Abstract
This study identified that the absolute abundance of 15 types of antibiotic resistance genes (ARGs) across 21 organic fertilizer samples ranged between 1.15 × 104 and 6.74 × 1010 copies/g, with sulfonamide ARGs and the intI1 gene exhibiting relatively higher residuals. Subsequent analyses delved into the evolutionary patterns and reduction mechanisms pertinent to sulfonamide ARGs throughout aerobic composting processes. Three bacteria, Bacillus amyloliquefaciens, Bacillus subtilis, and Bacillus velezensis, capable of significantly reducing sulfonamide-resistant bacteria and their sul1 gene were identified. The study revealed that adding composite microbial agent, lowering the pH, or increasing the temperature could inhibit the growth of sulfonamide-resistant bacteria and decrease the abundance of the sul1 gene. Additionally, it was ascertained that the optimization of initial compost pH levels or the incorporation of a compound microbial inoculant effectively reduced the abundance of intracellular and extracellular sulfonamide ARGs and the intI1 gene. The proliferation of Actinobacteria and certain genera during the maturation phase was closely associated with the enrichment of sulfonamide ARGs. This research provides references for the multi-pathway comprehensive control of sulfonamide ARG pollution in composting.
1. Introduction
The extensive use of antibiotics in livestock and poultry farming has led to the emergence of antibiotic-resistant bacteria and ARGs, making livestock and poultry manure the major reservoir of ARGs. Several studies have identified over 200 types of ARGs in swine and poultry manure [1,2,3], with the absolute abundance of ARGs in fresh pig manure reaching 1011–1013 copies per gram dry weight [4,5]. Sulfonamides, such as sulfadiazine and sulfamethoxazole, are among the most widely used antibiotics in livestock and poultry farming, and their extensive use has also led to the enrichment and contamination of sulfonamide ARGs (including genes like sul1, sul2, and sul3), making them one of the frequently detected ARGs in livestock manure [6]. ARGs in livestock and poultry manure are linked to mobile genetic elements (MGEs) like plasmids, integrons, and transposons. These elements help ARGs spread among environmental microbes via conjugation, transposition, and transformation [7]. This leads to contamination of air, soil, water, and crops, and ARGs can eventually reach humans through the food chain, posing health risks.
Aerobic composting is an effective way to reduce ARGs from livestock and poultry manure into the environment. The total ARGs in composting include intracellular antibiotic-resistant genes (iARGs) and extracellular antibiotic-resistant genes (eARGs). Current research mainly focuses on the fate of total ARGs in compost, with relatively less attention on eARGs and iARGs. Sulfonamide resistance genes sul1 and sul2 and tetracycline resistance gene tetX are the main co-existing ARGs in different livestock manure composts [8]. Numerous studies indicate that the diversity and abundance of most ARGs are effectively reduced during the aerobic composting process [9,10,11]. However, sulfonamide ARGs tend to increase during the cooling and maturation phases of composting, persistently remaining and difficult to remove effectively. Studies have found that the reduction in sul1 and sul2 genes during high-temperature composting and thermophilic bacteria-amended composting of pig manure was limited. sul1 and sul2 genes rebounded significantly after the thermophilic phase of pig manure composting, accounting for 98.4% of the total targeted ARGs, thus defining sulfonamide resistance genes sul1 and sul2 as persistent ARGs [12]. Qiu et al. and Deng et al. also found enrichment of sul1 and tetM during composting [13,14]. Cao et al. found that the absolute abundance of ARGs increased by 0.19–1.61 orders of magnitude after composting, mainly contributed by sul1, sul2, and dfrA1 genes [4]. Therefore, the risk of environmental pollution caused by the persistent residue of sulfonamide ARGs in compost should be taken seriously. It is urgent to comprehensively and deeply analyze the influencing factors and mechanisms of sulfonamide ARGs removal in aerobic composting.
Aerobic composting, as a complex physical, chemical, and biological process, is influenced by various factors, among which biological factors play a decisive role in the change in ARGs [8]. The abundance of ARGs is negatively correlated with temperature [15]. Different ARGs host bacteria with preferred different pH values, and sul1, sul2, and blaCTX-M were positively correlated with pH [14]. In high-temperature composting of pig manure and thermophilic bacteria inoculation composting, pH and electrical conductivity (EC) were the key factors affecting ARGs, whereas difficult-to-remove sulfonamide ARGs were significantly correlated with pH, EC, and water-soluble organic carbon and nitrogen ratio (DOC/DON) [12]. In summary, composting conditions and biological factors, among others, affect the fate of ARGs, and it is necessary to study the abatement mechanism of sulfonamide ARGs in depth from multiple pathways.
This study investigates the residual contamination of ARGs in industrial aerobic composting organic fertilizer products to clarify the residual contamination status of sulfonamide ARGs. It studies the effects of key environmental factors of composting on the cellular activity and sul1 gene expression of sulfonamide model bacteria and resistant bacteria at the cellular and molecular levels and screens for functional strains that can effectively inhibit sulfonamide-resistant bacteria and sul1. The changes in the abundance of sulfonamide iARGs, eARGs, and microbial communities in pig manure compost under altered key environmental factors or added functional microbial agents are detected using qPCR and 16S rRNA sequencing, aiming to elucidate the dynamic pattern and reduction mechanism of sulfonamide ARGs in compost.
2. Materials and Methods
2.1. Organic Fertilizer Sampling
Samples were collected from 21 organic fertilizer factories across 5 counties and cities from 23 March to 19 July 2023. Sterile spatulas were used to collect the organic fertilizer samples into sterile bags, resulting in a total of 21 samples, which were then stored at −20 °C. Detailed information on these 21 samples is provided in Table S1. DNA was extracted from the samples. Fifteen ARGs associated with sulfonamides, tetracyclines, macrolides, and β-lactams, which were identified as having higher residual levels during the composting maturity phase in prior studies, were selected for absolute quantitative analysis.
2.2. Isolation, Identification of Sulfonamide-Resistant Bacteria, and Construction of Model Bacteria
2.2.1. Isolation and Identification of Sulfonamide-Resistant Bacteria
To isolate and identify sulfonamide-resistant bacteria, 1 g of aerobic composted pig manure sample from the maturity phase was placed into a conical flask, to which 100 mL of sterile water and glass beads were added. The mixture was incubated at 180 rpm and 35 °C for 1 h. The solution was then serially diluted to 10−8, and 100 μL of each dilution was spread on a sulfonamide resistance screening medium and incubated at 37 °C for 24 h. Colonies with distinct macroscopic morphologies were individually transferred to fresh media, incubated for another 24 h in ZWY-211C incubator shaker (Zhicheng, Shanghai, China), and then sampled for whole genome sequencing. The sulfonamide resistance screening medium consisted of 10 g/L peptone, 5 g/L yeast extract, 10 g/L sodium chloride, and 10 g/L agar. The medium was sterilized at 121 °C for 20 min under high pressure. Before the medium solidified completely, a sulfamethoxazole stock solution was added to achieve a final concentration of 50 μg/mL. Genomic DNA was extracted from cultured sulfonamide-resistant strains using Simgen bacterial DNA kit (Simgen, Hangzhou, China). The presence of resistance genes (sul1, sul2, intI1) was qualitatively assessed by BIO-RAD CFX Opus 96 Real-Time PCR System, with primer sequences and annealing temperatures listed in Table S2. PCR products were analyzed by 1.5% agarose gel electrophoresis. Positive bands were visualized using a gel imaging system. The sequencing results were analyzed using the BLAST function in the NCBI database on 28 April 2022. The purified strains were preserved in a mixture containing 60% glycerol and stored in cryovials at −80 °C.
2.2.2. Construction of a Model Bacterium Containing the sul1 Gene
To construct a model bacterium containing the sul1 gene, total genomic DNA was first extracted from compost samples using Simgen fecal DNA purification kit (Simgen, Hangzhou, China). Primers designed for the full length of the sul1 gene, forward primer 3′ = ATGGTGACGGTGTTCGGCA and reverse primer 5′ = CTAGGCATGATCTAACCCTCGG, were used for PCR amplification.
After PCR amplification, the products were separated by DYY-6C electrophoresis(Liuyi, Beijing, China). Then, the gel recovery and purification were performed using Simgen rapid gel recovery kit (Simgen, Hangzhou, China). The purified PCR product was then cloned into TaKaRa pMD18-T vector (TaKaRa, Beijing, China) and transformed into TaKaRa E. coli DH5α Competent Cells (TaKaRa, Beijing, China) for propagation with overnight culture at 37 °C on ampicillin-containing media.
