Successive Harvesting Interval and Salinity Level Modulate Biomass Production and Nutritional Value in Sarcocornia fruticosa and Arthrocaulon macrostachyum
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Harvest Regime
2.3. Shoot Diameter
2.4. Chlorophyll and Total Carotenoid Content
- Chlorophyll A = [(12.21 × A664) − (2.81 × A647)/w] × (V/1000)
- Chlorophyll B = [(20.13 × A647) − (2.03 × A664)/w] × (V/1000)
2.5. Relative Water Content (Rwc) Measurement
2.6. Total Protein Content
2.7. Total Soluble Solids (Tss) Content and Electroconductivity (Ec) Level
2.8. Anthocyanin Contents
2.9. Total Polyphenol Content
2.10. Total Flavonoids
2.11. Radical Scavenging Assay
2.12. Malondialdehyde (MDA) Content
- [(Abs532+TBA − Abs600+TBA) − (Abs532−TBA − Abs600−TBA)] = A
- [(Abs440+TBA − Abs600+TBA) × 0.0571] = B
2.13. Data Analysis
3. Results
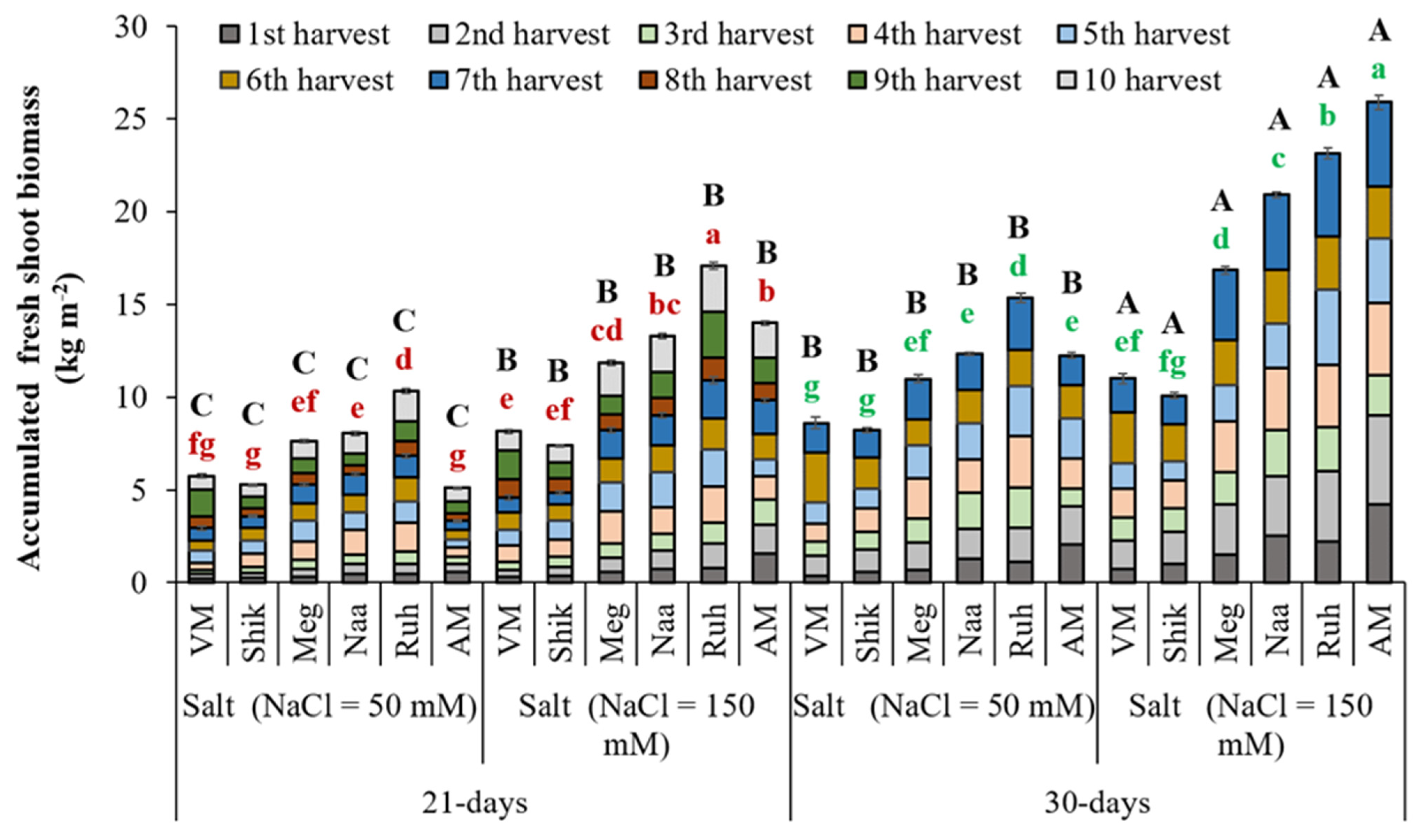
3.1. Successive Harvesting Interval and Salinity Level Affect Fresh Biomass Production
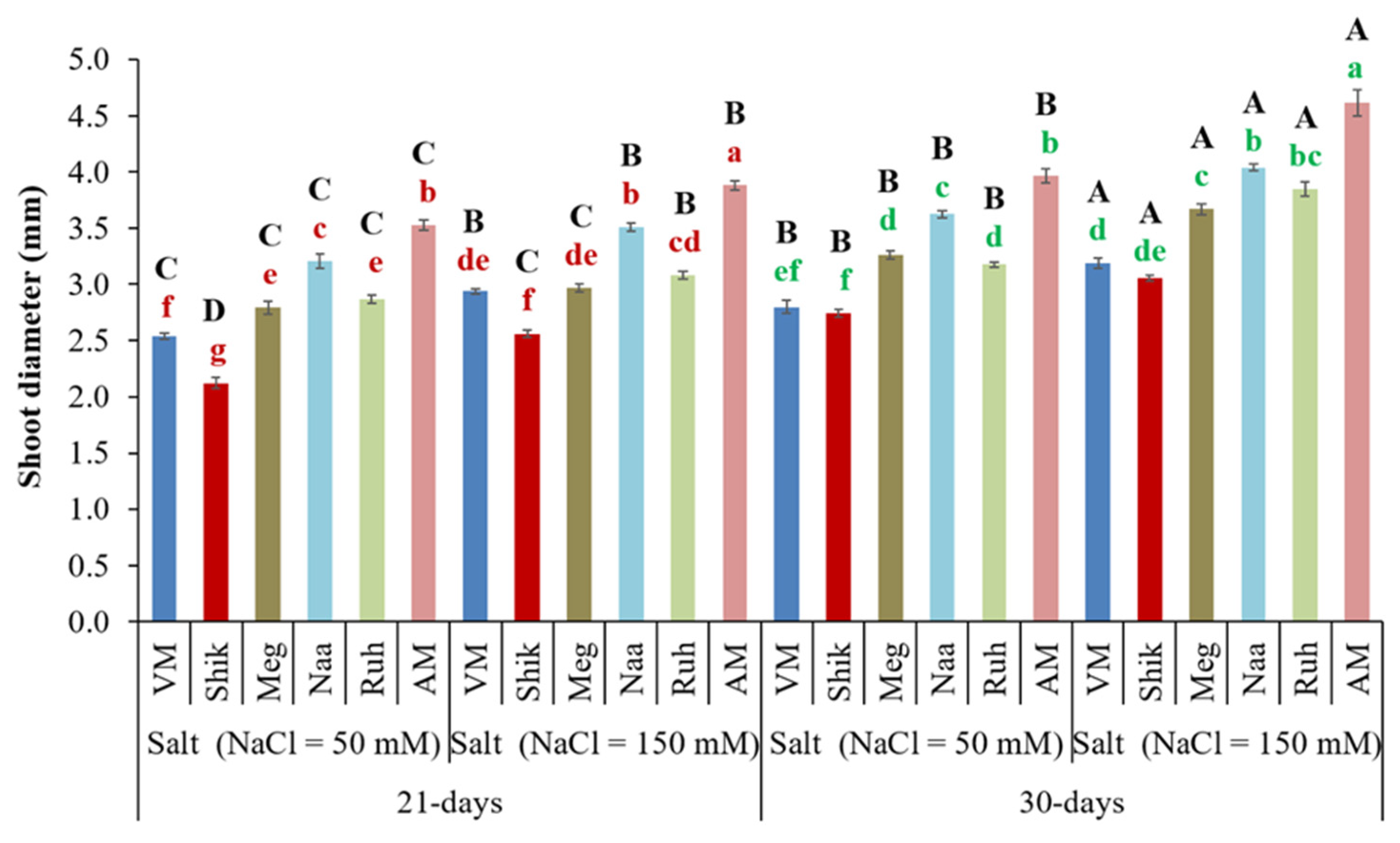
3.2. Successive Harvest Interval and Salinity Affect Shoot Diameter
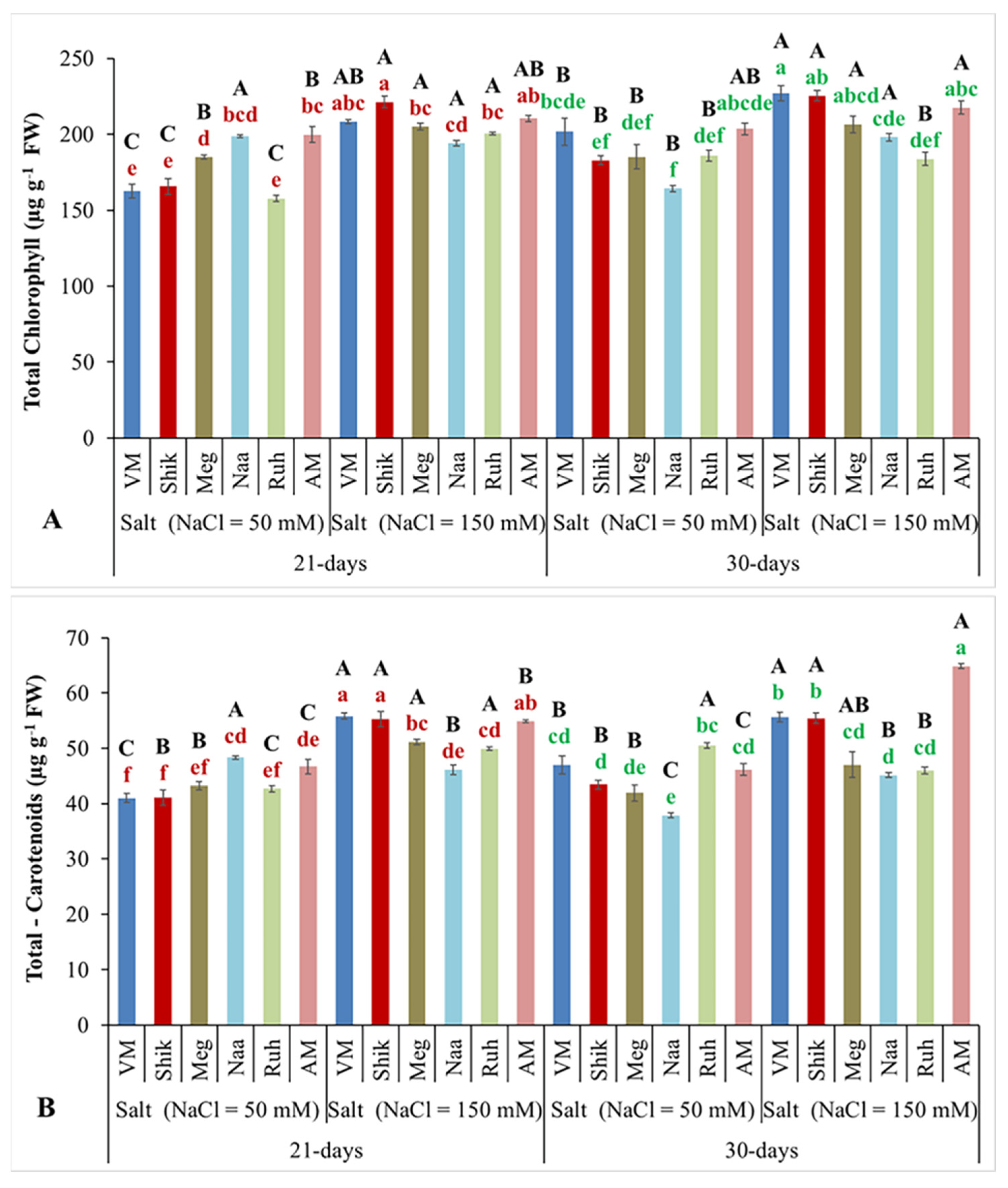
3.3. Total Chlorophyll and Carotenoid Contents
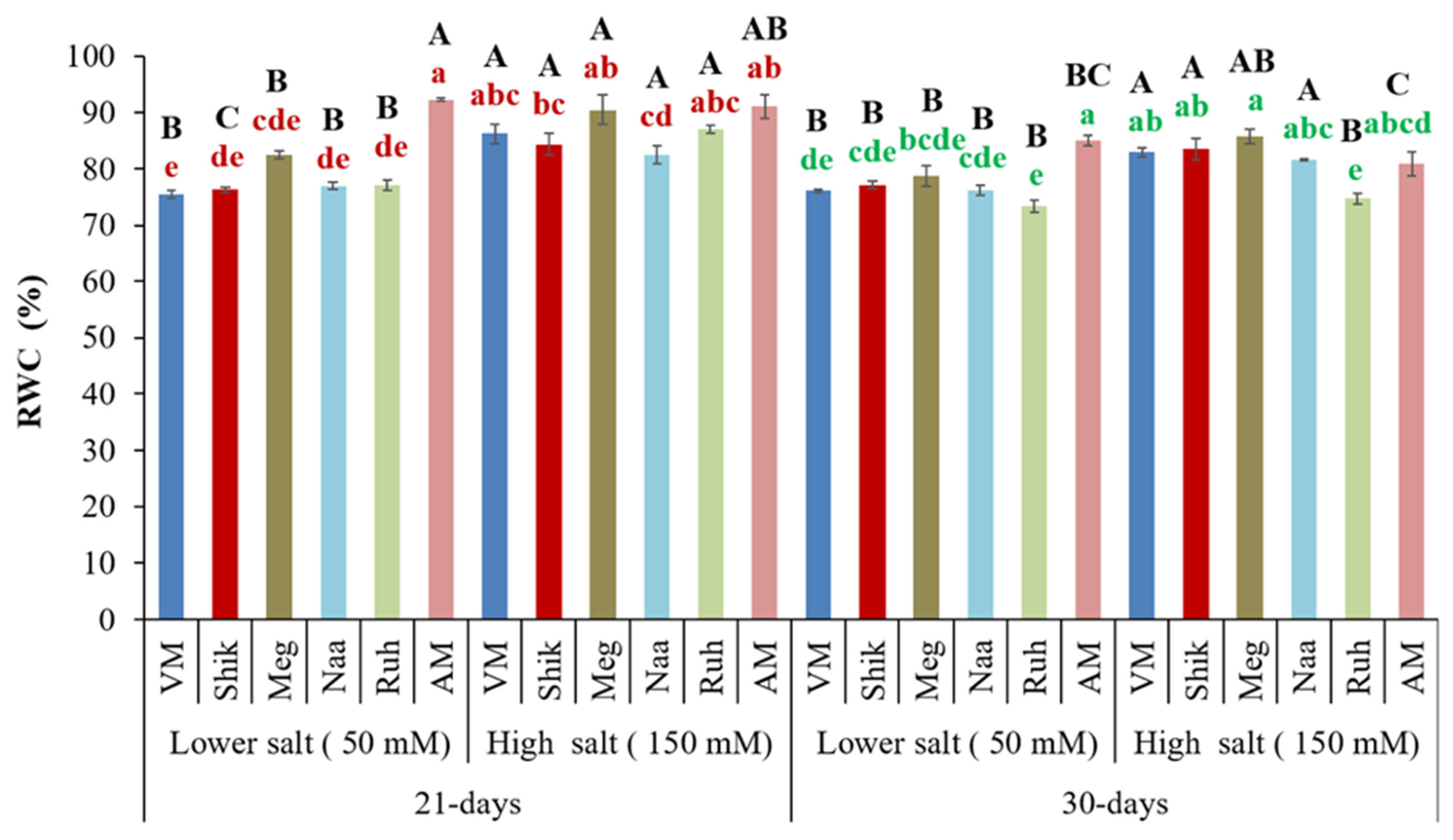
3.4. Relative Water Content (Rwc)
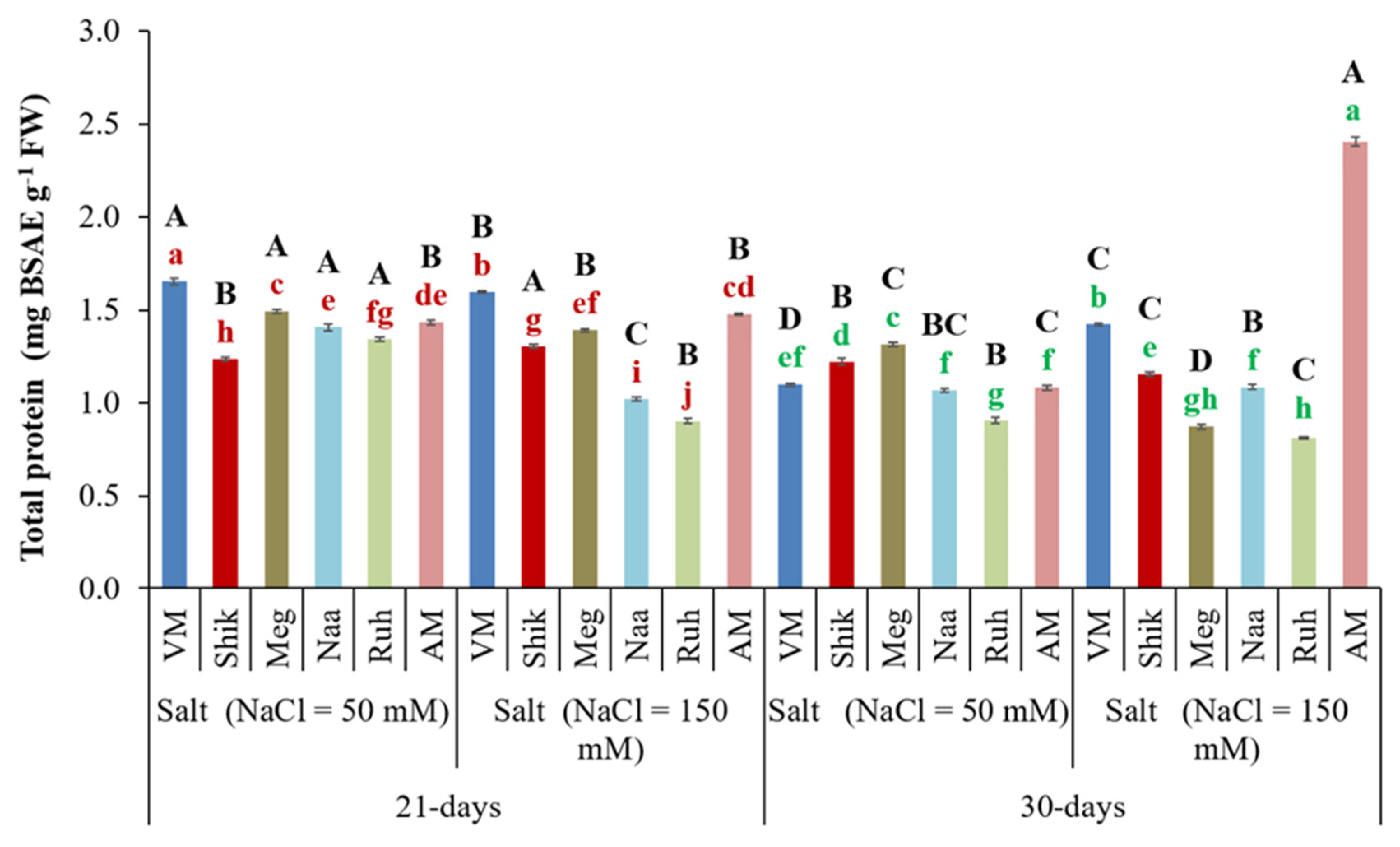
3.5. Successive Harvesting Interval and Salinity Level Affect Total Protein Content
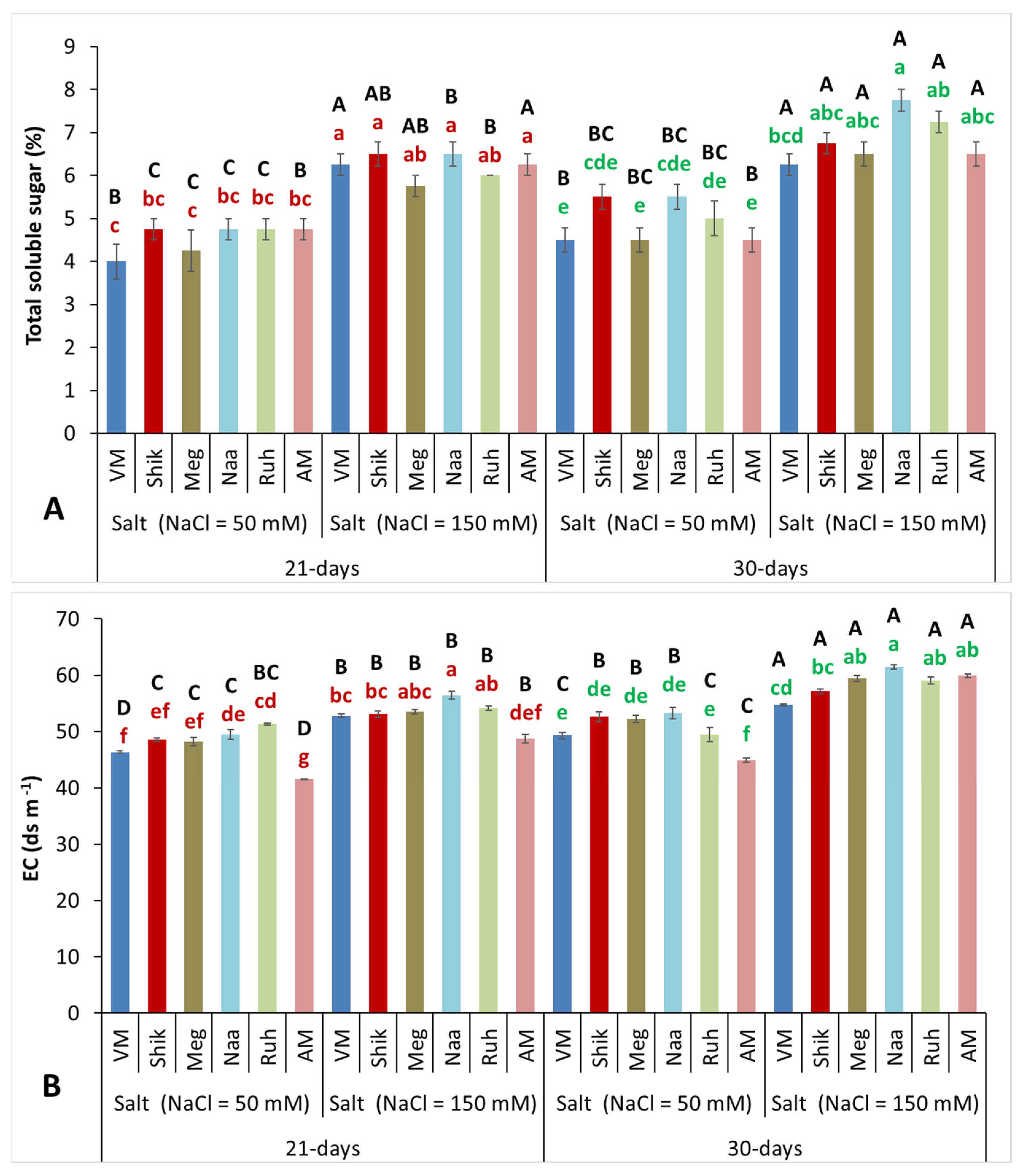
3.6. Successive Harvesting Interval and Salinity Level Significantly Influenced Total Soluble Sugar Content and Electroconductivity
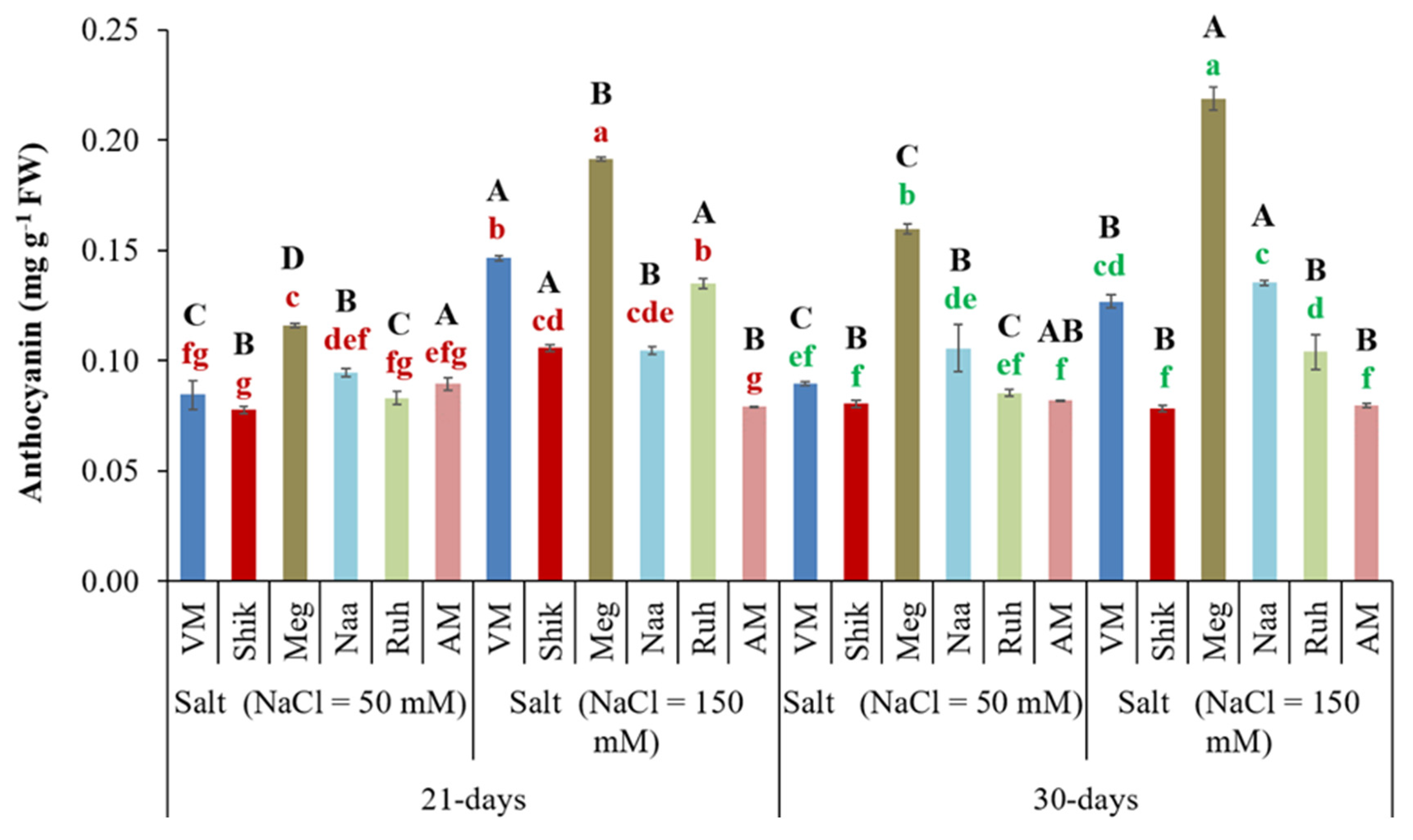
3.7. Harvest Interval and Salinity Level Significantly Influenced Anthocyanin Accumulation
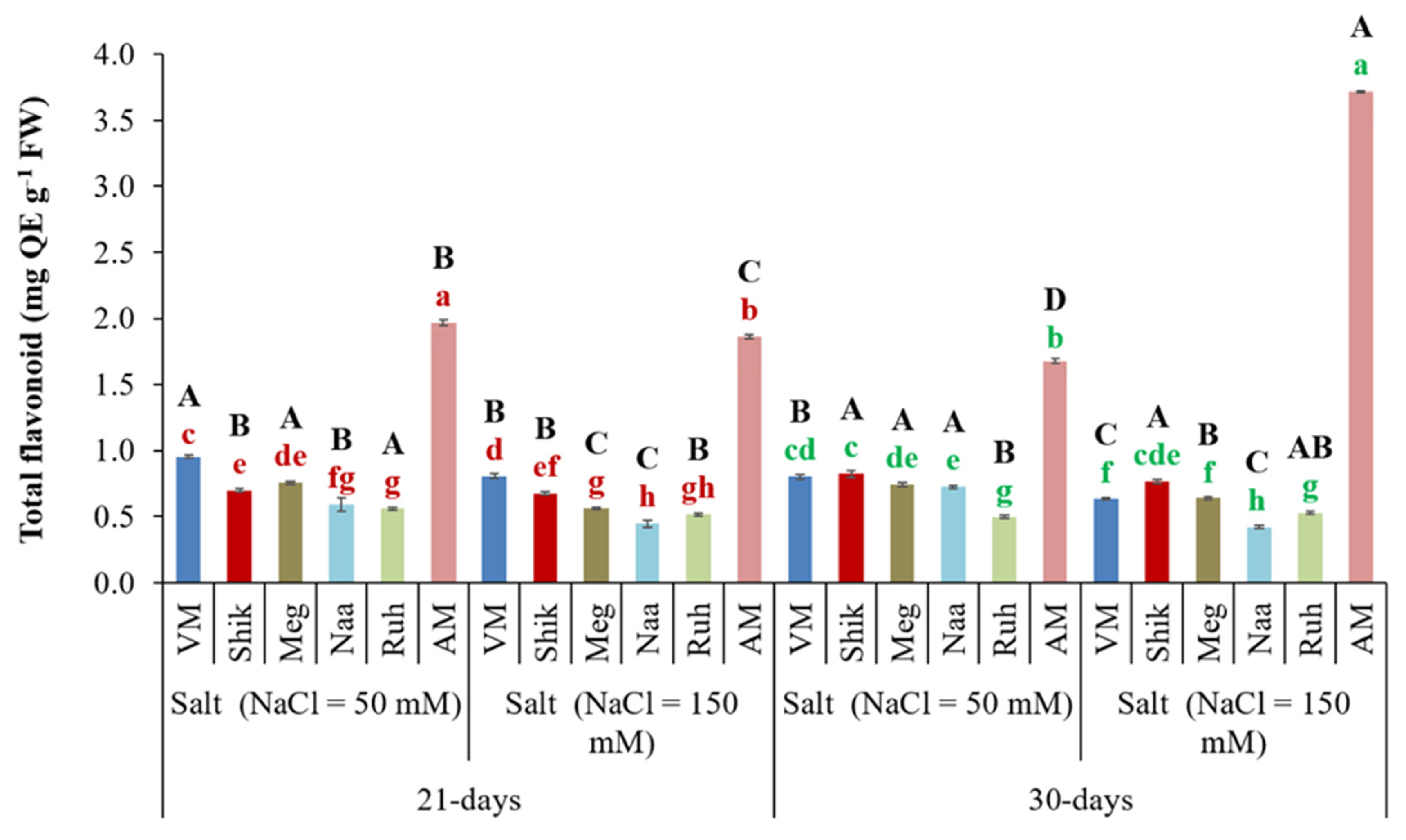
3.8. Total Flavonoid Content
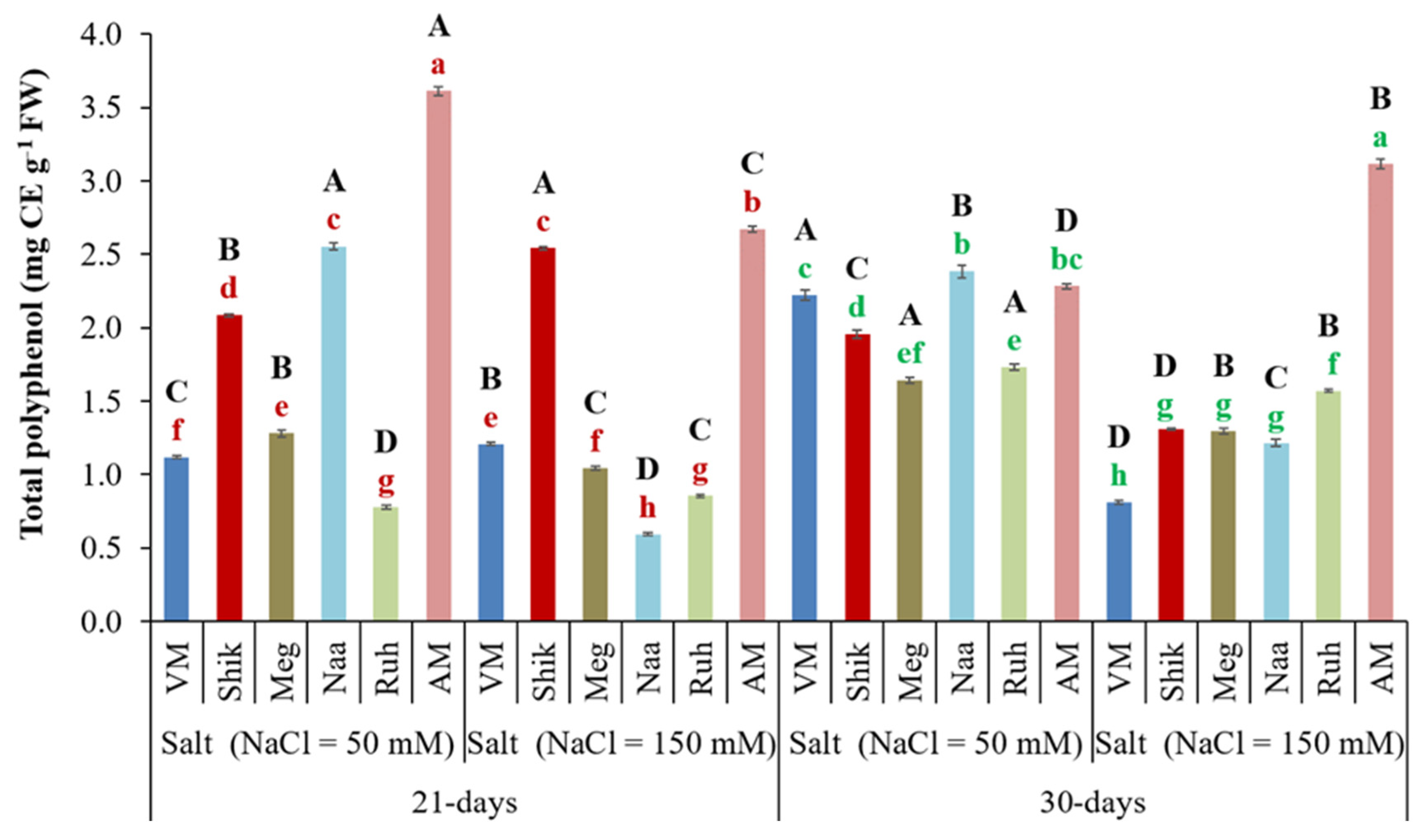
3.9. Successive Harvesting Interval and Salinity Affect Polyphenol Accumulations
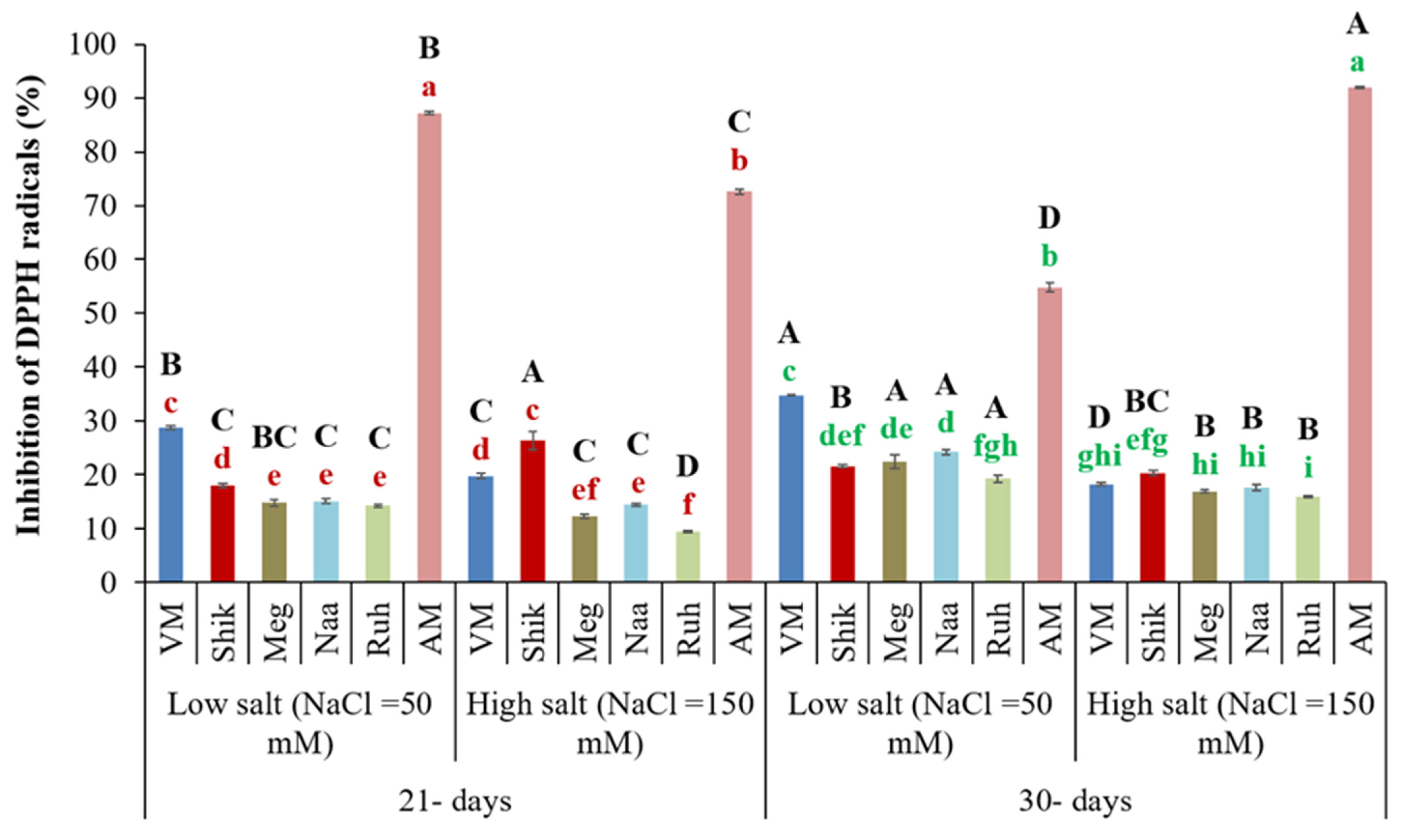
3.10. Successive Harvesting Interval and Salinity Affect 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity
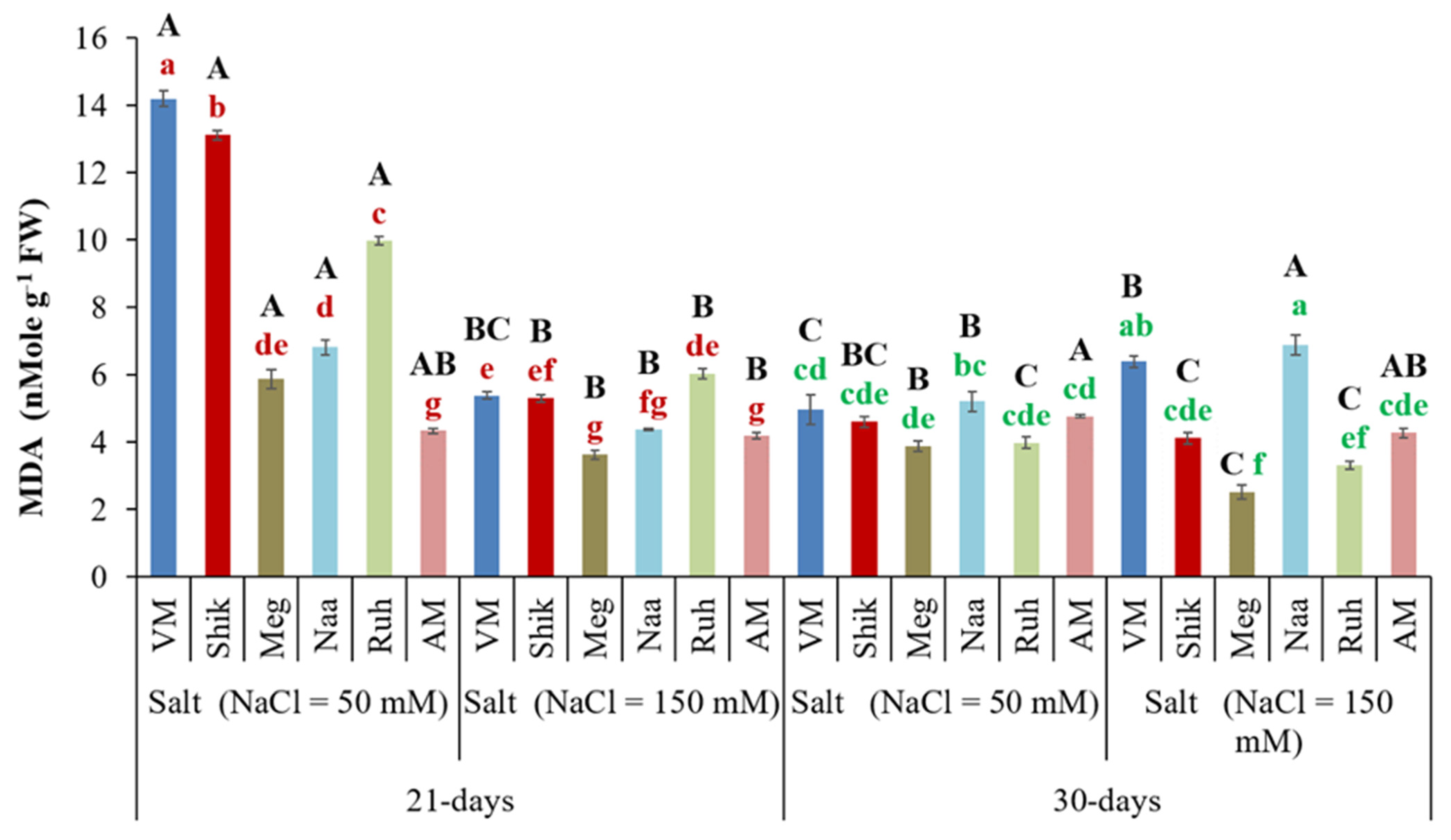
3.11. Successive Harvesting Interval and Salinity Affect Malondialdehyde (MDA) Content
4. Discussion
4.1. Growth Responses
4.2. Photosynthetic Pigments
4.3. Relative Water Content
