Tritordeum as a Habitat for the Development of the Rice Weevil Sitophilus oryzae L.—Analysis of Selected Properties of the Cereal Grains Determining Their Resistance Mechanisms
Abstract
1. Introduction
- The intensity of rice weevil development varies depending on the cereal species studied;
- The tested Tritordeum lines differ in the physicochemical properties of the grain;
- The intensity of S. oryzae development on the tested Tritordeum lines results from the different physicochemical characteristics of the grain.
2. Materials and Methods
2.1. Materials
2.2. Bioassays
2.3. Physicochemical Properties of Grain
2.4. Statistical Analysis
3. Results
3.1. Developmental Parameters of S. oryzae
3.2. Physicochemical Properties of the Hybrid Varieties of the Tested Grain
4. Discussion
5. Conclusions
- Tritordeum grain is a habitat in which S. oryzae can develop, although with varying intensity.
- The Tritordeum lines studied differed in their physicochemical grain characteristics.
- The physical properties of the kernels (seed coat hardness) did not influence the resistance of the studied cereals to S. oryzae feeding. Resistance is therefore determined by chemical factors or a combination of chemical factors.
- Resistance of Tritordeum to rice weevil feeding appears to result primarily from the grain’s chemical composition, particularly protein or associated fractions, rather than hardness.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pawlak, K.; Kołodziejczak, M. The Role of Agriculture in Ensuring Food Security in Developing Countries: Considerations in the Context of the Problem of Sustainable Food Production. Sustainability 2020, 12, 5488. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. The State of Food and Agriculture 2023—Revealing the True Cost of Food to Transform Agrifood Systems. In The State of Food and Agriculture 2023; FAO: Rome, Italy, 2023; 150p. [Google Scholar] [CrossRef]
- Varzakas, T.; Smaoui, S. Global Food Security and Sustainability Issues: The Road to 2030 from Nutrition and Sustainable Healthy Diets to Food Systems Change. Foods 2024, 13, 306. [Google Scholar] [CrossRef]
- Galanakis, C.M. The Future of Food. Foods 2024, 13, 506. [Google Scholar] [CrossRef]
- Sun, L.; Lai, M.; Ghouri, F.; Nawaz, M.A.; Ali, F.; Baloch, F.S.; Nadeem, M.A.; Aasim, M.; Shahid, M.Q. Modern Plant Breeding Techniques in Crop Improvement and Genetic Diversity: From Molecular Markers and Gene Editing to Artificial Intelligence—A Critical Review. Plants 2024, 13, 2676. [Google Scholar] [CrossRef]
