Seeds Mineral Profile and Ash Content of Thirteen Different Genotypes of Cultivated and Wild Cardoon over Three Growing Seasons
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Research Site, Crop Management and Plant Materials
2.2. Sampling and Determination of Seed, Ash, and Mineral Content
2.3. Statistical Analysis
3. Results
3.1. Influence of Genotype on Ash Content and Macro-Elements Content of Cardoon Seeds
3.2. Influence of Genotype on Micro-Elements Content of Cardoon Seeds
3.3. Influence of Different Growing Seasons on Ash Content and Mineral Content
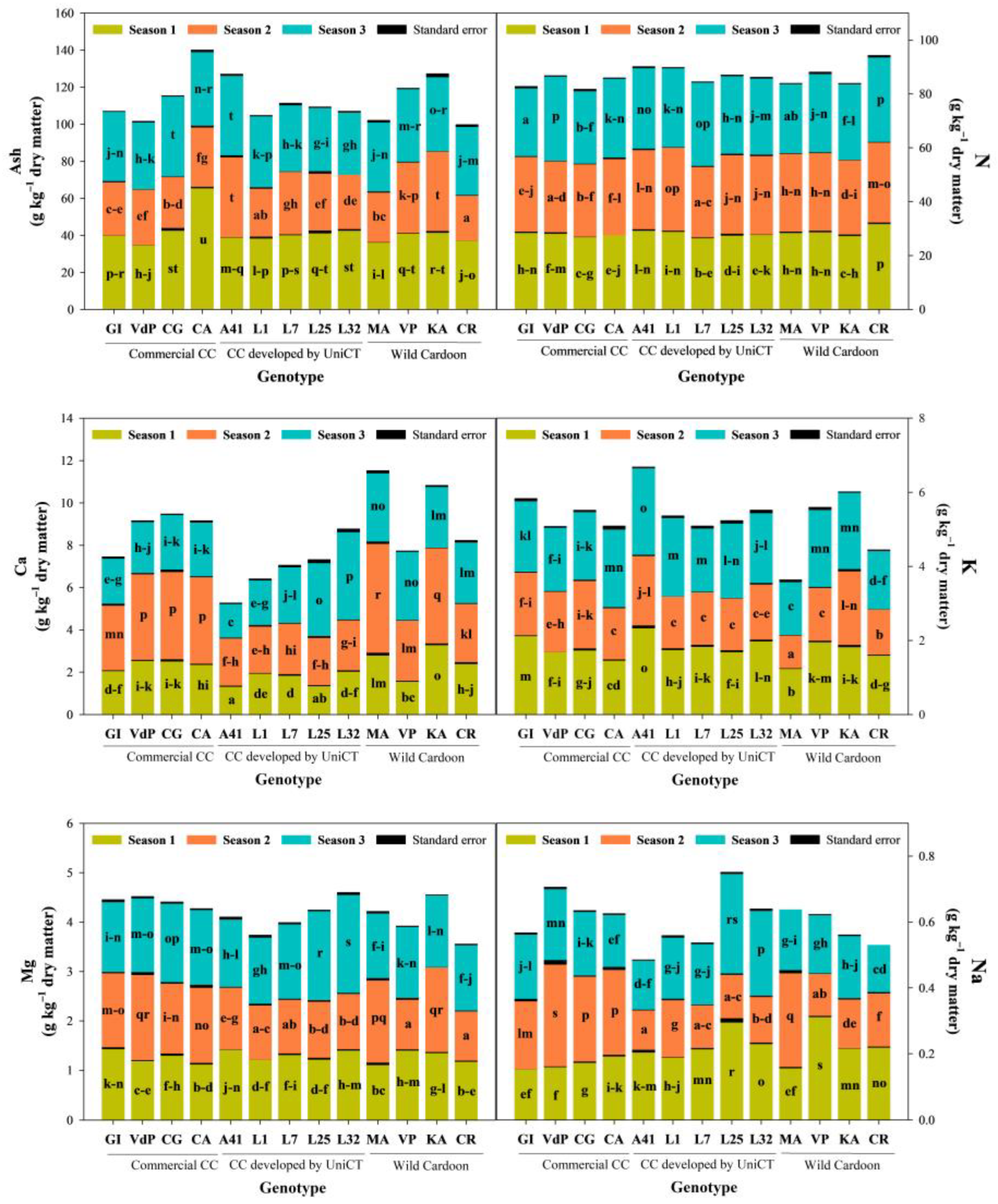
3.4. Influence of the ‘Genotype × Growing Season’ Interaction on Ash and Macro-Elements Content of Cardoon Seeds
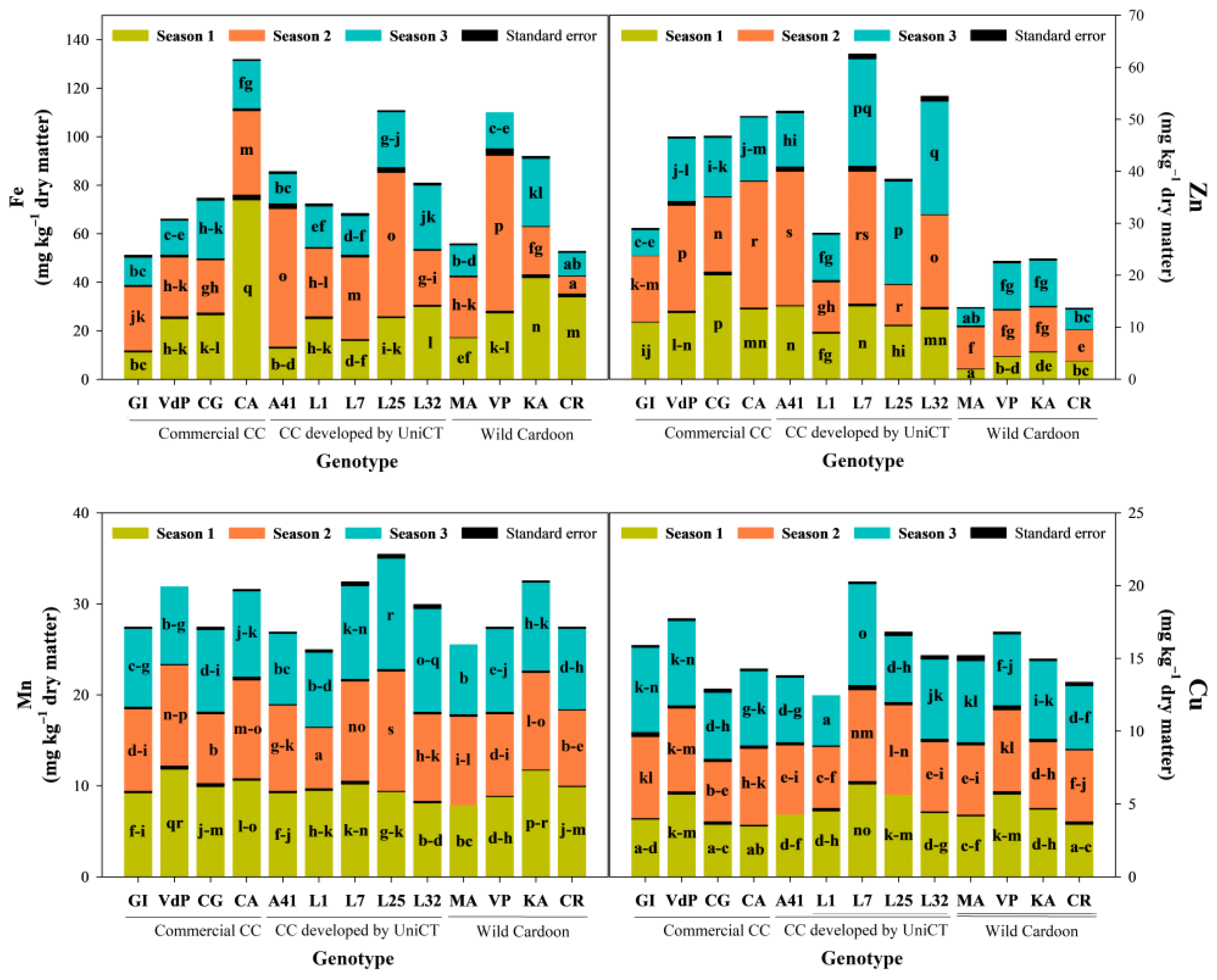
3.5. Influence of the ‘Genotype × Growing Season’ Interaction on Micro-Elements Content of Cardoon Seeds
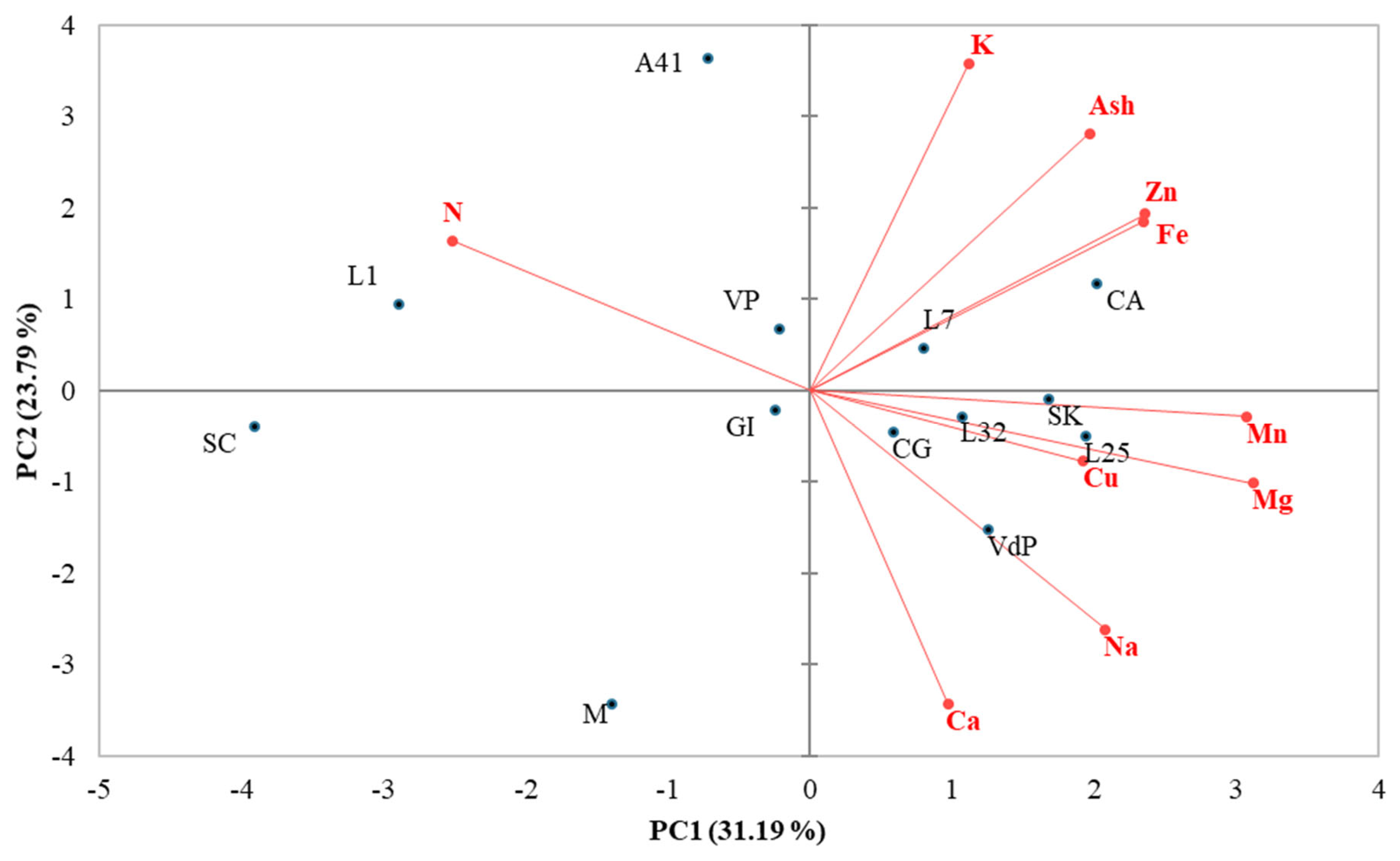
3.6. Principal Component Analysis and Coefficient of Variability on Mineral Elements Content of Cardoon Seeds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pandino, G.; Lombardo, S.; Mauromicale, G. Mineral profile in globe artichoke as affected by genotype, head part and environment. J. Sci. Food Agric. 2011, 91, 302–308. [Google Scholar] [CrossRef]
- Sałata, A.; Sękara, A.; Pandino, G.; Mauromicale, G.; Lombardo, S. Living Mulch as Sustainable Tool to Improve Leaf Biomass and Phytochemical Yield of Cynara cardunculus var. altilis. Agronomy 2023, 13, 1274. [Google Scholar] [CrossRef]
- Pandino, G.; Mauromicale, G. Globe artichoke and cardoon forms between traditional and modern uses. Acta Hort. 2020, 1284, 1–18. [Google Scholar] [CrossRef]
- Ben Salem, M.; Affes, H.; Athmouni, K.; Ksouda, K.; Dhouibi, R.; Sahnoun, Z.; Hammami, S.; Zeghal, K.M. Chemicals Compositions, Antioxidant and Anti-Inflammatory Activity of Cynara scolymus Leaves Extracts, and Analysis of Major Bioactive Polyphenols by HPLC. Evid. Based Complement. Alternat. Med. 2017, 2017, 4951937. [Google Scholar] [CrossRef]
- Gostin, A.I.; Waisundara, V.Y. Edible flowers as functional food: A review on artichoke (Cynara cardunculus L.). Trends Food Sci. Technol. 2019, 86, 381–391. [Google Scholar] [CrossRef]
- Mandim, F.; Petropoulos, S.A.; Pinela, J.; Dias, M.I.; Giannoulis, K.D.; Kostić, M.; Soković, M.; Queijo, B.; Santos-Buelga, C.; Ferreira, I.C.F.R.; et al. Chemical composition and biological activity of cardoon (Cynara cardunculus L. var. altilis) seeds harvested at different maturity stages. Food Chem. 2022, 369, 130875. [Google Scholar] [CrossRef]
- Scavo, A.; Rial, C.; Molinillo, J.M.G.; Varela, R.M.; Mauromicale, G.; Macias, F.A. The extraction procedure improves the allelopathic activity of cardoon (Cynara cardunculus var. altilis) leaf allelochemicals. Ind. Crops Prod. 2019, 128, 479–487. [Google Scholar] [CrossRef]
- Ben Amira, A.; Blecker, C.; Richel, A.; Arias, A.A.; Fickers, P.; Francis, F.; Besbes, S.; Attia, H. Influence of the ripening stage and the lyophilization of wild cardoon flowers on their chemical composition, enzymatic activities of extracts and technological properties of cheese curds. Food Chem. 2018, 245, 919–925. [Google Scholar] [CrossRef]
