Responses to the Interaction of Selenium and Zinc Through Foliar Fertilization in Processed Grains of Brazilian Upland Rice Genotypes
Abstract
1. Introduction
2. Materials and Methods
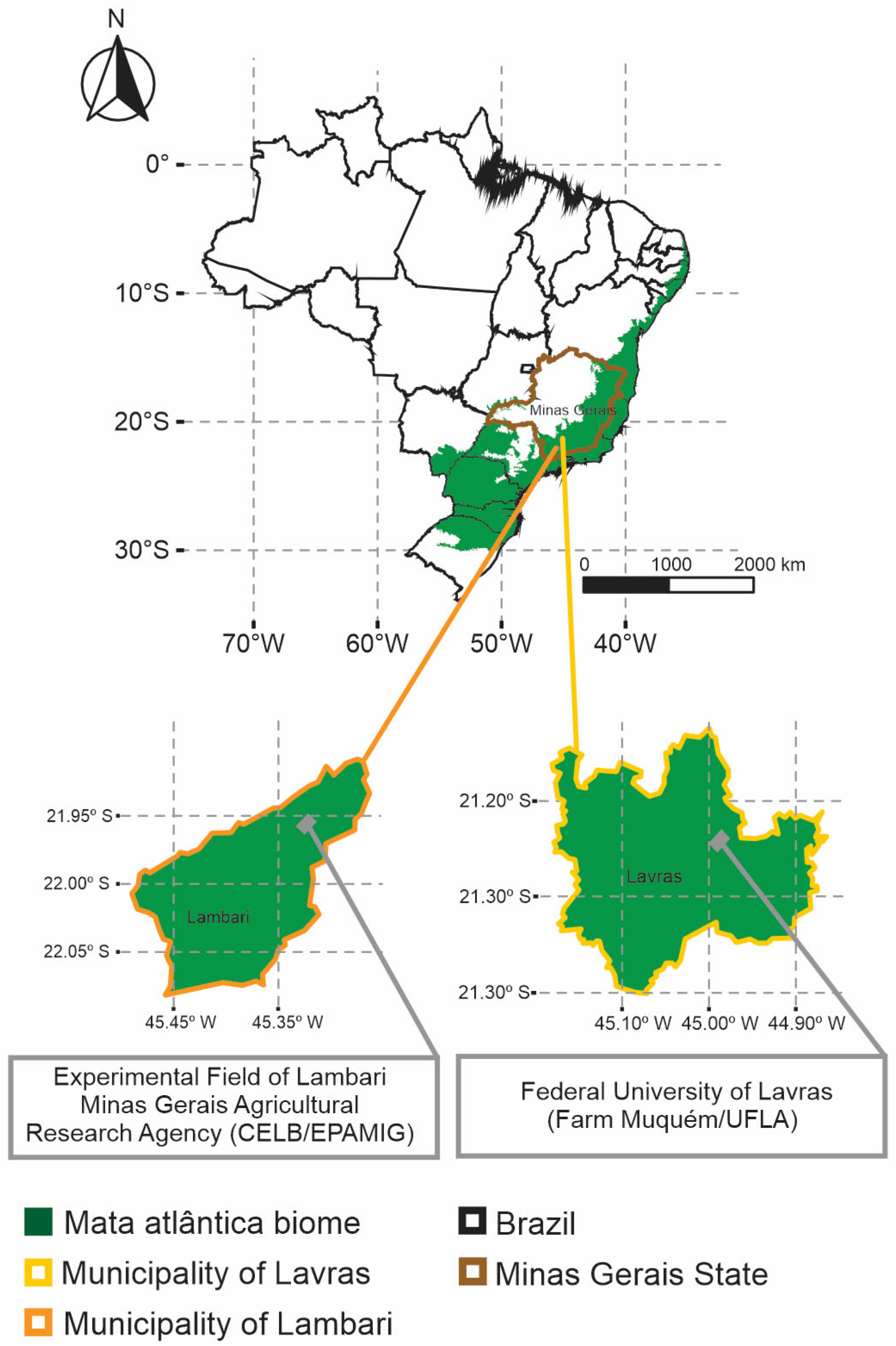
2.1. Field Growing Conditions and Experimental Design
2.2. Agronomic Parameters
2.3. Processing of Rice Grains (Polished and Whole)
2.4. Proteins and Amino Acids Determination
2.5. Selenium and Zinc: Content, Uptake, Intake
2.6. Statistical Analysis
3. Results
3.1. Agronomic Traits
3.2. Grain Contents of Zn and Se
3.3. Grain Uptake of Zn and Se
3.4. Grain Intake of Zn and Se
3.5. Grain Free Proteins and Free Amino Acids
3.6. Principal Component Analysis
4. Discussion
4.1. Agronomic Traits
4.2. Grain Contents of Zn and Se
4.3. Grain Uptake of Zn and Se
4.4. Grain Intake of Zn and Se
4.5. Grain Free Proteins and Free Amino Acids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA (United States Department of Agriculture). Food Data Central: United States Department of Agriculture; United States Department of Agriculture: Washington, DC, USA, 2019. Available online: https://fdc.nal.usda.gov/ (accessed on 2 February 2024).