- Martin, A.; Sanchez-Monge Laguna, E. Cytology and Morphology of the Amphiploid Hordeum chilense × Triticum turgidum Cony. Durum. Euphytica 1982, 31, 261–267. [Google Scholar] [CrossRef]
- Hiywotu, A.M. Advancing Sustainable Agriculture for Goal 2: Zero Hunger—A Comprehensive Overview of Practices, Policies, and Technologies. Agroecol. Sustain. Food Syst. 2025, 49, 1027–1055. [Google Scholar] [CrossRef]
- Islam, S. Agriculture, Food Security, and Sustainability: A Review. Explor. Foods Foodomics 2025, 3, 101082. [Google Scholar] [CrossRef]
- Martín, A.; Alvarez, J.B.; Martín, L.M.; Barro, F.; Ballesteros, J. The Development of Tritordeum: A Novel Cereal for Food Processing. J. Cereal Sci. 1999, 30, 85–95. [Google Scholar] [CrossRef]
- Polonskiy, V.I. Tritordeum—A Promising Grain Crop. Bull. KSAU 2025, 5, 74–90. [Google Scholar] [CrossRef]
- Villegas, D.; Casadesús, J.; Atienza, S.G.; Martos, V.; Maalouf, F.; Karam, F.; Aranjuelo, I.; Nogués, S. Tritordeum, Wheat and Triticale Yield Components under Multi-Local Mediterranean Drought Conditions. Field Crops Res. 2010, 116, 68–74. [Google Scholar] [CrossRef]
- Shewry, P.R.; Brouns, F.; Dunn, J.; Hood, J.; Burridge, A.J.; America, A.H.P.; Gilissen, L.; Proos-Huijsmans, Z.A.M.; Van Straaten, J.P.; Jonkers, D.; et al. Comparative Compositions of Grain of Tritordeum, Durum Wheat and Bread Wheat Grown in Multi-Environment Trials. Food Chem. 2023, 423, 136312. [Google Scholar] [CrossRef]
- Wiwart, M.; Szafrańska, A.; Suchowilska, E. Does the Use of the Tritordeum, an Alternative Cereal for Breadmaking, Can Be One of the Mitigation Strategies of Acrylamide in Bread? LWT 2025, 223, 117715. [Google Scholar] [CrossRef]
- Mellado-Ortega, E.; Hornero-Méndez, D. Carotenoid Evolution during Short-Storage Period of Durum Wheat (Triticum turgidum Conv. Durum) and Tritordeum (×Tritordeum Ascherson et Graebner) Whole-Grain Flours. Food Chem. 2016, 192, 714–723. [Google Scholar] [CrossRef]
- Suchowilska, E.; Wiwart, M.; Przybylska-Balcerek, A.; Stuper-Szablewska, K. The Profile of Bioactive Compounds in the Grain of Various x Tritordeum Genotypes. J. Cereal Sci. 2021, 102, 103352. [Google Scholar] [CrossRef]
- Visioli, G.; Lauro, M.; Vamerali, T.; Dal Cortivo, C.; Panozzo, A.; Folloni, S.; Piazza, C.; Ranieri, R. A Comparative Study of Organic and Conventional Management on the Rhizosphere Microbiome, Growth and Grain Quality Traits of Tritordeum. Agronomy 2020, 10, 1717. [Google Scholar] [CrossRef]
