Abstract
Reducing nitrogen excretion in dairy cattle is a critical factor for improving the environmental sustainability of the livestock industry. This research aimed to estimate the genetic parameters over time for the milk urea nitrogen (MUN) trait in Iranian Holstein dairy cattle. Data from 347,639 test-day records of 52,219 first-parity Iranian Holstein dairy cows (spanning 2018 to 2023), were sourced from the Iranian National Animal Breeding Center. A single-trait random regression test-day animal model was used for the genetic evaluation of MUN. Three orders of Legendre orthogonal polynomials (ranging from 1 to 3) were tested to fit the fixed curve, additive genetic effects, and permanent environmental effects. Based on the AIC, BIC, and residual variances to compare the models, the third order was considered as the appropriate order for this dataset. The average heritability and repeatability of the MUN trait were estimated to be 0.027 and 0.081, respectively. The average estimates for additive genetic variance, permanent environmental variance, and phenotypic variance were 0.14, 0.28, and 5.17, respectively. The genetic trend analysis revealed that the MUN trait exhibited fluctuations across birth years (2016–2021), with an overall negative trend. Importantly, the average MUN levels remained within the desirable range of 13–16 mg/dL for Iranian Holstein cows across calving years from 2019 to 2023. Despite the low heritability estimates, the genetic parameters obtained in this study are valuable for improving MUN in Iranian dairy cattle. These findings provide critical insights for designing effective breeding programs aimed at reducing nitrogen excretion and promoting environmental sustainability in the dairy industry.
1. Introduction
As an important source of basic nutrients and active substances, milk is considered a nearly perfect food [1]. It plays a significant role in meeting human nutritional needs and improving quality of life. However, the dairy industry faces increasing pressure to reduce production costs, driven by rising feed and labor expenses, which impact the profitability of commercial dairy farms. Additionally, the livestock industry is under growing pressure to mitigate environmental pollution associated with dairy farming. Recent studies indicate that the livestock sector and its related supply chains contribute approximately 30% of total nitrogen emissions [2,3,4].
Milk urea nitrogen (MUN) reflects the urea concentration in milk and serves as an indicator of protein metabolism in dairy cows. Conversely, blood urea nitrogen provides insights into overall nitrogen balance and renal function. Under normal circumstances, nearly 28% of the nitrogen ingested by dairy cows is secreted into milk, with 5% excreted as non-protein nitrogen and the remainder as true protein [5]. Reducing nitrogen excretion in milk production is crucial due to the environmental impact of animal husbandry. Livestock manure releases nitrogen, which can volatilize into the atmosphere [6] or leach into water sources [7,8]. Volatilized ammonia and leached nitrates pose significant environmental risks and human health hazards [9]. Animal agriculture is a major contributor to diffuse water pollution through ammonia volatilization, nitrate leaching, and nitrate runoff [10]. It reportedly accounts for 36% of ammonia emissions in Europe [10]. Therefore, improving nitrogen efficiency in milk production is essential for reducing greenhouse gas emissions and minimizing the environmental footprint of dairy farming [11].
The concentration of MUN is influenced by various factors, encompassing both nutritional and non-nutritional factors. Non-nutritional factors include breed, lactation stage, and parity, with breed being the most influential [12]. For instance, Holstein cows generally exhibit lower MUN concentrations compared with other dairy breeds [13,14]. Among the nutritional factors, the quantity and composition of dietary protein are the primary determinants of MUN concentration [15,16,17]. Therefore, establishing appropriate MUN thresholds is crucial for accurately assessing the nutritional balance of the diet and the post-feeding response of dairy cows. Based on the existing literature, MUN levels on commercial farms should not exceed 18 mg/dL [18]. If the MUN levels in the herd exceed or fall below this range, it is necessary to evaluate dietary protein and energy sources. For high-yielding dairy herds (annual milk production > 12,000 kg), the optimal MUN range is between 10 and 16 mg/dL [19,20]. Selecting cows with lower MUN concentrations could reduce the environmental impact of dairy farming by improving nitrogen utilization efficiency and minimizing agricultural nitrogen waste [21].
Previous studies have demonstrated considerable heterogeneity in genetic parameters for MUN across different populations and datasets [22,23,24]. For instance, heritability estimates for MUN have been reported to range widely, from 0.06 to 0.44, influenced by factors such as herd size, number of test-day records per animal, and period of data collection, e.g., [11,25,26,27]. Moreover, most of these studies have relied on repeatability models, which assume constant genetic parameters throughout lactation [28]. However, recent studies, including the findings of Chen et al. [29,30], have demonstrated significant variations in the genetic variance of milk urea concentration throughout lactation, underscoring the importance of accounting for temporal variation in genetic parameters when evaluating MUN.
Despite these advancements, our understanding of the genetic architecture of MUN in Iranian Holstein dairy cattle remains limited, particularly regarding how variance components and genetic parameters change over time. Considering the breed’s significant economic importance to Iran and the growing emphasis on improving nitrogen utilization efficiency to mitigate environmental impacts, a more precise understanding of MUN’s genetic basis is crucial. Random regression models (RRMs) offer a powerful tool to address this knowledge gap. By enabling the fitting of random genetic and environmental effects over time, RRMs provide higher accuracy of estimated breeding values compared with other statistical approaches. Furthermore, RRMs offer valuable insights into the temporal variation of biological processes and the underlying physiological implications associated with the studied trait [28]. Therefore, the primary objective of this study is to estimate variance components and genetic parameters for MUN over time using an RRM for Iranian Holstein dairy cattle.
2. Materials and Methods
Data were obtained from the Iranian National Animal Breeding Center’s existing database. Therefore, Animal Care and Use Committee approval was not needed for this study.
2.1. Data and Quality Control
In this study, 347,639 test-day records from 52,219 first-parity Iranian Holstein dairy cows raised in 297 different herds were analyzed. The data were collected and registered in the Iranian National Animal Breeding Center (NABC) database between 2018 and 2023. While many cows had multiple records, analyses were restricted to the first lactation per cow to reduce computational demands. Days in milk (DIM) intervals were categorized into ten groups: 5–30 DIM, 30–60 DIM, and so on, up to 270–305 DIM. The original dataset included information regarding animal identification, date of record, birth date, type of record, herd, sire, dam, calving date, DIM, age at the first calving, and MUN.
