Bacterial Abundance, Fermentation Pattern, and Chemical Composition of Oat Haylage Are Altered by the Forage Dehydration Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Material and Experimental Design
2.3. Dehydration Rate
2.4. Scanning Electron Microscopy
2.5. Microbiology
2.6. Organic Acid Analysis
2.7. Losses
2.8. Aerobic Stability Analysis
2.9. Chemical Analysis
2.10. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zamarchi, G.; Pavinato, P.S.; Menezes, L.F.G.; Martin, T.N. Silagem de aveia branca em função da adubação nitrogenada e pré-murchamento. Semin. Cienc. Agrar. 2014, 35, 2185–2196. [Google Scholar] [CrossRef]
- Soundharrajan, I.; Kim, D.H.; Srisesharam, S.; Kuppusamy, P.; Park, H.S.; Yoon, Y.H.; Kim, W.H.; Song, Y.G.; Choi, K.C. Application of customised bacterial inoculants for grass haylage production and its effectiveness on nutrient composition and fermentation quality of haylage. Biotech 2017, 7, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Keshri, J.; Chen, Y.; Pinto, R.; Kroupitski, Y.; Weinberg, Z.G.; Sela, S. Microbiome dynamics during ensiling of corn with and without Lactobacillus plantarum inoculant. Appl. Microbiol. Biotechnol. 2018, 102, 4025–4037. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, W.G.; Jungbeck, M.; Pereira, S.N.; Silveira, A.M.; Schons, C.L.; Tonin, T.J.; Skonieski, F.R.; Viegas, J. Evaluation of Winter Cereal Silages Subjected to Pre-Drying at Different Phenological Stages with and without the Use of Additives. J. Agric. Sci. Technol. 2022, 24, 337–350. [Google Scholar]
- Nascimento, M.C.O.; Souza, B.B.; Silva, F.V.; Melo, T.S. Armazenamento de forragem para caprinos e ovinos no semiárido do nordeste. Agropec. Cien. Semiárido 2013, 9, 20–27. [Google Scholar] [CrossRef]
- Ribas, W.F.G.; Monção, F.P.; Rocha, V.R.; Maranhão, C.M.D.A.; Ferreira, H.C.; Santos, A.S.D.; Gomes, V.M.; Rigueira, J.P.S. Effect of wilting time and enzymatic-bacterial inoculant on the fermentative profile, aerobic stability, and nutritional value of BRS capiaçu grass silage. Rev. Bras. Zootec. 2021, 50, e20200207. [Google Scholar] [CrossRef]
- Carneiro, M.K.; Neumann, M.; Junior, J.C.H.; Horst, E.H.; Leão, G.F.M.; Galbeiro, S.; Poczynek, M. Mechanical and chemical dehydration for pre- drying of black oat silage. Semin. Cienc. Agrar. 2017, 38, 981–995. [Google Scholar] [CrossRef]
- Bueno, A.V.I.; Jacovaci, F.A.; Ribeiro, M.G.; Jobim, C.C.; Daniel, J.L.P.; Tres, T.T.; Rossi, R.M. Chemical composition, aerobic stability, and fermentation pattern of white oat silage wilted with glyphosate. Semin. Cienc. Agrar. 2020, 41, 971–984. [Google Scholar] [CrossRef]
- Guo, X.S.; Ke, W.C.; Ding, W.R.; Ding, L.M.; Xu, D.M.; Wang, W.W.; Zhang, P.; Yang, F.Y. Profiling of metabolome and bacterial community dynamics in ensiled Medicago sativa inoculated without or with Lactobacillus plantarum or Lactobacillus buchneri. Sci. Rep. 2018, 8, 357. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Gou, W.; Cheng, Q.; Bai, S.; Cai, Y. Silage fermentation and bacterial community of bur clover, annual ryegrass and their mixtures prepared with microbial inoculant and chemical additive. Anim. Feed. Sci. Technol. 2019, 247, 285–293. [Google Scholar] [CrossRef]
- Gobbi, K.F.; Garcia, R.; Ventrella, M.C.; Neto, A.F.G.; Rocha, G.C. Specific leaf area and quantitative leaf anatomy of signalgrass and forage peanut submitted to shading. Rev. Bras. Zootec. 2011, 40, 1436–1444. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, P.Y. Conservative fragments in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA amplicons in metagenomic studies. PLoS ONE 2009, 4, e7401. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, L.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 18 October 2023).
