Effects of Microplastics on Bioavailability, Persistence and Toxicity of Plant Pesticides: An Agricultural Perspective
Abstract
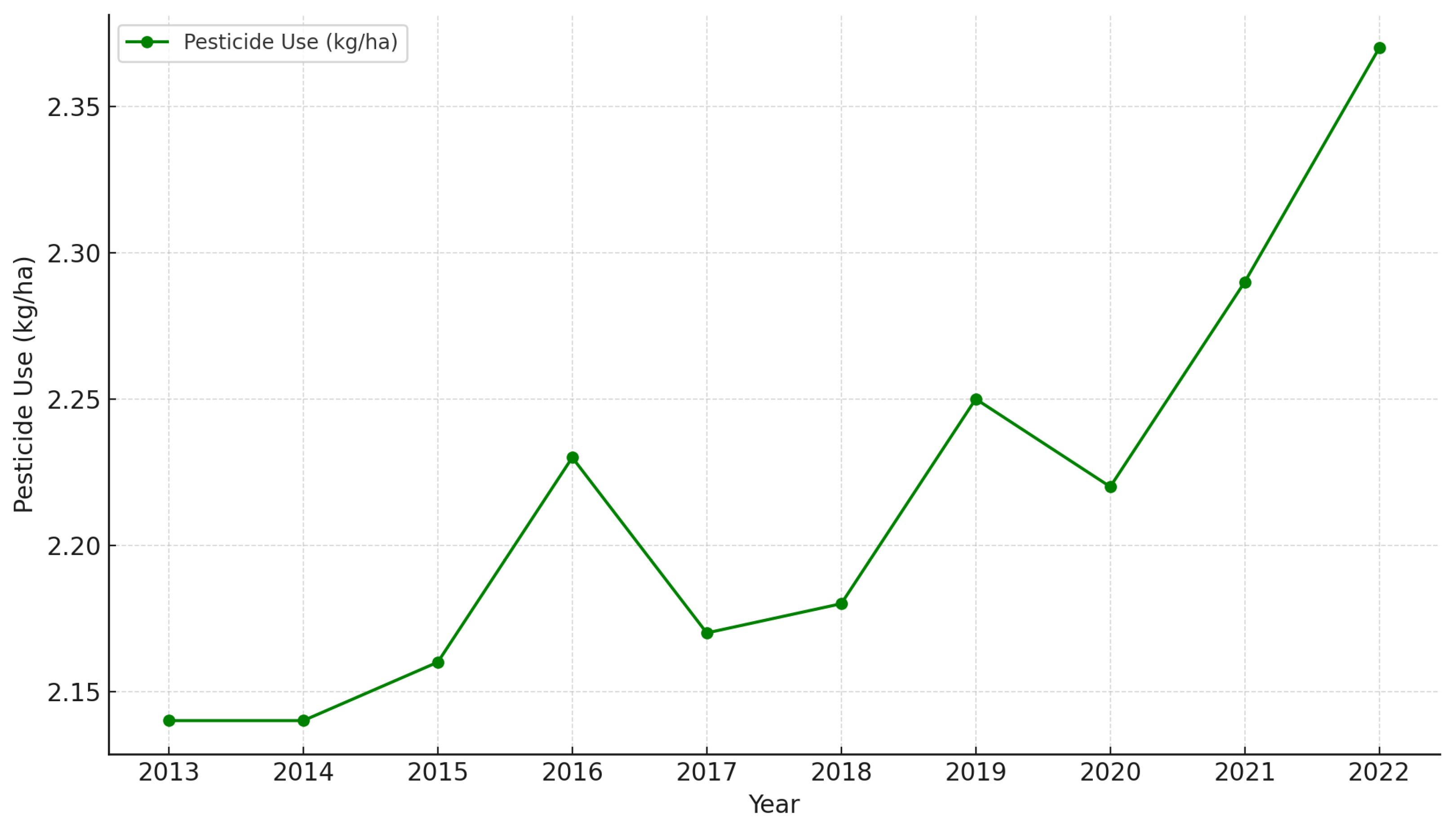
1. Introduction
2. Methodology for Literature Review
3. Types of Pesticides
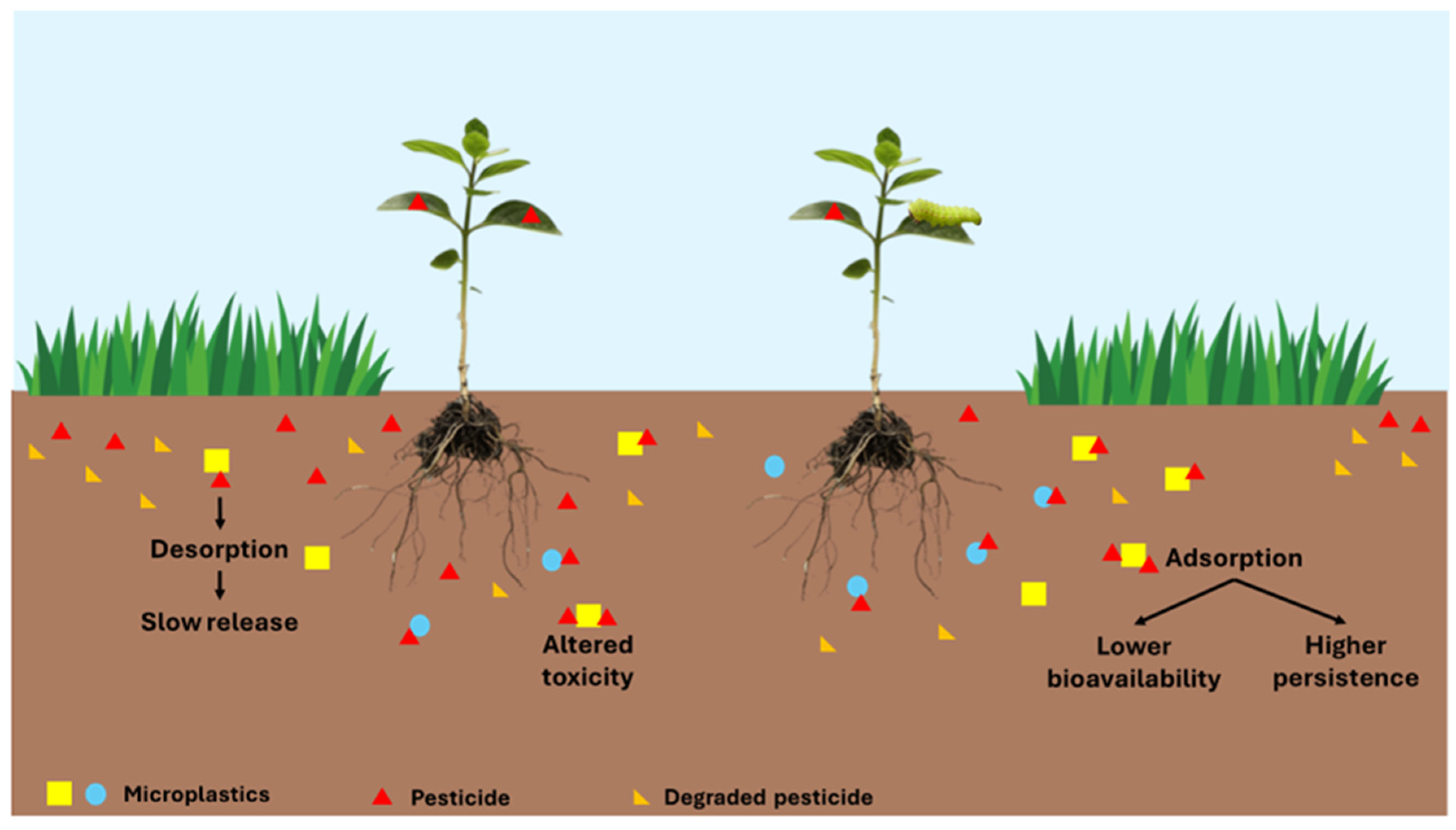
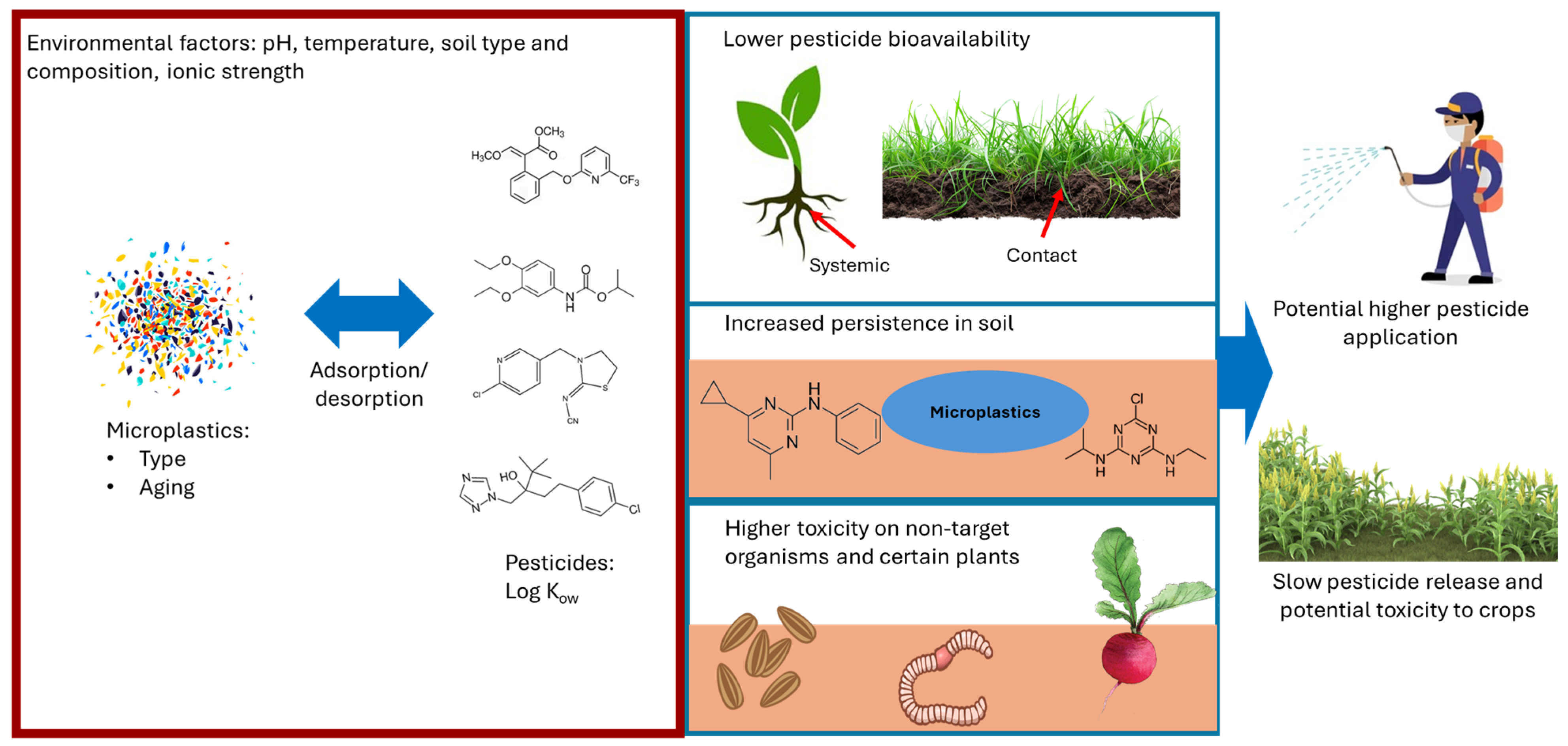
4. Effects on Pesticide Bioavailability
5. Effects on Pesticide Persistence
6. Effects on Pesticide Toxicity
7. Agricultural Implications
8. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, K.H.D.; Li, R. Aged Microplastics and Antibiotic Resistance Genes: A Review of Aging Effects on Their Interactions. Antibiotics 2024, 13, 941. [Google Scholar] [CrossRef]
- Tang, K.H.D. Microplastics in and Near Landlocked Countries of Central and East Asia: A Review of Occurrence and Characteristics. Trop. Aquat. Soil Pollut. 2023, 3, 120–130. [Google Scholar] [CrossRef]
- Tang, K.H.D. Interactions of microplastics with persistent organic pollutants and the ecotoxicological effects: A review. Trop. Aquat. Soil Pollut. 2021, 1, 24–34. [Google Scholar] [CrossRef]
- Sun, S.; Sidhu, V.; Rong, Y.; Zheng, Y. Pesticide Pollution in Agricultural Soils and Sustainable Remediation Methods: A Review. Curr. Pollut. Rep. 2018, 4, 240–250. [Google Scholar] [CrossRef]
- Hüesker, F.; Lepenies, R. Why does pesticide pollution in water persist? Environ. Sci. Policy 2022, 128, 185–193. [Google Scholar] [CrossRef]
- Tang, K.H.D. Microplastics and Antibiotics in Aquatic Environments: A Review of Their Interactions and Ecotoxicological Implications. Trop. Aquat. Soil Pollut. 2024, 4, 60–78. [Google Scholar] [CrossRef]
- Li, H.; Wang, F.; Li, J.; Deng, S.; Zhang, S. Adsorption of three pesticides on polyethylene microplastics in aqueous solutions: Kinetics, isotherms, thermodynamics, and molecular dynamics simulation. Chemosphere 2021, 264, 128556. [Google Scholar] [CrossRef]
- Wang, K.; Li, C.; Li, H.; Liu, Q.; Khan, K.; Li, F.; Chen, W.; Xu, L. Interactions of traditional and biodegradable microplastics with neonicotinoid pesticides. Sci. Total Environ. 2024, 947, 174512. [Google Scholar] [CrossRef] [PubMed]
