Animal Supplementation and Legume Pastures Enhance Nitrogen Balance and Efficiency in Integrated Crop-Livestock Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design and Treatments
2.3. Pasture Phase
2.4. Animal Evaluations
2.5. Nutrient Intake and N Excretion
2.6. Feces and Urine Analysis
2.7. N Retention by Beef Cattle
2.8. Crop Performance
2.9. Decomposition and N Release from Animal Feces and Post-Grazing Residue
2.10. Statistical Analyses
3. Results
3.1. Nitrogen in the Livestock Phase
3.2. Nitrogen Retained in the Beef Cattle Carcass
3.3. Nitrogen in Soybean Yield and Grain Off-Take
3.4. Release of N by Post-Grazing Residue and Animal Feces
4. Discussion
4.1. Nitrogen in the Livestock Phase
4.2. Nitrogen Retained in the Beef Cattle Carcass
4.3. Nitrogen in Soybean Yield and Grain Off-Take
4.4. Release of N by Post-Grazing Residue and Animal Excreta
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barłóg, P.; Grzebisz, W.; Łukowiak, R. Fertilizers and fertilization strategies mitigating soil factors constraining efficiency of nitrogen in plant production. Plants 2022, 11, 1855. [Google Scholar] [CrossRef]
- Oliveira, J.G.; Santana-Júnior, M.L.; Maia, N.J.C.; Dubeux-Junior, J.C.B.; Gameiro, A.H.; Kunrath, T.R.; Mendonça, G.G.; Simili, F.F. Nitrogen balance and efficiency as indicators for monitoring the proper use of fertilizers in agricultural and livestock systems. Sci. Rep. 2022, 12, 12021. [Google Scholar] [CrossRef]
- Haynes, R.J.; Williams, P.H. Nutrient cycling and soil fertility in the grazed pasture ecosystem. Adv. Agron. 1993, 49, 119–199. [Google Scholar] [CrossRef]
- Saggar, S.; Luo, J.; Kim, D.G.; Jha, N. Intensification in Pastoral Farming: Impacts on Soil Attributes and Gaseous Emissions. In Soil Health and Climate Change; Springer: Berlin/Heidelberg, Germany, 2011; pp. 207–236. [Google Scholar] [CrossRef]
- Sartor, L.R.; Sandini, I.E.; Adami, P.F.; Novakowiski, J.H.; Ruthes, B.E.S. Corn yield and grain nutritional status in a crop-livestock system with winter/summer nitrogen levels. Int. J. Plant Prod. 2018, 12, 309–314. [Google Scholar] [CrossRef]
- Garcia, L.; Dubeux, J.C.B.; Sollenberger, L.E.; Vendramini, J.M.B.; DiLorenzo, N.; Santos, E.R.S.; Jaramillo, D.M.; Ruiz-Moreno, M. Nutrient excretion from cattle grazing nitrogen-fertilized grass or grass–legume pastures. Agron. J. 2021, 113, 3110–3123. [Google Scholar] [CrossRef]
- Jensen, E.S.; Carlsson, G.; Hauggaard-Nielsen, H. Intercropping of grain legumes and cereals improves the use of soil n resources and reduces the requirement for synthetic fertilizer N: A global-scale analysis. Agron. Sustain. Dev. 2020, 40, 5. [Google Scholar] [CrossRef]
- Barbizan, M.; Valente, E.E.L.; Damasceno, M.L.; Lopes, S.A.; Tanaka, E.S.; Barros, C.P.; Melo, B.V.R. Balanced protein/energy supplementation plan for beef cattle on tropical pasture. Livest. Sci. 2020, 241, 104211. [Google Scholar] [CrossRef]
