Indirect Regulation of SOC by Different Land Uses in Karst Areas Through the Modulation of Soil Microbiomes and Aggregate Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Plot Setting and Sample Collection
2.3. Soil Analysis
2.4. Statistical Analysis
3. Results
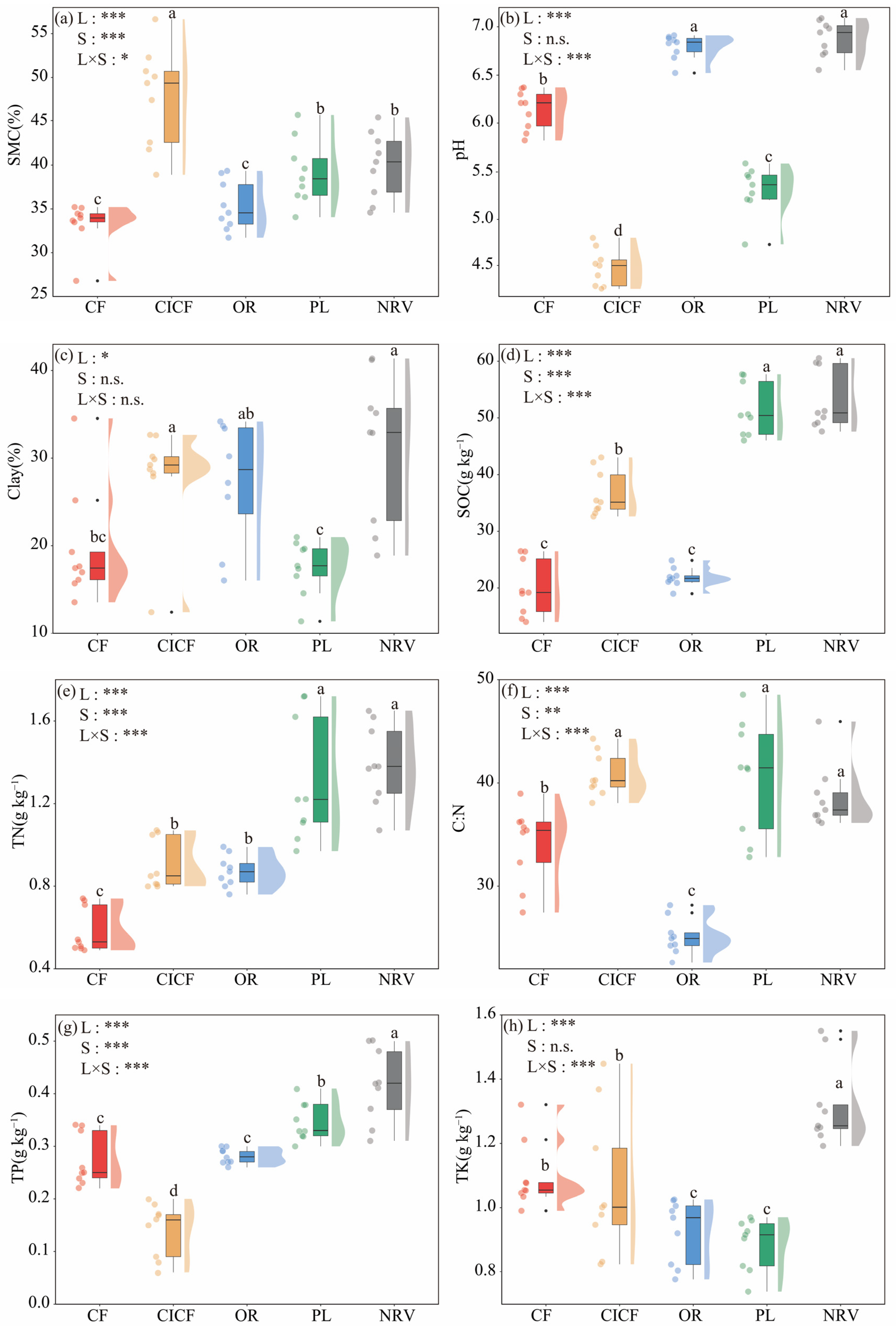
3.1. Impact of Different Land Uses on Soil Physicochemical Properties
3.2. Effects of Different Land Uses on Microbial Diversity and Community Composition
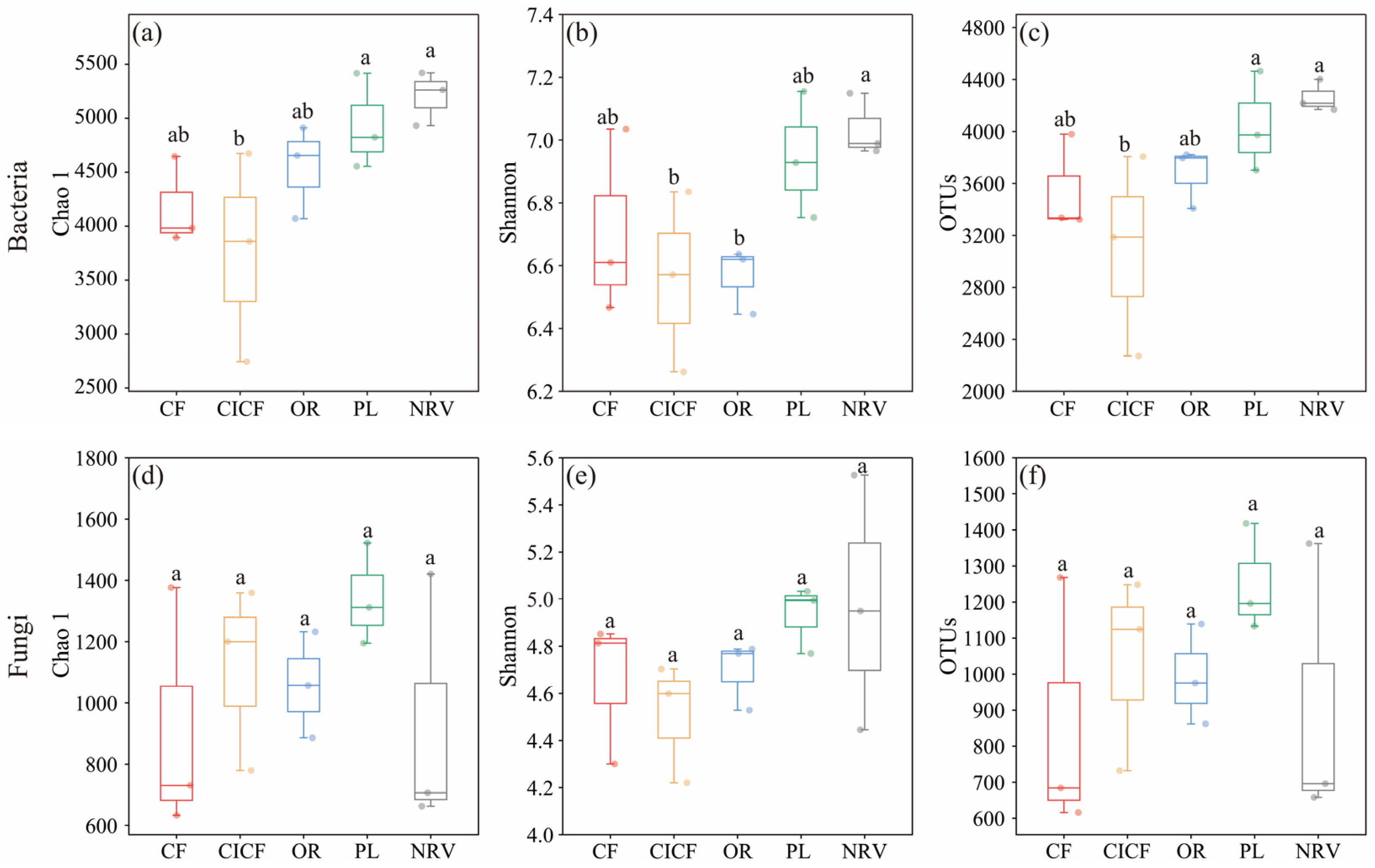
3.2.1. Microbial Alpha Diversity
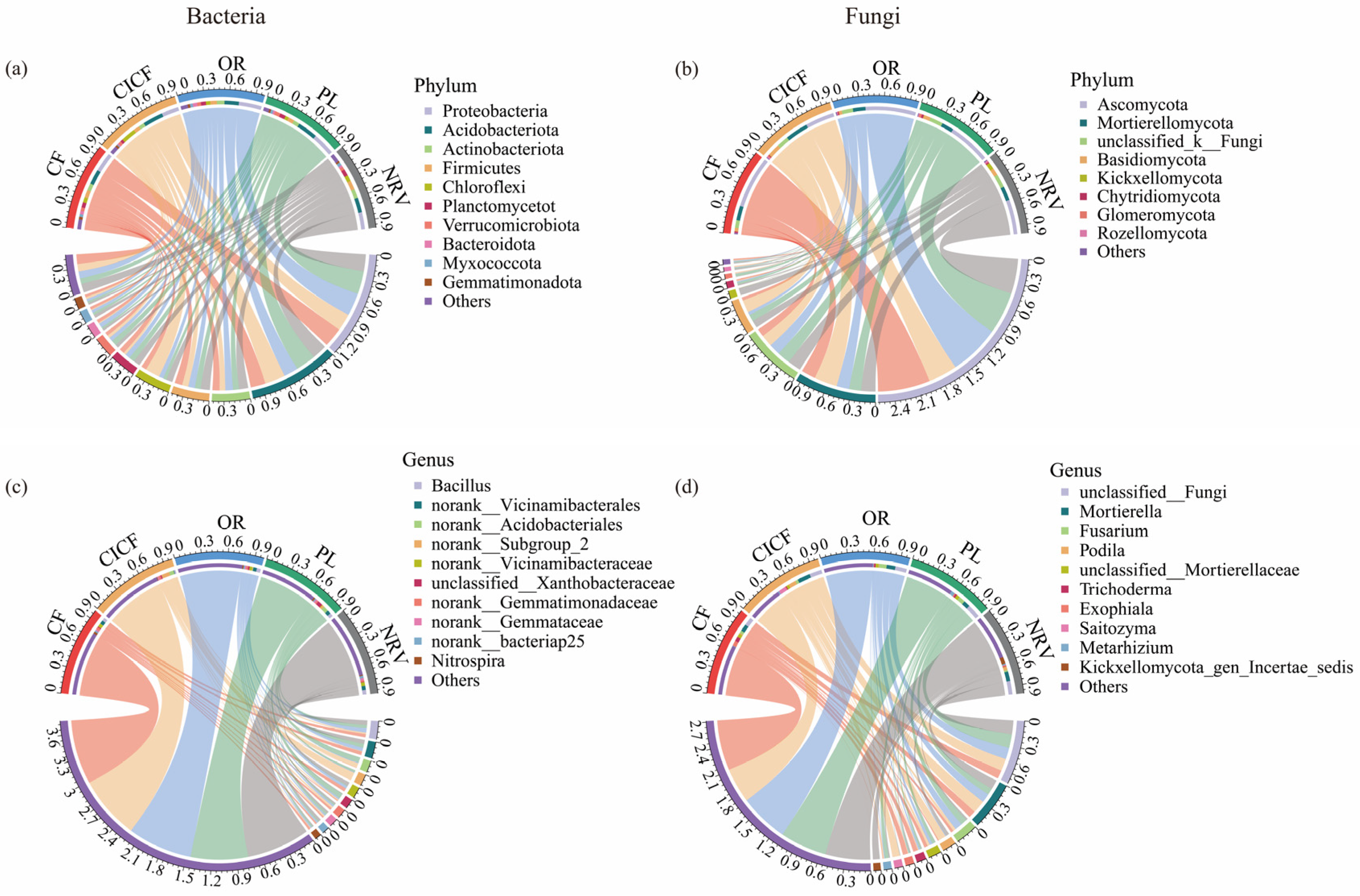
3.2.2. Microbial Community Composition
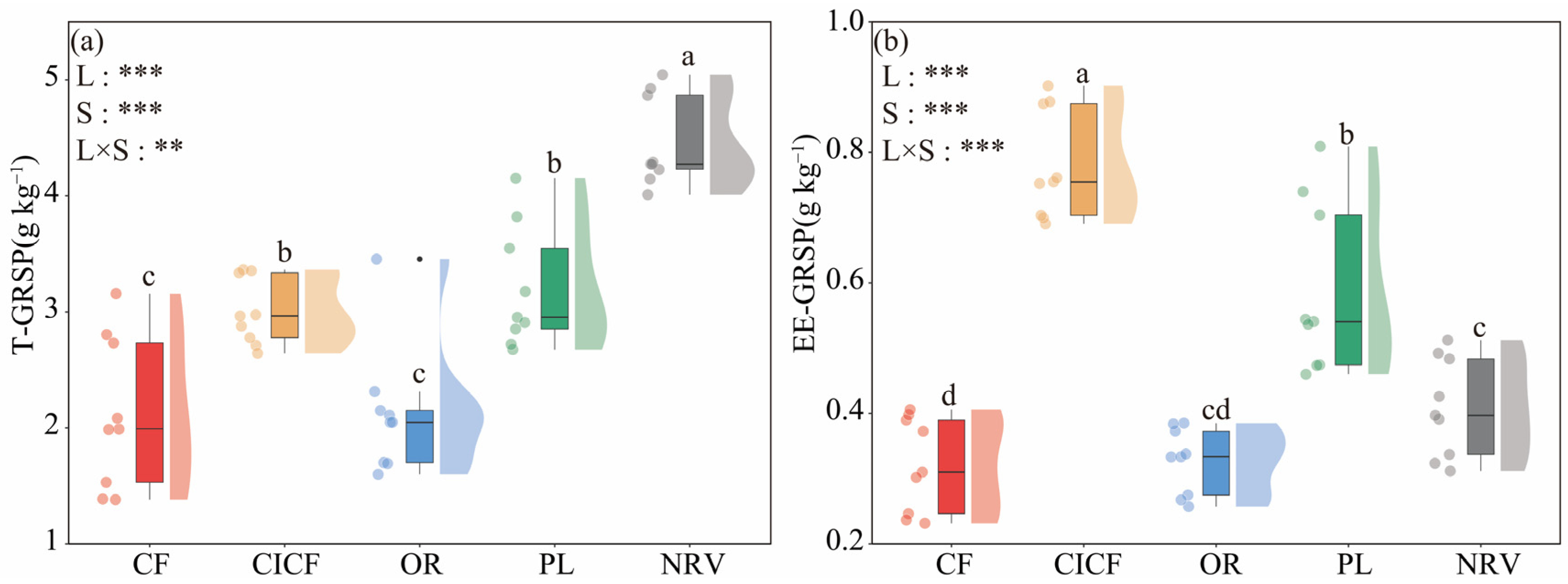
3.3. Effects of Different Land Uses on GRSP Content
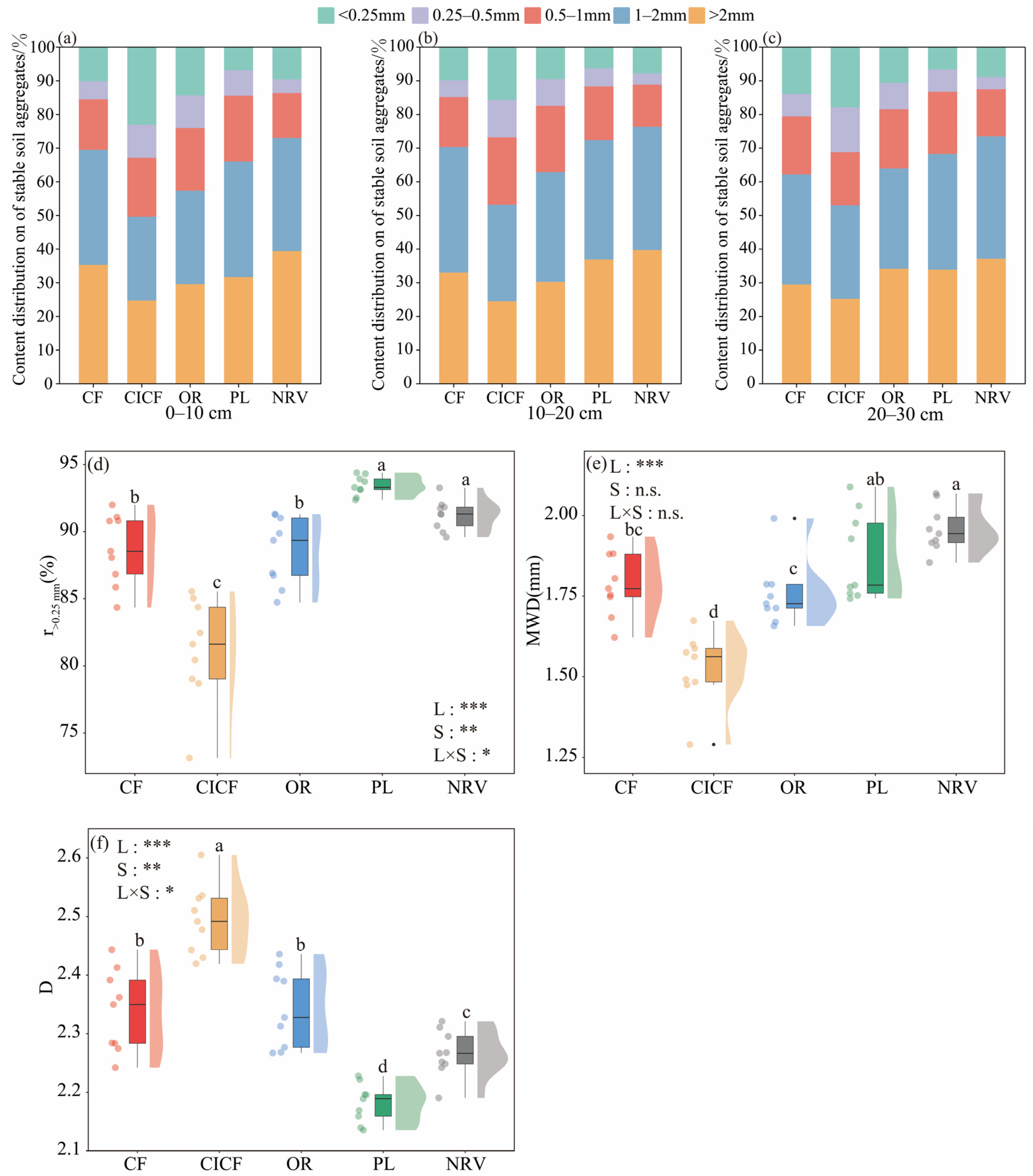
3.4. Effects of Different Land Uses on the Distribution of Soil Aggregate Content and Its Stability
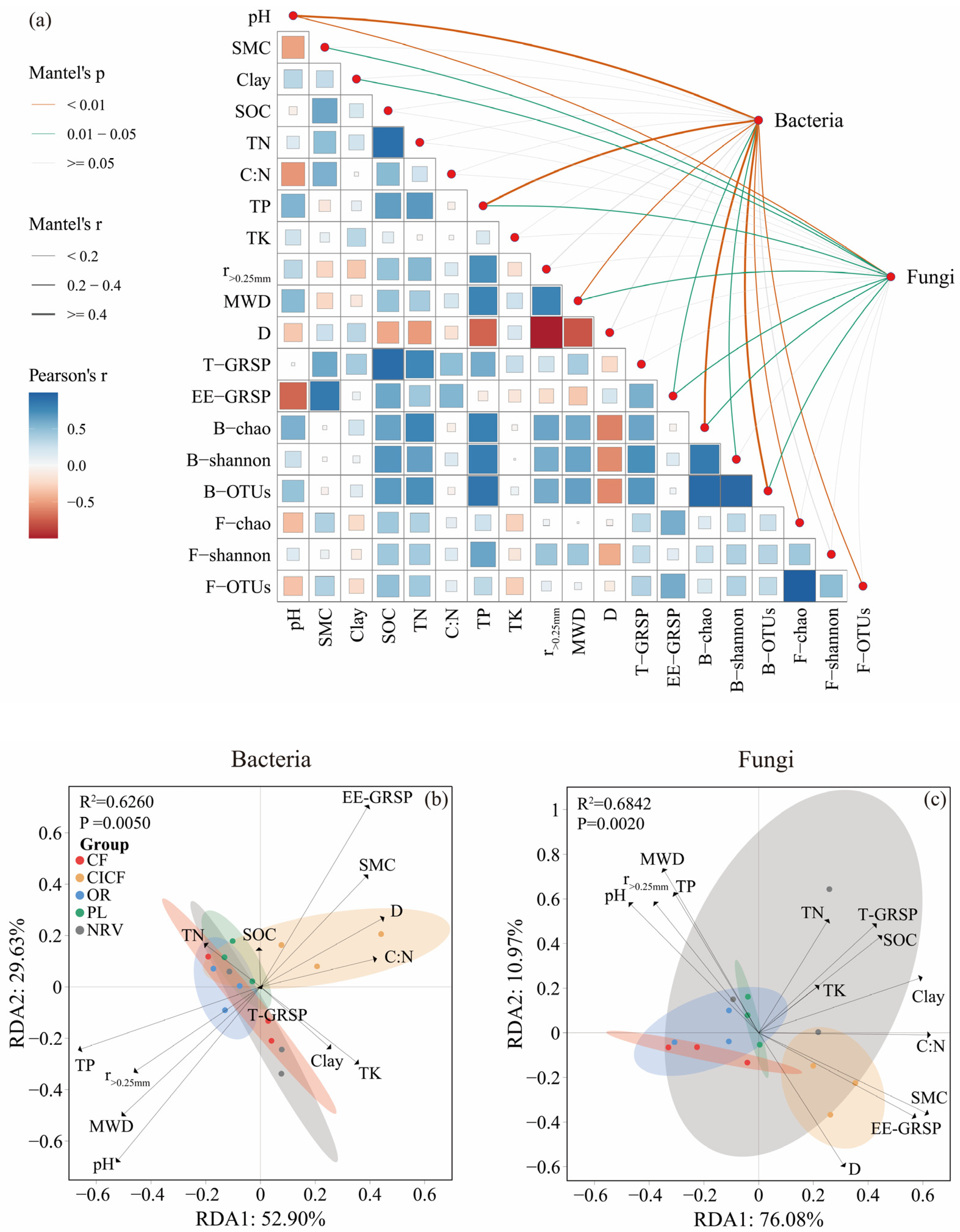
3.5. Correlation Between Microbial Diversity and Community Composition with Environmental Factors
3.6. Contribution of Soil Environmental Factors to SOC
4. Discussion
4.1. Response of Soil Physicochemical Properties as Well as Microbial Diversity and Community Composition to Different Land Uses
4.2. Differences in Land Use Affect GRSP Accumulation and Aggregate Stability
4.3. Regulatory Factors of Soil SOC Under Different Land Uses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, X.; Wang, Z.; Lu, C.; Huang, R.; Wang, F.; Gao, M. Effects of land use practices on soil aggregate stability and organic carbon content. Environ. Sci. 2019, 40, 3816–3824. [Google Scholar] [CrossRef]
- Morris, E.K.; Morris, D.J.P.; Voget, S.; Gleber, S.C.; Bigalke, M.; Wilcke, W.; Rillig, M.C. Visualizing the dynamics of soil aggregation as affected by arbuscular mycorrhizal fungi. ISME J. 2019, 13, 1639–1646. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Rabbi, S.M.F.; Zhang, Y.; Young, I.M.; Jones, A.R.; Dennis, P.G.; Menzies, N.W.; Kopittke, P.M.; Dalal, R.C. Soil organic carbon is significantly associated with the pore geometry, microbial diversity and enzyme activity of the macro-aggregates under different land uses. Sci. Total Environ. 2021, 778, 146286. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Six, J.; Zhang, H.X.; Zhang, Z.B.; Peng, X.H. Integrated aggregate turnover and soil organic carbon sequestration using rare earth oxides and 13C isotope as dual tracers. Geoderma 2023, 430, 116313. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Mao, Y.L.; Gao, S.F.; Lu, C.Y.; Pan, C.J.; Li, X.Y. Organic carbon accumulation and aggregate formation in soils under organic and inorganic fertilizer management practices in a rice-wheat cropping system. Sci. Rep. 2023, 13, 12. [Google Scholar] [CrossRef]
- Agnihotri, R.; Sharma, M.P.; Prakash, A.; Ramesh, A.; Bhattacharjya, S.; Patra, A.K.; Manna, M.C.; Kurganova, I.; Kuzyakov, Y. Glycoproteins of arbuscular mycorrhiza for soil carbon sequestration: Review of mechanisms and controls. Sci. Total Environ. 2022, 806, 150571. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 1998, 198, 97–107. [Google Scholar] [CrossRef]
- An, W.; Li, Z.; Wang, S.; Wu, X.; Lu, Y.; Liu, G.; Fu, B. Exploring the effects of the “Grain for Green” program on the differences in soil water in the semi-arid Loess Plateau of China. Ecol. Eng. 2017, 107, 144–151. [Google Scholar] [CrossRef]
- Huang, L.; Bai, J.; Chen, B.; Zhang, K.; Huang, C.; Liu, P. Two-decade wetland cultivation and its effects on soil properties in salt marshes in the Yellow River Delta, China. Ecol. Inform. 2012, 10, 49–55. [Google Scholar] [CrossRef]
- Schneider, D.; Engelhaupt, M.; Allen, K.; Kurniawan, S.; Krashevska, V.; Heinemann, M.; Nacke, H.; Wijayanti, M.; Meryandini, A.; Corre, M.D.; et al. Impact of Lowland Rainforest Transformation on Diversity and Composition of Soil Prokaryotic Communities in Sumatra (Indonesia). Front. Microbiol. 2015, 6, 1339. [Google Scholar] [CrossRef]
- Bai, Z.; Zheng, L.; Bai, Z.; Jia, A.; Wang, M. Long-term cultivation alter soil bacterial community in a forest-grassland transition zone. Front. Microbiol. 2022, 13, 1001781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, X.; Chen, K.; Shi, J.; Wang, Y.; Luo, P.; Yang, J.; Wang, Y.; Han, X. Long-term fertilization altered microbial community structure in an aeolian sandy soil in northeast China. Front. Microbiol. 2022, 13, 979759. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ge, T.; van Groenigen, K.J.; Yang, Y.; Wang, P.; Cheng, K.; Zhu, Z.; Wang, J.; Li, Y.; Guggenberger, G.; et al. Rice paddy soils are a quantitatively important carbon store according to a global synthesis. Commun. Earth Environ. 2021, 2, 2662–4435. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, M.; Shi, F. Impact of typical agricultural land use on the characteristics of soil microbial communities in the Nyingchi region of southeastern Tibet. J. Agro-Environ. Sci. 2020, 39, 331–342. [Google Scholar] [CrossRef]