- Mo, Q.; Yang, X.; Wang, J.; Xu, H.; Li, W.; Fan, Q.; Gao, S.; Yang, W.; Gao, C.; Liao, D.; et al. Adsorption mechanism of two pesticides on polyethylene and polypropylene microplastics: DFT calculations and particle size effects. Environ. Pollut. 2021, 291, 118120. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Wang, T.; Cao, F.; Yu, C.; Chu, Q.; Wang, F. A comparative study on the adsorption behavior of pesticides by pristine and aged microplastics from agricultural polyethylene soil films. Ecotoxicol. Environ. Saf. 2021, 209, 111781. [Google Scholar] [CrossRef]
- Barreto, M.; Lopes, I.; Oliveira, M. Micro(nano)plastics: A review on their interactions with pharmaceuticals and pesticides. TrAC Trends Anal. Chem. 2023, 169, 117307. [Google Scholar] [CrossRef]
- Bhagat, J.; Nishimura, N.; Shimada, Y. Toxicological interactions of microplastics/nanoplastics and environmental contaminants: Current knowledge and future perspectives. J. Hazard. Mater. 2021, 405, 123913. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Lehmann, A. Microplastic in terrestrial ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef]
- Lehmann, A.; Leifheit, E.F.; Gerdawischke, M.; Rillig, M.C. Microplastics have shape- and polymer-dependent effects on soil aggregation and organic matter loss—An experimental and meta-analytical approach. Microplastics Nanoplastics 2021, 1, 7. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of Microplastics on the Soil Biophysical Environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef]
- Zhang, G.S.; Zhang, F.X.; Li, X.T. Effects of polyester microfibers on soil physical properties: Perception from a field and a pot experiment. Sci. Total Environ. 2019, 670, 1–7. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Qiu, W.; Song, Z. Effect of microplastics and arsenic on nutrients and microorganisms in rice rhizosphere soil. Ecotoxicol. Environ. Saf. 2021, 211, 111899. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Q.; Hu, W.; Qin, J.; Zheng, Y.; Wang, J.; Wang, Q.; Xu, Y.; Guo, G.; Hu, S.; et al. Effects of plastic mulch film residues on soil-microbe-plant systems under different soil pH conditions. Chemosphere 2021, 267, 128901. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Sun, X.; Peng, Y.; Xiao, L. Mixing effect of polylactic acid microplastic and straw residue on soil property and ecological function. Chemosphere 2020, 243, 125271. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Z.; Zhu, D.; Lindhardt, J.H.; Lin, S.-M.; Ke, X.; Cui, L. Long-Term Fertilization History Alters Effects of Microplastics on Soil Properties, Microbial Communities, and Functions in Diverse Farmland Ecosystem. Environ. Sci. Technol. 2021, 55, 4658–4668. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, Z.; Zhu, F.; Zhu, C.; Wang, C.; Gu, C. Effect of Polyvinyl Chloride Microplastics on Bacterial Community and Nutrient Status in Two Agricultural Soils. Bull. Environ. Contam. Toxicol. 2021, 107, 602–609. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Q.; Sun, Y.; Zhang, S.; Wang, F. Microplastics change soil properties, heavy metal availability and bacterial community in a Pb-Zn-contaminated soil. J. Hazard. Mater. 2022, 424, 127364. [Google Scholar] [CrossRef]
- Hou, J.; Xu, X.; Yu, H.; Xi, B.; Tan, W. Comparing the long-term responses of soil microbial structures and diversities to polyethylene microplastics in different aggregate fractions. Environ. Int. 2021, 149, 106398. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Zhou, S.; Zhang, L.; Ding, S. The effects of three different microplastics on enzyme activities and microbial communities in soil. Water Environ. Res. 2021, 93, 24–32. [Google Scholar] [CrossRef]
- Sun, Y.; Duan, C.; Cao, N.; Li, X.; Li, X.; Chen, Y.; Huang, Y.; Wang, J. Effects of microplastics on soil microbiome: The impacts of polymer type, shape, and concentration. Sci. Total Environ. 2022, 806, 150516. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, X.; Liu, G.; Liang, C.; Xue, S.; Chen, H.; Ritsema, C.J.; Geissen, V. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere 2017, 185, 907–917. [Google Scholar] [CrossRef]
- Lian, J.; Liu, W.; Meng, L.; Wu, J.; Zeb, A.; Cheng, L.; Lian, Y.; Sun, H. Effects of microplastics derived from polymer-coated fertilizer on maize growth, rhizosphere, and soil properties. J. Clean. Prod. 2021, 318, 128571. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.X.F.; Doolette, C.L.; Vasileiadis, S.; Drigo, B.; Wyrsch, E.R.; Djordjevic, S.P.; Donner, E.; Karpouzas, D.G.; Lombi, E. Pesticide effects on nitrogen cycle related microbial functions and community composition. Sci. Total Environ. 2022, 807, 150734. [Google Scholar] [CrossRef] [PubMed]