- Muthayya, S.; Rah, J.H.; Sugimoto, J.D.; Roos, F.F.; Kraemer, K.; Black, R.E. The Global Hidden Hunger Indices and Maps: An Advocacy Tool for Action. PLoS ONE 2013, 8, e67860. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Zheng, S.; Xu, C.; Lv, G.; Shuai, H.; Zhang, Q.; Zhu, Q.; Zhu, H.; Huang, D. Foliar zinc reduced Cd accumulation in grains by inhibiting Cd mobility in the xylem and increasing Cd retention ability in roots1. Environ. Pollut. 2023, 333, 122046. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. Plant nutrition research: Priorities to meet human needs for food in sustainable ways. Plant Soil 2002, 247, 3–24. [Google Scholar] [CrossRef]
- Fordyce, F.M. Selenium Deficiency and Toxicity in the Environment. In Essentials of Medical Geology; Selinus, O., Ed.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Newman, R.; Waterland, N.; Moon, Y.; Tou, J.C. Selenium Biofortification of Agricultural Crops and Effects on Plant Nutrients and Bioactive Compounds Important for Human Health and Disease Prevention—A Review. Plant Foods Hum. Nutr. 2019, 74, 449–460. [Google Scholar] [CrossRef]
- Lowe, N.M.; Hall, A.G.; Broadley, M.R.; Foley, J.; Boy, E.; Bhutta, Z.A. Preventing and Controlling Zinc Deficiency Across the Life Course: A Call to Action. Adv. Nutr. 2024, 15, 100181. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, W.; Wang, M.; Chen, W.; Li, X.; Li, Y.; Cai, Y. Mechanisms and uncertainties of Zn supply on regulating rice Cd uptake. Environ. Pollut. 2019, 253, 959–965. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Z.F.; Yu, D.; Jiang, Z.; Wang, B.; Yuan, L. Geographical distribution of trace elements (selenium, zinc, iron, copper) and case fatality rate of COVID-19: A national analysis across conterminous USA. Environ. Geochem. Health 2022, 44, 4423–4436. [Google Scholar] [CrossRef]
- Krebs, N.F.; Miller, L.V.; Michael Hambidge, K. Zinc deficiency in infants and children: A review of its complex and synergistic interactions. Paediatr. Int. Child Health 2014, 34, 279–288. [Google Scholar] [CrossRef]
- Terrin, G.; Berni Canani, R.; Di Chiara, M.; Pietravalle, A.; Aleandri, V.; Conte, F.; De Curtis, M. Zinc in Early Life: A Key Element in the Fetus and Preterm Neonate. Nutrients 2015, 7, 10427–10446. [Google Scholar] [CrossRef]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, W.; Zhao, G.; Guo, Y. Progress of selenium biological transformation in animals, plants, and microorganisms. JARE 2018, 35, 189–198. [Google Scholar] [CrossRef]
- Roda, F.A.; Marques, I.; Esquível, M.G.; Ndayiragije, A.; Lidon, F.C.; Swamy, B.P.; Ramalho, J.C.; Ribeiro-Barros, A.I. Rice Biofortification With Zinc and Selenium: A Transcriptomic Approach to Understand Mineral Accumulation in Flag Leaves. Front. Genet. 2020, 11, 520396. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, S.B. The Copenhagen Consensus: Perspective Paper on Transnational Terrorism Policies. JSTOR 2008. Available online: https://www.jstor.org/stable/resrep25503 (accessed on 12 July 2024).
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Sec. 2017, 12, 49–58. [Google Scholar] [CrossRef]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are Improving Lives of Millions of People around the World. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef]
- USDA ERS (United States Department of Agriculture Economic Research Service). Rice Yearbook; United States Department of Agriculture: Washington, DC, USA, 2019. Available online: https://www.ers.usda.gov/data-products/rice-yearbook/ (accessed on 20 August 2023).
- FAOSTAT (Food and Agriculture Organization of the United Nations). Crops and Livestock Products; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2020; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 12 July 2024).
- FAO. The State of Food and Agriculture—Climate Change, Agriculture and Food Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef]
- Walter, M.; Marchezan, E.; Avila, L.A. Arroz: Composição e características nutricionais. Cienc. Rural. 2008, 38, 1184–1192. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Roda, F.A.; Pessoa, M.F.G.; Reboredo, F.H.; Pais, I.P.; Ndayiragije, A.; Lindo, F.C.; Ribeiro-Barros, A.I. Selenium agronomic biofortification in rice: Improving crop quality against malnutrition. In The Future of Rice Demand: Quality Beyond Productivity; Oliveira, A.C., Pegoraro, C., Viana, V.E., Eds.; Springer Nature: Cham, Switzerland, 2020; Chapter 8; pp. 179–203. [Google Scholar] [CrossRef]
- Martins, F.B.; Gonzaga, G.; Santos, D.F.; Reboita, M.S. Classificação climática de KÖPPEN e de THORNTHWAITE para Minas Gerais: Cenário atual e projeções futuras. Rev. Bras. Climatol. 2018, 1, 129–156. [Google Scholar] [CrossRef]
- Dos Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.D.; de Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; de Almeida, J.A.; de Araújo Filho, J.C.; de Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos; Embrapa Solos: Brasília, Brazil, 2018. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, Brazil, 2017. [Google Scholar]
- USEPA (United States Environmental Protection Agency). Microwave Assisted Acid Digestion of Sediments Sludge, Soils, and Oils; EPA SW 846:3051A; United States Environmental Protection Agency: Washington, DC, USA, 2007.