- Gu, R.; Xiao, K.; Zhu, Z.; He, X.; Li, D. Afforestation enhances glomalin-related soil protein content but decreases its contribution to soil organic carbon in a subtropical karst area. J. Environ. Manag. 2024, 356, 120754. [Google Scholar] [CrossRef]
- Zhang, M.; Che, R.; Cheng, Z.; Zhao, H.; Wu, C.; Hu, J.; Zhang, S.; Liu, D.; Cui, X.; Wu, Y. Decades of reforestation significantly change microbial necromass, glomalin, and their contributions to soil organic carbon. Agric. Ecosyst. Environ. 2023, 346, 108362. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Y.; Wang, W.; Zhong, Z.; Pei, Z.; Ren, J.; Wang, H.; Zu, Y. Spatial Variations in Concentration, Compositions of Glomalin Related Soil Protein in Poplar Plantations in Northeastern China, and Possible Relations with Soil Physicochemical Properties. Sci. World J. 2014, 2014, 160403. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Z.M.; Liu, Y.T.; Cui, Z.L.; Mo, Q.Y.; Zhang, C.; Sheng, H.Y.; Wang, W.; Zhang, Y.K. Variation in Soil Aggregate Stability Due to Land Use Changes from Alpine Grassland in a High-Altitude Watershed. Land 2023, 12, 393. [Google Scholar] [CrossRef]
- Yu, P.; Liu, J.; Tang, H.; Ci, E.; Tang, X.; Liu, S.; Ding, Z.; Ma, M. The increased soil aggregate stability and aggregate-associated carbon by farmland use change in a karst region of Southwest China. Catena 2023, 231, 107284. [Google Scholar] [CrossRef]
- Novara, A.; Gristina, L.; La Mantia, T.; Rühl, J. Carbon dynamics of soil organic matter in bulk soil and aggregate fraction during secondary succession in a Mediterranean environment. Geoderma 2013, 193, 213–221. [Google Scholar] [CrossRef]
- Zolfaghari, A.A.; Taghizadeh-Mehrjardi, R.; Asadzadeh, F.; Hajabbasi, M.-A. Soil structure changes due to different land-use practices in the central Zagruos region, Iran. Arch. Agron. Soil Sci. 2015, 62, 163–175. [Google Scholar] [CrossRef]
- Shepherd, T.; Saggar, S.; Newman, R.; Ross, C.; Dando, J. Tillage included changes in soil structure and soil organic matter fractions. Aust. J. Soil Res. 2001, 39, 465–489. [Google Scholar] [CrossRef]