- Scheeren, F.B.; Sartor, L.R.; Danna, M.; Kuss, F.; Paris, W.; Bianchin, A.; Andriotti, N.M.; De Menezes, L.F.G. Energy supplementation of beef steers or inclusion of legumes in temperate pastures in crop-livestock integration area. J. Agric. Sci. 2024, 162, 268–274. [Google Scholar] [CrossRef]
- Santos, A.D.; Fonseca, D.M.; Sousa, B.M.L.; Santos, M.E.R.; Carvalho, A.N. Estrutura do pasto e produção de bovinos suplementados em pastagens diferidas com capim braquiária. Ciência Anim. Bras. Braz. Anim. Sci. 2020, 21, e43578. [Google Scholar] [CrossRef]
- Reis, R.A.; Ruggieri, A.C.; Casagrande, D.R.; Páscoa, A.G. suplementação da dieta de bovinos de corte como estratégia do manejo das pastagens. Rev. Bras. Zootec. 2009, 38, 147–159. [Google Scholar] [CrossRef]
- Alves, L.A.; Denardin, L.G.d.O.; Martins, A.P.; Anghinoni, I.; Carvalho, P.C.d.F.; Tiecher, T. Soil Acidification and P, K, Ca and Mg budget as affected by sheep grazing and crop rotation in a long-term integrated crop-livestock system in southern brazil. Geoderma 2019, 351, 197–208. [Google Scholar] [CrossRef]
- Silva, L.S.; Laroca, J.V.d.S.; Coelho, A.P.; Gonçalves, E.C.; Gomes, R.P.; Pacheco, L.P.; Carvalho, P.C.d.F.; Pires, G.C.; Oliveira, R.L.; Souza, J.M.A.; et al. Does grass-legume intercropping change soil quality and grain yield in integrated crop-livestock systems? Appl. Soil Ecol. 2022, 170, 104257. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Mott, G.O.; Lucas, H.L. The design conduct and interpretation of grazing trials on cultivated and improved pastures. In Proceedings of the 6th Internacional Grassland Congress, Philadelphia, PA, USA, 17–23 August 1952; Wagner, R.E., Ed.; State College Press: State College, PA, USA, 1952; pp. 1380–1395. [Google Scholar]
- Sollenberger, L.E.; Moore, J.E.; Allen, V.G.; Pedreira, C.G.S. Reporting forage allowance in grazing experiments. Crop Sci. 2005, 45, 896–900. [Google Scholar] [CrossRef]
- Wilm, H.G.; Vm, D.A.; Costello, F.; Klipple, G.E. Estimating forage yield by the double-sampling Method1. Agron. J. 1944, 36, 194–203. [Google Scholar] [CrossRef]
- Campbell, A.G. Grazed Pasture Parameters. II. Pasture dry-matter use in a stocking rate and grazing management experiment with dairy cows. J. Agric. Sci. 1966, 67, 211–216. [Google Scholar] [CrossRef]
- Euclides, V.P.B.; Macedo, M.C.M.; Oliveira, M.P. Avaliação de diferentes métodos de amostragem para estimar o valor nutritivo de forragens sob pastejo. Rev. Bras. Zootec. 1992, 21, 691–701. [Google Scholar]
- Wiseman, J. Editorial: Digestibility and degradability in animal nutrition studies. J. Agric. Sci. 2018, 156, 1161–1162. [Google Scholar] [CrossRef]
