1. Introduction
The mulching practice through plastic films in agriculture has been widely adopted all over the world over the past several decades: it helps increase soil temperature and stimulate soluble nutrient release, preserve soil moisture and reduce water loss, control weeds and protect soil from erosion [
1]. The result is a higher crop yield [
2].
In the European Union, approximately 1.74 million tons of plastics were used in 2018 for the agricultural sector, that is between 3 and 4 percent of the total European plastic converter demand of 51.2 million tons FAO [
3]. The most used plastic polymer for soil mulching is black polyethylene (PE), which is considered the standard in agriculture due to its cheapness and good performance in terms of strength, transparency, insulation and water resistance. However, PE mulch films are not biodegradable, and due to the high cost for their proper disposal, they are frequently being buried in soil after their life service has expired. Therefore, they remain in the environment for a time that largely exceeds a human life [
4]. The soil burial of PE films brings about disintegration/fragmentation, the release of microplastics into environmental compartments (soil, water, air) and their consequent entering into the food chain which negatively affects not only soil quality and crop yield [
5,
6], but also animal and human health [
7,
8]. Zhang et al. [
9] reported that in agricultural soil up to 9708 particles of plastics/kg of soil can be found. Furthermore, polyethylene fragments release phthalates [
10] and contribute to the spread of pesticides into the soil [
11]. Last but not least, being a low density PE and a non-renewable fossil-based industrial product, its use is rising and increasing public concerns for its wide impact on environmental resources [
3].
To avoid the high costs of proper polyethylene disposal in terms of both time and expenses [
12,
13], and to make the burial of mulching films at the end of their life cycle feasible, while minimizing microplastics’ accumulation in agricultural soils, biodegradable mulching films have been developing for many years. They can be buried safely because after the physical degradation/disintegration has taken place, the microbial soil community is able to fully biodegrade the residual products into harmless carbon dioxide, water and biomass [
14].
The most common, and consequently the most easily available and cheap, naturally existing polymers are polysaccharides, widely present in plants and animals and frequently produced by algae and bacteria. In this class of naturally occurring biomolecules, it is possible to find chitin, cellulose and alginate: they consist of chains of monosaccharides linked by glycosidic bonds.
Carboxymethyl cellulose (CMC) is a water-soluble, non-toxic polymer obtained by the reaction between cellulose and chloroacetate, which is able to form films characterized by moderate physical strength and good flexibility; however, some researchers are concerned about the need to improve CMC mechanical properties by adding graphene [
15,
16].
Chitosan (CS) is a derivative of chitin, the second most abundant biopolymer in nature after cellulose, and it can form non-toxic, biodegradable and water-resistant films [
17].
Sodium alginate (SA) is a biodegradable biopolymer extracted from algae [
18]. Although sodium alginate films are water-soluble, this characteristic can be faded away by crosslinking with CaCl
2, making them usable as a component of mulching films [
19].
Despite previous advantages, these biodegradable mulch constituents show sensitivity to moisture and brittleness due to their mechanical behavior related to water adsorption and retention [
20]. Indeed, the strong presence of hydroxyl and carboxyl groups in CMC causes a great susceptibility to water [
21], while CS films are affected by low mechanical strength [
22].
The mechanical limits of CMC, CS and SA can be overcome by mixing them in proper ratios [
23]. The combination CMC-CS positively affects tensile strength and water resistance [
24]. Kulig et al. [
25] reported an improvement in flexibility and the thermal stability by CS-SA blending. A further strategy to enhance the required polymers’ mechanical properties is adding crosslinkers, such as CaCl
2, that act on the interconnections of non-covalent bonds and the entanglement of molecular chains, forming ionic bonds with the carboxylic groups (-COO
−) of polysaccharides [
26,
27]. On the other hand, an excessive CaCl
2 concentration causes an increase in the brittleness of the film [
28]. To further enhance the structural flexibility and the mobility of polymers chains, plasticizers like glycerol are therefore recommended [
29]. Unaffordable high costs are among the factors limiting the employment of biodegradable mulch films [
30]. An example is given by one of the more successful biodegradable biopolymers, compliant to the European standard EN 13432 [
31], known as Mater-Bi
® (Novamont s.p.a, Novara, Italy). This polymer is resistant to photodegradation, and, based on information released by the Italian patents database (
http://brevettidb.uibm.gov.it, accessed on 26 May 2025), the main components of the Mater-Bi
® formula are polymerized aliphatic and/or aromatic polyesters, at least a vegetal polyphenol and up to 10% in its weight of additives such as plasticizer agents, pigments and surfactants.