- Dong, S.K.; Zhang, J.; Li, Y.Y.; Liu, S.L.; Dong, Q.N.M.; Zhou, H.K.; Yeomans, J.; Li, Y.; Li, S.; Gao, X.X. Effect of grassland degradation on aggregate-associated soil organic carbon of alpine grassland ecosystems in the Qinghai-Tibetan Plateau. Eur. J. Soil Sci. 2020, 71, 69–79. [Google Scholar] [CrossRef]
- Garland, G.; Bünemann, E.K.; Oberson, A.; Frossard, E.; Snapp, S.; Chikowo, R.; Six, J. Phosphorus cycling within soil aggregate fractions of a highly weathered tropical soil: A conceptual model. Soil Biol. Biochem. 2018, 116, 91–98. [Google Scholar] [CrossRef]
- Gunina, A.; Kuzyakov, Y. Pathways of litter C by formation of aggregates and SOM density fractions: Implications from 13C natural abundance. Soil Biol. Biochem. 2014, 71, 95–104. [Google Scholar] [CrossRef]
- Wen, L.; Li, D.; Xiao, K.; Tang, H.; Li, C.; Xiao, X. Dynamics of aggregate-associated organic carbon after long-term cropland conversion in a karst region, southwest China. Sci. Rep. 2023, 13, 50759–50771. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, W.; Hu, P.; Xiao, D.; Yang, R.; Ye, Y.; Wang, K. The formation of large macroaggregates induces soil organic carbon sequestration in short-term cropland restoration in a typical karst area. Sci. Total Environ. 2021, 801, 149588. [Google Scholar] [CrossRef]
- Lan, J.; Long, Q.; Huang, M.; Jiang, Y.; Hu, N. Afforestation-induced large macroaggregate formation promotes soil organic carbon accumulation in degraded karst area. For. Ecol. Manag. 2022, 505, 119884. [Google Scholar] [CrossRef]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef]
- Li, Z.W.; Xu, X.L.; Wang, K.L. Effects of distribution patterns of karst landscapes on runoff and sediment yield in karst watersheds. Catena 2023, 223, 11. [Google Scholar] [CrossRef]
- Jianhua, C.; Daoxian, Y.; Liqiang, T.; Mallik, A.; Hui, Y.; Fen, H. An overview of karst ecosystem in Southwest China: Current state and future management. J. Resour. Ecol. 2015, 6, 247–256. [Google Scholar] [CrossRef]
- Yang, L.Y.; Li, Y.B.; Yu, L.M.; Chen, M.; Yu, M.; Zhang, Y.Y. Theory and case of land use transition promoting ecological restoration in karst mountain areas of Southwest China. Ecol. Indic. 2024, 158, 13. [Google Scholar] [CrossRef]