4.4. Antioxidant Responses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soltabayeva, A.; Ongaltay, A.; Omondi, J.O.O.; Srivastava, S. Morphological, physiological and molecular markers for salt-stressed plants. Plants 2021, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Ventura, Y.; Myrzabayeva, M.; Alikulov, Z.; Omarov, R.; Khozin-Goldberg, I.; Sagi, M. Effects of salinity on flowering, morphology, biomass accumulation and leaf metabolites in an edible halophyte. AoB Plants 2014, 6, plu053. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Loaiza, V.; Oliveira, M.; Santos, T.; Schüler, L.; Lima, A.R.; Gama, F.; Salazar, M.; Neng, N.R.; Nogueira, J.; Varela, J.; et al. Wild vs cultivated halophytes: Nutritional and functional differences. Food Chem. 2020, 333, 127536. [Google Scholar] [CrossRef]
- Ventura, Y.; Sagi, M. Halophyte crop cultivation: The case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Lombardi, T.; Bertacchi, A.; Pistelli, L.; Pardossi, A.; Pecchia, S.; Toffanin, A.; Sanmartin, C. Biological and Agronomic Traits of the Main Halophytes Widespread in the Mediterranean Region as Potential New Vegetable Crops. Horticulturae 2022, 8, 195. [Google Scholar] [CrossRef]
- Custódio, L.; Rodrigues, M.J.; Pereira, C.G.; Castañeda-Loaiza, V.; Fernandes, E.; Standing, D.; Neori, A.; Shpigel, M.; Sagi, M. A review on Sarcocornia Species: Ethnopharmacology, nutritional properties, phytochemistry, biological activities and propagation. Foods 2021, 10, 2778. [Google Scholar] [CrossRef]
- Ventura, Y.; Eshel, A.; Pasternak, D.; Sagi, M. The development of halophyte-based agriculture: Past and present. Ann. Bot. 2015, 115, 529–540. [Google Scholar] [CrossRef]
- Sisay, T.A.; Nurbekova, Z.; Oshanova, D.; Dubey, A.K.; Khatri, K.; Mudgal, V.; Mudgal, A.; Neori, A.; Shpigel, M.; Srivastava, R.K.; et al. Effect of Salinity and Nitrogen Fertilization Levels on Growth Parameters of Sarcocornia fruticosa, Salicornia brachiata, and Arthrocnemum macrostachyum. Agronomy 2022, 12, 1749. [Google Scholar] [CrossRef]
- Kaushal, S.S.; Likens, G.E.; Pace, M.L.; Utz, R.M.; Haq, S.; Gorman, J.; Grese, M. Freshwater salinization syndrome on a continental scale. Proc. Natl. Acad. Sci. USA 2018, 115, E574–E583. [Google Scholar] [CrossRef]
- Agudelo, A.; Carvajal, M.; Martinez-Ballesta, M.d.C. Halophytes of the mediterranean basin—Underutilized species with the potential to be nutritious crops in the scenario of the climate change. Foods 2021, 10, 119. [Google Scholar] [CrossRef]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet food with nutritional health benefits? J. Food Compos. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- ElNaker, N.A.; Yousef, A.F.; Yousef, L.F. A review of Arthrocnemum (Arthrocaulon) macrostachyum chemical content and bioactivity. Phytochem. Rev. 2020, 19, 1427–1448. [Google Scholar] [CrossRef]