- Astigarraga, L. Técnicas para la medición del consumo de rumiantes en pastoreo. In Proceedings of the Simpósio Sobre Avaliação de Pastagens com Animais, Anais, UEM, Maringá, Brazil, 13–14 May 1997; UEM Press: Maringá, Brazil, 1997; pp. 1–23. [Google Scholar]
- Myers, W.D.; Ludden, P.A.; Nayigihugu, V.; Hess, B.W. Technical note: A procedure for the preparation and quantitative analysis of samples for titanium dioxide. J. Anim. Sci. 2004, 82, 179–183. [Google Scholar] [CrossRef] [PubMed]
- AOAC (Association of Official Analytical Chemistry). Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemistry: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Chizzotti, M.L.; Valadares-Filho, S.d.C.; Valadares, R.F.D.; Chizzotti, F.H.M.; Tedeschi, L.O. Determination of creatinine excretion and evaluation of spot urine sampling in holstein cattle. Livest. Sci. 2008, 113, 218–225. [Google Scholar] [CrossRef]
- Hankins, O.G.; Howe, P.E. Estimation of the Composition of Beef Carcasses and Cuts; United States Department of Agriculture, Economic Research Service: Washington, DC, USA, 1946. [Google Scholar]
- Muller, L. Tecnicas para determinar la composicion de la canal. In Memoria de la Asociación Latinoamericana de Producción Animal; ALPA: Guadalajara, Mexico, 1973; p. 75. [Google Scholar]
- Wieder, R.K.; Lang, G.E. A critique of the analytical methods used in examining decomposition data obtained from litter bags. Ecology 1982, 63, 1636–1642. [Google Scholar] [CrossRef]
- Gurgel, A.; Gurgel, A.L.C.; Difante, G.d.S.; Roberto, F.F.d.S.; Dantas, J.L.S. Suplementação estratégica para animais em pasto. Pubvet 2018, 12, 147. [Google Scholar] [CrossRef]
- Rocha, M.G.; Restle, J.; Pilau, A.; Texeira, D.; Santos, D. Produção animal e retorno econômico da suplementação em pastagem de aveia e azevém. Ciência Rural. 2003, 33, 573–578. [Google Scholar] [CrossRef]
- Lüscher, A.; Mueller-Harvey, I.; Soussana, J.F.; Rees, R.M.; Peyraud, J.L. Potential of legume-based grassland–livestock systems in europe: A review. Grass Forage Sci. 2014, 69, 206–228. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, D.R.; Cardenas, L.M.; Dhanoa, M.S.; Donovan, N.; Misselbrook, T.; Williams, J.R.; Thorman, R.E.; McGeough, K.L.; Watson, C.J.; Bell, M.; et al. The contribution of cattle urine and dung to nitrous oxide emissions: Quantification of country specific emission factors and implications for national inventories. Sci. Total Environ. 2018, 635, 607–617. [Google Scholar] [CrossRef] [PubMed]
- National Research Council—NRC. Nutrient Requirements of Dairy Cattle, 7th revised ed.; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Sleugh, B.; Moore, K.J.; George, J.R.; Brummer, E.C. Binary legume–grass mixtures improve forage yield, quality, and seasonal distribution. Agron. J. 2000, 92, 24–29. [Google Scholar] [CrossRef]
- Bellows, B. Nutrient Cycling in Pastures; Livestock Systems Guide; ATTRA: Victoria, Australia, 2001. [Google Scholar]