- Stefan, L.; Hartmann, M.; Engbersen, N.; Six, J.; Schöb, C. Positive Effects of Crop Diversity on Productivity Driven by Changes in Soil Microbial Composition. Front. Microbiol. 2021, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Xu, M.G.; Shah, S.A.A.; Abrar, M.M.; Sun, N.; Wang, B.R.; Cai, Z.J.; Saeed, Q.; Naveed, M.; Mehmood, K.; et al. Soil aggregation and soil aggregate stability regulate organic carbon and nitrogen storage in a red soil of southern China. J. Environ. Manag. 2020, 270, 11. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Six, J.; Elliott, E.; Paustian, K.; Doran, J.W. Aggregation and Soil Organic Matter Accumulation in Cultivated and Native Grassland Soils. Soil Sci. Soc. Am. J. 1998, 62, 1367–1377. [Google Scholar] [CrossRef]
- Shi, K.; Liao, J.; Zou, X.; Chen, H.Y.H.; Delgado-Baquerizo, M.; Wanek, W.; Ni, J.; Ren, T.; Zhang, C.; Yan, Z.; et al. Forest development induces soil aggregate formation and stabilization: Implications for sequestration of soil carbon and nitrogen. Catena 2024, 246, 108363. [Google Scholar] [CrossRef]
- Tyler, S.W.; Wheatcraft, S.W. Fractal scaling of soil particle-size distributions: Analysis and Limitations. Soil Sci. Soc. Am. J. 1992, 56, 362–369. [Google Scholar] [CrossRef]
- Yu, P.J.; Liu, S.W.; Han, K.X.; Guan, S.C.; Zhou, D.W. Conversion of cropland to forage land and grassland increases soil labile carbon and enzyme activities in northeastern China. Agric. Ecosyst. Environ. 2017, 245, 83–91. [Google Scholar] [CrossRef]
- Briedis, C.; Baldock, J.; Sá, J.C.D.; dos Santos, J.B.; McGowan, J.; Milori, D. Organic carbon pools and organic matter chemical composition in response to different land uses in southern Brazil. Eur. J. Soil Sci. 2021, 72, 1083–1100. [Google Scholar] [CrossRef]
- Gans, J.; Wolinsky, M.; Dunbar, J. Computational improvements reveal great bacterial diversity and high metal toxicity in soil. Science 2005, 309, 1387–1390. [Google Scholar] [CrossRef]
- Treseder, K.K. Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol. Lett. 2008, 11, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Serna-Chavez, H.M.; Fierer, N.; van Bodegom, P.M. Global drivers and patterns of microbial abundance in soil. Glob. Ecol. Biogeogr. 2013, 22, 1162–1172. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Dunlap, P.V.; Clark, D.P. Brock Biology of Microorganisms, 12th ed.; Pearson Benjamin Cummings: Hoboken, NJ, USA, 2008. [Google Scholar]
