Performance of Georgian Grapevine Varieties in a Vineyard Infected by Flavescence Dorée Phytoplasma in Piedmont, Northwestern Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Case-Study Vineyard
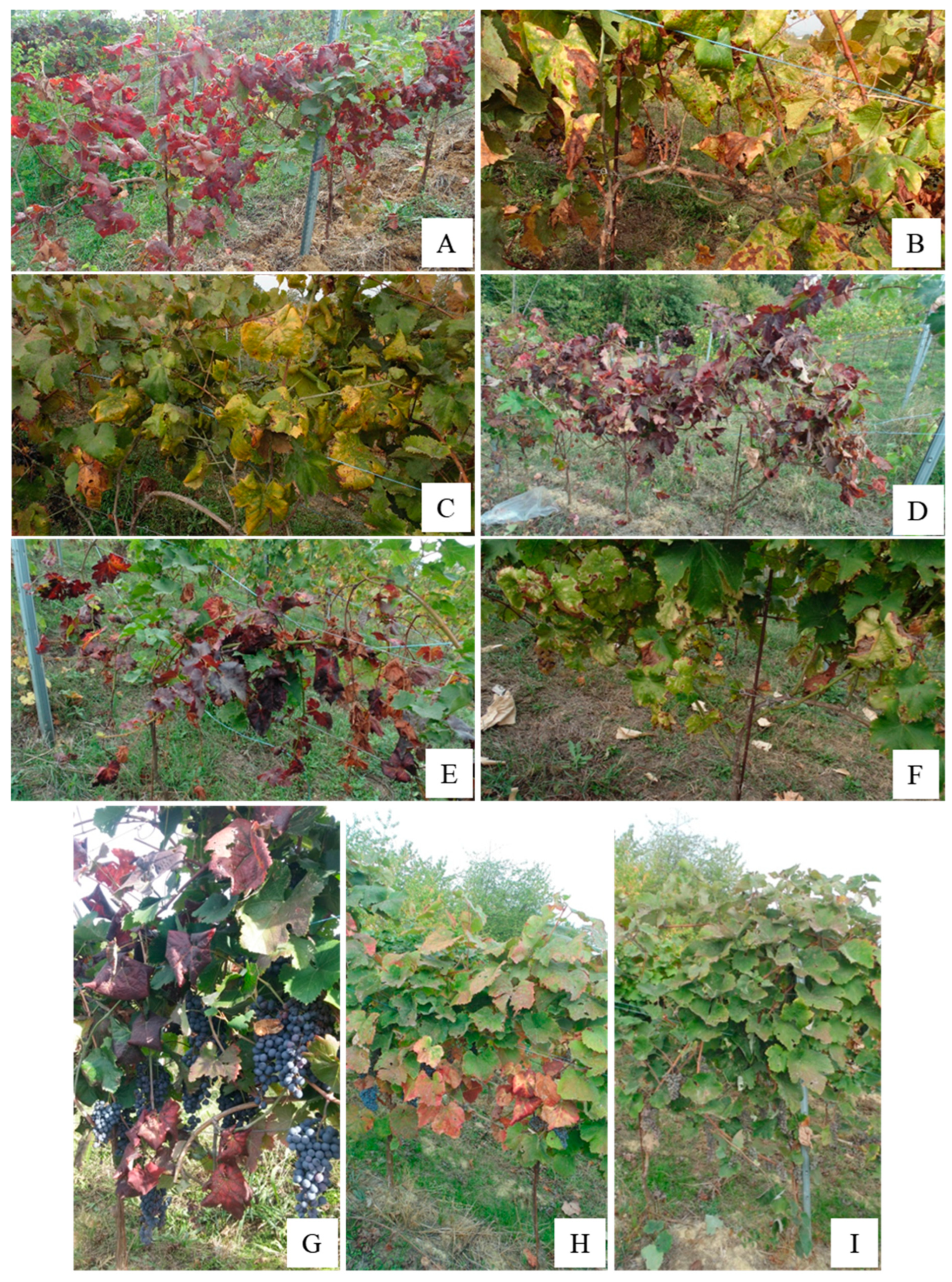
2.2. Symptom Observation and Plant Sampling
2.3. Identification and Genotyping of GY-Associated Phytoplasmas
2.4. Grape Sampling and Berry Analyses
2.5. Winemaking Protocol of Georgian Varieties
2.6. Wine Analysis
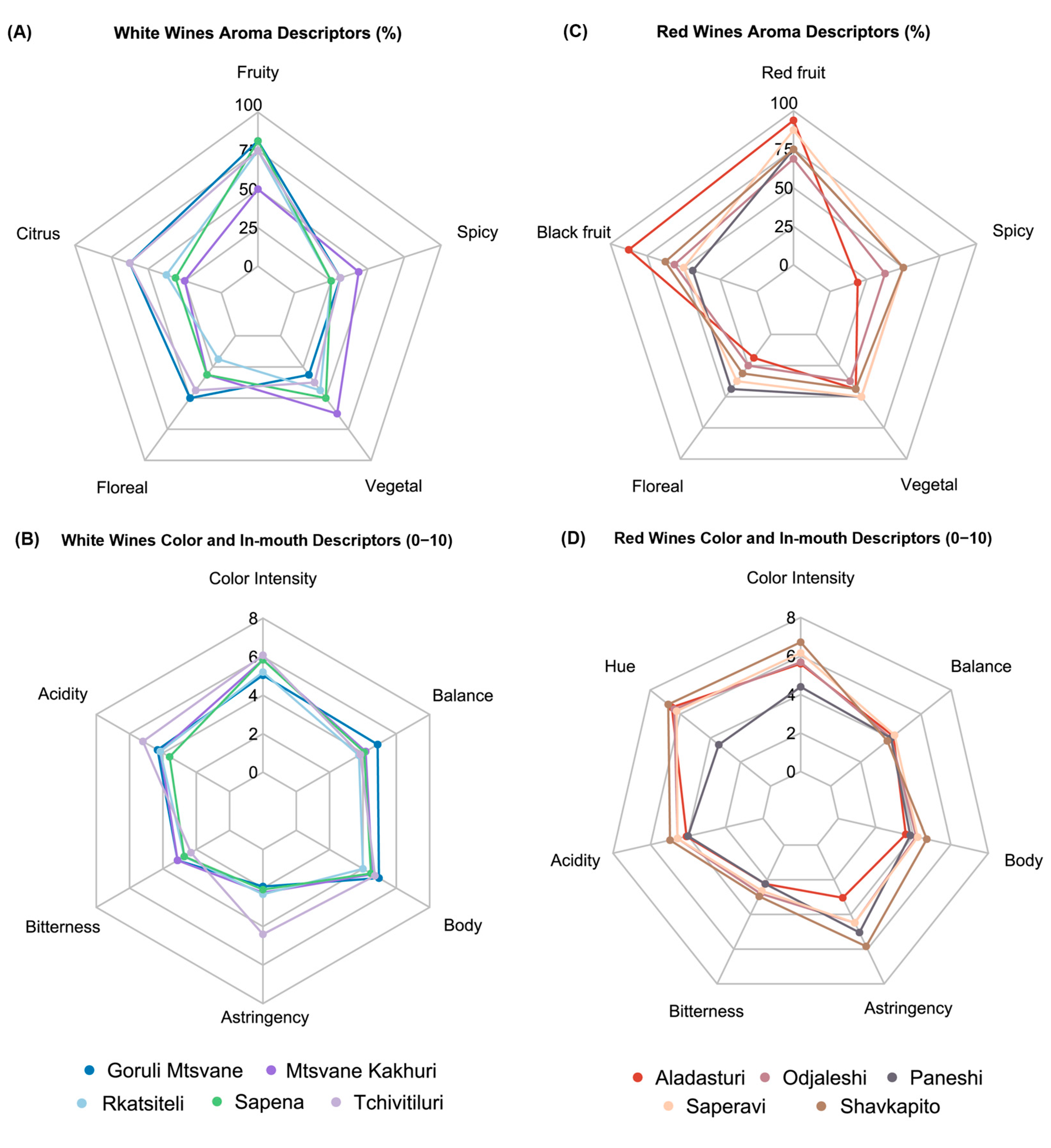
2.7. Sensory Analysis of Wines
3. Results
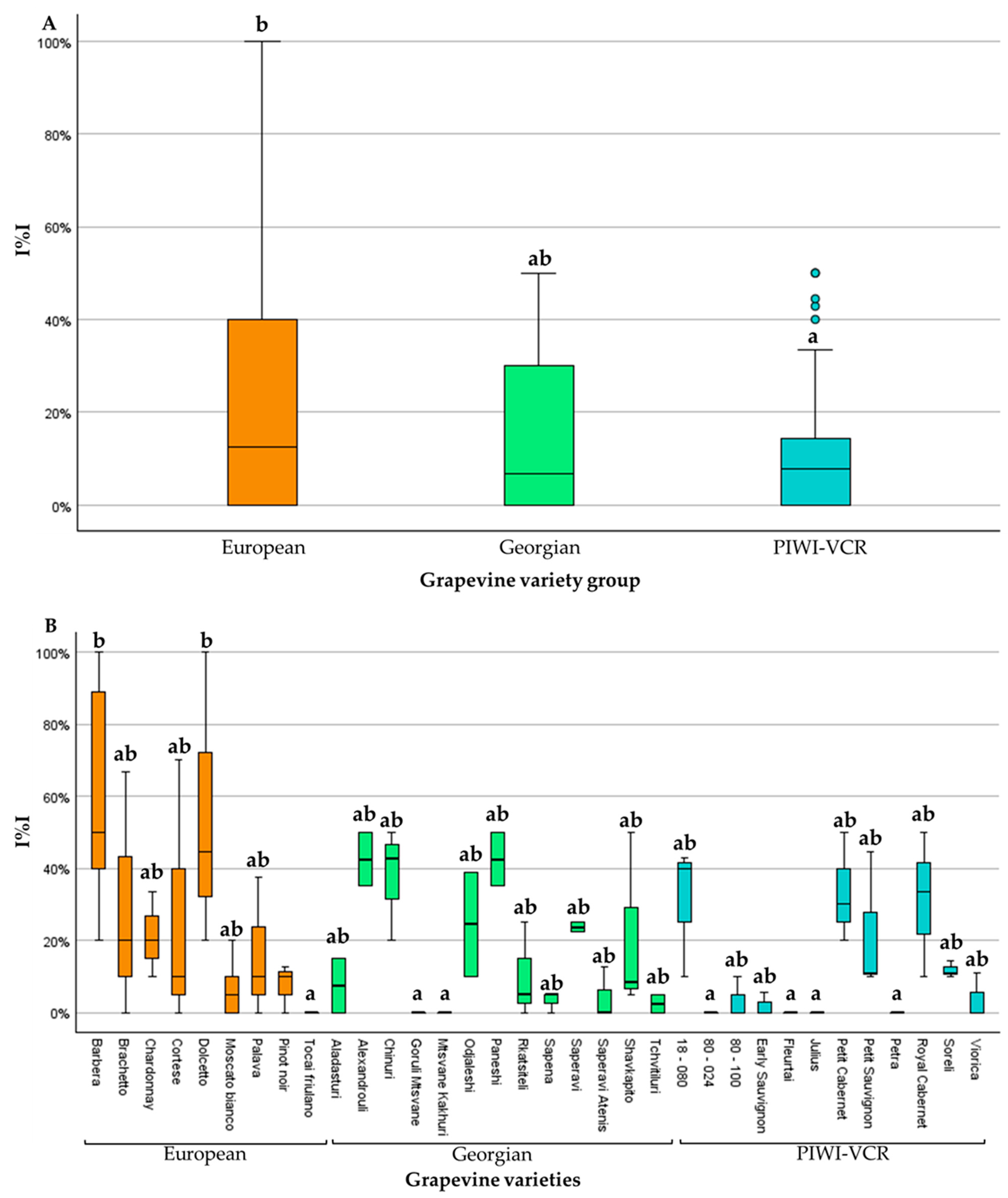
3.1. Incidence of Grapevine Yellows
3.2. Phytoplasmas Identified in Symptomatic Vines
3.3. Georgian Varieties Oenological Potential: Grapes and Wines Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bertaccini, A. Phytoplasmas: Diversity, taxonomy, and epidemiology. Front. Biosci. 2007, 12, 673–689. [Google Scholar] [CrossRef]
- Weintraub, P.G.; Beanland, L.A. Insect vectors of phytoplasmas. Ann. Rev. Entomol. 2006, 51, 91–111. [Google Scholar] [CrossRef]
- Bertaccini, A.; Arocha-Rosete, Y.; Contaldo, N.; Duduk, B.; Fiore, N.; Montano, H.G.; Kube, M.; Kuo, C.H.; Martini, M.; Oshima, K.; et al. Revision of the ‘Candidatus Phytoplasma’ species description guidelines. Int. J. Syst. Evol. Microbiol. 2022, 72, 005353. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhao, Y. Phytoplasma Taxonomy: Nomenclature, Classification, and Identification. Biology 2022, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Angelini, E.; Clair, D.; Borgo, M.; Bertaccini, A.; Boudon-Padieu, E. Flavescence dorée in France and Italy: Occurrence of closely related phytoplasma isolates and their near relationships to palatinate grapevine yellows and an alder yellows phytoplasma. Vitis 2001, 40, 79–86. [Google Scholar] [CrossRef]
- Davis, R.E.; Dally, E.L. Revised subgroup classification of group 16SrV phytoplasmas and placement of Flavescence dorée-associated phytoplasmas in two distinct subgroups. Plant Dis. 2001, 85, 790–797. [Google Scholar] [CrossRef]
- Belli, G.; Bianco, P.A.; Conti, M. Grapevine yellows: Past, present and future. J. Plant Pathol. 2010, 92, 303–326. [Google Scholar]
- Oliveira, M.J.R.A.; Castro, S.; Paltrinieri, S.; Bertaccini, A.; Sottomayor, M.; Santos, C.S.; Vasconcelos, M.W.; Carvalho, S.M.P. “Flavescence dorée” impacts growth, productivity and ultrastructure of Vitis vinifera plants in Portuguese “Vinhos Verdes” region. Sci. Hortic. 2020, 261, 108742. [Google Scholar] [CrossRef]
- Rizzoli, A.; Jelmini, L.; Pezzatti, G.B.; Jermini, M.; Schumpp, O.; Debonneville, C.; Marcolin, E.; Krebs, P.; Conedera, M. Impact of the “Flavescence Dorée” Phytoplasma on Xylem Growth and Anatomical Characteristics in Trunks of ‘Chardonnay’ Grapevines (Vitis vinifera). Biology 2022, 11, 978. [Google Scholar] [CrossRef]
- Quaglino, F.; Zhao, Y.; Casati, P.; Bulgari, D.; Bianco, P.A.; Wei, W.; Davis, R.E. ‘Candidatus Phytoplasma solani’, a novel taxon associated with stolbur- and bois noir-related diseases of plants. Int. J. Syst. Evol. Microbiol. 2013, 63, 2879–2894. [Google Scholar] [CrossRef]