Single colonies were picked for Sanger sequencing to confirm the presence of the sul1 gene in the constructed plasmid. The sequencing results were analyzed and compared using the BLAST function in the NCBI database on 27 April 2022, ensuring the correct insertion and orientation of the sul1 gene in the plasmid.
2.3. Selection of Functional Strains and Formulation of Compound Microbial Inoculant
2.3.1. Screening of Functional Strains
Five functional bacterial strains with antibacterial activity, isolated from soil, were selected: Bacillus amyloliquefaciens-8, Bacillus velezensis-17, Bacillus Siam-25, Bacillus subtilis-45, and Bacillus velezensis-51. Their antibacterial effects against sulfonamide-resistant bacteria were observed using the plate confrontation assay. After incubation at 37 °C for 24 h, the inhibition zones were examined and their diameters measured. Each functional strain was co-cultured with sulfonamide-resistant bacteria in liquid medium until the culture reached an OD600 of 1, and absolute quantitative PCR (qPCR) was subsequently performed to assess changes in the expression levels of the sul1 gene within the resistant bacteria.
2.3.2. Evaluation of Cellular Antagonism in Various Strain Combinations
The antagonism between strains was measured using the punch hole method. Strains were prepared as bacterial suspensions and smeared onto the surface of the culture medium. A puncher was used to create equidistant holes at four points and a central hole. The suspension of the bacteria to be tested was added into the holes. Blank liquid culture medium served as the control. After 24 h, the inhibition zones around the holes were observed and their diameters measured. Bacterial suspensions with an OD600 of 1.0 were taken (722E, Spectrum). An L9 (33) orthogonal experimental design was employed to optimize the formulation of mixed microbial agents. The experiment included three factors, each representing the addition level of one functional bacterial strain, tested at three levels: 0.25%, 0.5%, and 0.75% (v/w, relative to compost weight). This design yielded nine different microbial combinations to evaluate their effects on sulfonamide ARGs reduction during composting. The viable count of different combination mixed cultures was determined using the spread plate method, in which appropriately diluted samples were spread onto nutrient agar plates and incubated to count colony-forming units (CFUs). Optical density at 600 nm (OD600) was also measured to assess cell concentration, aiming to identify the optimal combination of functional strains.
2.4. Live/Dead Bacterial Staining
The bacterial suspensions treated under different pH conditions were stained using the Simgen Live/Dead Bacterial Staining Kit (Hangzhou, China). The staining and morphological observations of the bacteria were conducted using a fluorescence microscope (BX53, OLYMPUS, Tokyo, Japan) at a wavelength of 490 ± 10 nm, to observe the yellow-green live bacteria and the red dead bacteria.
2.5. Pig Manure Composting Experiment
Fresh pig manure was obtained from the National Livestock Engineering Research Center’s breeding pig farm at Huazhong Agricultural University. Sawdust was sourced from the Veterinary Hospital of Huazhong Agricultural University. The pig manure composting experiment was conducted in February 2023. The initial carbon (C), nitrogen (N) content, and moisture content of the materials (sawdust and pig manure) were measured. An elemental analyzer (PerkinElmer, Waltham, MA, USA) was used to determine the total nitrogen (TN), total carbon (TC) of the samples. The moisture content of the samples was determined by drying them in crucibles at 105 °C to constant weights and calculated based on the weight loss. Specifically, the C and N content and moisture content of the sawdust were 46.51%, 0.11%, and 24.59%, respectively, while those of the pig manure were 36.68%, 3.47%, and 73.1%, respectively. The composting C/N was adjusted to 25:1, with a moisture content of 60%, and the final mix ratio of pig manure to sawdust was determined to be 4:1. The composting experiment was conducted in a metal box measuring 550 mm in height, 250 mm in length, and 250 mm in width, which was wrapped in a foam box. The composting device was connected to a ventilation system for timed aeration, and the entire composting experiment was carried out in a greenhouse shed at a temperature of 17–20 °C.
To explore the effect of the compound microbial inoculant in pig manure composting, the agent—composed of the bacterial biomass obtained by centrifuging the culture suspensions of functional strains (see Section 2.3.2)—was added at 2% (wet weight) of the total compost mass. Three experimental groups were set up: CK as the blank control group (no microbial agent added); A as the compound microbial inoculant, in which the agent was added at the beginning of composting; and B as the repeated addition group, in which the agent was added both at the beginning and again during the cooling phase (on the eighth day of composting).
The composite Bacillus bioagent includes: Bacillus amyloliquefaciens, Bacillus subtilis, and Bacillus velezensis, with preservation numbers CCTCC NO: M 2023643, CCTCC NO: M 2023644, and CCTCC NO: M 2023645, respectively. Calcium hydroxide and calcium superphosphate were added at a content of 0.2% to alter the pH for the composting experiment, setting three experimental groups with pH 6.8, 7.7, and 8.2, respectively.
During the initial, heating, high-temperature, and maturity phases of composting, samples were collected from the compost pile using a sterile shovel and a mixed sampling method, placed in sample bags (repeated in triplicate), and stored at 4 °C and −80 °C for the analysis of physicochemical indicators, ARGs detection, and 16 s RNA sequencing. The temperature of the compost pile and room temperature were measured at 9:00 and 21:00 daily using a Kaitai B-8A thermometer (Kaitai, Ningbo, China). Compost samples were mixed with deionized water at a ratio of 1:10 W (g):V (mL), shaken at room temperature for 1 h, and then filtered to measure the pH, EC, and Germination Index (GI) of the leachate. The pH and EC of the extract were determined directly using a pH meter (FE28-standard, Mettler Toledo, Columbus, OH, USA) and a conductivity meter (TDS, Dongguan, China). GI measurement method referring to [16]. Moisture content, TC, TN, and the C/N were determined as described previously.
2.6. DNA Extraction from Samples and Absolute Quantification of ARGs
DNA from organic fertilizer samples, compost samples, and sulfonamide-resistant bacteria was extracted using the Simgen Bacteria/Fecal DNA Kit (Simgen, Hangzhou, China). The method for extracting extracellular and intracellular DNA from compost samples was adapted from the study by [17]. A sample equivalent to 1 g of dry weight was taken, to which 4 mL of NaH2PO4 and 0.2 g of PVPP were added. The mixture was then vortexed to mix thoroughly and incubated in a constant temperature shaker at 250 rpm and 25 °C for 10 min, followed by centrifugation at 10,000× g and 4 °C for 20 min (5424R, Eppendorf, Hamburg, Germany). This step was repeated 2–5 times. The supernatant was processed using the CTAB method to extract extracellular DNA [18], while the pellet was used with the Simgen Fecal DNA Kit to extract intracellular DNA.
The primers for the resistance genes used in this study are shown in Table S2. The 25 μL qPCR mix consisted of 10 μL of ABclonal qPCR SYBR Mix (ABclonal, Wuhan, China), 2 μL of template DNA, 0.4 μL of each forward and reverse primer (10 μM), and 7.2 μL of ddH2O. Each reaction was performed in triplicate. The qPCR conditions included an initial denaturation at 95 °C for 3 min, followed by 40 cycles of 5 s at 95 °C and 30 s at the annealing temperature (AT) indicated in Table S2. Standard curves for qPCR were generated using plasmids containing the target genes (Table S3). The copy numbers of ARGs were calculated using the internal reference method.
2.7. Microbial 16S rRNA Sequencing
In the compound microbial inoculant addition group, samples from the initial, warming, peak temperature, and maturation phases were selected across two categories, CK and B, totaling nine sets corresponding to days 0, 1, 7, and 14 for 16S rRNA sequencing. Additionally, twelve sets of samples from varying pH levels within this group during these same phases were chosen, corresponding to days 0, 1, 3, and 14, with each set having three replicates. The 16S rDNA V3-V4 variable regions were amplified using primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Libraries were prepared using the NEXTFLEX Rapid DNA-Seq Kit (Bioo Scientific, Austin, TX, USA) and sequenced on the Illumina MiseqPE300/NovaSeq PE250 platform (Meiji, Shanghai, China). The library construction and sequencing tasks were outsourced to Shanghai Majorbio Bio-pharm Technology Co., Ltd., Shanghai, China.