- Sousa, D.M.G.; Lobato, E. Cerrado: Correção do Solo e Adubação; Embrapa Informação Tecnológica: Planaltina, Brazil, 2004. [Google Scholar]
- Félix, M.R.; Zauza, S.B.; Namorato, F.A.; Castro, D.G.; Martins, F.A.D.; de Lima Lessa, J.H.; Guilherme, L.R.G.; Botelho, F.B.S. Biofortification of Upland Rice Using Selenium-Enriched Urea: Evaluation of Potential Genotypes. J. Food Compos. Anal. 2023, 122, 105409. [Google Scholar] [CrossRef]
- MAPA (Ministério da Agricultura, Pecuária e Abastecimento). Regras para Análises de Sementes; MAPA/ACS: Brasília, Brazil, 2009. [Google Scholar]
- Zanandrea, I.; Alves, J.D.; Deuner, S.; Goulart, P.F.P.; Henrique, P.C.; Silveira, N.M. Tolerance of Sesbania virgata plants to flooding. Aust. J. Bot. 2010, 57, 661–669. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Cocking, E.C.; Yemm, E.W. Estimation of amino acids by ninhydrin. Biochem. J. 1954, 58, 12. [Google Scholar]
- Ducsay, L.; Ložek, O.; Marček, M.; Varényiová, M.; Hozlár, P.; Lošák, T. Possibility of selenium biofortification of winter wheat grain. Plant Soil Environ. 2016, 62, 379–383. [Google Scholar] [CrossRef]
- Lessa, J.H.L.; Raymundo, J.F.; Corguinha, A.P.B.; Martins, F.A.D.; Araujo, A.M.; Santiago, F.E.M.; Carvalho, H.W.P.; Guilherme, L.R.G.; Lopes, G. Strategies for applying selenium for biofortification of rice in tropical soils and their effect on element accumulation and distribution in grains. J. Cereal Sci. 2020, 96, 103125. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Pimentel-Gomes, F. Curso de Estatística Experimental, 15th ed.; Fealq: Piracicaba, Brazil, 2009. [Google Scholar]
- Cao, Y.; Williams, D.D.; Williams, N.E. Data transformation and standardization in the multivariate analysis of river water quality. Ecol. Appl. 1999, 9, 669–677. [Google Scholar] [CrossRef]
- Hair Junior, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E.; Tatham, R.L. Análise Multivariada de Dados, 6th ed.; Bookman: Porto Alegre, Brazil, 2009. [Google Scholar]
- Shang, G.; Meng, Z.; Qinyue, Z.; Feng, X.; Zhang, W. Effects of exogenous zinc (ZnSO4·7H2O) on photosynthetic characteristics and grain quality of hybrid rice. Plant Physiol. Biochem. 2023, 205, 108049. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, M.; Xu, J.; Xu, F.; Zhang, W. The application of organic selenium (SeMet) improve the photosynthetic characteristics, yield and quality of hybrid rice. Plant Physiol. Biochem. 2024, 208, 108457. [Google Scholar] [CrossRef]
- Gao, S.; Tang, X.; Zhang, J.; Zhou, Q.; Peng, T.; Liu, A.; Xue, Y.; Xu, F.; Li, P. Zinc-selenium interaction regulates leaf photosynthesis and mediates grain sugar metabolism to improve the yield and quality of hybrid rice: A physiological perspective. Plant Physiol. Biochem. 2025, 221, 109611. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.A.S.; Gomes, F.T.L.; Antonio, J.R.R.; Guilherme, L.R.G.; Liu, J.; Li, L.; Silva, M.L.S. Sulfate availability and soil selenate adsorption alleviate selenium toxicity in rice plants. Environ. Exp. Bot. 2022, 201, 104971. [Google Scholar] [CrossRef]
- Gui, J.-Y.; Rao, S.; Huang, X.; Liu, X.; Cheng, S.; Xu, F. Interaction between selenium and essential micronutrient elements in plants: A systematic review. Sci. Total Environ. 2022, 853, 158673. [Google Scholar] [CrossRef]
- Kolbert, Z.; Molnár, Á.; Feigl, G.; Van Hoewyk, D. Plant Selenium Toxicity: Proteome in the Crosshairs. J. Plant Physiol. 2019, 232, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.A.S.; Rezende, P.M.; Alvarenga, A.A.; Andrade, M.J.B.; Passos, A.M.A. Doses and forms of selenium application on the culture of soybean. Cienc. E Agrotecnol. 2009, 33, 698–704. [Google Scholar] [CrossRef]