- Cock, P.J.; Antão, T.; Chang, J.T.; Chapman, B.A.; Cox, C.J.; Dalke, A.; Friedberg, I.; Hamelryck, T.; Kauff, F.; Wilczynski, B.; et al. Biopython: Freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 2009, 25, 1422–1423. [Google Scholar] [CrossRef]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: Paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef]
- Smyth, R.P.; Schlub, T.E.; Grimm, A.; Venturi, V.; Chopra, A.; Mallal, S.; Davemport, M.P.; Mak, J. Reduzindo a formação de quimeras durante a amplificação por PCR para garantir uma genotipagem precisa. Gene 2010, 469, 45–51. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Pryce, J.D.A. Modification of the Barker-Summerson Method for the Determination of Lactic Acid. Analyst 1969, 94, 1151–1152. [Google Scholar] [CrossRef]
- Del Valle, T.A.; Zenatti, T.F.; Antonio, G.; Campana, M.; Gandra, J.R.; Zilio, E.M.C.; Mattos, L.F.A.; Morais, J.G.P. Effect of chitosan on the preservation quality of sugarcane silage. Grass Forage Sci. 2018, 73, 630–638. [Google Scholar] [CrossRef]
- Jobim, C.C.; Nussio, L.G.; Reis, R.A.; Schmidt, P. Avanços metodológicos na avaliação da qualidade da forragem conservada. Rev. Bras. Zootec. 2007, 36, 101–119. [Google Scholar] [CrossRef]
- Taylor, C.C.; Kung Jr., L. The effect of Lactobacillus buchneri 40788 on the fermentation and aerobic stability of high moisture corn in laboratory silos. J. Dairy Sci. 2002, 85, 1526–1532. [Google Scholar] [CrossRef]
- Cherney, J.H.; Cherney, D.J.R. Assessing Silage Quality. In Silage Science and Technology; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 2003; Volume 1, pp. 141–198. [Google Scholar]
- O’kiely, P.; Moloney, A.; Keatin, T.; Shiels, P. Maximing Output of Beef Within Cost Efficient, Environmentally Compatible Forage Conservation Systems; Teagasc: Carlow, Ireland, 1999. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Hongyu, K.; Sandanielo, V.L.M.; Oliveira Junior, G.J. Principal Component Analysis: Theory, interpretations and applications. Eng. Sci. 2015, 1, 83–90. [Google Scholar] [CrossRef]