- Brás, T.; Guerreiro, O.; Duarte, M.F.; Neves, L.A. Impact of extraction parameters and concentration by nanofiltration on the recovery of phenolic compounds from Cynara cardunculus var. altilis: Assessment of antioxidant activity. Ind. Crops Prod. 2015, 67, 137–142. [Google Scholar] [CrossRef]
- Mandim, F.; Petropoulos, S.A.; Pinela, J.; Dias, M.I.; Kostic, M.; Soković, M.; Ferreira, I.C.F.R.; Santos-Buelga, C.; Barros, L. Phenolic Composition and Antioxidant, Anti-Inflammatory, Cytotoxic, and Antimicrobial Activities of Cardoon Blades at Different Growth Stages. Biology 2022, 11, 699. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Calhelha, R.C.; Danalatos, N.; Barros, L.; Ferreira, I.C.F.R. How extraction method affects yield, fatty acids composition and bioactive properties of cardoon seed oil. Ind. Crops Prod. 2018, 124, 459–465. [Google Scholar] [CrossRef]
- Portis, E.; Mauromicale, G.; Mauro, R.; Acquadro, A.; Scaglione, D.; Lanteri, S. Construction of a reference molecular linkage map of globe artichoke (Cynara cardunculus var. scolymus). Theor. Appl. Genet. 2009, 120, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Mandim, F.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Petropoulos, S.A.; Barros, L. The wide spectrum of industrial applications for cultivated cardoon (Cynara cardunculus L. var. Altilis DC.): A review. Food Chem. 2023, 423, 136275. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Berenguer, M.; Gutiérrez-Pozo, M.; Serna-Escolano, V.; Giménez, M.J.; Zapata, P.J. Influence of Artichoke Antioxidant Activity in Their Susceptibility to Suffer Frost Injury. Antioxidants 2023, 12, 1960. [Google Scholar] [CrossRef]
- Rossi, R.; Picuno, P.; Fagnano, M.; Amato, M. Soil reinforcement potential of cultivated cardoon (Cynara cardunculus L.): First data of root tensile strength and density. Catena 2022, 211, 106016. [Google Scholar] [CrossRef]
- Mauromicale, G.; Sortino, O.; Pesce, G.R.; Agnello, M.; Mauro, R.P. Suitability of cultivated and wild cardoon as a sustainable bioenergy crop for low input cultivation in low quality Mediterranean soils. Ind. Crops Prod. 2014, 57, 82–89. [Google Scholar] [CrossRef]
- La Iacona, M.; Lombardo, S.; Mauromicale, G.; Scavo, A.; Pandino, G. Allelopathic Activity of Three Wild Mediterranean Asteraceae: Silybum marianum, Cynara cardunculus var. sylvestris, Galactites tomentosus. Agronomy 2024, 14, 575. [Google Scholar] [CrossRef]
- Conceição, C.; Martins, P.; Alvarenga, N.; Dias, J.; Lamy, E.; Garrido, L.; Gomes, S.; Freitas, S.; Belo, A.; Brás, T.; et al. Cynara cardunculus: Use in Cheesemaking and Pharmaceutical Applications. In Technological Approaches for Novel Applications in Dairy Processing; InTech: Rang-du-Fliers, France, 2018. [Google Scholar] [CrossRef]
- Elsebai, M.F.; Mocan, A.; Atanasov, A.G. Cynaropicrin: A Comprehensive Research Review and Therapeutic Potential as an Anti-Hepatitis C Virus Agent. Front. Pharmacol. 2016, 7, 472. [Google Scholar] [CrossRef]
- Gominho, J.; Curt, M.D.; Lourenço, A.; Fernández, J.; Pereira, H. Cynara cardunculus L. as a biomass and multi-purpose crop: A review of 30 years of research. Biomass Bioenergy 2018, 109, 257–275. [Google Scholar] [CrossRef]
- Khaldi, S.; Naouari, M.; Ben Jemaa, A. Cardoon (Cynara cardunculus L.) oil from cultivated and wild Tunisian populations and its antimicrobial activity. Ind. Crops Prod. 2021, 171, 113852. [Google Scholar] [CrossRef]
