Combined Effects of Polyethylene and Bordeaux Mixture on the Soil–Plant System: Phytotoxicity, Copper Accumulation and Changes in Microbial Abundance
Abstract
1. Introduction
2. Materials and Methods
2.1. Contaminants and Test Soil
2.2. Soil Contamination
2.3. Seed Germination and Plant Growth Tests
2.4. Post-Harvest Treatment: Determination of Soil Properties and Plant Growth Parameters
2.5. Cu Bioaccumulation
2.6. Quantification of Photosynthetic Pigments
2.7. Microbial Community Abundance
2.8. Statistical Analysis
3. Results and Discussion
3.1. Impact of BM and MPs Contamination on Soil Properties After Plant Growth
3.2. Impact of BM and MPs Contamination on Plant Growth Parameters
3.3. Impact of BM and MPs on Metal Bioavailability and Plant Bioaccumulation
3.4. Impact of BM and MPs Contamination on Plant Photosynthetic Performance
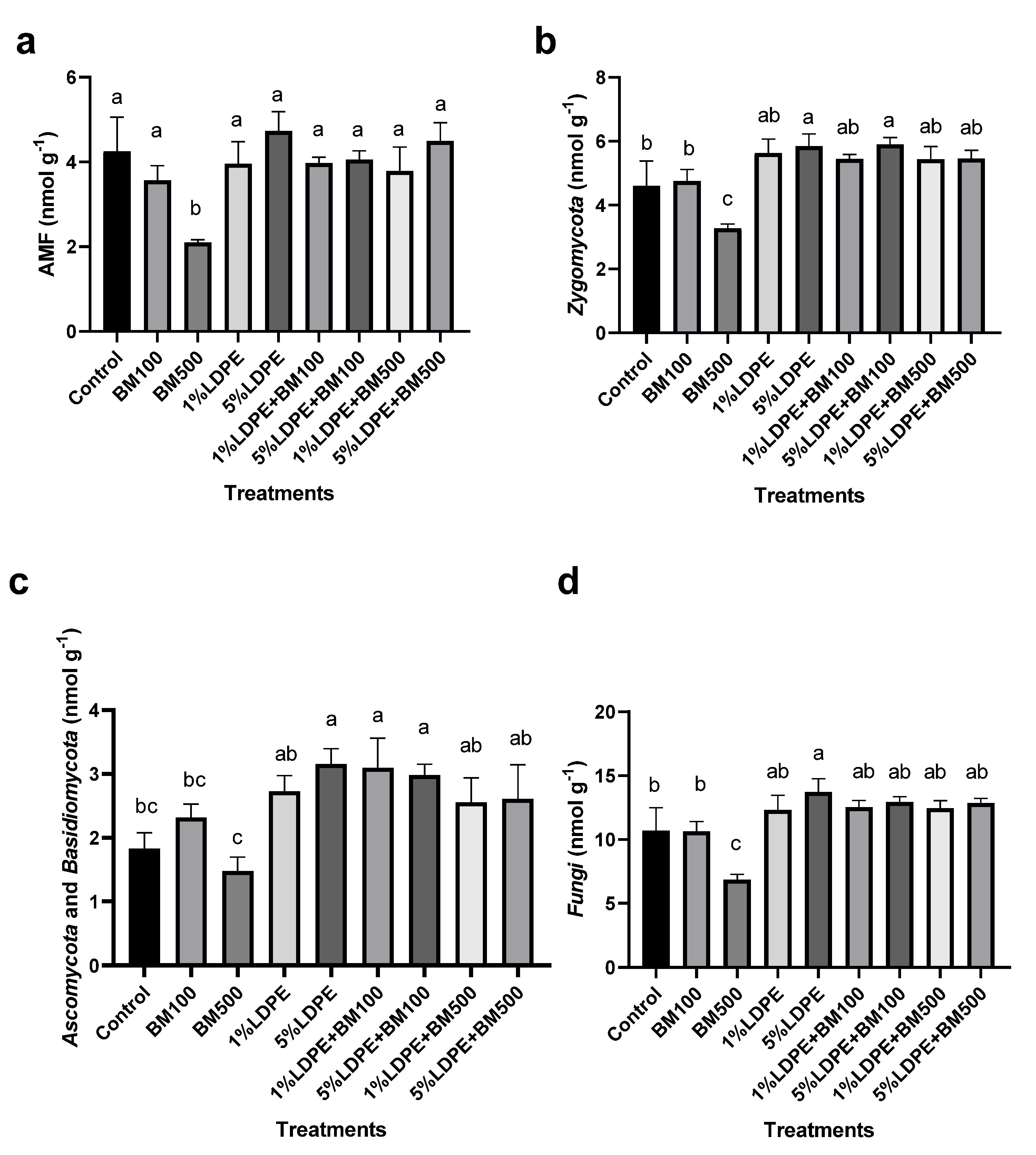
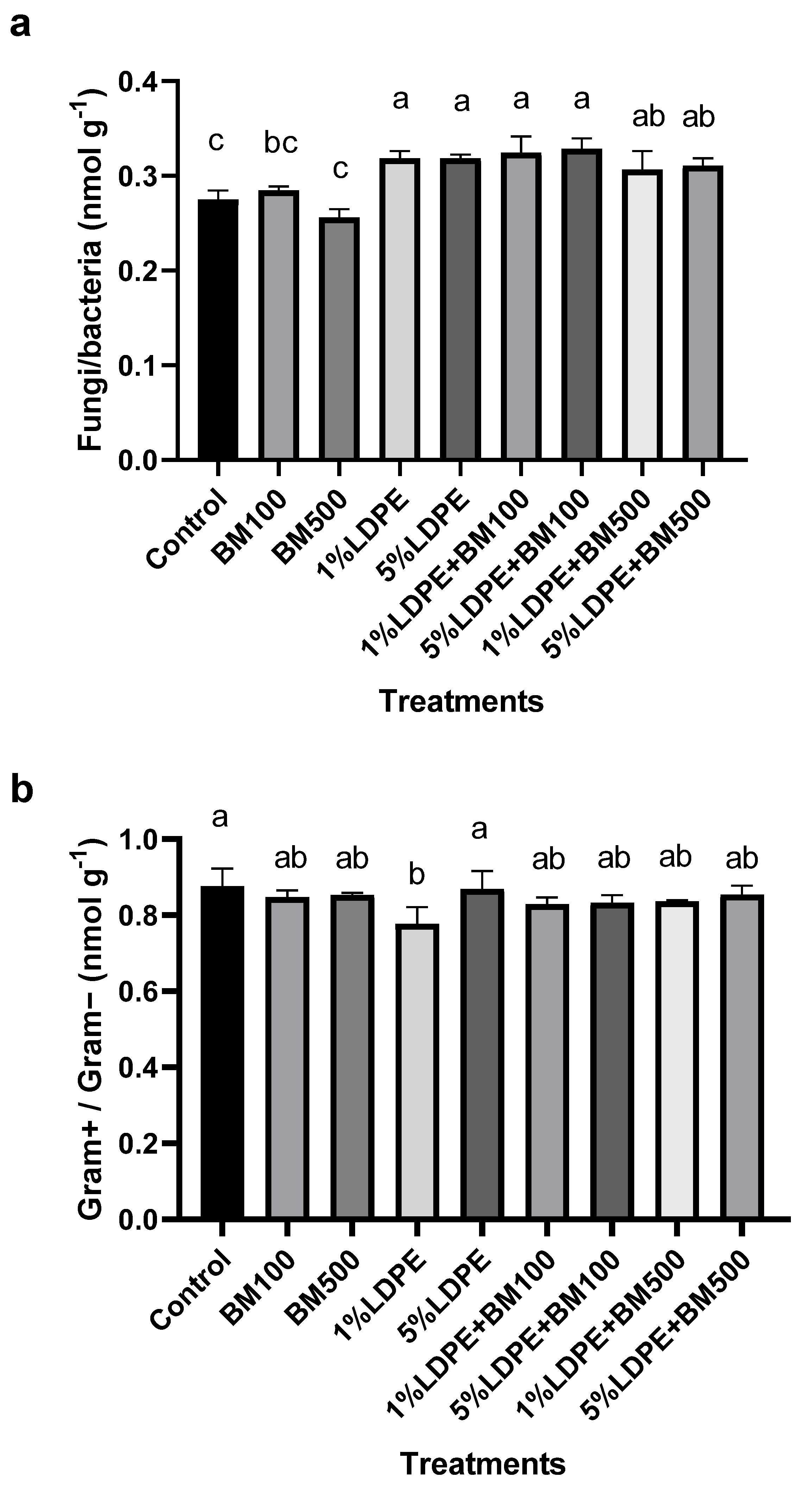
3.5. Impact of BM and MPs Contamination on Soil Microbial Structure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Evangeliou, N.; Grythe, H.; Klimont, Z.; Heyes, C.; Eckhardt, S.; Lopez-Aparicio, S.; Stohl, A. Atmospheric transport is a major pathway of microplastics to remote regions. Nature Comm. 2020, 11, 3381. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial eco-systems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [PubMed]
- Chia, R.W.; Lee, J.; Jang, J.; Kim, H.J.; Kwon, K.D. Soil health and microplastics: A review of the impacts of microplastic contamination on soil properties. J. Soil Sed. 2022, 22, 2690–2705. [Google Scholar] [CrossRef]