- Redondo-Gã3Mez, S.; Mateos-Naranjo, E.; Figueroa, M.E.; Davy, A.J. Salt stimulation of growth and photosynthesis in an extreme halophyte, Arthrocnemum macrostachyum. Plant Biol. 2010, 12, 79–87. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Gangadhar, K.N.; Vizetto-Duarte, C.; Wubshet, S.G.; Nyberg, N.T.; Barreira, L.; Varela, J.; Custódio, L. Maritime halophyte species from southern portugal as sources of bioactive molecules. Mar. Drugs 2014, 12, 2228–2244. [Google Scholar] [CrossRef] [PubMed]
- Ventura, Y.; Wuddineh, W.A.; Myrzabayeva, M.; Alikulov, Z.; Khozin-Goldberg, I.; Shpigel, M.; Samocha, T.M.; Sagi, M. Effect of seawater concentration on the productivity and nutritional value of annual Salicornia and perennial Sarcocornia halophytes as leafy vegetable crops. Sci. Hortic. 2011, 128, 189–196. [Google Scholar] [CrossRef]
- Ventura, Y.; Wuddineh, W.A.; Shpigel, M.; Samocha, T.M.; Klim, B.C.; Cohen, S.; Shemer, Z.; Santos, R.; Sagi, M. Effects of day length on flowering and yield production of Salicornia and Sarcocornia species. Sci. Hortic. 2011, 130, 510–516. [Google Scholar] [CrossRef]
- Antunes, M.D.; Gago, C.; Guerreiro, A.; Sousa, A.R.; Julião, M.; Miguel, M.G.; Faleiro, M.L.; Panagopoulos, T. Nutritional Characterization and Storage Ability of Salicornia ramosissima and Sarcocornia perennis for Fresh Vegetable Salads. Horticulturae 2021, 7, 6. [Google Scholar] [CrossRef]
- Mohammadi, H.; Kardan, J. Morphological and physiological responses of some halophytes to salinity stress. In Annales Universitatis Mariae Curie-Sklodowska, Sectio C–Biologia; Wydawnictwo Uniwersytetu Marii Curie-Skłodowskiej: Lublin, Poland, 2015; Volume 70. [Google Scholar]
- Patel, J.; Khatri, K.; Sisay, T.A.; Du Nja, Z.; Choudhary, B.; Nurbekova, Z.; Mishra, A.; Sikron, N.; Standing, D.; Mudgal, A.; et al. UV-C-induced reactive carbonyl species are better detoxified in the halophytic plants Salicornia brachiata and Arthrocnemum macrostachyum than in the halophytic Sarcocornia fruticosa plants. Plant J. 2025, 122, e70239. [Google Scholar] [CrossRef]
- Mishra, A.; Patel, M.K.; Jha, B. Non-targeted metabolomics and scavenging activity of reactive oxygen species reveal the potential of Salicornia brachiata as a functional food. J. Funct. Foods 2015, 13, 21–31. [Google Scholar] [CrossRef]