- Russo, F.; Riezzo, G.; Linsalata, M.; Orlando, A.; Tutino, V.; Prospero, L.; D’Attoma, B.; Giannelli, G. Managing Symptom Profile of IBS-D Patients with Tritordeum-Based Foods: Results from a Pilot Study. Front. Nutr. 2022, 9, 797192. [Google Scholar] [CrossRef]
- Giordano, D.; Reyneri, A.; Locatelli, M.; Coisson, J.D.; Blandino, M. Distribution of bioactive compounds in pearled fractions of tritordeum. Food Chem. 2019, 301, 125228. [Google Scholar] [CrossRef]
- Sánchez-León, S.; Haro, C.; Villatoro, M.; Vaquero, L.; Comino, I.; González-Amigo, A.B.; Vivas, S.; Pastor, J.; Sousa, C.; Landa, B.B.; et al. Tritordeum Breads Are Well Tolerated with Preference over Gluten-Free Breads in Non-Celiac Wheat-Sensitive Patients and Its Consumption Induce Changes in Gut Bacteria. J. Sci. Food Agric. 2021, 101, 3508–3517. [Google Scholar] [CrossRef]
- Vaquero, L.; Comino, I.; Vivas, S.; Rodríguez-Martín, L.; Giménez, M.J.; Pastor, J.; Sousa, C.; Barro, F. Tritordeum: A Novel Cereal for Food Processing with Good Acceptability and Significant Reduction in Gluten Immunogenic Peptides in Comparison with Wheat. J. Sci. Food Agric. 2017, 98, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Haro, C.; Guzmán-López, M.H.; Marín-Sanz, M.; Sánchez-León, S.; Vaquero, L.; Pastor, J.; Comino, I.; Sousa, C.; Vivas, S.; Landa, B.B.; et al. Consumption of Tritordeum Bread Reduces Immunogenic Gluten Intake without Altering the Gut Microbiota. Foods 2022, 11, 1439. [Google Scholar] [CrossRef]
- Rajnincová, D.; Gálová, Z. The Protein Profile of Cereals, Pseudocereals and Legumes. J. Food Sci. Technol.-Mysore 2019, 7, 49–53. [Google Scholar]
- Comino, I.; Moreno, M.D.L.; Real, A.; Rodríguez-Herrera, A.; Barro, F.; Sousa, C. The Gluten-Free Diet: Testing Alternative Cereals Tolerated by Celiac Patients. Nutrients 2013, 5, 4250–4268. [Google Scholar] [CrossRef]
- Nitride, C.; D’Auria, G.; Dente, A.; Landolfi, V.; Picariello, G.; Mamone, G.; Blandino, M.; Romano, R.; Ferranti, P. Tritordeum as an Innovative Alternative to Wheat: A Comparative Digestion Study on Bread. Molecules 2022, 27, 1308. [Google Scholar] [CrossRef]
- Kakabouki, I.; Beslemes, D.F.; Tigka, E.L.; Folina, A.; Karydogianni, S.; Zisi, C.; Papastylianou, P. Performance of Six Genotypes of Tritordeum Compare to Bread Wheat under East Mediterranean Condition. Sustainability 2020, 12, 9700. [Google Scholar] [CrossRef]
- Kumar, D.; Kalita, P. Reducing Postharvest Losses during Storage of Grain Crops to Strengthen Food Security in Developing Countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef]