- Daghighi, E.; Shah, T.; Chia, R.W.; Lee, J.Y.; Shang, J.; Rodríguez-Seijo, A. The forgotten impacts of plastic contamination on terrestrial micro- and mesofauna: A call for research. Environ. Res. 2023, 231, 116227. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Seijo, A.; Santos, B.; Ferreira da Silva, E.; Cachada, A.; Pereira, R. Low-density polyethylene microplastics as a source and carriers of agrochemicals to soil and earthworms. Environ. Chem. 2019, 16, 8–17. [Google Scholar] [CrossRef]
- Chang, J.; Fang, W.; Liang, J.; Zhang, P.; Zhang, G.; Zhang, H.; Zhang, Y.; Wang, Q. A critical review on interaction of microplastics with organic contaminants in soil and their ecological risks on soil organisms. Chemosphere 2022, 306, 135573. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Bao, Q.; Gao, M.; Qiu, W.; Song, Z. A novel mechanism study of microplastic and as co-contamination on indica rice (Oryza sativa L.). J. Hazard. Mater. 2022, 421, 126694. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Kim, S.; Sarkar, B.; Oleszczuk, P.; Sang, M.K.; Haque, M.N.; Ahn, J.; Bank, M.S.; Ok, Y.S. Effects of microplastics on the terrestrial environment: A critical review. Environ. Res. 2022, 209, 112734. [Google Scholar] [CrossRef]
- Khalid, N.; Aqeel, M.; Noman, A. Micro-plastics could be a threat to plants in terrestrial systems directly or indirectly. Environ. Pollut. 2020, 267, 115653. [Google Scholar] [CrossRef]
- Rodríguez-Seijo, A.; Pereira, R. Microplastics in Agricultural Soils: Are They a Real Environmental Hazard? In Bioremediation of Agricultural Soils; Sánchez-Hernández, J.C., Ed.; CRC Press: Boca Raton, FL, USA; pp. 45–60.