- Pes, L.Z.; Arenhardt, M.H. Fisiologia Vegetal, 1st. ed.; Universidade Federal de Santa Maria: Santa Maria, Brazil, 2015; pp. 1–81. [Google Scholar]
- Fracchia, L.; Banat, J.J.; Cavallo, M.; Ceresa, C.; Banat, I.M. Potential therapeutic applications of microbial surface-active compounds. AIMS Bioeng. 2015, 2, 144–162. [Google Scholar] [CrossRef]
- Zobiole, L.H.S.; Oliveira, R.S., Jr.; Kremer, R.J.; Constantin, J.; Bonato, C.M.; Muniz, A.S. Water use efficiency and photosynthesis of glyphosate-resistant soybean as affected by glyphosate. Pestic. Biochem. Physiol. 2010, 97, 182–193. [Google Scholar] [CrossRef]
- Nguyen, M.X.; Moon, S.; Jung, K.H. Genome-wide expression analysis of rice aquaporin genes and development of a functional gene network mediated by aquaporin expression in roots. Planta 2013, 238, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E. Fisiologia Vegetal, 3rd. ed.; Editora Artmed: Porto Alegre, Brazil, 2004; pp. 1–618. [Google Scholar]
- Zi, X.; Li, M.; Chen, Y.; Lv, R.; Zhou, H.; Tang, J. Effects of citric acid and Lactobacillus plantarum on silage quality and bacterial diversity of king grass silage. Front. Microbiol. 2021, 12, 631096. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.; Wu, S.; Zou, X.; Chen, X.; Ge, L.; Zhang, Q. Effects of gallic acid on fermentation parameters, protein fraction, and bacterial community of whole plant soybean silage. Front. Microbiol. 2021, 12, 662966. [Google Scholar] [CrossRef]
- Ogunade, I.M.; Jiang, Y.; Pech Cervantes, A.A.; Kim, D.H.; Oliveira, A.S.; Vyas, D.; Weinberg, Z.G.; Jeong, K.C.; Adesogan, A.T. Bacterial diversity and composition of alfalfa silage as analyzed by Illumina MiSeq sequencing: Effects of Escherichia coli. O157:H7 and silage additives. J. Dairy Sci. 2018, 101, 2048–2059. [Google Scholar] [CrossRef]
