Abstract
Mycorrhizal symbiosis relies on the host’s supply of carbohydrates, while sugar transport within plants is governed by the SWEET sugar transporter family. Although the symbiotic association between arbuscular mycorrhizal fungi (AMF) and maize is critical for its growth and sugar regulation, different AMF species have varying impacts on the host. The aim of this study was to analyze the effects of inoculating six different AMF species [Diversispora epigaea (De), Rhizophagus intraradices (Ri), Paraglomus occultum (Po), Entrophospora etunicata (Ee), Glomus heterosporum (Gh), and Funneliformis mosseae (Fm)] on plant growth, leaf photosynthetic capacity, glomalin-related soil protein content, leaf sugar content, and SWEET gene expression of maize under potted conditions for two months. AMF species colonize maize roots and showed significant species-specific variation, where Ri and Fm colonized treatment had the greatest rates (66~68%). All six fungi significantly increased biomass and stem diameter, with Ee treatment yielding the thickest stems, and enhanced leaf photosynthetic performance and glomalin-related soil protein fractions to some extent, with species-specific enhancements. All AMF species in particular significantly increased leaf sucrose; all except Ri treatment significantly increased fructose; while only Po and Fm treatments significantly increased glucose. AMF inoculations consistently upregulated the expression of ZmSWEET1b/3a/3b/4a/4b/14a and 16 genes, consistently downregulated the expression of ZmSWEET6b/11b/12a/13a/13b/13c and 17b genes, and induced treatment-specific regulation in the other gene expression. Root AMF colonization clustered with sugars and specific ZmSWEETs, with ZmSWEET4a/15b and 14b central to sucrose/glucose based on principal component analysis, indicating that these genes have specific regulatory effects in response to AMF treatments. In short, AMF inoculation reprogrammed ZmSWEET expression in a species-specific manner, with core ZmSWEET genes mediating sugar accumulation to support symbiosis.
1. Introduction
Maize (Zea mays L.) is one of the world’s most important and widely cultivated crops [1], leading global agricultural production in terms of annual yield, cultivated area, and economic value. Among all cereal grains, the importance of maize ranks second only to wheat and rice [2]. In China, the sown area of maize reached 44.7407 million hectares (ha) in 2024, with a yield of 6.5917 t/ha and a total output of 294.917 million tons, ranking first globally in maize production [3]. Arbuscular mycorrhizal fungi (AMF) could inhabit the maize rhizosphere, then colonizing the roots to establish mycorrhizal symbiosis [4]. Following symbiosis establishment, AMF hyphae could penetrate root cortical cells to form specialized structures including arbuscules (nutrient-exchange hub) and vesicles (storage and propagation) [5]. Within this symbiotic relationship, the host plant transfers photosynthates to the AMF to support fungal growth [6], while the AMF extends the absorption range of the host roots through extraradical hyphae to facilitates the transport of water and mineral nutrients (such as phosphorus and copper) from the soil to the host plant [7,8].
AMF have many important physiological functions in maize. AMF colonization causes morphological changes in maize roots, thus enhancing nutrient uptake [9]. AMF also significantly improve phosphorus (P) absorption, which is vital for maize growth, especially in P-limited soils [10]. AMF secrete glomalin, an insoluble glycoprotein that binds to soil humic-mineral complexes, forming glomalin-related soil proteins (GRSP) [11]. The GRSP can stabilize soil aggregates and contribute to soil organic carbon sequestration [12,13,14]. Inoculation with specific AMF, such as Rhizophagus irregularis, has been shown to increase the biomass and nutrient content of maize, enhancing the uptake of essential minerals like calcium, magnesium, and sulfur [15]. Mycorrhizal associations lead to denser and more branched root systems in maize, which facilitate better nutrient and water acquisition and plant performance under resource-limited conditions [15,16]. Although AMF are generally beneficial for maize, the specific type of AMF can lead to varying degrees of effectiveness in nutrient uptake and root growth [17,18,19]. Different indigenous strains of AMF significantly influenced maize yield and productivity, with co-inoculation of Funneliformis geosporum, Glomus caledonius, and Rhizophagus intraradices combined with reduced mineral fertilizers yielding a 62.5% increase in grain yield [17]. In ferruginous soil in Northern Benin, different AMF strains, particularly Funneliformis mosseae, significantly enhanced maize growth and yield [20]. This variability suggests that choosing appropriate AMF strains could optimize maize cultivation under different environmental conditions.
Sugar, as a substrate for the production of primary and secondary metabolites, is a crucial fundamental carbon source supporting plant morphogenesis and growth [21]. It participates in energy metabolism and signal transduction to sustain plant development [22]. The distribution of photoassimilates in plants relies on sugar transporters to control assimilation and the efficient transport of sugars to various tissues and cells [23]. The SWEET (Sugars Will Eventually Be Exported Transporters) family in plants has been identified as an unusually conserved form of sugar transporters, present in nearly all plants [24]. SWEET proteins can transport glucose, sucrose, fructose, and galactose, and are involved in sugar transport and accumulation within plants [25]. The observed increase in sucrose and glucose concentrations in AMF-colonized citrus seedlings reflects a reciprocal relationship between sugar accumulation and fungal colonization: AMF colonization triggers upregulation of plant photosynthetic genes and enhances carbon fixation, increasing root sugar availability; and these elevated sugar levels then sustain fungal growth and promote further colonization [26]. The interaction between AMF and host plants involves complex molecular signaling and transcriptional changes, including the regulation of SWEET genes, which are crucial for nutrient exchange and symbiotic maintenance [27]. The colonization of AMF could lead to significant transcriptional reprogramming of the SWEET sugar transporter family in plants like potatoes, with changes observed in 22 out of 35 SWEET genes in roots [28]. During AM symbiosis in soybeans, the SWEET transporter GmSWEET6 was upregulated, enhancing sucrose transfer towards AMF, which is crucial for maintaining the symbiotic association [29]. In Medicago truncatula, the SWEET1b transporter was upregulated in arbuscule-containing cells, facilitating glucose transport across the peri-arbuscular membrane, which is vital for arbuscule maintenance [30].
Current studies have shown that AMF are beneficial for promoting sugar synthesis (especially sucrose synthesis) in plants [31], but the effects of different AMF species on the host vary. Does this selectivity differ between species of AMF? Do certain AMFs have a greater tendency to regulate specific SWEET genes? So, the purpose of this study is to analyze the effects of six different AMF species on maize growth, glomalin levels, leaf gas exchange, sugar content, and the expression of the SWEET gene family members.
2. Materials and Methods
2.1. AMF Inoculants
Six AMF species were tested: Diversispora epigaea (B.A. Daniels & Trappe) C. Walker & A. Schüßler (De), Rhizophagus intraradices (N.C. Schenck & G.S. Sm.) Sieverd., G.A. Silva & Oehl (Ri), Paraglomus occultum (C. Walker) J.B. Morton & D. Redecker (Po), Entrophospora etunicata (W.N. Becker & Gerd.) Błaszk., Niezgoda, B.T. Goto & Magurno (Ee), Glomus heterosporum G.S. Sm. & N.C. Schenck (Gh), and Funneliformis mosseae (T.H. Nicolson & Gerd.) C. Walker & A. Schüßler (Fm). All AMF strains were provided by the Institute of Root Biology, Yangtze University. These strains were propagated for 70 days using white clover (Trifolium repens) as a host plant under potted conditions. After removing aboveground biomass, colonized root fragments and growth substrates were collected as AMF inoculants, in which each gram of inoculum contained approximately 20 spores.
