Comprehensive Assessment of Soil Heavy Metal Contamination in Agricultural and Protected Areas: A Case Study from Iași County, Romania
Abstract
1. Introduction
| Heavy Metal | Sources | Effects on Human Health | References |
|---|---|---|---|
| Aluminium (Al) | Steel industry cutlery; pots and pans | Nausea; vomiting; mouth and skin ulcers; skin rashes; arthritic pain; diarrhoeal disease; possible onset factor of Alzheimer’s disease; memory loss; vertigo and dizziness; bone and brain damage | [19] |
| Arsenic (As) | Pesticides; herbicides; insecticides; rodenticides; treated wood products; colouring agents for textiles; mining; wallpaper; toy industry; ash resulting from coal combustion | Visceral neoplastic pathologies: liver, kidney, lung, bladder; epithelial neoplastic pathologies; circulatory disorders | [22,23,24,25] |
| Barium (Ba) | Equipment for industrial control | Cardiac arrhythmias; respiratory failure; gastrointestinal dysfunction; muscle spasms; high blood pressure | [23] |
| Beryllium (Be) | Coal; rocket fuels | Carcinogenic compound in acute/accidental/chronic exposure | [23] |
| Cadmium (Cd) | Fertilisers; metallurgical industry; spoiled food; cigarettes; manufacture of plastic materials | Kidney damage; prostate dysfunction; bone pathologies; neoplasia; lung damage; disturbance of calcium metabolism | [23,26,27] |
| Chromium (Cr) | Leather tanning; metal refining; textile dyeing; pharmaceutical industry; ink and pigments; refractory materials; wood preservatives; fungicides | Neoplasms; nephritis; ulcerations; hair loss; diabetes; feeling of nausea; headaches; genetic/congenital diseases | [23,28,29,30] |
| Cobalt (Co) | Mining industry; wood conservation; metal industry; graphic industry; electronics; medical assistance | Diarrheal disease; arterial hypotension; paresis; damage to striated, smooth, and cardiac muscle tissue; muscle weakness; obtundation | [23,31,32] |
| Coper (Cu) | Pesticides; insecticides; treated wood products | Tissue destruction in the brain and kidneys; elevated levels are commonly associated with cirrhosis of the liver | [23,33] |