- Yue, Z.B.; Chen, R.; Yang, F.; James, M.; Terence, M.; Liu, Y.; Liao, W. Effects of dairy manure and corn stover co-digestion on anaerobic microbes and corresponding digestion performance. Bioresour. Technol. 2013, 128, 65–71. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, X.; Gu, Q.; Liang, M.; Mu, S.; Zhou, B.; Huang, F.; Lin, B.; Zou, C. Analysis of the correlation between bacteria and fungi in sugarcane tops silage prior to and after aerobic exposure. Bioresour. Technol. 2019, 291, 121835. [Google Scholar] [CrossRef]
- Graf, K.; Ulrich, A.; Idler, C.; Klocke, M. Bacterial community dynamics during ensiling of perennial ryegrass at two compaction levels monitored by terminal restriction fragment length polymorphism. J. Appl. Microbiol. 2016, 120, 1479–1491. [Google Scholar] [CrossRef]
- Guan, H.; Yan, Y.H.; Li, X.L.; Li, X.M.; Shuai, Y.; Feng, G.Y.; Ran, Q.; Cai, Y.; Li, Y.; Zhang, X. Microbial communities and natural fermentation of corn silages prepared with farm bunker-silo in Southwest China. Bioresour. Technol. 2018, 265, 282–290. [Google Scholar] [CrossRef]
- Li, M.; Lv, R.; Zhang, L.; Zi, X.; Zhou, H.; Tang, J. Melatonin is a promising silage additive: Evidence from microbiota and metabolites. Front. Microbiol. 2021, 12, 670764. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zheng, P.; Zou, X.; Chen, X.; Zhang, Q. Influence of Pyroligneous Acid on Fermentation Parameters, CO2 Production and Bacterial Communities of Rice Straw and Stylo Silage. Front. Microbiol. 2021, 8, 701434. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.K.; Zheng, M.Y.; Guo, X.; Chen, X.Y.; Zhang, Q. Altering bacterial community: A possible way of lactic acid bacteria inoculants reducing CO2 production and nutrient loss during fermentation. Bioresour. Technol. 2021, 329, 124915. [Google Scholar] [CrossRef] [PubMed]
- Bian, B.; Bajracharya, S.; Xu, J.J.; Pant, D. Microbial electrosynthesis from CO2: Challenges, opportunities and perspectives in the context of circular bioeconomy. Bioresour. Technol. 2020, 302, 122863. [Google Scholar] [CrossRef]