- Almeida, C.M.; Simões, I. Cardoon-based rennets for cheese production. Appl. Microbiol. Biotechnol. 2018, 102, 4675–4686. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.H.; Andrade, M.A.; Vilarinho, F.; Castanheira, I.; Fernando, A.L.; Loizzo, M.R.; Sanches Silva, A. A new insight on cardoon: Exploring new uses besides cheese making with a view to zero waste. Foods 2020, 9, 564. [Google Scholar] [CrossRef] [PubMed]
- Barracosa, P.; Barracosa, M.; Pires, E. Cardoon as a sustainable crop for biomass and bioactive compounds production. Chem. Biodivers. 2019, 16, e1900498. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Pereira, C.; Tzortzakis, N.; Barros, L.; Ferreira, I.C.F.R. Nutritional value and bioactive compounds characterization of plant parts from Cynara cardunculus L. (Asteraceae) cultivated in central Greece. Front. Plant Sci. 2018, 9, 459. [Google Scholar] [CrossRef]
- Zumbo, A.; Tardiolo, G.; Genovese, C.; Sutera, A.M.; Raccuia, S.A.; D’Alessandro, E. Cardoon (Cynara cardunculus L. var. altilis) seeds presscake: A natural by-product for pigs feeding. Nat. Prod. Res. 2022, 36, 4551–4556. [Google Scholar] [CrossRef]
- Mauromicale, G.; Pesce, G.R.; Curt, M.D.; Fernández, J.; González, J.; Gominho, J.; Tabla, R.; Roa, I.; Portis, E. Cynara cardunculus as a Multiuse Crop. In The Globe Artichoke Genome. Compendium of Plant Genomes; Portis, E., Acquadro, A., Lanteri, S., Eds.; Springer: Cham, Switzerland, 2019; pp. 65–98. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Ibrahim, M.; Rashid, U.; Nawaz, M.; Ali, S.; Hussain, A.; Gull, M. Biomass production for bioenergy using marginal lands. Sustain. Prod. Consum. 2017, 9, 3–21. [Google Scholar] [CrossRef]
- Pesce, G.R.; Negri, M.; Bacenetti, J.; Mauromicale, G. The biomethane, silage and biomass yield obtainable from three accessions of Cynara cardunculus. Ind. Crops Prod. 2017, 103, 233–239. [Google Scholar] [CrossRef]
- Lanteri, S.; Portis, E.; Acquadro, A.; Mauro, R.P.; Mauromicale, G. Morphology and SSR fingerprinting of newly developed Cynara cardunculus genotypes exploitable as ornamentals. Euphytica 2012, 184, 311–321. [Google Scholar] [CrossRef]
- Barbanera, M.; Castellini, M.; Tasselli, G.; Turchetti, B.; Cotana, F.; Buzzini, P. Prediction of the environmental impacts of yeast biodiesel production from cardoon stalks at industrial scale. Fuel 2021, 283, 118967. [Google Scholar] [CrossRef]
- Bartocci, P.; Bidini, G.I.; Cotana, F.; Fantozzi, F. Energy Balance of cardoon (Cynara cardunculus L.) cultivation and pyrolysis. In Perennial Biomass Crops for a Resource-Constrained; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- De Domenico, S.; Strafella, L.; D’Amico, L.; Mastrorilli, M.; Ficarella, A.; Carlucci, P.; Santino, A. Biodiesel production from Cynara cardunculus L. and Brassica carinata A. Braun seeds and their suitability as fuels in compression ignition engines. Ital. J. Agron. 2016, 11, 47–56. [Google Scholar] [CrossRef]
- Fernández, J.; Curt, M.D.; Aguado, P.L. Industrial applications of Cynara cardunculus L. for energy and other uses. Ind. Crop. Prod. 2006, 24, 222–229. [Google Scholar] [CrossRef]