- Junaid, M.; Abbas, Z.; Siddiqui, J.A.; Liu, S.; Tabraiz, S.; Yue, Q.; Wang, J. Ecotoxicological impacts associated with the interplay between micro(nano)plastics and pesticides in aquatic and terrestrial environments. TrAC Trends Anal. Chem. 2023, 165, 117133. [Google Scholar] [CrossRef]
- Yu, Y.; Mo, W.Y.; Luukkonen, T. Adsorption behaviour and interaction of organic micropollutants with nano and microplastics—A review. Sci. Total Environ. 2021, 797, 149140. [Google Scholar] [CrossRef]
- Tang, K.H.D. A review of the toxic effects of microplastics based on studies on mammals and mammalian cell lines. Environ. Sci. Adv. 2024, 3, 1669–1678. [Google Scholar] [CrossRef]
- Khalid, N.; Aqeel, M.; Noman, A.; Khan, S.M.; Akhter, N. Interactions and effects of microplastics with heavy metals in aquatic and terrestrial environments. Environ. Pollut. 2021, 290, 118104. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, M.; Ma, X.; Song, Y.; Zuo, S.; Li, H.; Deng, W. A critical review on the interactions of microplastics with heavy metals: Mechanism and their combined effect on organisms and humans. Sci. Total Environ. 2021, 788, 147620. [Google Scholar] [CrossRef]
- Sabzevari, S.; Hofman, J. A worldwide review of currently used pesticides’ monitoring in agricultural soils. Sci. Total Environ. 2022, 812, 152344. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, Y.; Tijjani, H.; Egbuna, C.; Adetunji, C.O.; Kala, S.; Kryeziu, T.L.; Ifemeje, J.C.; Patrick-Iwuanyanwu, K.C. Chapter 3—Pesticides, History, and Classification. In Natural Remedies for Pest, Disease and Weed Control; Egbuna, C., Sawicka, B., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 29–42. [Google Scholar]
- Statista. Agricultural Consumption of Pesticides per Area of Cropland Worldwide from 1990 to 2022. Available online: https://www.statista.com/statistics/1263369/global-pesticide-use-per-area/ (accessed on 20 December 2024).