- Lantinga, E.A.; Keuning, J.A. Distribution of excreted nitrogen by grazing cattle and its effects on sward quality, herbage production and utilization. In Animal Manure on Grassland and Fodder Crops. Fertilizer or Waste?, Proceedings of an International Symposium of the European Grassland Federation, Wageningen, The Netherlands, 31 August–3 September 1987; Van der Meer, H.G., Unwin, R.J., Van Dijk, T.A., Ennik, G.C., Eds.; Springer: Dordrecht, The Netherlands, 1987; pp. 103–117. [Google Scholar]
- Richards, I.R.; Wolton, K.M. The spatial distribution of excreta under intensive cattle grazing. Grass Forage Sci. 1976, 31, 89–92. [Google Scholar] [CrossRef]
- Menezes, A.C.B.; Valadares Filho, S.C.; Costa e Silva, L.F.; Pacheco, M.V.C.; Pereira, J.M.V.; Rotta, P.P.; Zanetti, D.; Detmann, E.; Silva, F.A.S.; Godoi, L.A.; et al. Does a reduction in dietary crude protein content affect performance, nutrient requirements, nitrogen losses, and methane emissions in finishing nellore bulls? Agric. Ecosyst. Environ. 2016, 223, 239–249. [Google Scholar] [CrossRef]
- Reis, J.C.; Rodrigues, G.S.; de Barros, I.; Rodrigues, R.d.A.R.; Garrett, R.D.; Valentim, J.F.; Kamoi, M.Y.T.; Michetti, M.; Wruck, F.J.; Rodrigues-Filho, S.; et al. Integrated crop-livestock systems: A sustainable land-use alternative for food production in the brazilian Cerrado and Amazon. J. Clean. Prod. 2021, 283, 124580. [Google Scholar] [CrossRef]
- Chiriacò, M.V.; Valentini, R. A land-based approach for climate change mitigation in the livestock sector. J. Clean. Prod. 2021, 283, 124622. [Google Scholar] [CrossRef]
- Macitelli, F.; Berchielli, T.T.; Da Silveira, R.N.; Andrade, P.; Lopes, A.D.; Sato, K.J.; Barbosa, J.C. Effects of feeding different forage and protein sources on carcass biometry and organ and internal organs weights for steers. Rev. Bras. Zootec. 2005, 34, 1751–1762. [Google Scholar] [CrossRef]
- Rodrigues, V.C.; De Andrade, I.F. Chemical physical meat characteristics of buffaloes and cattle entire and castrated. Rev. Bras. Zootec. 2004, 33, 1839–1849. [Google Scholar] [CrossRef]
- Williams, P. Nutritional composition of red meat. Nutr. Diet. 2007, 64, 113–119. [Google Scholar] [CrossRef]
- Jayawardena, S.R.; Morton, J.D.; Brennan, C.S.; Bekhit, A.E.D.A. Utilisation of beef lung protein powder as a functional ingredient to enhance protein and iron content of fresh pasta. Int. J. Food Sci. Technol. 2019, 54, 610–618. [Google Scholar] [CrossRef]
- Pezzato, A.C.; Narváez-Solarte, W.V.; Pezzato, L.E.; Barros, M.M.; Koch, J.F.A.; Fernandes, A.C. Nutritional evaluation, in nile-tilapia, of bovine blood meals obtained by three processing methods. Rev. Bras. Zootec. 2012, 41, 491–500. [Google Scholar] [CrossRef]