Quality control was performed based on the following criteria: (1) only first-lactation dairy cows with at least three test-day records were included; (2) data for milk urea nitrogen (MUN) were restricted to the lactation period between 5 and 305 days in milk (DIM); (3) MUN values were restricted to a range from 7 to 26 mg/dL; i.e., only records within the range of the mean ± 3 standard deviations (SDs) were kept in the data set. Moreover, only cows that calved between 18 and 41 months of age were included in the analysis. Quality control procedures were performed using R software (version 4.4.1; [31]). A few packages were used for data cleaning and filtering, including dplyr and data.table [32]. Variance components were then estimated using the restricted maximum likelihood (REML) method (AI-REML), with a single-trait animal linear model implemented in the BLUPf90 software (version 1.71; [33]).
2.2. Pedigree File and Fixed Effects
The original pedigree file included 3,309,990 individuals, spanning 51 generations. Their birthdates ranged from 1986 to 2021. Pedigree structure and connectivity were analyzed using R [34] and CFC software version 1.0 [35], respectively. Animals unrelated to the animals with MUN records were removed from the main pedigree, resulting in a final pedigree file containing 235,277 individuals. This method, developed by Sewall Wright in 1922, involves quantifying the likelihood that two alleles at a given locus in an individual are identical by descent from a common ancestor. By analyzing the pedigree data and applying Wright’s inbreeding coefficient formula, we were able to estimate the level of inbreeding within our population [36]. A summary of the pedigree file (before and after quality control) is shown in Table 1.

Table 1.
Summary of the pedigree file used in this study.
2.3. Phenotypic Analyses of Fixed Effects
For the phenotypic statistical analysis, the lm function available in the R software was used. A fixed effects model was fitted with herd–year–month of test day (HTD) as a time-independent effect and age–year–season (AYS) as a regression effect. Effects were considered significant when p-value < 0.05.
2.4. Random Regression Models and Estimation of Variance Components
Variance components were estimated using the BLUPf90 family software (AIREMLf90; [33]), based on a single-trait random regression test-day animal model. A total of three orders of Legendre orthogonal polynomials were tested, ranging from the first to third order. The general random regression model can be described as follows:
where is the test-day record j for cattle k in the ith herd test-date subclass. HTDi is the fixed effect of herd test-day classes, independent of time. is the lth fixed regression coefficient for the effect of age–year–season of calving k. is the nth random regression coefficient for the additive genetic effect of animal k. is the oth random regression coefficient for the permanent environmental effect of animal k. Φx is Legendre’s polynomial coefficient for standardized DIM, p is the order of the Legendre polynomials used for the fixed and random regressions (assumed all the same order), and is the random residual effect (error). In matrix form, this model can be defined as:
In this model, y is the vector of test-day MUN records, b is the vector of the fixed effects (HTD and the AYS fixed regression), a and pe are the vectors of the random additive genetic and permanent environmental effects, respectively, and e is the vector of residual effects. X, Z, and W are the corresponding design matrices for the fixed and random effects. The (co)variance structure of the above model is defined as follows:
where G is the genetic covariance matrix of random regression coefficients, ⊗ denotes the Kronecker product, A is the pedigree-based relationship matrix among animals, containing up to 22 generations of the phenotyped animals, P is the permanent environmental covariance matrix of the random regression coefficients, is the identity matrix of dimension w (number of cows with records), and R is the diagonal matrix of residual variances.
2.5. Polynomial Order Comparison
In this study, a random regression model using Legendre polynomials of the first to third order was applied for fixed effects, additive genetic effects, and permanent environmental effects. To identify the best fit model, both residual variance and the Akaike information criterion (AIC) related to BLUPf90 output (blupf90.log), as well as the Bayesian Information Criterion (BIC), were considered. The fitted model was selected based on the lowest residual variance, AIC, and BIC value. The BIC was calculated as follows [37]:
where −2logL is the restricted maximum log likelihood value, p is the total number of parameters estimated in the corresponding model, and n is the difference between the number of test-day records and the rank of the fixed effects design matrix.
BIC = −2logL + plog(n),
2.6. Genetic Parameters
The optimal model included the fixed herd test-day effect, a third-order Legendre polynomial for age at calving, and the random additive genetic effects of the animals and permanent environmental effects. Therefore, genetic parameters (i.e., genetic correlations among different DIM, heritability, and repeatability) were estimated using this optimal model. The genetic correlation between test days was calculated as follows [38]:
where Ka represents the additive genetic covariance structure, Φi denotes Legendre’s polynomial coefficients for day i, and Φj is Legendre’s polynomial coefficients on day j. Heritability for each day of lactation was estimated as follows [38]:
where represents the heritability for test day i, Ka is the additive genetic covariance matrix, Kpe is the permanent environmental covariance matrix, Φi denotes Legendre’s polynomial coefficients for day i, and is the residual variance. Repeatability was estimated as follows [38]:
where Ri is the repeatability, Ka is the additive genetic covariance matrix, Kpe is the permanent environmental covariance matrix, Φi is Legendre’s polynomial coefficients for day i, and indicates the residual variance.
2.7. Estimated Breeding Values and Genetic Trends
Estimated breeding values (EBVs) were predicted using the BLUPf90 program. Therefore, the coefficients estimated for the additive genetic effect were paired with the corresponding animal codes and Legendre’s polynomial coefficients. The model used can be described as follows:
where BVij is the estimated breeding value for animal i on day j; Φ0j, Φ1j, and Φ2j are the auxiliary variables of the Legendre polynomials; and ai0, ai1, and ai2 denote the estimated regression coefficients for the breeding value of animal i. Finally, the total (accumulated) breeding value for MUN for each animal over a lactation period was estimated using the following procedure:
where all terms are as previously described. Subsequently, the calculated value was divided by 300 (average MUN).