- Fang, S.; Yu, W.; Li, C.; Liu, Y.; Qiu, J.; Kong, F. Adsorption behavior of three triazole fungicides on polystyrene microplastics. Sci. Total Environ. 2019, 691, 1119–1126. [Google Scholar] [CrossRef]
- Qiu, S.; Shen, H.; Song, J.; Fang, H.; Yu, Y.; Zhang, L. Different effects of polyethylene microplastics on bioaccumulation of three fungicides in maize (Zea mays L.). Crop Health 2024, 2, 7. [Google Scholar] [CrossRef]
- Hai, N.; Liu, X.; Li, Y.; Kong, F.; Zhang, Y.; Fang, S. Effects of Microplastics on the Adsorption and Bioavailability of Three Strobilurin Fungicides. ACS Omega 2020, 5, 30679–30686. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, P.; Yan, N.; Ren, Y.; Liang, X.; Guo, X. Adsorption of neonicotinoid insecticides by mulch film-derived microplastics and their combined toxicity. Sci. Total Environ. 2024, 955, 177238. [Google Scholar] [CrossRef]
- Ju, H.; Yang, X.; Tang, D.; Osman, R.; Geissen, V. Pesticide bioaccumulation in radish produced from soil contaminated with microplastics. Sci. Total Environ. 2024, 910, 168395. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Du, Y.; Yang, L.; Luo, Y.; Zhong, G.; Zhao, H.-M.; Liu, J. Effects of microplastics on the environmental behaviors of the herbicide atrazine in soil: Dissipation, adsorption, and bioconcentration. J. Hazard. Mater. 2024, 465, 133085. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Wu, Y.; Song, R.; Gao, Y.; Zhang, S.; Zhao, K.; Wu, T.; Zhang, C.; Du, F. The simple strategy to improve pesticide bioavailability and minimize environmental risk by effective and ecofriendly surfactants. Sci. Total Environ. 2022, 851, 158169. [Google Scholar] [CrossRef]
- Miranda, M.N.; Lado Ribeiro, A.R.; Silva, A.M.T.; Pereira, M.F.R. Can aged microplastics be transport vectors for organic micropollutants?—Sorption and phytotoxicity tests. Sci. Total Environ. 2022, 850, 158073. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.; Braunbeck, T. Bioavailability of microplastic-bound pollutants in vitro: The role of adsorbate lipophilicity and surfactants. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 221, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Zhao, D.; Wang, H.; Wei, T. Residues and Bioavailability of Neonicotinoid Pesticide in Shaanxi Agricultural Soil. Water Air Soil Pollut. 2023, 234, 129. [Google Scholar] [CrossRef]
- Chen, D.; Hao, G.; Song, B. Finding the Missing Property Concepts in Pesticide-Likeness. J. Agric. Food Chem. 2022, 70, 10090–10099. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, D.; Pan, Y.; Shen, Y.; Wang, X.; Xiong, F.; Han, J.; Zhang, Z.; Chen, Y.; Chen, Z.; et al. Interaction behavior, mechanisms and hazardous changes of microplastics on single and binary component pesticide in the environment and food: Diethofencarb and pyrimethanil. J. Hazard. Mater. 2024, 475, 134809. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Chen, B.; Yang, M.; Gao, S.; Wang, H.; Gu, W.; Li, X.; Zhang, B. A critical review on the interaction of polymer particles and co-existing contaminants: Adsorption mechanism, exposure factors, effects on plankton species. J. Hazard. Mater. 2023, 445, 130463. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.-X.; He, S.-X.; Li, G.; Teng, H.H.; Ma, L.Q. Application of diffusive gradients in thin-films technique for speciation, bioavailability, modeling and mapping of nutrients and contaminants in soils. Crit. Rev. Environ. Sci. Technol. 2022, 52, 3035–3079. [Google Scholar] [CrossRef]
- Song, M.; Su, Y.; Jiang, L.; Peng, K.; Li, J.; Liu, S.; Sun, Y.; Chen, C.-E.; Luo, C. Assessing the bioavailability of antibiotics in soil with the diffusive gradients in thin films (DGT). J. Hazard. Mater. 2023, 448, 130935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Han, B.; Sun, Y.; Wang, F. Microplastics influence the adsorption and desorption characteristics of Cd in an agricultural soil. J. Hazard. Mater. 2020, 388, 121775. [Google Scholar] [CrossRef] [PubMed]
- Ni, N.; Shi, R.; Meng, J.; Guo, X.; Shi, M.; Zhang, X.; Yao, S.; Nkoh, J.N.; Wang, F.; Song, Y.; et al. Comparative analysis of the sorption behaviors and mechanisms of amide herbicides on biodegradable and nondegradable microplastics derived from agricultural plastic products. Environ. Pollut. 2023, 318, 120865. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, J.; Lu, Y.; Hua, D.; Wang, X.; Zou, X. Biodegradable microplastics (BMPs): A new cause for concern? Environ. Sci. Pollut. Res. 2021, 28, 66511–66518. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Jiang, M.; Han, P.; Liang, G.; Zhang, T.; Liu, G. Comparative analysis on the sorption kinetics and isotherms of fipronil on nondegradable and biodegradable microplastics. Environ. Pollut. 2019, 254, 112927. [Google Scholar] [CrossRef]
