Abstract
This study investigates the recovery of bioactive polyphenolic compounds from the pomace of two white winemaking grape varieties, Moschofilero and Rhoditis. The pomace was subjected to two drying techniques: air drying (AD) and solar drying (SD). Extraction methods included microwave-assisted extraction (MW), ultrasound-assisted extraction (US), and Soxhlet extraction (S), using water and water–ethanol (WE) solvents. Antioxidant activity (IC50), total phenolic content (TPC), and total flavan-3-ol content (TFC) were determined. For Moschofilero, SD pomace extracted with US-WE showed the highest antioxidant activity (IC50: 0.59 mg/mL) and the highest phenolic recovery (TPC: 285.76 mg gallic acid equivalents (GAE)/g) and flavan-3-ol content (TFC: 46.21 mg catechin equivalents (CE)/g). For Rhoditis, AD pomace extracted with US-WE demonstrated superior antioxidant activity (IC50: 1.08 mg/mL), phenolic content (TPC: 216.51 mg GAE/g), and flavan-3-ol content (TFC: 35.96 mg CE/g). HPLC analysis identified quercetin-3-glucuronide, myricetin, and quercetin as the main flavonols in both grape varieties, with Moschofilero also containing isorhamnetin-3-glucoside and syringetin-3-glucoside. Drying and extraction methods significantly influenced the recovery of bioactive compounds, with US combined with AD or SD yielding the best results for both grape varieties These findings show that improved drying and extraction methods can add value to grape pomace for use in functional foods and nutraceuticals.
1. Introduction
Vitis vinifera L. is one of the most widely cultivated fruit crops globally, with over 70% of the grape harvest dedicated to wine and grape juice production. In 2022, global grape production reached approximately 75 million tons, with Europe accounting for over 60% of the total output. Notably, five European countries—France, Italy, Spain, Portugal, and Greece—are key contributors to global wine production [1]. In this context, the wine industry generates substantial byproducts, with grape pomace constituting around 20–30% of the total grape weight used during vinification [2]. The environmental and economic challenges posed by these byproducts have driven efforts to explore their valorization as a source of bioactive compounds, including polyphenols, which have significant applications in the pharmaceutical, cosmetic, and food industries.
White wine vinification results in a byproduct known as grape pomace, composed primarily of grape skins, seeds, and stems. This material, often discarded as waste or repurposed for animal feed, compost, or ethanol production, retains a substantial proportion of the grapes’ original phenolic compounds, as the extraction process during fermentation is incomplete [3]. Recent studies highlight grape pomace as a rich source of polyphenols, including flavonoids (e.g., flavonols, flavan-3-ols, anthocyanins, and flavanones) and non-flavonoid polyphenols such as phenolic acids (e.g., hydroxycinnamic acids and hydroxybenzoic acids) and stilbenes (e.g., trans-resveratrol) [4].
Due to the seasonal nature of grape harvesting and winemaking, large quantities of grape pomace are produced in a short period of time each year. This makes it essential to apply preservation methods, such as drying, to stabilize the pomace for long-term storage and prevent microbial growth and degradation of valuable bioactive compounds. Drying is one of the most common preservation methods used to extend the shelf life of agricultural byproducts like grape pomace [5]. Air drying (AD) and solar drying (SD) are widely applied due to their simplicity and low cost; however, the drying process can significantly influence the polyphenolic composition of the pomace [6].
Heat-sensitive compounds, such as polyphenols, can degrade during drying, especially when high temperatures are used. Studies have shown that mild drying conditions are preferable to preserve the antioxidant capacity and phenolic content of grape pomace [7]. Therefore, the choice of drying treatment plays a crucial role in optimizing the preservation and subsequent recovery of bioactive compounds from grape pomace, which is valuable for producing high-quality extracts for nutraceuticals, cosmetics, and other functional applications [6,8].
Extracting bioactive compounds from grape pomace is a critical step in utilizing these byproducts. Traditional extraction methods, such as Soxhlet extraction (S), are often inefficient, requiring large amounts of solvent and extended extraction times [9]. In recent years, microwave-assisted extraction (MW) and ultrasound-assisted extraction (US) have gained significant attention as innovative techniques for the efficient recovery of bioactive compounds from grape pomace, including that from white wine production. These methods improve polyphenol extraction yields while being more eco-friendly and time-efficient than Soxhlet extraction [10,11,12]. The growing interest in these methods stems from their potential to improve the sustainability of industrial processes while ensuring higher recovery of valuable compounds like flavonols, flavan-3-ols, and phenolic acids.
MW has been extensively studied for its ability to extract phenolic compounds from grape pomace with minimal degradation of heat-sensitive compounds [13]. Several studies have demonstrated that MAE can efficiently extract a wide range of bioactive compounds from white grape pomace, including flavonols (e.g., quercetin, kaempferol, and myricetin) and phenolic acids (e.g., gallic acid and ferulic acid) [14]. For example, Brahim et al. (2014) demonstrated that the optimized microwave-assisted extraction in water, with or without sodium carbonate, significantly increased phenolic compound yields from grape residues compared to traditional methods. This makes it an effective alternative for extracting polyphenolics from grape pomace and marcs [15]. Similarly, Moutinho et al. (2023) optimized the extraction of bioactive compounds from grape pomace using MW, identifying key molecules such as phenolic acids, flavonoids, and anthocyanins, with significant antioxidant activity. These results further support the use of efficient extraction methods for recovering valuable phytochemicals from agro-industrial byproducts [16].
Similarly, US has shown great promise for the extraction of polyphenols from grape pomace. US is particularly noted for its ability to efficiently extract flavan-3-ols (e.g., catechin, epicatechin, and proanthocyanidins), which are present in significant quantities in both white and red grape pomace [17]. For instance, Liu et al. (2024) demonstrated that US, optimized for efficiency and energy savings, is a more suitable method for recovering high-value bioactive compounds from grape marc compared to conventional extraction methods [18]. In another study, González et al. (2020) demonstrated that US increased the yield of total polyphenols and total monomeric anthocyanins in both fresh and freeze-dried grape pomace by 50% and 180%, respectively, when compared to conventional extraction methods. Additionally, the study highlighted US’ ability to maintain the antioxidant capacity of the extracts, making it a valuable technique for the valorization of grape pomace in various industries [19]. Another study by Ferri et al. (2017) focused on white grape pomace and confirmed that US, combined with ethanol-based extraction, effectively released polyphenols, which exhibited strong antioxidant, anti-tyrosinase, and anti-inflammatory activities [20].
These findings underline the growing interest in MW and US for the valorization of white grape pomace as a sustainable source of polyphenols with potential uses in the food, nutraceutical, and cosmetic industries. The integration of these modern extraction techniques with grape pomace treatment not only enhances the recovery of bioactive compounds but also aligns with the industry’s focus on green, sustainable processes. Moreover, the promising results from studies on white grape pomace suggest that MW and US could further be optimized for specific industrial applications, contributing to the creation of high-value products from agricultural byproducts [3].
Moschofilero and Rhoditis, two significant Greek white winemaking grape varieties, represent a substantial portion of the country’s white wine production and are known for their distinctive polyphenolic profiles. Despite their economic and cultural importance, these varieties remain underexplored in terms of their polyphenolic composition and the impact of processing techniques on the recovery of bioactive compounds. While extensive research has been conducted on polyphenol extraction from grape pomace, studies addressing the specific impact of drying methods remain scarce [7,21,22,23], and no previous work has systematically examined Moschofilero and Rhoditis. This study addresses this critical gap by systematically evaluating the influence of drying techniques (air drying and solar drying) in combination with advanced extraction methods, namely MW and US, on the recovery of polyphenols from Moschofilero and Rhoditis grape pomace. Unlike prior research, this study provides the first comprehensive assessment of varietal-specific differences in polyphenolic composition and their response to drying treatments. Additionally, this study provides novel insights into how drying treatments and advanced extraction techniques can optimize bioactive compound recovery, contributing to a more targeted and sustainable approach to grape pomace valorization. These findings contribute novel insights into sustainable and industrially applicable methods for valorizing grape pomace, offering practical applications in the food, nutraceutical, and cosmetic industries.
This study explores the effects of two drying techniques, air drying and solar drying, on the recovery of bioactive compounds from the pomace of two Greek white winemaking grape varieties, Moschofilero and Rhoditis. The grape pomace underwent extraction using both US and MW, with Soxhlet extraction (S) serving as a reference method. The extraction efficiency was evaluated by measuring antioxidant activity (IC50), total phenolic content (TPC), and total flavan-3-ol content (TFC). The most effective extracts were further analyzed using HPLC analysis to identify the predominant flavonols. This approach provides valuable insights into optimizing the recovery of high-value compounds from grape pomace, with potential applications in various industries, including nutraceuticals, cosmetics, and food products.
2. Materials and Methods
2.1. Plant Material
The pomace used in this study was obtained from Moschofilero and Rhoditis (Vitis vinifera L.) grapes, harvested in 2022 and donated by Oinopraxia Voiotias—INO S.A. (Thiva, Central Greece). Rhoditis is a pink-skinned Greek wine grape, while Moschofilero grapes have a gray/purple colored skin. The pomace samples were collected after typical white winemaking processes that utilized a pneumatic press with three distinct cycles. The free-run juice was collected without applied pressure and used for premium wine production. In the first press cycle, a pressure of 0.5–0.6 bar was applied to extract high-quality juice for standard white wine production. Subsequently, a second press cycle was performed with pressures reaching 1.7–1.8 bar, producing juice intended for lower-quality wines, such as bulk or bag-in-box products. The pomace was collected immediately after the completion of the second press cycle, ensuring consistency in the degree of juice exhaustion. No fermentation or maceration processes occurred prior to the collection of the pomace, preserving its phenolic content. The moisture content of the fresh pomace was 62.42 ± 3.30% for Rhoditis and 65.60 ± 2.29% for Moschofilero. To preserve its phenolic integrity, the pomace was immediately transferred to insulated containers on-site and stored at −30 °C to prevent enzymatic degradation of polyphenols prior to further use.
2.2. Chemicals
All reagents and solvents used for the extraction processes were of analytical grade, while those employed for the analytical procedures were of HPLC grade. Gallic acid, Folin–Ciocalteu reagent, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were obtained from Sigma–Aldrich (St. Louis, MO, USA), and p-dimethylaminocinnamaldehyde (DMACA) was sourced from Acros Organics (Morris Plains, NJ, USA). Additional chemicals such as water, acetonitrile, ethanol, methanol, sodium carbonate (Na2CO3), and hydrochloric acid (32% (w/w)) were supplied by Fisher Scientific (Leicestershire, UK).