Depending on the country, amount and employment, Mater-Bi
® plastic film can cost, in the European market, from 4.70 to 5.50 EUR/kg versus 0.5 to 1.5 EUR/kg of polyethylene. In the US market, depending on the roll dimension and film thickness, biodegradable mulch films cost between USD 212 and USD 250 per roll, whereas the cost of polyethylene mulches ranges from USD 135 to USD 154 per roll [
13].
In order to achieve a decisive cost cut off, more simple production processes, including a low number of cheap and easily accessible materials, such as the CMC, SA and CS described above, together with the adoption of specific crop management practices can represent a more economically and technically feasible strategy among those being developed.
Indeed, although biodegradable mulch films must be strong enough to be laid out on the field, according to European standards EN 17033 [
32], the requirement of a durability covering for the entire crop cycle could be not so necessary in some cases, opening the possibility of the employment of cheaper and more simple, in terms of components, short-life degradable mulch films for a specific step of a crop cycle and/or for short crop cycles.
The employment of a planting density higher than that commonly considered as optimal for lettuce cropping [
33] is still debated as a possible way to grow several varieties of
Lactuca sativa L. It is important to note that while Tarara [
34] underlined the beneficial effects of less-dense plant spacing in terms of the quality of the product, Fekadu et al. [
35] reported a higher fresh leaf yield per unit area, although the fresh leaf weight per plant decreased. Yet, Jenni et al. [
36] found that the combination of higher density and white mulching increased the head weight, while the percentage of the marketable heads was like that of low-density cropping.
Given this premise, the focus of this study was to assess the performances of an innovative CMC-CS-SA mulch film, enriched or not with the monoammonium phosphate (MAP, NH
4H
2PO
4) provided as a fertilizer/microbial activity primer, on soil, and plants’ responses during an entire lettuce (
Lactuca sativa L. var.
Romana) crop cycle grown in soil mesocosms under field conditions. The two versions of the experimental mulcher (with and without MAP) evaluated only the mechanical resistance and the consequent effects on the mulching action. The experimental mulching film, named BPMF (BioPolymer for Mulching Films), was pre-tested in lab conditions showing fair/good performances related to EN 17033 standards [
32]. It was then tested in field conditions in a Mediterranean environment without any crops, showing a 15/20 day long effective mulching action before a clear mechanical structural collapse as briefly reported in Materials and Methods. This work is embedded in a wider project focused on the effects of the degradation of the experimental bio-based mulcher once buried. The aspects related to the response of the microbial soil community and N and C cycling will be considered in future papers.
Based on the field pre-test performances, due to the simple composition of this polymer, including the absence of coloring, and its potential low cost once produced at an industrial scale, the main aim of this work was to test the effects on a lettuce yield. The nutraceutical quality of a short mulching action by a white biodegradable film on the early stages of lettuce growth was compared to a mulching action covering the entire crop cycle with black-colored Mater-Bi® and black-colored polyethylene (PE) mulching films in a Mediterranean environment, taking into account both the positive and negative effects of combined early mulching and high crop density.