- Boughalleb, F.; Denden, M. Physiological and Biochemical Changes of Two Halophytes, Nitraria retusa (Forssk.) and Atriplex halimus (L.) Under Increasing Salinity. Agric. J. 2011, 6, 327–339. [Google Scholar] [CrossRef]
- Forghani, A.H.; Mohebatinejad, H.; Fazilati, M. The Effect of Salt Stress on Antimicrobial Activity and Potential Production of Anthocyanin and Total Phenolic of Salicornia in Hydroponic Culture. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2024, 94, 793–801. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Bin Dukhyil, A.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Khlestkina, E. The adaptive role of flavonoids: Emphasis on cereals. Cereal Res. Commun. 2013, 41, 185–198. [Google Scholar] [CrossRef]
- Marone, D.; Mastrangelo, A.M.; Borrelli, G.M.; Mores, A.; Laidò, G.; Russo, M.A.; Ficco, D.B.M. Specialized metabolites: Physiological and biochemical role in stress resistance, strategies to improve their accumulation, and new applications in crop breeding and management. Plant Physiol. Biochem. 2022, 172, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Kadereit, G.; Ball, P.; Beer, S.; Mucina, L.; Sokoloff, D.; Teege, P.; Yaprak, A.E.; Freitag, H. A taxonomic nightmare comes true: Phylogeny and biogeography of glassworts (Salicornia L., Chenopodiaceae). Taxon 2007, 56, 1143–1170. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic Acids Symposium Series; Oxford University Press: Oxford, UK, 1999; Volume 41, pp. 95–98. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Ramírez, E.; Sánchez-Gavilán, D.; Rufo, L.; Sánchez-Mata, D.; De la Fuente, V. Morphology, anatomy and phylogeny of the two sister halophytic genera Microcnemum and Arthrocnemum (Salicornioideae/Amaranthaceae). Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2022, 156, 1422–1437. [Google Scholar]
- Ben-Amotz, A.; Lers, A.; Avron, M. Stereoisomers of β-Carotene and Phytoene in the Alga Dunaliella bardawil. Plant Physiol. 1988, 86, 1286–1291. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar] [CrossRef]
- Sarkar, S.; Mondal, M.; Ghosh, P.; Saha, M.; Chatterjee, S. Quantification of total protein content from some traditionally used edible plant leaves: A comparative study. J. Med. Plants Stud. 2020, 8, 166–170. [Google Scholar] [CrossRef]
- Khatri, K.; Rathore, M.S. Salt and osmotic stress-induced changes in physio-chemical responses, PSII photochemistry and chlorophyll a fluorescence in peanut. Plant Stress 2022, 3, 100063. [Google Scholar] [CrossRef]