- Anghinoni, I.; Carvalho, P.C.F.; Costa, S.E.V.G.A. Abordagem sistêmica do solo em sistemas integrados de produção agrícola e pecuária no subtrópico brasileiro. In Tópicos em Ciência do Solo; Araújo, A.P., Avelar, B.J.R., Eds.; UFV: Viçosa, Brazil, 2013; pp. 221–278. [Google Scholar]
- Alves, L.A.; De Denardin, L.G.O.; Farias, G.D.; Flores, J.P.M.; Filippi, D.; Bremm, C.; De Carvalho, P.C.F.; Martins, A.P.; Gatiboni, L.C.; Tiecher, T. Fertilization strategies and liming in no-till integrated crop–livestock systems: Effects on phosphorus and potassium use efficiency. Rev. Bras. Cienc. Solo 2022, 46, e0210125. [Google Scholar] [CrossRef]
- Phelan, P.; Moloney, A.P.; McGeough, E.J.; Humphreys, J.; Bertilsson, J.; O’Riordan, E.G.; O’Kiely, P. forage legumes for grazing and conserving in ruminant production systems. CRC Crit. Rev. Plant Sci. 2015, 34, 281–326. [Google Scholar] [CrossRef]
- Bosshard, C.; Oberson, A.; Leinweber, P.; Jandl, G.; Knicker, H.; Wettstein, H.R.; Kreuzer, M.; Frossard, E. Characterization of fecal nitrogen forms produced by a sheep fed with 15N labeled ryegrass. Nutr. Cycl. Agroecosyst. 2011, 90, 355–368. [Google Scholar] [CrossRef]
- Assmann, A.L.; Pelissari, A.; De Moraes, A.; Assmann, T.S.; De Oliveira, E.B.; Sandini, I. Produção de gado de corte e acúmulo de matéria seca em sistema de integração lavoura-pecuária em presença e ausência de trevo branco e nitrogênio. Rev. Bras. De Zootec. 2004, 33, 37–44. [Google Scholar] [CrossRef]
- Szymczak, L.S.; Carvalho, P.C.d.F.; Lurette, A.; de Moraes, A.; Nunes, P.A.d.A.; Martins, A.P.; Moulin, C.H. System diversification and grazing management as resilience-enhancing agricultural practices: The case of crop-livestock integration. Agric. Syst. 2020, 184, 102904. [Google Scholar] [CrossRef]
- Bernués, A.; Ruiz, R.; Olaizola, A.; Villalba, D.; Casasús, I. Sustainability of pasture-based livestock farming systems in the European mediterranean context: Synergies and trade-offs. Livest. Sci. 2011, 139, 44–57. [Google Scholar] [CrossRef]
- Luz, P.A.C.; Andrighetto, C.; Lupatini, G.C.; Aranha, H.S.; Trivelin, G.A.; Mateus, G.P.; Santos, C.T.; Francisco, C.d.L.; Castilhos, A.M.; Jorge, A.M. Effect of integrated crop-livestock systems in carcass and meat quality of nellore cattle. Livest. Sci. 2019, 220, 83–92. [Google Scholar] [CrossRef]
- Muniz, M.P.; Costa, K.A.D.P.; Severiano, E.D.C.; Bilego, U.O.; Almeida, D.P.; Furtini Neto, A.E.; Vilela, L.; Lana, M.A.; Leandro, W.M.; Dias, M.B.D.C. Soybean yield in integrated crop–livestock system in comparison to soybean–maize succession system. J. Agric. Sci. 2021, 159, 188–198. [Google Scholar] [CrossRef]
- Pitta, C.S.R.; Pelissari, A.; Da Silveira, A.L.F.; Adami, P.F.; Sartor, L.R.; Assmann, T.S.; Migliorini, F. Decomposition and nitrogen release in areas with and without grazing and its influence on corn. Semin. Cienc. Agrar. 2013, 34, 905–920. [Google Scholar] [CrossRef]