2.8. Data Statistics and Analysis
Pearson correlation analysis was conducted using IBM SPSS Statistics 20 to design orthogonal experimental groups. The significance of experimental data differences was analyzed through homogeneity of variance tests, independent samples t-tests using F and t values, and ANOVA single-factor analysis. GraphPad Prism 9.0 was used to calculate the mean and standard deviation for each group and to create bar and line graphs. Excel was employed to generate fluorescent quantitative standard curves. Microbial diversity analysis and significance of differences were performed on the Majorbio Cloud platform (https://cloud.majorbio.com (accessed on 15 Marth 2023)).
3. Results and Discussion
3.1. Investigation of Residual Quantities of ARGs and MGEs in Organic Fertilizer Products
While numerous studies have indicated that aerobic composting processes can reduce the risk of ARG transmission, they do not entirely eliminate ARGs [2]. Research has shown that the ARGs removal rate of conventional pig manure composting hovers around a mere 30% [19]. Within the scope of this study, among the 21 organic fertilizer samples tested, 14 samples were found to contain ARGs and MGEs, with a detection rate ranging between 33.3% and 66.7%. Sulfonamide ARGs and the intI1 gene dominated in absolute abundance (Figure 1), likely due to the use of sulfonamide antibiotics as therapeutic drugs, which increases the concentration of antibiotics in livestock and poultry manure [20]. It has been suggested that elevated residual antibiotic levels may inhibit the growth of some bacteria capable of degrading these antibiotics [21], leading to the persistent residue of sulfonamide ARGs in compost. Similarly, these antibiotics have a relatively high detection rate in agricultural soil [21]. The absolute abundance of the four classes of ARGs in organic fertilizers ranged from 1.15 × 104 to 6.74 × 1010 copies/g, with the average detection rate and absolute abundance of the four major classes of ARGs in descending order being: sulfonamides > tetracyclines > macrolides > β-lactams. The concentration range of sulfonamide ARGs was 2.04 × 104 to 6.74 × 1010 copies/g, with the mean absolute abundance significantly higher than the other classes of ARGs. The content of the sul1 gene ranged between 6.72 × 104 and 1.41 × 1010, the absolute abundance of sul2 ranged from 2.33 × 105 to 6.74 × 1010, and sul3 from 2.04 × 104 to 1.60 × 106. The abundance of the sul2 gene reached up to 6.74 × 1010 copies/g. Among the four MGEs, the intI1 gene had the highest absolute abundance, ranging from 6.61 × 104 to 7.31 × 109. The significant variation in ARG residues among different organic fertilizer products could be related to the raw materials and manufacturing processes of the organic fertilizers.
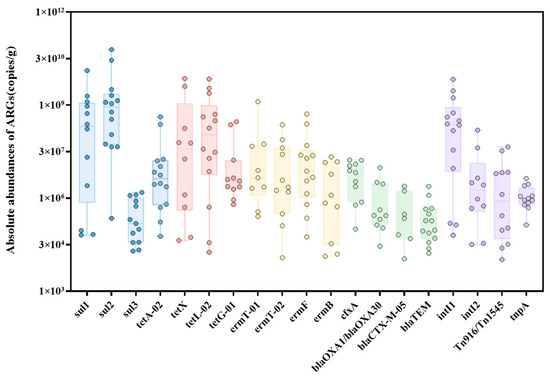
Figure 1.
Absolute abundance of ARGs and MGEs in organic fertilizer samples.
3.2. Selection of Functional Strains Inhibiting Sulfonamide-Resistant Bacteria
The E. coli model bacteria containing sul1 gene was successfully constructed by amplifying the sul1 gene fragment with specific primers. Two resistant strains containing sulfonamide ARGs were screened from the late samples of pig manure compost using sulfonamide antibiotic resistance panel, which were identified as Bacillus pseudomycoides and Alcaligenes faecalis.
In this study, the inhibitory effect of five strains of functional bacteria (as identified in Section 2.3.1) on the resistant bacteria of Bacillus pseudomycoides and Alcaligenes faecalis was observed by plate standoff test. The statistical results of the mean diameter of the hyaline circle were shown in Table S4. All five functional strains exhibited varying degrees of inhibition against the two sulfonamide-resistant bacteria, with Bacillus amyloliquefaciens-8 and Bacillus velezensis-51 showing relatively stronger inhibitory effects. Co-cultivation of the sul1 gene model bacterium, Bacillus pseudomycoides, and Alcaligenes faecalis with the five functional strains significantly downregulated the expression of the sul1 gene in the three sulfonamide-resistant bacteria (Figure S1).
The results of the antagonism tests indicated that no antagonistic effects were observed among Bacillus amyloliquefaciens-8, Bacillus subtilis-45, and Bacillus velezensis-51, suggesting their compatibility for use in a compound microbial inoculant. The optimal ratio for the compound microbial inoculant was determined to be Bacillus amyloliquefaciens-8:Bacillus subtilis-45:Bacillus velezensis-51 = 2:1:2. Antibiotic sensitivity tests for sulfonamides were conducted on Bacillus amyloliquefaciens 8, Bacillus subtilis-45, and Bacillus velezensis-51 used in the microbial preparation, showing high sensitivity to sulfonamide antibiotics (the diameter of the transparent circle was greater than 15 mm), and none of them carried sulfonamide resistance genes as verified by PCR.
3.3. The Reductive Effect of Compound Microbial Inoculant on Sulfonamide-Resistant Bacteria and Their ARGs
After co-cultivation with the compound microbial inoculant, the quantity of sulfonamide-resistant bacteria, including the sul1 gene model bacterium, Bacillus pseudomycoides, and Alcaligenes faecalis, significantly decreased compared to the control group (Figure S2), with a reduction of over 80% in the expression levels of the sul1 gene across all sulfonamide-resistant bacteria (Figure 2). The notable decrease in the quantity of sulfonamide-resistant bacteria following the addition of the compound microbial inoculant may be attributed to the antagonism between different bacterial species affecting both bacterial counts and the proliferation of sul1 content. Research indicates that Bacillus amyloliquefaciens can significantly inhibit the growth of Staphylococcus aureus [22]. Antimicrobial substances produced by Bacillus amyloliquefaciens, such as biosurfactants and lipopeptide mixtures, exhibit broad and potent antimicrobial activity against Gram-positive pathogens with multidrug resistance [23,24].
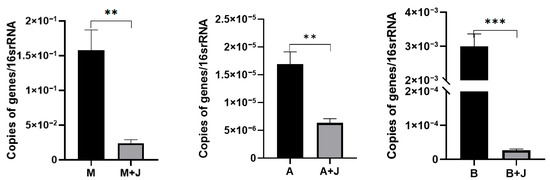
Figure 2.
Effects of compound microbial inoculant on sul1 gene expression in bacterial strains M, A, and B. ‘M’ represents a group of the model bacteria; ‘A’ denotes a group of Bacillus pseudomycoides; ‘B’ indicates a group of Alcaligenes faecalis. The black bars indicate the basal expression levels of the sul1 gene, while the gray bars show the expression levels post-treatment with the compound microbial inoculant ‘J’. Statistical significance of differences in gene expression relative to the control is denoted by asterisks (** p < 0.01, *** p < 0.001).
3.4. The Impact of Temperature and pH on Sulfonamide-Resistant Bacteria and Their ARGs
The initial pH of the medium was changed for sulfonamide-resistant as well as model bacteria, respectively, and the fluid was collected for live–dead bacterial staining. The proportion of dead bacteria were higher in the suspensions of Bacillus pseudomycoides and Alcaligenes faecalis at a pH of 5–6.5, indicating that lowering the pH significantly inhibits the growth of sulfonamide-resistant bacteria. In contrast, at a pH of 7–9, a higher number of live bacteria were present. The sul1 gene model bacterium showed higher adaptability in relatively neutral environments, experiencing significant mortality at pH values of 5 or 9 (Figure 3).
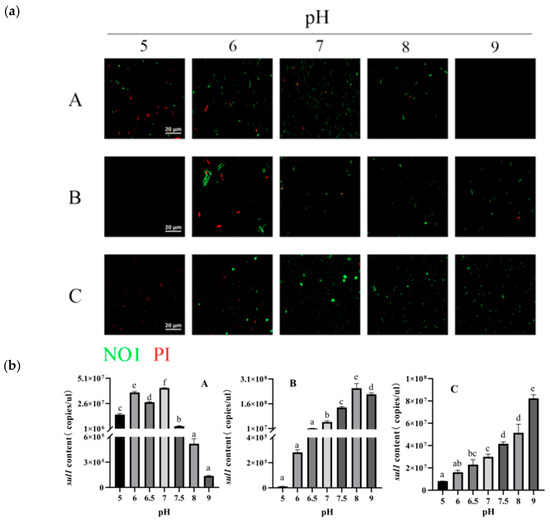
Figure 3.