- Klejdysz, T.; Mrówczyński, M. Metodyka Integrowanej Ochrony Magazynów Zbożowych Dla Doradców [Methodology of Integrated Protection of Grain Storage for Advisers]; Instytut Ochrony Roślin—Państwowy Instytut Badawczy: Poznań, Poland, 2007; ISBN 978-83-64655-31-9. [Google Scholar]
- Jalaeian, M.; Mohammadzadeh, M.; Mohammadzadeh, M.; Borzoui, E. Rice Cultivars Affect Fitness-Related Characteristics and Digestive Physiology of the Rice Weevil, Sitophilus oryzae (L.) (Coleoptera: Curculionidae). J. Stored Prod. Res. 2021, 93, 101821. [Google Scholar] [CrossRef]
- Baker, J.E. Development of Four Strains of Sitophilus oryzae (L.) (Coleoptera: Curculionidae) on Barley, Corn (Maize), Rice, and Wheat. J. Stored Prod. Res. 1988, 24, 193–198. [Google Scholar] [CrossRef]
- Raghuraman, M.; Sharma, V.; Chaudhary, T.; Raghuraman, M.; Meena, R.S.; Kumar, P. Biology of Sitophilus oryzae (L.) on Stored Rice Chaudhary et al Biology of Sitophilus oryzae (L.) on Stored Rice. Biol. Forum 2025, 17, 33–36. [Google Scholar]
- Suchowilska, E.; Radawiec, W.; Wiwart, M. Tritordeum—The Content of Basic Nutrients in Grain and the Morphological and Anatomical Features of Kernels. Int. Agrophysics 2021, 35, 343–355. [Google Scholar] [CrossRef]
- Halstead, D.G.H. External Sex Differences in Stored-Products Coleoptera. Bull. Entomol. Res. 1963, 54, 119–134. [Google Scholar] [CrossRef]
- Kosewska, O.; Przemieniecki, S.W.; Nietupski, M. The Effect of Antibiotics on Bacteriome of Sitophilus oryzae and Rhyzopertha dominica as a Factor Determining the Success of Foraging: A Chance for Antibiotic Therapy in Grain Stores. Appl. Sci. 2023, 13, 1576. [Google Scholar] [CrossRef]
- Kosewska, O.; Przemieniecki, S.W.; Koronkiewicz, S.; Nietupski, M. Effect of Different Chemical Properties of Cereal Grains on the Foraging and Microbiome of the Rice Weevil (Sitophilus oryzae L.). Int. Agrophys. 2024, 38, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Kosewska, O.; Nietupski, M.; Koronkiewicz, S.; Przemieniecki, S.W. The Chemical Grain Composition of Wheat and Barley Affects the Development of the Lesser Grain Borer (Rhyzopertha dominica F.) and the Rice Weevil (Sitophilus oryzae L.). J. Plant Prot. Res. 2024, 64, 275–287. [Google Scholar] [CrossRef]
- Hoseney, R.C. Principles of Cereal Science and Technology, 3rd ed.; Cereals & Grains Association: St. Paul, MN, USA, 2014. [Google Scholar]