2.2. Plant Culture
The maize cultivar ‘Zhengdan 958’ was provided by the Food Crops Research Institute of the Henan Academy of Agricultural Sciences. The pot experiment was conducted in a greenhouse at the West Campus of Yangtze University from 15 July to 15 September, 2024. Pots (2.4 L) with dimensions of 16.5 cm top diameter, 14.5 cm bottom diameter, and 12.5 cm depth were used. Six AM fungal treatments were established: De, Ri, Po, Ee, Gh, and Fm. Inoculation treatments were carried out when maize was sown. Each treatment pot received 400 g of respective inoculum containing approximately 200 spores per 10 g, which was thoroughly mixed with 800 g of autoclaved growth substrate comprising soil and sand (3:1, v/v). Control (CK) pots were filled with 800 g of autoclaved substrate and 400 g of autoclaved inoculums plus of 3 mL of 20-μm-passed inoculum filters.
Prior to sowing, maize seeds were soaked in distilled water for 24 h. Four seeds were sown per pot. After seedling emergence, plants were thinned to two uniform seedlings per pot. Throughout the maize growth period, daily irrigation was applied to maintain well-watered moisture levels. The experiment used a completely randomized design with seven treatments (CK, De, Ri, Po, Ee, Gh, and Fm) and four replicates (pots) per treatment, totaling 28 pots. All the plants were grown in an environmentally controlled growth chamber, with the specific environmental conditions detailed in Liang et al. [32].
Pots were randomly arranged in the chamber to minimize positional effects (e.g., light/temperature gradients).
2.3. Determination of Plant Growth, Leaf Gas Exchange, and Root Mycorrhizal Colonization Rate
On the day of harvest, maize plant height was measured using a measuring tape, and stem diameter was determined with Vernier calipers. Subsequently, shoot and root biomass was dried at 80 °C for 48 h and then weighed. Prior to harvest, leaf gas exchange parameters were measured on the 3rd fully expanded leaves from the apex of two representative plants per treatment, with one leaf measured per plant. Between 9:30 and 11:30 a.m. on clear days, net photosynthetic rate (Pn), transpiration rate (Tr), and intercellular CO2 concentration (Ci) were determined using a Yaxin-1102G portable photosynthesis system. The system was operated under the following conditions: photosynthetic photon flux density of 1000 μmol/m2/s provided by an integrated red/blue LED light source, ambient CO2 concentration (400 ± 10 μmol/mol), and a constant flow rate of 500 μmol/s. Leaf chamber temperature was maintained at ambient levels (25 °C) with relative humidity of 56%. Prior to data collection, each leaf was allowed to equilibrate for 60 s, followed by three consecutive measurements taken at 30 s intervals, with the average value recorded.
Harvested roots were thoroughly rinsed with tap water and cut into approximately 1.5 cm segments. Root colonization assessment was performed using trypan blue staining [33]. Root mycorrhizal colonization was examined under a microscope, and the root colonization rate was calculated as number of colonized root segments against total observed root segments.
2.4. Determinations of GRSP Levels
Following harvest, the growth substrate adhering to the root surfaces was gently shaken off and collected for the determination of GRSP levels. The contents of easily extractable GRSP (EEG) and difficultly extractable GRSP (DEG) were measured according to the method described by Wu et al. [34]. EEG was extracted by autoclaving 1.0 g soil in 20 mM citrate (pH 7.0) at 121 °C for 30 min, then centrifuging at 10,000× g for 3 min. The residues were subsequently extracted with 50 mM citrate (pH 8.0) for 60 min and centrifuged at 10,000× g for 3 min for DEG isolation, with both fractions analyzed using Bradford method with bovine serum albumin standards. Total GRSP (TG) content was calculated as the sum of EEG and DEG.
2.5. Determinations of Leaf Sugar Levels
Leaf sugar levels were assayed as per the protocol of Wen et al. [35]. Leaf samples (50 mg dry weight, dried at 65 °C and sieved at 0.5 mm) underwent dual extraction with 4 mL 80% ethanol at 80 °C for 40 min followed by centrifugation at 2500× g/min for 5 min. Combined supernatants were decolorized with 10 mg activated charcoal at 80 °C for 30 min and filtered. Sucrose quantification used resorcinol after alkaline hydrolysis: 150 µL extract + 150 µL 2 M NaOH (100 °C, 5 min), cooled, mixed with 2.1 mL 10 M HCl and 0.6 mL 0.1% ethanolic resorcinol, and incubated (80 °C, 10 min). The absorbance was measured at 480 nm against sucrose standards. Fructose quantification employed direct resorcinol reaction: 150 µL extract + 2.8 mL 10 M HCl + 0.8 mL 0.1% resorcinol (80 °C, 10 min). The absorbance was read at 480 nm using fructose standards. Glucose quantification followed enzymatic oxidation: 500 µL extract + 1 mL pre-warmed (30 °C, 2 min) enzyme reagent (glucose oxidase/peroxidase with o-dianisidine in acetate buffer, pH 5.5) and incubated (30 °C, 5 min). The reaction was terminated with 2 mL 10 M H2SO4, and the absorbance read at 460 nm against glucose standards.
2.6. ZmSWEET Gene Expression Analysis
Total RNA in leaf samples was isolated using the FastPure® Plant Total RNA Isolation Kit (Vazyme Biotech Co., Ltd., Nanjing, China), with RNA integrity verified through 1.2% agarose gel electrophoresis and concentration quantified via NanoDrop™ 2000 spectrophotometer. The RNA was reverse-transcribed into first-strand cDNA using the HiScript® II 1st Strand cDNA Synthesis Kit (Vazyme Biotech Co., Ltd., Nanjing, China), including a no-template control and a no-reverse-transcriptase control to monitor genomic DNA contamination and reagent purity. Quantitative real-time PCR (qRT-PCR) was performed using a CFX96 Touch™ system (Bio-Rad, Hercules, CA, USA) with Taq Pro Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd., Nanjing, China) in 20 μL reactions containing 50 ng cDNA and 0.4 μM gene-specific primers. Each qRT-PCR run incorporated no-template controls and no-amplification controls to detect potential contamination or nonspecific amplification. Expression profiles of 24 ZmSWEET homologs [36] were analyzed using primers designed via PrimerQuest™ Tool (Supplementary Material Table S1). The reference gene ZmTubulin was simultaneously amplified for normalization. Relative gene expression was calculated using the 2−ΔΔCt method [37], with data log2-transformed for statistical analysis, after validation of amplification specificity through single-peak melt curves and confirmation of primer efficiencies via standard curve dilution series.
2.7. Statistical Analysis
Data processing was performed using Microsoft Excel 2016. Prior to statistical analysis, all datasets were tested for normality (Shapiro-Wilt test) and homogeneity of variance (Levene’s test). If data violate these assumptions, logarithmic transformations were applied. Statistical significance was assessed through one-way analysis of variance (ANOVA) in IBM SPSS Statistics 27 (IBM, Armonk, NY, USA), followed by Fisher’s Least Significant Difference (LSD) post hoc test for pairwise comparisons (p < 0.05). Experimental results were visualized using GraphPad Prism 8 (GraphPad, San Diego, CA, USA) and Origin 2024 software (OriginLab Corporation, Northampton, MA, USA).