- Cipriano, P.E.; da Silva, R.F.; de Oliveira, C.; de Lima, A.B.; Martins, F.A.D.; Celante, G.; dos Santos, A.A.; Archilha, M.V.L.R.; Pinatto Botelho, M.F.; Faquin, V.; et al. Sodium Selenate, Potassium Hydroxy-Selenide, Acetylselenide and Their Effect on Antioxidant Metabolism and Plant Nutrition and Yield in Sorghum Genotypes. Foods 2023, 12, 2034. [Google Scholar] [CrossRef] [PubMed]
- Lessa, J.H.L.; Araujo, A.M.; Ferreira, L.A.; da Silva Júnior, E.C.; de Oliveira, C.; Corguinha, A.P.B.; Martins, F.A.D.; de Carvalho, H.W.P.; Guilherme, L.R.G.; Lopes, G. Agronomic Biofortification of Rice (Oryza sativa L.) with Selenium and Its Effect on Element Distributions in Biofortified Grains. Plant Soil 2019, 444, 331–342. [Google Scholar] [CrossRef]
- Yuan, Z.; Long, W.; Liang, T.; Zhu, M.; Zhu, A.; Luo, X.; Fu, L.; Hu, Z.; Zhu, R.; Wu, X. Effect of foliar spraying of organic and inorganic selenium fertilizers during different growth stages on selenium accumulation and speciation in rice. Plant Soil 2023, 486, 87–101. [Google Scholar] [CrossRef]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Palmer, C.M.; Guerinot, M.L. Facing the challenges of Cu, Fe, and Zn homeostasis in plants. Nat. Chem. Biol. 2009, 5, 333–340. [Google Scholar] [CrossRef]
- Al-Zahrani, H.S.; Alharby, H.F.; Hakeem, K.R.; Rehman, R.U. Exogenous Application of Zinc to Mitigate the Salt Stress in Vigna radiata (L.) Wilczek—Evaluation of Physiological and Biochemical Processes. Plants 2021, 10, 1005. [Google Scholar] [CrossRef]
- Hussein, M.M.; Abou-Baker, N.H. The contribution of nano-zinc to alleviate salinity stress on cotton plants. R. Soc. Open Sci. 2018, 5, 171809. [Google Scholar] [CrossRef]
- Mabesa, R.L.; Impa, S.M.; Grewal, D.; Johnson-Beebout, S.E. Contrasting grain-Zn response of biofortification rice (Oryza sativa L.) breeding lines to foliar Zn application. F. Crop. Res. 2013, 149, 223–233. [Google Scholar] [CrossRef]
- Phattarakul, N.; Rerkasem, B.; Li, L.J.; Wu, L.H.; Zou, C.Q.; Ram, H.; Sohu, V.S.; Kang, B.S.; Surek, H.; Kalayci, M.; et al. Biofortification of rice grain with zinc through zinc fertilization in different countries. Plant Soil 2012, 361, 131–141. [Google Scholar] [CrossRef]
- Brinch-Pedersen, H.; Borg, S.; Tauris, B.; Holm, P.B. Molecular genetic approaches to increasing mineral availability and vitamin content of cereals. J. Cereal Sci. 2007, 46, 308–326. [Google Scholar] [CrossRef]
- Boonchuay, P.; Cakmak, I.; Rerkasem, B.; Prom-U-Thai, C. Effect of different foliar zinc application at different growth stages on seed zinc concentration and its impact on seedling vigor in rice. Soil Sci. Plant Nutr. 2013, 59, 180–188. [Google Scholar] [CrossRef]
- Rao, S.D.; Neeraja, C.N.; Babu, P.M.; Nirmala, B.; Suman, K.; Rao, L.V.S.; Surekha, K.; Raghu, P.; Longvah, T.; Surendra, P.; et al. Zinc biofortified rice varieties: Challenges, possibilities, and progress in India. Front Nutr. 2020, 7, 26. [Google Scholar] [CrossRef]
- Farooq, M.U.; Zhu, J. The paradox in accumulation behavior of cadmium and selenium at different planting times in rice. Environ. Sci. Pollut. Res. 2019, 26, 22421–22430. [Google Scholar] [CrossRef]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Kronzucker, H.J.; Shi, W. Selenium Biofortification and Interaction with Other Elements in Plants: A Review. Front. Plant Sci. 2020, 11, 586421. [Google Scholar] [CrossRef]
- Barwinska-Sendra, A.; Waldron, K.J. The Role of Intermetal Competition and Mis-Metalation in Metal Toxicity. In Advances in Microbial Physiology; Poole, R.K., Ed.; Academic Press: London, UK, 2017; pp. 315–379. [Google Scholar] [CrossRef]