| Iron (Fe) | Building materials (asbestos); mining industry (iron smelting and steel smelting); car construction | Iron toxicity depends on the dose/chemical form and the exposure period: 6 h after the overdose, gastrointestinal/local effects appear (gastrointestinal bleeding, vomiting, and diarrhoeal disease); 6–24 h after the overdose, the latency period sets in (apparent improvement of local symptoms); at 12–96 h systemic and hepatic toxicity sets in (shocks, lethargy, tachycardia, arterial hypotension, hepatic necrosis, metabolic acidosis, and death); within 2–6 weeks of administration late gastrointestinal effects appear (ulcerations and strictures/obstructions); asbestosis (second leading cause of lung cancer); cell death | [19,34] |
| Manganese (Mn) | Foundries; smelters; steel industry; ceramics; fireworks; dry cell batteries; fertilisers; fungicides; paints; medical imaging agent; cosmetics; additive in gasoline to improve the octane number; food (cereals, beans, nuts, and tea); mining activities; car exhaust; cigarette smoke | Neurological disorders, the combination of neuropsychiatric symptoms = ‘manganism’; inhalation causes lung damage (pneumonia); crosses the blood–brain barrier and the placental barrier; infertility; nephropathies and renal lithiasis; carcinogenic potential; associated with Parkinson’s disease, schizophrenia, and hypertension | [35] |
| Mercury (Hg) | Thermometers and barometers; tensiometers; amalgam for dental restoration; fluorescent lighting; in the production of caustic soda; in the preservation of pharmaceutical products; nuclear reactors; antifungal agents for the woodworking industry | Gastrointestinal toxicity; neurotoxicity; nephrotoxicity; depression; sleepiness; asthenia; hair loss; insomnia; memory loss; restlessness; visual disturbances; tremors; tantrums; brain injuries; renal and respiratory failure | [23,36] |
| Nickel (Ni) | Plating industry; fossil fuel burning; mining; cigarette smoke; jewellery; shampoos; detergents; coins | Carcinogenic embryotoxicity; teratogenesis | [23,37] |
| Lead (Pb) | Plastic materials; (automatic) vehicles; hair dyes; paints; paint varnish; pipes; batteries; gasoline; enamelled products | Decreased intelligence quotient (mental retardation); memory loss; infertility; alternating mood; sterility; risk of cardiovascular disease | [23,38,39] |