2. Materials and Methods
2.1. Mulching Films
The experimental bio-based and biodegradable mulch film (BPMF) was prepared in the laboratory by the researchers from the University of Palermo (Italy) by using the solvent casting technique as reported by Ciaramitaro et al. [
37]. Briefly, dispersion A (CMC) prepared by dissolving 6.75 g of CMC (1.5%
w/
v) in 450 mL of deionized water was mixed with dispersion B (CS/SA) prepared by dissolving 6.75 g of CS (1.5%
w/
v), 6.75 g of SA (1.5%
w/
v) and 9 g of glycerol (2.0%
w/
v; used as plasticizer agent) in 450 mL of a 2.0%
v/
v aqueous acetic acid solution. The CMC and the CS/SA suspensions were mixed at a 1:1 mass weight ratio, with the mixed solution gently poured over a flat and clean glass area and left drying at room temperature. The MAP-enriched bioplastic film (BPMF + MAP) was produced the same way as above, with the addition of 5.0 g of monoammonium phosphate (MAP) to the CMC dispersion (25% salt percentage relative to the 20 g mass weight of the dry polymer). This MAP addition, initially set at a 90% salt percentage, was then lowered to increase the tensile strength of the film. The thickness of the resulting film, albeit irregular, was approximately 50 µm. No colorants were added. All the reagents used for BPMF preparation were purchased from Merck Life Science Srl (Milan, Italy).
The commercial biodegradable (according to DIN EN 17033:2018 [
32]) and compostable (according to EN 13432 [
31]) black-colored mulch film used in the mesocosm experiment (Mater-Bi
®) was an ECOPAC BIO BLACK film (thickness 15–18 µm), kindly provided by Guarniflon SpA PATI division (San Zenone degli Ezzelini, Treviso, Italy), and it was made of Mater-Bi grade EF04P converted into a film with the addition of black masterbatch (FDM 324006 BK BIOE MASTERBATCH provided by AVIENT).
A commercial, black-colored non-degradable polyethylene (PE, 50 µm thickness) mulch film was purchased from Si.Sac. S.p.a. (Ragusa, Italy). The film PE was removed at the end of the crop cycle without being buried into the soil.
2.2. Soil and Substrates
The soil used for the mesocosms’ filling was a Calcaric-Fluvi Cambisol [
38] collected from the surface (0–20 cm) Ap horizon of a citrus orchard located in the Sibari plain (Northern Calabria Region, sampling date October 2022). After coarse sieving (4 mm), soil was thoroughly mixed with commercial perlite (Agrilit
®3, purchased from Perlite Italiana s.r.l., Milan, Italy) to prepare an 80/20 (
v/
v) soil/perlite mixture. Soon after mixing, the mixture was amended with an equivalent amount of 1 kg dry weight of municipal solid waste compost (TerrasanaBio
®, Calabra Maceri S.p.A., Rende, Cosenza, Italy). Major characteristics of soil, perlite and compost are reported in
Table A1,
Table A2 and
Table A3, respectively (
Appendix A). The choice to carry on the trial using outdoor mesocosms characterized by a soil type different from that of the experimental location was driven by the need to guarantee a good degree of soil fertility.
2.3. Plant Material
Lettuce (Lactuca sativa L. var. Romana) was purchased from Fratelli Ingegnoli (Milan, Italy), germinated and grown under greenhouse conditions for four weeks before transplanting.
2.4. Chemicals
Analytical standards 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), rutin, gallic acid, DPPH and ethanol were purchased from Sigma-Aldrich (St. Louis, MO, USA). Folin–Ciocalteu’s reagent, NaOH and AlCl3 were purchased from Carlo Erba (Cornaredo, Milan, Italy). All chemicals were of analytical grade.
2.5. Mesocosms and Experimental Set-Up
The experimental plan consisted of twenty-four mesocosms filled with the compost-amended soil/perlite mixture and buried up to the top in three 40 cm deep dugouts, leaving their surface unburied. Outdoor mesocosms were located close to the Department of Agricultural Sciences at the Mediterranean University of Reggio Calabria (38°07′13.6″ N, 15°40′10.3″ E, 150 m a.s.l.). The experimental site was surrounded by a protective enclosure to avoid wild animal grazing. The experimental area was 24 m2 wide, and the buckets were placed in 3 lines of eight buckets each. The buckets were distanced at 30 cm from one another.