- Huang, S.; Sasaki, A.; Yamaji, N.; Okada, H.; Mitani-Ueno, N.; Ma, J.F. The ZIP Transporter Family Member OsZIP9 Contributes to Root Zinc Uptake in Rice under Zinc-Limited Conditions. Plant Physiol. 2020, 183, 1224–1234. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Huang, Q.; Gill, R.A.; Ali, S.; Mwamba, T.M.; Ali, B.; Hina, F.; Zhou, W. Protective mechanisms of melatonin against selenium toxicity in Brassica napus: Insights into physiological traits, thiol biosynthesis and antioxidant machinery. BMC Plant Biol. 2019, 19, 507. [Google Scholar] [CrossRef]
- Wu, M.; Cong, X.; Li, M.; Rao, S.; Liu, Y.; Guo, J.; Zhu, S.; Chen, S.; Xu, F.; Cheng, S.; et al. Effects of different exogenous selenium on Se accumulation, nutrition quality, elements uptake, and antioxidant response in the hyperaccumulation plant Cardamine violifolia. Ecotoxicol. Environ. Saf. 2020, 204, 111045. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, L.; Xin, Z.; Zhao, L.; An, X.; Hu, Q. Effect of Foliar Application of Zinc, Selenium, and Iron Fertilizers on Nutrients Concentration and Yield of Rice Grain in China. J. Agric. Food Chem. 2008, 56, 2079–2084. [Google Scholar] [CrossRef] [PubMed]
- Ei, H.H.; Zheng, T.; Farooq, M.U.; Zeng, R.; Su, Y.; Zhang, Y.; Liang, Y.; Tang, Z.; Ye, X.; Jia, X.; et al. Impact of selenium, zinc and their interaction on key enzymes, grain yield, selenium, zinc concentrations, and seedling vigor of biofortified rice. Environ. Sci. Pollut. Res. 2020, 27, 16940–16949. [Google Scholar] [CrossRef]
- Germ, M.; Pongrac, P.; Regvar, M.; Vogel-Mikuš, K.; Stibilj, V.; Jaćimović, R.; Kreft, I. Impact of double Zn and Se biofortification of wheat plants on the element concentrations in the grain. Plant, Soil Environ. 2013, 59, 316–321. [Google Scholar] [CrossRef]
- Poblaciones, M.J.; Rengel, Z. Combined foliar selenium and zinc biofortification in field pea (Pisum sativum): Accumulation and bioavailability in raw and cooked grains. Crop Pasture Sci. 2017, 68, 265. [Google Scholar] [CrossRef]
- Germ, M.; Kreft, I.; Stibilj, V.; Urbanc-Berčič, O. Combined effects of selenium and drought on photosynthesis and mitochondrial respiration in potato. Plant Physiol. Biochem. 2007, 45, 162–167. [Google Scholar] [CrossRef]
- Adamczyk-Szabela, D.; Wolf, W.M. The Influence of Copper and Zinc on Photosynthesis and Phenolic Levels in Basil (Ocimum basilicum L.), Borage (Borago officinalis L.), Common Nettle (Urtica dioica L.) and Peppermint (Mentha piperita L.). Int. J. Mol. Sci. 2024, 25, 3612. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Verma, R.; Singh, A.; Chandra, A.; Solomon, S. Influence of selenium on metallothionein gene expression and physiological characteristics of sugarcane plants. Plant Growth Regul. 2015, 77, 109–115. [Google Scholar] [CrossRef]
- Gao, S.; Cai, L.P.; Shan, C.J. Effect of selenomethionine on photosynthetic characteristics and chlorophyll fluorescence parameters of strawberries. J. Irrig. Drain. 2020, 39, 16–23. [Google Scholar]
- Qiao, X.; He, Y.; Wang, Z.; Li, X.; Zhang, K.; Zeng, H. Effect of foliar spray of zinc on chloroplast β-carbonic anhydrase expression and enzyme activity in rice (Oryza sativa L.) leaves. Acta Physiol. Plant 2014, 36, 263–272. [Google Scholar] [CrossRef]
- Ren, D.; Ding, C.; Qian, Q. Molecular bases of rice grain size and quality for optimized productivity. Sci. Bull. 2023, 68, 314–350. [Google Scholar] [CrossRef]
- Monsen, E.R. Dietary Reference Intakes for The Antioxidant Nutrients. J. Am. Diet. Assoc. 2000, 100, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary Reference Intakes. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.A.; Shrift, A. Selenium: Toxicity and tolerance in higher plants. Biol. Rev. 1982, 57, 59–84. [Google Scholar] [CrossRef]
- Stanton, C.; Sanders, D.; Krämer, U.; Podar, D. Zinc in plants: Integrating homeostasis and biofortification. Mol. Plant 2022, 15, 65–85. [Google Scholar] [CrossRef]