- Ember, I.; Bodor, P.; Zsófi, Z.; Pálfi, Z.; Ladányi, M.; Pásti, G.; Deák, T.; Nyitrainé, D.S.; Bálo, B.; Szekeres, A.; et al. Bois noir affects the yield and wine quality of Vitis vinifera L. cv. ‘Chardonnay’. Eur. J. Plant Pathol. 2018, 152, 185–197. [Google Scholar] [CrossRef]
- Chuche, J.; Thiéry, D. Biology and ecology of the flavescence dorée vector Scaphoideus titanus: A review. Agr. Sustain. Dev. 2014, 34, 381–403. [Google Scholar] [CrossRef]
- Arnaud, G.; Malembic-Maher, S.; Salar, P.; Bonnet, P.; Maixner, M.; Marcone, C.; Boudon-Padieu, E.; Foissac, X. Multilocus sequence typing confirms the close genetic interrelatedness of three distinct Flavescence dorée phytoplasma strain clusters and group 16SrV phytoplasmas infecting grapevine and alder in Europe. Appl. Environ. Microbiol. 2007, 73, 4001–4010. [Google Scholar] [CrossRef]
- Casati, P.; Jermini, M.; Quaglino, F.; Corbani, G.; Schaerer, S.; Passera, A.; Bianco, P.A.; Rigamonti, I.E. New insights on Flavescence dorée phytoplasma ecology in the vineyard agro-ecosystem in southern Switzerland. Ann. Appl. Biol. 2017, 171, 37–51. [Google Scholar] [CrossRef]
- Plavec, J.; Budinšćak, Ž.; Križanac, I.; Škorić, D.; Foissac, X.; Šeruga Musić, M. Multilocus sequence typing reveals the presence of three distinct flavescence dorée phytoplasma genetic clusters in Croatian vineyards. Plant Pathol. 2019, 68, 18–30. [Google Scholar] [CrossRef]
- Rossi, M.; Pegoraro, M.; Ripamonti, M.; Abbà, S.; Beal, D.; Giraudo, A.; Veratti, F.; Malembic-Maher, S.; Salar, P.; Bosco, D.; et al. Genetic diversity of flavescence dorée phytoplasmas at the vineyard scale. Appl. Environ. Microbiol. 2019, 85, e03123-18. [Google Scholar] [CrossRef]
- Malembic-Maher, S.; Desqué, D.; KhaliI, D.; Salar, P.; Bergey, B.; Danet, J.-L.; Duret, S.; Dubrana-Ourabah, M.-P.; Beven, L.; Ember, I.; et al. When a Palearctic bacterium meets a Nearctic insect vector: Genetic and ecological insights into the emergence of the grapevine Flavescence dorée epidemics in Europe. PLoS Pathog. 2020, 16, e1007967. [Google Scholar] [CrossRef]
- Krstić, O.; Cvrković, T.; Marinković, S.; Jakovljević, M.; Mitrović, M.; Toševski, I.; Jović, J. Genetic diversity of flavescence dorée phytoplasmas in vineyards of Serbia: From the widespread occurrence of autochthonous Map-M51 to the emergence of endemic Map-FD2 (Vectotype II) and new Map-FD3 (Vectotype III) epidemic genotypes. Agronomy 2022, 12, 448. [Google Scholar] [CrossRef]
- Filippin, L.; Jović, J.; Cvrković, T.; Forte, V.; Clair, D.; Toševski, I.; Boudon-Padieu, E.; Borgo, M.; Angelini, E. Molecular characteristics of phytoplasmas associated with Flavescence dorée in clematis and grapevine and preliminary results on the role of Dictyophara europaea as a vector. Plant Pathol. 2009, 58, 826–837. [Google Scholar] [CrossRef]
- Strauss, G.; Reisenzein, H. First detection of Flavescence dorée phytoplasma in Phlogotettix cyclops (Hemiptera, Cicadellidae) and considerations on its possible role as vector in Austrian vineyards. Integr. Prot. Viticult IOBC-WPRS Bull. 2018, 139, 12–21. [Google Scholar]
- Belgeri, E.; Rizzoli, A.; Jermini, M.; Angelini, E.; Filippin, L.; Rigamonti, I.E. First report of Flavescence dorée phytoplasma identification and characterization in three species of leafhoppers. J. Plant Pathol. 2022, 104, 375–379. [Google Scholar] [CrossRef]