- Clarke, K.R. Non-parametric Multivariate Analyses of Changes in Community Structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- ter Braak, C.J.R.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide Software for Canonical Community Ordination; Version 4.5; Microcomputer Power: Ithaca, NY, USA, 1998. [Google Scholar]
- Ávila, C.M.; Rodríguez-Suárez, C.; Atienza, S.G. Tritordeum: Creating a New Crop Species—The Successful Use of Plant Genetic Resources. Plants 2021, 10, 1029. [Google Scholar] [CrossRef]
- Papadopoulos, G.; Mavroeidis, A.; Stavropoulos, P.; Anastasopoulos, V.; Beslemes, D.; Tigka, E.; Kakabouki, I. Tritordeum: A Versatile and Resilient Cereal for Mediterranean Agriculture and Sustainable Food Production. Cereal Res. Commun. 2024, 52, 323–331. [Google Scholar] [CrossRef]
- Selwet, M. Maize Plants Infestation by Fusarium spp. and Deoxynivalenol in Genetically Modified Corn Hybrid and Traditional Maize Cultivars. Pol. J. Microbiol. 2011, 60, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.M.; Vasicec, A.; Ramos, S.; Martin, A.; Martin, L.M.; Dixon, A.F.G. Resistance against Greenbug, Schizuphis graminum Rond., and Russian Wheat Aphid, Diuraphis Noxia Mordvilko, in Tritordeum Amphiploids. Plant Breed. 1998, 117, 515–522. [Google Scholar] [CrossRef]
- Kumar, C.; Ram, C.L.; Jha, S.N.; Vishwakarma, R.K. Warehouse Storage Management of Wheat and Their Role in Food Security. Front. Sustain. Food Syst. 2021, 5, 675626. [Google Scholar] [CrossRef]
- Jayas, D.S. Storing Grains for Food Security and Sustainability. Agric. Res. 2012, 1, 21–24. [Google Scholar] [CrossRef]
- Das, S.; Barve, A.; Sahu, N.C.; Muduli, K.; Kumar, A.; Luthra, S. Analysing the Challenges to Sustainable Food Grain Storage Management: A Path to Food Security in Emerging Nations. Int. J. Food Sci. Technol. 2023, 58, 5501–5509. [Google Scholar] [CrossRef]
- Manandhar, A.; Milindi, P.; Shah, A. An Overview of the Post-Harvest Grain Storage Practices of Smallholder Farmers in Developing Countries. Agriculture 2018, 8, 57. [Google Scholar] [CrossRef]
- Zhao, Y.; Lv, H.; Li, Y. Grain Storage: Theory, Technology and Equipment. Foods 2023, 12, 3792. [Google Scholar] [CrossRef] [PubMed]
- Gouthami, D. Storage Insect Pests and Their Management. In Insights into Agroculture Sciences; Kripa Drishti Publications: Pune, India, 2024; Volume II, pp. 111–128. [Google Scholar]
- Balaji, K.; Murugesan, S.; Sruthi, A.B.; Sarika, M. Pest Management in Stored Grains: Challenges and Innovations. In Integrated Plant Protection: Safeguarding Agricultural Productivity; Paper Trial Publications: Thanesar, India, 2025; pp. 306–324. [Google Scholar]