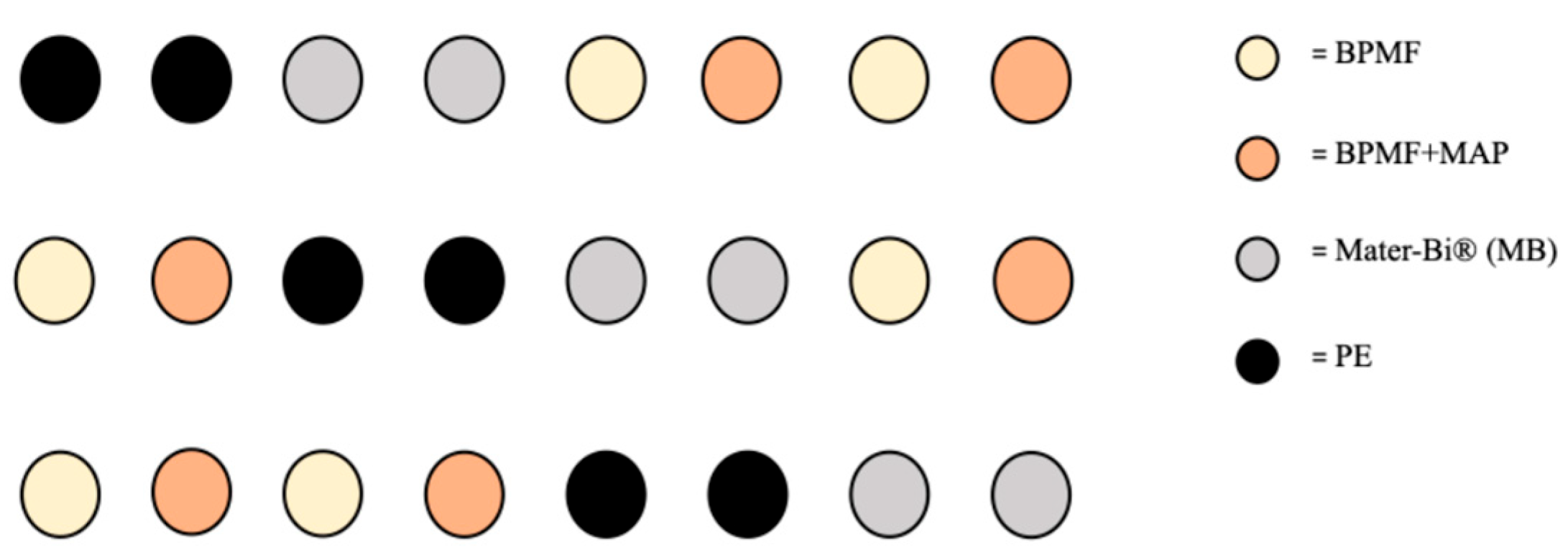
The experimental design was built by dividing the twenty-four buckets in four theses characterized by two versions of BPMF and the other two plastic mulchers, with six replicates for each one: (1) white BPMF; (2) white BPMF + MAP; (3) black Mater-Bi® (MB); (4) conventional non-degradable black polyethylene (PE), which was considered the control because of its well-known performances in several decades of employment.
The theses replicates were distributed through a semi-randomized geometric distribution as shown in
Figure 1.
The pre-trial focused on the mechanical resistance of BPMF was carried on as follows: the BPMF disks (six added with MAP and six without MAP) were placed on the top of the bucket covering the entire mesocosm surface. The test was 8-months long, crossing all the seasons from May 2023 to January 2024, and included three consecutive mulching cycles: at the end of each one, the films were buried, and after 1 week, they were replaced with new ones. The remaining twelve buckets were mulched with six MB and six PE disks, in agreement with the experimental design, to avoid different management before the beginning of the main experiment. Each mulching cycle was on average 70 days ± 7. The water supply consisted of 5 L of tap water per bucket every 20 days plus rainfall.
Differently from PE and MB, BPMF and BPMF + MAP, both in cold and hot seasons, started quickly to show physical changes in two steps: the quick water accumulation and loss led at first from a white/semi-transparent to an opaque white color, then to a crinkling of the structure followed by a breakage (
Figure 2). Therefore, the phenomenon must be intended not as chemical or bio-chemical degradation but only as a result of mechanical stress. The entire process made clear the inefficacy of the mulching action after about 20 days.