The genetic trend for MUN was evaluated by calculating the mean EBV for animals born each year. The EBV of MUN was obtained from the genetic parameter estimation of the BLUPF90 results. Data were prepared and analyzed using R software. Data were filtered to include only animals with complete records for MUN and their birth year. The mean EBV for MUN was calculated for the entire population, males and females, for each year using the dplyr R package. The genetic trend was visualized by plotting the mean EBV for MUN against the year of birth for each group using the ggplot2 R package [39]. The plots were arranged using the gridExtra R package [40].
3. Results and Discussion
3.1. Descriptive Statistics
After data quality control, a total of 347,639 observations from 52,219 cows remained in the data file. The trend of MUN over DIM is shown in Figure 1, and the descriptive statistics of MUN are shown in Table 2. Ma et al. [11] reported that MUN levels in dairy cows typically exhibit a gradual increase during early lactation (0–100 DIM), peaking between 100 and 150 DIM, and then declining in late lactation for first- and second-parity cows. However, they observed an upward trend in MUN for third-parity cows, potentially attributed to a reduced number of MUN records after 300 DIM in this parity group. This finding aligns with the observed upward phenotypic trend of MUN in Holstein cows in Iran, which could be influenced by factors such as genetic diversity within the cattle population, fluctuations in herd management practices, measurement errors, and environmental conditions.
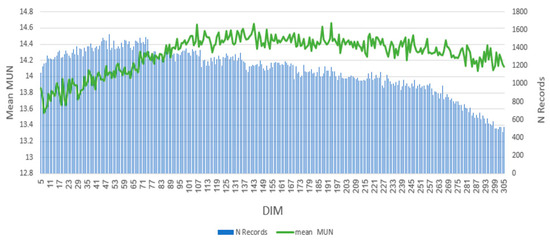
Figure 1.
Concentrations of milk urea nitrogen (MUN) on different days in milk (DIM).

Table 2.
Descriptive statistics of phenotypic records used in in this study.
A previous study [41] reported that the lowest MUN values were observed at the beginning of lactation, followed by a rapid increase with increasing DIM. The MUN peaked at mid-lactation and then slightly decreased toward the end of lactation. The coefficient of variation (CV %) of milk urea nitrogen (MUN) was 32% in both lactations. Environmental factors, including herd, calving year, calving season, age at first calving, parity, and lactation stage significantly influenced MUN (p < 0.05) [41]. In our study, the phenotypic mean (SD) of MUN in first-parity cows was 14.27 (2.47) mg/dL (Table 2). Consistent with previous findings, MUN levels in our study population also increased during early lactation (5–105 DIM), reaching a maximum within 105–205 DIM, and subsequently declined in late lactation.
The declining number of records with increasing DIM, especially toward the end of lactation, could influence the precision of the estimated mean MUN. This indicates that the results from these later stages may be subject to higher levels of uncertainty. A more in-depth analysis of the peak mean MUN and the rate of its decline in the later stages of lactation could offer valuable insights into protein metabolism and overall cow performance during the lactation period [42]. Furthermore, exploring the correlation between changes in mean MUN and other reproductive and health variables could contribute to a better understanding of the factors affecting this indicator. These findings could inform future studies aimed at improving our understanding of MUN dynamics in dairy cows.
3.2. Phenotypic Analyses of Fixed Effects
The results of the statistical analysis and significant test of fixed effects is shown in Table 3. In summary, the herd test-day effect is one of the most important factors affecting MUN levels. In a study aimed at estimating the genetic parameters of MUN, it was concluded that one of the most important environmental factors affecting this trait was herd test day (HTD), and its effect was reported to be highly significant [22]. In other studies that aimed to analyze the genetic parameters of MUN, the herd test-day effect was considered as a fixed effect independent of time in the random regression model [23,26].

Table 3.
Information on the fixed effects and their significance.
Table 3 shows the results of the statistical analysis and significant test of fixed effects. The results indicate that state, herd test days (HTD), age at calving (AC), calving year (CY), season, age–year–season (AYS), and days in milk (DIM) had highly significant effects on the trait (p < 0.001). It has been reported that MUN levels in the first month of lactation were higher in cows that calved in summer compared to other seasons, while in the fourth month of lactation, MUN levels in cows that calved in autumn were significantly lower than those that calved in other seasons. According to the result of Hojman et al. [18], MUN levels exhibit a high genetic correlation between different lactation periods and are also influenced by season, with concentrations reported to decrease to 11.8 mg/dL in winter and increase to 18.1 mg/dL in summer and spring. Abdouli et al. [43] reported that the highest MUN level in summer was 13.17 mg, and the lowest was 12.82 in winter [43]. In the studies of Wattiaux et al. [44] and Fatehi at al. [45], it was reported that MUN concentration varies not only in different seasons of the year, but also throughout each season [37,38]. Different results have been reported regarding the relationship between dairy cow breed and MUN levels. For example, Kauffman and St-Pierre [46] reported that the effects of breed on MUN and BUN were non-significant, but this effect was significant on other milk components such as fat and protein [46]. However, Doska et al. [47], using the analysis of test-day records from different herds, proved that there are different MUN values among herds with different breeds, and the amount of MUN was estimated to be higher in Ayrshire and Brown Swiss breeds than in Holstein [47]. Also, Kessler et al. [48] proved that Brown Swiss cows have higher MUN compared to Holstein under completely identical management conditions, and this difference is not due to milk production but due to the genetic difference between the two breeds [48]. Other factors affecting MUN levels include milk yield, milking frequency, calving age, milk protein, milk fat, and milk volume [49]. In the study of Mutsvangwa et al. [50] and also of Guliński et al. [51], crude protein intake and digestible and indigestible protein in the rumen were identified as influencing factors [50,51].
Figure 2a illustrates the average values for the MUN trait across different seasons of the year. The results of this study indicated significant seasonal variations in MUN levels in Holstein dairy cows in Iran. The highest MUN levels were observed in spring, followed by summer and winter, with the lowest values occurring in autumn. These findings suggest that dietary protein requirements were not always met throughout the year, leading to suboptimal nitrogen utilization. The seasonal variations in MUN can be attributed to several factors, including changes in dietary protein content, ambient temperature, and photoperiod. To improve nitrogen utilization and reduce environmental pollution, it is recommended to adjust dietary protein levels based on the seasonal changes in feed quality and cow requirements.