2.3. Drying Treatment of Grape Pomace
Grape pomace samples from Moschofilero and Rhoditis were subjected to two drying methods—air drying and accelerated solar drying—to evaluate the impact of the drying procedure on the material. For the AD process, the pomace samples were dried at 55 ± 2 °C with an air velocity of 1.0 m/s for 24 h under atmospheric pressure, using an experimental air dryer equipped with controls for airflow rate, heating, humidity, and drying test compartments. The samples were placed on perforated trays positioned perpendicular to the airflow. In the SD process, the pomace samples were dried for 24 h using a laboratory-scale artificial solar dryer, which delivered a total light intensity of 1000 W·m−2 via eight 300 W OSRAM-ultravitalux lamps, producing an energy spectrum similar to natural sunlight. All drying experiments were conducted in triplicate, and the moisture content of the samples was monitored throughout the process to determine drying kinetics. The dried samples were subsequently stored in plastic containers at −30 °C until further analysis.
Drying Kinetics and Moisture Content
The moisture content of both fresh and dried grape pomace was determined using a vacuum oven (Sanyo Gallenkamp PLC, Leicester, UK) at 70 ± 0.2 °C, following the procedure recommended by AOAC (1980) [24]. The samples were dried until a constant weight was achieved. The moisture content (MC) was calculated on a wet basis using the following equation:
where MC is the moisture content on wet basis (g/g), mw is the wet weight of the sample (g) and md is the dry weight of the sample (g). All experiments were conducted in triplicate.
To determine the drying kinetics, a first-order kinetic model was applied:
where X is the material moisture content on dry basis during drying (kg water/kg dry solids), Xe is the equilibrium moisture content of dehydrated material (kg water/kg dry solids), k is the drying rate (min−1), and t is the time of drying (min) [25].
The drying rate was calculated from the slope of the falling rate-drying curve. At time zero, the initial moisture content Xi is equal to the dry material’s moisture content, and by integrating Equation (2), the following equation is derived:
2.4. Extraction of Grape Pomace
Fresh and dried pomace samples were subjected to three different extraction techniques: MW, US, and conventional Soxhlet extraction (S). All experiments were conducted in triplicate. Prior to extraction, the dried pomace samples were ground using a knife mill (Pulverisette 11, Fritsch, Thessaloniki, Greece) to obtain fractions with different particle sizes. The ground samples were then sieved manually using 500 μm sieves to ensure uniform particle size distribution for the subsequent extraction processes.
2.4.1. Microwave-Assisted Extraction (MW)
MW was performed using a XO-SM50 Ultrasonic Microwave Reaction System (Nanjing Xianou Instruments Manufacture Co., Ltd., Nanjing, China). A 2 g sample of fresh or dried grape pomace was extracted with 100 mL of either water or a water–ethanol (1:1) mixture. The temperature was set to 50 °C, while the system operated at a power of 200 W for a total duration of 60 min. Temperature was continuously recorded in real-time using the system’s built-in thermometer, ensuring precise monitoring and automatic adjustment of microwave irradiation cycles to maintain stable extraction conditions.
2.4.2. Ultrasound-Assisted Extraction (US)
US was conducted using the same XO-SM50 Ultrasonic Microwave Reaction System. Samples of fresh and dried grape pomace (1 g) were placed in a beaker containing 70 mL of the chosen solvent (either water or water–ethanol (1:1) mixture). The ultrasonic probe operated at a fixed frequency of 25 kHz with a power output of 300 W to ensure consistent sonication conditions. The extraction process lasted for 60 min, with the temperature maintained at 20 °C using a cooling bath to ensure thermal stability during the procedure.
2.4.3. Soxhlet Extraction (S)
A Soxhlet extraction apparatus, comprising a 100 mL extraction flask, a Soxhlet chamber, and a condenser, was utilized for the extraction of fresh or dried grape pomace samples. For each extraction, 3 g of Rhoditis or Moschofilero pomace was placed in the extraction thimble, and 100 mL of the chosen solvent (either ethanol or water) was added to the flask. The extraction process involved refluxing the ethanol for 2–3 h and water for 5–6 h, completing ten full extraction cycles in each case.
2.5. Characterization of the Extracts
2.5.1. Extraction Yield (Y, %)
The extraction yield for each procedure was calculated as the percentage of the mass of the extracted material relative to the dry weight of the sample used in the extraction. This is represented by Equation (4):
2.5.2. Extraction Kinetics
The Peleg model was employed to describe the extraction kinetics from Moschofilero and Rhoditis grape pomace under different drying and extraction conditions. The extraction curves (Y (%) vs. time) were validated using the Peleg (1988) sorption model, which, in the case of solid–liquid extraction processes, assumes the following form:
where Y(t) is the yield of extract at time t (g extract/g grape pomace), Y0 is the initial yield of the extract at t = 0 (g extract/g grape pomace), t is the extraction time (min), K1 is the Peleg rate constant (min·g extract/g grape pomace), K2 is the Peleg capacity constant (g extract/g pomace), indicating the extraction capacity of the system over time [26].
The equation was fitted to the experimental data using non-linear regression to determine the values of K1 and K2 for each combination of drying and extraction methods. The model allows for the calculation of the equilibrium yield Ye, representing the maximum extractable yield and the initial extraction rate B0, calculated by the following equations:
The performance of the model was evaluated using the correlation coefficient (q) and the root mean square deviation (RMSD) between the predicted and experimental values. Higher values of q and lower RMSD values indicate a better fit of the model to the experimental data.
2.5.3. Antioxidant Activity (IC50)
The antioxidant activity of the grape pomace extracts was evaluated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging method, as described by Brand-Williams et al. (1995) [27]. In this assay, 0.1 mL of appropriately diluted grape pomace extracts were added to 3.9 mL of a DPPH solution (0.03 mg/mL in methanol). The reaction mixtures were vortexed and incubated in the dark for 30 min at room temperature (25 °C). After this incubation period, the absorbance of the samples was measured at 515 nm using a UV-Vis spectrophotometer (Bel Photonics M51, Monza, Italy). Methanol was used as a blank to zero the instrument.
The reduction in absorbance at 515 nm, corresponding to the scavenging of DPPH radicals by the extracts, was recorded, and the percentage of remaining DPPH radicals (% DPPH rem) was calculated using the following equation:
where At is the absorbance after 30 min of reaction, and At=0 is the initial absorbance of the DPPH solution.
The antioxidant activity of the extracts was further quantified by determining the IC50 value, which represents the concentration of extract required to scavenge 50% of the DPPH radicals. The IC50 value was determined by plotting the percentage of remaining DPPH against the concentration of the extract and applying a linear regression model. The concentration needed to achieve 50% inhibition was interpolated from the resulting equation. The IC50 is expressed as the ratio of the mass of extract solids to the initial mass of DPPH (g/g DPPH).
2.5.4. Total Phenolic Content (TPC)
The total phenolic content (TPC) of the Rhoditis and Moschofilero grape pomace extracts was determined using the Folin–Ciocalteu method. In brief, 100 μL of appropriately diluted extract was added to 7.9 mL of distilled water and 500 μL of Folin–Ciocalteu reagent in a test tube. The mixture was vortexed, and after 1.5 mL of saturated Na2CO3 solution was added, the samples were incubated at 40 °C for 30 min. Absorbance was measured at 765 nm using a UV-Vis spectrophotometer (Bel Engineering s.r.l., Monza, Italy). For further quantification, a gallic acid calibration curve was constructed by plotting absorbance versus known concentrations of gallic acid. The TPC of the extracts was calculated by interpolating the average absorbance values of the samples against the calibration curve, and the results were expressed as mg gallic acid equivalents (GAE) per gram of dry extract (mg GAE/g dry extract). All measurements were performed in triplicate to ensure accuracy.
2.5.5. Total Flavan-3-ol Content (TFC)
The total flavan-3-ol content (TFC) of the grape pomace extracts was determined using the DMACA method, as originally proposed by Vivas et al. (1994) [28]. In this procedure, 0.6 mL of appropriately diluted sample was mixed with 3 mL of DMACA solution (0.1% in 1 N HCl in methanol). The mixture was vortexed and allowed to incubate at room temperature for 10 min. After incubation, the absorbance was measured at 640 nm using a UV-Vis spectrophotometer (Bel Engineering s.r.l., Monza, Italy). A calibration curve was constructed using (+)-catechin as the standard, with concentrations ranging from 1 to 16 mg/L. The total flavan-3-ol content was calculated by interpolating the absorbance values of the samples against the calibration curve. Results were expressed as mg catechin equivalents (CE) per gram of dry extract (mg CE/g dry extract). All measurements were performed in duplicate to ensure accuracy.
2.5.6. HPLC Analysis
The identification and quantification of grape pomace flavonols was performed according to the method of Bimpilas et al. (2015) [29]. A Varian 212-LC chromatography system, coupled to an ion trap mass spectrometer equipped with an electrospray interface and a diode array detector, was used for the analyses. System control and data acquisition were performed using the Varian Workstation software version 6 (Varian Inc., Palo Alto, CA, USA) and coupled to Varian Workstation data processing software. The grape pomace extracts were injected after filtration (0.2 μm, PVDF syringe filters, Teknokroma, Barcelona, Spain) on a reversed-phase Hypersil C18 column (ODS 5 μm, 250 × 4.6 mm, MZ Analysentechnik, Mainz, Germany). The quantification of flavonols was based on their response at 360 nm, and expressed as quercetin equivalents, according to the calibration curve of the respective standard compound.
2.5.7. Statistical Analysis
All experiments were performed in triplicate, and the results are presented as mean ± standard deviation (SD). Statistical analyses were conducted using the StatSoft STATISTICA 12.0 software (Hamburg, Germany). Statistical analysis was performed using one-way ANOVA to assess the effects of extraction method and drying treatment on yield, IC50, TPC, and TFC. Differences between means were evaluated using analysis of variance (ANOVA) and Tukey’s post hoc test for significance (p < 0.05).