2.6. Mesocosm Soil Analysis
On 10 April 2024, five days before the start, and on 5 June 2024, the day of the end of the main trial, twenty-four composite soil samples, each consisting of three samples pooled together, were surface (0–10 cm) collected per mesocosm, air dried, sieved at <2 mm and then stored before analysis.
The soil texture was determined by the Bouyoucos method [
39], while the chemical properties were determined as described in [
40] (
Appendix A,
Table A1 and
Table A2): the pH was potentiometrically measured in a 1:2.5 (
w/
v) soil-to-0.01 M CaCl
2 solution mixture (pH); electrical conductivity was measured at 25 °C in a 1:2 (
w/
v) soil-to-water ratio (EC
1:2). Total organic C (TOC) and N (TN) were analyzed by an elemental analyzer LECO CN628 (LECO Corporation, St. Joseph, MI, USA). TOC/TN provided the C/N ratio (C/N). The cation exchange capacity (CEC) was determined accordingly by the Mehlich method [
41].
2.7. Main Experiment
The trial started on 15 April 2024: in the twenty-four buckets, seventy-two 30-day old lettuce seedlings were transplanted, that is three seedlings per mesocosm, 15 cm apart from each other and 10 cm apart from the bucket border to avoid potential leaves burning and guarantee the highest feasible plant density that resulted in 19 seedling/m2. After those twelve BPMF disks, six BPMF and six BPMF + MAP were drilled with three 6 cm diameter holes according to the seedling’s placement.