- Patel, J.; Khandwal, D.; Choudhary, B.; Ardeshana, D.; Jha, R.K.; Tanna, B.; Yadav, S.; Mishra, A.; Varshney, R.K.; Siddique, K.H.M. Differential Physio-Biochemical and Metabolic Responses of Peanut (Arachis hypogaea L.) under Multiple Abiotic Stress Conditions. Int. J. Mol. Sci. 2022, 23, 660. [Google Scholar] [CrossRef]
- Choudhary, B.; Khandwal, D.; Gupta, N.K.; Patel, J.; Mishra, A. Nutrient Composition, Physicobiochemical Analyses, Oxidative Stability and Antinutritional Assessment of Abundant Tropical Seaweeds from the Arabian Sea. Plants 2023, 12, 2302. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Srivastava, R. Physicochemical, antioxidant properties of carotenoids and its optoelectronic and interaction studies with chlorophyll pigments. Sci. Rep. 2021, 11, 18365. [Google Scholar] [CrossRef] [PubMed]
- Pincus, M.R. Physiological Structure and Function of Proteins. In Cell Physiology Source Book; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N.; Boulaaba, M.; Smaoui, A.; Abdelly, C. Influence of biological, environmental, and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. Comptes Rendus Biol. 2008, 331, 865–873. [Google Scholar] [CrossRef]
- Soltabayeva, A.; Bekturova, A.; Kurmanbayeva, A.; Oshanova, D.; Nurbekova, Z.; Srivastava, S.; Standing, D.; Sagi, M. Ureides are accumulated similarly in response to UV-C irradiation and wounding in Arabidopsis leaves but are remobilized differently during recovery. J. Exp. Bot. 2022, 73, 1016–1032. [Google Scholar] [CrossRef]
- Katschnig, D.; Broekman, R.; Rozema, J. Salt tolerance in the halophyte salicornia dolichostachya moss: Growth, morphology and physiology. Environ. Exp. Bot. 2013, 92, 32–42. [Google Scholar] [CrossRef]
- Aghaleh, M.; Niknam, V.; Ebrahimzadeh, H.; Razavi, K. Salt stress effects on growth, pigments, proteins and lipid peroxidation in Salicornia persica and S. europaea. Biol. Plant. 2009, 53, 243–248. [Google Scholar] [CrossRef]
- Salazar, O.R.; Chen, K.; Melino, V.J.; Reddy, M.P.; Hřibová, E.; Čížková, J.; Beránková, D.; Vega, J.P.A.; Leal, L.M.C.; Aranda, M.; et al. SOS1 tonoplast neo-localization and the RGG protein SALTY are important in the extreme salinity tolerance of Salicornia bigelovii. Nat. Commun. 2024, 15, 4279. [Google Scholar] [CrossRef]
- Ibraheem, F.; Albaqami, M.; Elghareeb, E.M. Halophytic resilience in extreme environments: Adaptive strategies of Suaeda schimperi in the Red Sea’s hyper-arid salt marshes. Plant Soil Environ. 2025, 71, 320–337. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Potential of Producing Salicornia bigelovii Hydroponically as a Vegetable at Moderate NaCl Salinity. HortScience 2014, 49, 1154–1157. [Google Scholar] [CrossRef]