| Attributes (0–20 cm) | Lavras | Lambari |
|---|---|---|
| pH—active acidity | 4.9 | 4.7 |
| Potassiumavailable (mg dm−3) | 86.85 | 62.07 |
| Phosphorusavailable (mg dm−3) | 20.64 | 12.49 |
| Calciumavailable (cmolc dm−3) | 2.26 | 1.55 |
| Magnesiumavailable (cmolc dm−3) | 0.59 | 0.21 |
| Aluminumavailable (cmolc dm−3) | 0.30 | 1.10 |
| H+Al—potential acidity (cmolc dm−3) | 7.50 | 8 |
| SB—Sum of bases (cmolc dm−3) | 3.07 | 1.92 |
| t—Effective cation exchange capacity (cmolc dm−3) | 3.37 | 3.02 |
| T—Total cation exchange capacity (cmolc dm−3) | 10.57 | 9.92 |
| V—base saturation (%) | 29.07 | 19.35 |
| m—Aluminum saturation (%) | 8.90 | 36.42 |
| OM—Organic matter (dag kg−1) | 2.64 | 2.63 |
| Zincavailable (mg dm−3) | 2.70 | 1.20 |
| Ironavailable (mg dm−3) | 30.60 | 66.90 |
| Manganeseavailable (mg dm−3) | 12 | 17.10 |
| Copperavailable (mg dm−3) | 0.40 | 69.50 |
| Boronavailable (mg dm−3) | 0.08 | 0.01 |
| Sulfuravailable (mg dm−3) | 5.30 | 4.80 |
| Clay (dag kg−1) | 46 | 36 |
| Silt (dag kg−1) | 13 | 32 |
| Sand (dag kg−1) | 41 | 32 |
| Seleniumtotal (mg kg−1) | 0.29 | 0.18 |
| Soil Amendment Before Planting (60 Days Before Sowing). | |||
| Nutrients | Lavras | Lambari | Sources |
| Calcium and magnesium | V% = 50 | Not applied | CaCO3/MgCO3 Dolomitic limestone |
| Fertilization at planting | |||
| Nitrogen | 20 kg ha−1 | 20 kg ha−1 | *8-28-16 (Urea—Simple superphosphate— potassium chloride) |
| Phosphorus | 35 kg ha−1 | 70 kg ha−1 | |
| Potassium | 20 kg ha−1 | 40 kg ha−1 | |
| Top-dressing fertilization (40 days after sowing) | |||
| Nitrogen | 50 kg ha−1 | 50 kg ha−1 | Ammonium sulfate |
| Potassium | 20 kg ha−1 | 20 kg ha−1 | Potassium chloride |
| F-Test | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source of Variation | Lambari | Lavras | ||||||||
| B | G | T | G × T | CV | B | G | T | G × T | CV | |
| Milling yield | 3.26 * | 22.20 ** | 1.93 ns | 3.86 ** | 10.87 | 1.01 ns | 48.26 ** | 4.95 ** | 1.01 ns | 9.03 |
| Grain yield | 5.17 * | 25.19 ** | 3.33 * | 6.06 ** | 14.15 | 2.44 ns | 15.52 ** | 5.60 ** | 3.92 ** | 16.38 |
| Whole Grain | ||||||||||
| Hulling | 1.27 ns | 4.02 ** | 3.69 * | 5.31 ** | 2.56 | 0.71 ns | 1.33 ns | 2.12 ns | 1.27 ns | 2.23 |
| Se content | 1.55 ns | 7.59 ** | 59.36 ** | 3.50 ** | 18.03 | 1.48 ns | 29.57 ** | 131.68 ** | 7.35 ** | 12.15 |
| Zn content | 12.69 ** | 20.49 ** | 52.34 ** | 2.57 * | 8.47 | 1.72 ns | 16.88 ** | 39.08 ** | 2.29 * | 8.25 |
| Se uptake | 0.23 ns | 48.92 ** | 82.51 ** | 8.87 ** | 16.00 | 1.84 ns | 50.20 ** | 55.46 ** | 20.25 ** | 13.82 |
| Zn uptake | 3.25 * | 25.11 ** | 38.72 ** | 5.08 ** | 12.88 | 5.17 * | 49.56 ** | 51.51 ** | 17.78 ** | 9.64 |
| Se intake | 1.26 ns | 16.23 ** | 128.79 ** | 7.76 ** | 12.25 | 1.79 ns | 54.87 ** | 241.68 ** | 13.66 ** | 8.90 |
| Zinc intake | 10.42 ** | 13.63 ** | 34.76 ** | 1.71 ns | 10.39 | 4.16 * | 15.66 ** | 36.30 ** | 2.13 * | 8.56 |
| Free proteins | 1.32 ns | 23.28 ** | 3.12 * | 4.47 ** | 19.19 | 0.09 ns | 22.35 ** | 3.01 * | 9.23 ** | 11.41 |
| Free amino acids | 0.15 ns | 38.63 ** | 5.64 ** | 13.34 ** | 11.04 | 0.70 ns | 4.74 ** | 4.83 ** | 4.89 ** | 13.77 |
| Polished Grain | ||||||||||
| Hulling | 0.19 ns | 9.74 ** | 0.59 ns | 1.89 ns | 3.73 | 0.17 ns | 2.49 ns | 0.80 ns | 1.47 ns | 2.59 |
| Se content | 1.95 ns | 6.96 ** | 52.35 ** | 2.21 * | 13.96 | 2.58 ns | 2.74 * | 54.29 ** | 11.23 ** | 12.96 |
| Zn content | 15.77 ** | 16.34 ** | 20.20 ** | 2.73 ** | 9.80 | 3.07 ns | 7.37 ** | 22.96 ** | 1.42 ns | 9.08 |
| Se uptake | 1.28 ns | 43.08 ** | 68.31 ** | 8.95 ** | 13.00 | 4.61 * | 23.99 ** | 19.48 ** | 23.29 ** | 14.15 |
| Zn uptake | 0.37 ns | 23.12 ** | 17.45 ** | 3.59 ** | 17.50 | 1.59ns | 9.90 ** | 14.18 ** | 5.54 ** | 17.27 |