3. Results
3.1. Effects of Different AMF Species on Maize Root Colonization
Root colonization analysis revealed significant variation in mycorrhizal colonization rates among AMF treatments. Characteristic symbiotic structures–including vesicles, hyphae, and arbuscules–were observed in all AMF-inoculated maize roots (Figure 1b–g), confirming successful colonization, while non-inoculated controls (CK) showed no colonization (Figure 1a). Colonization rates exhibited species-specific patterns: Ri and Fm demonstrated the highest colonization efficiencies at 66.88% and 68.33%, respectively, followed by De at 34.83% (Figure 1h). In contrast, significantly lower colonization rates were observed in Po (20.43%), Ee (21.30%), and Gh (25.50%), with a significant difference between Po and Gh inoculations.

Figure 1.
The root mycorrhizal colonization of maize (a–g) and the changes in root mycorrhizal colonization rate in maize colonized by six AMF (h). Data (means ± SD, n = 4) showed significant (p < 0.05; LSD tests) differences represented by different letters above the bars. (a): CK, inoculation without arbuscular mycorrhizal fungi; (b): De, Diversispora epigaea; (c): Ri, Rhizophagus intraradices; (d): Po, Paraglomus occultum; (e): Ee, Entrophospora etunicata; (f): Gh, Glomus heterosporum; (g): Fm, Funneliformis mosseae.
3.2. Effects of Different AMF Species on Maize Growth
Maize growth responses to AMF inoculation exhibited significant variation across different fungal species (Figure 2a). Inoculation with Ri, Po, Ee, or Fm did not significantly affect maize plant height compared to CK (Figure 2b). However, maize inoculated with De exhibited 21.31% significantly greater plant height than CK. Among all treatments, De-inoculated plants achieved the maximum height (37.0 cm), with De treatments yielding significantly taller (12.03−33.31%) plants than those inoculated with Po, Ee, Gh, or Fm. For stem diameter, all six AMF-inoculated treatments showed significantly increased values relative to CK (Figure 2c). Notably, Ee inoculation produced the thickest stems, surpassing De, Po, Gh, and Fm by 31.47%, 29.89%, 17.21%, and 23.37%, respectively. Inoculation with AMF significantly enhanced both shoot and root biomass in maize plants relative to non-inoculated controls (Figure 2d,e). Shoot biomass exhibited significant increases in De (22.75%), Ri (51.82%), Po (36.94%), and Ee (32.47%) (Figure 2d), while root biomass showed a significant increase of De (32.39%), Ri (198.51%), Po (130.33%), Ee (64.75%), Gh (122.71%), and Fm (125.17%) (Figure 2e).
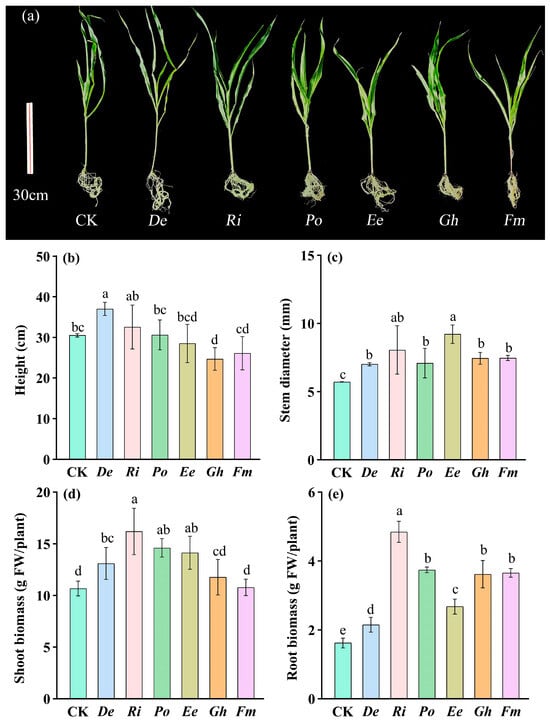
Figure 2.
Plant growth behavior of different treated maize (a) and effects of six arbuscular mycorrhizal fungi on height (b), stem diameter (c), shoot biomass (d), and root biomass (e) of maize. Data (means ± SD, n = 4) showed significant (p < 0.05; LSD tests) differences represented by different letters above the bars. Abbreviation: CK, inoculation without arbuscular mycorrhizal fungi; De, Diversispora epigaea; Ri, Rhizophagus intraradices; Po, Paraglomus occultum; Ee, Entrophospora etunicata; Gh, Glomus heterosporum; Fm, Funneliformis mosseae.
3.3. Effects of Different AMF Species on Leaf Gas Exchange Parameters
All AMF inoculations enhanced photosynthetic performance to a certain extent relative to CK, though species-specific variations were prominent (Figure 3a–c). Ri, Ee, and Fm significantly increased Pn by 39.24%, 91.73%, and 83.67%, respectively, while De, Po, and Gh showed no significant differences (Figure 3a). Tr increased significantly with Ri (80.78%), Ee (43.59%), and Fm (62.03%) inoculations, whereas De and Gh had no notable impact (Figure 3b). De, Ri, Po, and Gh elevated Ci levels by 53.18%, 40.04%, 39.01%, and 43.12%, while Ee and Fm treatments remained statistically unchanged (Figure 3c).

Figure 3.
Effects of six arbuscular mycorrhizal fungi on leaf net photosynthetic rate (a), transpiration rate (b), and intercellular CO2 concentration (c) of maize. Data (means ± SD, n = 4) showed significant (p < 0.05; LSD tests) differences represented by different letters above the bars. Refer to Figure 2 for the abbreviations.
3.4. Effects of Different AMF Species on GRSP Levels of Substrate Soil
AMF inoculation significantly modulated GRSP accumulation in the maize substrate soil compared to CK (Figure 4a–c). Significant increases in EEG occurred with De (9.55%), Ri (11.04%), Po (14.02%), Ee (5.97%), and Gh (11.63%), compared to CK. Fm showed a non-significant reduction in EEG. In addition, De, Ri, and Po significantly enhanced DEG levels by 3.10%, 4.53%, and 6.56%, respectively, while Ee markedly reduced DEG by 9.97%, compared to CK. Gh and Fm exhibited non-significant alterations in DEG relative to CK. TG was highest in De, Ri, and Po inoculated substrate soils, significantly surpassing levels in Ee, CK, and Fm treatments.
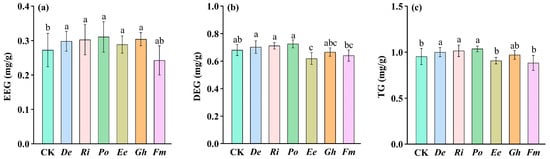
Figure 4.
Effects of six arbuscular mycorrhizal fungi on substrate soil EEG (a), DEG (b), and TG (c) levels of maize. Data (means ± SD, n = 4) showed significant (p < 0.05; LSD tests) differences represented by different letters above the bars. Abbreviation: EEG, easily extractable glomalin-related soil protein; DEG, difficultly extractable glomalin-related soil protein; TG, total glomalin-related soil protein. Refer to Figure 2 for the other abbreviations.