3. Results and Discussion
3.1. Drying Kinetics of Grape Pomace Through AD and SD
Figure 1a,b illustrate the drying kinetics of Moschofilero and Rhoditis grape pomace, respectively, using AD and SD treatments. The moisture content (g H2O/g dry matter) is plotted against the drying time (min), with the experimental (exp) and theoretical (theor) data for both drying methods. Based on Figure 1a, for Mosch-AD, the experimental data showed a rapid reduction in moisture content, with the initial moisture content, XoMosch-AD, at 1.92 g H2O/g dry matter. The drying rate constant kMosch-AD was 0.04 min−1, the equilibrium moisture content, XeMosch-AD, was 0.07 g H2O/g dry matter, and the correlation coefficient (R2) was 0.995, indicating a strong fit between the experimental data and the theoretical model. For Mosch-SD, the experimental data showed a slower drying rate compared to air drying, with a drying rate constant, kMosch-SD, of 0.01 min−1 and an initial moisture content, XoMosch-SD, of 2.13 g H2O/g dry matter. The equilibrium moisture content (XeMosch-SD) was 0.06 g H2O/g dry matter, with a similarly high correlation coefficient (R2 = 0.993), indicating a good fit with the theoretical model. Based on Figure 1b, for Rho-AD, the experimental data indicated a relatively rapid moisture removal with an initial moisture content (XoRho-AD) of 1.68 g H2O/g dry matter. The drying rate constant kRho-AD was 0.05 min−1, and the equilibrium moisture content (XeRho-AD) was 0.06 g H2O/g dry matter. The high correlation coefficient (R2 = 0.994) shows a strong fit between the experimental and theoretical data. For Rho-SD, the experimental data showed a slower drying process compared to AD with a drying rate constant, kRho-SD, of 0.01 min−1 and an initial moisture content (XoRho-SD) of 1.75 g H2O/g dry matter. The equilibrium moisture content (XeRho-SD) for AD was 0.09 g H2O/g dry matter, with a correlation coefficient of 0.997, indicating a good fit with the theoretical model. The results obtained in this study are consistent with findings from other research evaluating the drying kinetics of grape pomace. Particularly, the drying rate constant values in this study align with previously reported values for grape pomace [23].
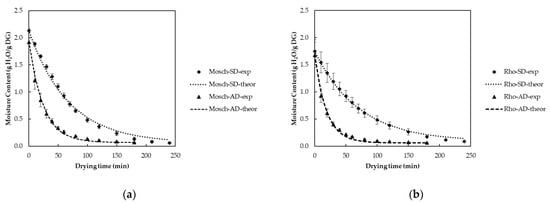
Figure 1.
Drying kinetic plots of (a) Moschofilero and (b) Rhoditis grape pomace through AD and SD.
In comparing the drying kinetics of Moschofilero and Rhoditis grape pomace, it is evident that AD resulted in faster moisture removal compared to SD for both varieties. Moschofilero exhibited a slightly higher initial moisture content than Rhoditis under both drying treatments. However, Rhoditis demonstrated a higher drying rate constant under AD than Moschofilero, indicating that Rhoditis dried more rapidly under these conditions. In contrast, under SD, both varieties exhibited similar drying rate constants, suggesting that SD was similarly effective for both pomace types but significantly slower than AD.
3.2. Extraction Kinetics of Grape Pomace Through MAE and UAE
For Moschofilero grape pomace extracts, the extraction yield varied from 10.12 ± 0.40 to 19.38 ± 0.60, 8.20 ± 0.24 to 18.61 ± 0.58, and 10.40 ± 0.32 to 20.21 ± 0.63 g extract/100 g of dry material for AD, SD, and F samples, respectively. Figure 2 presents the extraction yield (% d.b) kinetic plots of Moschofilero extracts recovered using MW and US for fresh and dried samples. Based on these results, drying treatment significantly influences the extraction yield in Moschofilero grape pomace (p < 0.05). This trend aligns with studies by Sokač et al. (2022), which demonstrated that drying methods such as vacuum drying and open sun drying enhance the concentration of bioactive compounds. The increased extraction yields are primarily attributed to moisture reduction, which improves extraction efficiency [22]. Furthermore, Peternel et al. (2024) found that dried grape pomace, especially when processed under controlled drying conditions, leads to improved extraction yields compared to undried samples, due to the higher concentration of extractable compounds [30].
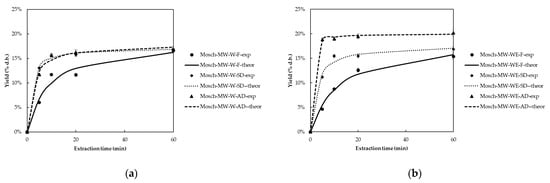
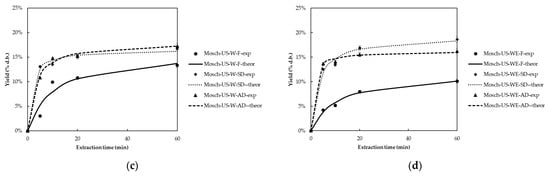
Figure 2.
Extraction yield (% d.b) kinetic plots of fresh (F) and dried (SD, AD) Moschofilero grape pomace using (a) MW and water (W), (b) MW and water:ethanol (1:1) (WE), (c) US and water (W), and (d) US and water:ethanol (1:1) (WE) as solvent.
The choice of extraction method had a substantial impact on the extraction yield. Among extraction methods, MW-WE and US-WE provided the highest yields, with MW-WE being more effective in AD samples (20.21%) and US-WE in SD samples (18.61%) (p < 0.05). These results are in agreement with findings from Ameer et al. (2017), which showed that MW and US techniques enhance the extraction efficiency of polyphenolic compounds from plant matrices by improving cell wall disruption, leading to faster and more efficient extraction [31].
The Peleg model parameters for Moschofilero grape pomace, as presented in Table 1, illustrate how the drying and extraction methods significantly affect the extraction kinetics and efficiency. Peleg’s rate constant (K1), which represents the initial extraction rate, was notably lower for AD and SD samples compared to F samples across all extraction methods. For instance, for MW-WE extraction, the K1 value for AD samples was 1.83 min g grape pomace/g extract, indicating a faster initial extraction rate than for fresh samples, where K1 was 84.47 min g grape pomace/g extract. This suggests that drying enhances the accessibility of bioactive compounds for extraction. In particular, the drying process promotes the breakdown of cell walls, making the compounds more accessible, while the grinding of samples increases the surface area, further facilitating extraction. Peleg’s capacity constant (K2), which is related to the maximum extraction yield, was lower for AD and SD samples. In general, lower K2 values are preferred because they signify a greater extraction yield, meaning more bioactive compounds are efficiently recovered before reaching saturation. For example, in US-WE extraction, K2 for AD samples was 6.14 g grape pomace/g extract, indicating a higher extraction capacity compared to fresh samples, which had a K2 of 8.36 g grape pomace/g extract. This suggests that drying treatments improve the maximum yield of extractable compounds. The initial extraction rate (B0) is another important parameter, with dried samples showing consistently higher B0 values, further demonstrating the enhanced extraction efficiency of dried samples. The equilibrium yield (Ye), representing the final extract yield, was also significantly higher in dried samples. In US-WE extraction, for example, SD samples reached a Ye of 0.19 g extract/g grape pomace, compared to 0.12 g extract/g grape pomace for fresh samples. This indicates that AD and SD allows for a greater total extraction yield, which aligns with prior studies that emphasize the efficiency of drying in improving the recovery of phenolic compounds [5]. Additionally, based on the results, among extraction methods, both MW and US extraction techniques are effective, but their relative performance depends on the specific combination of solvent, extraction method, and drying treatment. Finally, the correlation coefficient (q) values for all models were consistently close to 1.00, demonstrating a strong fit of the Peleg model to the experimental data. The RMSD values were very low (ranging from 0.00 to 0.01), further confirming the accuracy of the model in predicting extraction kinetics. These results demonstrate that drying enhances both the rate and yield of polyphenol extraction from Moschofilero grape pomace.

Table 1.
Peleg’s rate constant K1 (min g grape pomace/g extract), Peleg’s capacity constant K2 (g grape pomace/g extract), extraction rate at the very beginning B0 (min g extract/g grape pomace), equilibrium yield of the extract obtained from grape pomace (g extract/g grape pomace) (Ye), correlation coefficient (q), and root-mean-squared deviation (RMSD) for Moschofilero and Rhoditis grape pomace using different extraction and drying methods.
For Rhoditis grape pomace extracts, the extraction yield varied from 16.70 ± 0.31 to 25.07 ± 0.63, from 13.70 ± 0.22 to 23.93 ± 0.62, and from 10.80 ± 0.35 to 25.60 ± 0.64 g extract/100 g of dry material for AD, SD, and F samples, respectively. Figure 3 presents the extraction yield (% d.b) kinetic plots of Rhoditis extracts recovered using MW and US for fresh and dried samples. Based on these results, the drying treatment significantly impacts the extraction yield (p < 0.05). AD samples consistently exhibited higher extraction yields compared to both SD and F samples. This increase in Y for dried samples is primarily due to the reduction in moisture content, which facilitates improved solvent penetration into the plant matrix and enhances the release of bound phenolic compounds. These results align with studies by Sokač et al. (2022), who highlighted that drying enhances the recovery of polyphenols and other bioactive compounds by increasing their concentration and improving solvent penetration [22].
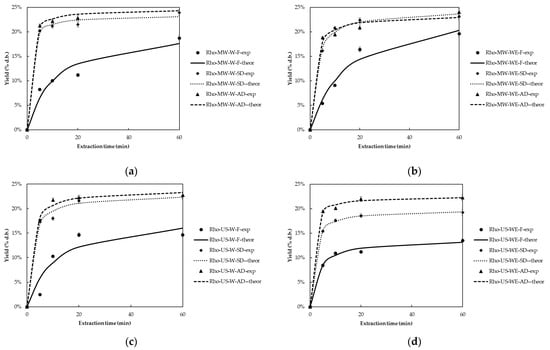
Figure 3.
Extraction yield (% d.b) kinetic plots of fresh (F) and dried (SD, AD) Rhoditis grape pomace using: (a) MW and water (W), (b) MW and water:ethanol (1:1) (WE), (c) US and water (W), and (d) US and water:ethanol (1:1) (WE) as solvent.