- Rigamonti, I.E.; Salvetti, M.; Girgenti, P.; Bianco, P.A.; Quaglino, F. Investigation on flavescence dorée in north-western Italy identifies map-M54 (16SrV-D/Map-FD2) as the only phytoplasma genotype in Vitis vinifera L. and reveals the presence of new putative reservoir plants. Biology 2023, 12, 1216. [Google Scholar] [CrossRef]
- Ripamonti, M.; Pegoraro, M.; Morabito, C.; Gribaudo, I.; Schubert, A.; Bosco, D.; Marzachì, C. Susceptibility to flavescence dorée of different Vitis vinifera genotypes from north-western Italy. Plant Pathol. 2021, 70, 511–520. [Google Scholar] [CrossRef]
- Laimer, M.; Lemaire, O.; Herrbach, E.; Goldschmidt, V.; Minafra, A.; Bianco, P.A.; Wetzel, T. Resistance to viruses, phytoplasmas and their vectors in the grapevine in Europe: A review. J. Plant Pathol. 2009, 91, 7–23. [Google Scholar]
- Imazio, S.; Maghradze, D.; De Lorenzis, G.; Bacilieri, R.; Laucou, V.; This, P.; Scienza, A.; Failla, O. From the cradle of grapevine domestication: Molecular overview and description of Georgian grapevine (Vitis vinifera L.) germplasm. Tree Genet. Genom. 2013, 9, 641–658. [Google Scholar] [CrossRef]
- Bacilieri, R.; Lacombe, T.; Le Cunff, L.; Di Vecchi-Staraz, M.; Lacouu, V.; Genna, B.; Péros, J.-P.; This, P.; Boursiquot, J.-M. Genetic structure in cultivated grapevines is linked to geography and human selection. BMC Plant Biol. 2013, 13, 1–12. [Google Scholar] [CrossRef]
- Chkhartishvili, N.; Maghradze, D. Viticulture and winemaking in Georgia. Vitis 2012, 51, 169–176. [Google Scholar] [CrossRef]
- Bitsadze, N.; Aznarashvili, M.A.; Vercesi, A.; Chipashvili, R.; Failla, O.; Maghradze, D. Screening of Georgian grape germplasm for susceptibility to downy mildew. Vitis 2015, 54, 193–196. [Google Scholar] [CrossRef]
- Toffolatti, S.L.; De Lorenzis, G.; Costa, A.; Maddalena, G.; Passera, A.; Bonza, M.C.; Pindo, M.; Stefani, E.; Cestaro, A.; Casati, P.; et al. Unique resistance traits against downy mildew from the center of origin of grapevine (Vitis vinifera). Sci. Rep. 2018, 8, 12523. [Google Scholar] [CrossRef] [PubMed]
- Possamai, T.; Wiedemann-Merdinoglu, S.; Merdinoglu, D.; Migliaro, D.; De Mori, G.; Cipriani, G. Construction of a high-density genetic map and detection of a major QTL of resistance to powdery mildew (Erysiphe necator Sch.) in Caucasian grapes (Vitis vinifera L.). BMC Plant Biol. 2021, 21, 528. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, F.; Maghradze, D.; Casati, P.; Chkhaidze, N.; Lobjanidze, M.; Ravasio, A.; Passera, A.; Venturini, G.; Failla, O.; Bianco, P.A. Identification and characterization of new ‘Candidatus Phytoplasma solani’ strains associated with bois noir disease in Vitis vinifera L. cultivars showing a range of symptoms severity in Georgia, the Caucasus region. Plant Dis. 2016, 100, 904–915. [Google Scholar] [CrossRef]
- Kison, H.; Seemüller, E. Differences in strain virulence of the European stone fruit yellows phytoplasma and susceptibility of stone fruit trees on various rootstocks to this pathogen. J. Phytopath 2011, 149, 533–541. [Google Scholar] [CrossRef]
- Seemüller, E.; Schneider, B. Differences in virulence and genomic features of strains of ‘Candidatus Phytoplasma mali’, the apple proliferation agent. Phytopathology 2007, 97, 964–970. [Google Scholar] [CrossRef]