- Trematerra, P.; Fontana, F.; Mancini, M. Analysis of Development Rates of Sitophilus oryzae (L.) in Five Cereals of the Genus Triticum. J. Stored Prod. Res. 1996, 32, 315–322. [Google Scholar] [CrossRef]
- Gvozdenac, S.; Tanasković, S.; Vukajlović, F.; Prvulović, D.; Ovuka, J.; Višacki, V.; Sedlar, A. Host and Ovipositional Preference of Rice Weevil (Sitophilus oryzae) Depending on Feeding Experience. Appl. Ecol. Environ. Res. 2020, 18, 6663–6673. [Google Scholar] [CrossRef]
- Kordan, B.; Nietupski, M.; Ludwiczak, E.; Gabryś, B.; Cabaj, R. Selected Cultivar-Specific Parameters of Wheat Grain as Factors Influencing Intensity of Development of Grain Weevil Sitophilus granarius (L.). Agriculture 2023, 13, 1492. [Google Scholar] [CrossRef]
- Nietupski, M.; Ludwiczak, E.; Cabaj, R.; Purwin, C.; Kordan, B. Fatty Acids Present in Wheat Kernels Influence the Development of the Grain Weevil (Sitophilus granarius L.). Insects 2021, 12, 806. [Google Scholar] [CrossRef]
- Kordan, B.; Gabryś, B.; Załuski, D.; Zdunowski, K. Podatność Wybranych Gatunków Zbóż Na Żerowanie Trojszyka Ulca (Tribolium Confusum Duv.). Prog. Plant Prot./Postępy Ochr. Roślin 2011, 51, 1559–1562. [Google Scholar]
- Warchalewski, J.R.; Gralik, J.; Nawrot, J. Możliwości Zmniejszania Powodowanych Przez Szkodniki Owadzie Strat Magazynowanego Ziarna Zbóż. Zesz. Probl. Postępów Nauk Rol. 2000, 47, 85–96. [Google Scholar]
- Hassan, M.W.; Hashmi, M.A.; Sarwar, G.; Mehmood, Z.; Saleem, W.; Farooqi, M.A. Damage Assessment of Stored Grain Pests against Rice Grains Types and Wheat. Int. J. Trop. Insect Sci. 2023, 43, 35–41. [Google Scholar] [CrossRef]
- Mebarkia, A.; Rahbé, Y.; Guechi, A.; Bouras, A.; Makhlouf, M. Susceptibility of Twelve Soft Wheat Varieties (Triticum aestivum) to Sitophilus granarius (L.) (Coleoptera: Curculionidae). Agric. Biol. J. N. Am. 2010, 1, 571–578. [Google Scholar]
- Sahoo, G.; Sahoo, B.K. Grain Hardness and Protein Content of Milled Rice Grains and Their Relationship with Infestation of Rice Weevil Sitophilus oryzae L., (Coleoptera: Curculionidae). ORYZA-Int. J. Rice 2016, 53, 332–336. [Google Scholar]
- Nawrot, J.; Warchlewski, J.R.; Piasecka-Kwiatkowska, D.; Niewiada, A. The Effect of Some Biochemical and Technological Properties of Wheat Grain on Granary Weevil (Sitophilus granarius L.) (Coleoptera: Curculionidae) Development. In Proceedings of the 9th International Working Conference on Stored Product Protection, São Paulo, Brazil, 15–18 October 2006. [Google Scholar]