- Yang, C.; Shi, D.; Wang, D. Comparative effects of salt and alkali stresses on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte Suaeda glauca (Bge.). Plant Growth Regul. 2008, 56, 179–190. [Google Scholar] [CrossRef]
- Homayouni, H.; Razi, H.; Izadi, M.; Alemzadeh, A.; Kazemeini, S.A.; Niazi, A.; Vicente, O. Temporal Changes in Biochemical Responses to Salt Stress in Three Salicornia Species. Plants 2024, 13, 979. [Google Scholar] [CrossRef]
- Lee, G.; Carrow, R.N.; Duncan, R.R. Growth and water relation responses to salinity stress in halophytic seashore paspalum ecotypes. Sci. Hortic. 2005, 104, 221–236. [Google Scholar] [CrossRef]
- Kumar, H.; Lal, S.B.; Wani, A.M. Correlation Studies for Morphological and Biomass Traits in Half Sib Families of Terminalia Arjuna (L.). Curr. World Environ. J. 2017, 12, 345–354. [Google Scholar] [CrossRef]
- Saadati, S.; Borzouei, A.; Rahemi, M.R.; Khiabani, B.N. Alteration of physiological and biochemical properties in leaves and fruits of pomegranate in response to gamma irradiation. Sci. Rep. 2022, 12, 4312. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Dutta, G. From photosynthesis to biosensing: Chlorophyll proves to be a versatile molecule. Sens. Int. 2020, 1, 100058. [Google Scholar] [CrossRef]
- Tan, X.; Zhu, J.; Wakisaka, M. Effect of protocatechuic acid on Euglena gracilis growth and accumulation of metabolites. Sustainability 2020, 12, 9158. [Google Scholar] [CrossRef]
- Jameel, J.; Anwar, T.; Majeed, S.; Qureshi, H.; Siddiqi, E.H.; Sana, S.; Zaman, W.; Ali, H.M. Effect of salinity on growth and biochemical responses of brinjal varieties: Implications for salt tolerance and antioxidant mechanisms. BMC Plant Biol. 2024, 24, 128. [Google Scholar] [CrossRef]
- Taïbi, K.; Taïbi, F.; Abderrahim, L.A.; Ennajah, A.; Belkhodja, M.; Mulet, J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- Türkan, I.; Demiral, T. Recent developments in understanding salinity tolerance. Environ. Exp. Bot. 2009, 67, 2–9. [Google Scholar] [CrossRef]
- Pavlovic, D.; Nikolic, B.; Djurovic, S.; Waisi, H.; Andjelkovic, A.; Marisavljevic, D. Chlorophyll as a measure of plant health: Agroecological aspects. Pestic. I Fitomed. 2014, 29, 21–34. [Google Scholar] [CrossRef]
- Talebzadeh, F.; Valeo, C. Evaluating the Effects of Environmental Stress on Leaf Chlorophyll Content as an Index for Tree Health. In IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Bristol, UK, 2022. [Google Scholar]