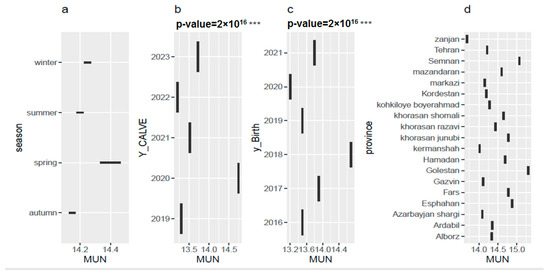
Figure 2.
Average milk urea nitrogen (MUN) levels (a) across different seasons, (b) according to the calving year, (c) according to the year of birth, and (d) in different provinces of Iran, based on model number 3. *** means that this effect are significant in p-value under 0.001.
Figure 2b depicts the average values of MUN across calving years in Iranian Holstein cows. The MUN levels remained within the desirable range of 13 to 15 mg/dL between 2019 and 2023. Interestingly, a slight downward trend was observed, with MUN levels decreasing from 14.74 mg/dL in 2020 to 13.72 mg/dL in 2023. This trend suggests a potential improvement in nitrogen utilization efficiency by the cows. Dietary changes, enhanced cow performance, or improved herd management practices could be contributing factors to this observation.
Figure 2c shows average MUN levels in Iranian Holsteins across birth years (2016–2021) for a 305-day lactation period. The average MUN (14.40 mg/dL) in this study falls within the range reported for Holstein Friesian, Jersey, and their crosses by Beatson et al. [52], but is lower than values observed in New Zealand pasture-fed cattle with a minimum of 17% crude protein [53]. This suggests potential differences in feed management practices between these populations.
Figure 2d presents the average MUN trait across different provinces of Iran. Golestan province exhibits the highest MUN levels compared to the other provinces, while Zanjan province records the lowest MUN levels. Factors such as diet, production system, dairy farm health care, climate, management system and genetics of the animals may influence MUN variations among the provinces of Iran. A full understanding of the relationship between genetics and MUN can help develop new strategies to improve the nutritional efficiency and health of dairy cows.
3.3. Polynomial Order and Fixed Effects
Our analysis explored the optimal polynomial order for modeling MUN in the Iranian Holstein population. Previous studies have reported varying degrees of polynomial complexity for modeling MUN. For instance, Mitchell et al. [23] employed a third-degree function for fixed effects and a fourth-degree function for random effects. Similarly, Mucha and Strandberg [26] used a fourth-degree polynomial order for random effects and an eighth-degree polynomial order for fixed effects. In our study, the model with a third-order Legendre polynomial (Model 3) exhibited the lowest residual variance (Table 4), suggesting a better goodness of fit to the data. This finding aligns with previous research that has demonstrated the effectiveness of higher-order polynomials in capturing complex relationships between predictors and MUN. Table 4 provides a summary of the model comparison, including residual variance, −2logL, AIC, and BIC values; so, in the model number 3 estimate the lower values of −2logL, AIC, and BIC and indicates a better goodness of fit.

Table 4.
Model comparison criteria based on a different Legendre polynomial order for fixed effect (nFE), additive genetic effect (nAE), and permanent environmental effect (nPE).
3.4. Variance Components
The range of additive genetic variance for MUN has been estimated to fluctuate between 0.06 and 0.48, with an average of 0.14. The highest values were observed during the initial and final days of the lactation period, while the values remained relatively stable from approximately day 70 to day 270 of lactation (Figure 3A). This value is lower than the average reported by Ma et al. [11] of 0.47. Moreover, in the study conducted by Bobbo et al. [27], which aimed to estimate genetic parameters for Brown Swiss cattle, the average additive genetic variance was estimated to be 4.03, which is higher than the values estimated for the Holstein breed. This suggests that there may be greater genetic variability for MUN within the Brown Swiss population compared to Holsteins. The beginning and end of lactation are critical periods for data collection, as they provide valuable insights into the animal’s physiological changes. More data points from these stages will enhance the precision of our estimates.
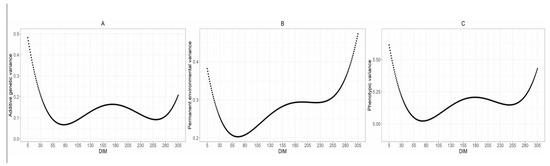
Figure 3.
(A) Additive genetic variance, (B) permanent environmental variance, and (C) phenotypic variance of milk urea nitrogen across different days in milk (DIM).
Permanent environmental variance, a component of phenotypic variance, represents the effects of environmental factors that remain relatively constant over time and influence the trait of interest. In this study, MUN levels peaked during the late lactation period (Figure 3B). The estimated range of permanent environmental variance fluctuated between 0.20 and 0.47, with an average of 0.28. These findings can provide valuable insights for dairy farmers and nutritionists in monitoring cow health and optimizing feeding strategies. The pattern of the permanent environmental variance curve at the beginning of lactation aligns with trends reported by Miglior et al. [54]. However, our study observed a wider range (0.15–0.23) compared to the narrower range (0.01–0.03) reported by Miglior et al. [54]. Additionally, the average permanent environmental variance reported for the Brown Swiss breed by Bobbo et al. [27] (i.e., 2.10) was significantly higher than the average value observed in this study for Iranian Holstein dairy cattle.
The phenotypic variance is the sum of additive genetic, permanent environmental, and residual variances. Since residual variance was assumed to be homogeneous, it was estimated at 4.75 for all DIM. Figure 3C represent the phenotypic variance of the MUN trait as a function of lactation days. The shape of the phenotypic variance curve resembles that of the additive genetic variance curve, with an average of 5.17. This value is lower than the average reported by Stoop et al. [25] of 18.64.