Additionally, the extraction method played a crucial role in determining the overall yield, with MW using water as the solvent consistently achieving the highest efficiencies for both AD and SD samples, reaching up to 25.07 g extract/100 g dry material in AD samples (p < 0.05). This highlights the effectiveness of MW in enhancing polyphenol release through cell disruption and improved solvent penetration, as supported by previous studies [32]. US also provided high yields, though slightly lower than MW, with US-WE extraction reaching up to 22.24 g extract/100 g dry material in dried samples, confirming the efficacy of ultrasound-assisted extraction in promoting solvent interaction and extract recovery.
The Peleg model parameters for Rhoditis grape pomace, as shown in Table 1, reveal the influence of drying methods and extraction techniques on the extraction kinetics and yields. As in Moschofilero extracts, Peleg’s rate constant (K1) is significantly lower for SD and AD samples compared to F samples. For instance, in MW-WE extraction, the K1 value for AD samples is 6.53 min g grape pomace/g extract, indicating a faster extraction rate compared to fresh samples, which had a K1 of 60.76 min g grape pomace/g extract. Additionally, Peleg’s capacity constant (K2) is lower for SD and AD samples compared to F samples, indicating increased extract recovery efficiency before reaching saturation. For example, in US-WE extraction, K2 for AD samples is 4.44 g grape pomace/g extract, compared to 7.22 g grape pomace/g extract in F samples. This indicates that drying treatments improve the overall capacity for recovery of extracts, leading to higher yields in dried samples. The initial extraction rate (B0) is notably higher for dried samples compared to fresh samples. For instance, MW-W in SD samples achieved a B0 value of 0.26 min g extract/g grape pomace, whereas fresh samples had a B0 of 0.02 min g extract/g grape pomace. Ye also showed an increase in AD and SD samples compared to F. This demonstrates the effectiveness of drying in improving the yield of extracts. Similar to Moschofilero, the correlation coefficients (q) for Rhoditis samples were close to 1.00, indicating a strong fit of the Peleg model to the experimental data. The RMSD values were also very low, ranging from 0.00 to 0.03, indicating that the Peleg model accurately predicts extraction behavior across different drying methods and extraction techniques. This confirms that drying is highly beneficial for enhancing both the extraction rate and the final yield of extracts from Rhoditis grape pomace. Finally, similar to Moschofilero, among extraction methods, MW and US are effective, but their relative performance depends on the specific combination of solvent, extraction method, and drying treatment.
Table 2 presents the Y (%) of fresh and dried Moschofilero and Rhoditis grape pomace using Soxhlet as the extraction method and W and E as solvents. Based on the results, W as a solvent consistently led to higher extraction yields in both grape varieties (p < 0.05). For instance, Rhoditis F samples exhibited the highest Y of 24.2% when water was used as the solvent. Similarly, Moschofilero F samples extracted with water achieved 16.2%, though this was lower than Rhoditis. This suggests that water is more effective at extracting a broad range of phenolic compounds and other bioactives compared to ethanol, which likely targets more specific compounds. Studies have shown that water-based extractions generally result in higher total polyphenol content, as many phenolic compounds, particularly hydrophilic procyanidins, phenolic acids, flavonol glucosides, and stilbenoids, exhibit higher solubility in aqueous media [33]. Ethanol, on the other hand, showed lower yields in both grape varieties (p < 0.05). For Moschofilero, the highest Y with ethanol was 18.8% in F samples, while for Rhoditis, the highest was only 16.7% in AD samples. Ethanol’s lower yield compared to water suggests it may not extract as wide a range of phenolic compounds but is more selective for specific bioactives, potentially flavonoids or smaller phenolics [34]. Furthermore, the drying method also played a significant role in extraction efficiency (p < 0.05). AD samples tended to show improved extraction yields compared to SD and F samples, particularly when water was used as the solvent. This pattern is consistent with the notion that drying treatments help break down cell walls and concentrate bioactive compounds, making them more accessible during extraction. In contrast, ethanol extraction, especially in dried samples, resulted in lower yields. For instance, Moschofilero AD samples extracted with ethanol showed a yield of only 10.4%, significantly lower than the 18.8% achieved with F samples. This can be attributed to the presence of water in F samples acting as a co-solvent, promoting higher solubility and better mass transfer during extraction. Previous studies have shown that ethanol–water mixtures optimize polyphenol extraction efficiency, as ethanol alone may not fully solubilize all classes of polyphenols [35].

Table 2.
Extraction yield (% d.b) of fresh (F) and dried (SD, AD) Moschofilero and Rhoditis grape pomace.
While Soxhlet extraction provided insights into solvent selectivity, modern non-conventional techniques, including MW and US extractions, exhibited significantly higher Y (p < 0.05), as shown in Table 2. In Moschofilero, MW-WE in AD samples yielded the highest Y (20.21 ± 0.63%), surpassing US-WE (16.22 ± 0.50%) and S-E (10.4 ± 0.32%) (p < 0.05), indicating that MW enhances polyphenol recovery when coupled with AD. Similarly, for Rhoditis, MW-WE resulted in the highest Y in AD samples (24.20 ± 0.58%), significantly higher than US-WE (22.44 ± 0.58%) and S-E (16.7 ± 0.31%) (p < 0.05), supporting findings that MW improves solvent penetration and mass transfer efficiency, leading to increased polyphenol recovery. In general, MW is based on rapid internal heating of the plant matrix and solvent, which generates high pressure within cells that leads to cell wall rupture and facilitates the release of bioactive compounds [13]. In contrast, S consistently exhibited the lowest Y across all treatments, confirming its lower efficiency in polyphenol extraction compared to modern, energy-efficient techniques.
The extraction yield alone is not a definitive measure of a method’s efficiency, as the bioactivity of the extracted compounds should also be considered to fully assess the effectiveness of the extraction process. In the following sections, the characterization of the Moschofilero and Rhoditis grape pomace extracts with respect to IC50 (antioxidant activity), total phenolic content (TPC), and total flavan-3-ol content (TFC) was performed following an extraction time of 60 min. This duration was used for all extraction methods (MW and US) to ensure consistency in the evaluation of the bioactive compound recovery and antioxidant properties in both grape varieties.
3.3. Antioxidant Activity (IC50) of Moschofilero and Rhoditis Grape Pomace Extracts
Table 3 presents the IC50 values (mg/mL) of Moschofilero and Rhoditis grape pomace extracts under different extraction and drying methods. IC50 values reflect the concentration of extract required to scavenge 50% of DPPH free radicals, with lower IC50 values indicating stronger antioxidant activity. For Moschofilero grape pomace extracts, the IC50 values ranged from 0.92 mg/mL to 3.01 mg/mL, from 0.59 mg/mL to 6.12 mg/mL, and from 2.88 mg/mL to 8.32 mg/mL for AD, SD, and F samples, respectively. In particular, dried samples consistently showed the lowest IC50 values across all extraction methods, indicating stronger antioxidant activity compared to F samples (p < 0.05). Fresh samples exhibited the weakest antioxidant activity, with significantly higher IC50 values, such as 8.23 mg/mL for MW-W and 6.36 mg/mL for MW-WE, likely due to the higher moisture content hindering phenolic extraction (p < 0.05). These findings are in agreement with Rajha et al. (2014) who demonstrated that drying processes can significantly influence phenolic recovery and antioxidant activity in grape pomace. For instance, dried grape pomace extracts showed better free radical scavenging activities compared to wet pomace when subjected to accelerated solvent extraction [36]. Additionally, the extraction method had a significant impact on IC50 values, with MW and US paired with WE as the solvent showing the strongest antioxidant activity across all samples (p < 0.05). Specifically, MW-WE achieved IC50 values ranging from 0.92 mg/mL (AD) to 1.08 mg/mL (SD), while US-WE resulted in even lower IC50 values, reaching 0.59 mg/mL (SD) and 1.05 mg/mL (AD), confirming the high efficiency of both techniques in recovering antioxidant phenolic compounds. In contrast, S-W extracts exhibited the weakest antioxidant activity, with IC50 values of 3.01 mg/mL (AD) and 6.12 mg/mL (SD). Wang et al. (2019) also observed that MW enhanced the polyphenolic yield, while US provided superior antioxidant activity. Their study revealed that combining microwave and ultrasound techniques yielded the best results for Merlot seed pomace, significantly surpassing SE in terms of both antioxidant capacity and extraction efficiency [37].

Table 3.
Free radical scavenging activity of Moschofilero and Rhoditis grape pomace extracts of AD, SD, and F (IC50 (mg/mL)).
For Rhoditis grape pomace extracts, the IC50 values ranged from 1.41 mg/mL to 5.30 mg/mL, from 1.22 mg/mL to 6.08 mg/mL, and from 3.10 mg/mL to 11.25 mg/mL for AD, SD, and F samples, respectively. In particular, as in Moschofilero extracts, dried samples exhibited the strongest antioxidant activity compared to fresh ones, with AD showing the lowest IC50 values across all extraction methods (p < 0.05). For instance, SD resulted in higher IC50 values, such as 5.97 mg/mL for MW-W and 1.22 mg/mL for MW-WE, compared to AD samples. As with Moschofilero, fresh Rhoditis samples showed the weakest antioxidant activity, with IC50 values reaching 22.14 mg/mL for US-W and 8.41 mg/mL for S-E (p < 0.05), further indicating that drying treatments enhance the recovery of bioactive compounds with antioxidant properties. Similarly to our findings, Ferri et al. (2017) reported that drying grape pomace significantly enhances polyphenol extraction and antioxidant activity. Their study on white grape pomace found that dried pomace extracts consistently outperformed fresh samples, particularly using ethanol-based extractions, which supports the stronger antioxidant activity in dried samples [20]. MW and US provided the best antioxidant activity, particularly when combined with water–ethanol, with IC50 values of 1.22 mg/mL for MW-WE-SD and 1.08 mg/mL for US-WE-AD (p < 0.05). This trend, consistent with Moschofilero, confirms the superior performance of microwave and ultrasound extractions. In contrast, Soxhlet extracts showed considerably weaker antioxidant activity, with IC50 values reaching 5.30 mg/mL (S-W) and 6.11 mg/mL (S-E) in AD samples. These results are in line with previous studies, such as Rocha and Noreña (2020) which found that MW at 1000 W for 10 min significantly increased the extraction of phenolic compounds and antioxidant activity in grape pomace, with MW outperforming Soxhlet extraction in terms of antioxidant efficiency [17]. Additionally, Drosou et al. (2015) demonstrated that UAE with a water–ethanol solvent achieved the highest total phenolic content and antioxidant activity (as measured by antiradical activity), outperforming both MW and Soxhlet extraction in their study on red grape pomace [38]. In summary, for both Moschofilero and Rhoditis, MW and US, particularly with water–ethanol (WE), was the most efficient extraction technique for maximizing phenolic recovery and antioxidant potential.