3.5. Effects of Different AMF Species on Leaf Sucrose, Fructose, and Glucose Concentrations
Inoculation with different AMF species had varying effects on sugar component concentrations in maize leaves (Figure 5a–c). Compared with CK, sucrose concentrations significantly increased in the De (326.86%), Ri (93.04%), Po (544.50%), Ee (132.69%), Gh (179.61%), and Fm (184.47%) treatments. Glucose concentrations significantly increased in the De (369.85%), Po (709.74%), Ee (191.95%), Gh (471.91%), and Fm (524.34%) treatments. The Ri treatment showed no significant increase in glucose concentrations, compared with CK. Compared with CK, fructose concentrations significantly increased in the Po (148.20%) and Fm (77.62%) treatments, while the De, Ri, Ee, and Gh treatments had no significant effect on fructose concentrations (Figure 5c). Additionally, Po treatment exhibited the greatest leaf sucrose, glucose, and fructose concentrations compared with the other inoculations and CK.

Figure 5.
Effects of six arbuscular mycorrhizal fungi on leaf sucrose (a), glucose (b), and fructose (c) levels of maize. Data (means ± SD, n = 4) showed significant (p < 0.05; LSD tests) differences represented by different letters above the bars. Refer to Figure 2 for the abbreviations.
3.6. Effects of Different AMF Species on the Expression of Leaf ZmSWEETs
Following the removal of genes ZmSWEET6a, ZmSWEET15a, and ZmSWEET17a (which showed no detectable signals across all treatments), the expression levels of the remaining 21 genes were log2-transformed for standardization. The expression patterns of ZmSWEET genes varied significantly under different AMF treatments (Figure 6). Specifically, ZmSWEET1b, ZmSWEET3a, ZmSWEET3b, ZmSWEET4a, ZmSWEET4b, ZmSWEET14a, and ZmSWEET16 were consistently upregulated in AMF-inoculated maize leaves. Conversely, ZmSWEET6b, ZmSWEET11b, ZmSWEET12a, ZmSWEET13a, ZmSWEET13b, ZmSWEET13c, and ZmSWEET17b exhibited consistent downregulation under mycorrhization conditions. The remaining genes showed either up- or downregulation patterns depending on the specific AMF treatment applied.
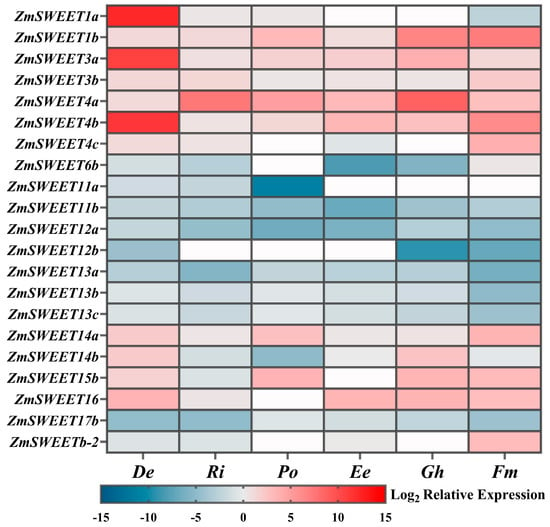
Figure 6.
Effects of six arbuscular mycorrhizal fungi on the expression of leaf ZmSWEETs of maize. White indicates undetected the gene expression. Refer to Figure 2 for the abbreviations.
3.7. Results Analysis of the PCA
The principal component analysis (PCA) showed the relationships between 21 ZmSWEET gene expressions, sugar (sucrose, fructose, and glucose), root AMF colonization, and different experimental groups (Figure 7). PC1 (42.87%) and PC2 (19.65%) collectively account for 62.52% of the total variance. Samples form separate three clusters, where CK and Fm each formed separate clusters, while De, Ee, Gh, Ri, and Po grouped together. It suggested different treatments (or microbial inoculations) induce unique responses in ZmSWEET expression and carbon metabolism. Sucrose, glucose, and fructose strongly correlate with PC1 (positive axis), clustering with ZmSWEET4a, ZmSWEET15b, ZmSWEET14b, etc. This suggested these genes were closely linked to sugar accumulation under Po, Gh, Ee and Fm treatments. Root colonization associated with PC1 (positive axis), clustering with sugar-related ZmSWEETs (such as ZmSWEET1b, ZmSWEET14a and ZmSWEETb-2) and sugars. Correlation analysis further revealed that the root AMF colonization rate was significantly positively correlated with ZmSWEET3a (r = 0.55, p < 0.01), ZmSWEET3b (r = 0.51, p < 0.01), ZmSWEET4c (r = 0.58, p < 0.01), and ZmSWEET14a (r = 0.48, p < 0.01), but significantly negatively correlated with ZmSWEET1a (r = −0.64, p < 0.01), ZmSWEET11a (r = −0.46, p < 0.05), ZmSWEET11b (r = −0.50, p < 0.01), ZmSWEET12a (r = −0.60, p < 0.01), ZmSWEET12b (r = −0.59, p < 0.01), ZmSWEET13a (r = −0.73, p < 0.01), ZmSWEET13b (r = −0.82, p < 0.01), ZmSWEET13c (r = −0.79, p < 0.01), and ZmSWEET17b (r = −0.82, p < 0.01). This hinted that root AMF colonization may directly/indirectly regulate specific SWEET-mediated sugar transport/accumulation. Groups like Gh, Po, and Ee clustered near sugars, suggesting these fungi enhanced SWEET-driven sugar uptake/transport in roots. ZmSWEET4a, ZmSWEET4b, ZmSWEET14b ZmSWEET15b, and ZmSWEET16 emerged as central to sugar metabolism under symbiotic conditions. Sucrose and glucose were pivotal, driving PC1 variability and linking mycorrhizal colonization to SWEET expression.
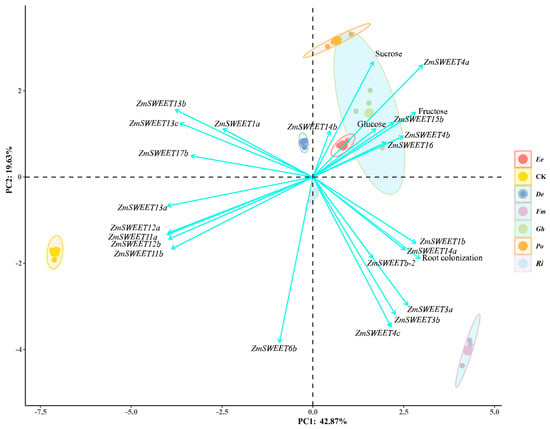
Figure 7.
Principal component analysis of root mycorrhizal colonization, sugars, and ZmSWEETs in maize under different AMF inoculations.
4. Discussion
AMF can colonize plant roots to form symbiotic associations that regulate plant growth, while the magnitude of mycorrhizal effects is influenced by multiple factors, including AMF species, soil environment, host plant type, and human activities [38]. Mycorrhizal colonization rate serves as a critical indicator of AMF expansion capacity within roots and reflects the symbiotic compatibility between the fungi and host plants [39,40]. In our study, all inoculated AMF strains successfully established symbiosis with maize roots, with Ri and Fm achieving peak colonization (66.88% and 68.33%) and Po exhibiting the lowest colonization (20.43%). These results demonstrate distinct host-symbiont compatibility hierarchies, identifying Ri and Fm as the most effective colonizers of maize roots under experimental conditions. This reflected that strain-dependent host recognition in AMF-maize systems [41]. The superior colonization rates of Ri and Fm likely result from strain-specific adaptations that enhance symbiotic compatibility including host recognition efficiency, cell invasion strategies, and nutrient-exchange synergy, which must be further studied. Ri and Fm achieved high colonization rates, whereas their functional performance (such as growth promotion) were not consistent, dependent on the combination of the plant and fungal genotypes and on environmental conditions [38,42].