The effect of pH on the sul1 model bacterium, Bacillus pseudomycoides and Alcaligenes faecalis content of sul1. (a): Representative immunofluorescence images (×20) of live (green) and dead (red) bacteria following culture under different pH conditions. Panels A, B, and C show the sul1 model bacterium, Bacillus pseudomycoides, and Blcaligenes faecalis, respectively. (b): Absolute sul1 content in each group. Significant differences between groups are indicated by different lowercase letters. Panels A, B, and C show the sul1 model bacterium, Bacillus pseudomycoides, and Blcaligenes faecalis, respectively.
Meanwhile, the influence of different pH levels on the abundance of the sul1 gene was detected. Results indicated that the sul1 gene content in the sul1 model bacterium gradually decreased with increasing pH values between 7 and 9, reaching its lowest value of 1.36 × 105 copies/μL at a pH of 9. For Bacillus pseudomycoides and Alcaligenes faecalis, the sul1 content decreased with the lowering of pH; at pH 5, the sul1 content reached its lowest, with Bacillus pseudomycoides’s sul1 gene content decreasing to 1.08 × 104 copies/μL and Alcaligenes faecalis’s sul1 gene content to 1.67 × 107 copies/μL (Figure 3). The activity of two strains of sulfonamide-resistant bacteria was significantly reduced under acidic conditions under different pH incubation conditions, likely due to the fact that the two strains were sieved from the compost rotting period and were suitable for survival under alkaline conditions. Therefore, in this study, it was hypothesized that changing the pH of the pile could affect the activity of sulfonamide-resistant bacteria in compost, thereby reducing the abundance of sulfonamide ARGs.
Furthermore, this study explored the effect of different cultivation temperatures on the abundance of the sul1 gene. The three types of sulfonamide-resistant bacteria could not tolerate temperatures above 45 °C. At 50 °C, the sul1 gene content in the sul1 gene model bacterium significantly decreased, reaching its lowest value of 4.49 × 105 copies/μL. The trend in Alcaligenes faecalis and Bacillus pseudomycoides was similar to that in the sul1 gene model bacterium with significant reductions in sul1 content at temperatures above 40 °C, and the sul1 expression decreasing to 3.72 × 103 copies/μL and 1.67 × 103 copies/μL at 50 °C, respectively (Figure S3).
3.5. The Reductive Effect of Compound Microbial Inoculant on Sulfonamide ARGs in Aerobic Composting of Pig Manure
3.5.1. Changes in Conventional Composting Indicators
The temperature of the compost pile remained above 55 °C for more than 3 days. The addition of the compound microbial inoculant was found to increase the composting temperature, with the A and B groups reaching a maximum temperature of 60 °C, compared to the maximum temperature of 58 °C in the CK (control) group. The addition of the compound microbial inoculant enhanced the composting process, improving the maturity and fertilizing effectiveness of the compost product. The seed germination index, nitrogen content, and organic matter content in the A and B groups during the maturation phase were significantly higher than those in the control group, while the C/N ratio was significantly lower than that in the control group. Group B with intensive addition of compound microbial inoculant during the cooling phase was more effective (Figure 4).
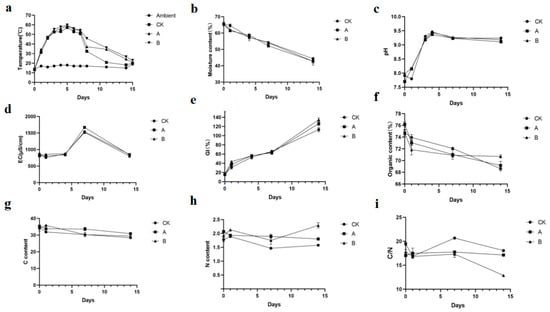
Figure 4.
Physicochemical changes during pig manure composting. The parameters measured include (a) temperature, (b) moisture content, (c) pH, (d) electrical conductivity (EC), (e) germination index (GI), (f) organic matter content, (g) carbon (C), (h) nitrogen (N), and (i) carbon-to-nitrogen ratio (C/N). ‘CK’ denotes the control composting process with pig manure, ‘A’ represents the addition of 2% compound microbial agents once, and ‘B’ indicates repeated additions of 2% compound microbial agents.
3.5.2. Changes in the Expression of Intracellular and Extracellular Sulfonamide ARGs and the inti1 Gene During Composting
Research has discovered that extracellular DNA (eDNA) accounts for 3.7% of the total DNA in pig manure, with eARGs being three orders of magnitude lower than iARGs, yet the ermB gene in eDNA exhibits relatively high abundance [25]. Throughout the composting phase, the content of sulfonamide ARGs and the intI1 gene initially increased and then decreased, still leaving significant residues, with extracellular ARGs significantly lower than intracellular ARGs. The intracellular sul1 gene content ranged from 3.81 × 106 to 4.20 × 107 copies/g, while the extracellular sul1 gene content ranged from 1.12 × 104 to 2.07 × 107 copies/g. The addition of the compound microbial inoculant in groups A and B significantly reduced both intracellular and extracellular sul1 content during the high-temperature phase, with group B showing a more pronounced reduction effect. The intracellular sul2 gene content was relatively higher, ranging from 6.56 × 106 to 5.48 × 108, while the extracellular sul2 gene content ranged from 9.68 × 102 to 8.53 × 106, with the extracellular sul2 gene in group B significantly reduced during the high-temperature phase, whereas the intracellular sul2 gene showed no significant difference from the control group. The intracellular intI1 gene content ranged from 1.71 × 106 to 3.32 × 107 copies/g, and the extracellular intI1 gene content ranged from 5.63 × 102 to 6.93 × 106 copies/g (Figure 5). Intensive addition of the compound microbial inoculant facilitated the removal of both intracellular and extracellular intI1 genes. Studies have shown that the relative abundance of sulfonamide ARGs (sul1, sul2) in compost increases after high-temperature treatment during the maturation phase [12]. The compound microbial inoculant selectively screened in this study has shown some effectiveness in reducing sulfonamide ARGs and the intI1 gene in compost.
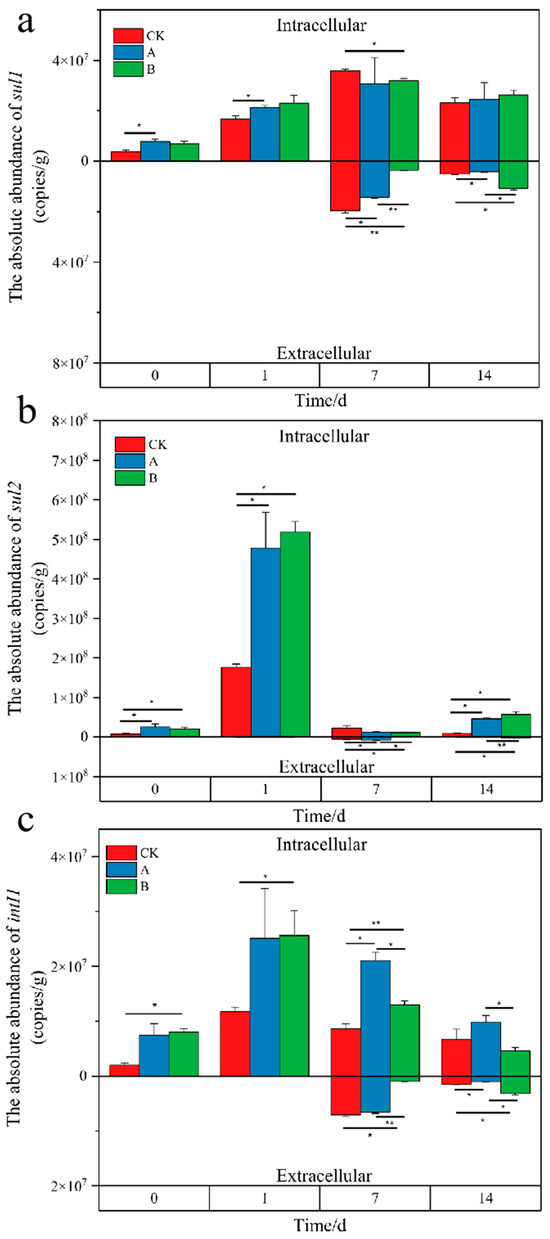
Figure 5.