3.5. Genetic Parameters
The average heritability of the MUN trait during the first lactation period was estimated to be 0.027 (Figure 4A). Heritability of MUN is highest in the initial and final days of the lactation period, with a moderate trend observed between 70 and 270 days. These findings suggest lower heritability estimates compared to previous studies. For instance, Miglior et al. [54] reported heritability estimates of 0.394, 0.384, and 0.414 for MUN during the first, second, and third lactation periods, respectively, in Canadian Holsteins. Wood et al. [22] reported estimates between 0.44 and 0.59 for Ontario cattle. Mitchell et al. [23] obtained values of 0.22 and 0.14 using infrared and wet chemistry methods, respectively. Additionally, Van den Berg et al. [55] documented a range of 0.08 to 0.32 for various urea traits, with Jerseys and New Zealand cows exhibiting higher heritability compared with Holsteins and Australian cows, respectively. Mucha and Strandberg [26] attributed the variation in heritability estimates across studies to factors such as herd size, the number of test-day records per animal, the number of progeny per sire, and the duration of data collection. Further research is warranted to investigate the underlying causes of these discrepancies, evaluate the accuracy of different reference populations, and estimate genetic correlations between MUN and other traits, such as fertility and feed intake [55].
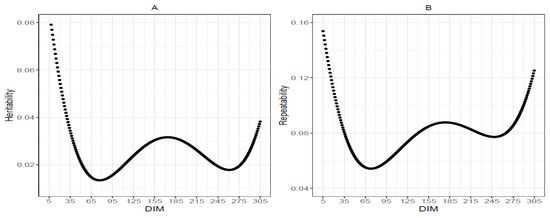
Figure 4.
(A) Heritability and (B) Repeatability of milk urea nitrogen over different Days In Milk (DIM).
Repeatability is defined as the reliability of measurements for the same trait taken from the same animal over time. It is typically expressed as a range or an average of differences between replicate measurements. A lower range and average indicate higher repeatability, meaning the measurements are more consistent. In this study, the repeatability of MUN ranged from 0.05 to 0.15, with a mean value of 0.08 (Figure 4B). This value is lower than those reported in previous studies, which were 0.38 [52] and 0.43 [25]. Similarly, Mitchell et al. [23] reported higher repeatability of 0.46 and 0.37 using infrared and wet chemistry methods, respectively, for the first-lactation cattle. Ma et al. [11] also reported higher repeatability of 0.12 for MUN. Several factors can influence repeatability, including measurement methods, sample type, and laboratory conditions. Accurate interpretation of the study’s results necessitates careful consideration of the various factors that can influence repeatability. Comparing repeatability values across different studies without accounting for these factors may lead to misleading conclusions.
Figure 5A,B illustrate the additive genetic and permanent environmental correlations across different lactation days. The highest genetic correlations are observed between consecutive days, while the lowest correlations are found between days that are farther apart. This pattern suggests that days with similar additive genetic variances exhibit higher correlations. Furthermore, the proximity of lactation days leads to increased permanent environmental correlations, indicating that environmental factors affecting one day are likely to influence consecutive days more strongly.
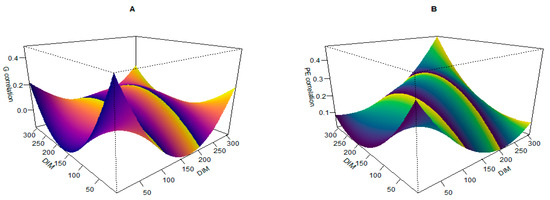
Figure 5.
(A) Correlation curve of genetic variance and (B) correlation curve of permanent environmental variance of milk urea nitrogen over different days in milk (DIM).
In a study performed in 2007, the correlation between test-day records of the MUN trait was estimated to be lower than other production traits [25]. In another study, analyses were conducted for different lactation periods: <50 DIM, 50–100 DIM, 100–150 DIM, >150 DIM, or the first record per cow. Data were analyzed for all breeds and crossbreds (AU_ALL), and all breeds and crossbreds in New Zealand (NZ_ALL). Significant correlations are highlighted in bold intervals. For example, the genetic correlation between MUN measured between 100–150 DIM and >150 DIM was 0.85 in AU_ALL and 0.63 in NZ_ALL [56]. These findings emphasize the importance of considering both genetic and environmental factors in the analysis of lactation data, as these factors can significantly influence the observed correlations and overall interpretation of the study results.
3.6. Genetic Trends for MUN
Three separate plots were created to evaluate genetic trends for MUN in the Iranian Holstein dairy herd (Figure 6). The linear regression line for the total population (y = 0.0017x − 0.0449, R2 = 0.5533) indicates a slight positive genetic trend, suggesting a gradual increase in MUN levels over the years. Similarly, the regression line for males (y = 0.0016x − 0.0442, R2 = 0.5389) also demonstrates a positive genetic trend. In contrast, the regression line for females (y = −0.0001x − 0.0219, R2 = 0.0014) suggests a very slight negative or negligible trend in MUN levels. These findings indicate that the breeding program has not been consistently effective in reducing MUN levels. The overall trends for the total population and males show a slight increase in MUN, whereas the minimal change observed in females suggests potential sex-specific genetic effects or environmental factors influencing MUN levels.
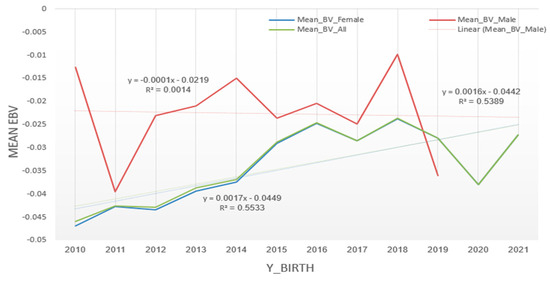
Figure 6.
Plot of average of genetic trends for MUN for the whole population, males, and females born in the same year, and the average EBV (y-axis) and year of birth (x-axis).
The accuracy of EBV estimates can be influenced by factors such as the size and structure of the pedigree, the number of records, and the genetic parameters applied in the model. Environmental factors such as nutrition, management, and health can also affect MUN levels and may mask the underlying genetics. Further research is needed to investigate the impact of environmental factors, refine selection criteria, and explore genomic selection to improve the accuracy of EBV prediction and accelerate genetic progress for MUN reduction.