- Kumar, R.; Ivy, N.; Bhattacharya, S.; Dey, A.; Sharma, P. Coupled effects of microplastics and heavy metals on plants: Uptake, bioaccumulation, and environmental health perspectives. Sci. Total Environ. 2022, 836, 155619. [Google Scholar] [CrossRef]
- Botyanszká, L.; Šurda, P.; Vitková, J.; Lichner, Ľ.; Igaz, D. Effect of microplastics on silty loam soil properties and radish growth. J. Hydrol. Hydromech. 2022, 70, 321–329. [Google Scholar] [CrossRef]
- Zong, X.; Zhang, J.; Zhu, J.; Zhang, L.; Jiang, L.; Yin, Y.; Guo, H. Effects of polystyrene microplastic on uptake and toxicity of copper and cadmium in hydroponic wheat seedlings (Triticum aestivum L.). Ecotox. Environ. Saf. 2021, 217, 112217. [Google Scholar] [CrossRef]
- Jia, H.; Wu, D.; Yu, Y.; Han, S.; Sun, L.; Li, M. Impact of microplastics on bioaccumulation of heavy metals in rape (Brassica napus L.). Chemosphere 2022, 288, 132576. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Aqeel, M.; Khalid, N.; Nazir, A.; Alzuaibr, F.M.; Al-Mushhin, A.; Hakami, O.; Iqbal, M.F.; Chen, F.; Alamri, S.; et al. Effects of microplastics on growth and metabolism of rice (Oryza sativa L.). Chemosphere 2022, 307, 135749. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, B.; Zhao, T.; Yin, W.; Zhao, X.; Xie, Q.J.; Khan, K.Y.; Zhao, X.; Nazar, M.; Li, G.; Du, D. Impacts of soil microplastics on crops: A review. Appl. Soil Ecol. 2023, 181, 104680. [Google Scholar] [CrossRef]
- Roy, T.; Dey, T.K.; Jamal, M. Microplastic/nanoplastic toxicity in plants: An imminent concern. Environ. Monit. Assess. 2023, 195, 27. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef]
- Rong, L.; Zhao, L.; Zhao, L.; Cheng, Z.; Yao, Y.; Yuan, C.; Wang, L.; Sun, H. LDPE microplastics affect soil microbial communities and nitrogen cycling. Sci. Total Environ. 2021, 773, 145640. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Q.; Sun, Y.; Zhang, S.; Wang, F. Microplastics change soil properties, heavy metal availability and bacterial community in a Pb-Zn-contaminated soil. J. Hazard. Mater. 2022, 424, 127364. [Google Scholar] [CrossRef]
- Yin, W.; Zhang, B.; Zhang, H.; Zhang, D.; Leiviskä, T. Vertically co-distributed vanadium and microplastics drive distinct microbial community composition and assembly in soil. J. Hazard. Mater. 2022, 440, 129700. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.W.; Chadwick, D.R.; Thornton, H.; Marshall, M.R.; Bei, S.; Distaso, M.A.; Bargiela, R.; Marsden, K.A.; Clode, P.L.; Murphy, D.V.; et al. Field application of pure polyethylene microplastic has no significant short-term effect on soil biological quality and function. Soil Biol. Biochem. 2022, 165, 108496. [Google Scholar] [CrossRef]
- Chen, X.; Xie, Y.; Wang, J.; Shi, Z.; Zhang, J.; Wei, H.; Ma, Y. Presence of different microplastics promotes greenhouse gas emissions and alters the microbial community composition of farmland soil. Sci. Total Environ. 2023, 879, 162967. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Ali, A.; Haider, G.; Asad, M.; Munsif, F. Microplastics alter soil enzyme activities and microbial community structure without negatively affecting plant growth in an agroecosystem. Chemosphere 2023, 322, 138188. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Soler-Rovira, P.; Polo, A.; Díaz-Raviña, M.; Arias-Estévez, M.; Plaza, C. Enzyme activities in vineyard soils long-term treated with copper-based fungicides. Soil Biol. Biochem. 2010, 42, 2119–2127. [Google Scholar] [CrossRef]