- Prückler, M.; Lorenz, C.; Endo, A.; Kraler, M.; Dürrschmid, K.; Hendriks, K.F.S.; Auterith, E.; Kneifel, W.; Michlmayr, H. Comparison of homo- and heterofermentative lactic acid bactéria for implementation of fermented wheat bran in bread. Food Microbiol. 2015, 49, 211–219. [Google Scholar] [CrossRef]
- Muck, R. Silage microbiology and its control through additives. Rev. Bras. Zootec. 2010, 39, 183–191. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Muck, R.E.; Nadeau, E.M.G.; Mcallister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung Junior, L. Silage review: Recent Advances and Future Uses of Silage Additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Xu, X.; Williams, T.C.; Divne, C.; Pretorius, I.S.; Paulsen, I.T. Evolutionary engineering in Saccharomyces cerevisiae reveals a TRK1-dependent potassium influx mechanism for propionic acid tolerance. Biotechnol. Biofuels 2019, 12, 97. [Google Scholar] [CrossRef]
- Diepersloot, E.C.; Pupo, M.R.; Ghizzi, L.G.; Gusmão, J.O.; Heinzen, C.; McCary, C.L.; Wallau, M.O.; Ferraretto, L.F. Effects of Microbial Inoculation and Storage Length on Fermentation Profile and Nutrient Composition of Whole-Plant Sorghum Silage of Different Varieties. Front. Microbiol. 2021, 12, 660567. [Google Scholar] [CrossRef]
- Sun, L.; Bai, C.; Xu, H.; Na, N.; Jiang, Y.; Yin, G.; Liu, S.; Xue, Y. Succession of bacterial community during the initial aerobic, intense fermentation, and stable phases of whole-plant corn silages treated with lactic acid bacteria suspensions prepared from other silages. Front. Microbiol. 2021, 12, 655095. [Google Scholar] [CrossRef]
- Ding, Z.; Bai, J.; Xu, D.; Li, F.; Zhang, Y.; Guo, X. Microbial community dynamics and natural fermentation profiles of ensiled alpine grass Elymus nutans prepared from different regions of the Qinghai-Tibetan plateau. Front. Microbiol. 2020, 11, 855. [Google Scholar] [CrossRef]
- Simionatto, M.; Maeda, E.M.; Fluck, A.C.; Silveira, A.P.; Piran Filho, F.A.; Paula, F.L.M.; Costa, O.A.D.; Mayer, L.R.R.; Paulo Macedo, V. Nutritional and morphostructural characterization of pre-dried winter grass silage. Semin. Cienc. Agrar. 2019, 40, 2375–2386. [Google Scholar] [CrossRef]