| Tin (Sn) | Preservatives and dyes for wood; biocides; food industry; brazing alloys; dental amalgams; aircraft engineering (titanium alloys); foil; reducing agents in the manufacture of polymers, toothpaste, ceramics, porcelain, enamel, drill glass, and inks; pigments in the ceramic and textile industry (purple tin); manufacture of tin salts for chemical plating reagents; perfume stabiliser; as SnO2 for glass making | High concentrations: haemolysis; ecotoxicity; skin rashes; stomach ache; vomiting; diarrhoea; abdominal pain; headaches; palpitations; possible cytotoxicity Low concentrations: fatigue; depression; low adrenals; breathing difficulties; asthma; headache; insomnia | [40] |
| Zinc (Zn) | Fertilisers; paints; rubber; cosmetics; plastic products; pharmaceuticals; inks; soaps; textiles; batteries; electrical equipment | Dizziness; fatigue; vomiting; kidney damage; cramps | [23,38,41] |
2. Materials and Methods
2.1. Climate and Environmental Conditions of the Study Area
2.2. Soil Sample Collection
2.3. Physicochemical Parameters
2.3.1. Determination of Soil Humidity
2.3.2. Determination of Organic Carbon and Estimation of Soil Humus Content
2.3.3. pH Evaluation
2.4. Heavy Metal Analysis
2.4.1. Micro-Imaging of Soil Samples Using Scanning Electron Microscopy
2.4.2. Initial Evaluation of Elemental Composition Using Energy-Dispersive X-Ray Spectroscopy
2.4.3. Determination of Heavy Metal Content by X-Ray Fluorescence Spectrometry
2.5. Pollution Assessment Methods of Heavy Metals
2.5.1. Contamination Factor and Degree of Contamination as Indicators of Soil Pollution—Method Description
2.5.2. Assessment of Soil Contamination Using the Pollution Load Index
2.5.3. Geo-Accumulation Index as a Tool for Assessing Soil Pollution
2.6. Statistical Analysis
3. Results and Discussions
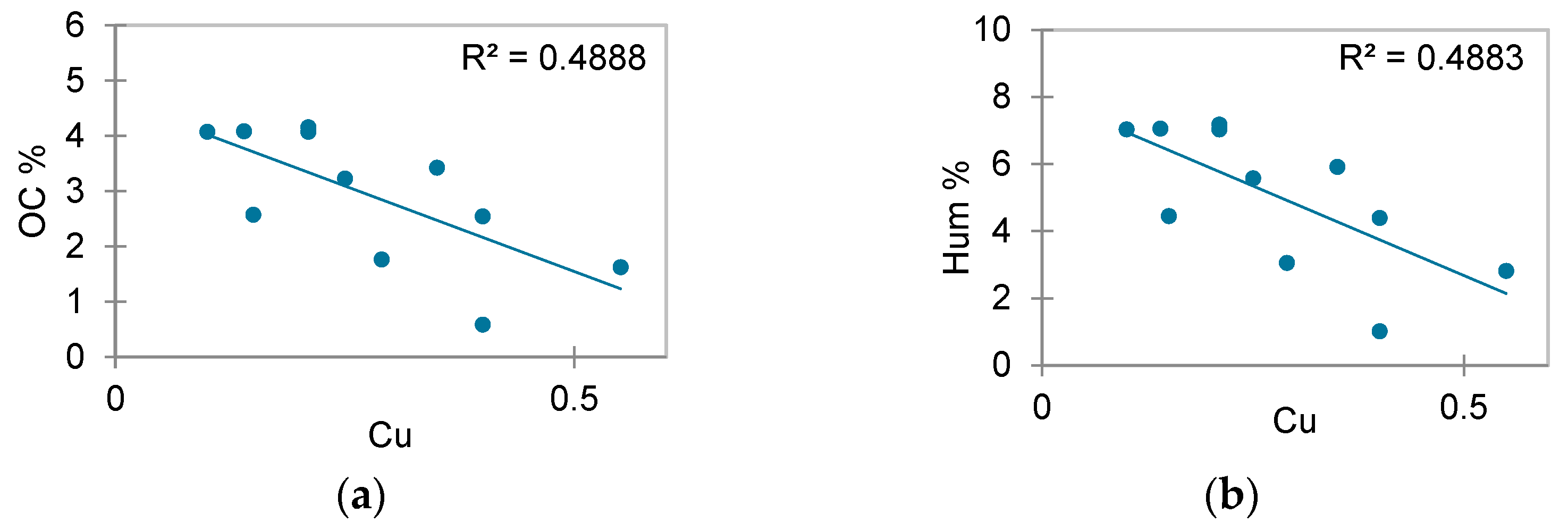
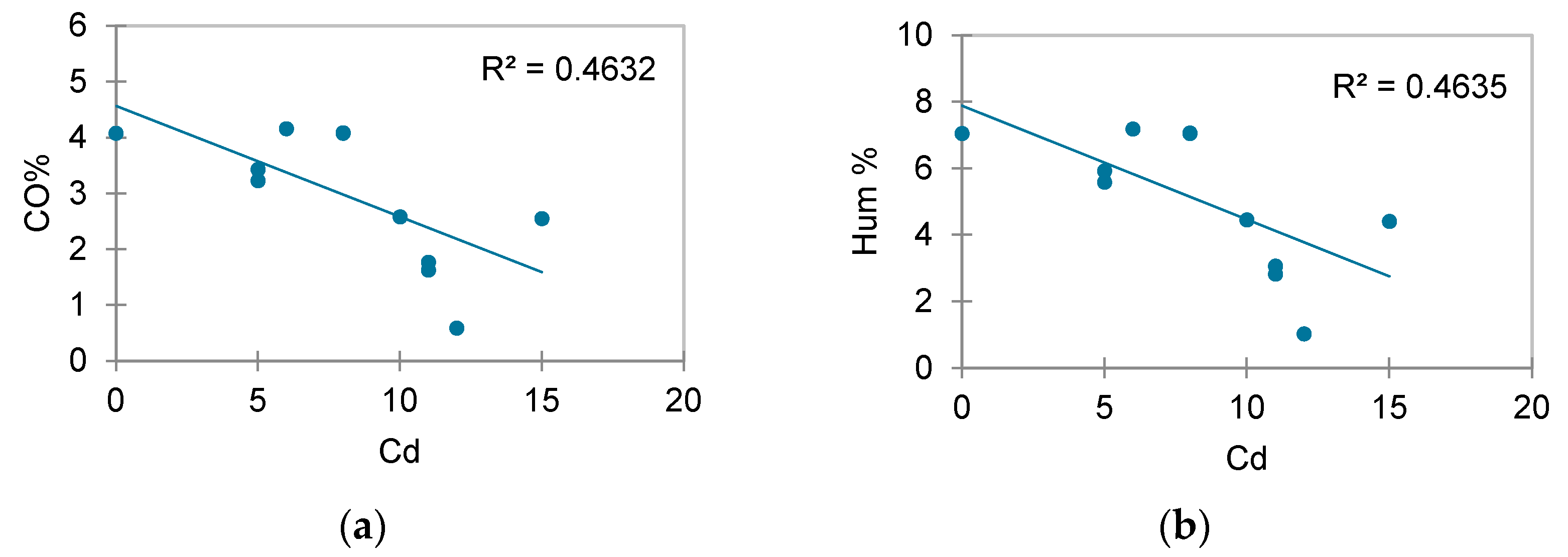
3.1. Physicochemical Properties
3.2. Heavy Metal Quantification
3.2.1. Soil Characteristics Using SEM
3.2.2. EDS Results
3.2.3. XRFS Results
| Samples | Al | Cr | Mn | Fe | Co | Ni | Cu | Zn | As | Cd | Hg | Pb | Sn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 2893 ± 534 | <LOD * | 40 ± 5 | 150 ± 5 | 18 ± 2 | <LOD * | 53 ± 2 | 27 ± 1 | 6 ± 1 | 5 ± 1 | <LOD * | 2 ± 1 | <LOD * |