Finally, the comparison of the IC50 values between Moschofilero and Rhoditis grape pomace extracts indicates that Moschofilero generally exhibits stronger antioxidant activity (lower IC50 values) compared to Rhoditis across most extraction methods and drying treatments (p < 0.05). The differences could be attributed to the distinct phenolic profiles of each variety, as reported in previous studies [39,40]. These findings are consistent with research demonstrating that extraction efficiency and phenolic retention are highly dependent on both grape variety and drying method. However, while varietal composition plays a key role, it is important to consider that differences in extraction efficiency may also contribute to these findings. The US-WE method, which was the most effective for Moschofilero, likely facilitated higher polyphenol recovery, leading to greater antioxidant potential. Additionally, variations in total phenolic content (TPC) and total flavonoid content (TFC) may have influenced antioxidant capacity, as higher bioactive compound recovery correlates with stronger radical scavenging activity. The phenolic compounds in Moschofilero appear to be more stable and better preserved, leading to enhanced antioxidant activity as measured by IC50, when compared to Rhoditis. Therefore, while varietal differences remain a key factor, the extraction method also plays a crucial role in optimizing polyphenol yield and antioxidant activity.
3.4. Total Phenolic Content (TPC) of Moschofilero and Rhoditis Grape Pomace Extracts
Table 4 presents the TPC values (mg GAE/g dry extract) of Moschofilero and Rhoditis grape pomace extracts under different extraction and drying methods. For Moschofilero grape pomace extracts, the TPC values ranged from 67.89 mg GAE/g to 245.61 mg GAE/g for AD samples, from 41.57 mg GAE/g to 285.76 mg GAE/g for SD samples, and from 28.33 mg GAE/g to 124.88 mg GAE/g for F samples, respectively. Based on the results, the TPC values varied significantly depending on the drying method used (p < 0.05). SD samples exhibited the highest TPC value, particularly for the US-WE extraction method, where the TPC reached 285.76 mg GAE/g dry extract (p < 0.05). In comparison, AD samples showed slightly lower TPC values, such as 245.61 mg GAE/g for US-WE (p < 0.05), though air drying still preserved high phenolic content across most extraction methods. F samples consistently had the lowest TPC values, with a maximum of 124.88 mg GAE/g for MW-WE, demonstrating that the drying process enhances phenolic extraction. These results align with Tomaz et al. (2021), who demonstrated that different drying methods significantly affect polyphenol recovery in grape skins, with oven drying preserving the highest levels of specific polyphenols [41].

Table 4.
Total phenolic content (TPC) of Moschofilero and Rhoditis grape pomace extracts of AD, SD, and F (mg GAE/g dry extract).
Additionally, the extraction method had a pronounced effect on the TPC values, with US and MW paired with water–ethanol yielding significantly higher TPC compared to Soxhlet extraction (p < 0.05). US-WE provided the highest TPC, particularly in SD samples (285.76 mg GAE/g), benefiting from cavitation-induced cell wall disruption that enhances phenolic release (p < 0.05). Similarly, MW-WE produced high TPC, especially in AD samples (220.89 mg GAE/g), where rapid microwave heating and pressure-assisted cell disruption improved extraction efficiency. In contrast, Soxhlet extraction resulted in the lowest TPC values, reaching only 41.57 mg GAE/g in SD samples, likely due to prolonged heat exposure causing phenolic degradation. These results are consistent with findings from Alara et al. (2018), who also reported that modern extraction techniques like MW and US are more efficient in extracting phenolics from plant materials compared to traditional methods [42].
For Rhoditis grape pomace extracts, the TPC values ranged from 35.23 mg GAE/g to 216.51 mg GAE/g for AD samples, from 22.84 mg GAE/g to 99.24 mg GAE/g for SD samples, and from 17.08 mg GAE/g to 66.85 mg GAE/g for F samples, respectively. For Rhoditis, AD samples generally showing the highest TPC values across most extraction methods (p < 0.05). The maximum TPC for Rhoditis was 216.51 mg GAE/g in AD samples with US-WE extraction. SD samples had moderate TPC values, with a maximum of 99.24 mg GAE/g for MW-WE, while F samples showed consistently lower phenolic content, peaking at 66.85 mg GAE/g for US-WE (p < 0.05). This trend confirms the importance of drying for the effective recovery of phenolic compounds. As observed in studies by Sokač et al. (2022), controlled drying methods, such as vacuum drying and solar drying, enhance the phenolic content by breaking down cell walls and facilitating extraction [22]. In addition, the highest TPC was achieved with US-WE extraction in AD samples (216.51 mg GAE/g), followed by MW-WE, which also produced high values (156.58 mg GAE/g in AD samples) (p < 0.05). As with Moschofilero, Soxhlet extraction yielded the lowest TPC, with values as low as 22.84 mg GAE/g in SD samples (S-E), confirming the superior efficiency of modern extraction methods.
The TPC values comparison between Moschofilero and Rhoditis grape pomace extracts showed that Moschofilero generally exhibits higher TPC values across most drying and extraction methods (p < 0.05). For instance, in AD samples, Moschofilero using MW-WE yielded 220.89 mg GAE/g, whereas Rhoditis under the same conditions had a lower TPC of 156.58 mg GAE/g. Similarly, in SD samples, Moschofilero consistently exhibited higher TPC values, such as 173.29 mg GAE/g for MW-WE compared to 99.24 mg GAE/g for Rhoditis. These results indicate that Moschofilero retains and extracts more phenolic compounds than Rhoditis across different drying and extraction methods. This trend could be due to varietal differences in the phenolic composition of the grape pomace, with Moschofilero potentially containing a richer or more extractable phenolic profile. Similar observations have been reported in other studies comparing phenolic content across different grape varieties [43,44]. This highlights the significance of grape variety in determining the efficiency of phenolic recovery in byproducts like grape pomace.
3.5. Total Flavan-3-ol Content (TFC) of Moschofilero and Rhoditis Grape Pomace Extracts
Table 5 presents the TFC values (mg CE/g dry extract) of Moschofilero and Rhoditis grape pomace extracts under different extraction and drying methods. For Moschofilero, TFC values ranged from 7.48 mg CE/g to 33.31 mg CE/g for AD samples, 6.63 mg CE/g to 46.21 mg CE/g for SD samples, and 0.67 mg CE/g to 9.41 mg CE/g for F samples, respectively. For Moschofilero, SD samples consistently exhibited higher TFC compared to AD and F samples across most extraction methods (p < 0.05). For example, TFC values reached 46.21 mg CE/g for US-WE in SD samples, whereas AD samples showed lower values of 33.31 mg/g. Fresh samples consistently had the lowest TFC values, with a maximum of 9.41 mg CE/g for MW-WE. This trend aligns with studies showing that drying, particularly solar drying, enhances phenolic and flavonoid recovery by reducing moisture content and improving extraction efficiency [22]. In Moschofilero pomace, US-WE extraction yielded the highest TFC, reaching 46.21 mg CE/g in SD samples and 33.31 mg CE/g in AD samples, both significantly higher than Soxhlet extracts, which showed values as low as 7.48 mg CE/g (p < 0.05). These results are consistent with studies by Alara et al. (2018), which reported the superior efficiency of ultrasound extraction in recovering flavonoids from plant materials [42].

Table 5.
Total flavan-3-ol content (TFC) of Moschofilero and Rhoditis grape pomace extracts of AD, SD, and F (mg (+)-catechin (CE)/g dry extract).
For Rhoditis, TFC values ranged from 0.35 mg CE/g to 4.26 mg CE/g for AD samples, 0.26 mg CE/g to 7.22 mg CE/g for SD samples, and 0.15 mg CE/g to 5.19 mg CE/g for F samples, respectively. For Rhoditis, the highest TFC values were found in AD samples, reaching 35.96 mg CE/g for US-WE, while SD samples showed lower values, such as 7.22 mg CE/g for US-WE (p < 0.05). Fresh samples again exhibited the lowest TFC values, with a maximum of 6.31 mg CE/g for US-WE. Furthermore, US-WE achieved the highest TFC values in AD samples (35.96 mg CE/g), followed by MW-WE with 24.01 mg CE/g, confirming the superior efficiency of both modern techniques over Soxhlet extraction, which produced TFC values as low as 0.26 mg CE/g in SD samples (p < 0.05). This confirms the findings by Chaves et al. (2020), which highlight the efficiency of modern extraction methods in preserving bioactive compounds like flavonoids [45].
The comparison of TFC values between Moschofilero and Rhoditis grape pomace extracts shows that Moschofilero generally has higher TFC values across most extraction and drying methods. For example, in SD samples, Moschofilero TFC values reach 46.21 mg CE/g for US-WE, while Rhoditis under the same conditions only reaches 7.22 mg CE/g. This indicates that Moschofilero retains and extracts more flavan-3-ols compared to Rhoditis across different methods, likely due to varietal differences in flavonoid composition.
In summary, for both grape pomace varieties, the use of WE as a solvent resulted in the highest extraction yields in both MW and US methods, and this can be attributed to several factors. Ethanol, when combined with water, acts as a polar solvent that efficiently penetrates plant cell walls, enabling the extraction of a wide range of phenolic compounds, including both polar and slightly non-polar polyphenols such as flavonoids, tannins, and phenolic acids. Ethanol helps to enhance the solubility of bioactive compounds, while water assists in breaking down cell structures and improving solvent diffusion. The synergy between the two solvents allows for the extraction of a broader spectrum of polyphenols, leading to higher yields compared to the use of water or ethanol alone [46].