- Vázquez-Blanco, R.; Arias-Estévez, M.; Bååth, E.; Fernández-Calviño, D. Comparing the effect of Cu-based fungicides and pure Cu salts on microbial biomass, microbial community structure and bacterial community tolerance to Cu. J. Hazard. Mater. 2021, 409, 124960. [Google Scholar] [CrossRef]
- OECD. Test No. 208: Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test. In OECD Guidelines for the Testing of Chemicals; Section 2; OECD Publishing: Paris, France, 2006. [Google Scholar] [CrossRef]
- Rodríguez-Seijo, A.; Soares, C.; Ribeiro, S.; Amil, B.F.; Patinha, C.; Cachada, A.; Fidalgo, F.; Pereira, R. Nano-Fe2O3 as a tool to restore plant growth in contaminated soils—Assessment of potentially toxic elements (bio)availability and redox homeostasis in Hordeum vulgare L. J. Hazard Mater. 2022, 425, 127999. [Google Scholar] [CrossRef]
- Maraveas, C.; Hahladakis, J.N. A scoping review on the European Union agricultural plastic waste management strategies: Focusing on liquefaction. J. Hazard. Mater. Adv. 2025, 18, 100727. [Google Scholar] [CrossRef]
- Mansoor, Z.; Tchuenbou-Magaia, F.; Kowalczuk, M.; Adamus, G.; Manning, G.; Parati, M.; Radecka, I.; Khan, H. Polymers Use as Mulch Films in Agriculture—A Review of History, Problems and Current Trends. Polymers 2022, 14, 5062. [Google Scholar] [CrossRef]
- Büks, F.; Kaupenjohann, M. Global concentrations of microplastics in soils—A review. Soil 2020, 6, 649–662. [Google Scholar] [CrossRef]
- Vázquez-Blanco, R.; Arias-Estévez, M.; Bååth, E.; Fernández-Calviño, D. Comparison of Cu salts and commercial Cu based fungicides on toxicity towards microorganisms in soil. Environ. Pollut. 2020, 257, 113585. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef]
- Vázquez-Blanco, R.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Calviño, D.; Perez-Rodriguez, P. Changes in Cu accu-mulation and fractionation along soil depth in acid soils of vineyards and abandoned vineyards (now forests). Agric. Ecosyst. Environ. 2022, 339, 108146. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norwell, W.A. Development of DTPA of Soil Test for Zn, Fe, Mn and Cu. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Rodríguez-Seijo, A.; Lago-Vila, M.; Andrade, M.L.; Vega, F.A. Pb pollution in soils from a trap shooting range and the phy-toremediation ability of Agrostis capillaris L. Environ. Sci. Pollut. Res. 2016, 23, 1312–1323. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzim. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Lichtenthaler, H.; Babani, F.; Langsdorf, G.; Buschmann, C. Measurement of Differences in Red Chlorophyll Fluorescence and Photosynthetic Activity between Sun and Shade Leaves by Fluorescence Imaging. Photosynthetica 2000, 38, 521–529. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Babani, F. Contents of photosynthetic pigments and ratios of chlorophyll a/b and chlorophylls to carot-enoids (a + b)/(x + c) in C4 plants as compared to C3 plants. Photosynthetica 2022, 60, 3–9. [Google Scholar] [CrossRef]
- Marschall, M.; Proctor, M.C. Are bryophytes shade plants? Photosynthetic light responses and proportions of chlorophyll a, chlorophyll b and total carotenoids. Ann. Bot. 2004, 94, 593–603. [Google Scholar] [CrossRef]
- Frostegård, Å.; Bååth, E.; Tunlio, A. Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol. Biochem. 1993, 25, 723–730. [Google Scholar] [CrossRef]
- Joergensen, R.G. Phospholipid fatty acids in soil—Drawbacks and future prospects. Biol. Fertil. Soils 2022, 58, 1–6. [Google Scholar] [CrossRef]