- Marasca, I.; Costa Medeiros, L.; Jose Araújo, M.; Perin, A.; Alberto Alves de Oliveira, C. Teores e Acúmulo de Nitrogênio, Fósforo e Potássio e Decomposição da Biomassa De Braquiária em Sistema Santa Fé. Enciclopédia Biosf. 2011, 7, 1–9. [Google Scholar]
- Wilson, J.R.; Mannetje, L. Senescence, digestibility and carbohydrate content of buffel grass and green panic leaves in swards. Aust. J. Agric. Res. 1978, 29, 503–516. [Google Scholar] [CrossRef]
- Lang, C.R.; Pelissari, A.; De Moraes, A.; Sulc, R.M.; Batista De Oliveira, E.; César, P.; Carvalho, F. Aerial biomass residue of winter annual forages in an integrated crop-livestock system. Sci. Agrar. 2004, 5, 43–48. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminanti, 2nd ed.; Comstock Pub: Ithaca, NY, USA, 1994. [Google Scholar]
- Hentz, P.; Carvalho, N.L.; Luz, L.V.; Barcellos, A.L. Ciclagem de nitrogênio em sistemas de integração lavoura-pecuaria. Ciência Nat. 2014, 36, 663–676. [Google Scholar] [CrossRef]
- Heinrichs, R.; Aita, C.; Amado, T.J.C.; Fancelli, A.L. Cultivo consorciado de aveia e ervilhaca: Relação c/n da fitomassa e produtividade do milho em sucessão. Rev. Bras. Cienc. Solo 2001, 25, 331–340. [Google Scholar] [CrossRef]
- Paulino, M.F.; de Figueiredo, D.M.; de Moraes, E.H.B.K.; Porto, M.O.; Sales, M.F.L.; Acedo, T.S.; Villela, S.D.J.; Filho, S.d.C.V. Suplementação de bovinos em pastagens: Uma visão sistêmica. In Proceedings of the IV Simpósio de Produção de Gado de Corte, Viçosa, Brazil, 10–12 June 2004; UFV: Viçosa, Brazil, 2004. [Google Scholar]
- De Oliveira Junior, A.; de Castro, C.; Pereira, L.R.; Domingos, C.D.S. Estádios Fenológicos e Marcha de Absorção de Nutrientes da Soja; Fortgreen: Paiçandu, Brazil; Embrapa Soja: Londrina, Brazil, 2016. [Google Scholar]
- Hungria, M.; Campo, R.J.; Mendes, I.C. Fixação Biológica do Nitrogênio na Cultura da Soja; Embrapa Soja: Londrina, Brazil, 2001; Volume 35. [Google Scholar]



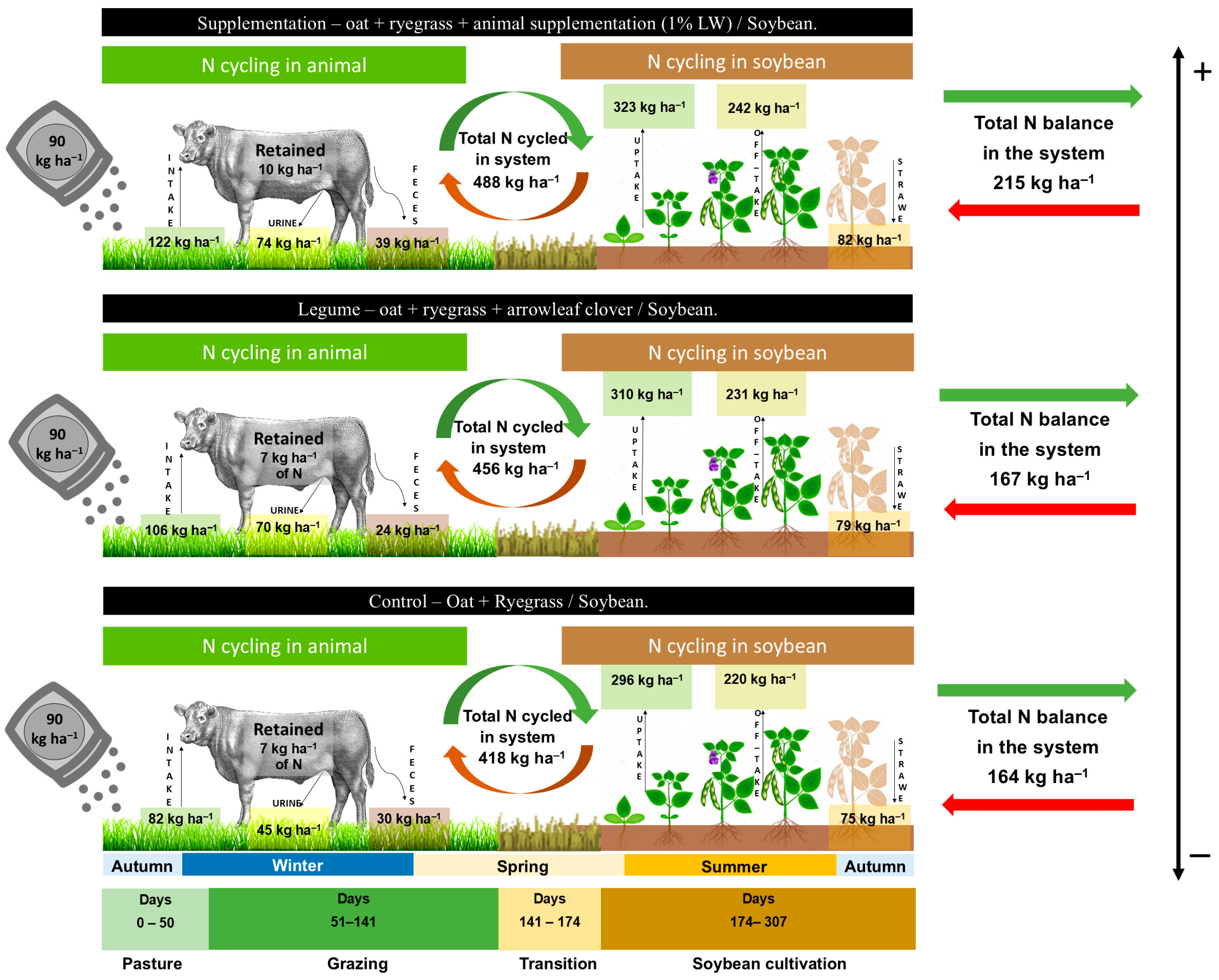
| Variables | Control | Legume | Supplementation | p-Value |
|---|---|---|---|---|
| Oat leaf (g kg−1 DM) | 219.13 ± 29.6 ns | 234.60 ± 31.7 | 242.18 ± 32.7 | 0.5265 |
| Ryegrass leaf (g kg−1 DM) | 182.03 ± 10.5 ns | 187.80 ± 10.8 | 194.67 ± 11.2 | 0.2103 |
| Grass stems (g kg−1 DM) | 354.80 ± 33.5 ns | 364.50 ± 34.4 | 369.63 ± 34.9 | 0.9328 |
| Leaf: stem ratio (g kg−1 DM) | 1.15 ± 0.07 ns | 1.17 ± 0.07 | 1.20 ± 0.07 | 0.9337 |
| Forage allowance (kg DM kg−1 BW) | 8.60 ± 1.29 ns | 7.90 ± 1.18 | 7.50 ± 1.12 | 0.3579 |
| Legume (g kg−1 DM) | - | 212.64 | - | |
| Live weight (kg) | 482.8 ± 10.4 ns | 510.0 ± 12.1 | 506.8 ± 5.0 | 0.0685 |
| Stocking rate (kg LW ha−1 d−1) | 1370.2 ± 189.1 b | 1472.3 ± 40.1 ab | 1666.1 ± 82.1 a | 0.0320 |
| N supplementation (g kg−1 DM) | - | - | 31.6 | - |
| N grazing simulation (g kg−1 DM) | 26.9 ± 1.21 ns | 29.4 ± 1.32 | 28.0 ± 1.26 | 0.3508 |