Our results showed that AMF inoculation could significantly increase plant biomass, indicating that AMF could promote the absorption of nutrients by plants and increase plant biomass. Ri, Po, Ee, and Fm did not significantly alter plant height compared to CK, while De uniquely promoted plant height, yielding the tallest plants among all treatments. The differential influence of AMF species on maize growth reveals a complex interplay between symbiotic efficiency and functional specialization. This height advantage was particularly pronounced relative to Po, Ee, Gh, and Fm inoculations, suggesting that De may enhance auxin signaling or improve photosynthate allocation toward stem elongation [43,44]. In contrast, all AMF-inoculated plants exhibited significantly thicker stems than CK, underscoring a universal benefit of mycorrhization on radial development. Remarkably, Ee—despite its low colonization rate (21.30%)—produced the stoutest stems, exceeding other AMF treatments. This implies distinct physiological mechanisms: Ee may optimize carbon partitioning to stem vasculature or enhance water/nutrient flux through specialized hyphal networks. The stem response divergence highlights that colonization extent alone cannot predict functional outcomes; Gh showed higher colonization than Ee yet lower stem enhancement, indicating strain-specific functional hierarchies.
Photosynthetic capacity serves as a critical physiological foundation for plant growth and yield formation [45]. Singh et al. pointed out that inoculation with AMF could enhance photosynthetic capacity in leaves of host plants [46]. In our study, Ri, Ee, and Fm drove substantial increases in Pn (39.24–91.73%), reflecting superior efficiency in facilitating Rubisco activation and electron transport chain coordination [47]. The concurrent Tr elevation in these treatments (43.59–80.78%) aligns with AMF-mediated improvement in stomatal conductance and hydraulic conductivity, a well-documented mechanism for enhancing CO2 diffusion into mesophyll cells [48]. Notably, Ee’s exceptional Pn boost (91.73%)—despite its low root colonization rate (21.30%)—suggests that certain AMF strains prioritize the modulation of chloroplast development or photophosphorylation efficiency [49]. Conversely, the lack of significant Pn and Tr response in De and Gh indicates functional trade-offs; these strains may allocate resources toward growth structural investment (e.g., stem thickening) rather than photosynthetic optimization, as evidenced by De’s height promotion and Gh’s stem diameter neutrality. The observed 39.01–53.18% significant increase in Ci under De, Ri, Po, and Gh treatments could reflect a limitation in carboxylation or stomatal conductance, which warrants in-depth study.
GRSP, which is a metal-ion-containing glycoprotein produced by AMF, could enter the soil through the mycelium and spores of AMF. Subsequently, it will bind the micro-aggregates together, while also increasing the organic carbon content in the soil and enhancing the structural stability of the soil [34,50]. Our study revealed that the EEG elevation across De, Ri, Po, Ee, and Gh treatments (5.97–14.02%) demonstrated a photosynthate-derived carbon toward fresh glomalin production. However, the sharp divergence in DEG responses—where De, Ri, and Po increased DEG (3.10–6.56%) while Ee reduced it markedly—suggests distinct glomalin stabilization pathways among AMF taxa. Notably, the triad of De, Ri, and Po emerged as optimal GRSP producers, driving parallel increases in EEG and TG. Conversely, Ee’s strategy—elevating EEG while suppressing DEG—may represent an ecological adaptation. The non-responsiveness of Fm across GRSP fractions reinforces its earlier observed metabolic economy; despite high root colonization, it minimizes glomalin production, possibly redirecting carbon toward hyphal network expansion. This GRSP differentiation underscores that AMF functional traits extend beyond plant growth modulation to direct soil engineering—a critical consideration when selecting strains for ecosystem restoration versus crop productivity [51].
Sugar is a secondary messenger and an important organic permeate in plants. AMF could obtain hexose, mainly glucose, which is one of the most important categories of sugar, by the hydrolysis of sucrose from host plants. They could also convert these sugars into trehalose and glycogen, which are typical fungal carbohydrates [26,52]. Hyphal carbon demand alters source leaf sugar equilibrium [53]. In our study, a significant increase in sucrose content (93.04–544.50%) was observed in all AMF treatment groups, indicating an enhanced load capacity of the phloem and an improved coordination between the source and sink. Similar results were also reported by Wu et al. [52]. However, the extreme sucrose accumulation in Po-inoculated leaves (544.50%)—coupled with its parallel fructose surge (709.74%)—suggests exceptional carbon fixation efficiency. The elevated sucrose levels could potentially support increased growth and development by providing more carbon resources for processes such as root development, reproductive growth, or defense mechanisms [54]. Fructose responses exhibited greater AMF specificity: Po and Fm drove substantial increases, while De, Ri, Ee, and Gh exhibited no significant change, reflecting differential fructose modulation. The absence of fructose response in De, Ri, Ee, and Gh despite its sucrose elevation indicates preferential carbon channeling toward root allocation rather than hexose accumulation. The dynamic changes in glucose levels further highlighted the functional differences, for Po and Fm significantly increased the glucose content (77.62–148.20%), indicating the existence of glucose-induced effects specific to different strains. The change in fructose and glucose is due to the accelerated cleavage of sucrose into glucose and fructose by mycorrhization [55]. In our study, sucrose, glucose and fructose have a high correlation with the root colonization parameters in the PC1 cluster analysis, confirming that AMF symbionts actively adjust the carbon allocation pathways. However, more research needs to focus on how much sugars are distributed from the leaves to the roots and the root mycorrhizae.
It is known that the ZmSWEET family is phylogenetically divided into four clades. Clades I (SWEETs1–3) and II (SWEETs4–8) contain genes with glucose transport activity, Clade III (SWEETs9–15) genes mediate sucrose transport, and Clade IV (SWEETs16–17) includes fructose transport [56,57]. The consistent upregulation of ZmSWEET1b/3a/3b/4a/4b/14a and 16 across all AMF treatments reveals a core transcriptional reprogramming essential for mycorrhizal symbiosis. The conserved response may explain the evolutionary conservation among these ZmSWEETs, functional specialization, and host-fungal coordination. These genes likely mediate symbiotic carbon allocation by facilitating sucrose efflux toward fungal interfaces, as SWEET transporters are established regulators of apoplastic sugar transfer [58]. Notably, ZmSWEET4a/b homologs in rice (OsSWEET11/15) similarly exhibited AMF-inducible expression, suggesting conserved roles in delivering carbon rewards to fungal partners [59]. SWEET4 homologs in other species mediate sucrose efflux to support microbial symbionts (e.g., arbuscular mycorrhizae or rhizobia) [60]. In maize, the upregulation of the ZmSWEET gene indicates that it can facilitate the transportation of more sugar to the AMF, thereby promoting the growth of the AMF and the exchange of nutrients. The association of ZmSWEET4a/15b and 14b expression with sugars in PCA suggests that these transporters served as central metabolic hubs for photoassimilates toward mycorrhizal interfaces. This coupling implied that AMF colonization directly activates SWEET mediated sucrose efflux into the apoplast, where fungal hyphae access these carbon resources [61]. Some SWEET genes may have been recruited for mycorrhizal symbiosis in the early stage of plant evolution [28].