- Zhang, P.; Yuan, Y.; Zhang, J.; Wen, T.; Wang, H.; Qu, C.; Tan, W.; Xi, B.; Hui, K.; Tang, J. Specific response of soil properties to microplastics pollution: A review. Environ. Res. 2023, 232, 116427. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Wang, J.; Wu, L.; Liu, Z.; Wei, H.; Zhang, J. Unraveling consequences of the co-exposure of polyethylene micro-plastics and acid rain on plant-microbe-soil system. Chemosphere 2022, 307, 135941. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, F.; Rejeb, S.; Ghorbal, M.H.; Morel, J. Plant Response to Copper Toxicity as Affected by Plant Species and Soil Type. J. Plant Nutr. 2005, 28, 379–392. [Google Scholar] [CrossRef]
- Shams, M.; Ekinci, M.; Turan, M.; Dursun, A.; Kul, R.; Yildrim, E. Growth, nutrient uptake and enzyme activity response of Lettuce (Lactuca sativa L.) to excess copper. Environ. Sus. 2019, 2, 67–73. [Google Scholar] [CrossRef]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef]
- Lian, J.; Wu, J.; Xiong, H.; Zeb, A.; Yang, T.; Su, X.; Su, L.; Liu, W. Impact of polystyrene nanoplastics (PSNPs) on seed germination and seedling growth of wheat (Triticum aestivum L.). J. Hazard. Mater. 2020, 385, 121620. [Google Scholar] [CrossRef]
- Li, B.; Huang, S.; Wang, H.; Liu, M.; Xue, S.; Tang, D.; Cheng, W.; Fan, T.; Yang, X. Effects of plastic particles on germination and growth of soybean (Glycine max): A pot experiment under field condition. Environ. Pollut. 2021, 272, 116418. [Google Scholar] [CrossRef]
- Zantis, L.J.; Borchi, C.; Vijver, M.G.; Peijnenburg, W.J.; Di Lonardo, S.; Bosker, T. Nano- and microplastics commonly cause adverse impacts on plants at environmentally relevant levels: A systematic review. Sci. Total Environ. 2023, 867, 161211. [Google Scholar] [CrossRef]
- Li, Z.; Li, Q.; Li, R.; Zhao, Y.; Geng, J.; Wang, G. Physiological responses of lettuce (Lactuca sativa L.) to microplastic pollution. Environ. Sci. Pollut. Res. 2020, 27, 30306–30314. [Google Scholar] [CrossRef] [PubMed]
- Lozano, Y.M.; Lehnert, T.; Linck, L.T.; Lehmann, A.; Rillig, M.C. Microplastic Shape, Polymer Type, and Concentration Affect Soil Properties and Plant Biomass. Front. Plant Sci. 2021, 12, 616645. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Belz, R.G.; Kitao, M.; Koike, T.; Calabrese, E.J. Does the root to shoot ratio show a hormetic response to stress? An ecological and environmental perspective. J. For. Res. 2019, 30, 1569–1580. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Prinsen, E.; Veselov, S.U.; Martinenko, E.V.; Melentiev, A.I.; Kudoyarova, G.R. Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil 2007, 292, 305–315. [Google Scholar] [CrossRef]
- Takoutsing, B.; Tchoundjeu, Z.; Degrande, A.; Asaah, E.; Gyau, A.; Nkeumoe, F.; Tsobeng, A. Assessing the Quality of Seedlings in Small-scale Nurseries in the Highlands of Cameroon: The Use of Growth Characteristics and Quality Thresholds as Indicators. Small-Scale For. 2014, 13, 65–77. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Rillig, M.C. Effects of Microplastic Fibers and Drought on Plant Communities. Environ. Sci. Technol. 2020, 54, 6166–6173. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, M.; Shahbaz, M.; Hu, Z.; Zhu, Z.; Lu, S.; Yu, Y.; Yao, H.; Chen, J.; Ge, T. Microplastics in soil can increase nutrient uptake by wheat. J. Hazard. Mater. 2022, 438, 129547. [Google Scholar] [CrossRef]
- Tumwet, F.C.; Richter, A.; Kleint, T.; Scheytt, T. Vertical movement of microplastics by roots of wheat plant (Triticum aestivum) and the plant response in sandy soil. Micropl. Nanopl. 2024, 4, 15. [Google Scholar] [CrossRef]