- Cabiddu, A.; Contini, S.; Gallo, A.; Lucini, L.; Bani, P.; Decandia, M.; Molle, G.; Piluzza, G.; Sulas, L. In vitro fermentation of cardoon seed press cake—A valuable byproduct from biorefinery as a novel supplement for small ruminants. Ind. Crops Prod. 2019, 130, 420–427. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Giosafatto, C.V.L.; Porta, R. Biorefining of seed oil cakes as industrial co-streams for production of innovative bioplastics. A review. Trends Food Sci. Technol. 2021, 109, 259–270. [Google Scholar] [CrossRef]
- Silva, L.R.; Jacinto, T.A.; Coutinho, P. Bioactive Compounds from Cardoon as Health Promoters in Metabolic Disorders. Foods 2022, 11, 336. [Google Scholar] [CrossRef]
- Zayed, A.; Serag, A.; Farag, M.A. Cynara cardunculus L.: Outgoing and potential trends of phytochemical, industrial, nutritive and medicinal merits. J. Funct. Foods 2020, 69, 103937. [Google Scholar] [CrossRef]
- Mandim, F.; Dias, M.I.; Pinela, J.; Barracosa, P.; Ivanov, M.; Stojković, D.; Soković, M.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C.F.R. Chemical composition and in vitro biological activities of cardoon (Cynara cardunculus L. var. altilis DC.) seeds as influenced by viability: Chemical prospection and bioactivity of cardoon seeds. Food Chem. 2020, 323, 126838. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.; Fernandes, Â.; Pereira, C.; Tzortzakis, N.; Vaz, J.; Soković, M.; Barros, L.; Ferreira, I.C.F.R. Bioactivities, chemical composition and nutritional value of Cynara cardunculus L. seeds. Food Chem. 2019, 289, 404–412. [Google Scholar] [CrossRef]
- Angelova, V.; Nemska, M.P.; Uzunova, G.; Krustev, L. Chemical composition of cardoon (Cynara Cardunculus L.) grown in south bulgaria. Agrofor 2019, 4, 100–110. [Google Scholar] [CrossRef]
- Piluzza, G.; Molinu, M.G.; Re, G.A.; Sulas, L. Phenolic compounds content and antioxidant capacity in cardoon achenes from different head orders. Nat. Prod. Res. 2020, 34, 2071–2075. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Moglia, A.; Portis, E.; Lanteri, S.; Mauromicale, G. Leaf polyphenol profile and SSR-based fingerprinting of new segregant Cynara cardunculus genotypes. Front. Plant Sci. 2015, 5, 800. [Google Scholar] [CrossRef]
- Matus, I.M.; Gonzales, G.; del Poso, A. Evaluation of phenotypic variation in a Chilean collection of garlic (Allium sativum L.) clones using multivariate analysis. Plant Genet. Resour. Newsl. 1996, 117, 31–36. [Google Scholar]
- Cajarville, C.; González, J.; Repetto, J.L.; Alvir, M.R.; Rodríguez, C.A. Nutritional evaluation of cardoon (Cynara cardunculus) seed for ruminants. Anim. Feed Sci. Technol. 2000, 87, 203–213. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Campos, G.; Pinto, E.; Oliveira, A.S.; Almeida, A.; de Pinho, P.G.; Alves, G.; Silva, L.R. Essential and non-essential elements, and volatile organic compounds for the discrimination of twenty-three sweet cherry cultivars from Fundão, Portugal. Food Chem. 2022, 367, 130503. [Google Scholar] [CrossRef] [PubMed]
- Delgado, C.; Rosegrant, M.; Steinfeld, H.; Ehui, S.; Courbois, C. Livestock to 2020: The Next Food Revolution. Outlook Agric. 2001, 30, 27–29. [Google Scholar] [CrossRef]
- López-Alonso, M. Trace Minerals and Livestock: Not Too Much Not Too Little. ISRN Vet. Sci. 2012, 2012, 704825. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G. Minerals profile of two globe artichoke cultivars as affected by NPK fertilizer regimes. Food Res. Int. 2017, 100, 95–99. [Google Scholar] [CrossRef]