- Liu, S.E.; Wang, H.; Tian, P.; Yao, X.; Sun, H.; Wang, Q.K.; Delgado-Baquerizo, M. Decoupled diversity patterns in bacteria and fungi across continental forest ecosystems. Soil Biol. Biochem. 2020, 144, 9. [Google Scholar] [CrossRef]
- Holden, S.R.; Treseder, K.K. A meta-analysis of soil microbial biomass responses to forest disturbances. Front. Microbiol. 2013, 4, 163. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Ren, C.J.; Han, X.H.; Yang, G.H.; Wang, J.; Doughty, R.; Wang, Z.T. Trends in soil microbial communities in afforestation ecosystem modulated by aggradation phase. For. Ecol. Manag. 2019, 441, 167–175. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Xiang, W.H.; Ouyang, S.; Zhang, T.D.; Zhang, X.L.; Zeng, Y.L.; Hu, Y.T.; Luo, G.W.; Kuzyakov, Y. Forest conversion to plantations: A meta-analysis of consequences for soil and microbial properties and functions. Glob. Change Biol. 2021, 27, 5643–5656. [Google Scholar] [CrossRef]
- Pei, G.; Li, X.; He, T.; Li, J.; Pang, Y.; Hu, B.; Zhang, W.; Sun, J. Characterization and driving factors of soil microbial diversity and community structure under different vegetation restoration modes in the karst desertification area of Guangxi. Geoscience 2024, 44, 1630–1642. [Google Scholar] [CrossRef]
- Zhou, J.; Guan, D.W.; Zhou, B.K.; Zhao, B.S.; Ma, M.C.; Qin, J.; Jiang, X.; Chen, S.F.; Cao, F.M.; Shen, D.L.; et al. Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- George, P.B.L.; Lallias, D.; Creer, S.; Seaton, F.M.; Kenny, J.G.; Eccles, R.M.; Griffiths, R.I.; Lebron, I.; Emmett, B.A.; Robinson, D.A.; et al. Divergent national-scale trends of microbial and animal biodiversity revealed across diverse temperate soil ecosystems. Nat. Commun. 2019, 10, 1107. [Google Scholar] [CrossRef]
- Luo, X.Z.; Wen, D.Z.; Hou, E.Q.; Zhang, L.L.; Li, Y.; He, X.J. Changes in the composition of soil microbial communities and their carbon-cycle genes following the conversion of primary broadleaf forests to plantations and secondary forests. Land Degrad. Dev. 2022, 33, 974–985. [Google Scholar] [CrossRef]
- Hartmann, M.; Widmer, F. Community structure analyses are more sensitive to differences in soil bacterial communities than anonymous diversity indices. Appl. Environ. Microbiol. 2006, 72, 7804–7812. [Google Scholar] [CrossRef] [PubMed]