- Riedle-Bauer, M.; Hanak, K.; Regner, F.; Tiefenbrunner, W. Influence of pruning measures on recovery of bois noir-infected grapevines. J. Phytopathol. 2010, 158, 628–632. [Google Scholar] [CrossRef]
- Pierro, R.; Passera, A.; Panattoni, A.; Casati, P.; Luvisi, A.; Rizzo, D.; Bianco, P.A.; Quaglino, F.; Materazzi, A. Molecular typing of ‘bois noir’ phytoplasma strains in the Chianti Classico area (Tuscany, central Italy) and their association with symptom severity in Vitis vinifera L. cv. Sangiovese. Phytopathology 2018, 108, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Townsend, G.R.; Heuberger, J.W. Methods for estimating losses caused by diseases in fungicide experiments. Plant Dis. Rep. 1943, 27, 340–343. [Google Scholar]
- Fabre, A.; Danet, J.-L.; Foissac, X. The stolbur phytoplasma antigenic membrane protein gene stamp is submitted to diversifying positive selection. Gene 2010, 475, 7–13. [Google Scholar] [CrossRef]
- Lee, I.-M.; Gundersen-Rindal, D.E.; Davis, R.E.; Bottner, K.D.; Marcone, C.; Seemüller, E. ‘Candidatus Phytoplasma asteris’, a novel phytoplasma taxon associated with aster yellows and related diseases. Int. J. Syst. Evol. Microbiol. 2004, 54, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-M.; Martini, M.; Marcone, C.; Zhu, S.F. Classification of phytoplasma strains in the elm yellows group (16SrV) and proposal of ‘Candidatus Phytoplasma ulmi’ for the phytoplasma associated with elm yellows. Int. J. Syst. Evol. Microbiol. 2004, 54, 337–347. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98 NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Rizzoli, A.; Belgeri, E.; Jermini, M.; Conedera, M.; Filippin, L.; Angelini, E. Alnus glutinosa and Orientus ishidae (Matsumura, 1902) share phytoplasma genotypes linked to the ‘Flavescence dorée’ epidemics. J. Appl. Entomol. 2021, 145, 1015–1028. [Google Scholar] [CrossRef]
- Radonjić, S.; Krstić, O.; Cvrković, T.; Hrnčić, S.; Marinković, S.; Mitrović, M.; Toševski, I.; Jović, J. The first report on the occurrence of Flavescence dorée phytoplasma affecting grapevine in vineyards of Montenegro and an overview of epidemic genotypes in natural plant reservoirs. J. Plant Pathol. 2023, 105, 419–427. [Google Scholar] [CrossRef]
- OIV-MA-AS313-15; Compendium of International Methods of Analysis of Wines and Musts. pH. International Organisation of Vine and Wine: Paris, France, 2011.
- OIV-MA-AS313-01; Compendium of International Methods of Analysis of Wines and Musts. Total Acidity. International Organisation of Vine and Wine: Paris, France, 2015.
- Di Stefano, R.; Cravero, M.C. Metodi per lo studio dei polifenoli dell’uva. Riv. Di Vitic. Ed Enol. 1991, 44, 37–45. [Google Scholar]
- Giordano, M.; Rolle, L.; Zeppa, G.; Gerbi, V. Chemical and volatile composition of three Italian sweet white Passito wines. OENO One 2009, 43, 159–170. [Google Scholar] [CrossRef]
- Di Stefano, R.; Cravero, M.C.; Gentilini, N. Metodi per lo studio dei polifenoli dei vini. L’Enotecnico 1989, 5, 83–89. [Google Scholar]
- OIV-MA-AS2-07B; Compendium of International Methods of Analysis of Wines and Musts. Chromatic Characteristics. International Organisation of Vine and Wine: Paris, France, 2009.