The localization results processed by Ri and Gh indicated that both of these two AMF strains exhibited distinct mycelial and sugar cluster growth characteristics. This suggests that these two AMF species perform exceptionally well in jointly establishing absorption capabilities. They could promote the growth of mycelia in the roots, then simultaneously upregulated the key ZmSWEET type proteins to facilitate carbon transfer. This aligned with sucrose accumulation in earlier observed of Ri treatment and EEG elevation in Gh treatment, revealing a unified strategy where fungal partners manipulate host transport machinery to optimize resource exchange. The dominant role of sucrose in driving the PC1 variation highlights its role as the main sugar species in leaves responsible for long-distance transport in the phloem of plants to roots and cleave glucose for root mycorrhizas [62]. Conversely, the uniform downregulation of ZmSWEET6b/11b/12a/13a-c and 17b indicated suppression of alternative carbon sinks—potentially redirecting photoassimilates away from non-symbiotic tissues to fuel mycorrhizal networks. Correlation analysis also presented a significantly negative correlation between root AMF colonization rates with ZmSWEET11b, ZmSWEET12a, ZmSWEET13a-c, and ZmSWEET17b. This transcriptional dichotomy aligns with the “symbiotic trade-off” model, where plants prioritize carbon investment into AMF-colonized roots over endogenous metabolic pathways [62]. The strain-dependent expression of remaining ZmSWEET genes highlighted AMF-specific modulation of host sugar transporters [63]. Their co-regulation of ZmSWEETs with root colonization further supports a feedback loop where AMF-derived signals (e.g., Myc-LCOs) transcriptionally reprogram sugar transport to sustain symbiosis [64]. Based on these, we can speculate that the inoculation of AMF reprogrammed ZmSWEET expression in maize by a species-specific manner, with core genes mediating carbon allocation to support symbiosis. This specific mechanism and which are core genes still requires further in-depth study.
5. Conclusions
In short, the inoculation of AMF had a positive promoting effect on the growth and leaf photosynthetic capacity of maize, and also had a positive promoting effect on the substrate GRSP levels. More importantly, AMF could affect the expression of the ZmSWEET sugar transporter genes in maize by consistently upregulating ZmSWEET1b/3a/3b/4a/4b/14a/16, and downregulating ZmSWEET6b/11b/12a/13a/13b/13c/17b. The colonization of AMF and sugar accumulation was closely related to the expression of specific ZmSWEET genes. High colonization reflects compatibility, but not necessarily functionality. Strain selection for agriculture should prioritize performance metrics (yield and stress resilience) over colonization rates alone. These findings disclosed that AMF mediates the accumulation of leaf sugar in maize through species-specific reprogramming of the expression of SWEET sugar transporter genes, so as to support the establishment and maintenance of the symbiotic relationship. Our study laid a foundation for further exploration of the signal pathways involved in the interaction of AMF and SWEET genes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture15161790/s1, Table S1: Specific primer sequences of selected genes in qRT-PCR.
Author Contributions
Conceptualization, Q.-S.W.; Methodology, G.-X.H.; Material preparation, G.-X.H. and C.G.; Data determination and statistical analysis, G.-X.H., F.-L.Z. and C.G.; Supervision, Q.-S.W. and C.G.; Writing—original draft preparation, G.-X.H.; Writing—review and editing, C.G., X.-B.G., F.-L.Z., Y.-N.Z. and Q.-S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31960616), the Construction of Modern Agriculture (tea) Industry Technology System (CARS-19) (China), Guizhou Key Laboratory of Agricultural Microbiology Construction Project (Qiankehe-pingtai[2025]029), Tea Science and Technology Experimental Demonstration Base Construction in Sinan County for 2025 ZSYS (Guizhou Province), and Research and Development of Special Microbial Fertilizer for Tea Seedling Cultivation Using Arbuscular Mycorrhizal Fungi [2025520101000147].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data supporting the findings of this study are included in this article.
Acknowledgments
The authors gratefully acknowledge support from as shown under Funding. Special thanks support from Guizhou Yibai Billion Biotechnology Co., Ltd., China (2025520101000147).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, J.J.; Yan, D.; Liu, R.; Wang, T.; Lian, Y.J.; Lu, Z.; Hong, Y.; Wang, Y.; Li, R. The physiological and molecular mechanisms of exogenous melatonin promote the seed germination of maize (Zea mays L.) under salt stress. Plants 2024, 13, 2142. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, D.P.; Kumar, S.; Yadav, O.P. Nutritive value of maize: Improvements, applications and constraints. In Maize: Nutrition Dynamics and Novel Uses; Chaudhary, D.P., Kumar, S., Langyan, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–17. [Google Scholar]
- National Bureau of Statistics of China. National Grain Production Data for 2024. Available online: https://www.stats.gov.cn/ (accessed on 20 February 2025).
- Borriello, R.; Lumini, E.; Girlanda, M.; Bonfante, P.; Bianciotto, V. Effects of different management practices on arbuscular mycorrhizal fungal diversity in maize fields by a molecular approach. Biol. Fert. Soils 2012, 48, 911–922. [Google Scholar] [CrossRef]
- Chen, X.; Wu, X.L.; Liu, S.R.; Hu, X.C.; Liu, C.Y. Effects of AMF on photosynthetic characteristics and gene expressions of tea plants under drought stress. Acta Hortic. Sin. 2024, 51, 2358–2370. [Google Scholar]
- Bunn, R.A.; Corrêa, A.; Joshi, J.; Kaiser, C.; Lekberg, Y.; Prescott, C.E.; Sala, A.; Karst, J. What determines transfer of carbon from plants to mycorrhizal fungi? New Phytol. 2024, 244, 1199–1215. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, X.F.; Zou, Y.N.; Srivastava, A.K.; Alqahtani, M.D.; Wu, Q.S. Negotiating soil water deficit in mycorrhizal trifoliate orange plants: A gibberellin pathway. Environ. Exp. Bot. 2024, 219, 105658. [Google Scholar] [CrossRef]
- Zou, Y.N.; Wu, Q.S.; Kuča, K. Unravelling the role of arbuscular mycorrhizal fungi in mitigating the oxidative burst of plants under drought stress. Plant Biol. 2021, 23, 50–57. [Google Scholar] [CrossRef]