- Hasan, M.M.; Jho, E.H. Effect of different types and shapes of microplastics on the growth of lettuce. Chemosphere 2023, 339, 139660. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xie, Y.; Li, H.; Dong, H.; Li, B.; Guo, Y.; Wang, Y.; Guo, X.; Yin, T.; Liu, X.; et al. Responses of lettuce (Lactuca sativa L.) growth and soil properties to conventional non-biodegradable and new biodegradable microplastics. Environ. Pollut. 2024, 341, 122897. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Xu, G.; Wang, Y.; Yu, Y. Impacts of polyethylene microplastics on bioavailability and toxicity of metals in soil. Sci. Total Environ. 2021, 760, 144037. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, X.; Song, N. Polyethylene microplastics increase cadmium uptake in lettuce (Lactuca sativa L.) by altering the soil microenvironment. Sci. Total Environ. 2021, 784, 147133. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Poblete, A.; Retamal-Salgado, J.; Zapata, N.; Sierra-Almeida, Á.; Schoebitz, M. Impact of polyethylene microplastics and copper nanoparticles: Responses of soil microbiological properties and strawberry growth. Appl. Soil Ecol. 2023, 184, 104773. [Google Scholar] [CrossRef]
- Gan, C.D.; Liao, Y.L.; Liu, H.B.; Yang, J.Y.; Nikitin, A. Microplastic-induced changes in Cd and Cr behavior in the agricultural soil-wheat system: Insights into metal bioavailability and phytotoxicity. J. Hazard. Mater. 2025, 482, 136592. [Google Scholar] [CrossRef]
- Erdem, H.; Gence, C.Ç.; Öztürk, M.; Buhan, E.; Kholikulov, S.T.; Kaya, Y. Microplastics in Soil Increase Cadmium Toxicity: Im-plications for Plant Growth and Nutrient Imbalance. Water Air Soil Pollut. 2025, 236, 575. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Khozin-Goldberg, I.; Cohen, Z.; Merzlyak, M.N. Carotenoid-to-chlorophyll ratio as a proxy for assay of total fatty acids and arachidonic acid content in the green microalga Parietochloris incisa. J. Appl. Phycol. 2009, 21, 361–366. [Google Scholar] [CrossRef]
- Srivastava, S.; Mishra, S.; Tripathi, R.D.; Dwivedi, S.; Gupta, D.K. Copper-induced oxidative stress and responses of antioxidants and phytochelatins in Hydrilla verticillata (L.f.) Royle. Aquat. Toxicol. 2006, 80, 405–415. [Google Scholar] [CrossRef]
- Xu, E.; Liu, Y.; Gu, D.; Zhan, X.; Li, J.; Zhou, K.; Zhang, P.; Zou, Y. Molecular Mechanisms of Plant Responses to Copper: From Deficiency to Excess. Int. J. Mol. Sci. 2024, 25, 6993. [Google Scholar] [CrossRef]
- Trifunović-Momčilov, M.; Milošević, S.; Marković, M.; Đurić, M.; Jevremović, S.; Dragićević, I.Č.; Subotić, A.R. Changes in Pho-tosynthetic Pigments Content in Non-Transformed and AtCKX Transgenic Centaury (Centaurium erythraea Rafn) Shoots Grown under Salt Stress In Vitro. Agronomy 2021, 11, 2056. [Google Scholar] [CrossRef]
- Cunha Neto, A.R.; Ambrósio, A.D.; Wolowski, M.; Westin, T.B.; Govêa, K.P.; Carvalho, M.M.; Barbosa, S. Negative effects on photosynthesis and chloroplast pigments exposed to lead and aluminum: A Meta-analysis. CERNE 2020, 26, 232–237. [Google Scholar] [CrossRef]
- Colzi, I.; Renna, L.; Bianchi, E.; Castellani, M.B.; Coppi, A.; Pignattelli, S.; Loppi, S.; Gonnelli, C. Impact of microplastics on growth, photosynthesis and essential elements in Cucurbita pepo L. J. Hazard. Mater. 2022, 423, 127238. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Zhu, Y.; Li, H.; Song, X.; Shi, L. The phytotoxicity of microplastics to the photosynthetic performance and tran-scriptome profiling of Nicotiana tabacum seedlings. Ecotoxicol. Environ. Saf. 2022, 231, 113155. [Google Scholar] [CrossRef]