Six Mater-Bi® disks of the same size of BPMF were cut out and drilled in correspondence to the seedling’s position. The same procedure was carried out to obtain six black-colored 50 µm-tick polyethylene drilled disks. All the mulching film disks were placed on the same day as the seedling transplanting.
All theses were irrigated with 5 L of tap water two times a week, and supplied two times during the entire crop cycle (on 29 April and 16 May 2024) with 2 g per plant of a commercial Ca(NO3)2 (1.2% NH4+-N, 14.4% NO3−-N, 26.6% CaO) (Agrisol, Siriac, Ragusa, Italy), with inorganic fertilizers supplying in total 0.048 g of NH4+-N, 0.560 g of NO3−-N and 0.761 g of Ca per lettuce plant.
On 19 May 2024, an antifungal treatment (propamocarb hydrochloride) was supplied by spraying Previcur Flex (Bayer) both on the soil and leaf surface.
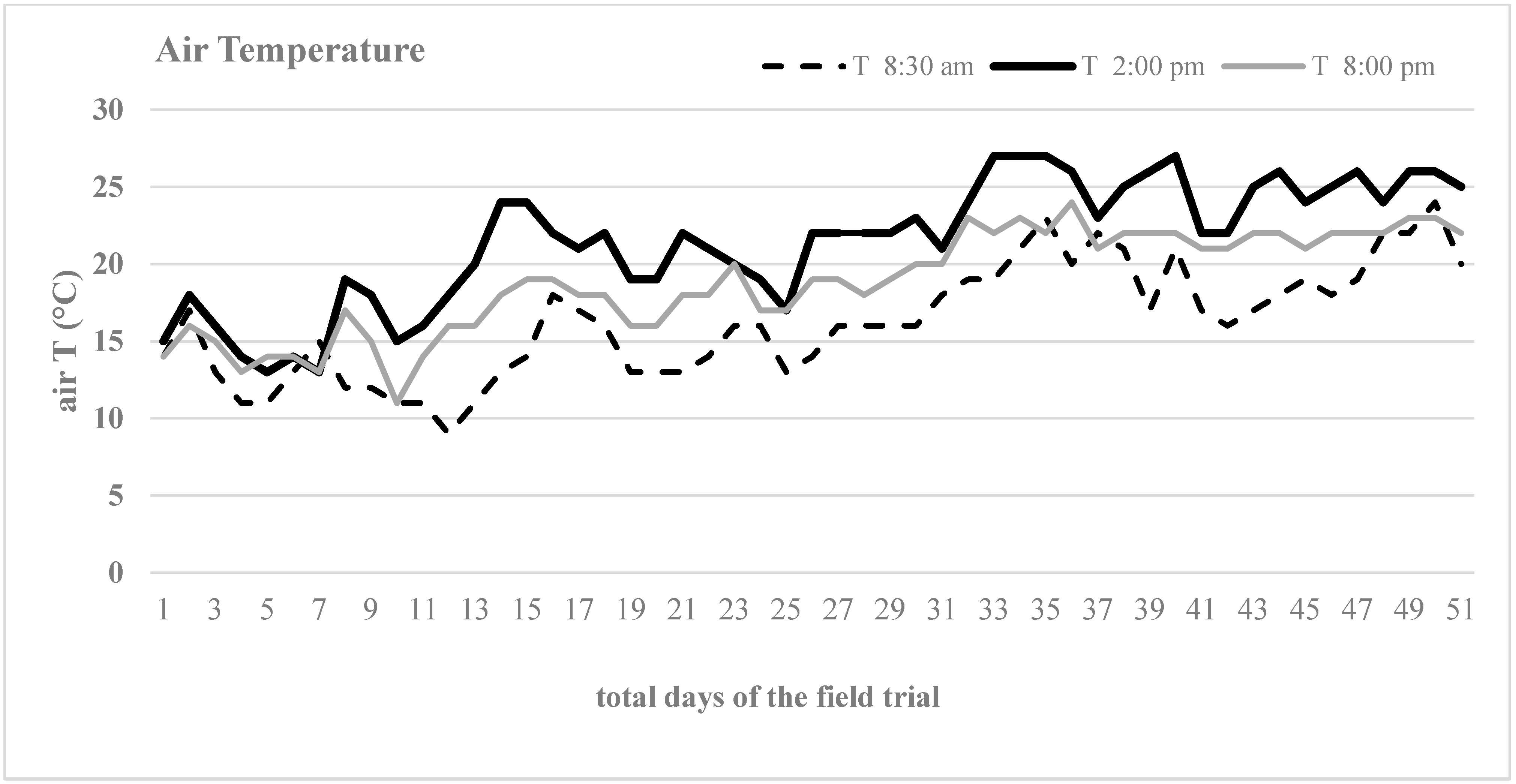
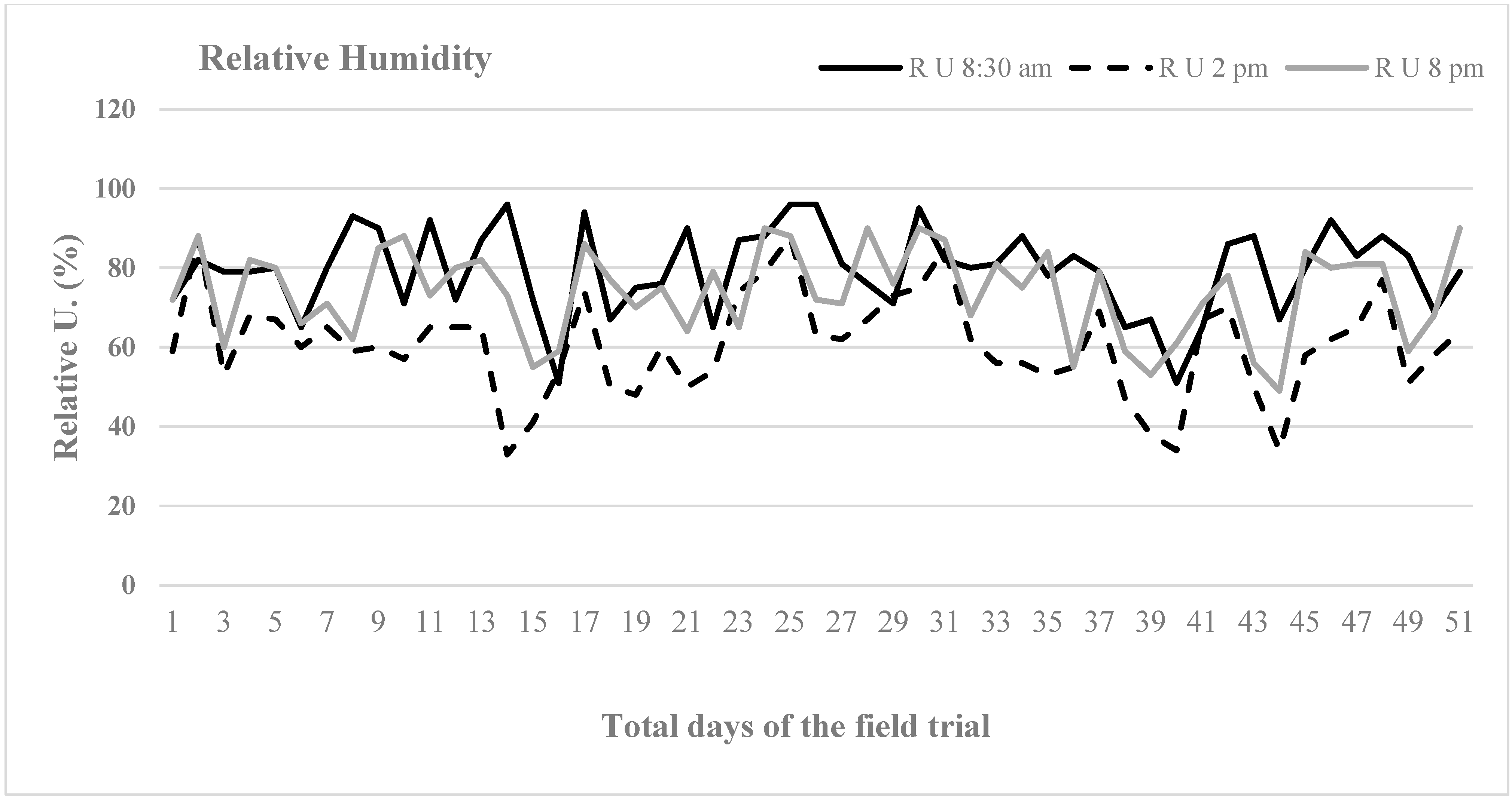
During the field trial, the air temperature and humidity of the site were recorded daily through the meteorological station located at San Brunello in Reggio Calabria, Italy (
https://wheather.com).
On 3 June 2024, the lettuce was harvested, leaving in the soil only the root systems to avoid any unnecessary perturbation to the mesocosms. For each bucket, all three plants were immediately weighed to record the fresh weight. One plant per bucket was oven dried at 60 °C for 48 h to record the dry weight. From the remaining two plants, three or four intermediate leaves, avoiding the oldest and the youngest ones, were stored at −20 °C for further analysis.