| S2 | 1312 ± 323 | 23 ± 7 | 40 ± 4 | <LOD * | 16 ± 2 | <LOD * | 36 ± 1 | 12 ± 1 | 959 ± 9 | <LOD * | 78 ± 2 | 10,176 ± 32 | <LOD * |
| S3 | 7546 ± 816 | 85 ± 13 | 866 ± 16 | 26,884 ± 112 | 174 ± 16 | 47 ± 3 | 46 ± 3 | 117 ± 3 | 21 ± 1 | 11 ± 1 | <LOD * | 14 ± 1 | 68 ± 7 |
| S4 | 8580 ± 809 | 119 ± 13 | 816 ± 15 | 30,571 ± 118 | 209 ± 16 | 55 ± 3 | 56 ± 3 | 153 ± 3 | 15 ± 1 | 15 ± 1 | <LOD * | 24 ± 1 | 66 ± 7 |
| S5 | 2743 ± 716 | 122 ± 11 | 730 ± 13 | 25,108 ± 96 | 163 ± 14 | 39 ± 3 | 52 ± 3 | 116 ± 3 | 13 ± 1 | 8 ± 1 | <LOD * | 16 ± 1 | 39 ± 6 |
| S6 | 10,904 ± 790 | 96 ± 13 | 803 ± 15 | 28,921 ± 113 | 184 ± 16 | 41 ± 3 | 63 ± 3 | 107 ± 3 | 18 ± 1 | 12 ± 1 | <LOD * | 20 ± 1 | 54 ± 7 |
| S7 | 12,544 ± 806 | 78 ± 13 | 834 ± 15 | 31,587 ± 122 | 139 ± 17 | 39 ± 3 | 48 ± 3 | 116 ± 3 | 19 ± 1 | 11 ± 1 | <LOD * | 15 ± 1 | 64 ± 7 |
| S8 | 8596 ± 747 | 89 ± 13 | 750 ± 14 | 28,264 ± 108 | 174 ± 16 | 46 ± 3 | 61 ± 3 | 101 ± 2 | 17 ± 1 | 6 ± 1 | <LOD * | 29 ± 1 | 28 ± 6 |
| S9 | 7380 ± 728 | 137 ± 13 | 787 ± 14 | 26,517 ± 101 | 110 ± 15 | 28 ± 3 | 40 ± 3 | 100 ± 2 | 10 ± 1 | 8 ± 1 | <LOD * | 22 ± 1 | 56 ± 6 |
| S10 | 12,888 ± 761 | 106 ± 13 | 757 ± 14 | 27,960 ± 106 | 248 ± 16 | 27 ± 3 | 74 ± 3 | 118 ± 3 | 18 ± 1 | 10 ± 1 | 5 ± 1 | 16 ± 1 | <LOD * |
| S11 | 14,377 ± 777 | 186 ± 15 | 828 ± 14 | 29,004 ± 113 | 166 ± 17 | 54 ± 3 | 52 ± 3 | 113 ± 3 | 19 ± 1 | 5 ± 1 | <LOD * | 20 ± 1 | 73 ± 7 |
| Normal values in soil [83] | - | 30 | 900 | - | 15 | 20 | 20 | 100 | 5 | 1 | 0.1 | 20 | 20 |
| Alert threshold, sensitive soil type [83] | - | 100 | 1500 | - | 30 | 75 | 100 | 300 | 15 | 3 | 1 | 50 | 35 |
| Alert threshold, less sensitive soil type [83] | - | 300 | 2000 | - | 100 | 200 | 250 | 700 | 25 | 5 | 4 | 250 | 100 |
| European soils | |||||||||||||
| [67] | - | 21.72 | 237.68 | - | 6.35 | 18.15 | 13.01 | - | - | 0.09 | 0.04 | 8.33 | - |
| [79] | - | 94.8 | 524 | - | 10.4 | 37 | 17.3 | 68.1 | 11.6 | 0.28 | 0.061 | 32 | 4.5 |
| [80] | - | 26.5 | 295 | 13,608 | 7.1 | 20.9 | 22.5 | 52.8 | - | 0.34 | - | 22.8 | - |
| National baseline in the Netherlands [81] | - | 55 | - | - | 15 | 35 | 40 | 140 | 20 | 0.6 | 0.15 | 50 | 6.5 |
| World soils | |||||||||||||
| [3] | - | 59.5 | 488 | - | 11.3 | 29 | 38.9 | 70 | 6.83 | 0.41 | 0.07 | 27 | 2.5 |
| [82] | - | 42 | 418 | - | 6.9 | 18 | 14 | 62 | 4.7 | 1.1 | 0.1 | 25 | - |