4. Conclusions
This study investigated the genetic parameters of milk urea nitrogen (MUN) in Holstein cattle using a single-trait random regression model. Results indicate that MUN is a trait with low heritability and repeatability, consistent with previous findings. Despite these challenges, the high and consistent genetic correlations observed across lactation suggest that genetic selection for MUN may be feasible. Genomic approaches offer potential for improving the accuracy of genetic evaluations for MUN, even with low heritability. By utilizing genomic information, we may be able to identify specific genomic regions that contribute to MUN variation. However, it is crucial to consider the potential trade-offs associated with selecting for MUN. Careful consideration must be given to ensure that selection for MUN does not adversely impact other economically important traits such as milk yield, milk protein content, and animal health. Further research is warranted to investigate the feasibility and potential consequences of MUN-based selection programs in more detail. Integrating these findings with a comprehensive understanding of MUN physiology and its relationships with other traits will be essential for developing effective and sustainable breeding strategies for optimizing MUN levels in dairy cattle.
Author Contributions
M.M., M.B.Z., R.P. and M.E.N. contributed to the design of the study. M.M. and R.P. assisted in data collection. M.M., M.B.Z., R.P. and H.R.d.O. assisted in statistical analyses and visualization. M.M., M.B.Z., R.P., M.E.N. and H.R.d.O. discussed the results and contributed to the final manuscript. M.M. carried out the analyses and drafted the manuscript. H.R.d.O., M.B.Z. and R.P. contributed to revising the main manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study, as all data was obtained from existing datasets.
Data Availability Statement
Restrictions apply to the availability of these data. Data were obtained from the Iranian National Animal Breeding Center and are available from M.B.Z. with the permission of the Iranian National Animal Breeding Center.
Acknowledgments
The authors appreciate the officials at the Iranian National Animal Breeding Center for providing data for this research.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Abbreviations
The following abbreviations are used in this manuscript:
| MUN | Milk urea nitrogen |
| RRM | Random regression model |
| NABC | Iranian National Animal Breeding Center |
| DIM | Days in milk |
| IBD | Identity by descent |
| HTD | herd–year–month of test day |
| AYS | Age–year–season |
| AIC | Akaike information criterion |
| BIC | Bayesian information criterion |
References
- Perrault, E.E. Melanie DuPuis: Nature’s perfect food: How milk became America’s drink. Agric. Hum. Values 2011, 28, 583–584. [Google Scholar] [CrossRef]
- Oita, A.; Malik, A.; Kanemoto, K.; Geschke, A.; Nishijima, S.; Lenzen, M. Substantial nitrogen pollution embedded in international trade. Nat. Geosci. 2016, 9, 111–115. [Google Scholar] [CrossRef]
- Uwizeye, A.; de Boer, I.J.M.; Opio, C.I.; Schulte, R.P.O.; Falcucci, A.; Tempio, G.; Teillard, F.; Casu, F.; Rulli, M.; Galloway, J.N. Nitrogen emissions along global livestock supply chains. Nature Food 2020, 1, 437–446. [Google Scholar] [CrossRef]
- Yu, C.; Huang, X.; Chen, H.; Godfray, H.C.J.; Wright, J.S.; Hall, J.W.; Gong, P.; Ni, S.; Qiao, S.; Huang, G. Managing nitrogen to restore water quality in China. Nature 2019, 567, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Spek, J.W.; Bannink, A.; Gort, G.; Hendriks, W.H.; Dijkstra, J. Interaction between dietary content of protein and sodium chloride on milk urea concentration, urinary urea excretion, renal recycling of urea, and urea transfer to the gastrointestinal tract in dairy cows. J. Dairy Sci. 2013, 96, 5734–5745. [Google Scholar] [CrossRef]
- Ahmed, M.; Rauf, M.; Mukhtar, Z.; Saeed, N.A. Excessive use of nitrogenous fertilizers: An unawareness causing serious threats to environment and human health. Environ. Sci. Pollut. Res. 2017, 24, 26983–26987. [Google Scholar] [CrossRef]
- Wattiaux, M.A.; Uddin, M.E.; Letelier, P.; Jackson, R.D.; Larson, R.A. Invited Review: Emission and mitigation of greenhouse gases from dairy farms: The cow, the manure, and the field. Appl. Anim. Sci. 2019, 35, 238–254. [Google Scholar] [CrossRef]
- Aguirre-Villegas, H.A.; Larson, R.A. Evaluating greenhouse gas emissions from dairy manure management practices using survey data and lifecycle tools. J. Clean. Prod. 2017, 143, 169–179. [Google Scholar] [CrossRef]
- Thomann, R.V.; Collier, J.R.; Butt, A.; Casman, E.; Linker, L.C. Response of the Chesapeake Bay Water Quality Model to Loading Scenarios; (a report of the modeling Subcommittee, Chesapeake Bay Program Office, Annapolis, MD). Technology transfer report; Environmental Protection Agency: Annapolis, MD, USA, 1994. [Google Scholar]
- Müller, C.B.M.; Görs, S.; Derno, M.; Tuchscherer, A.; Wimmers, K.; Zeyner, A.; Kuhla, B. Differences between Holstein dairy cows in renal clearance rate of urea affect milk urea concentration and the relationship between milk urea and urinary nitrogen excretion. Sci. Total Environ. 2021, 755, 143198. [Google Scholar] [CrossRef]