- OIV-MA-AS2-11; Determination of chromatic characteristics according to CIELab. Compendium of International Methods of Analysis of Wines and Musts. International Organisation of Vine and Wine: Paris, France, 2006.
- Paissoni, M.A.; Río Segade, S.; Carrero-Carralero, C.; Montanini, C.; Giacosa, S.; Rolle, L. Role of anthocyanin traits on the impact of oenological tannins addition in the first stage of red winegrape skin simulated maceration. Food Chem. 2020, 320, 126633. [Google Scholar] [CrossRef]
- Failla, O.; Maghradze, D. Alle origini della viticoltura e dell’enologia: La Georgia. I Tempi Della Terra 2022, 13, 41–56. [Google Scholar]
- Sergazy, S.; Gulyayev, A.; Dudikova, G.; Chulenbayeva, L.; Nurgaziyev, M.; Elena, K.; Nurgozhoina, A.; Ziyat, A.; Tritek, V.; Kozhakhmetov, S.; et al. Comparison of phenolic content in Cabernet Sauvignon and Saperavi wines. J. Microbiol. Biotech. Food Scie 2019, 20, 557–561. [Google Scholar] [CrossRef]
- Casarin, S.; Vincenzi, S.; Esposito, A.; Filippin, L.; Forte, V.; Angelini, E.; Bertazzon, N. A successful defence strategy in grapevine cultivar ‘Tocai friulano’ provides compartmentation of grapevine Flavescence dorée phytoplasma. BMC Plant Biol. 2023, 23, 161. [Google Scholar] [CrossRef]
- Constable, F.E.; Gibb, K.S.; Symons, R.H. Seasonal distribution of phytoplasmas in Australian grapevines. Plant Pathol. 2003, 52, 267–276. [Google Scholar] [CrossRef]
- Martini, M.; Ermacora, P.; Magris, G.; Ferrini, F.; Loi, N. Symptom expression and ‘Candidatus Phytoplasma prunorum’ concentration in different Prunus species. Bull. Insect 2011, 64, S171–S172. [Google Scholar]
- Deboneville, C.; Mandelli, L.; Brodard, J.; Groux, R.; Roquis, D.; Schumpp, O. The complete genome of the “flavescence dorée” phytoplasma reveals characteristics of low genome plasticity. Biology 2022, 11, 953. [Google Scholar] [CrossRef] [PubMed]
- Kharadze, M.; Japaridze, I.; Kalandia, A.; Vanidze, M. Anthocyanins and antioxidant activity of red wines made from endemic grape varieties. Ann. Agrar. Sci. 2018, 16, 181–184. [Google Scholar] [CrossRef]
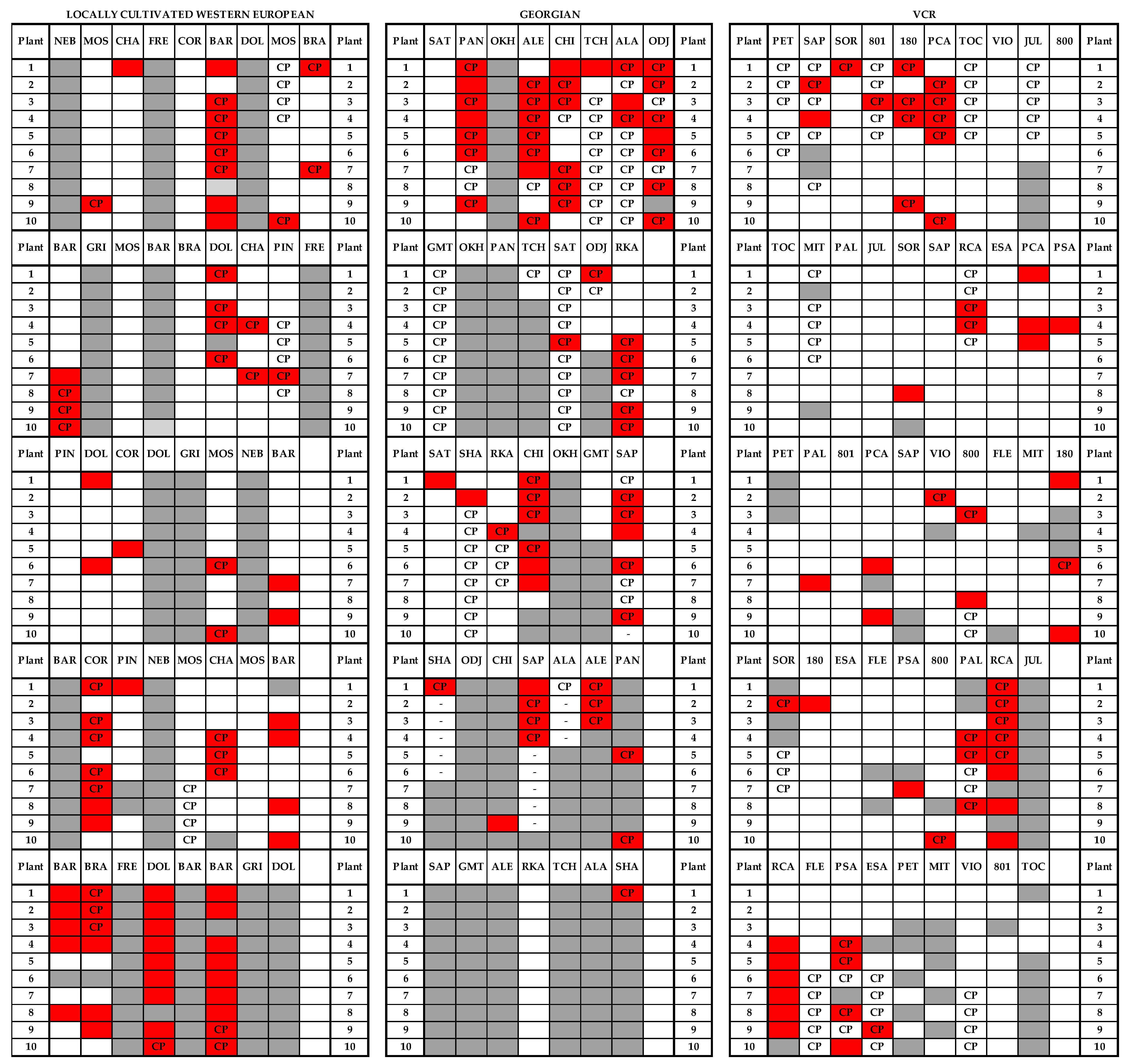
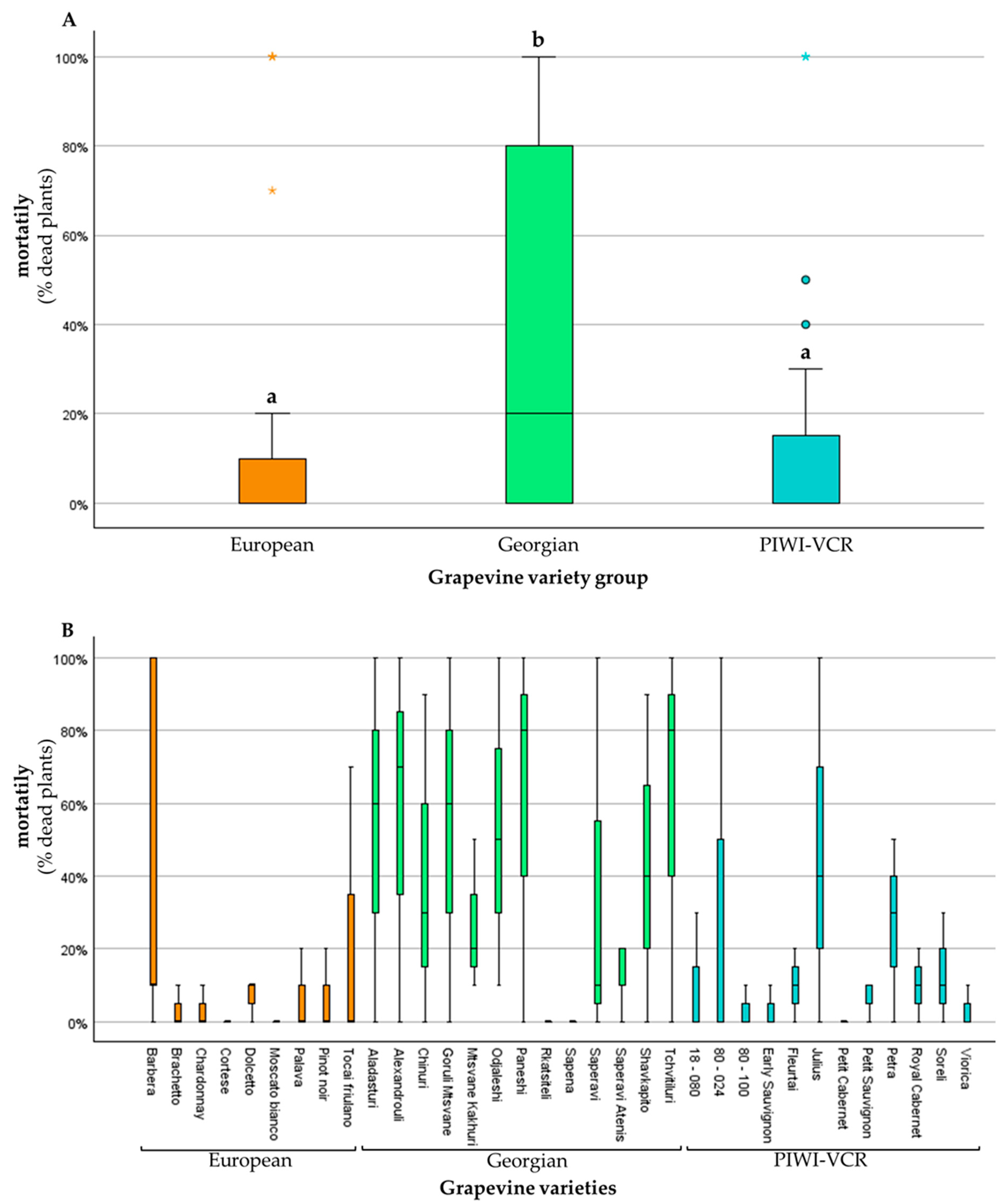

| Group | Variety | Number of Grapevines | Mortality | GY | Symptom | |||
|---|---|---|---|---|---|---|---|---|
| Planted | Dead | Observed | Symptomatic | (%) | Incidence (%) | Severity Class | ||
| European | Barbera | 90 | 34 | 56 | 32 | 38 | 57 | 2 |
| Brachetto | 30 | 1 | 29 | 8 | 3 | 28 | 2 | |
| Chardonnay | 30 | 1 | 29 | 6 | 3 | 21 | 2 | |
| Cortese | 30 | 0 | 30 | 8 | 0 | 27 | 2 | |
| Dolcetto | 60 | 32 | 28 | 15 | 53 | 54 | 2 | |
| Freisa | 30 | 30 | 0 | n.a. | 100 | n.a. | n.a. | |
| Grignolino | 30 | 30 | 0 | n.a. | 100 | n.a. | n.a. | |
| Moscato bianco | 60 | 0 | 60 | 4 | 0 | 7 | 2 | |
| Nebbiolo | 30 | 30 | 0 | n.a. | 100 | n.a. | n.a. | |
| Palava_VCR | 30 | 2 | 28 | 4 | 7 | 14 | 2 | |
| Pinot noir | 30 | 2 | 28 | 2 | 7 | 7 | 2 | |
| Tocai friulano_VCR | 30 | 7 | 23 | 0 | 23 | 0 | 0 | |
| Total | 480 | 169 | 311 | 79 | 35 | 25 | ||
| Georgian | Aladasturi | 30 | 16 | 14 | 3 | 53 | 21 | 1 |
| Alexandrouli | 30 | 17 | 13 | 10 | 57 | 77 | 1 | |
