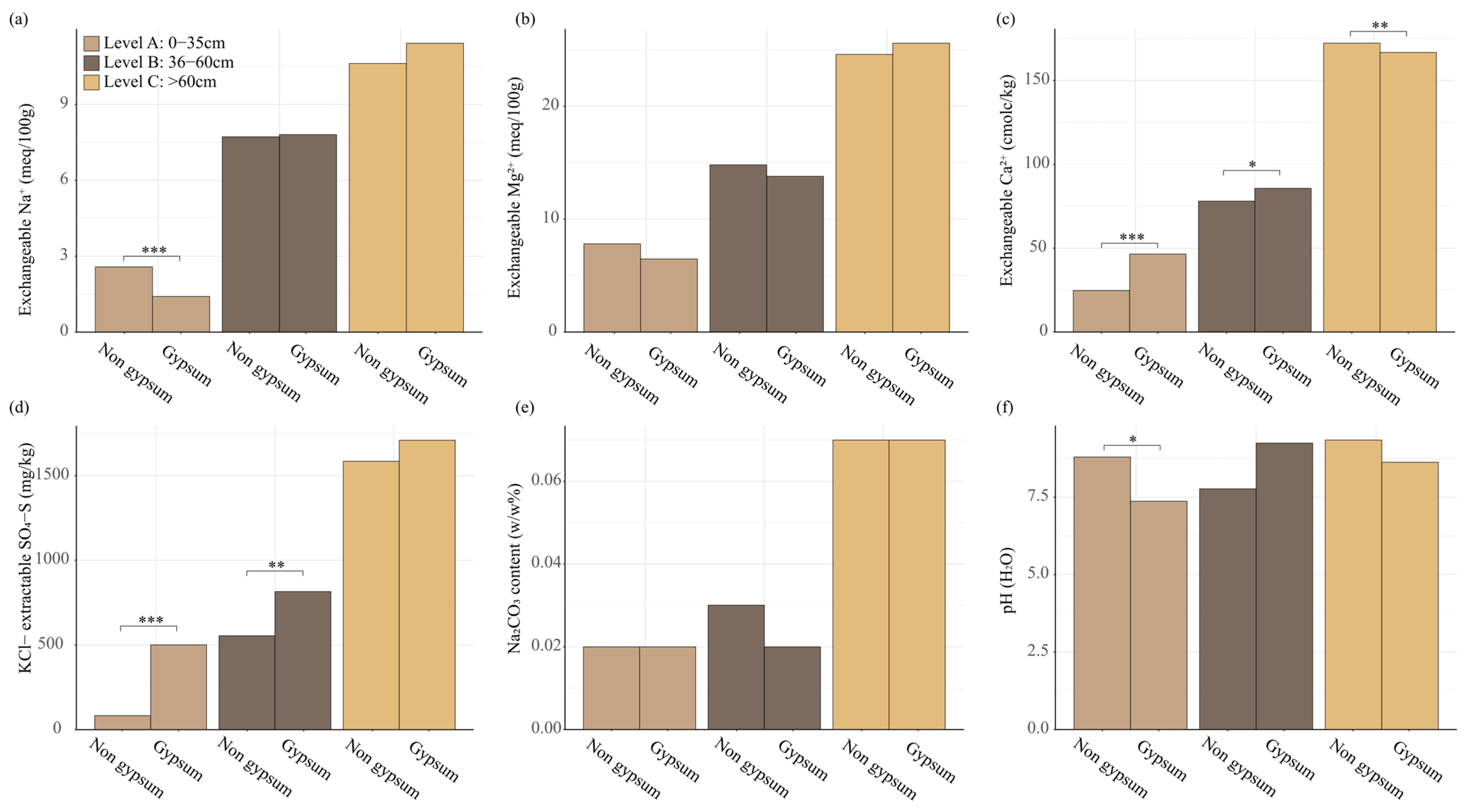
| Soil Properties Analyzed 1 | Measured Parameters | ||
|---|---|---|---|
| A Soil Level | B Soil Level | C Soil Level | |
| Arany’s plasticity index | 56 | 73 | 80 |
| Bulk density (g/cm3) | 1.367 | 1.485 | 1.472 |
| Humus content (w/w%) | 2.07 | 1.17 | 0.46 |
| Na2CO3 content (w/w%) | <0.2 | 0.033 | 0.72 |
| CaCO3 content (w/w%) | 0.086 | 3.8 | 7.16 |
| pH (KCl) | 5.75 | 7.73 | 8.11 |
| pH (H2O) | 7.25 | 8.37 | 9.05 |
| Exchangeable K+ (meq/100 g) | 0.532 | 0.522 | 0.501 |
| Exchangeable Mg2+ (meq/100 g) | 5.43 | 12.11 | 17.8 |
| Exchangeable Ca2+ (cmolc/kg) | 16.51 | 84.5 | 171 |
| Exchangeable Na+ (meq/100 g) | 1.87 | 5.11 | 8.22 |
| Parameters 16S | Metrics |
|---|---|
| Total yield | 2.91 Gb |
| Read length | N50 = 1620 bp |
| Number of reads | |
| Total | 1,770,000 |
| Pass | 1,248,244 |
| Average reads per sample (min–max) | 104,020 (45,146–227,130) |
| Reads mapped to reference | 1,245,630 |
| Average reads per amplicon (min–max) | 1583 (134.3–18,265.5) |
| Mean qscore | 9.7 |
| Parameters ITS | Metrics |
|---|---|
| Total yield | 2.27 Gb |
| Read length | N50 = 927 bp |
| Number of reads | |
| Total | 2,140,000 |
| Pass | 1,612,448 |
| Average reads per sample (min–max) | 134,371 (49,696–175,370) |
| Reads mapped to reference | 1,575,532 |
| Average reads per amplicon (min–max) | 963.3 (112.7–9437.3) |
| Mean qscore | 13.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gashi, N.; Szőke, Z.; Czakó, A.; Fauszt, P.; Dávid, P.; Mikolás, M.; Stündl, L.; Gál, F.; Remenyik, J.; Sándor, Z.; et al. Gypsum and Tillage Practices for Combating Soil Salinity and Enhancing Crop Productivity. Agriculture 2025, 15, 658. https://doi.org/10.3390/agriculture15060658
Gashi N, Szőke Z, Czakó A, Fauszt P, Dávid P, Mikolás M, Stündl L, Gál F, Remenyik J, Sándor Z, et al. Gypsum and Tillage Practices for Combating Soil Salinity and Enhancing Crop Productivity. Agriculture. 2025; 15(6):658. https://doi.org/10.3390/agriculture15060658
Chicago/Turabian StyleGashi, Njomza, Zsombor Szőke, Antal Czakó, Péter Fauszt, Péter Dávid, Maja Mikolás, László Stündl, Ferenc Gál, Judit Remenyik, Zsolt Sándor, and et al. 2025. "Gypsum and Tillage Practices for Combating Soil Salinity and Enhancing Crop Productivity" Agriculture 15, no. 6: 658. https://doi.org/10.3390/agriculture15060658
APA StyleGashi, N., Szőke, Z., Czakó, A., Fauszt, P., Dávid, P., Mikolás, M., Stündl, L., Gál, F., Remenyik, J., Sándor, Z., & Paholcsek, M. (2025). Gypsum and Tillage Practices for Combating Soil Salinity and Enhancing Crop Productivity. Agriculture, 15(6), 658. https://doi.org/10.3390/agriculture15060658