- Mondal, N.K.; Kundu, S.; Debnath, P.; Mondal, A.; Sen, K. Effects of polyethylene terephthalate microplastic on germination, biochemistry and phytotoxicity of Cicer arietinum L. and cytotoxicity study on Allium cepa L. Environ. Toxicol. Pharmacol. 2022, 94, 103908. [Google Scholar] [CrossRef]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M.A. The potential of microplastics as carriers of metals. Environ. Pollut. 2020, 255, 113363. [Google Scholar] [CrossRef]
- Jia, L.; Liu, L.; Zhang, Y.; Fu, W.; Liu, X.; Wang, Q.; Tanveer, M.; Huang, L. Microplastic stress in plants: Effects on plant growth and their remediations. Front. Plant Sci. 2023, 14, 1226484. [Google Scholar] [CrossRef]
- Schloter, M.; Dilly, O.; Munch, J.C. Indicators for evaluating soil quality. Agric. Ecosyst. Environ. 2003, 98, 255–262. [Google Scholar] [CrossRef]
- Xue, D.; Yao, H.Y.; Ge, D.Y.; Huang, C.Y. Soil microbial community structure in diverse land use systems: A comparative study using Biolog, DGGE, and PLFA analyses. Pedosphere 2008, 18, 653–663. [Google Scholar] [CrossRef]
- Ekelund, F.; Olsson, S.; Johansen, A. Changes in succession and diversity of protozoan and microbial populations in soil spiked with a renge of copper concentrations. Soil Biol. Biochem. 2003, 35, 1507–1516. [Google Scholar] [CrossRef]
- Turpeinen, R.; Kairesalo, T.; Häggblom, M.M. Microbial community structure and activity in arsenic-, chromium- and cop-per-contaminated soils. FEMS Microbiol. Ecol. 2024, 47, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Wilke, B.M.; Mai, M.; Gattinger, A.; Schloter, M.; Gong, P. Effects of fresh and aged copper contaminations on soil microorganisms. J. Plant Nutr. Soil Sci. 2005, 168, 668–675. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Martín, A.; Arias-Estévez, M.; Bååth, E.; Díaz-Raviña, M. Microbial community structure of vineyard soils with different pH and copper content. Appl. Soil Ecol. 2010, 46, 276–282. [Google Scholar] [CrossRef]
- Wiedner, K.; Polifka, S. Effects of microplastic and microglass particles on soil microbial community structure in an arable soil (Chernozem). Soil 2020, 6, 315–324. [Google Scholar] [CrossRef]
- Gao, B.; Yao, H.; Li, Y.; Zhu, Y. Microplastic addition alters the microbial community structure and stimulates soil carbon dioxide emissions in vegetable-growing soil. Environ. Toxicol. 2020, 40, 352–365. [Google Scholar] [CrossRef]
- Schöpfer, L.; Schnepf, U.; Marhan, S.; Brümmer, F.; Kandeler, E.; Pagel, H. Hydrolyzable microplastics in soil—Low biodegradation but formation of a specific microbial habitat? Biol. Fertil. Soils 2022, 58, 471–486. [Google Scholar] [CrossRef]
- Zang, H.; Zhou, J.; Marshall, M.R.; Chadwick, D.R.; Wen, Y.; Jones, D.L. Microplastics in the agroecosystem: Are they an emerging threat to the plant-soil system? Soil Biol. Biochem. 2020, 148, 107926. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, W.; Jia, J.; Kong, P.; Tong, X.; Lu, Y.; Xie, L.; Ma, F.; Giesy, J.P. Effects of pig manure containing copper and zinc on microbial community assessed via phospholipid in soils. Environ. Monit. Asses. 2014, 186, 5297–5306. [Google Scholar] [CrossRef]
- Ranjard, L.; Echairi, A.; Nowak, V.; Lejon, D.P.; Nouaïm, R.; Chaussod, R. Field and microcosm experiments to evaluate the effects of agricultural Cu treatment on the density and genetic structure of microbial communities in two different soils. FEMS Microbiol. Ecol. 2006, 58, 303–315. [Google Scholar] [CrossRef]