- Kir, H. Yield and quality traits of some silage maize cultivars. Fresenius Environ. Bull. 2020, 20, 2843–2849. [Google Scholar]
- Garcez, B.S.; Alves, A.A.; Araújo, D.L.C.; Lacerda, M.D.S.B.; Souza, L.G.C.; Carvalho, L.F. Degradabilidade ruminal do capim colonião (Panicum maximum jacq. cv. colonião) em três idades pós-rebrota. Acta Vet. Bras. 2016, 10, 130–134. [Google Scholar] [CrossRef]
- Fasolo, D.J.; Carvalho, A.F.G. Uso de Diferentes Inoculantes Bacterianos Isolados e em Associação para Silagem de Milho. Rev. Técnico-Cient. 2021, 27, 1–20. [Google Scholar]
- Gayer, T.O.; Kasper, N.F.; Tadielo, L.E.; Krolow, R.H.; Azevedo, E.B.; Oaigen, R.P.; Castagnara, D.D. Different dry matters content used for the conservation of annual ryegrass (Lolium multiflorum Lam.) in anaerobic environment. Afr. J. Agric. Res. 2019, 14, 369–378. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage review: Factors affecting dry matter and quality losses in silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.L.; Ribeiro, K.G.; Marcondes, M.I.; Pereira, O.G.; Weiβ, K. Chemical composition and production of ethanol and other volatile organic compounds in sugarcane silage treated with chemical and microbial additives. Anim. Prod. Sci. 2019, 59, 721–728. [Google Scholar] [CrossRef]
- Ren, H.; Wang, C.; Fan, W.; Zhang, B.; Li, Z.; Li, D. Effects of formic or acetic acid on the storage quality of mixed air-dried corn stover and cabbage waste, and microbial community analysis. Food Technol. Biotechnol. 2018, 56, 71–82. [Google Scholar] [CrossRef]
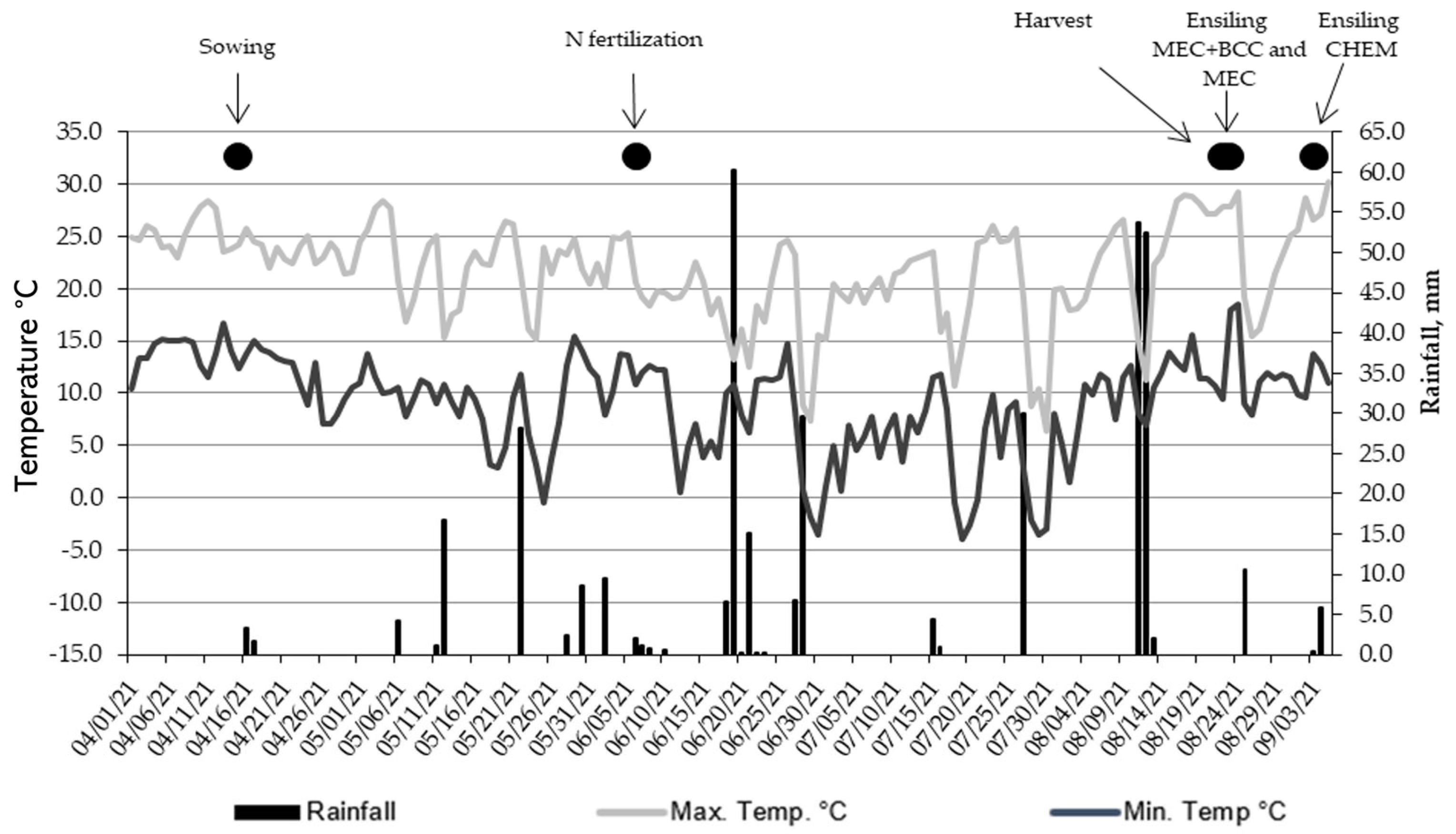
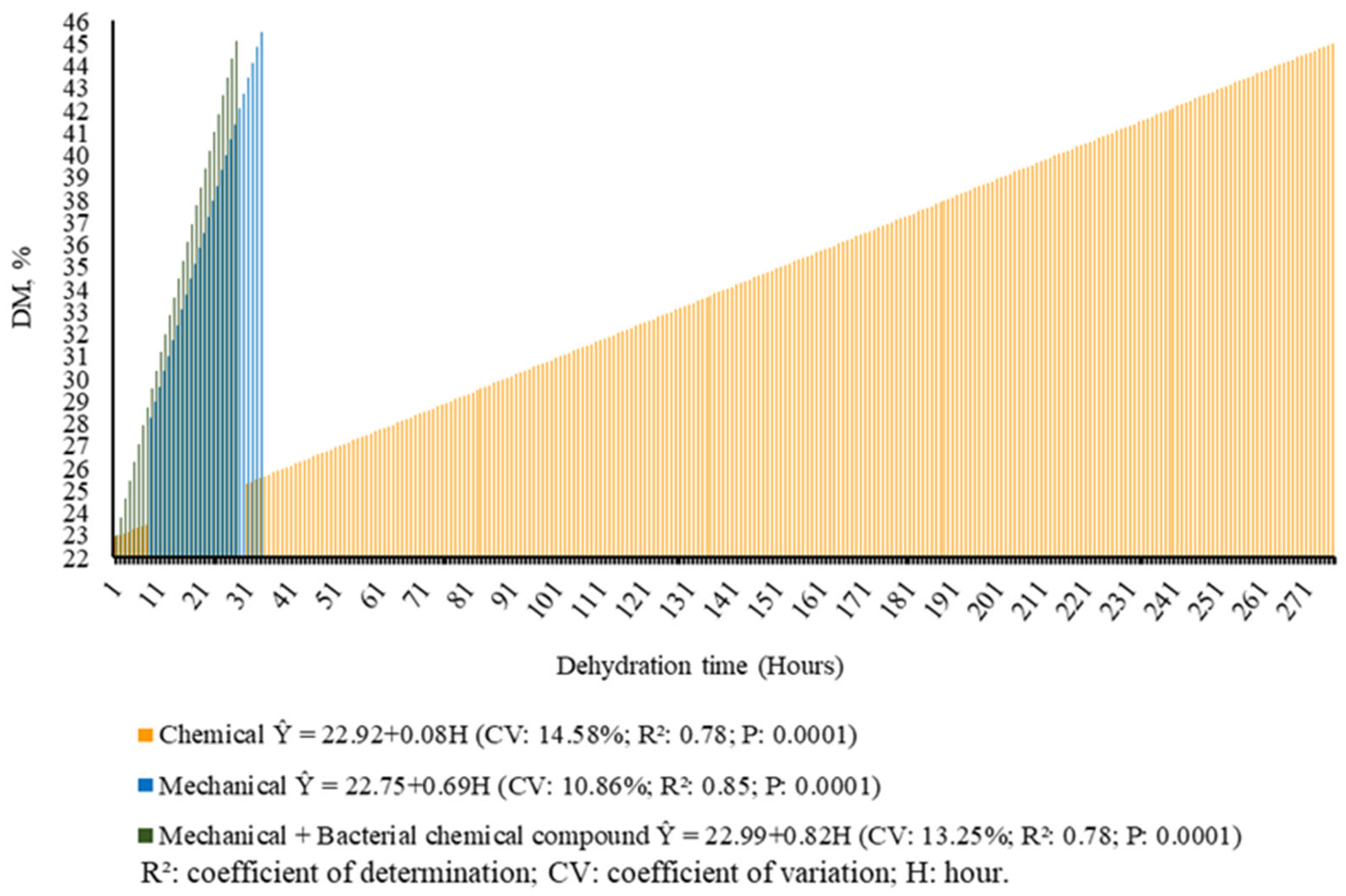


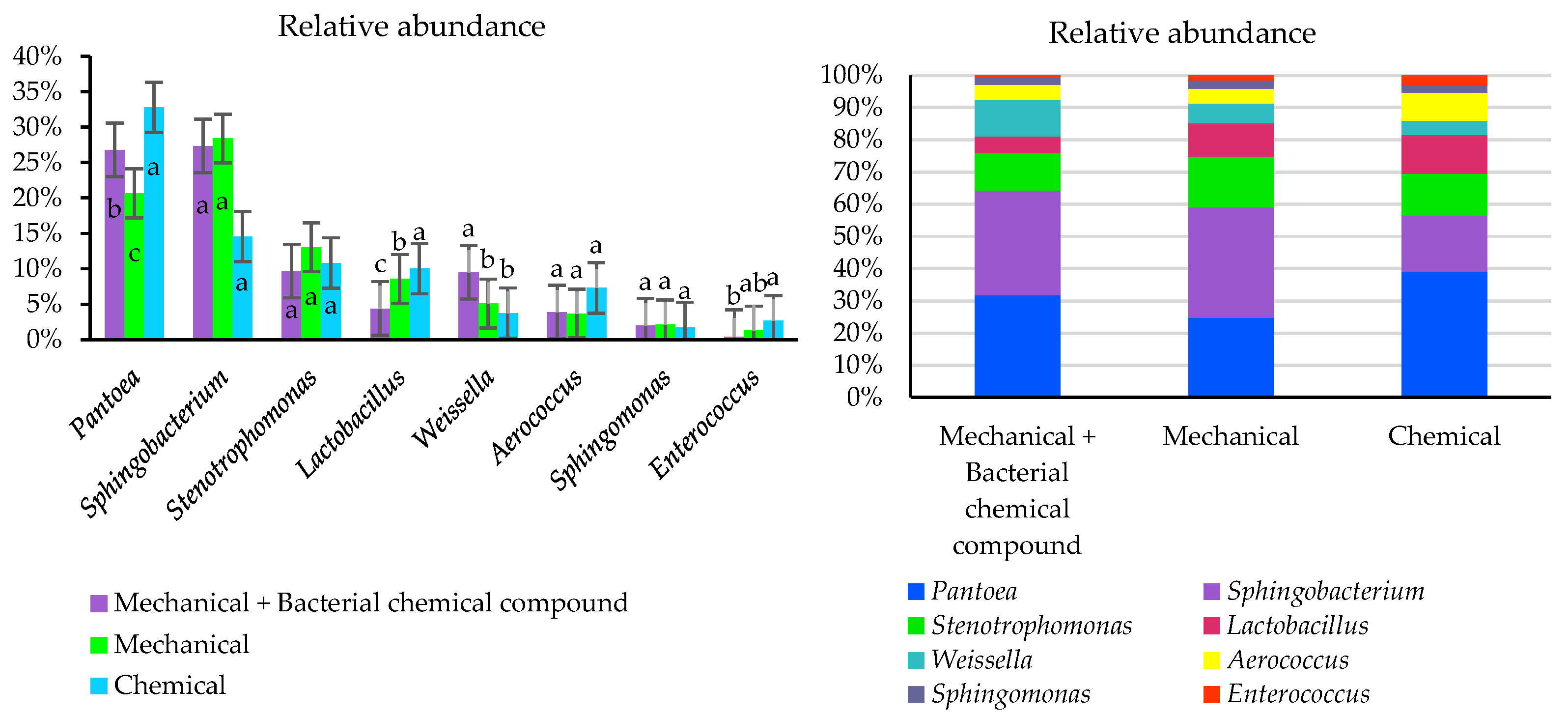

| Population, plants ha−1 | 1,930,000 |
| Green forage mass production, kg ha−1 | 20,707 |
| Dry forage mass production, kg ha−1 | 4448 |
| DM (%) | 21.48 |
| L:S ratio (%) | 74.82 |
| Parameters | Dehydration Method | ||||
|---|---|---|---|---|---|
| MEC | MEC + BCC | CHE | SEM | p-Value | |
| Phylum | |||||
| Richness | 4 | 4 | 4 | - | - |