| Chinuri | 30 | 12 | 18 | 11 | 40 | 61 | 1 | |
| Goruli Mtsvane | 30 | 16 | 14 | 0 | 53 | 0 | 0 | |
| Mtsvane Kakhuri_VCR | 30 | 8 | 22 | 0 | 27 | 0 | 0 | |
| Odjaleshi | 30 | 16 | 14 | 8 | 53 | 57 | 1 | |
| Okhtoura | 30 | 30 | 0 | n.a. | 100 | n.a. | n.a. | |
| Paneshi | 30 | 18 | 12 | 9 | 60 | 75 | 1 | |
| Rkatsiteli | 30 | 0 | 30 | 6 | 0 | 20 | 1 | |
| Sapena | 30 | 11 | 19 | 9 | 37 | 47 | 1 | |
| Saperavi_VCR | 30 | 4 | 26 | 2 | 13 | 8 | 1 | |
| Saperavi Atenis | 30 | 0 | 30 | 2 | 0 | 7 | 1 | |
| Shavkapito | 30 | 13 | 17 | 3 | 43 | 18 | 1 | |
| Tchvitiluri | 30 | 18 | 12 | 1 | 60 | 8 | 1 | |
| Total | 420 | 179 | 241 | 64 | 43 | 27 | ||
| PIWI-VCR | 18-080 | 30 | 3 | 27 | 8 | 10 | 30 | 2 |
| 80-024 | 30 | 10 | 20 | 0 | 33 | 0 | 0 | |
| 80-100 | 30 | 1 | 29 | 1 | 3 | 3 | 2 | |
| Early Sauvignon | 30 | 1 | 29 | 1 | 3 | 3 | 1 | |
| Fleurtai | 30 | 3 | 27 | 0 | 10 | 0 | 0 | |
| Julius | 30 | 14 | 16 | 0 | 47 | 0 | 0 | |
| Petit Cabernet | 30 | 0 | 30 | 10 | 0 | 33 | 2 | |
| Petit Sauvignon | 30 | 2 | 28 | 6 | 7 | 21 | 2 | |
| Petra | 30 | 8 | 22 | 0 | 27 | 0 | 0 | |
| Royal Cabernet | 30 | 3 | 27 | 16 | 10 | 59 | 1 | |
| Soreli | 30 | 4 | 26 | 3 | 13 | 12 | 2 | |
| Viorica | 30 | 1 | 29 | 1 | 3 | 3 | 2 | |
| Total | 360 | 50 | 310 | 46 | 14 | 15 | ||
| Group | Variety | Status | N. of Plants | 16SrVp |
|---|---|---|---|---|
| 16SrVp-Infected/Sampled | Map Genotype | |||
| European | Barbera | S | 10/10 | M54 (FDp) |
| Brachetto | S | 3/5 | M54 (FDp) | |
| Chardonnay | S | 3/5 | M54 (FDp) | |
| Cortese | S | 5/5 | M54 (FDp) | |
| Dolcetto | S | 5/5 | M54 (FDp) | |
| Moscato bianco | S | 3/3 | M54 (FDp) | |
| A | 0/12 | |||
| Palava_VCR | S | 1/3 | M54 (FDp) | |
| A | 0/2 | |||
| Pinot noir | S | 0/1 | ||
| A | 0/4 | |||
| Tocai friulano_VCR | A | 1/5 | M54 (FDp) | |
| Total | S | 30/37 | ||
| A | 1/23 | |||
| Georgian | Aladasturi | S | 1/2 | M54 (FDp) |
| A | 1/8 | M54 (FDp) | ||
| Alexandrouli | S | 8/9 | M54 (FDp) | |
| A | 0/1 | |||
| Chinuri | S | 4/9 | M54 (FDp) | |
| A | 0/1 | |||
| Goruli Mtsvane | A | 4/10 | M54 (FDp) | |
| Mtsvane Kakhuri_VCR | A | 0/5 | ||
| Odjaleshi | S | 4/7 | M54 (FDp) | |
| A | 0/3 | |||
| Paneshi | S | 3/7 | M54 (FDp) | |
| A | 0/3 | |||
| Rkatsiteli | S | 6/6 | M54 (FDp) | |
| A | 0/4 | |||
| Saperavi_VCR | S | 0/1 | ||
| A | 0/4 | |||
| Saperavi Atenis | S | 1/1 | M54 (FDp) | |
| A | 1/9 | M54 (FDp) | ||
| Sapeva | S | 4/6 | M54 (FDp) | |
| A | 0/4 | |||
| Shavkapito | S | 1/2 | M54 (FDp) | |
| A | 4/8 | M54 (FDp) | ||
| Tchvitiluri | A | 2/10 | M54 (FDp) | |
| Total | S | 32/50 | ||
| A | 12/70 | |||
| PIWI-VCR | 18-080 | S | 5/5 | M54 (FDp) |
| 80-024 | S | 0/2 | ||
| A | 0/3 | |||
| 80-100 | S | 0/1 | ||
| A | 0/4 | |||
| Early Sauvignon | S | 0/1 | ||
| A | 0/4 | |||
| Fleurtai | A | 0/5 | ||
| Julius | A | 0/5 | ||
| Petit Cabernet | S | 2/5 | M54 (FDp) | |
| Petit Sauvignon | S | 1/3 | M54 (FDp) | |
| A | 0/2 | |||
| Petra | A | 1/5 | M54 (FDp) | |
| Royal Cabernet | S | 3/7 | M54 (FDp) | |
| A | 0/3 | |||
| Soreli | S | 2/2 | M54 (FDp) | |
| A | 0/3 | |||
| Viorica | S | 1/1 | M54 (FDp) | |