- Sahai, H.; Hernando, M.D.; Martínez Bueno, M.J.; Aguilera del Real, A.M.; Fernández- Alba, A.R. Evaluation of the sorption/desorption processes of pesticides in biodegradable mulch films used in agriculture. Chemosphere 2024, 351, 141183. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.M.; da Rocha, G.O.; de Andrade, J.B. Customized dispersive micro-solid-phase extraction device combined with micro-desorption for the simultaneous determination of 39 multiclass pesticides in environmental water samples. J. Chromatogr. A 2021, 1639, 461781. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, P.; Berenstein, G.; Montserrat, J.M. Factors that influence the migration of sorbed pesticides in polyethylene and biodegradable mesoplastics. Environ. Pollut. 2025, 364, 125205. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.F.; Rohan, M.; Stodart, B.J.; Chen, C.; Wu, H.; Doran, G.S. Persistence of atrazine and trifluralin in a clay loam soil undergoing different temperature and moisture conditions. Environ. Pollut. 2021, 276, 116687. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Lacroix, C.; González Fernández, C.; Hégaret, H.; Lambert, C.; Le Goïc, N.; Frère, L.; Cassone, A.-L.; Sussarellu, R.; Fabioux, C.; et al. Exposure of marine mussels Mytilus spp. to polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environ. Pollut. 2016, 216, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Eckert, E.M.; Di Cesare, A.; Kettner, M.T.; Arias-Andres, M.; Fontaneto, D.; Grossart, H.-P.; Corno, G. Microplastics increase impact of treated wastewater on freshwater microbial community. Environ. Pollut. 2018, 234, 495–502. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Wang, F.; Sun, Q. Behavior and mechanism of atrazine adsorption on pristine and aged microplastics in the aquatic environment: Kinetic and thermodynamic studies. Chemosphere 2022, 292, 133425. [Google Scholar] [CrossRef] [PubMed]
- Junck, J.; Diagboya, P.N.; Peqini, A.; Rohnke, M.; Düring, R.-A. Mechanistic interpretation of the sorption of terbuthylazine pesticide onto aged microplastics. Environ. Pollut. 2024, 345, 123502. [Google Scholar] [CrossRef] [PubMed]
- Martinho, S.D.; Fernandes, V.C.; Figueiredo, S.A.; Vilarinho, R.; Moreira, J.A.; Delerue-Matos, C. Laboratory Studies about Microplastic Aging and Its Effects on the Adsorption of Chlorpyrifos. Polymers 2023, 15, 3468. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Dong, Z.; Li, Z.; Zhou, W.; Li, Y.; Xing, L.; Wu, T.; Lin, W.; Chang, H.; Li, B. Adsorption-desorption behavior of florpyrauxifen-benzyl on three microplastics in aqueous environment as well as its mechanism and various influencing factors. Ecotoxicol. Environ. Saf. 2024, 272, 116066. [Google Scholar] [CrossRef]
- Wang, F.; Gao, J.; Zhai, W.; Liu, D.; Zhou, Z.; Wang, P. The influence of polyethylene microplastics on pesticide residue and degradation in the aquatic environment. J. Hazard. Mater. 2020, 394, 122517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, N.; Hou, L.; Li, C.; Li, C. Adsorption behaviors of chlorpyrifos on UV aged microplastics. Mar. Pollut. Bull. 2023, 190, 114852. [Google Scholar] [CrossRef]
- Nie, E.; Guo, L.; Zhou, X.; Zhou, D.; Wang, H.; Ye, Q.; Yang, Z. Effects of charged polystyrene microplastics on the bioavailability of dufulin in tomato plant. J. Hazard. Mater. 2024, 467, 133748. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Qian, X.; Wang, C.; Zhang, C.; Tang, T.; Zhao, X.; Li, L. Environmentally relevant concentrations of microplastic exhibits negligible impacts on thiacloprid dissipation and enzyme activity in soil. Environ. Res. 2020, 189, 109892. [Google Scholar] [CrossRef] [PubMed]
- Schäffer, A.; Fenner, K.; Wang, Z.; Scheringer, M. To be or not to be degraded: In defense of persistence assessment of chemicals. Environ. Sci. Process. Impacts 2022, 24, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, Y.; Ju, H.; Tang, D.W.S.; Xue, S.; Geissen, V.; Yang, X. Chlorpyrifos degradation and its impacts on phosphorus bioavailability in microplastic-contaminated soil. Ecotoxicol. Environ. Saf. 2024, 277, 116378. [Google Scholar] [CrossRef]