- Ma, J.Q.; Wang, W.Q.; Yang, J.; Qin, S.F.; Yang, Y.S.; Sun, C.; Pei, G.; Zeeshan, M.; Liao, H.; Liu, L. Mycorrhizal symbiosis promotes the nutrient content accumulation and affects the root exudates in maize. BMC Plant Biol. 2022, 22, 64. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Feng, G.; George, T.S. Arbuscular mycorrhizal fungi have a greater role than root hairs of maize for priming the rhizosphere microbial community and enhancing rhizosphere organic P mineralization. Soil Biol. Biochem. 2022, 171, 108713. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Cao, M.A.; Wang, P.; Hashem, A.; Wirth, S.; Abd_Allah, E.F.; Wu, Q.S. Field inoculation of arbuscular mycorrhizal fungi improves fruit quality and root physiological activity of citrus. Agriculture 2021, 11, 1297. [Google Scholar] [CrossRef]
- Meng, L.L.; He, J.D.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Mycorrhiza-released glomalin-related soil protein fractions contribute to soil total nitrogen in trifoliate orange. Plant Soil Environ. 2020, 66, 183–189. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Z.; Han, Y.; Cao, J. Impact of silver nanoparticles on arbuscular mycorrhizal fungi and glomalin-related soil proteins in the rhizosphere of maize seedlings. Diversity 2024, 16, 273. [Google Scholar] [CrossRef]
- Ramírez-Flores, M.R.; Bello-Bello, E.; Rellán-Álvarez, R.; Sawers, R.J.; Olalde-Portugal, V. Inoculation with the mycorrhizal fungus Rhizophagus irregularis modulates the relationship between root growth and nutrient content in maize (Zea mays ssp. mays L.). Plant Direct 2019, 3, e00192. [Google Scholar] [CrossRef]
- Bisht, A.; Gupta, M.M. Arbuscular mycorrhiza fungi resources for sustainable and climate-smart cultivation of maize. In Fungal Resources for Sustainable Economy: Current Status and Future Perspectives; Singh, I., Rajpal, V.R., Navi, S.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2023; pp. 299–317. [Google Scholar]
- Aguégué, M.R.; Ahoyo Adjovi, N.R.; Agbodjato, N.A.; Noumavo, P.A.; Assogba, S.; Salami, H.; Salako, V.K.; Ramón, R.; Baba-Moussa, F.; Adjanohoun, A. Efficacy of native strains of arbuscular mycorrhizal fungi on maize productivity on ferralitic soil in Benin. Agric. Res. 2021, 11, 627–641. [Google Scholar] [CrossRef]
- Assogba, S.; Noumavo, P.A.; Dagbenonbakin, G.; Agbodjato, N.; Akpode, C.; Koda, A.D.; Aguegue, R.M.; Bade, F.; Adjanohoun, A.; Rodriguez, A.F.; et al. Improvement of maize productivity (Zea mays L.) by mycorrhizal inoculation on ferruginous soil in center of Benin. Int. J. Sustain. Agric. Res. 2017, 4, 63–76. [Google Scholar] [CrossRef][Green Version]
- Wu, X.J.; Li, Z.F.; Guo, P.P.; Zhang, L. Effects of different arbuscular mycorrhizal fungi on the growth and the nitrogen and phosphorus absorption of sweet corn seedlings. J. Trop. Biol. 2023, 14, 167–172. [Google Scholar][Green Version]
- Koda, A.D.; Dagbenonbakin, G.; Assogba, F.; Noumavo, P.A.; Agbodjato, N.A.; Assogba, S.; Aguegue, R.M.; Adjanohoun, A.; Rivera, R.; de la Noval Pons, B.M.; et al. Maize (Zea mays L.) response to mycorrhizal fertilization on ferruginous soil of northern Benin. J. Exp. Biol. Agric. Sci. 2018, 6, 919–928. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wu, Q.S. Influence of sugar metabolism on the dialogue between arbuscular mycorrhizal fungi and plants. Hortic. Adv. 2023, 1, 2. [Google Scholar] [CrossRef]
- Walmsley, A.R.; Barrett, M.P.; Bringaud, F.; Gould, G.W. Sugar transporters from bacteria, parasites and mammals: Structure-Activity relationships. Trend. Biochem. Sci. 1998, 23, 476–481. [Google Scholar] [CrossRef]
- Julius, B.T.; Leach, K.A.; Tran, T.M.; Mertz, R.A.; Braun, D.M. Sugar transporters in plants: New insights and discoveries. Plant Cell Physiol. 2017, 58, 1442–1460. [Google Scholar] [CrossRef]
- Chen, L.Q.; Hou, B.H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.Q.; Guo, W.J.; Kim, J.G.; Underwood, W.; Chaudhuri, B. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef]
- Ji, J.L.; Yang, L.M.; Fang, Z.Y.; Zhang, Y.Y.; Zhuang, M.; Lv, H.H.; Wang, Y. Plant SWEET family of sugar transporters: Structure, evolution and biological functions. Biomolecules 2022, 12, 205. [Google Scholar] [CrossRef]
- Zheng, F.L.; Wang, Y.J.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Mycorrhizae with Funneliformis mosseae regulate the trehalose synthesis and sucrose cleavage for enhancing drought tolerance in trifoliate orange. Sci. Hortic. 2024, 337, 113486. [Google Scholar] [CrossRef]
- Díaz, V.; Villalobos, M.; Arriaza, K.; Flores, K.; Hernández-Saravia, L.P.; Velásquez, A. Decoding the dialog between plants and arbuscular mycorrhizal fungi: A molecular genetic perspective. Genes 2025, 16, 143. [Google Scholar] [CrossRef] [PubMed]
- Manck-Götzenberger, J.; Requena, N. Arbuscular mycorrhiza symbiosis induces a major transcriptional reprogramming of the potato SWEET sugar transporter family. Front. Plant Sci. 2016, 7, 487. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhao, S.; Zhou, Y.; Yang, G.; Chen, A.; Li, X.; Wang, J.; Tian, J.; Liao, H.; Wang, X. The soybean sugar transporter GmSWEET6 participates in sucrose transport towards fungi during arbuscular mycorrhizal symbiosis. Plant Cell Environ. 2024, 47, 1041–1052. [Google Scholar] [CrossRef]
- An, J.; Zeng, T.; Ji, C.; de Graaf, S.; Zheng, Z.; Xiao, T.T.; Deng, X.; Xiao, S.; Bisseling, T.; Limpens, E. A Medicago truncatula SWEET transporter implicated in arbuscule maintenance during arbuscular mycorrhizal symbiosis. New Phytol. 2019, 224, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.H.; Duncan, L.W.; Eissenstat, D.M. Carbohydrate allocation patterns in citrus genotypes as affected by phosphorus nutrition, mycorrhizal colonization and mycorrhizal dependency. New Phytol. 1997, 135, 335–343. [Google Scholar] [CrossRef][Green Version]
- Liang, S.M.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Transcriptomic analysis reveals potential roles of polyamine and proline metabolism in waterlogged peach roots inoculated with Funneliformis mosseae and Serendipita indica. Tree Physiol. 2025, 45, tpaf013. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Wu, Q.S.; Li, Y.; Zou, Y.N.; He, X.H. Arbuscular mycorrhiza mediates glomalin-related soil protein production and soil enzyme activities in the rhizosphere of trifoliate orange grown under different P levels. Mycorrhiza 2015, 25, 121–130. [Google Scholar] [CrossRef]
- Wen, Y.; Zhou, L.J.; Xu, Y.J.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Growth performance and osmolyte regulation of drought-stressed walnut plants are improved by mycorrhiza. Agriculture 2024, 14, 367. [Google Scholar] [CrossRef]