| N post-grazing residue (g kg−1 DM) | 21.7 ± 2.2 ns | 24.2 ± 2.5 | 22.0 ± 2.3 | 0.4100 |
| Variables | Control | Legume | Supplementation | p-Value |
|---|---|---|---|---|
| Daily intake of forage DM (g kg−1 LW d−1) | 24.99 ± 0.10 a | 27.25 ± 0.73 a | 15.79 ± 0.42 b | 0.0001 |
| Daily intake of supplementation DM (g kg−1 LW d−1) | -- | -- | 10.00 | -- |
| Daily intake of total DM (g kg−1 LW d−1) | 24.99 ± 1.16 ns | 27.25 ± 1.27 | 25.79 ± 1.21 | 0.1141 |
| N intake of forage (g kg−1 LW d−1) | 0.67 ± 0.15 b | 0.80 ± 0.19 a | 0.50 ± 0.12 c | 0.0010 |
| N intake of supplementation (g kg−1 LW d−1) | -- | -- | 0.32 | -- |
| Total daily N intake (g kg−1 LW d−1) | 0.67 ± 0.07 b | 0.80 ± 0.09 a | 0.82 ± 0.09 a | 0.0002 |
| Total daily N intake per hectare (kg LW ha−1 d−1) | 0.92 ± 0.13 b | 1.18 ± 0.03 a | 1.36 ± 0.07 a | 0.0257 |
| Total N intake 90-day (kg ha−1) | 82.51 ± 11.3 b | 106.2 ± 2.9 a | 122.4 ± 6.0 a | 0.0234 |
| Variables | Control | Legume | Supplementation | p-Value |
|---|---|---|---|---|
| N Feces (g kg−1 DM) | 34.6 ± 0.69 ns | 34.3 ± 0.69 | 33.3 ± 0.67 | 0.5000 |
| N Urine (g L−1) | 5.8 ± 1.11 b | 8.5 ± 1.61 a | 7.9 ± 1.50 a | 0.0010 |
| Daily urine residual N (g kg LW−1) | 0.36 ± 0.05 b | 0.53 ± 0.03 a | 0.49 ± 0.04 a | 0.0001 |
| Daily residual N from feces (g kg LW−1) | 0.25 ± 0.02 a | 0.22 ± 0.02 a | 0.27 ± 0.03 a | 0.0091 |
| Total residual N from urine (kg ha−1 day) | 0.50 ± 0.07 b | 0.78 ± 0.02 a | 0.82 ± 0.04 a | 0.0078 |
| Total residual N from feces (kg ha−1 day) | 0.34 ± 0.05 b | 0.33 ± 0.01 b | 0.44 ± 0.02 a | 0.0490 |
| Daily of total residual N (U + F) excreted ha−1 | 0.84 ± 0.12 b | 1.11 ± 0.03 a | 1.27 ± 0.06 a | 0.0180 |
| Total residual N urine (kg ha−1 90 days) | 44.95 ± 6.2 b | 70.15 ± 1.9 a | 74.03 ± 3.6 a | 0.0071 |
| Total residual N feces (kg ha−1 90 days) | 30.70 ± 4.2 b | 29.54 ± 0.8 b | 39.83 ± 6.2 a | 0.0500 |
| Total residual N of excreted (kg ha−1 90 days) | 75.65 ± 10.4 b | 100.04 ± 2.7 a | 113.86 ± 5.6 a | 0.0199 |
| Total residual N of post-grazing residues (kg ha−1) | 39.77 ± 1.25 ns | 40.10 ± 1.26 | 39.92 ± 0.72 | 0.5012 |
| Total residual N during winter (kg ha−1) | 115.42 ± 5.7 b | 140.14 ± 7.0 a | 153.78 ± 7.7 a | 0.0120 |
| Variables | Control | Legume | Supplementation |
|---|---|---|---|
| N Blood (g kg−1 LW) | 2.31 ± 1.2 ns | 0.98 ± 0.51 | 1.04 ± 0.54 |
| N Muscle + diaphragm (g kg−1 LW) | 11.91 ± 0.24 ns | 12.13 ± 0.25 | 12.4 ± 0.25 |
| N Carcass fat (g kg−1 LW) | 1.04 ± 0.2 ns | 0.96 ± 0.18 | 1.36 ± 0.26 |
| N GIT fat (g kg−1 LW) | 0.13 ± 0.07 ns | 0.29 ± 0.16 | 0.11 ± 0.06 |
| N Lung + trachea (g kg−1 LW) | 0.16 ± 0.01 ns | 0.16 ± 0.01 | 0.18 ± 0.01 |
| N Tail (g kg−1 LW) | 0.09 ± 0.01 ns | 0.09 ± 0.01 | 0.11 ± 0.01 |
| N GIT (g kg−1 LW) | 1.71 ± 0.42 ns | 2.82 ± 0.69 | 2.58 ± 0.64 |