- Borah, S.; Baruah, A.M.; Das, A.K.; Borah, J. Determination of Mineral Content in Commonly Consumed Leafy Vegetables. Food Anal. Methods 2009, 2, 226–230. [Google Scholar] [CrossRef]
- Kopsell, D.E.; Kopsell, D.A.; Lefsrud, M.G.; Curran-Celentano, J. Variability in Elemental Accumulations Among Leafy Brassica oleracea Cultivars and Selections. J. Plant Nutr. 2004, 27, 1813–1826. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Phenolic acids and flavonoids in leaf and floral stem of cultivated and wild Cynara cardunculus L. genotypes. Food Chem. 2011, 126, 417–422. [Google Scholar] [CrossRef]
- Raigón, M.D.; Prohens, J.; Muñoz-Falcón, J.E.; Nuez, F. Comparison of eggplant landraces and commercial varieties for fruit content of phenolics, minerals, dry matter and protein. J. Food Compost. Anal. 2008, 21, 370–376. [Google Scholar] [CrossRef]
- Silva, C.A.; Abreu, Â. de F.B.; Ramalho, M.A.P.; Corrêa, A.D. Interaction Genotype by Season and Its Influence on the Identification of Beans with High Content of Zinc and Iron. Bragantia 2012, 71, 336–341. [Google Scholar] [CrossRef]
- Tziouvalekas, M.; Tigka, E.; Kargiotidou, A.; Beslemes, D.; Irakli, M.; Pankou, C.; Arabatzi, P.; Aggelakoudi, M.; Tokatlidis, I.; Mavromatis, A.; et al. Seed Yield, Crude Protein and Mineral Nutrients of Lentil Genotypes Evaluated across Diverse Environments under Organic and Conventional Farming. Plants 2022, 11, 3328. [Google Scholar] [CrossRef] [PubMed]
- Ierna, A.; Sortino, O.; Mauromicale, G. Biomass, Seed and Energy Yield of Cynara Cardunculus L. as Affected by Environment and Season. Agronomy 2020, 10, 1548. [Google Scholar] [CrossRef]
- Elia, A.; Conversa, G. Mineral nutrition aspects in artichoke growing. Acta Hortic. 2007, 730, 239–249. [Google Scholar] [CrossRef]
- Scavo, A.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Mauromicale, G.; Macias, F.A. Influence of Genotype and Harvest Time on the Cynara Cardunculus L. Sesquiterpene Lactone Profile. J. Agric. Food Chem. 2019, 67, 6487–6496. [Google Scholar] [CrossRef]
- Mandim, F.; Petropoulos, S.A.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Effect of Harvesting Time on the Chemical Composition of Cynara Cardunculus L. Var. Altilis Blades. Agronomy 2022, 12, 1705. [Google Scholar] [CrossRef]
- Borgognone, D.; Rouphael, Y.; Cardarelli, M.; Lucini, L.; Colla, G. Changes in Biomass, Mineral Composition, and Quality of Cardoon in Response to NO3-:Cl- Ratio and Nitrate Deprivation from the Nutrient Solution. Front. Plant Sci. 2016, 7, 978. [Google Scholar] [CrossRef]
- Sorooshzadeh, A.; Barthakur, N.N.; Isobe, S.; Sase, S. Water stress and photoperiod during seed-filling affect calcium distribution in soybean. Environ. Control. Biol. 1999, 37, 49–56. [Google Scholar] [CrossRef]
- Nwosu, D.J.; Olubiyi, M.R.; Aladele, S.E.; Apuyor, B.; Okere, A.U.; Lawal, A.I.; Afolayan, G.; Ojo, A.O.; Nwadike, C.; Lee, M.-C.; et al. Proximate And Mineral Composition Of Selected Soybean Genotypes In Nigeria. J. Plant Dev. 2019, 26, 67–76. [Google Scholar] [CrossRef]
- Xu, Q.; Fu, H.; Zhu, B.; Hussain, H.A.; Zhang, K.; Tian, X.; Duan, M.; Xie, X.; Wang, L. Potassium improves drought stress tolerance in plants by affecting root morphology, root exudates and microbial diversity. Metabolites 2021, 11, 131. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 3rd ed.; Academic: San Diego, CA, USA, 2012; 643p. [Google Scholar]