- Ludwiczak, E.; Nietupski, M.; Laszczak-Dawid, A.; Gabryś, B.; Kordan, B.; Purwin, C. Influence of Chemical Composition and Degree of Fragmentation of Millet Grain on Confused Flour Beetle (Tribolium confusum Duv.) Infestation. Agriculture 2023, 13, 2178. [Google Scholar] [CrossRef]
- Ludwiczak, E.; Nietupski, M.; Gabryś, B.; Purwin, C.; Kordan, B. Selected Chemical Parameters of Cereal Grain Influencing the Development of Rhyzopertha dominica F. Sustainability 2024, 16, 7178. [Google Scholar] [CrossRef]
- Abedi, Z.; Razmjou, J.; Rafiee Dastjerdi, H.; Ebadollahi, A. Physical and Biochemical Characteristics of Cereal Grains Affect Population Growth Parameters of Sitophilus oryzae (L.) (Coleoptera: Curculionidae). J. Stored Prod. Res. 2024, 109, 102459. [Google Scholar] [CrossRef]
- Krzymańska, J.G.Z. Biochemical Composition of Wheat Grani as Influenced by Some Beetles Feeding. Prace Naukowe Istytutu Ochrony Roślin 1987, XXVIII, 87–105. [Google Scholar]
- Arya, P.S.; Srivastava, C.; Rajna, S.; Ranjith, H.V.; Subramanian, S. Physicochemical Grain Properties of Wheat Cultivars and Insect Biology Drive Resistance to Sitophilus oryzae L. Sci. Rep. 2025, 15, 35308. [Google Scholar] [CrossRef] [PubMed]
- Nietupski, M.; Ciepielewska, D.; Fornal, L. Wpływ zróżnicowania chemicznego białek w ziarnie wybranych odmian pszenicy na rozwój szkodników magazynowych. Prog. Plant Prot./Postępy Ochr. Roślin 2006, 2, 420–423. [Google Scholar]
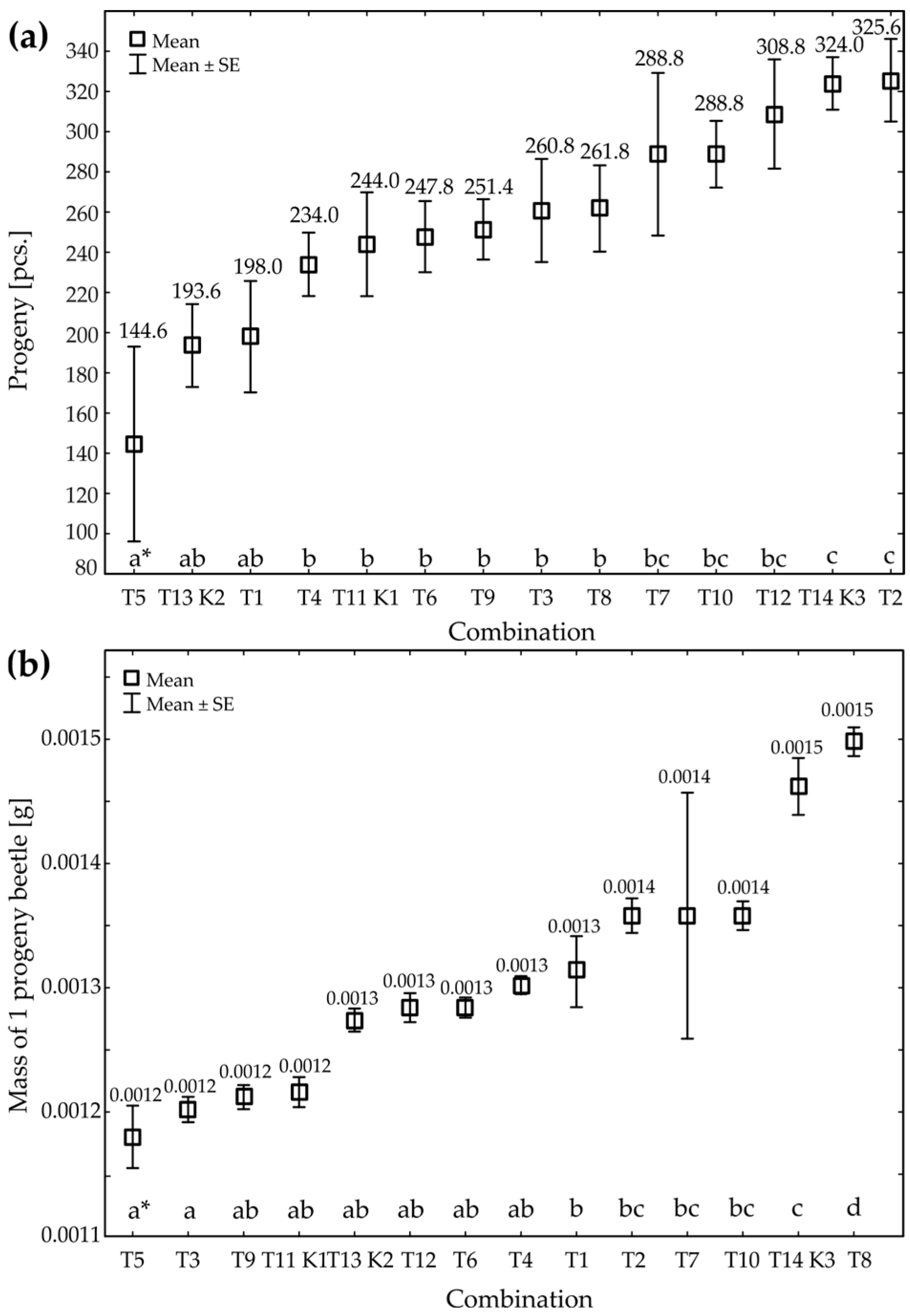
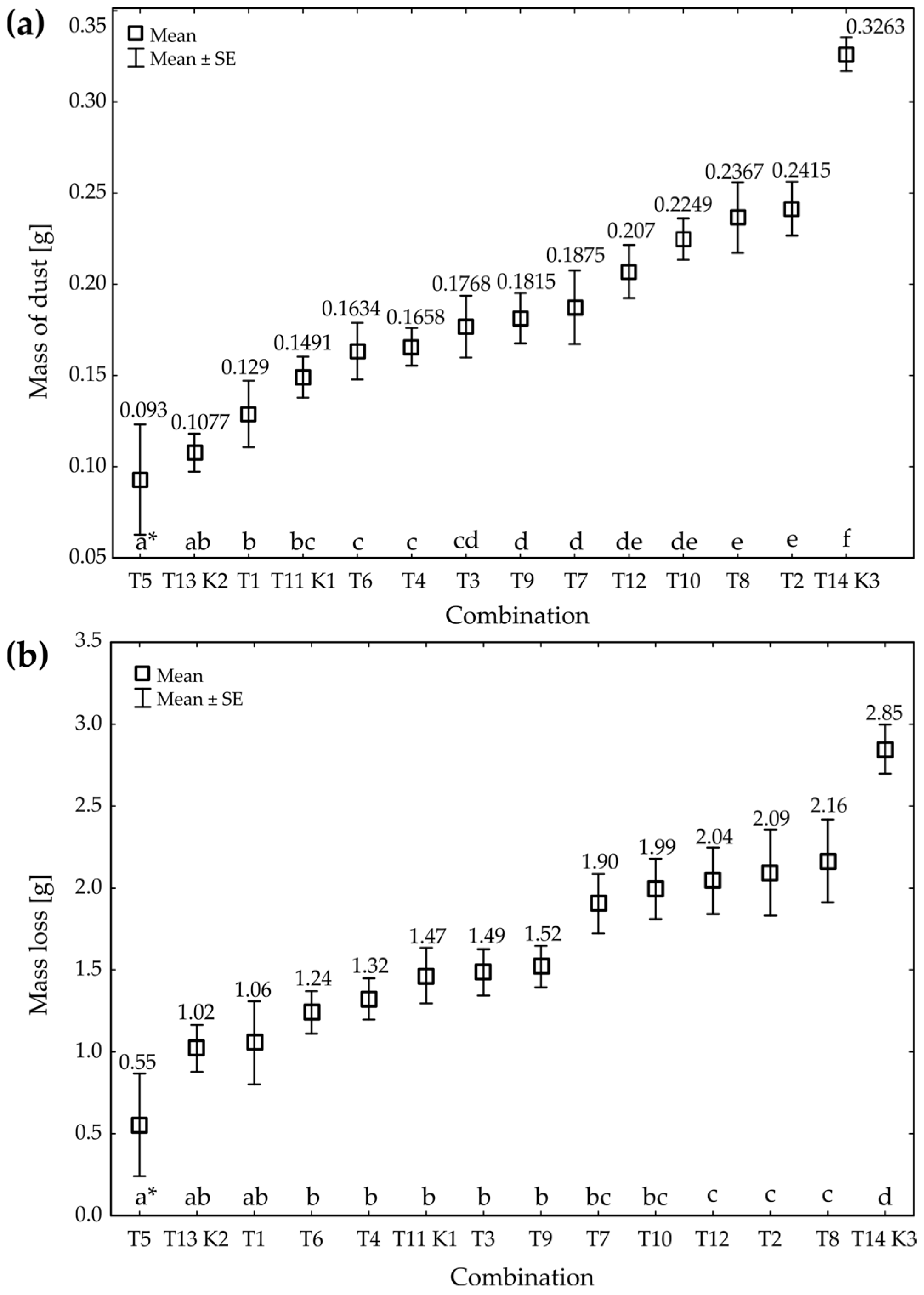
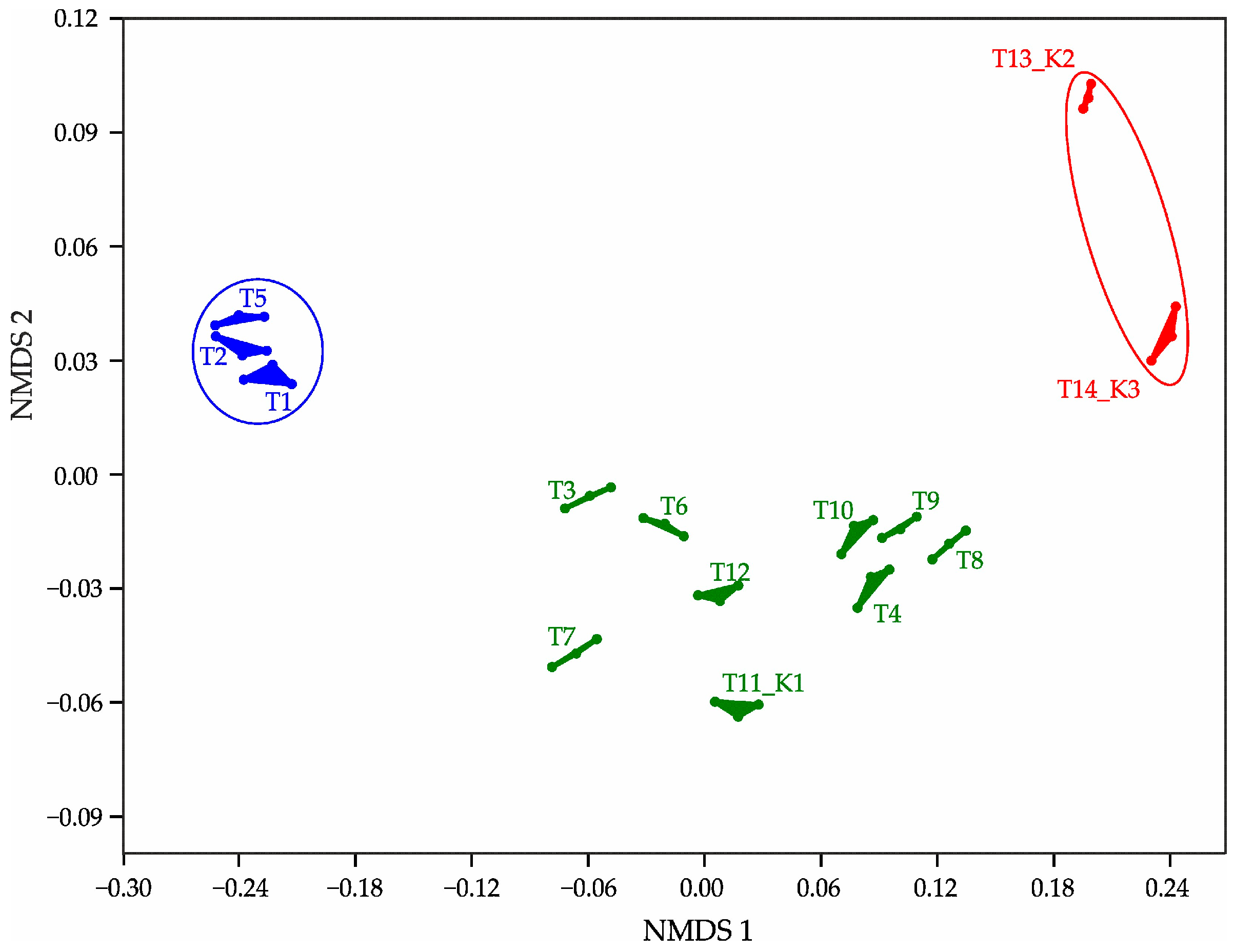
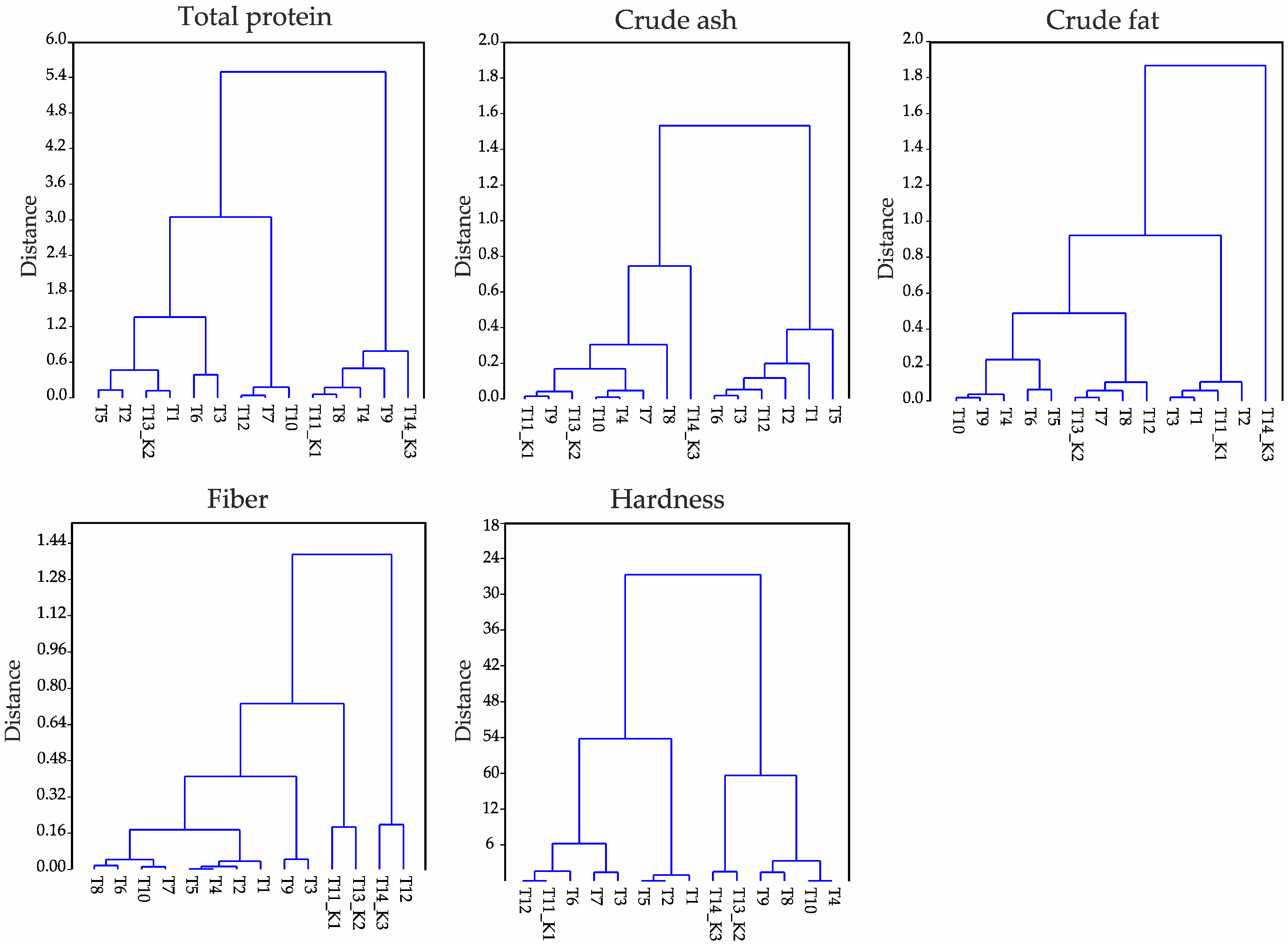

| df * | ANOVA | p ** | |
|---|---|---|---|
| F Value | |||
| Progeny of beetles | 13 | 4.01 | 0.00 |
| Mass of 1 progeny beetle | 13 | 9.62 | 0.00 |
| Mass of dust | 13 | 13.77 | 0.00 |
| Loss of grain mass | 13 | 8.97 | 0.00 |
| df * | ANOVA | p ** | |
|---|---|---|---|
| F Value | |||
| Crude ash | 13 | 468.74 | 0.00 |
| Fiber | 13 | 103.79 | 0.00 |
| Total protein | 13 | 2154.65 | 0.00 |
| Crude fat | 13 | 395.33 | 0.00 |
| Hardness | 13 | 574.77 | 0.00 |
| Combinations | Crude Ash | Fiber | Total Protein | Crude Fat | Hardness Score |
|---|---|---|---|---|---|
| T1 HT 440 | 2.52 ± 0.5 e * | 2.11 ± 0.06 bc | 18.29 ± 0.10 i | 2.59 ± 0.05 cd | 41 ± 1.0 a |
| T2 HT 129 | 2.79 ± 0.03 f | 2.14 ± 0.05 c | 18.54 ± 0.06 j | 2.52 ± 0.01 cd | 40 ± 1.0 a |
| T3 HTC 2060 | 2.71 ± 0.02 ef | 2.52 ± 0.04 d | 17.64 ± 0.03 h | 2.62 ± 0.05 d | 55 ± 1.0 b |