- Soudzilovskaia, N.A.; van Bodegom, P.M.; Terrer, C.; van’t Zelfde, M.; McCallum, I.; McCormack, M.L.; Fisher, J.B.; Brundrett, M.C.; de Sá, N.C.; Tedersoo, L. Global mycorrhizal plant distribution linked to terrestrial carbon stocks. Nat. Commun. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, N.; Liu, H.; Huang, W. Effect of Different Land Use Type on Distribution of Glomalin-related Soil Protein on Hilly Slope with Purple Soils in Hengyang of Hunan Province, China. Acta Agrestia Sin. 2020, 28, 1260–1265. [Google Scholar]
- Tang, H.-L.; Liu, L.; Wang, L.; Ba, C.-J. Effect of land use type on profile distribution of glomalin. Chin. J. Eco-Agric. 2009, 17, 1137–1142. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Zhong, Z.; Wang, H.; Fu, Y. Variation in glomalin in soil profiles and its association with climatic conditions, shelterbelt characteristics, and soil properties in poplar shelterbelts of Northeast China. J. For. Res. 2020, 31, 279–290. [Google Scholar] [CrossRef]
- Engelhardt, I.C.; Welty, A.; Blazewicz, S.J.; Bru, D.; Rouard, N.; Breuil, M.-C.; Gessler, A.; Galiano, L.; Miranda, J.C.; Spor, A.; et al. Depth matters: Effects of precipitation regime on soil microbial activity upon rewetting of a plant-soil system. ISME J. 2018, 12, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.X.; Wang, C.; Feng, M.C.; Zhang, M.J.; Song, X.Y.; Xiao, L.J.; Li, G.X.; Jin, X.L.; Anwar, S.; Yang, W.D. Hyperspectral response and quantitative estimation on soil aggregate characters. Catena 2021, 202, 11. [Google Scholar] [CrossRef]
- Dou, Y.; Yang, Y.; An, S.; Zhu, Z. Effects of different vegetation restoration measures on soil aggregate stability and erodibility on the Loess Plateau, China. Catena 2020, 185, 104294. [Google Scholar] [CrossRef]
- An, J.; Wu, Y.; Wu, X.; Wang, L.; Xiao, P. Soil aggregate loss affected by raindrop impact and runoff under surface hydrologic conditions within contour ridge systems. Soil Tillage Res. 2021, 209, 104937. [Google Scholar] [CrossRef]
- Zhong, Z.K.; Wu, S.J.; Lu, X.Q.; Ren, Z.X.; Wu, Q.M.; Xu, M.P.; Ren, C.J.; Yang, G.H.; Han, X.H. Organic carbon, nitrogen accumulation, and soil aggregate dynamics as affected by vegetation restoration patterns in the Loess Plateau of China. Catena 2021, 196, 9. [Google Scholar] [CrossRef]
- Dorji, T.; Field, D.J.; Odeh, I.O.A. Soil aggregate stability and aggregate-associated organic carbon under different land use or land cover types. Soil Use Manag. 2020, 36, 308–319. [Google Scholar] [CrossRef]