3.6. HPLC Analysis of Moschofilero and Rhoditis Grape Pomace Extracts
According to the results of TPC, TFC, and IC50, the superiority of specific extracts was pointed out. Particularly, the MW-WE and US-WE extracts after air drying and solar drying of both Moschofilero and Rhoditis grape pomaces (with the exception of US-WE after solar drying of Rhoditis) exhibited high content of total phenolics and flavan-3-ols accompanied with the lowest IC50 values, i.e., high antiradical activity. Additionally, HPLC-DAD analyses at various wavelengths were used for preliminary characterization of the extracts. The chromatograms were monitored at 280, 360, and 520 nm to detect the full range of components. The 280 nm wavelength provided an overall view of the phenolic composition of the extracts. Several minor peaks corresponding to small quantities of condensed tannins were detected at 280 nm. This group of compounds, consisting of oligomers of proanthocyanidins, produced low and broad peaks due to the co-elution of different oligomers. Therefore, the quantification of proanthocyanidins was performed based on the TFC protocol, utilizing p-dimethylaminocinnamaldehyde (DMACA), a highly selective reagent for this group of compounds. At 280 nm, flavonols were also detected; however, to avoid interference from minor proanthocyanidin peaks and baseline increases, the 360 nm wavelength was preferred, as it produced clearer chromatograms with higher compound responses. The 520 nm wavelength, highly sensitive for anthocyanins, detected only trace amounts of these compounds. Consequently, aside from proanthocyanidins, flavonols were the only group of phenolics identified.
According to the above observations, the specific seven extracts were further analyzed with HPLC-DAD-ESI-MS/MS for the detection of micromolecular phenolic compounds. The analyses revealed 3 common flavonols in both Moschofilero and Rhoditis pomaces, namely quercetin-3-glucoronide, myricetin, and quercetin. Moschofilero exhibited two additional flavonols, isorhamnetin-3-glucoside and syringetin-3-glucoside, compounds that were not identified in Rhoditis. Overall the identified flavonols are known components of grape cultivars [38].
The quantifications of the respective compounds are presented in Table 6. Quercetin-3-glucuronide, myricetin, and quercetin could be characterized as the most significant compounds in terms of antiradical activity, since they possess free ortho hydroxyls in the B-ring of the flavonol skeleton. Focusing on the specific compounds, the MW-WE extract after solar drying of Rhoditis presents the highest concentration in the highly antiradical flavonols, with the compounds amounting to 3.34 mg quercetin equivalents/g dry extract. In terms of total flavonol content, US-WE extract after air drying of Moschofilero presents the highest concentration, with 3.74 mg quercetin equivalents/g dry extract.

Table 6.
The quantification of the identified individual flavonols of Moschofilero and Rhoditis grape pomace WE extracts of AD and SD (mg quercetin equivalents/g dry extract).
Table 7 presents the IC50 values and quantification of major polyphenol groups in Moschofilero and Rhoditis grape pomace extracts, comparing different extraction methods (MW and US) and drying treatments (AD and SD) using water–ethanol as the solvent. For Moschofilero, SD generally resulted in higher TPC and TFC extraction, while also demonstrating better antioxidant activity, as indicated by the lower IC50 value (0.59 mg/mL) compared to AD (p < 0.05). In contrast, for Rhoditis, AD proved to be the superior drying method, leading to higher TPC and TFC extraction, and more potent antioxidant activity, especially with US extraction (p < 0.05). For both Moschofilero and Rhoditis, US-WE extracts demonstrated superior performance compared to MW-WE, exhibiting higher total phenolic content, stronger antioxidant activity, and greater concentrations of polyphenol subclasses such as flavan-3-ols and flavonols. In general, US relies on acoustic cavitation, where the formation and collapse of microbubbles generate localized shear forces that break down cell walls facilitating the release of phenolic compounds [13].

Table 7.
IC50 values and the quantification of the major polyphenol groups of Moschofilero and Rhoditis grape pomace WE extracts of AD and SD.
The impact of drying methods on the stability and retention of polyphenolic compounds was evaluated by comparing AD and SD in Moschofilero and Rhoditis grape pomace, with detailed results on extraction yield, antioxidant activity, and polyphenolic content presented in Appendix A. Our results demonstrate that different polyphenolic groups exhibited varied responses based on grape variety and drying method.
Specifically, flavan-3-ols responded differently to drying conditions depending on the grape variety. In Moschofilero, SD samples showed higher flavan-3-ol content (46.21 mg CE/g in US-WE extracts) compared to AD (33.31 mg CE/g), suggesting that solar exposure may facilitate the release of these compounds, possibly due to enhanced extraction from cellular breakdown. This phenomenon has been observed in certain drying conditions where controlled solar drying can lead to the breakdown of polyphenol–protein complexes, increasing extractability [6]. Conversely, in Rhoditis, flavan-3-ol content was significantly lower in SD samples (7.22 mg CE/g) compared to AD (35.96 mg CE/g), indicating a higher susceptibility to degradation under solar drying. Studies have shown that flavan-3-ols degrade more rapidly when exposed to prolonged solar radiation and oxidative conditions, particularly in high-exposure drying environments [47].
Flavonols exhibited different stability patterns based on grape variety. In Moschofilero, total flavonol content remained relatively stable between AD (3.74 mg/g) and SD (3.59 mg/g) for US-WE extracts, suggesting a higher resilience to solar drying. However, in Rhoditis, flavonol degradation was more pronounced, with a decrease from 3.34 mg/g in AD to 1.13 mg/g in SD, suggesting that increased UV exposure and prolonged drying time may have contributed to flavonol degradation through photo-oxidation and glycosidic bond cleavage, ultimately reducing their content [48].
TPC also showed significant differences between drying methods and grape varieties. In Moschofilero, TPC was higher in SD samples (285.76 mg GAE/g in US-WE extracts) compared to AD (245.61 mg GAE/g), suggesting that SD may enhance polyphenol extractability, possibly due to enhanced cellular breakdown. However, in Rhoditis, AD was more effective in preserving TPC (216.51 mg GAE/g) compared to SD (68.23 mg GAE/g), indicating that total polyphenol retention in Rhoditis is more negatively affected by solar drying. This supports findings that AD preserves more phenolic compounds in certain grape varieties due to reduced oxidative stress, whereas SD can lead to significant degradation depending on temperature and exposure duration [22,49].
These results highlight the importance of selecting appropriate drying methods based on the target polyphenolic profile and grape variety. While SD improved flavan-3-ol extractability in Moschofilero, it significantly accelerated the degradation of flavan-3-ols and flavonols in Rhoditis. These findings provide valuable guidance for optimizing drying processes in the food and nutraceutical industries to maximize polyphenol retention.
4. Conclusions
This study highlights the significant impact of both drying and extraction methods on the recovery of bioactive polyphenols and the antioxidant potential of grape pomace from two white wine varieties, Moschofilero and Rhoditis. The findings indicate that drying treatments and extraction techniques significantly affect the yield and quality of the extracted compounds. In the case of Moschofilero, SD proved to be more effective in enhancing TPC and TFC, resulting in extracts with higher antioxidant activity, particularly when combined with US-WE. This combination yielded the best results in terms of both phenolic recovery (TPC: 285.76 mg GAE/g) and antioxidant activity (IC50: 0.59 mg/mL), suggesting that solar drying helps retain polyphenolic compounds, which are better extracted using ultrasound. HPLC analysis further identified quercetin-3-glucuronide, myricetin, and quercetin as the main flavonols, with Moschofilero SD-US-WE extracts showing the highest total flavonol content at 3.59 mg QE/g dry extract. Additionally, isorhamnetin-3-glucoside and syringetin-3-glucoside were found exclusively in Moschofilero.
For Rhoditis, however, AD demonstrated superior performance, especially in terms of antioxidant activity and phenolic recovery when combined with US-WE. The highest values for TPC (216.51 mg GAE/g), TFC (35.96 mg CE/g), and antioxidant activity (IC50: 1.08 mg/mL) were achieved in AD samples, showing that air drying is more suitable for preserving bioactive compounds in Rhoditis pomace. In contrast, HPLC analysis of Rhoditis showed the highest flavonol concentration of 3.34 mg QE/g dry extract in SD-MW-WE extracts, with quercetin-3-glucuronide, myricetin, and quercetin being the primary flavonols identified.
In both grape varieties, US-WE consistently outperformed MW-WE and Soxhlet extraction, especially when water–ethanol (1:1) was used as the solvent. Ultrasound-assisted extraction is known for its ability to disrupt cell walls and enhance solvent penetration, leading to higher extraction efficiency and improved recovery of bioactive compounds. This technique, coupled with optimized drying treatments, showed the highest yields in both antioxidant activity and polyphenolic content, making it the most effective method for valorizing grape pomace.
The selection of a 1:1 water–ethanol ratio was based on its ability to effectively extract a broad range of polyphenols, balancing hydrophilic and lipophilic compound solubility. However, as phenolic compounds exhibit varying solubilities, the ethanol concentration plays a crucial role in their selective extraction. Future studies could explore different ethanol–water proportions to selectively enhance the recovery of specific phenolic subgroups, optimizing extraction efficiency for targeted applications in functional foods and nutraceuticals.
Overall, this study underscores the importance of selecting appropriate drying and extraction methods to maximize the recovery of valuable polyphenols and flavan-3-ols from grape pomace. Solar drying combined with ultrasound-assisted extraction was particularly effective for Moschofilero, while air drying paired with ultrasound-assisted extraction was more suitable for Rhoditis. These optimized methods can significantly enhance the potential for grape pomace utilization in functional foods, nutraceuticals, and other bioactive-rich products. They also promote sustainability in the winemaking industry by transforming waste into high-value resources.
Our findings show that MW and US extractions are efficient, reduce solvent use, and improve polyphenol recovery, making them suitable for large-scale valorization. While MW extraction requires specialized equipment and higher initial investment, it provides rapid extraction with high reproducibility. In contrast, US extraction is more cost-effective and easier to integrate into existing processing lines, offering a scalable and energy-efficient solution. These aspects highlight the industrial potential of MW and US extractions for sustainable polyphenol recovery, provided that process optimization and economic feasibility assessments are considered for large-scale applications.
Additionally, while this study demonstrates the beneficial effects of drying on polyphenol retention in Moschofilero and Rhoditis grape pomace, further research is needed to assess whether these findings hold across a broader range of grape cultivars with distinct polyphenolic profiles. This would provide a more comprehensive understanding of how drying conditions influence bioactive compound stability in diverse grape varieties.
These findings have important real-world applications, supporting the valorization of grape pomace as a sustainable and low-cost source of bioactive compounds for the development of functional foods, dietary supplements, and cosmetic formulations. The use of water–ethanol as a green solvent further enhances the environmental compatibility of the process, aligning with circular economy principles and industrial sustainability goals. Future work could explore alternative eco-friendly solvents, such as deep eutectic solvents (DESs), to improve the extraction of specific phenolic subclasses while reducing environmental impact. Moreover, advances in low-energy and scalable extraction technologies, including hybrid techniques (e.g., ultrasound–microwave combined extraction), may further optimize polyphenol recovery with minimal energy input. These directions offer promising opportunities for industrial-scale applications, promoting the full utilization of winery byproducts in a sustainable manner.