| A | 0/4 | |||
| Total | S | 14/27 | ||
| A | 1/38 |
| Parameter (Unit) | White Winegrapes | Red Winegrapes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Goruli Mtsvane | Mtsvane Kakhuri | Rkatsiteli | Sapena | Tchivitiluri | Aladasturi | Odjaleshi | Paneshi | Saperavi | Shavkapito | |
| Grape juice basic parameters | ||||||||||
| Total soluble solids (Brix) | 22.2 | nd | 22.3 | 21.9 | 21.5 | 25.6 | 25.2 | 24.2 | 24.3 | 23.6 |
| Sugars (g/L) | 222 | nd | 198 | 223 | 182 | 227 | 222 | 207 | 211 | 200 |
| pH | 3.13 | nd | 3.22 | 3.16 | 3.10 | 3.25 | 3.05 | 3.42 | 3.16 | 3.36 |
| Total acidity (g/L tartaric acid) | 6.5 | nd | 7.2 | 8.5 | 10.7 | 7.2 | 8.8 | 5.0 | 6.1 | 6.7 |
| Grape characteristics | ||||||||||
| Bunch weight (g) | 211.83 | nd | 185.44 | 271.67 | 134.50 | 257.00 | 127.17 | 161.39 | 126.25 | 237.59 |
| Berry weight (g) | 2.38 | nd | 2.56 | 3.19 | 1.83 | 2.61 | 1.67 | 2.16 | 1.70 | 2.79 |
| Average number of seeds/berries | nd | nd | nd | nd | nd | 3.2 | 2.4 | 1.8 | 1.9 | 3.0 |
| Seeds weight (g) | nd | nd | nd | nd | nd | 2.08 | 1.07 | 0.79 | 0.84 | 1.45 |
| Skins weight (% w/w) | nd | nd | nd | nd | nd | 26.82 | 40.37 | 27.39 | 31.91 | 19.10 |
| Seeds weight (% w/w) | nd | nd | nd | nd | nd | 7.96 | 6.39 | 3.71 | 4.90 | 5.90 |
| Phenolics determination | ||||||||||
| TA (mg/kg malvidin-3-O-glucoside chloride) | nd | nd | nd | nd | nd | 1082 b | 1974 c | 452 a | 1348 b | 980 b |
| TPI seeds (mg/kg (+)-catechin) | nd | nd | nd | nd | nd | 295 bc | 360 c | 152 ab | 238 b | 51 a |
| TPI skins (mg/kg (+)-catechin) | nd | nd | nd | nd | nd | 1475 a | 3399 c | 2278 b | 2495 b | 2292 b |
| TPI from seeds (% w/w) | nd | nd | nd | nd | nd | 16.6% | 9.6% | 6.3% | 8.7% | 2.2% |
| Harvest date | 21 Sept, 2018 | 21 Sept, 2018 | 27 Sept, 2018 | 21 Sept, 2018 | 27 Sept, 2018 | 23 Oct, 2018 | 23 Oct, 2018 | 27 Sept, 2018 | 08 Oct, 2018 | 27 Sept, 2018 |
| Harvest weight (kg) | 23 | 24 | 12 | 16 | 10 | 21 | 12 | 12 | 25 | 25 |
| Parameter (unit) | White Wines | Red Wines | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Goruli Mtsvane | Mtsvane Kakhuri | Rkatsiteli | Sapena | Tchvitiluri | Aladasturi | Odjaleshi | Paneshi | Saperavi | Shavkapito | |
| Basic parameters | ||||||||||
| Ethanol (% v/v) | 13.0 | 13.8 | 12.2 | 14.1 | 12.3 | 11.2 | 12.8 | 13.7 | 13.0 | 12.7 |
| Residual sugars (g/L) | 2.33 | 2.25 | 2.61 | 3.11 | 3.19 | 0.22 | nd | 0.23 | 0.11 | 0.16 |
| pH | 3.71 | 3.75 | 3.43 | 3.65 | 3.16 | 3.48 | 3.38 | 3.55 | 3.53 | 3.63 |
| Total acidity (g/L as tartaric acid) | 6.2 | 5.8 | 6.8 | 7.0 | 7.9 | 6.0 | 7.1 | 7.1 | 7.1 | 7.3 |
| Acetic acid (g/L) | 0.26 | 0.31 | 0.16 | 0.30 | 0.25 | 0.43 | 0.36 | 0.64 | 0.44 | 0.58 |
| Tartaric acid (g/L) | 1.83 | 1.71 | 1.77 | 1.51 | 2.35 | 2.13 | 1.79 | 1.15 | 1.26 | 1.05 |
| Malic acid (g/L) | 2.16 | 1.81 | 1.82 | 3.11 | 2.26 | nd | nd | nd | nd | nd |
| Citric acid (g/L) | 0.47 | 0.14 | 0.36 | 0.42 | 0.23 | nd | 0.07 | nd | 0.06 | nd |
| Lactic acid (g/L) | 0.29 | 0.29 | 0.28 | 0.29 | 0.30 | 1.77 | 1.59 | 1.36 | 1.83 | 1.96 |
| Glycerol (g/L) | 10.17 | 10.16 | 9.20 | 10.49 | 9.31 | 8.76 | 9.95 | 11.65 | 10.80 | 10.78 |