- Zhu, J.L.; Zhou, L.; Li, T.F.; Ruan, Y.Y.; Zhang, A.; Dong, X.; Zhu, Y.; Li, C.; Fan, J. Genome-wide investigation and characterization of SWEET gene family with focus on their evolution and expression during hormone and abiotic stress response in maize. Genes 2022, 13, 1682. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Berger, F.; Gutjahr, C. Factors affecting plant responsiveness to arbuscular mycorrhiza. Curr. Opin. Plant Biol. 2021, 59, 101994. [Google Scholar] [CrossRef]
- Gange, A.C.; Ayres, R.L. On the relation between arbuscular mycorrhizal colonization and plant ‘benefit’. Oikos 1999, 87, 615–621. [Google Scholar] [CrossRef]
- Lu, J.-N. The Effects of Arbuscular Mycorrhizal Fungi on the Growth of Three Grassland Plants. Master’s Thesis, Hebei Agricultural University, Baoding, China, 2023. [Google Scholar]
- Ren, Z.; Xia, T.Y.; Chen, L.J.; Han, L.; Chen, Z.B.; Bai, H.L. Effect of different AMF on physiological related indexes of corn. Southwest China J. Agric. Sci. 2015, 28, 563–568. [Google Scholar]
- Ramírez-Flores, M.R.; Perez-Limon, S.; Li, M.; Barrales-Gamez, B.; Albinsky, D.; Paszkowski, U.; Olalde-Portugal, V.; Sawers, R.J. The genetic architecture of host response reveals the importance of arbuscular mycorrhizae to maize cultivation. eLIFE 2020, 9, e61701. [Google Scholar] [CrossRef]
- Liu, R.C.; Yang, L.; Zou, Y.N.; Wu, Q.S. Root-associated endophytic fungi modulate endogenous auxin and cytokinin levels to improve plant biomass and root morphology of trifoliate orange. Hortic. Plant J. 2023, 9, 463–472. [Google Scholar] [CrossRef]
- Mathur, S.; Sharma, M.P.; Jajoo, A. Improved photosynthetic efficacy of maize (Zea mays L.) plants with arbuscular mycorrhizal fungi (AMF) under high temperature stress. J. Photochem. Photobiol. B Biol. 2018, 180, 149–154. [Google Scholar] [CrossRef]
- Chen, F.; Yan, S.; Wang, H.L.; Zhang, K.; Zhao, F.N.; Huang, X.Y. Study on gas exchange parameters and water use efficiency of spring wheat leaves under different levels of water stress. Arid. Zone Res. 2021, 38, 821–832. [Google Scholar]
- Singh, M.; Sharma, J.G.; Giri, B. Augmentative role of arbuscular mycorrhizal fungi, Piriformospora indica, and plant growth-promoting bacteria in mitigating salinity stress in maize (Zea mays L.). J. Plant Growth Regul. 2024, 43, 1195–1215. [Google Scholar] [CrossRef]
- Zhu, X.C.; Song, F.B.; Liu, S.Q.; Liu, T.D. Effects of arbuscular mycorrhizal fungus on photosynthesis and water status of maize under high temperature stress. Plant Soil 2011, 346, 189–199. [Google Scholar] [CrossRef]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Boldt, K.; Pörs, Y.; Haupt, B.; Bitterlich, M.; Kühn, C.; Grimm, B.; Franken, P. Photochemical processes, carbon assimilation and RNA accumulation of sucrose transporter genes in tomato arbuscular mycorrhiza. J. Plant Physiol. 2011, 168, 1256–1263. [Google Scholar] [CrossRef]
- Liu, R.C.; Gao, W.Q.; Srivastava, A.K.; Zou, Y.N.; Kuča, K.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Differential effects of exogenous glomalin-related soil proteins on plant growth of trifoliate orange through regulating auxin changes. Front. Plant Sci. 2021, 12, 745402. [Google Scholar] [CrossRef]
- Holátko, J.; Brtnický, M.; Kučerík, J.; Kotianová, M.; Elbl, J.; Kintl, A.; Kynický, J.; Benada, O.; Datta, R.; Jansa, J. Glomalin–truths, myths, and the future of this elusive soil glycoprotein. Soil Biol. Biochem. 2021, 153, 108116. [Google Scholar] [CrossRef]
- Wu, Q.S.; Srivastava, A.K.; Li, Y. Effects of mycorrhizal symbiosis on growth behavior and carbohydrate metabolism of trifoliate orange under different substrate P levels. J. Plant Growth Regul. 2015, 34, 499–508. [Google Scholar] [CrossRef]
- Salmeron-Santiago, I.A.; Martínez-Trujillo, M.; Valdez-Alarcón, J.J.; Pedraza-Santos, M.E.; Santoyo, G.; Pozo, M.J.; Chávez-Bárcenas, A.T. An updated review on the modulation of carbon partitioning and allocation in arbuscular mycorrhizal plants. Microorganisms 2021, 10, 75. [Google Scholar] [CrossRef]
- Göbel, M.; Fichtner, F. Functions of sucrose and trehalose 6-phosphate in controlling plant development. J. Plant Physiol. 2023, 291, 154140. [Google Scholar] [CrossRef]
- Du, Y.L.; Zhao, Q.; Chen, L.R.; Yao, X.D.; Zhang, W.; Zhang, B.; Xie, F. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef]
- Eom, J.S.; Chen, L.Q.; Sosso, D.; Julius, B.T.; Lin, I.W.; Qu, X.Q.; Braun, D.M.; Frommer, W.B. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr. Opin. Plant Biol. 2015, 25, 53–62. [Google Scholar] [CrossRef]
- Feng, L.; Frommer, W.B. Structure and function of SemiSWEET and SWEET sugar transporters. Trends Biochem. Sci. 2015, 40, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Saida, Y.; Yoshimizu, M.; Takanashi, K.; Sosso, D.; Frommer, W.B.; Yazaki, K. Molecular characterization of LjSWEET3, a sugar transporter in nodules of Lotus japonicus. Plant Cell Physiol. 2017, 58, 298–306. [Google Scholar] [PubMed]
- Yang, J.; Luo, D.; Yang, B.; Frommer, W.B.; Eom, J.S. SWEET 11 and 15 as key players in seed filling in rice. New Phytol. 2018, 218, 604–615. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Yang, C.; Tian, Z.; Li, J. AtSWEET4, a hexose facilitator, mediates sugar transport to axial sinks and affects plant development. Sci. Rep. 2016, 6, 24563. [Google Scholar] [CrossRef]
- Kryukov, A.A.; Gorbunova, A.O.; Kudriashova, T.R.; Yakhin, O.I.; Lubyanov, A.A.; Malikov, U.M.; Shishova, M.F.; Kozhemyakov, A.P.; Yurkov, A.P. Sugar transporters of the SWEET family and their role in arbuscular mycorrhiza. Vavilov J. Genet. Breed. 2021, 25, 754. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, W.; Xie, Q.; Liu, N.A.; Liu, L.; Wang, D.; Zhang, X.; Yang, C.; Chen, X.; Tang, D. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175. [Google Scholar] [CrossRef]
- Li, Q.S.; Srivastava, A.K.; Zou, Y.N.; Wu, Q.S. Field inoculation responses of arbuscular mycorrhizal fungi versus endophytic fungi on sugar metabolism associated changes in fruit quality of lane late navel orange. Sci. Hortic. 2023, 308, 111587. [Google Scholar] [CrossRef]
- Rich, M.K.; Vigneron, N.; Libourel, C.; Keller, J.; Xue, L.; Hajheidari, M.; Radhakrishnan, G.V.; Le Ru, A.; Diop, S.I.; Potente, G.; et al. Lipid exchanges drove the evolution of mutualism during plant terrestrialization. Science 2021, 372, 864–868. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).