- Ma, L.; Luo, H.; Brito, L.F.; Chang, Y.; Chen, Z.; Lou, W.; Zhang, F.; Wang, L.; Guo, G.; Wang, Y. Estimation of genetic parameters and single-step genome-wide association studies for milk urea nitrogen in Holstein cattle. J. Dairy Sci. 2023, 106, 352–363. [Google Scholar] [CrossRef]
- Ishler, V. Interpretation of Milk Urea Nitrogen (MUN) Values; Penn State Extension, DAS: University Park, PA, USA, 2008; pp. 2001–2134. [Google Scholar]
- Bittante, G. Effects of breed, farm intensiveness, and cow productivity on infrared predicted milk urea. J. Dairy Sci. 2022, 105, 5084–5096. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.G.; Young, A.J. The association between milk urea nitrogen and DHI production variables in western commercial dairy herds. J. Dairy Sci. 2003, 86, 3008–3015. [Google Scholar] [CrossRef]
- Barros, T.; Quaassdorff, M.A.; Aguerre, M.J.; Colmenero, J.J.O.; Bertics, S.J.; Crump, P.M.; Wattiaux, M.A. Effects of dietary crude protein concentration on late-lactation dairy cow performance and indicators of nitrogen utilization. J. Dairy Sci. 2017, 100, 5434–5448. [Google Scholar] [CrossRef]
- Nousiainen, J.; Shingfield, K.J.; Huhtanen, P. Evaluation of milk urea nitrogen as a diagnostic of protein feeding. J. Dairy Sci. 2004, 87, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Wattiaux, M.; Aguere, M.; Powell, J. Background and overview on the contribution of dairy nutrition to addressing environmental concerns in Wisconsin: Nitrogen, phosphorus, and methane. In La Ganadéría ante el Agotamiento de los Paradigas Dominantes; Universidad Autoónoma Chapingo: Chapingo, Mexico, 2011; pp. 111–139. [Google Scholar]
- Hojman, D.; Kroll, O.; Adin, G.; Gips, M.; Hanochi, B.; Ezra, E. Relationships between milk urea and production, nutrition, and fertility traits in Israeli dairy herds. J. Dairy Sci. 2004, 87, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Kohn, R. Use of milk or blood urea nitrogen to identify feed management inefficiencies and estimate nitrogen excretion by dairy cattle and other animals. In Proceedings of the Florida Ruminant Nutrition Symposium, Gainesville, FL, USA, 30–31 January 2007. [Google Scholar]
- Wattiaux, M.A.; Ranathunga, S.D. Milk urea Nitrogen as a tool to assess efficiency of Nitrogen utilization in dairy cows. In Proceedings of the Four-State Dairy Nutrition and Management Conference, Dubuque, IA, USA, 15–16 June 2016. [Google Scholar]
- Kohn, R.; High, J. Milk urea nitrogen: Theory and practice. In Proceedings of the Maryland Nutrition Conference for Feed Manufacturers, College Park, MD, USA, 20–23 March 1997; pp. 83–90. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=b7d8dfbad73fa57a994703edcb6767b40a54ddef (accessed on 9 January 2025).
- Wood, G.M.; Boettcher, P.J.; Jamrozik, J.; Jansen, G.B.; Kelton, D.F. Estimation of genetic parameters for concentrations of milk urea nitrogen. J. Dairy Sci. 2003, 86, 2462–2469. [Google Scholar] [CrossRef]
- Mitchell, R.G.; Rogers, G.W.; Dechow, C.D.; Vallimont, J.E.; Cooper, J.B.; Sander-Nielsen, U.; Clay, J.S. Milk urea nitrogen concentration: Heritability and genetic correlations with reproductive performance and disease. J. Dairy Sci. 2005, 88, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Wenninger, A.; Distl, O. Analysis of Environmental and Genetic Influences on the Urea and Acetone Content in Milk from the Breeds German Spotted Cattle and German Brown Cattle. DTW—Dtsch. Tierarztl. Wochenschr. 1993, 100, 405–410. [Google Scholar]
- Stoop, W.M.; Bovenhuis, H.; Van Arendonk, J.A.M. Genetic parameters for milk urea nitrogen in relation to milk production traits. J. Dairy Sci. 2007, 90, 1981–1986. [Google Scholar] [CrossRef]
- Mucha, S.; Strandberg, E. Genetic analysis of milk urea nitrogen and relationships with yield and fertility across lactation. J. Dairy Sci. 2011, 94, 5665–5672. [Google Scholar] [CrossRef]
- Bobbo, T.; Penasa, M.; Rossoni, A.; Cassandro, M. Genetic aspects of milk urea nitrogen and new indicators of nitrogen efficiency in dairy cows. J. Dairy Sci. 2020, 103, 9207–9212. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.R.; Brito, L.F.; Lourenco DA, L.; Silva, F.F.; Jamrozik, J.; Schaeffer, L.R.; Schenkel, F.S. Invited review: Advances and applications of random regression models: From quantitative genetics to genomics. J. Dairy Sci. 2019, 102, 7664–7683. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. Genetic Analyses of Different Nitrogen Use Efficiency Proxies and Their Relationships with Other Traits for Holstein Cows. Ph.D. Thesis, Gembloux Agro-Bio Tech—Université de Liège, Gembloux, Belgium, 2023. [Google Scholar]
- Chen, Y.; Atashi, H.; Vanderick, S.; Mota, R.R.; Soyeurt, H.; Hammami, H.; Gengler, N. Genetic analysis of milk urea concentration and its genetic relationship with selected traits of interest in dairy cows. J. Dairy Sci. 2021, 104, 12741–12755. [Google Scholar] [CrossRef]
- Simpson, G.L.; Bates, D.M.; Oksanen, J.; R Core Team. Permute: Functions for Generating Restricted Permutations of Data, R package version 0.9-7; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H.; Bryan, J. R Packages; O’Reilly Media, Inc.: Sebastopol, CA, USA, 2023. [Google Scholar]
- Misztal, I.; Tsuruta, S.; Lourenco, D.A.L.; Masuda, Y.; Aguilar, I.; Legarra, A.; Vitezica, Z. Manual for BLUPF90 Family of Programs; University of Georgia: Athens, GA, USA, 2018. [Google Scholar]