- Gan, J.; Han, X.; Zou, W. Globularin and its role in soil ecosystems. Soil Crops 2022, 11, 41–53. [Google Scholar] [CrossRef]
- Mendez-Millan, M.; Tu, T.T.N.; Balesdent, J.; Derenne, S.; Derrien, D.; Egasse, C.; M’Bou, A.T.; Zeller, B.; Hatté, C. Compound-specific 13C and 14C measurements improve the understanding of soil organic matter dynamics. Biogeochemistry 2014, 118, 205–223. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Li, T.; Yuan, Y.; Mou, Z.; Li, Y.; Kuang, L.; Zhang, J.; Wu, W.; Wang, F.; Wang, J.; Lambers, H.; et al. Faster accumulation and greater contribution of glomalin to the soil organic carbon pool than amino sugars do under tropical coastal forest restoration. Glob. Change Biol. 2022, 29, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Muller, L.A.H.; Lehmann, A. Soil aggregates as massively concurrent evolutionary incubators. ISME J. 2017, 11, 1943–1948. [Google Scholar] [CrossRef]
- Nichols, K.A.; Halvorson, J.J. Roles of biology, chemistry, and physics in soil macroaggregate formation and stabilization. Open Agric. J. 2013, 7, 107. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, H.; Liang, X.; Hou, L.; Li, M.; Zhang, L.; Zhang, W.; Song, Y. Indirect Regulation of SOC by Different Land Uses in Karst Areas Through the Modulation of Soil Microbiomes and Aggregate Stability. Agriculture 2025, 15, 1220. https://doi.org/10.3390/agriculture15111220
Shu H, Liang X, Hou L, Li M, Zhang L, Zhang W, Song Y. Indirect Regulation of SOC by Different Land Uses in Karst Areas Through the Modulation of Soil Microbiomes and Aggregate Stability. Agriculture. 2025; 15(11):1220. https://doi.org/10.3390/agriculture15111220
Chicago/Turabian StyleShu, Haiyuan, Xiaoling Liang, Lei Hou, Meiting Li, Long Zhang, Wei Zhang, and Yali Song. 2025. "Indirect Regulation of SOC by Different Land Uses in Karst Areas Through the Modulation of Soil Microbiomes and Aggregate Stability" Agriculture 15, no. 11: 1220. https://doi.org/10.3390/agriculture15111220
APA StyleShu, H., Liang, X., Hou, L., Li, M., Zhang, L., Zhang, W., & Song, Y. (2025). Indirect Regulation of SOC by Different Land Uses in Karst Areas Through the Modulation of Soil Microbiomes and Aggregate Stability. Agriculture, 15(11), 1220. https://doi.org/10.3390/agriculture15111220