- Bååth, E.; Díaz-Raviña, M.; Frostegård, A.; Campbell, C. Effect of metal-rich sludge amendments on the soil microbial com-munity. Appl. Environ. Microb. 1998, 64, 238–245. [Google Scholar] [CrossRef]
- Blöcker, L.; Watson, C.; Wichern, F. Living in the plastic age-different short-term microbial response to microplastics addition to arable soils with contrasting soil organic matter content and farm management legacy. Environ. Pollut. 2020, 267, 115468. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Ma, Y.; Yu, H.; Lv, B.; Tan, W. Effects of microplastics on soil microbial necromass carbon and plant residual carbon. Appl. Soil Ecol. 2025, 210, 106097. [Google Scholar] [CrossRef]
- Krueger, M.C.; Harms, H.; Schlosser, D. Prospects for microbiological solutions to environmental pollution with plastics. Appl. Microbiol. Biotechnol. 2015, 99, 8857–8874. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.L.; Huerta, L.E.; Eldridge, S.M.; Johnston, P.; Hu, H.W.; Geissen, V.; Chen, D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef]
- Kumar Sen, S.; Raut, S. Microbial degradation of low density polyethylene (LDPE): A review. J. Environ. Chem. Eng. 2015, 3, 462–473. [Google Scholar] [CrossRef]
- de Vries, F.T.; Hofland, E.; van Eekeren, N.; Brussaard, L.; Bloem, J. Fungal/bacterial ratios in grasslands with contrasting nitrogen management. Soil Biol. Biochem. 2006, 38, 2092–2103. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, L.; Zhang, S.; Zhou, M.; Huang, W.; Zou, X.; He, Z.; Shu, L. Soil protists are more resilient to the combined effect of microplastics and heavy metals than bacterial communities. Sci. Total Environ. 2024, 906, 167645. [Google Scholar] [CrossRef]

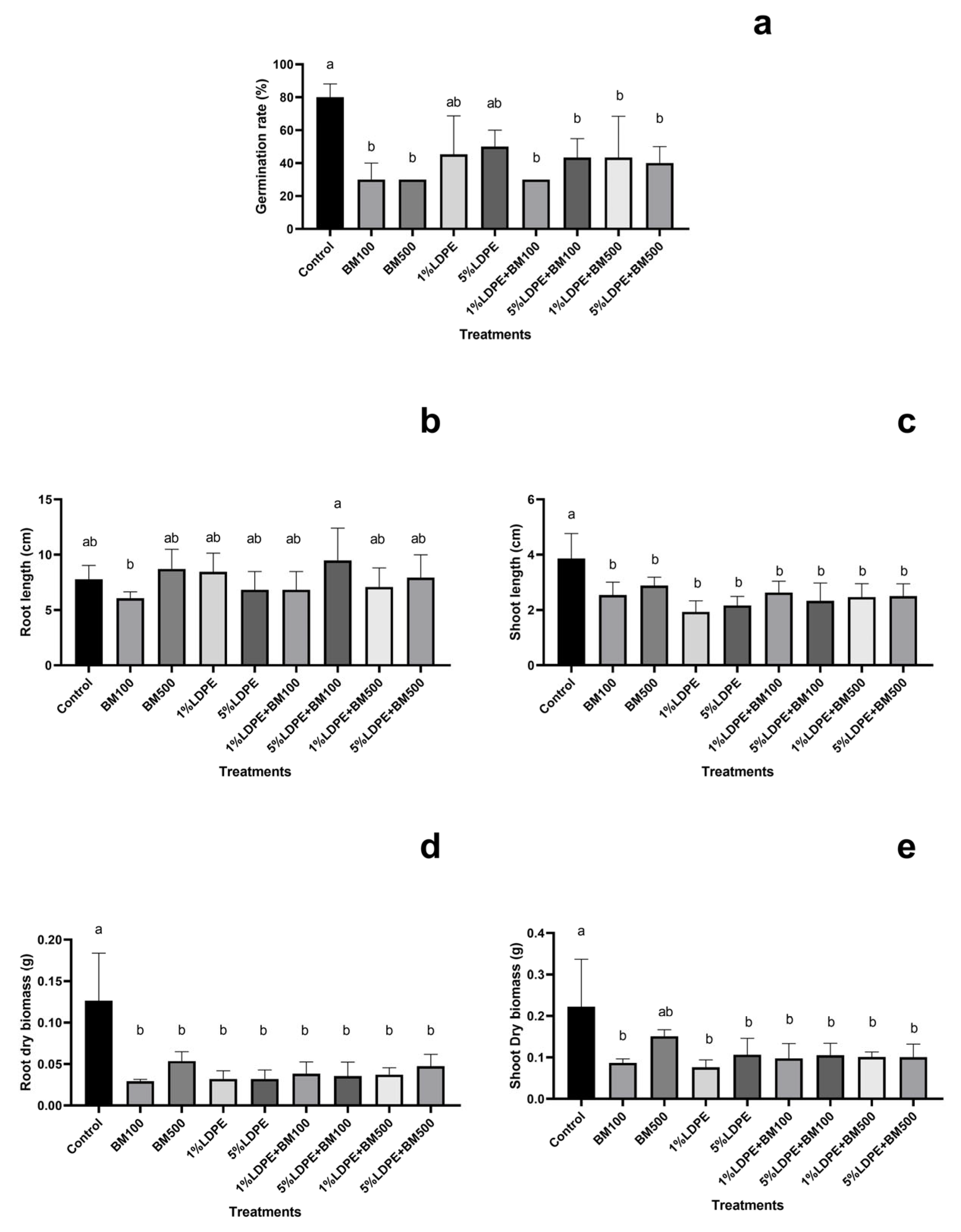
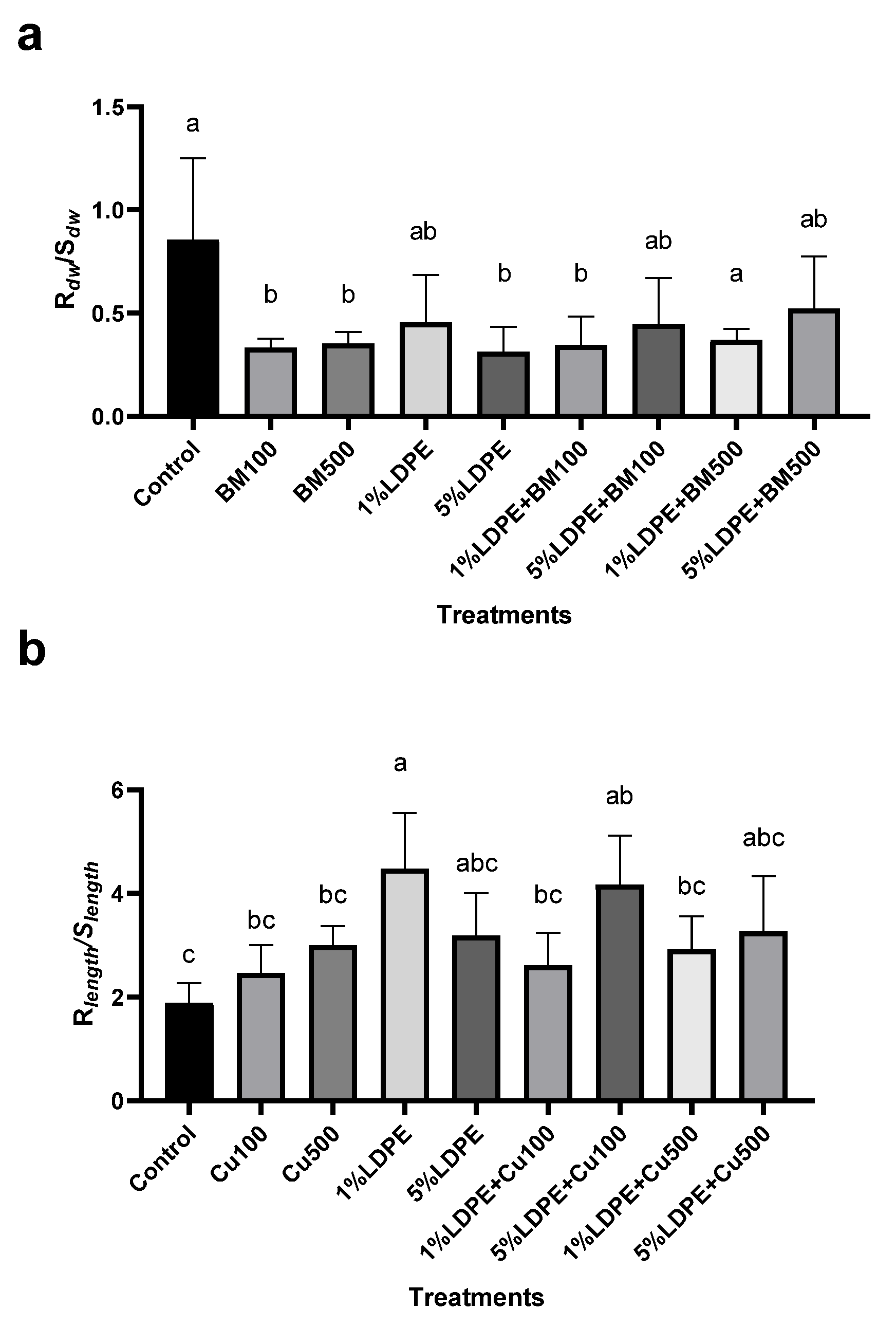
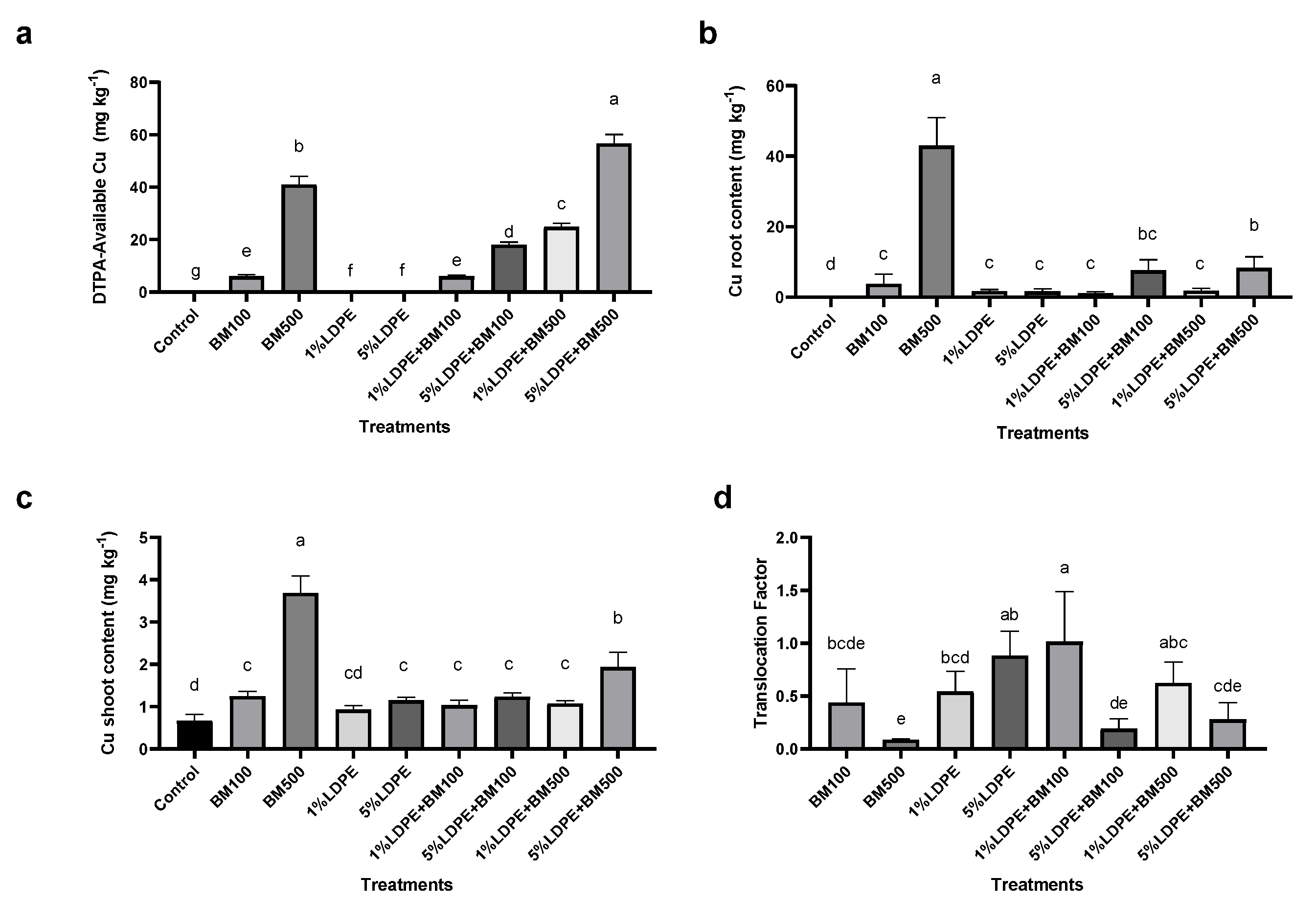

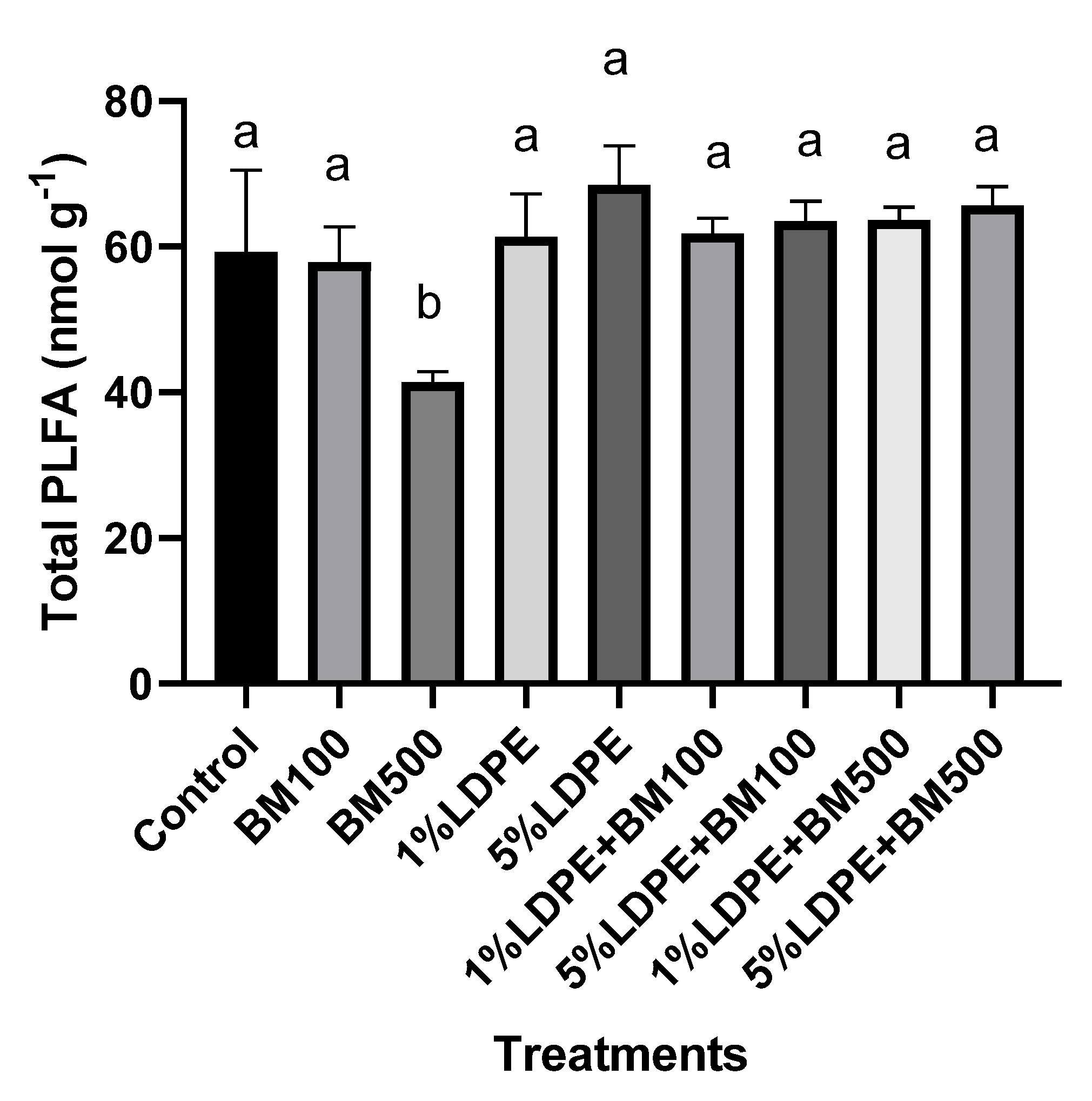

| Treatment | Cu(II) [mg kg−1] | Polyethylene [% v/v] |
|---|---|---|
| Control | 0 | 0 |
| BM100 | 17 mg kg−1 | 0 |
| BM500 | 80 mg kg−1 | 0 |
| 1%LDPE | 0 | 1% |
| 5%LDPE | 0 | 5% |
| 1%LDPE + BM100 | 17 mg kg−1 | 1% |
| 5%LDPE + BM100 | 17 mg kg−1 | 5% |
| 1%LDPE + BM500 | 80 mg kg−1 | 1% |
| 5%LDPE + BM500 | 80 mg kg−1 | 5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeo-Río, S.; Foguieng, H.M.; Gómez-Armesto, A.; Conde-Cid, M.; Fernández-Calviño, D.; Rodríguez-Seijo, A. Combined Effects of Polyethylene and Bordeaux Mixture on the Soil–Plant System: Phytotoxicity, Copper Accumulation and Changes in Microbial Abundance. Agriculture 2025, 15, 1657. https://doi.org/10.3390/agriculture15151657
Romeo-Río S, Foguieng HM, Gómez-Armesto A, Conde-Cid M, Fernández-Calviño D, Rodríguez-Seijo A. Combined Effects of Polyethylene and Bordeaux Mixture on the Soil–Plant System: Phytotoxicity, Copper Accumulation and Changes in Microbial Abundance. Agriculture. 2025; 15(15):1657. https://doi.org/10.3390/agriculture15151657
Chicago/Turabian StyleRomeo-Río, Silvia, Huguette Meta Foguieng, Antía Gómez-Armesto, Manuel Conde-Cid, David Fernández-Calviño, and Andrés Rodríguez-Seijo. 2025. "Combined Effects of Polyethylene and Bordeaux Mixture on the Soil–Plant System: Phytotoxicity, Copper Accumulation and Changes in Microbial Abundance" Agriculture 15, no. 15: 1657. https://doi.org/10.3390/agriculture15151657
APA StyleRomeo-Río, S., Foguieng, H. M., Gómez-Armesto, A., Conde-Cid, M., Fernández-Calviño, D., & Rodríguez-Seijo, A. (2025). Combined Effects of Polyethylene and Bordeaux Mixture on the Soil–Plant System: Phytotoxicity, Copper Accumulation and Changes in Microbial Abundance. Agriculture, 15(15), 1657. https://doi.org/10.3390/agriculture15151657