Changes in absolute abundance of intracellular and extracellular sul1 (a), sul2 (b), intI1 (c) genes during composting. Above the 0 scale are intracellular genes, below the 0 scale are extracellular genes. CK represents the control group (no microbial agent added); A represents the single inoculation group (agent added at the start of composting); and B represents the repeated inoculation group (agent added at the start and again on the eighth day of composting). Statistical significance between treatments and the control group at each time point is denoted by asterisks (* p < 0.05, ** p < 0.01).
In this study, the absolute abundance of both intracellular and extracellular sulfonamide ARGs and intI1 were observed an upward trend, possibly due to the thermophilic characteristics of their host bacteria. High-temperature aerobic composting has minimal impact on reducing certain heat-resistant genes [21,26]. The abundance of eARGs dramatically increases in the later stages of composting, possibly due to the high temperatures causing the death of resistant bacteria. Cell lysis from dead bacteria typically produces eDNA, and thermophilic bacteria can integrate homologous or heterologous eDNA into their chromosomes, making it part of their own DNA or using it as a component of their biofilm [27,28], thereby increasing the abundance of intracellular ARGs.
3.5.3. Impact of Compound Microbial Inoculant on Microbial Communities in Compost
This study utilized high-throughput 16S rRNA gene sequencing technology to explore the changes in microbial community structure, the interrelationships between environmental factors, and sulfonamide ARGs during the composting process. Firmicutes, Proteobacteria, Actinobacteria, and Bacteroidetes were the dominant phyla throughout the composting process. At the initial stage, Firmicutes accounted for 62.93% and 49.7% in the CK and A groups, respectively, followed by Proteobacteria at 18.78% and 36.74%. During the thermophilic phase, the abundance of Firmicutes increased to its peak, reaching 68.99% in the CK group and 78.20% in the A group, and then decreased markedly to 38.04% in the maturation phase of the A group. After the addition of microbial preparations, the abundance of Bacteroidetes and Proteobacteria increased, while Firmicutes decreased during the maturation phase (Figure 6). At the genus level (Figure S4), dominant genera throughout the composting process included Clostridium_sensu_stricto_1 and Terrisporobacter from the Firmicutes phylum, Lactobacillus, and Turicibacter. During the maturation phase, the abundance of genera such as Ureibacillus, Bacillus, Chryseobacterium, Sphingomonas, and Monascus increased, while the abundance of Lactobacillus, Acinetobacter, Bacillus, and Streptococcus decreased as the compost matured. Additionally, the abundance of these genera in the microbial preparation-added groups was lower than in the control group (CK).
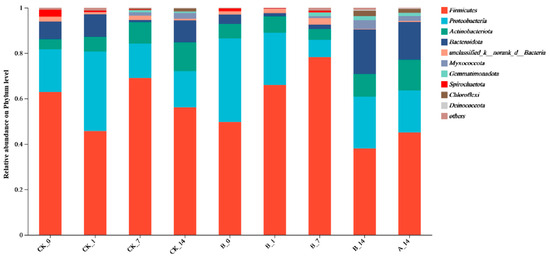
Figure 6.
Structure and abundance of flora at the phylum and genus level. ‘CK’ denotes the control composting process with pig manure, ‘A’ represents the addition of 2% compound microbial inoculant once, and ‘B’ indicates repeated additions of 2% compound microbial inoculant. The number corresponds to a sampling point during the composting timeline: ‘0’ (initial phase), ‘1’ (thermophilic phase), ‘7’ (high-temperature phase), and ‘14’ (maturation phase).
Phylum-level correlation analysis revealed that Actinobacteria showed a significant positive correlation with both intracellular and extracellular sulfonamide ARGs and the intI1 gene, while Spirochaetota exhibited a negative correlation (Figure 7a). At the genus level, during the maturation phase, significant increases were observed in the abundance of genera such as Ureibacillus, Bacillus, Luteimonas, Saccharomonospora, and Persicitalea, among others, which showed a positive correlation with sulfonamide ARGs and the intI1 gene. These microbial groups could be potential hosts for sulfonamide ARGs and the intI1 gene, with their abundance reduced after the addition of microbial preparations. Genera such as Streptococcus, Corynebacterium, Acinetobacter, and Lactobacillus exhibited a significant negative correlation with intracellular and extracellular sul1 and the intI1 gene, and their abundance was also reduced in the later stages (Figure 7b). Intracellular sul2 (isul2) showed a significant negative correlation with Streptococcus and so on.
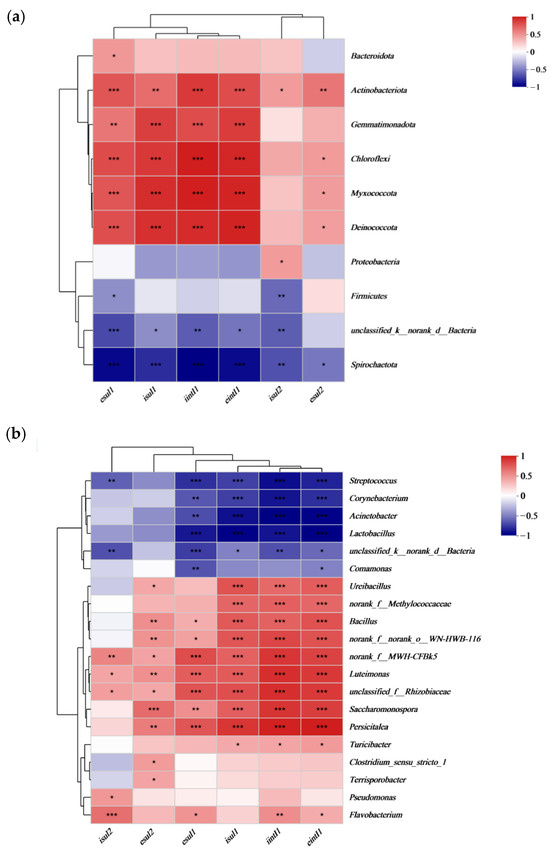
Figure 7.
Correlation of microorganisms at the phylum (a) and genus (b) level with sulfonamide ARGs. The letters ‘i’ and ‘e’ indicate intracellular and extracellular resistance genes, respectively. The color gradient from red to blue represents the spectrum of correlation, with red indicating a positive correlation and blue indicating a negative correlation. Statistical significance is denoted by asterisks, with * p < 0.05, ** p < 0.01, and *** p < 0.001.
3.6. The Impact of pH on Sulfonamide Resistance Genes in Aerobic Composting of Pig Manure
3.6.1. Changes in Conventional Composting Indicators
The results of conventional indicator tests for aerobic composting at different initial pH levels are shown in Figure 8. The temperature changes among the three groups were not significantly different, with all reaching the high-temperature phase by the second day, and the pH 7.7 group reaching the highest temperature of 57.8 °C. The pH exhibited a trend of initially increasing and then decreasing, ultimately stabilizing between 8 and 9. The compost pile with an initial pH of 8.2 generally had a relatively higher overall pH. During the maturation phase of composting, the nitrogen content of the pH 7.7 compost group was higher than the other two groups, and its carbon-to-nitrogen ratio was lower than the other two groups, indicating that starting the compost with a slightly acidic or alkaline pH led to more nitrogen loss and was not conducive to maturation. However, the organic matter content in the pH 8.2 group was higher than in the other two groups.
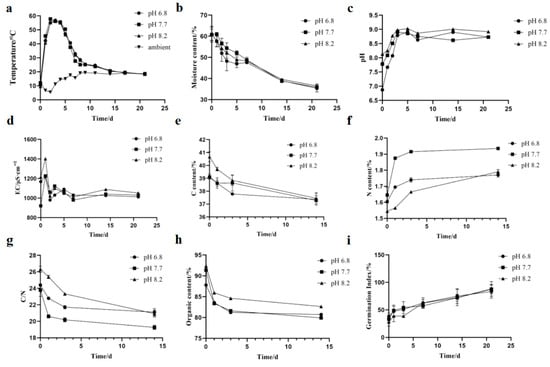
Figure 8.