- Choukri, H.; Hejjaoui, K.; El-Baouchi, A.; El haddad, N.; Smouni, A.; Maalouf, F.; Thavarajah, D.; Kumar, S. Heat and drought stress impact on phenoly, grain yield, and nutritional quality of lentil (Lens culinaris Medikus). Front. Nutr. 2020, 7, 596307. [Google Scholar] [CrossRef] [PubMed]
- Pandino, G.; Lombardo, S.; Williamson, G.; Mauromicale, G. Flavonoids content of Cynara cardunculus L. wild and cultivated germplasm accessions. Acta Hort. 2013, 983, 81–86. [Google Scholar] [CrossRef]
- Briat, J.F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef]
| Parameter | |
|---|---|
| Meteorological variables | |
| Temperature min (°C) 2017 a | 13.2 |
| Temperature min (°C) 2018 b | 13.1 |
| Temperature min (°C) 2019 c | 13.4 |
| Temperature max (°C) 2017 a | 23.8 |
| Temperature max (°C) 2018 b | 23.4 |
| Temperature max (°C) 2019 c | 24.0 |
| Rainfall (mm) 2017 d | 448.2 |
| Rainfall (mm) 2018 e | 758.1 |
| Rainfall (mm) 2019 d | 571.3 |
| Soil characteristics | |
| Clay (<0.002 mm) (%) | 46 |
| Silt (0.02–0.002 mm) (%) | 36 |
| Sand (2–0.02 mm) (%) | 18 |
| Total N (%) | 0.1 |
| Organic Matter (%) | 1.1 |
| P2O5 available (ppm) | 11 |
| K2O (exchangeable) (ppm) | 704 |
| Electrical conductivity (mS cm−1) | 0.2 |
| pH | 7.2 |
| Cation exchange capacity (meq 100 g−1) | 32.4 |
| Ca (ppm) | 5435 |
| Mg (ppm) | 308 |
| Na (ppm) | 216 |
| K (ppm) | 577 |
| Fe (ppm) | 118 |
| Zn (ppm) | 3.0 |
| Mn (ppm) | 162 |
| Cu (ppm) | 4.0 |
| Plant Material | Genotype | Acronyms | |
|---|---|---|---|
| C. cardunculus L. var. altilis DC. | Commercial CC | Gigante Inerme | GI |
| Verde di Peralta | VdP | ||
| Cardo Gobbo | CG | ||
| Cardo Avorio | CA | ||
| CC developed by Catania University (UniCt) | Altilis 41 | A41 | |
| Linea 1 | L1 | ||
| Linea 7 | L7 | ||
| Linea 25 | L25 | ||
| Linea 32 | L32 | ||
| C. cardunculus L. var. sylvestris (Lamk) Fiori | Wild Cardoon | Marsala | MA |
| Val Paraiso | VP | ||
| Kamarina | KA | ||
| Creta | CR | ||
| Source of Variation | Degree of Freedom | Ash Content | Mineral Element | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Ca | K | Mg | Na | Fe | Zn | Mn | Cu | |||
| Genotype (G) | 12 | 11.4 *** | 58.7 *** | 19.1 *** | 23.0 *** | 14.0 *** | 23.1 *** | 16.5 *** | 50.5 *** | 57.6 *** | 54.1 *** |
| Growing season (S) | 2 | 81.5 *** | 11.1 * | 72.5 *** | 73.0 *** | 68.0 *** | 23.1 *** | 66.4 *** | 38.7 *** | 16.9 *** | 33.9 *** |
| (G) × (S) | 24 | 7.1 *** | 30.1 *** | 8.2 *** | 4.1 *** | 18.0 *** | 53.8 *** | 17.1 *** | 10.8 *** | 25.4 *** | 12.0 *** |
| Plant Material | Genotype | Ash Content | Macro-Elements | ||||
|---|---|---|---|---|---|---|---|
| N | Ca | K | Mg | Na | |||
| Commercial CC | Gigante Inerme | 35.36 ± 2.18 cd | 27.13 ± 0.67 ab | 2.41 ± 0.20 c | 1.91 ± 0.08 f | 1.45 ± 0.02 fg | 0.19 ± 0.01 c |
| Verde di Peralta | 33.60 ± 1.18 ab | 28.58 ± 0.92 de | 3.00 ± 0.33 g | 1.68 ± 0.02 c | 1.47 ± 0.10 fg | 0.23 ± 0.03 e | |
| Cardo Gobbo | 37.78 ± 3.30 e | 26.90 ± 0.17 a | 3.08 ± 0.33 g | 1.79 ± 0.03 e | 1.43 ± 0.05 ef | 0.21 ± 0.02 d | |
| Cardo Avorio | 45.78 ± 6.44 h | 28.39 ± 0.32 c–e | 3.00 ± 0.35 g | 1.65 ± 0.15 c | 1.39 ± 0.08 de | 0.20 ± 0.02 d | |
| Mean ± SE | 38.13 ± 2.03 | 27.75 ± 0.32 | 2.87 ± 0.15 | 1.76 ± 0.04 | 1.43 ± 0.08 | 0.21 ± 0.01 | |