| Se intake | 2.04 ns | 22.29 ** | 172.89 ** | 7.56 ** | 7.62 | 2.22 ns | 7.46 ** | 151.76 ** | 32.48 ** | 7.67 |
| Zn intake | 15.23 ** | 21.90 ** | 27.14 ** | 3.65 ** | 8.46 | 3.07 ns | 7.37 ** | 22.99 ** | 1.42 ns | 9.08 |
| Free proteins | 0.51 ns | 28.02 ** | 4.15 * | 7.69 ** | 12.80 | 1.02 ns | 109.31 ** | 11.34 ** | 2.83 ** | 11.28 |
| Free amino acids | 0.69 ns | 75.94 ** | 28.34 ** | 13.60 ** | 12.80 | 1.31 ns | 72.03 ** | 72.16 ** | 24.12 ** | 10.51 |
| Source of Variation | FMax | F-Test | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B | G | T | L | B × L | G × T | L × G | L × T | L × G × T | CV (%) | ||
| Milling yield | 0.26 | 2.48 ns | 56.62 ** | 6.36 ** | 1913.26 ** | 0.48 ns | 1.67 ns | 29.03 ** | 2.29 ns | 1.54 ns | 10.07 |
| Grain yield | 0.48 | 6.04 ** | 12.53 ** | 6.75 ** | 57.44 ** | 0.63 ns | 3.15 ** | 24.82 ** | 2.98 * | 6.08 ** | 15.63 |
| Whole Grain | |||||||||||
| Hulling | 0.94 | 0.10 ns | 4.33 ** | 5.23 ** | 598.56 ** | 1.90 ns | 3.18 ** | 1.10 ns | 0.63 ns | 3.53 ** | 2.39 |
| Se content | 0.70 | 2.94 ns | 24.24 ** | 175.54 ** | 64.38 ** | 0.10 ns | 7.48 ** | 9.08 ** | 2.88 * | 2.69 ** | 14.83 |
| Zn content | 0.67 | 3.50 * | 30.28 ** | 86.02 ** | 212.34 ** | 8.76 ** | 1.37 ns | 6.38 ** | 2.80 * | 3.44 ** | 8.39 |
| Se uptake | 0.49 | 1.66 ns | 53.60 ** | 124.90 ** | 328.30 ** | 0.96 ns | 24.78 ** | 45.96 ** | 3.84 * | 8.24 ** | 14.87 |
| Zinc uptake | 0.61 | 6.67 ** | 52.16 ** | 70.10 ** | 697.79 ** | 2.22 ns | 10.42 ** | 28.48 ** | 23.25 ** | 15.54 ** | 10.91 |
| Se intake | 0.81 | 2.97 ns | 48.18 ** | 353.65 ** | 126.62 ** | 0.02 ns | 14.73 ** | 18.91 ** | 5.12 ** | 6.08 ** | 10.41 |
| Zinc intake | 0.94 | 2.46 ns | 24.23 ** | 68.87 ** | 169.80 ** | 11.93 ** | 1.09 ns | 5.12 ** | 2.24 ns | 2.75 ** | 9.38 |
| Free proteins | 0.36 | 0.83 ns | 39.64 ** | 3.65 * | 0.07 ns | 1.16 ns | 7.84 ** | 6.42 ** | 2.53 ns | 3.62 ** | 15.76 |
| Free amino acids | 0.77 | 0.14 ns | 29.69 ** | 6.79 ** | 15.02 ** | 0.78 ns | 5.65 ** | 9.18 ** | 3.58 * | 11.46 ** | 12.37 |
| Polished Grain | |||||||||||
| Hulling | 0.59 | 0.64 ns | 9.33 ** | 0.53 ns | 334.64 ** | 0.30 ns | 2.00 * | 4.75 ** | 0.81 ns | 1.46 ns | 3.15 |
| Se content | 0.92 | 0.30 ns | 5.04 ** | 102.96 ** | 21.91 ** | 4.26 * | 7.46 ** | 4.48 ** | 3.76 * | 6.35 ** | 13.44 |
| Zn content | 0.94 | 3.14 * | 15.29 ** | 42.05 ** | 37.47 ** | 15.33 ** | 1.90 * | 8.15 ** | 1.18 ns | 2.21 * | 9.42 |
| Se uptake | 0.33 | 3.78 * | 15.90 ** | 54.28 ** | 312.10 ** | 3.78 * | 20.41 ** | 41.61 ** | 90.06 ** | 19.00 ** | 14.19 |
| Zn uptake | 0.39 | 1.53 ns | 11.82 ** | 23.71 ** | 212.86 ** | 0.97 ns | 5.18 ** | 15.40 ** | 6.48 ** | 4.80 ** | 17.82 |
| Se intake | 0.79 | 2.06 ns | 14.06 ** | 310.81 ** | 65.32 ** | 2.22 ns | 23.59 ** | 13.92 ** | 11.33 ** | 19.42 ** | 7.66 |
| Zn intake | 0.70 | 2.46 ns | 17.45 ** | 48.05 ** | 42.80 ** | 13.72 ** | 2.17 * | 9.29 ** | 1.36 ns | 2.51 ** | 8.82 |
| Free amino acids | 0.39 | 1.69 ns | 138.29 ** | 67.59 ** | 155.85 ** | 0.04 ns | 29.74 ** | 11.40 ** | 13.52 ** | 3.33 ** | 12.13 |
| Free proteins | 0.44 | 1.31 ns | 96.91 ** | 2.56 ns | 158.52 ** | 0.02 ns | 6.14 ** | 8.59 ** | 10.12 ** | 6.28 ** | 12.40 |
| BRS Esmeralda | CMG 2188 | CMG ERF 85-15 | CMG ERF 221-16 | CMG ERF 221-19 | |
|---|---|---|---|---|---|
| Zn Whole (Lambari) | ↑22.7% (Zn+Se vs. Ctrl) | ↑28.3% (Zn+Se vs. Ctrl) | ↑34.5% (Zn vs. ERF 221-16) | — | — |
| Zn Whole (Lavras) | ↑16.0% (Zn+Se vs. Se) | ↑33.7% (Zn+Se vs. Ctrl) | — | ↑34.5% (Zn+Se vs. Se) | ↑27.6% (Zn+Se vs. Se) |
| Zn Polished (Lambari) | — | ↑27.9% (Zn vs. Ctrl) | ↑30.6% (Zn vs. ERF 221-16) | ↓33.3% (Zn+Se vs. others) | — |
| Zn Polished (Lavras) | — | ↑26.6% (Zn+Se vs. Ctrl) | — | ↑24.2% (Zn+Se vs. Se) | ↑23.7% (Zn+Se vs. Se) |
| Se Whole (Lambari) | ↑30.4% (Zn+Se vs. Zn) | ↑48.0% (Zn+Se vs. Ctrl) | ↑55.0% (Zn vs. Ctrl) | ↑50.0% (Zn+Se vs. Ctrl) | ↑68.2% (Zn+Se vs. Ctrl) |