| N Vitals (g kg−1 LW) | 0.69 ± 0.01 ns | 0.71 ± 0.01 | 0.71 ± 0.01 |
| N Bone + foot + hide (g kg−1 LW) | 7.58 ± 0.38 ns | 7.24 ± 0.36 | 8 ± 0.4 |
| N Leather + ear (g kg−1 LW) | 4.07 ± 0.21 ns | 3.8 ± 0.19 | 4.2 ± 0.21 |
| N Total organs (g kg−1 LW) | 29.73 ± 0.75 ns | 29.2 ± 0.74 | 30.69 ± 0.78 |
| N Total organ input (kg animal−1) | 11.83 ± 0.33 ns | 12.26 ± 0.34 | 12.49 ± 0.34 |
| N Total organ output (kg animal−1) | 14.35 ± 0.58 ns | 14.89 ± 0.6 | 15.56 ± 0.63 |
| Average live weight output (kg) | 482.8 ± 14.36 ns | 510 ± 15.17 | 506.8 ± 15.07 |
| N Total retained: animal output (kg) | 2.52 ± 0.3 ns | 2.51 ± 0.3 | 3.07 ± 0.36 |
| N Animal retention in 90 days of grazing (g kg−1 LW) | 5.22 ± 0.56 ns | 4.93 ± 0.53 | 6.04 ± 0.64 |
| N Exported animal LW (kg ha−1) | 7.15 ± 1.47 ns | 7.27 ± 1.5 | 10.13 ± 2.09 |
| Variables | Control | Legume | Supplementation | p-Value |
|---|---|---|---|---|
| Grain yield (Mg ha−1) | 3.97 ± 0.27 a | 4.19 ± 0.28 a | 4.30 ± 0.29 a | 0.4134 |
| N uptake (kg ha−1) | 296.25 ± 6.8 c | 310.46 ± 7.1 b | 323.88 ± 7.4 a | 0.0001 |
| N content in grains (g kg−1) | 5.56 ± 0.13 b | 5.51 ± 0.09 b | 5.62 ± 0.07 a | 0.0696 |
| N off-take in grains (kg ha−1) | 220.77 ± 5.10 c | 230.87 ± 3.59 b | 242.11 ± 3.04 a | 0.0001 |
| N straw return (kg ha−1) | 75.47 ± 4.1 ns | 79.59 ± 4.3 | 81.77 ± 4.5 | 0.4510 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danna, M.; Scheeren, F.B.; Luz, J.H.S.d.; Menezes, L.F.G.d.; Paris, W.; Amadori, C.; Andriotti, N.; Garrett, C.E.; Putti, F.F.; Sartor, L.R. Animal Supplementation and Legume Pastures Enhance Nitrogen Balance and Efficiency in Integrated Crop-Livestock Systems. Agriculture 2025, 15, 2394. https://doi.org/10.3390/agriculture15222394
Danna M, Scheeren FB, Luz JHSd, Menezes LFGd, Paris W, Amadori C, Andriotti N, Garrett CE, Putti FF, Sartor LR. Animal Supplementation and Legume Pastures Enhance Nitrogen Balance and Efficiency in Integrated Crop-Livestock Systems. Agriculture. 2025; 15(22):2394. https://doi.org/10.3390/agriculture15222394
Chicago/Turabian StyleDanna, Mirella, Fernanda Bernardi Scheeren, João Henrique Silva da Luz, Luis Fernando Glasenapp de Menezes, Wagner Paris, Caroline Amadori, Nathalia Andriotti, Caio Emanuell Garrett, Fernando Ferrari Putti, and Laercio Ricardo Sartor. 2025. "Animal Supplementation and Legume Pastures Enhance Nitrogen Balance and Efficiency in Integrated Crop-Livestock Systems" Agriculture 15, no. 22: 2394. https://doi.org/10.3390/agriculture15222394
APA StyleDanna, M., Scheeren, F. B., Luz, J. H. S. d., Menezes, L. F. G. d., Paris, W., Amadori, C., Andriotti, N., Garrett, C. E., Putti, F. F., & Sartor, L. R. (2025). Animal Supplementation and Legume Pastures Enhance Nitrogen Balance and Efficiency in Integrated Crop-Livestock Systems. Agriculture, 15(22), 2394. https://doi.org/10.3390/agriculture15222394