- Elhaissoufi, W.; Ghoulam, C.; Barakat, A.; Zeroual, Y.; Bargaz, A. Phosphate bacterial solubilization: A key rhizosphere driving force enabling higher P use efficiency and crop productivity. J. Adv. Res. 2022, 38, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Concha-Graña, E.; Moscoso-Pérez, C.M.; López-Mahía, P.; Muniategui-Lorenzo, S. Adsorption of pesticides and personal care products on pristine and weathered microplastics in the marine environment. Comparison between bio-based and conventional plastics. Sci. Total Environ. 2022, 848, 157703. [Google Scholar] [CrossRef]
- Hu, M.; Huang, L.; Wang, Y.; Tan, H.; Yu, X. Insight into the effect of microplastics on the adsorption and degradation behavior of thiamethoxam in agricultural soils. Chemosphere 2023, 337, 139262. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, H.; Banfield, C.C.; Wen, Y.; Zang, H.; Dippold, M.A.; Charlton, A.; Jones, D.L. The microplastisphere: Biodegradable microplastics addition alters soil microbial community structure and function. Soil Biol. Biochem. 2021, 156, 108211. [Google Scholar] [CrossRef]
- Hu, M.; Hou, N.; Li, Y.; Liu, Y.; Zhang, H.; Zeng, D.; Tan, H. The effect of microplastics on behaviors of chiral imidazolinone herbicides in the aquatic environment: Residue, degradation and distribution. J. Hazard. Mater. 2021, 418, 126176. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Guo, Z.; Dong, Y.; Wu, X.; Zhang, W. Effects of microplastics on 3,5-dichloroaniline adsorption, degradation, bioaccumulation and phytotoxicity in soil-chive systems. Environ. Geochem. Health 2024, 46, 519. [Google Scholar] [CrossRef]
- Beriot, N.; Zornoza, R.; Lwanga, E.H.; Zomer, P.; van Schothorst, B.; Ozbolat, O.; Lloret, E.; Ortega, R.; Miralles, I.; Harkes, P.; et al. Intensive vegetable production under plastic mulch: A field study on soil plastic and pesticide residues and their effects on the soil microbiome. Sci. Total Environ. 2023, 900, 165179. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Pan, S.; Shan, Y.; Ma, Y.; Wang, D.; Song, X.; Hu, H.; Ren, X.; Ma, X.; Cui, J.; et al. Microplastics mulch film affects the environmental behavior of adsorption and degradation of pesticide residues in soil. Environ. Res. 2022, 214, 114133. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Bento, C.P.M.; Chen, H.; Zhang, H.; Xue, S.; Lwanga, E.H.; Zomer, P.; Ritsema, C.J.; Geissen, V. Influence of microplastic addition on glyphosate decay and soil microbial activities in Chinese loess soil. Environ. Pollut. 2018, 242, 338–347. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, Y.; Li, J.; Wang, F.; Xia, S.; Zhao, J. Biofilm alters tetracycline and copper adsorption behaviors onto polyethylene microplastics. Chem. Eng. J. 2020, 392, 123808. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Li, R. The effects of plastisphere on the physicochemical properties of microplastics. Bioprocess Biosyst. Eng. 2024, 48, 1–15. [Google Scholar] [CrossRef]
- Lin, Y.; Xie, J.; Xiang, Q.; Liu, Y.; Wang, P.; Wu, Y.; Zhou, Y. Effect of propiconazole on plastic film microplastic degradation: Focusing on the change in microplastic morphology and heavy metal distribution. Sci. Total Environ. 2022, 822, 153609. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.H.D. Effects of microplastics on agriculture: A mini-review. Asian J. Environ. Ecol. 2020, 13, 1–9. [Google Scholar] [CrossRef]
- Zeng, Z.; Jia, B.; Liu, X.; Chen, L.; Zhang, P.; Qing, T.; Feng, B. Adsorption behavior of triazine pesticides on polystyrene microplastics aging with different processes in natural environment. Environ. Pollut. 2024, 356, 124319. [Google Scholar] [CrossRef]
- Yu, H.; Peng, J.; Cao, X.; Wang, Y.; Zhang, Z.; Xu, Y.; Qi, W. Effects of microplastics and glyphosate on growth rate, morphological plasticity, photosynthesis, and oxidative stress in the aquatic species Salvinia cucullata. Environ. Pollut. 2021, 279, 116900. [Google Scholar] [CrossRef]
- Shorobi, F.M.; Vyavahare, G.D.; Seok, Y.J.; Park, J.H. Effect of polypropylene microplastics on seed germination and nutrient uptake of tomato and cherry tomato plants. Chemosphere 2023, 329, 138679. [Google Scholar] [CrossRef]
- Schon, N.L.; Fraser, P.M.; Mackay, A.D. Earthworms for inclusion as an indicator of soil biological health in New Zealand pastures. New Zealand J. Agric. Res. 2023, 66, 208–223. [Google Scholar] [CrossRef]
- Fu, H.; Zhu, L.; Mao, L.; Zhang, L.; Zhang, Y.; Chang, Y.; Liu, X.; Jiang, H. Combined ecotoxicological effects of different-sized polyethylene microplastics and imidacloprid on the earthworms (Eisenia fetida). Sci. Total Environ. 2023, 870, 161795. [Google Scholar] [CrossRef] [PubMed]