| Shannon Diversity | 1.07 | 1.04 | 1.04 | 0.0122 | 0.3816 |
| Genus | |||||
| Richness | 16 | 16 | 17 | 0.2427 | 0.1278 |
| Shannon Diversity | 2.07 | 1.97 | 2.05 | 0.0258 | 0.3170 |
| Parameters | Dehydration Method | |||||
|---|---|---|---|---|---|---|
| MEC | MEC + BCC | CHE | Mean | SEM | p-Value | |
| Acetic, g kg−1 | 2.88 c | 3.36 b | 3.96 a | 3.40 | 0.1188 | 0.0001 |
| Lactic, g kg−1 | 28.54 | 31.17 | 27.26 | 28.98 | 0.8288 | 0.2309 |
| Butyric, g kg−1 | ND | ND | ND | - | - | - |
| Propionic, g kg−1 | ND | ND | 0.08 a | 0.03 | 0.0077 | 0.0001 |
| Isobutyric, g kg−1 | 0.66 a | 0.48 b | 0.78 a | 0.64 | 0.0336 | 0.0003 |
| pH | 4.91 | 4.73 | 4.62 | 4.75 | 0.0826 | 0.3899 |
| Parameters | Dehydration Methods | |||||
|---|---|---|---|---|---|---|
| MEC | MEC + BCC | CHE | Mean | SEM | p-Value | |
| AE, hours | 69.20 c | 78.20 b | 127.0 a | 91.47 | 6.8190 | 0.0001 |
| Cumulative temp., °C | ||||||
| 0 to 84 h | 12.73 a | 8.85 b | 6.90 c | 9.49 | 0.6507 | 0.0001 |
| 0 to 168 h | 25.91 a | 22.43 b | 17.52 c | 21.95 | 0.9295 | 0.0001 |
| pH | ||||||
| Initial AE | 4.91 | 4.73 | 4.62 | 4.75 | 0.0826 | 0.3899 |
| Final AE | 5.75 | 5.58 | 5.72 | 5.68 | 0.0413 | 0.2168 |
| Parameters | Dehydration Methods | |||||
|---|---|---|---|---|---|---|
| MEC | MEC + BCC | CHE | Mean | SEM | p-Value | |
| Chemical composition | ||||||
| DM (g kg−1) | 445.82 | 441.78 | 441.48 | 443.02 | 4.1065 | 0.8915 |
| MM (g kg−1) | 107.06 | 103.93 | 106.07 | 105.68 | 1.1815 | 0.5602 |
| NDF (g kg−1) | 521.04 b | 516.36 b | 585.81 a | 541.07 | 2.8639 | 0.0001 |
| ADF (g kg−1) | 328.83 a | 304.64 b | 339.22 a | 324.23 | 2.3691 | 0.0002 |
| ADL (g kg−1) | 39.88 a | 31.97 b | 42.25 a | 38.03 | 0.6520 | 0.0001 |
| HEM (g kg−1) | 192.21 b | 211.72 b | 246.59 a | 216.84 | 4.3223 | 0.0008 |
| CEL (g kg−1) | 288.96 a | 272.66 b | 296.96 a | 286.20 | 2.4754 | 0.0054 |
| CP (g kg−1) | 140.41 a | 145.33 a | 117.64 b | 134.46 | 1.4894 | 0.0001 |
| NDN (g kg−1) | 28.56 | 28.27 | 29.00 | 28.61 | 0.4469 | 0.8012 |