- Kumar, S.; Li, G.; Yang, J.; Huang, X.; Ji, Q.; Liu, Z.; Ke, W.; Hou, H. Effect of Salt Stress on Growth, Physiological Parameters, and Ionic Concentration of Water Dropwort (Oenanthe javanica) Cultivars. Front. Plant Sci. 2021, 12, 660409. [Google Scholar] [CrossRef]
- Rabhi, M.; Castagna, A.; Remorini, D.; Scattino, C.; Smaoui, A.; Ranieri, A.; Abdelly, C. Photosynthetic responses to salinity in two obligate halophytes: Sesuvium portulacastrum and Tecticornia indica. S. Afr. J. Bot. 2012, 79, 39–47. [Google Scholar] [CrossRef]
- Shah, S.H.; Houborg, R.; McCabe, M.F. Response of chlorophyll, carotenoid and SPAD-502 measurement to salinity and nutrient stress in wheat (Triticum aestivum L.). Agronomy 2017, 7, 61. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Isayenkov, S.V. The Role of Anthocyanins in Plant Tolerance to Drought and Salt Stresses. Plants 2023, 12, 2558. [Google Scholar] [CrossRef]
- Li, Z.; Ahammed, G.J. Hormonal regulation of anthocyanin biosynthesis for improved stress tolerance in plants. Plant Physiol. Biochem. 2023, 201, 107835. [Google Scholar] [CrossRef] [PubMed]
- Kovinich, N.; Kayanja, G.; Chanoca, A.; Riedl, K.; Otegui, M.S.; Grotewold, E. Not all anthocyanins are born equal: Distinct patterns induced by stress in Arabidopsis. Planta 2014, 240, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Maduraimuthu, D.; Agric, M.J.; Djanaguiraman, M.; Ramadass, R.; Devi, D.D. Effect of Salinity on Chlorophyll Content of Rice Genotypes Effect of Salt Stress on Germination and Seedling Growth in Rice Genotypes. 2004. Available online: https://www.researchgate.net/publication/284041307 (accessed on 1 January 2024).
- Hurkman, W.J.; Fornari, C.S.; Tanaka, C.K. A Comparison of the Effect of Salt on Polypeptides and Translatable mRNAs in Roots of a Salt-Tolerant and a Salt-Sensitive Cultivar of Barley. Plant Physiol. 1989, 90, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Collini, E. Carotenoids in Photosynthesis: The Revenge of the “Accessory” Pigments. Chem 2019, 5, 494–495. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J. Leaf pigment content. In Comprehensive Remote Sensing; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1–9, pp. 117–142. [Google Scholar]
- Demmig-Adams, B.; Adams, W.W. Antioxidants in Photosynthesis and Human Nutrition. Available online: https://www.science.org (accessed on 1 January 2024).