2.8. Chlorophylls and Carotenoids’ Determination
Chlorophylls and carotenoids were determined as described in Papalia et al. [
42]. Briefly, after a gentle crumbling in small pieces, 50 mg of fresh leaf tissue was mixed with 2.5 mL of absolute ethanol and left in the dark at 4 °C for 24 h. After that, the samples were centrifuged for 10 min at 7000×
g. Chlorophyll a and b, and carotenoids’ content were determined spectrophotometrically at 649, 665 and 470 nm, respectively, employing Lichtenthaler’s equation. Absorbance, like for all of the following colorimetric assays, was taken by a double ray spectrophotometer Perkin Elmer Lambda 35 (Perkin Elmer, Shelton, CT, USA). Chlorophylls and carotenoid content were expressed as mg kg
−1 FW.
2.9. Determination of Total Phenols, Total Flavonoids and Radical Scavenging Activity
Ethanolic extracts were prepared according to Muscolo et al. [
43] with the following modifications: frozen (−20 °C) pieces of lettuce leaves were dehydrated for 96 h through a freeze dryer Alpha 1-2 LDplus (Martin Christ GmbH, Osterode am Harz, Germany), and then were gently (to avoid increasing the temperature and phenols’ degradation) crumbled. An amount of 500 mg of each sample was extracted with absolute ethanol (10 mL) (1:20,
w/
v) under continuous stirring at room temperature for 90 min. The process was repeated once. Pooled extracts were centrifuged at 2365×
g for 15 min, and the supernatants were filtered with Whatman™ n. 42 filter papers. The final volume of the two extractions was stored at −20 °C.