| ADN (g kg−1) | 8.13 b | 7.54 c | 10.68 a | 8.78 | 0.0682 | 0.0001 |
| EE (g kg−1) | 40.40 b | 45.29 a | 34.10 c | 30.93 | 0.4433 | 0.0001 |
| NFC (g kg−1) | 318.12 a | 322.38 a | 278.23 b | 306.24 | 2.7279 | 0.0001 |
| NH3-N (% TN) | 0.16 b | 0.18 b | 0.23 a | 0.19 | 0.0051 | 0.0008 |
| Fermentation losses | ||||||
| DM losses (%) | 5.33 b | 3.04 c | 18.18 a | 8.85 | 1.7842 | 0.0001 |
| Gas losses (%) | 2.09 a | 1.37 b | 2.20 a | 1.88 | 0.1007 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, A.M.; Neumann, M.; Prado Calixto, O.P.; Gonçalves de Oliveira Júnior, A.; Baldissera, E.; Soethe Mokochinski, N.; Alessi Ienke, L.; Harry Bumbieris Junior, V. Bacterial Abundance, Fermentation Pattern, and Chemical Composition of Oat Haylage Are Altered by the Forage Dehydration Method. Agriculture 2025, 15, 2056. https://doi.org/10.3390/agriculture15192056
de Souza AM, Neumann M, Prado Calixto OP, Gonçalves de Oliveira Júnior A, Baldissera E, Soethe Mokochinski N, Alessi Ienke L, Harry Bumbieris Junior V. Bacterial Abundance, Fermentation Pattern, and Chemical Composition of Oat Haylage Are Altered by the Forage Dehydration Method. Agriculture. 2025; 15(19):2056. https://doi.org/10.3390/agriculture15192056
Chicago/Turabian Stylede Souza, André Martins, Mikael Neumann, Odimari Pricila Prado Calixto, Admilton Gonçalves de Oliveira Júnior, Ellen Baldissera, Nicolli Soethe Mokochinski, Livia Alessi Ienke, and Valter Harry Bumbieris Junior. 2025. "Bacterial Abundance, Fermentation Pattern, and Chemical Composition of Oat Haylage Are Altered by the Forage Dehydration Method" Agriculture 15, no. 19: 2056. https://doi.org/10.3390/agriculture15192056
APA Stylede Souza, A. M., Neumann, M., Prado Calixto, O. P., Gonçalves de Oliveira Júnior, A., Baldissera, E., Soethe Mokochinski, N., Alessi Ienke, L., & Harry Bumbieris Junior, V. (2025). Bacterial Abundance, Fermentation Pattern, and Chemical Composition of Oat Haylage Are Altered by the Forage Dehydration Method. Agriculture, 15(19), 2056. https://doi.org/10.3390/agriculture15192056