- Hyndman, R.; Booth, H.; Tickle, L.; Maindonald, J.; Wood, S.; Team, R.C.; Hyndman, M.R. Demography: Forecasting Mortality, Fertility, Migration and Population Data, R package version 1.22; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Sargolzaei, M.; Iwaisaki, H.; Colleau, J.J. CFC: A tool for monitoring genetic diversity. In Proceedings of the 8th World Congress on Genetics Applied to Livestock Production, Belo Horizonte, Brazil, 13–18 August 2006. [Google Scholar]
- Wright, S. Coefficients of inbreeding and relationship. Am. Nat. 1922, 56, 330–338. [Google Scholar] [CrossRef]
- Li, J.; Gao, H.; Madsen, P.; Li, R.; Liu, W.; Bao, P.; Xue, G.; Gao, Y.; Di, X.; Su, G. Impact of the order of Legendre polynomials in random regression model on genetic evaluation for milk yield in dairy cattle population. Front. Genet. 2020, 11, 586155. [Google Scholar] [CrossRef]
- Jamrozik, J.; Schaeffer, L.R. Estimates of genetic parameters for a test day model with random regressions for yield traits of first lactation Holsteins. J. Dairy Sci. 1997, 80, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Wickham, H. Getting Started with Ggplot2. In ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; pp. 11–31. [Google Scholar]
- Auguie, B.; Antonov, A. gridExtra: Miscellaneous Functions for “Grid” Graphics, R package version 2.3; R Core Team: Vienna, Austria, 2017. [Google Scholar]
- Atashi, H.; Chen, Y.; Vanderick, S.; Hubin, X.; Gengler, N. Single-step genome-wide association analyses for milk urea concentration in Walloon Holstein cows. J. Dairy Sci. 2024, 107, 3020–3031. [Google Scholar] [CrossRef]
- Ruban, S.; Viktor, D.; Mykhailo, M.; Oleksandr, O.B.; Oleksandr, V.B.; Lesia, K. Characteristics of Lactation Curve and Reproduction in Dairy Cattle. Acta Univ. Agric. Silvic. Mendel. Brun. 2022, 70, 373–381. (In Czech) [Google Scholar] [CrossRef]
- Abdouli, H.; Rekik, B.; Haddad-Boubaker, A. Non-nutritional factors associated with milk urea concentrations under Mediterranean conditions. World J. Agric. Sci. 2008, 4, 183–188. [Google Scholar]
- Wattiaux, M.A.; Nordheim, E.V.; Crump, P. Statistical evaluation of factors and interactions affecting dairy herd improvement milk urea nitrogen in commercial Midwest dairy herds. J. Dairy Sci. 2005, 88, 3020–3035. [Google Scholar] [CrossRef]
- Fatehi, F.; Zali, A.; Honarvar, M.; Dehghan-Banadaky, M.; Young, A.J.; Ghiasvand, M.; Eftekhari, M. Review of the relationship between milk urea nitrogen and days in milk, parity, and monthly temperature mean in Iranian Holstein cows. J. Dairy Sci. 2012, 95, 5156–5163. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, A.J.; St-Pierre, N.R. The relationship of milk urea nitrogen to urine nitrogen excretion in Holstein and Jersey cows. J. Dairy Sci. 2001, 84, 2284–2294. [Google Scholar] [CrossRef] [PubMed]
- Doska, M.C.; Silva, D.F.F.D.; Horst, J.A.; Valloto, A.A.; Rossi Junior, P.; Almeida, R.D. Sources of variation in milk urea nitrogen in Paraná dairy cows. Rev. Bras. De Zootec. 2012, 41, 692–697. [Google Scholar] [CrossRef]
- Kessler, E.C.; Bruckmaier, R.M.; Gross, J.J. Milk urea nitrogen concentration is higher in Brown Swiss than in Holstein dairy cows despite identical feeding. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1671–1677. [Google Scholar] [CrossRef]
- Kgole, M.L.; Visser, C.; Banga, C.B. Environmental factors influencing milk urea nitrogen in South African Holstein cattle. S. Afr. J. Anim. Sci. 2012, 42, 459–463. [Google Scholar] [CrossRef][Green Version]
- Mutsvangwa, T.; Davies, K.L.; McKinnon, J.J.; Christensen, D.A. Effects of dietary crude protein and rumen-degradable protein concentrations on urea recycling, nitrogen balance, omasal nutrient flow, and milk production in dairy cows. J. Dairy Sci. 2016, 99, 6298–6310. [Google Scholar] [CrossRef] [PubMed]
- Guliński, P.; Salamończyk, E.; Młynek, K. Improving nitrogen use efficiency of dairy cows in relation to urea in milk—A review. Anim. Sci. Pap. Rep. 2016, 34, 5–24. [Google Scholar]
- Beatson, P.R.; Meier, S.; Cullen, N.G.; Eding, H. Genetic variation in milk urea nitrogen concentration of dairy cattle and its implications for reducing urinary nitrogen excretion. Animal 2019, 13, 2164–2171. [Google Scholar] [CrossRef]
- Garcia-Muniz, J.G.; Lopez-Villalobos, N.; Burke, J.L.; Sandbrok, T.; Vazquez-Pelaez, C.G. Spatial-time correlation between milk urea with milk components and somatic cell score of bulk milk samples from farms supplying milk for cheese and milk powder manufacturing. Proc. N. Z. Soc. Anim. Prod. 2013, 73, 108–113. [Google Scholar]
- Miglior, F.; Sewalem, A.; Jamrozik, J.; Bohmanova, J.; Lefebvre, D.M.; Moore, R.K. Genetic analysis of milk urea nitrogen and lactose and their relationships with other production traits in Canadian Holstein cattle. J. Dairy Sci. 2007, 90, 2468–2479. [Google Scholar] [CrossRef]
- Van den Berg, I.; Ho, P.N.; Luke, T.D.W.; Haile-Mariam, M.; Bolormaa, S.; Pryce, J.E. The use of milk mid-infrared spectroscopy to improve genomic prediction accuracy of serum biomarkers. J. Dairy Sci. 2021, 104, 2008–2017. [Google Scholar] [CrossRef]
- Van den Berg, I.; Ho, P.N.; Haile-Mariam, M.; Beatson, P.R.; O’Connor, E.; Pryce, J.E. Genetic parameters of blood urea nitrogen and milk urea nitrogen concentration in dairy cattle managed in pasture-based production systems of New Zealand and Australia. Anim. Prod. Sci. 2021, 61, 1833–1842. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).