- Sarker, U.; Oba, S. Salinity stress enhances color parameters, bioactive leaf pigments, vitamins, polyphenols, flavonoids and antioxidant activity in selected Amaranthus leafy vegetables. J. Sci. Food Agric. 2019, 99, 2275–2284. [Google Scholar] [CrossRef]
- Parida, A.K.; Veerabathini, S.K.; Kumari, A.; Agarwal, P.K. Physiological, anatomical and metabolic implications of salt tolerance in the halophyte salvadora persica under hydroponic culture condition. Front. Plant Sci. 2016, 7, 351. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Ali, G.; Srivastava, P.; Iqbal, M. Proline accumulation, protein pattern and photosynthesis in Bacopa Monniera regenerants grown under nacl stress. Biol. Plant. 1999, 42, 89–95. [Google Scholar] [CrossRef]
- Pareek, A.; Singla, S.L.; Kush, A.K.; Grover, A. Distribution patterns of HSP 90 protein in rice. Plant Sci. 1997, 125, 221–230. [Google Scholar] [CrossRef]
- Sagi, M.; Davydov, O.; Orazova, S.; Yesbergenova, Z.; Ophir, R.; Stratmann, J.W.; Fluhr, R. Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell 2004, 16, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Al Hassan, M.; Naranjo, M.A.; Agrawal, V.; Boscaiu, M.; Vicente, O. Effects of salinity and drought on growth, ionic relations, compatible solutes and activation of antioxidant systems in oleander (Nerium oleander L.). PLoS ONE 2017, 12, e0185017. [Google Scholar] [CrossRef] [PubMed]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Romano, A. Nutrient Deficiency-Induced Stress Improves Skincare Effects and Phytochemical Content of Green Extracts from Lamiaceae In Vitro Cultures. Horticulturae 2024, 10, 947. [Google Scholar] [CrossRef]
- Harboub, N.; Mighri, H.; Bennour, N.; Dbara, M.; Pereira, C.; Chouikhi, N.; Custódio, L.; Abdellaoui, R.; Akrout, A. Nutritional profile, chemical composition and health promoting properties of Salicornia emerici Duval-Jouve and Sarcocornia alpini (Lag.) Rivas Mart. from southern Tunisia. Biocatal. Agric. Biotechnol. 2025, 64, 103502. [Google Scholar] [CrossRef]
- Esmaeili, K.A.; Taha, R.M.; Mohajer, S.; Banisalam, B. Antioxidant Activity and Total Phenolic and Flavonoid Content of Various Solvent Extracts from In Vivo and In Vitro Grown Trifolium pratense L. (Red Clover). Biomed. Res. Int. 2015, 2015, 643285. [Google Scholar] [CrossRef]

| Species | Ecotype Designation | Map Coordinates of Sampling Sites | Date of Collection | Collectors or Source Reference | Habitat | |
|---|---|---|---|---|---|---|
| 1 | Sarcocornia fruticosa | VM | 31° N | 2011 | [15] | Inland salt pan, Negev area |
| 2 | Sarcocornia fruticosa | Shikmona (Shik) | 32.8261, 34.95768 | 10 November 2020 | Sagi and Shpigel laboratories | Coastal, tidal area |
| 3 | Sarcocornia fruticosa | Megadim (Meg) | 32.73940, 34.95067 | 10 November 2020 | Sagi and Shpigel laboratories | Coastal, supratidal area, no tidal flooding |
| 4 | Sarcocornia fruticosa | Naaman (Naa) | 32.91284, 35.08551 | 10 November 2020 | Sagi and Shpigel laboratories | Coastal, tidal area |
| 5 | Sarcocornia fruticosa | Ruhama (Ruh) | 32.71746, 34.94855 | 10 November 2020 | Sagi and Shpigel laboratories | Coastal, tidal area |
| 6 | A. macrostachyum | A. macrostachyum (AM) | 30.96463, 35.37196 | 6 August 2020 | Sagi and Shpigel laboratories | Dead Sea shore |
| Variable | Correlations Marked with Asterisks Are Significant at p < 0.05000 N = 72. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Chlorophyll | Total Carotenoid | Total Polyphenol | Total Flavonoid | DPPH | Total Protein | Shoot Diameter | TSS | RWC | Anthocyanin | EC | FW | MDA | |
| Total chlorophyll | 1.000 | 0.908 * | 0.166 | 0.337 * | 0.313 * | 0.179 | 0.397 * | 0.411 * | 0.548 * | 0.319 * | 0.333 * | 0.257 * | −0.645 * |
| Total carotenoid | 0.908 * | 1.000 | 0.151 | 0.432 * | 0.364 * | 0.317 * | 0.367 * | 0.475 * | 0.468 * | 0.212 | 0.365 * | 0.330 * | −0.496 * |
| Total polyphenol | 0.166 | 0.151 | 1.000 | 0.645 * | 0.763 * | 0.333 * | 0.286 * | −0.115 | 0.077 | −0.410 * | −0.388 * | −0.064 | −0.182 |
| Total flavonoid | 0.337 * | 0.432 * | 0.645 * | 1.000 | 0.919 * | 0.732 * | 0.538 * | 0.025 | 0.215 | −0.329 * | −0.102 | 0.285 * | −0.137 |
| DPPH | 0.313 * | 0.364 * | 0.763 * | 0.919 * | 1.000 | 0.565 * | 0.517 * | −0.015 | 0.320 * | −0.379 * | −0.293 * | 0.144 | −0.183 |
| Total protein | 0.179 | 0.317 * | 0.333 * | 0.732 * | 0.565 * | 1.000 | 0.114 | −0.117 | 0.087 | −0.207 | −0.098 | −0.031 | 0.205 |
| Shoot diameter | 0.397 * | 0.367 * | 0.286 * | 0.5388 * | 0.517 * | 0.114 | 1.000 | 0.417 * | 0.172 | 0.063 | 0.366 * | 0.774 * | −0.532 * |
| TSS | 0.411 * | 0.475 * | −0.115 | 0.025 | −0.015 | −0.117 | 0.417 * | 1.000 | 0.186 | 0.213 | 0.719 * | 0.601 * | −0.354 * |
| RWC | 0.548 * | 0.468 * | 0.077 | 0.215 | 0.320 * | 0.087 | 0.172 | 0.186 | 1.000 | 0.278 * | −0.084 | −0.070 | −0.333 * |
| Anthocyanin | 0.319 * | 0.212 | −0.410 * | −0.329 * | −0.379 * | −0.207 | 0.063 | 0.213 | 0.278 * | 1.000 | 0.402 * | 0.193 | −0.342 * |
| EC | 0.333 * | 0.365 * | −0.388 * | −0.102 | −0.293 * | −0.098 | 0.366 * | 0.719 * | −0.084 | 0.402 * | 1.000 | 0.703 * | −0.312 * |
| FW | 0.257 * | 0.330 * | −0.064 | 0.285 * | 0.144 | −0.031 | 0.774 * | 0.601 * | −0.070 | 0.193 | 0.703 * | 1.000 | −0.410 * |
| MDA | −0.645 * | −0.496 * | −0.182 | −0.137 | −0.183 | 0.205 | −0.532 * | −0.354 * | −0.333 * | −0.342 * | −0.312 * | −0.410 * | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sisay, T.A.; Patel, J.; Khatri, K.; Choudhary, B.; Standing, D.; Nja, Z.D.; Shpigel, M.; Custódio, L.M.B.; Gelfand, I.; Sagi, M. Successive Harvesting Interval and Salinity Level Modulate Biomass Production and Nutritional Value in Sarcocornia fruticosa and Arthrocaulon macrostachyum. Agriculture 2025, 15, 2182. https://doi.org/10.3390/agriculture15212182
Sisay TA, Patel J, Khatri K, Choudhary B, Standing D, Nja ZD, Shpigel M, Custódio LMB, Gelfand I, Sagi M. Successive Harvesting Interval and Salinity Level Modulate Biomass Production and Nutritional Value in Sarcocornia fruticosa and Arthrocaulon macrostachyum. Agriculture. 2025; 15(21):2182. https://doi.org/10.3390/agriculture15212182
Chicago/Turabian StyleSisay, Tesfaye Asmare, Jaykumar Patel, Kusum Khatri, Babita Choudhary, Dominic Standing, Zai Du Nja, Muki Shpigel, Luísa Margarida Batista Custódio, Ilya Gelfand, and Moshe Sagi. 2025. "Successive Harvesting Interval and Salinity Level Modulate Biomass Production and Nutritional Value in Sarcocornia fruticosa and Arthrocaulon macrostachyum" Agriculture 15, no. 21: 2182. https://doi.org/10.3390/agriculture15212182
APA StyleSisay, T. A., Patel, J., Khatri, K., Choudhary, B., Standing, D., Nja, Z. D., Shpigel, M., Custódio, L. M. B., Gelfand, I., & Sagi, M. (2025). Successive Harvesting Interval and Salinity Level Modulate Biomass Production and Nutritional Value in Sarcocornia fruticosa and Arthrocaulon macrostachyum. Agriculture, 15(21), 2182. https://doi.org/10.3390/agriculture15212182