The total phenols were spectrophotometrically determined following the Folin–Ciocalteu assay with some modifications: in a 5 mL Eppendorf tube, 2.5 mL of ultrapure deionized water, 1 mL of extract, 500 μL of Folin–Ciocalteu reagent and last, to start the reaction, 1 mL of 0.33 M NaOH were added. The mixture was left to incubate at room temperature in the dark for two hours. The absorbance of the samples was recorded at 725 nm. A calibration curve was constructed with gallic acid, and the results were expressed as µg of gallic acid equivalent (GAE) g−1 DW.
The total flavonoid content in extracts was measured spectrophotometrically by mixing 1 mL of extract with 1 mL of 0.15 M AlCl
3 ethanolic solution, as described in [
43]. After 15 min of incubation at room temperature, the absorbance was measured at 430 nm. The flavonoid content was calculated from a calibration curve of rutin and expressed as µg rutin equivalent (RUE) g
−1 DW.
The DPPH radical scavenging assay was performed following the method described by Muscolo et al. [
43] with some modifications: the DPPH concentration in the cuvette was chosen to give absorbance values close to 1.0. In a 5 mL Eppendorf tube, 100 μL of ethanolic extract was added to 2.9 mL of 0.063 M DPPH. Immediately after the extract addition, the reaction mixture absorbance was spectrophotometrically measured at 517 mm: this value worked as the “control”, and it was thought to reduce as much as possible the differences in composition between the control and incubated mixture in comparison with a pure DPPH solution. After 30 min of incubation at room temperature in the dark, a second absorbance reading was taken at 517 nm. The inhibition I (%) of radical scavenging activity was calculated as I (%) = [(A0 − AS)/A0] × 100, where A0 is the absorbance of the control (value at T0) and AS is the absorbance of the sample after 30 min of incubation. Results were expressed as µmol of the Trolox equivalent (TE) g
−1 DW.
2.10. Statistics
A Shapiro–Wilk normality test and Levene’s test for the homogeneity of variance were performed. Depending on the test results, a one-way ANOVA plus Tukey post hoc test or Welch one-way ANOVA plus Games–Howell post hoc test was applied to the plant parameters’ dataset. The soil dataset corresponding to the beginning and the end of the mesocosm trial was analyzed with a t-test for independent samples to compare each soil parameter at the beginning and at the end of the experiment, while potential differences among the theses within the same sampling time were analyzed through a one-way ANOVA. All analyses were performed by the open-source software Jamovi 2.5.1 (the Jamovi project, 2024).
5. Conclusions
Agricultural mulching is an important practice to guarantee protection against weed competition, a stable upper soil temperature and to avoid excessive soil water loss. PE and MB-based polymers help achieve these targets. However, the first one is fossil-derived, non-biodegradable and non-renewable, thus requiring an additional cost for proper disposal after its life service. The second one is biodegradable and compostable and it can be soil-incorporated at the end of its life cycle; however, it is also more expensive, as most biodegradable mulches are.
The BPMF, constituted of a reduced number of easily available and economically affordable components, effectively maintained its mulching action for the first 3 weeks of the lettuce life cycle. Despite the different plastic color (milky white vs. black), it did not negatively affect the crop yield and the nutraceutical values of lettuce plants compared to those treated with Mater-Bi® and PE.
In a Mediterranean environment, the lettuce var. Romana can grow in an ideal range of air temperature and air humidity, and, under a regular water supply, a high crop density helps plants to control weed competition in the second half of their life cycle when the mulching film has become ineffective. Furthermore, irrespective of the high planting density, no fungi and mildews attacks were found.
Due to contradicting results found in the literature about the crop density and nutraceutical values of the lettuce, further investigation is required to refine this potential agricultural strategy that could give access to cheap and less durable biodegradable films to a new market niche and introduce a win–win strategy in intensive horticulture, namely, cutting down expenses for Mater-Bi® purchase and PE disposal without negatively affecting the environmental quality and soil health.
Despite their service life being shorter (~3 weeks) than that of the commercial mulching films actually on the market, the innovative composite polysaccharide-based mulching films have shown to promote comparative yields and competitive nutraceutical features when tested in a lettuce cropping system; therefore, they offer great potential for further developments.