| CC developed by Catania University | Altilis 41 | 41.65 ± 0.89 g | 29.51 ± 0.25 fg | 1.72 ± 0.17 a | 2.18 ± 0.10 g | 1.34 ± 0.03 cd | 0.16 ± 0.02 a |
| Linea 1 | 34.19 ± 2.66 bc | 29.65 ± 0.38 g | 2.08 ± 0.05 b | 1.75 ± 0.13 de | 1.22 ± 0.05 a | 0.18 ± 0.00 c | |
| Linea 7 | 36.60 ± 1.26 de | 27.88 ± 0.97 cd | 2.29 ± 0.15 c | 1.65 ± 0.08 c | 1.30 ± 0.08 bc | 0.18 ± 0.02 b | |
| Linea 25 | 35.38 ± 2.05 cd | 28.49 ± 0.39 de | 2.34 ± 0.39 c | 1.70 ± 0.11 cd | 1.38 ± 0.13 de | 0.24 ± 0.04 f | |
| Linea 32 | 35.19 ± 2.53 c | 28.39 ± 0.28 c-e | 2.85 ± 0.41 f | 1.78 ± 0.10 e | 1.50 ± 0.16 g | 0.21 ± 0.02 d | |
| Mean ± SE | 36.60 ± 0.96 | 28.78 ± 0.25 | 2.26 ± 0.13 | 1.82 ± 0.06 | 1.35 ± 0.05 | 0.19 ± 0.01 | |
| Wild Cardoon | Marsala | 33.44 ± 2.15 ab | 27.64 ± 0.67 bc | 3.73 ± 0.46 i | 1.18 ± 0.10 a | 1.36 ± 0.10 d | 0.21 ± 0.02 d |
| Val Paraiso | 39.44 ± 0.62 f | 28.90 ± 0.22 ef | 2.54 ± 0.32 d | 1.82 ± 0.13 e | 1.28 ± 0.09 b | 0.21 ± 0.04 d | |
| Kamarina | 41.46 ± 0.75 g | 27.68 ± 0.20 bc | 3.55 ± 0.31 h | 1.96 ± 0.05 f | 1.51 ± 0.07 g | 0.18 ± 0.01 c | |
| Creta | 32.77 ± 2.73 a | 30.96 ± 0.47 h | 2.67 ± 0.09 e | 1.46 ± 0.08 b | 1.17 ± 0.06 a | 0.17 ± 0.02 b | |
| Mean ± SE | 36.78 ± 1.15 | 28.79 ± 0.35 | 3.12 ± 0.19 | 1.61 ± 0.08 | 1.33 ± 0.05 | 0.19 ± 0.01 | |
| Plant Material | Genotype | Micro-Elements | |||
|---|---|---|---|---|---|
| Fe | Zn | Mn | Cu | ||
| Commercial CC | Gigante Inerme | 16.25 ± 3.16 a | 9.51 ± 1.48 c | 8.92 ± 0.17 c | 5.10 ± 0.38 ef |
| Verde di Peralta | 21.09 ± 2.28 b | 15.01 ± 1.67 e | 10.43 ± 0.67 e | 5.71 ± 0.09 g | |
| Cardo Gobbo | 23.96 ± 1.02 d | 15.18 ± 1.65 e | 8.82 ± 0.46 bc | 4.07 ± 0.20 a | |
| Cardo Avorio | 42.65 ± 10.31 h | 16.61 ± 2.41 f | 10.25 ± 0.31 e | 4.60 ± 0.36 b–d | |
| Mean ± SE | 25.99 ± 3.32 | 14.08 ± 1.03 | 9.60 ± 0.26 | 4.87 ± 0.18 | |
| CC developed by Catania University | Altilis 41 | 27.23 ± 9.36 e | 16.69 ± 2.95 f | 8.78 ± 0.35 bc | 4.49 ± 0.11 bc |
| Linea 1 | 23.18 ± 2.13 cd | 9.01 ± 0.23 c | 8.09 ± 0.54 a | 4.03 ± 0.23 a | |
| Linea 7 | 21.87 ± 3.74 bc | 20.00 ± 2.10 h | 10.43 ± 0.22 e | 6.52 ± 0.17 h | |
| Linea 25 | 35.72 ± 7.44 g | 12.52 ± 2.32 d | 11.53 ± 0.72 f | 5.45 ± 0.31 fg | |
| Linea 32 | 26.18 ± 1.47 e | 17.61 ± 1.52 g | 9.65 ± 0.60 d | 4.87 ± 0.21 de | |
| Mean ± SE | 26.84 ± 2.54 | 15.17 ± 1.12 | 9.70 ± 0.31 | 5.07 ± 0.18 | |
| Wild Cardoon | Marsala | 18.08 ± 2.25 a | 4.38 ± 1.15 a | 8.41 ± 0.44 ab | 4.83 ± 0.28 c–e |
| Val Paraiso | 35.20 ± 9.39 g | 7.31 ± 0.93 b | 8.96 ± 0.10 c | 5.37 ± 0.19 fg | |
| Kamarina | 29.71 ± 4.16 f | 7.46 ± 0.72 b | 10.66 ± 0.39 e | 4.83 ± 0.17 c–e | |
| Creta | 16.78 ± 5.49 a | 4.42 ± 0.47 a | 9.01 ± 0.31 c | 4.26 ± 0.25 ab | |
| Mean ± SE | 24.94 ± 3.20 | 5.89 ± 0.51 | 9.26 ± 0.24 | 4.82 ± 0.13 | |
| Growing Season | ||||
|---|---|---|---|---|
| Season 1 | Season 2 | Season 3 | ||
| Ash Content (g kg−1 DM) | 41.70 ± 1.47 c | 35.73 ± 1.18 a | 37.95 ± 0.59 b | |
| Macro-elements (g kg−1 DM) | N | 28.33 ± 0.27 a | 28.34 ± 0.27 a | 28.74 ± 0.38 b |
| Ca | 2.16 ± 0.11 a | 3.22 ± 0.20 c | 2.75 ± 0.13 b | |
| K | 1.79 ± 0.05 b | 1.51 ± 0.06 a | 1.90 ± 0.05 c | |
| Mg | 1.28 ± 0.02 a | 1.32 ± 0.05 b | 1.50 ± 0.04 c | |
| Na | 0.21 ± 0.01 b | 0.19 ± 0.01 a | 0.19 ± 0.01 a | |
| Micro-elements (mg kg−1 DM) | Fe | 28.21 ± 3.12 b | 32.36 ± 3.30 c | 17.41 ± 1.16 a |