| Mean values for chernozems on the world scale [3] | - | 77 | 480 | - | 7.5 | 25 | 24 | 65 | 8.5 | 0.44 | 0.1 | 23 | - |
| Samples | CF Cr | CF Mn | CF Co | CF Ni | CF Cu | CF Zn | CF As | CF Cd | CF Hg | CF Pb | CF Sn | DC | DC status | PLI | PLI Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 0.01 | 0.04 | 1.20 | 0.01 | 2.65 | 0.27 | 1.20 | 5.00 | 0.01 | 0.01 | 0.01 | 10.4 | Moderate | 0.11 | No pollution |
| S2 | 0.77 | 0.04 | 1.07 | 0.01 | 1.80 | 0.12 | 191.80 | 0.01 | 780.00 | 508.80 | 0.01 | 1484.4 | Very high | 0.95 | No pollution |
| S3 | 2.83 | 0.96 | 11.60 | 2.35 | 2.30 | 1.17 | 4.20 | 11.00 | 0.01 | 0.70 | 3.40 | 40.5 | Very high | 1.63 | None to medium pollution |
| S4 | 3.97 | 0.91 | 13.93 | 2.75 | 2.80 | 1.53 | 3.00 | 15.00 | 0.01 | 1.20 | 3.30 | 48.4 | Very high | 1.88 | None to medium pollution |
| S5 | 4.07 | 0.81 | 10.87 | 1.95 | 2.60 | 1.16 | 2.60 | 8.00 | 0.01 | 0.80 | 1.95 | 34.8 | Very high | 1.47 | None to medium pollution |
| S6 | 3.20 | 0.89 | 12.27 | 2.05 | 3.15 | 1.07 | 3.60 | 12.00 | 0.01 | 1.00 | 2.70 | 41.9 | Very high | 1.67 | None to medium pollution |
| S7 | 2.60 | 0.93 | 9.27 | 1.95 | 2.40 | 1.16 | 3.80 | 11.00 | 0.01 | 0.75 | 3.20 | 37.0 | Very high | 1.55 | None to medium pollution |
| S8 | 2.97 | 0.83 | 11.60 | 2.30 | 3.05 | 1.01 | 3.40 | 6.00 | 0.01 | 1.45 | 1.40 | 34.0 | Very high | 1.50 | None to medium pollution |
| S9 | 4.57 | 0.87 | 7.33 | 1.40 | 2.00 | 1.00 | 2.00 | 8.00 | 0.01 | 1.10 | 2.80 | 31.0 | Very high | 1.40 | None to medium pollution |
| S10 | 3.53 | 0.84 | 16.53 | 1.35 | 3.70 | 1.18 | 3.60 | 10.00 | 50.00 | 0.80 | 0.01 | 91.5 | Very high | 2.13 | Moderate pollution |
| S11 | 6.20 | 0.92 | 11.07 | 2.70 | 2.60 | 1.13 | 3.80 | 5.00 | 0.01 | 1.00 | 3.65 | 38 | Very high | 1.70 | None to medium pollution |
3.3. Pollution Assessment Methods
3.3.1. Degree of Contamination and Contamination Factor
3.3.2. Pollution Load Index
3.3.3. Geo-Accumulation Index
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Q.; Song, Y.; An, Y.; Lu, Y.; Zhong, G. Soil microorganisms: Their role in enhancing crop nutrition and health. Diversity 2024, 16, 734. [Google Scholar] [CrossRef]