Author Contributions
Conceptualization, C.D., K.K. and M.K.; methodology, C.D., K.K., K.T.L., A.B., D.T. and M.K.; software, C.D. and K.K.; validation, C.D., K.K., K.T.L., A.B., D.T. and M.K.; formal analysis, C.D. and K.K.; investigation, C.D. and K.K.; resources, M.K.; data curation, C.D., K.K., K.T.L., A.B., D.T. and M.K.; writing—original draft preparation, C.D., K.T.L. and D.T.; writing—review and editing, C.D., K.K., K.T.L., A.B., D.T. and M.K.; visualization, C.D.; supervision, M.K.; project administration, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Extraction yield (% d.b), free radical scavenging activity (IC50, (mg/mL)), total phenolic content (TPC, (mg GAE/g dry extract)), total flavan-3-ol content (TFC, (mg (+)-catechin (CE)/g dry extract) of Moschofilero and Rhoditis grape pomace extracts of AD, SD, and F across different extraction techniques.
Table A1.
Extraction yield (% d.b), free radical scavenging activity (IC50, (mg/mL)), total phenolic content (TPC, (mg GAE/g dry extract)), total flavan-3-ol content (TFC, (mg (+)-catechin (CE)/g dry extract) of Moschofilero and Rhoditis grape pomace extracts of AD, SD, and F across different extraction techniques.
| Extracts | Moschofilero | Rhoditis | ||||
|---|---|---|---|---|---|---|
| AD | SD | F | AD | SD | F | |
| Y (%) | ||||||
| MW-W | 17.34 ± 0.54 b,i | 16.84 ± 0.54 b,i | 16.58 ± 0.55 b,i | 25.07 ± 0.63 a,i | 23.93 ± 0.62 a,i | 18.70 ± 0.56 b,ii |
| MW-WE | 20.21 ± 0.63 a,i | 16.85 ± 0.52 b,ii | 19.38 ± 0.60 a,i | 24.06 ± 0.63 a,b,i,ii | 23.10 ± 0.55 a,ii | 25.60 ± 0.64 a,i |
| US-W | 17.28 ± 0.50 b,i | 16.74 ± 0.43 b,i | 13.33 ± 0.43 c,ii | 22.76 ± 0.59 b,i | 22.59 ± 0.66 a,b,i | 14.62 ± 0.42 c,ii |
| US-WE | 16.22 ± 0.50 b,ii | 18.61 ± 0.58 a,i | 10.12 ± 0.40 d,iii | 22.24 ± 0.58 b,i | 19.24 ± 0.52 c,ii | 13.51 ± 0.41 c,iii |
| S-W | 17.00 ± 0.35 b,i | 14.00 ± 0.27 c,ii | 16.20 ± 0.32 b,i | 22.40 ± 0.35 b,ii | 20.90 ± 0.28 b,c,iii | 24.20 ± 0.37 a,i |
| S-E | 10.40 ± 0.32 c,ii | 8.20 ± 0.24 d,iii | 18.80 ± 0.28 a,i | 16.70 ± 0.31 c,i | 13.70 ± 0.22 d,ii | 10.80 ± 0.35 d,iii |
| IC50 (mg/mL) | ||||||
| MW-W | 2.16 ± 0.03 d,i | 3.52 ± 0.03 d,ii | 8.23 ± 0.04 e,iii | 4.42 ± 0.02 d,i | 5.97 ± 0.04 c,ii | 5.90 ± 0.08 c,ii |
| MW-WE | 0.92 ± 0.01 a,i | 1.08 ± 0.01 a,ii | 6.36 ± 0.03 d,iii | 1.75 ± 0.01 b,ii | 1.22 ± 0.02 a,i | 3.10 ± 0.01 a,iii |
| US-W | 2.36 ± 0.02 e,ii | 1.80 ± 0.02 c,i | 9.60 ± 0.02 f,iii | 4.30 ± 0.01 c,i | 11.25 ± 0.06 f,ii | 22.14 ± 0.05 e,iii |
| US-WE | 1.05 ± 0.01 b,ii | 0.59 ± 0.01 b,i | 3.88 ± 0.01 b,iii | 1.08 ± 0.01 a,i | 2.47 ± 0.03 b,ii | 3.01 ± 0.09 a,iii |
| S-W | 3.01 ± 0.02 f,ii | 6.12 ± 0.05 e,iii | 2.88 ± 0.02 a,i | 5.30 ± 0.01 e,i | 6.08 ± 0.04 d,ii | 5.32 ± 0.02 b,i |
| S-E | 1.65 ± 0.01 c,ii | 1.08 ± 0.03 a,i | 4.23 ± 0.04 c,iii | 6.11 ± 0.03 f,i | 7.10 ± 0.01 e,ii | 8.41 ± 0.04 d,iii |
| TPC (mg GAE/g dry extract) | ||||||
| MW-W | 82.56 ± 0.18 e,i | 75.77 ± 0.21 e,ii | 28.33 ± 0.22 e,iii | 50.30 ± 0.37 c,i | 50.29 ± 0.22 c,i | 17.08 ± 0.18 f,ii |
| MW-WE | 220.89 ± 1.62 b,i | 173.29 ± 0.26 b,ii | 124.88 ± 0.26 a,iii | 156.58 ± 0.88 b,i | 99.24 ± 0.29 a,ii | 43.75 ± 0.23 c,iii |
| US-W | 90.61 ± 0.35 c,ii | 115.89 ± 0.22 c,i | 28.11 ± 0.88 e,iii | 40.36 ± 2.10 d,i | 30.39 ± 0.19 e,ii | 25.02 ± 0.18 e,iii |
| US-WE | 245.61 ± 0.30 a,ii | 285.76 ± 0.28 a,i | 46.11 ± 0.97 d,iii | 216.51 ± 0.81 a,i | 68.23 ± 1.04 b,ii | 66.85 ± 0.23 a,ii |
| S-W | 67.89 ± 0.16 f,ii | 41.57 ± 0.21 f,iii | 96.55 ± 1.05 b,i | 35.23 ± 0.20 e,iii | 38.01 ± 0.18 d,ii | 50.72 ± 0.95 b,i |
| S-E | 86.83 ± 0.25 d,ii | 105.96 ± 0.84 d,i | 51.50 ± 0.56 c,iii | 49.50 ± 1.15 c,i | 22.84 ± 1.22 f,iii | 40.23 ± 1.10 d,ii |
| TFC (mg (+)-catechin (CE)/g dry extract) | ||||||
| MW-W | 10.61 ± 0.35 c,i | 10.21 ± 0.02 d,i | 1.11 ± 0.04 c,ii | 4.26 ± 0.02 c,ii | 4.25 ± 0.12 c,ii | 5.19 ± 0.01 b,i |
| MW-WE | 17.46 ± 0.05 b,ii | 27.35 ± 0.43 b,i | 9.41 ± 0.02 a,iii | 24.01 ± 0.05 b,i | 12.03 ± 0.38 a,ii | 2.26 ± 0.09 d,iii |
| US-W | 9.95 ± 0.15 d,ii | 14.29 ± 0.04 c,i | 0.67 ± 0.04 d,iii | 2.34 ± 0.33 d,i | 2.10 ± 0.05 e,i,ii | 2.05 ± 0.07 d,ii |
| US-WE | 33.31 ± 0.68 a,ii | 46.21 ± 0.99 a,i | 0.73 ± 0.06 d,iii | 35.96 ± 0.08 a,i | 7.22 ± 0.04 b,ii | 6.31 ± 0.08 a,iii |
| S-W | 3.23 ± 0.01 f,ii | 0.63 ± 0.02 f,iii | 5.91 ± 0.05 b,i | 4.16 ± 0.15 c,i | 3.14 ± 0.06 d,ii | 4.12 ± 0.02 c,i |
| S-E | 7.48 ± 0.19 e,i | 7.60 ± 0.16 e,i | 0.74 ± 0.15 d,ii | 0.35 ± 0.11 e,i | 0.26 ± 0.05 f,i | 0.15 ± 0.02 e,ii |
For each grape pomace variety, different superscript letters indicate significant differences between Y, IC50, TPC, and TFC values under different extraction methods with the specified drying treatment (p < 0.05). Different superscript numbers show significant differences between Y, IC50, TPC, and TFC values under different drying treatments with the specified extraction method (p < 0.05).
References
- Available online: https://www.oiv.int/sites/default/files/documents/OIV_Annual_Assessment-2023.pdf (accessed on 13 January 2025).