| Zn | 10.26 ± 1.01 a | 14.47 ± 1.41 c | 11.20 ± 1.18 b | |
| Mn | 9.73 ± 0.24 b | 9.65 ± 0.33 b | 9.23 ± 0.26 a | |
| Cu | 4.62 ± 0.18 a | 5.10 ± 0.17 b | 5.07 ± 0.17 b | |
| Trait | Common Principal Component Coefficients | |
|---|---|---|
| PC1 | PC2 | |
| Ash | 0.488 | 0.609 |
| Ca | 0.241 | −0.742 |
| K | 0.277 | 0.773 |
| Mg | 0.774 | −0.220 |
| Na | 0.516 | −0.566 |
| N | −0.624 | 0.354 |
| Fe | 0.583 | 0.400 |
| Zn | 0.585 | 0.418 |
| Mn | 0.763 | −0.062 |
| Cu | 0.477 | −0.168 |
| Eigenvalue | 3.119 | 2.379 |
| Variability (%) | 31.19 | 23.79 |
| Cumulative (%) | 31.19 | 54.98 |
| Plant Material | Genotype | Ash Content | Mineral Element | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Ca | K | Mg | Na | Fe | Zn | Mn | Cu | |||
| Commercial CC | Gigante Inerme | 15.1 | 6.1 | 20.6 | 10.5 | 3.8 | 13.6 | 47.6 | 38.2 | 4.6 | 18.2 |
| Verde di Peralta | 8.6 | 7.8 | 27.2 | 2.8 | 16.4 | 29.6 | 26.5 | 27.3 | 15.6 | 4.0 | |
| Cardo Gobbo | 21.4 | 1.5 | 26.6 | 3.7 | 9.4 | 19.0 | 10.4 | 26.6 | 12.6 | 11.8 | |
| Cardo Avorio | 34.5 | 2.8 | 28.2 | 21.6 | 15.0 | 22.5 | 59.2 | 35.5 | 7.3 | 19.1 | |
| CC developed by Catania University | Altilis 41 | 5.2 | 2.1 | 24.1 | 11.2 | 6.2 | 24.6 | 84.2 | 43.2 | 9.7 | 6.0 |
| Linea 1 | 19.1 | 3.2 | 5.9 | 18.7 | 9.4 | 4.3 | 22.5 | 6.2 | 16.3 | 13.7 | |
| Linea 7 | 8.5 | 8.5 | 15.9 | 11.9 | 14.8 | 22.0 | 41.9 | 25.8 | 5.2 | 6.6 | |
| Linea 25 | 14.2 | 3.3 | 40.4 | 16.4 | 23.5 | 35.7 | 51.0 | 45.3 | 15.3 | 14.1 | |
| Linea 32 | 17.6 | 2.5 | 35.6 | 13.5 | 26.2 | 26.8 | 13.8 | 21.2 | 15.2 | 10.7 | |
| Wild Cardoon | Marsala | 15.8 | 5.9 | 30.1 | 21.3 | 18.3 | 29.2 | 30.5 | 64.3 | 12.9 | 14.0 |
| Val Paraiso | 3.8 | 1.9 | 30.6 | 17.3 | 17.0 | 41.7 | 65.3 | 31.1 | 2.7 | 8.8 | |
| Kamarina | 4.4 | 1.8 | 21.4 | 6.2 | 10.9 | 17.1 | 34.3 | 23.5 | 9.0 | 8.8 | |
| Creta | 20.4 | 3.8 | 8.4 | 13.0 | 12.6 | 21.2 | 80.1 | 26.2 | 8.5 | 14.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giménez-Berenguer, M.; Salicola, S.A.; Formenti, C.; Giménez, M.J.; Mauromicale, G.; Zapata, P.J.; Lombardo, S.; Pandino, G. Seeds Mineral Profile and Ash Content of Thirteen Different Genotypes of Cultivated and Wild Cardoon over Three Growing Seasons. Agriculture 2025, 15, 1228. https://doi.org/10.3390/agriculture15111228
Giménez-Berenguer M, Salicola SA, Formenti C, Giménez MJ, Mauromicale G, Zapata PJ, Lombardo S, Pandino G. Seeds Mineral Profile and Ash Content of Thirteen Different Genotypes of Cultivated and Wild Cardoon over Three Growing Seasons. Agriculture. 2025; 15(11):1228. https://doi.org/10.3390/agriculture15111228
Chicago/Turabian StyleGiménez-Berenguer, Marina, Salvatore Alfio Salicola, Claudia Formenti, María José Giménez, Giovanni Mauromicale, Pedro Javier Zapata, Sara Lombardo, and Gaetano Pandino. 2025. "Seeds Mineral Profile and Ash Content of Thirteen Different Genotypes of Cultivated and Wild Cardoon over Three Growing Seasons" Agriculture 15, no. 11: 1228. https://doi.org/10.3390/agriculture15111228
APA StyleGiménez-Berenguer, M., Salicola, S. A., Formenti, C., Giménez, M. J., Mauromicale, G., Zapata, P. J., Lombardo, S., & Pandino, G. (2025). Seeds Mineral Profile and Ash Content of Thirteen Different Genotypes of Cultivated and Wild Cardoon over Three Growing Seasons. Agriculture, 15(11), 1228. https://doi.org/10.3390/agriculture15111228