Physicochemical changes during pig manure composting. The parameters measured include (a) Temperature, (b) Moisture content, (c) pH, (d) EC, (e) TC (f) TN, (g) C/N, (h) Organic content, (i) GI.
3.6.2. Changes in the Content of Intracellular and Extracellular Sulfonamide ARGs and the intI1 Gene During Composting
Throughout the aerobic composting process of pig manure with different initial pH levels, the abundance of intracellular sul1 ranged between 1.05 × 105 and 2.99 × 107 copies/g (Figure 9). During the maturation phase, both the low and high pH groups saw a 31% decrease in abundance compared to the high-temperature phase. The decrease in extracellular sul1 abundance during the maturation phase was most significant in the low pH group, dropping by 35% compared to the high-temperature phase. Similarly to composting with the addition of compound microbial inoculant, the intracellular sul2 gene abundance was relatively higher and peaked during the warming phase, reaching up to 1.2 × 108 copies/g, and significantly decreased during the maturation phase, showing a 63% reduction compared to the high-temperature phase. The intracellular abundance of the intI1 gene exhibited an increasing and then decreasing trend, with the high pH group seeing a reduction from 1.67 × 107 copies/g during the high-temperature phase to 8.50 × 106 copies/g during the maturation phase, a 48% decrease, marking the most significant reduction. During the maturation phase, the extracellular abundance of intI1 in the high pH group was the lowest among the three groups, reaching 3.30 × 105 copies/g.
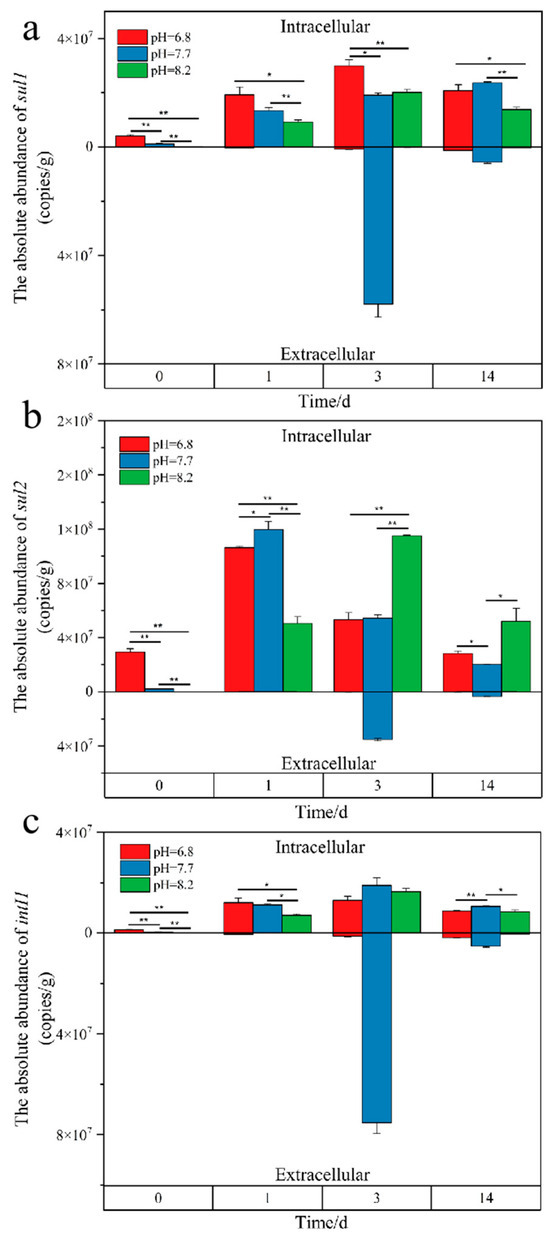
Figure 9.
Temporal changes in absolute abundance of intracellular and extracellular sul1 (a), sul2 (b), intI1 (c) Genes during composting. Values above the zero line on the y-axis indicate intracellular gene abundance, while values below represent extracellular abundance. The time points on the x-axis (0, 1, 3, and 14 days) correspond to the initial, thermophilic, high-temperature, and maturation phases of composting, respectively. Statistical significance is indicated by asterisks directly above the bars, with * p < 0.05, and ** p < 0.01.
Temperature, pH, moisture, antibiotics, and heavy metals, as abiotic indicators in aerobic composting, indirectly interfere with ARG abundance by affecting bacterial community evolution and the abundance of MGEs [29,30,31]. The impact of pH on ARGs during composting is primarily controlled by the host bacteria and horizontal gene transfer [32]. This study found that adjusting the compost pH to acidic conditions and adding compound microbial inoculant could reduce the abundance of sulfonamide ARGs. Due to the diversity of composting materials and conditions, changing the pH might not significantly impact ARGs [8]. This research observed that although altering the compost pH and composting with compound microbial inoculant could not completely eliminate sulfonamide ARGs, they could significantly suppress the proliferation of sulfonamide ARGs during the compost maturation phase.
3.6.3. The Impact of pH on the Microbial Community Structure in Pig Manure Composting
Significant differences in microbial community structure were observed across different stages of composting. Firmicutes, Proteobacteria, Actinobacteriota, and Bacteroidota were the dominant phyla throughout the composting process, with Firmicutes being the absolutely dominant phylum during the high-temperature and maturation phases in the pH 7.7 group. Different pH treatments markedly influenced the relative abundances of Firmicutes, Proteobacteria, and Actinobacteriota. During the heating phase, the abundances of Proteobacteria increased sharply from 0.45%, 9.94%, and 0.72% to 47.31%, 47.21%, and 37.65% in the pH 6.8, 7.7, and 8.2 groups, respectively, while Firmicutes decreased from 84.14%, 81.10%, and 89.49% to 33.78%, 45.40%, and 54.44%. Actinobacteriota increased from 0.46%, 1.10%, and 0.63% to 11.55%, 4.12%, and 5.11%, whereas Bacteroidota showed a slight decline from 9.38%, 2.63%, and 6.68% to 6.83%, 2.04%, and 2.16%, respectively. As composting progressed, the abundance of Firmicutes in the pH 6.8 and 8.2 groups first increased and then decreased, dropping from 75.04% and 63.90% in the thermophilic phase to 45.26% and 41.02% in the maturation phase. Notably, Firmicutes remained dominant in the pH 7.7 group during both the thermophilic and maturation phases, reaching 89.78% and 90.97%, respectively. In contrast, during the maturation phase, the abundances of Proteobacteria, Actinobacteriota, and Bacteroidota in the pH 7.7 group were significantly lower than in the other two groups (Figure 10). Genus-level analysis (Figure S5) revealed that Streptococcus, Lactobacillus, Clostridium_sensu_stricto_1, and Terrisporobacter were the predominant genera throughout the composting process across all treatment groups. During the heating phase, the relative abundances of 22 genera, including Acinetobacter, Corynebacterium, Stenotrophomonas, Glutamicibacter, and Flavobacterium, increased markedly. pH adjustment significantly affected genus composition during the maturation phase, with Ureibacillus showing enrichment in the pH 7.7 group, while Dietzia and Sphingobacterium were more abundant in the pH 6.8 and 8.2 groups. Pseudomonas exhibited increased abundance across all treatments. In contrast, the pH 7.7 group showed lower abundances of Timonella, Luteimonas, Sphingobacterium, Stenotrophomonas, and Glutamicibacter, but higher levels of Eptostreptococcus and Acinetobacter compared to the other groups.
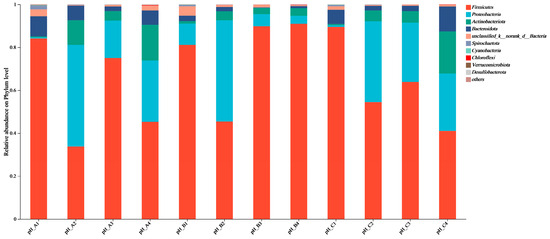
Figure 10.
Structure and abundance of flora at the phylum and genus level. The pH treatment groups are labeled as ‘A’ for pH 6.8, ‘B’ for pH 7.7, and ‘C’ for pH 8.2. Within each pH group, the numbers 1, 2, 3, and 4 denote the composting phases: ‘1’ for the initial phase, ‘2’ for the thermophilic phase, ‘3’ for the high-temperature phase, and ‘4’ for the maturation phase.