- Chowdhary, P.; Gupta, A.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Current trends and possibilities for exploitation of Grape pomace as a potential source for value addition. Environ. Pollut. 2021, 278, 116796. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green processing and biotechnological potential of grape pomace: Current trends and opportunities for sustainable biorefinery. Bioresour. Technol. 2020, 314, 123771. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; You, Y.; Huang, W.; Zhan, J. The high-value and sustainable utilization of grape pomace: A review. Food Chem. X 2024, 24, 101845. [Google Scholar] [CrossRef]
- Goula, A.M.; Thymiatis, K.; Kaderides, K. Valorization of grape pomace: Drying behavior and ultrasound extraction of phenolics. Food Bioprod. Process. 2016, 100, 132–144. [Google Scholar] [CrossRef]
- Guaita, M.; Panero, L.; Motta, S.; Mangione, B.; Bosso, A. Effects of high-temperature drying on the polyphenolic composition of skins and seeds from red grape pomace. LWT 2021, 145, 111323. [Google Scholar] [CrossRef]
- Teles, A.S.C.; Chávez, D.W.H.; Gomes, F.d.S.; Cabral, L.M.C.; Tonon, R.V. Effect of temperature on the degradation of bioactive compounds of Pinot Noir grape pomace during drying. Braz. J. Food Technol. 2018, 21. [Google Scholar] [CrossRef]
- Ortiz-Rodríguez, N.M.; Condorí, M.; Durán, G.; García-Valladares, O. Solar drying Technologies: A review and future research directions with a focus on agroindustrial applications in medium and large scale. Appl. Therm. Eng. 2022, 215, 118993. [Google Scholar] [CrossRef]
- Daniela, T.-R.; del Socorro, L.-C.M.; Fortunata, S.-T.; Patricia, R.-M.; Felipe, G.-O.; Teresa, H.-B.M.; de la Paz, S.-C.M. Optimization of the Extraction of Bioactive Compounds from Cabernet Sauvignon Grape Pomace from Querétaro, Mexico, Using MSPD. Separations 2024, 11, 13. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Tsakiri-Mantzorou, Z.; Drosou, C.; Mari, A.; Stramarkou, M.; Laina, K.T.; Krokida, M. Edible Coating with Encapsulated Antimicrobial and Antibrowning Agents via the Emerging Electrospinning Process and the Conventional Spray Drying: Effect on Quality and Shelf Life of Fresh-Cut Potatoes. Potato Res. 2024. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Laina, K.T.; Drosou, C.; Stergiopoulos, C.; Eleni, P.M.; Krokida, M. Optimization of Combined Ultrasound and Microwave-Assisted Extraction for Enhanced Bioactive Compounds Recovery from Four Medicinal Plants: Oregano, Rosemary, Hypericum, and Chamomile. Molecules 2024, 29, 5773. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, H.; Wang, M.; Zhao, S.; Sun, G.; Li, Z. Recent Advancements in Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Technol. 2024, 18, 2083–2100. [Google Scholar] [CrossRef]
- Brahim, M.; Gambier, F.; Brosse, N. Optimization of polyphenols extraction from grape residues in water medium. Ind. Crops Prod. 2014, 52, 18–22. [Google Scholar] [CrossRef]
- Moutinho, J.; Gouvinhas, I.; Domínguez-Perles, R.; Barros, A. Optimization of the Extraction Methodology of Grape Pomace Polyphenols for Food Applications. Molecules 2023, 28, 3885. [Google Scholar] [CrossRef]
- da Rocha, C.B.; Noreña, C.P.Z. Microwave-Assisted Extraction and Ultrasound-Assisted Extraction of Bioactive Compounds from Grape Pomace. Int. J. Food Eng. 2020, 16, 20190191. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, H.; Holland, B.; Barrow, C.J.; Suleria, H.A.R. An Optimization of the Extraction of Phenolic Compounds from Grape Marc: A Comparison between Conventional and Ultrasound-Assisted Methods. Chemosensors 2024, 12, 177. [Google Scholar] [CrossRef]
- González, M.; Barrios, S.; Budelli, E.; Pérez, N.; Lema, P.; Heinzen, H. Ultrasound assisted extraction of bioactive compounds in fresh and freeze-dried Vitis vinifera cv Tannat grape pomace. Food Bioprod. Process. 2020, 124, 378–386. [Google Scholar] [CrossRef]
- Ferri, M.; Rondini, G.; Calabretta, M.M.; Michelini, E.; Vallini, V.; Fava, F.; Roda, A.; Minnucci, G.; Tassoni, A. White grape pomace extracts, obtained by a sequential enzymatic plus ethanol-based extraction, exert antioxidant, anti-tyrosinase and anti-inflammatory activities. New Biotechnol. 2017, 39, 51–58. [Google Scholar] [CrossRef]
- Vashisth, T.; Singh, R.K.; Pegg, R.B. Effects of drying on the phenolics content and antioxidant activity of muscadine pomace. LWT-Food Sci. Technol. 2011, 44, 1649–1657. [Google Scholar] [CrossRef]
- Sokač, T.; Gunjević, V.; Pušek, A.; Tušek, A.J.; Dujmić, F.; Brnčić, M.; Ganić, K.K.; Jakovljević, T.; Uher, D.; Mitrić, G.; et al. Comparison of Drying Methods and Their Effect on the Stability of Graševina Grape Pomace Biologically Active Compounds. Foods 2022, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Khalangre, A.; Mirza, A.; Chavan, R.; Sharma, A.K.; Shaikh, N.; Tp, A.S. Drying and degradation kinetics of red grape pomace with special emphasis on degradation of anthocyanins using liquid chromatography-orbitrap-mass spectrometry. Biomass Convers. Biorefinery 2024. [Google Scholar] [CrossRef]
- Cunniff, P.; Washington, D. Official methods of analysis of AOAC International. J. AOAC Int. 1997, 80, 127A. [Google Scholar]
- Krokida, M.K.; Karathanos, V.T.; Maroulis, Z.B.; Marinos-Kouris, D. Drying kinetics of some vegetables. J. Food Eng. 2003, 59, 391–403. [Google Scholar] [CrossRef]
- Peleg, M. An Empirical Model for the Description of Moisture Sorption Curves. J. Food Sci. 1988, 53, 1216–1217. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Vivas, N.; Glories, Y.; Lagune, L.; Cédric, S.; Augustin, M. Estimation du degré de polymérisation des procyanidines du raisin et du vin par la méthode au p-dimethylaminocinnamaldéhyde. OENO One 1994, 28, 319–336. [Google Scholar] [CrossRef]
- Bimpilas, A.; Tsimogiannis, D.; Balta-Brouma, K.; Lymperopoulou, T.; Oreopoulou, V. Evolution of phenolic compounds and metal content of wine during alcoholic fermentation and storage. Food Chem. 2015, 178, 164–171. [Google Scholar] [CrossRef]
- Peternel, L.; Sokač Cvetnić, T.; Gajdoš Kljusurić, J.; Jurina, T.; Benković, M.; Radojčić Redovniković, I.; Jurinjak Tušek, A.; Valinger, D. The Effects of Drying and Grinding on the Extraction Efficiency of Polyphenols from Grape Skin: Process Optimization. Processes 2024, 12, 1100. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.-H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Popovic, B.M.; Micic, N.; Potkonjak, A.; Blagojevic, B.; Pavlovic, K.; Milanov, D.; Juric, T. Novel extraction of polyphenols from sour cherry pomace using natural deep eutectic solvents—Ultrafast microwave-assisted NADES preparation and extraction. Food Chem. 2022, 366, 130562. [Google Scholar] [CrossRef] [PubMed]
- Baron, G.; Ferrario, G.; Marinello, C.; Carini, M.; Morazzoni, P.; Aldini, G. Effect of Extraction Solvent and Temperature on Polyphenol Profiles, Antioxidant and Anti-Inflammatory Effects of Red Grape Skin By-Product. Molecules 2021, 26, 5454. [Google Scholar] [CrossRef] [PubMed]
- Mikucka, W. Effect of the solvent on the extraction of polyphenols from distillery stillage and on their antioxidant activity. Folia Biol. Oecologica 2021, 17, 54–62. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Pappas, V.M.; Palaiogiannis, D.; Chatzimitakos, T.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Pulsed Electric Field-Based Extraction of Total Polyphenols from Sideritis raiseri Using Hydroethanolic Mixtures. Oxygen 2022, 2, 91–98. [Google Scholar] [CrossRef]
- Rajha, H.N.; Ziegler, W.; Louka, N.; Hobaika, Z.; Vorobiev, E.; Boechzelt, H.G.; Maroun, R.G. Effect of the Drying Process on the Intensification of Phenolic Compounds Recovery from Grape Pomace Using Accelerated Solvent Extraction. Int. J. Mol. Sci. 2014, 15, 18640–18658. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Wang, H.; Huo, S. Evaluation of extraction technologies and optimization of microwave and ultrasonic assisted consecutive extraction of phenolic antioxidants from winery byproducts. J. Food Process Eng. 2019, 42, e13064. [Google Scholar] [CrossRef]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Esparza, I.; Moler, J.A.; Arteta, M.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Phenolic Composition of Grape Stems from Different Spanish Varieties and Vintages. Biomolecules 2021, 11, 1221. [Google Scholar] [CrossRef]
- Meng, J.-F.; Fang, Y.-L.; Qin, M.-Y.; Zhuang, X.-F.; Zhang, Z.-W. Varietal differences among the phenolic profiles and antioxidant properties of four cultivars of spine grape (Vitis davidii Foex) in Chongyi County (China). Food Chem. 2012, 134, 2049–2056. [Google Scholar] [CrossRef]
- Tomaz, I.; Štambuk, P.; Anić, M.; Šikuten, I.; Huzanić, N.; Karoglan, M.; Maletić, E.; Kontić, J.K.; Preiner, D. Effect of different drying methods on the content of polyphenolic compounds of red grape skins. J. Central Eur. Agric. 2021, 22, 429–442. [Google Scholar] [CrossRef]
- Alara, O.; Nour, A.; Olalere, O. Ethanolic extraction of flavonoids, phenolics and antioxidants from Vernonia amygdalina leaf using two-level factorial design. J. King Saud Univ.-Sci. 2017, 32, 7–16. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef] [PubMed]
- de la Cerda-Carrasco, A.; López-Solís, R.; Nuñez-Kalasic, H.; Peña-Neira, Á.; Obreque-Slier, E. Phenolic composition and antioxidant capacity of pomaces from four grape varieties (Vitis vinifera L.). J. Sci. Food Agric. 2015, 95, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.d.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Lim, K.J.A.; Cabajar, A.A.; Lobarbio, C.F.Y.; Taboada, E.B.; Lacks, D.J. Extraction of bioactive compounds from mango (Mangifera indica L. var. Carabao) seed kernel with ethanol–water binary solvent systems. J. Food Sci. Technol. 2019, 56, 2536–2544. [Google Scholar] [CrossRef]
- Alibade, A.; Lalas, S.; Lakka, A.; Chatzilazarou, A.; Makris, D. The combined effect of time and temperature during oven drying on red grape pomace polyphenols, pigments, and antioxidant properties. Acta Univ. Sapientiae Aliment. 2022, 15, 11–26. [Google Scholar] [CrossRef]
- Liu, L.; Gregan, S.M.; Winefield, C.; Jordan, B. Comparisons of controlled environment and vineyard experiments in Sauvignon blanc grapes reveal similar UV-B signal transduction pathways for flavonol biosynthesis. Plant Sci. 2018, 276, 44–53. [Google Scholar] [CrossRef]
- Corte-Real, J.; Archaimbault, A.; Schleeh, T.; Cocco, E.; Herrmann, M.; Guignard, C.; Hausman, J.-F.; Iken, M.; Legay, S. Handling wine pomace: The importance of drying to preserve phenolic profile and antioxidant capacity for product valorization. J. Food Sci. 2021, 86, 892–900. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).