| Phenolics and color characteristics | ||||||||||
| Total phenolics (mg/L (+)-catechin) | - | - | - | - | - | 1050 | 1487 | 1872 | 1431 | 2249 |
| Total flavonoids (mg/L (+)-catechin) | - | - | - | - | - | 1170 | 1903 | 2044 | 1458 | 2554 |
| Total anthocyanins (mg/L malvidin-3-O-glucoside chloride) | - | - | - | - | - | 325 | 428 | 120 | 283 | 274 |
| L* | 96.1 | 96.9 | 95.5 | 96.8 | 96.5 | 23.1 | 21.5 | 31.8 | 26.5 | 22.5 |
| a* | 0.34 | 0.01 | 0.25 | 0.63 | 0.03 | 54.96 | 53.73 | 55.47 | 55.14 | 52.98 |
| b* | 9.60 | 12.97 | 12.83 | 11.15 | 15.53 | 35.18 | 35.33 | 38.19 | 35.86 | 32.86 |
| A420 nm (A.U., O.P. 10 mm) | 0.161 | 0.182 | 0.207 | 0.163 | 0.231 | - | - | - | - | - |
| Color intensity (A.U., O.P. 10 mm) | - | - | - | - | - | 7.410 | 8.070 | 4.800 | 5.690 | 6.310 |
| Hue | - | - | - | - | - | 0.541 | 0.647 | 0.886 | 0.789 | 0.760 |
| Visible color transposition | ||||||||||
| Anthocyanin profile | ||||||||||
| Delphinidin-3-O-glucoside (%) | - | - | - | - | - | 3.52 | 5.50 | 6.51 | 6.77 | 12.87 |
| Cyanidin-3-O-glucoside (%) | - | - | - | - | - | 0.30 | 3.63 | 2.21 | 4.29 | 1.76 |
| Petunidin-3-O-glucoside (%) | - | - | - | - | - | 5.97 | 8.52 | 10.44 | 9.84 | 13.94 |
| Peonidin-3-O-glucoside (%) | - | - | - | - | - | 6.57 | 17.61 | 12.22 | 21.97 | 7.16 |
| Malvidin-3-O-glucoside (%) | - | - | - | - | - | 68.11 | 41.01 | 49.15 | 44.69 | 42.89 |
| ∑ acetylated anthocyanins (%) | - | - | - | - | - | 10.57 | 14.15 | 7.22 | 3.89 | 3.88 |
| ∑ cinnamoylated anthocyanins (%) | - | - | - | - | - | 4.97 | 9.58 | 12.26 | 8.55 | 17.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portaccio, L.; Paissoni, M.A.; Giacosa, S.; Passera, A.; Barbieri, C.; Maghradze, D.; Rolle, L.; Gerbi, V.; Failla, O.; Bianco, P.A.; et al. Performance of Georgian Grapevine Varieties in a Vineyard Infected by Flavescence Dorée Phytoplasma in Piedmont, Northwestern Italy. Agriculture 2025, 15, 1988. https://doi.org/10.3390/agriculture15181988
Portaccio L, Paissoni MA, Giacosa S, Passera A, Barbieri C, Maghradze D, Rolle L, Gerbi V, Failla O, Bianco PA, et al. Performance of Georgian Grapevine Varieties in a Vineyard Infected by Flavescence Dorée Phytoplasma in Piedmont, Northwestern Italy. Agriculture. 2025; 15(18):1988. https://doi.org/10.3390/agriculture15181988
Chicago/Turabian StylePortaccio, Letizia, Maria Alessandra Paissoni, Simone Giacosa, Alessandro Passera, Camilla Barbieri, David Maghradze, Luca Rolle, Vincenzo Gerbi, Osvaldo Failla, Piero Attilio Bianco, and et al. 2025. "Performance of Georgian Grapevine Varieties in a Vineyard Infected by Flavescence Dorée Phytoplasma in Piedmont, Northwestern Italy" Agriculture 15, no. 18: 1988. https://doi.org/10.3390/agriculture15181988
APA StylePortaccio, L., Paissoni, M. A., Giacosa, S., Passera, A., Barbieri, C., Maghradze, D., Rolle, L., Gerbi, V., Failla, O., Bianco, P. A., & Quaglino, F. (2025). Performance of Georgian Grapevine Varieties in a Vineyard Infected by Flavescence Dorée Phytoplasma in Piedmont, Northwestern Italy. Agriculture, 15(18), 1988. https://doi.org/10.3390/agriculture15181988