Correlation analysis revealed that Chloroflexi and Actinobacteriota were significantly positively correlated with both intracellular and extracellular ARGs and the intI1 gene (Figure 11a). Genera such as Ureibacillus, Dietzia, Pseudomonas, Sphingobacterium, Corynebacterium, Stenotrophomonas, Glutamicibacter, and Flavobacterium showed significant positive correlations with both intracellular and extracellular ARGs and the intI1 gene (Figure 11b). The abundance of these genera increased during the maturation phase of composting across different groups, leading to an increase in the absolute abundance of ARGs. Conversely, Lactobacillus was negatively correlated with all ARGs, and Psychrobacter showed significant negative correlations with extracellular sul1, sul2, intI1 genes, and intracellular sul1.
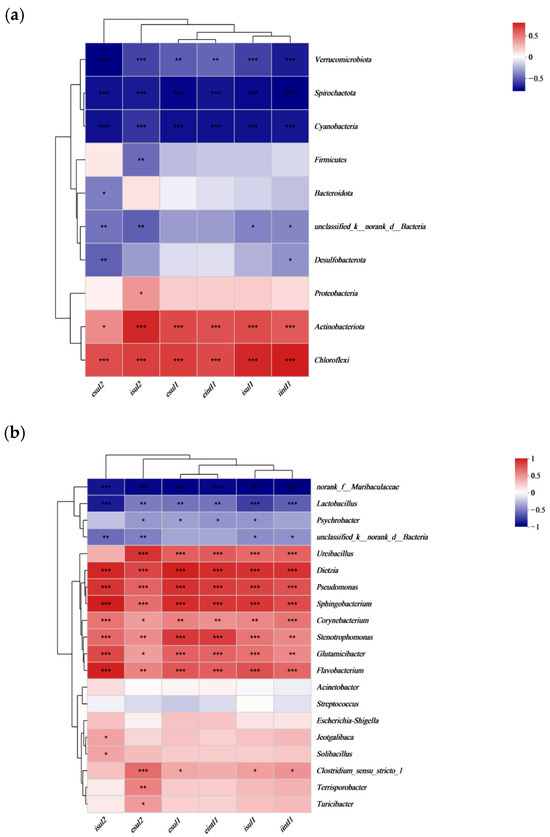
Figure 11.
Correlation of microorganisms at the phylum (a) and genus (b) level with sulfonamide ARGs and intI1 genes. The letters ‘i’ and ‘e’ indicate intracellular and extracellular resistance genes, respectively. The color gradient from red to blue represents the spectrum of correlation, with red indicating a positive correlation and blue indicating a negative correlation. Statistical significance is denoted by asterisks, with * p < 0.05, ** p < 0.01, and *** p < 0.001.
Although some studies have pointed out that temperature is a key factor in the removal of ARGs, this study found that temperature was only significantly correlated with extracellular sul1, sul2, and intI1 genes (p < 0.05) (Figure 12). This may suggest that the effect of temperature on intracellular ARGs is indirect, mediated through the influence on other factors, while its impact on extracellular ARGs is direct. Research indicates that high temperatures can directly promote the decomposition of eARGs and reduce the abundance of iARGs through mechanisms such as promoting the degradation of organic matter and antibiotics, killing heat-sensitive ARG hosts, and weakening horizontal gene transfer of ARGs [8]. Furthermore, adjustments to the C/N ratio and pH were found to aid in the elimination of sulfonamide ARGs, aligning with previous research findings [12].
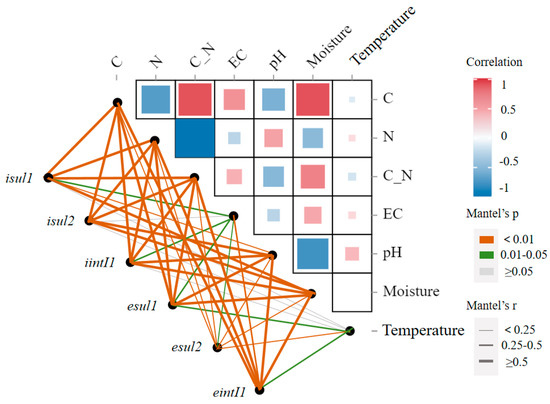
Figure 12.
Mantel test of sulfonamide resistance genes and physiochemical parameters. The heatmap shows Spearman’s correlation coefficients for pairwise comparisons, and the width of the lines indicates the magnitude of Mantel’s r statistic for corresponding distance correlations, while the color signifies statistical significance.
During the composting process, the composition and evolution of bacterial communities, along with MGEs, directly determine the production and dissemination potential of ARGs [4,31,33]. In the two experimental composting groups, Firmicutes, Proteobacteria, Actinobacteriota, and Bacteroidota were identified as the dominant phyla throughout the composting process, consistent with findings from other studies [14,34]. Firmicutes, abundant during the warming and high-temperature phases, are considered potential hosts for ARGs during the mesophilic to thermophilic stages [15,21]. Actinobacteria showed a significant positive correlation with both intracellular and extracellular sulfonamide ARGs and the intI1 gene, with an increase in abundance during the cooling phase of composting. This result suggests that the late proliferation of sulfonamide ARGs and MGEs may be related to Actinobacteria. Actinobacteria are often detected in strains with multiple resistances and intrinsic resistance [35,36]. Therefore, the proliferation of Actinobacteria in the later stages of pig manure composting and its involvement with sulfonamide ARGs warrant attention.
4. Conclusions
Among the 21 organic fertilizer samples, 14 samples were found to contain ARGs, with sulfonamide ARGs and the intI1 gene showing relatively high abundances and strong persistence, reaching up to 6.74 × 1010 copies/g. Reducing the initial pH of the compost pile or adding compound microbial inoculant can reduce the abundance of both intracellular and extracellular sulfonamide ARGs, as well as the mobile genetic element intI1 gene. The proliferation of Actinobacteriota during the compost maturation phase may contribute to the persistence of sulfonamide ARGs. The significant increase in genera such as Pseudomonas and Ureibacillus during the maturation phase, which are positively correlated with sulfonamide ARGs and the intI1 gene, suggests that they could be potential hosts for sulfonamide ARGs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture15202161/s1, Figure S1: The Impact of functional strains on the sul1 gene expression in resistant bacterial isolates; Figure S2: Influence of compound bacterial treatments on the growth of strains M, A, and B; Figure S3: Impact of temperature on sul1 model bacteria, Bacillus Pseudomycoides and Alcaligenes faecalis content of sul1; Figure S4: Structure and abundance of flora at the phylum and genus level; Figure S5: Structure and abundance of flora at the phylum and genus level; Table S1: Organic fertilizer production information; Table S2: PCR primer sequences and annealing temperature; Table S3: ARGs real-time PCR standard curve equation; Table S4: The colony morphology of the strain was screened.