- Gautam, K.; Dwivedi, S.; Verma, R.; Vamadevan, B.; Patnaik, S.; Anbumani, S. Combined effects of polyethylene microplastics and carbendazim on Eisenia fetida: A comprehensive ecotoxicological study. Environ. Pollut. 2024, 348, 123854. [Google Scholar] [CrossRef]
- Selonen, S.; Jemec Kokalj, A.; Benguedouar, H.; Alavian Petroody, S.S.; Dolar, A.; Drobne, D.; van Gestel, C.A.M. Modulation of chlorpyrifos toxicity to soil arthropods by simultaneous exposure to polyester microfibers or tire particle microplastics. Appl. Soil Ecol. 2023, 181, 104657. [Google Scholar] [CrossRef]
- Zhang, N.; Xie, F.; Guo, Q.N.; Yang, H. Environmental disappearance of acetochlor and its bioavailability to weed: A general prototype for reduced herbicide application instruction. Chemosphere 2021, 265, 129108. [Google Scholar] [CrossRef] [PubMed]
- Felten, V.; Toumi, H.; Masfaraud, J.-F.; Billoir, E.; Camara, B.I.; Férard, J.-F. Microplastics enhance Daphnia magna sensitivity to the pyrethroid insecticide deltamethrin: Effects on life history traits. Sci. Total Environ. 2020, 714, 136567. [Google Scholar] [CrossRef]

| Pesticide | Mode of Action | Specific Action |
|---|---|---|
| Fungicide | ||
| Azoxystrobin | Systemic | Strobilurin; inhibits mitochondrial respiration |
| Picoxystrobin | Systemic | |
| Pyraclostrobin | Systemic | |
| Metalaxyl | Systemic | Targets RNA polymerase; used against oomycetes |
| Tebuconazole | Systemic | Triazole; inhibits sterol biosynthesis |
| Diethofencarb | Contact | Carbamate fungicide; inhibits fungal respiration |
| Pyrimethanil | Systemic | Anilinopyrimidine; inhibits methionine biosynthesis |
| Cyprodinil | Systemic | |
| Difenoconazole | Systemic | Triazole; inhibits sterol biosynthesis |
| Myclobutanil | Systemic | |
| Flutriafol | Systemic | |
| Hexaconazole | Systemic | |
| Epoxiconazole | Systemic | |
| Triadimenol | Systemic | |
| Procymidone | Contact | Dicarboximide; inhibits lipid peroxidation and fungal cell membrane function |
| Fluxapyroxad | Systemic | Inhibits mitochondrial respiration |
| Fluopyram | Systemic | |
| Insecticide | ||
| Acetamiprid | Systemic | Neonicotinoid; acts as a nicotinic acetylcholine receptor agonist |
| Thiacloprid | Systemic | |
| Fipronil | Systemic | Phenylpyrazole; GABA receptor antagonist, causing hyperexcitation |
| Bifenthrin | Contact | Pyrethroid; disrupts sodium channel function in the nervous system |
| Etofenprox | Contact | Pyrethroid-like action; disrupts sodium channel function |
| Fenazaquin | Contact | Mitochondrial electron transport inhibitor |
| Lufenuron | Systemic | Benzoylurea; inhibits chitin synthesis |
| Pyridaben | Contact | Mitochondrial complex I electron transport inhibitor |
| Pyridalyl | Contact | Disrupts protein synthesis in insect cells |
| Pyriproxyfen | Contact | Juvenile hormone analog; disrupts insect development and reproduction |
| Carbenzapim | Contact | Neurotoxin; specific mode of action not well documented |
| Chlorantraniliprole | Systemic | Diamide; activates ryanodine receptors, causing calcium release and muscle paralysis |
| Chlorpyrifos | Contact | Organophosphate; acetylcholinesterase inhibitor |
| Herbicide | ||
| Acetochlor | Systemic | Chloroacetamide; inhibits cell division |
| Napropamide | Systemic | Amide herbicide; inhibits root and shoot growth |
| Atrazine | Systemic | Triazine; inhibits photosystem II |
| Simazine | Systemic | |
| Terbuthylazine | Systemic | |
| Metolachlor | Systemic | Chloroacetamide; inhibits cell division |
| Trifluralin | Systemic | Dinitroaniline; prevents cell division |
| Imazamox | Systemic | ALS inhibitor; blocks branched-chain amino acid synthesis |
| Imazapic | Systemic | |
| Imazethapyr | Systemic | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, K.H.D. Effects of Microplastics on Bioavailability, Persistence and Toxicity of Plant Pesticides: An Agricultural Perspective. Agriculture 2025, 15, 356. https://doi.org/10.3390/agriculture15040356
Tang KHD. Effects of Microplastics on Bioavailability, Persistence and Toxicity of Plant Pesticides: An Agricultural Perspective. Agriculture. 2025; 15(4):356. https://doi.org/10.3390/agriculture15040356
Chicago/Turabian StyleTang, Kuok Ho Daniel. 2025. "Effects of Microplastics on Bioavailability, Persistence and Toxicity of Plant Pesticides: An Agricultural Perspective" Agriculture 15, no. 4: 356. https://doi.org/10.3390/agriculture15040356
APA StyleTang, K. H. D. (2025). Effects of Microplastics on Bioavailability, Persistence and Toxicity of Plant Pesticides: An Agricultural Perspective. Agriculture, 15(4), 356. https://doi.org/10.3390/agriculture15040356