| T4 HT 157 | 2.11 ± 0.02 dc | 2.15 ± 0.07 c | 15.06 ± 0.06 c | 2.15 ± 0.05 b | 67 ± 1.0 d |
| T5 HT 352 | 3.04 ± 0.01 g | 2.15 ± 0.01 c | 18.73 ± 0.07 j | 2.42 ± 0.07 c | 40 ± 1.0 a |
| T6 JB3 | 2.68 ± 0.03 ef | 2.25 ± 0.07 c | 17.09 ± 0.01 g | 2.33 ± 0.01 bc | 58 ± 1.0 c |
| T7 HT 438 | 2.06 ± 0.08 c | 2.28 ± 0.06 cd | 16.25 ± 0.06 f | 1.97 ± 0.01 b | 53 ± 1.0 b |
| T8 HTC 1324 | 1.89 ± 0.02 b | 2.22 ± 0.05 c | 14.89 ± 0.04 b | 2.05 ± 0.01 ab | 71 ± 1.0 e |
| T9 HT 444 | 2.20 ± 0.04 cd | 2.46 ± 0.05 d | 15.46 ± 0.01 d | 2.20 ± 0.03 b | 69 ± 1.0 de |
| T10 HTC 2083 | 2.13 ± 0.06 cd | 2.27 ± 0.03 cd | 16.00 ± 0.08 e | 2.18 ± 0.02 b | 67 ± 1.0 d |
| T11 Gawrosz K1 | 2.23 ± 0.01 cd | 1.67 ± 0.08 a | 14.81 ± 0.07 b | 2.67 ± 0.01 d | 60 ± 1.0 c |
| T12 ‘60’ HTC 2083’ | 2.63 ± 0.01 ef | 2.93 ± 0.02 e | 16.19 ± 0.05 f | 1.90 ± 0.05 b | 60 ± 1.0 c |
| T13 Duragold K2 | 2.26 ± 0.01 d | 1.94 ± 0.01 b | 18.12 ± 0.02 i | 1.99 ± 0.5 ab | 84.5 ± 0.5 f |
| T14 Control K3 | 1.40 ± 0.01 a | 3.21 ± 0.18 f | 14.37 ± 0.04 a | 0.59 ± 0.06 a | 82.4 ± 1.0 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nietupski, M.; Ludwiczak, E.; Suchowilska, E.; Kordan, B.; Foltyński, M. Tritordeum as a Habitat for the Development of the Rice Weevil Sitophilus oryzae L.—Analysis of Selected Properties of the Cereal Grains Determining Their Resistance Mechanisms. Agriculture 2025, 15, 2395. https://doi.org/10.3390/agriculture15222395
Nietupski M, Ludwiczak E, Suchowilska E, Kordan B, Foltyński M. Tritordeum as a Habitat for the Development of the Rice Weevil Sitophilus oryzae L.—Analysis of Selected Properties of the Cereal Grains Determining Their Resistance Mechanisms. Agriculture. 2025; 15(22):2395. https://doi.org/10.3390/agriculture15222395
Chicago/Turabian StyleNietupski, Mariusz, Emilia Ludwiczak, Elżbieta Suchowilska, Bożena Kordan, and Mariusz Foltyński. 2025. "Tritordeum as a Habitat for the Development of the Rice Weevil Sitophilus oryzae L.—Analysis of Selected Properties of the Cereal Grains Determining Their Resistance Mechanisms" Agriculture 15, no. 22: 2395. https://doi.org/10.3390/agriculture15222395
APA StyleNietupski, M., Ludwiczak, E., Suchowilska, E., Kordan, B., & Foltyński, M. (2025). Tritordeum as a Habitat for the Development of the Rice Weevil Sitophilus oryzae L.—Analysis of Selected Properties of the Cereal Grains Determining Their Resistance Mechanisms. Agriculture, 15(22), 2395. https://doi.org/10.3390/agriculture15222395