Author Contributions
J.W.: Conceptualization; Writing—Original Draft; Writing—Review & Editing. Z.R.: Conceptualization. Y.H.: Formal analysis; Investigation; Writing—Original Draft; Writing—Review & Editing. P.W.: Formal analysis; Investigation; Writing—Original Draft; Writing—Review & Editing. S.Z.: Formal analysis; Investigation; Writing—Original Draft; Writing—Review & Editing. S.L.: Writing—Review & Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Key R&D Program of China (Program No. 2023YFD1301904), the Key Research and Development Project of Hubei Province (Program No. 2024BBB067), the Natural Science Foundation of Hubei Province (Program No. 2022CFB126, Program No. 2022CFD033), and the Fundamental Research Funds for the Central Universities (Program No. 2662023DKPY003).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ARGs | Antibiotic resistance genes |
| EC | Electrical conductivity |
| CFUs | Colony-forming units |
| GI | Germination Index |
| C/N | Carbon-nitrogen ratios |
References
- Pu, C.; Yu, Y.; Diao, J.; Gong, X.; Li, J.; Sun, Y. Exploring the persistence and spreading of antibiotic resistance from manure to biocompost, soils and vegetables. Sci. Total Environ. 2019, 688, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Gu, J.; Sun, W.; Wang, X.J.; Su, J.Q.; Stedfeld, R. Diversity, abundance, and persistence of antibiotic resistance genes in various types of animal manure following industrial composting. J. Hazard. Mater. 2018, 344, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, L.; Sha, G.; Liu, C.; Wang, Z.; Wang, L. Aerobic composting as an effective cow manure management strategy for reducing the dissemination of antibiotic resistance genes: An integrated meta-omics study. J. Hazard. Mater. 2020, 386, 121895. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Ben, W.; Qiang, Z.; Zhang, J. Removal of antibiotic resistance genes in pig manure composting influenced by inoculation of compound microbial agents. Bioresour. Technol. 2020, 317, 123966. [Google Scholar] [CrossRef] [PubMed]
- Sardar, M.F.; Zhu, C.; Geng, B.; Ahmad, H.R.; Song, T.; Li, H. The fate of antibiotic resistance genes in cow manure composting: Shaped by temperature-controlled composting stages. Bioresour. Technol. 2021, 320 Pt B, 124403. [Google Scholar] [CrossRef] [PubMed]
- Kenneth, M.J.; Koner, S.; Hsu, G.J.; Chen, J.S.; Hsu, B.M. A review on the effects of discharging conventionally treated livestock waste to the environmental resistome. Environ. Pollut. 2023, 338, 122643. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, J.R. Horizontal gene transfer in the human gastrointestinal tract: Potential spread of antibiotic resistance genes. Infect. Drug Resist. 2014, 7, 167–176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, B.; Yu, K.; Ahmed, I.; Gin, K.; Xi, B.; Wei, Z.; He, Y.; Zhang, B. Key factors driving the fate of antibiotic resistance genes and controlling strategies during aerobic composting of animal manure: A review. Sci. Total Environ. 2021, 791, 148372. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.; Hu, H.W.; Zhang, Y.J.; Wang, J.T.; Hayden, H.; Tang, Y.Q.; He, J.Z. Aerobic composting reduces antibiotic resistance genes in cattle manure and the resistome dissemination in agricultural soils. Sci. Total Environ. 2018, 612, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Yuan, Y.; Wang, Y.; Liu, D.; Shen, Q.; Zhao, F. Hazard reduction and persistence of risk of antibiotic resistance during thermophilic composting of animal waste. J. Environ. Manag. 2023, 330, 117249. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, X.; Qiu, X.; Zhang, J. Biochar influences the succession of microbial communities and the metabolic functions during rice straw composting with pig manure. Bioresour. Technol. 2019, 272, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fu, Q.; Wen, Q.; Wu, Y.; Bao, H.; Guo, J. Microbial community competition rather than high-temperature predominates ARGs elimination in swine manure composting. J. Hazard. Mater. 2022, 423 Pt B, 127149. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Wu, D.; Zhang, L.; Zou, D.; Sun, Y.; Gao, M.; Wang, X. A comparison of antibiotics, antibiotic resistance genes, and bacterial community in broiler and layer manure following composting. Environ. Sci. Pollut. Res. Int. 2021, 28, 14707–14719. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Zhang, A.; Chen, S.; He, X.; Jin, L.; Yu, X.; Yang, S.; Li, B.; Fan, L.; Ji, L.; et al. Heavy metals, antibiotics and nutrients affect the bacterial community and resistance genes in chicken manure composting and fertilized soil. J. Environ. Manag. 2020, 257, 109980. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Li, H.; Song, D.; Lin, X.; Wang, Y. Influence of zeolite and superphosphate as additives on antibiotic resistance genes and bacterial communities during factory-scale chicken manure composting. Bioresour. Technol. 2018, 263, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liang, F.; Yu, A.; Li, B.; Yang, L. Evaluation of stability and maturity during forced-aeration composting of chicken manure and sawdust at different C/N ratios. Chemosphere 2010, 78, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Luo, Y.; Mathieu, J.; Wang, Q.; Feng, L.; Mu, Q.; Feng, C.; Alvarez, P.J. Persistence of extracellular DNA in river sediment facilitates antibiotic resistance gene propagation. Environ. Sci. Technol. 2014, 48, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Schenk, J.J.; Becklund, L.E.; Carey, S.J.; Fabre, P.P. What is the “modified” CTAB protocol? Characterizing modifications to the CTAB DNA extraction protocol. Appl. Plant Sci. 2023, 11, e11517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, G.Y.; Yan, P.Y.; Liu, W.; Liu, L.K.; Li, J.P.; Zeng, Y. Potentilla bifurca flavonoids effectively improve insulin resistance. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8358–8369. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Song, W.; Cooper, W.J.; Jung, J.; Greaves, J. Degradation of tetracycline antibiotics: Mechanisms and kinetic studies for advanced oxidation/reduction processes. Chemosphere 2010, 78, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, M.; Sui, Q.; Tong, J.; Jiang, C.; Lu, X.; Zhang, Y.; Wei, Y. Impacts of addition of natural zeolite or a nitrification inhibitor on antibiotic resistance genes during sludge composting. Water Res. 2016, 91, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Rajan, L.; Chakraborty, K.; Chakraborty, R.D. Pharmacological properties of some mangrove sediment-associated bacillus isolates. Arch. Microbiol. 2021, 203, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ashengroph, M.; Hosseini, S.R. A newly isolated Bacillus amyloliquefaciens SRB04 for the synthesis of selenium nanoparticles with potential antibacterial properties. Int. Microbiol. 2021, 24, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Zheng, Q.W.; Wei, T.; Zhang, Z.Q.; Zhao, C.F.; Zhong, H.; Xu, Q.Y.; Lin, J.F.; Guo, L.Q. Isolation and Characterization of Fengycins Produced by Bacillus amyloliquefaciens JFL21 and Its Broad-Spectrum Antimicrobial Potential Against Multidrug-Resistant Foodborne Pathogens. Front. Microbiol. 2020, 11, 579621. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dong, P.; Wang, H.; Fang, T.; Wang, Y.; Ye, Q. Assessment of extracellular antibiotic resistance genes (eARGs) in typical environmental samples and the transforming ability of eARG. Environ. Int. 2019, 125, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Su, J.Q.; Wei, B.; Ou-Yang, W.Y.; Huang, F.Y.; Zhao, Y.; Xu, H.J.; Zhu, Y.G. Antibiotic resistome and its association with bacterial communities during sewage sludge composting. Environ. Sci. Technol. 2015, 49, 7356–7363. [Google Scholar] [CrossRef] [PubMed]
- Pietramellara, G.; Ceccherini, M.T.; Ascher, J.; Nannipieri, P. Persistence of transgenic and not transgenic extracellular DNA in soil and bacterial transformation. Riv. Biol. 2006, 99, 37–68. [Google Scholar] [PubMed]
- Zawadzki, P.; Cohan, F.M. The size and continuity of DNA segments integrated in Bacillus transformation. Genetics 1995, 141, 1231–1243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, H.; Gu, J.; Wang, X.; Wang, Q.; Sun, W.; Hu, T.; Guo, H.; Ma, J.; Bao, J. Insight into the fate of antibiotic resistance genes and bacterial community in co-composting green tea residues with swine manure. J. Environ. Manag. 2020, 266, 110581. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Zhu, C.; Xue, S.; Li, B.; Ye, J.; Geng, B.; Li, L.; Fahad Sardar, M.; Li, N.; Feng, S.; et al. Comparative effects of different antibiotics on antibiotic resistance during swine manure composting. Bioresour. Technol. 2020, 315, 123820. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, G.; Chang, J.; Kong, Y.; Jiang, T.; Wang, J.; Yuan, J. Enrichment of antibiotic resistance genes after sheep manure aerobic heap composting. Bioresour. Technol. 2021, 323, 124620. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, Y.; Zheng, X.; Su, Y.; Wan, R.; Yang, S. Distribution of tetracycline resistance genes in anaerobic treatment of waste sludge: The role of pH in regulating tetracycline resistant bacteria and horizontal gene transfer. Bioresour. Technol. 2016, 218, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Wang, X.; Gu, J.; Dai, X.; Zhang, K.; Wang, Q.; Ma, J.; Peng, H. Effects of macroporous adsorption resin on antibiotic resistance genes and the bacterial community during composting. Bioresour. Technol. 2020, 295, 121997. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gu, J.; Wang, X.; Ma, J.; Hu, T.; Peng, H.; Bao, J.; Zhang, R. Effects of nano-zerovalent iron on antibiotic resistance genes and mobile genetic elements during swine manure composting. Environ. Pollut. 2020, 258, 113654. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, V.M.; McGrann, K.M.; Hughes, D.W.; Wright, G.D. Sampling the antibiotic resistome. Science 2006, 311, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Stegmann, E.; Frasch, H.J.; Kilian, R.; Pozzi, R. Self-resistance mechanisms of actinomycetes producing lipid II-targeting antibiotics. Int. J. Med. Microbiol. 2015, 305, 190–195. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).