| Se Whole (Lavras) | ↑50.0% (Se vs. Ctrl) | ↑46.4% (Zn+Se vs. Ctrl) | ↑52.3% (Se vs. ERF 221-16) | ↑46.9% (Zn+Se vs. Ctrl) | ↑64.0% (Zn+Se vs. Ctrl) |
| Se Polished (Lambari) | ↑39.2% (Zn+Se vs. Ctrl) | ↑33.3% (Zn+Se vs. Ctrl) | ↑40.0% (Se vs. ERF 221-16) | ↑43.0% (Zn+Se vs. Zn) | ↑47.2% (Zn+Se vs. Ctrl) |
| Se Polished (Lavras) | ↓66.7% (Zn+Se vs. others) | ↑55.6% (Zn+Se vs. Ctrl) | ↑46.2% (Zn+Se vs. Zn) | ↑45.8% (Zn+Se vs. Zn) | ↑50.0% (Zn+Se vs. Ctrl) |
| Genotype | Better Treatment | % Increase over Control/Compared Treatment | |
|---|---|---|---|
| Whole grain (Lambari) | BRS Esmeralda | Zn+Se | +46.2% (vs. control); +36.5% (vs. Se) |
| CMG 2188 | Zn | +38.1% (vs. control); +34.5% (vs. Zn+Se) | |
| CMG ERF 221-16 | Zn | +55.7% (vs. control); +27.1% (vs. Zn+Se) | |
| Polished Grain (Lambari) | CMG ERF 221-16 | Zn | +54.3% (vs. controle); +38.0% (vs. Zn+Se) |
| CMG ERF 221-19 | Se | significant increase (no exact value mentioned) | |
| Whole grain (Lavras) | CMG 2188 | Zn | +51.3% (vs. control); +48.3% (vs. Zn+Se) |
| CMG ERF 85-15 | Se | +66.0% (vs. other genotypes) | |
| Polished Grain (Lavras) | CMG ERF 221-19 | Zn+Se | +49% (vs. BRS Esmeralda) |
| Genotype | Better Treatment | % Increase over Control/Compared Treatment | |
|---|---|---|---|
| Whole grain (Lambari) | BRS Esmeralda | Zn+Se | +50.3% (vs. control); +43.0% (vs. Zn) |
| CMG ERF 221-19 | Zn+Se | +61.7% (vs. control) | |
| CMG 2188 | Se | +50.3% (vs. control) | |
| Polished Grain (Lambari) | BRS Esmeralda | Zn+Se | +55.8% (vs. control) |
| CMG ERF 221-16 | Zn+Se | +55.8% (vs. control) | |
| CMG ERF 85-15 | Se | +63.7% (vs. CMG 2188) | |
| Whole grain (Lavras) | CMG ERF 221-19 | Zn+Se | +65.8% (vs. BRS Esmeralda) |
| CMG ERF 85-15 | Se | +74.5% (vs. other genotypes) | |
| Polished Grain (Lavras) | CMG ERF 221-19 | Zn+Se | +82.8% (vs. BRS Esmeralda) |
| CMG ERF 85-15 | Zn+Se | +79.7% (vs. BRS Esmeralda) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namorato, F.A.; Cipriano, P.E.; Benevenute, P.A.N.; de Morais, E.G.; Cardoso, F.P.; Corguinha, A.P.B.; Zauza, S.B.; de Sousa, G.F.; Silva, M.A.; Figueredo, E.S.S.; et al. Responses to the Interaction of Selenium and Zinc Through Foliar Fertilization in Processed Grains of Brazilian Upland Rice Genotypes. Agriculture 2025, 15, 1186. https://doi.org/10.3390/agriculture15111186
Namorato FA, Cipriano PE, Benevenute PAN, de Morais EG, Cardoso FP, Corguinha APB, Zauza SB, de Sousa GF, Silva MA, Figueredo ESS, et al. Responses to the Interaction of Selenium and Zinc Through Foliar Fertilization in Processed Grains of Brazilian Upland Rice Genotypes. Agriculture. 2025; 15(11):1186. https://doi.org/10.3390/agriculture15111186
Chicago/Turabian StyleNamorato, Filipe Aiura, Patriciani Estela Cipriano, Pedro Antônio Namorato Benevenute, Everton Geraldo de Morais, Felipe Pereira Cardoso, Ana Paula Branco Corguinha, Stefânia Barros Zauza, Gustavo Ferreira de Sousa, Maila Adriely Silva, Eduardo Sobrinho Santos Figueredo, and et al. 2025. "Responses to the Interaction of Selenium and Zinc Through Foliar Fertilization in Processed Grains of Brazilian Upland Rice Genotypes" Agriculture 15, no. 11: 1186. https://doi.org/10.3390/agriculture15111186
APA StyleNamorato, F. A., Cipriano, P. E., Benevenute, P. A. N., de Morais, E. G., Cardoso, F. P., Corguinha, A. P. B., Zauza, S. B., de Sousa, G. F., Silva, M. A., Figueredo, E. S. S., Correia, R. F. R., Martins, F. A. D., Botelho, F. B. S., & Guilherme, L. R. G. (2025). Responses to the Interaction of Selenium and Zinc Through Foliar Fertilization in Processed Grains of Brazilian Upland Rice Genotypes. Agriculture, 15(11), 1186. https://doi.org/10.3390/agriculture15111186