- Food and Agriculture Organisation of the United Nations. Available online: https://openknowledge.fao.org/bitstreams/4fb89216-b131-4809-bbed-b91850738fa1/download (accessed on 12 December 2024).
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Alloway, B.J. Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Pierzynski, G.M.; Vance, G.F.; Sims, J.T. Soils and Environmental Quality, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar] [CrossRef]
- Shi, H.; He, Z.; Deng, C.; Liu, A.; Feng, Y.; Li, L.; Ji, G.; Xie, M.; Liu, X. How has the source apportionment of heavy metals in soil and water evolved over the past 20 years? A bibliometric perspective. Water 2024, 16, 3171. [Google Scholar] [CrossRef]
- Pacyna, J.M. Atmospheric deposition. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 275–285. [Google Scholar] [CrossRef]
- Wang, M.; Markert, B.; Chen, W.; Peng, C.; Ouyang, Z. Identification of heavy metal pollutants using multivariate analysis and effects of land uses on their accumulation in urban soils in Beijing, China. Environ. Monit. Assess. 2012, 184, 5889–5897. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Kaur, P.; Sidhu, G.P.S.; Bali, A.S.; Bhardwaj, R.; Thukral, A.K.; Cerda, A. Pollution assessment of heavy metals in soils of India and ecological risk assessment: A state-of-the-art. Chemosphere 2019, 216, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S.; Lai, Y.; Wang, L.; Wang, F.; Chen, Z. Predicting future contents of soil heavy metals and related health risks by combining the models of source apportionment, soil metal accumulation and industrial economic theory. Ecotoxicol. Environ. Saf. 2019, 171, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Naila, A.; Meerdink, G.; Jayasena, V.; Sulaiman, A.Z.; Ajit, A.B.; Berta, G. A review on global metal accumulators—Mechanism, enhancement, commercial application, and research trend. Environ. Sci. Pollut. Res. 2019, 26, 26449–26471. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, Z.; Li, F. Using ensemble models to identify and apportion heavy metal pollution sources in agricultural soils on a local scale. Environ. Poll. 2015, 206, 227–235. [Google Scholar] [CrossRef]
- Lin, X.; Gu, Y.; Zhou, Q.; Mao, G.; Zou, B.; Zhao, J. Combined toxicity of heavy metal mixtures in liver cells. J. Appl. Toxicol. 2016, 36, 1163–1172. [Google Scholar] [CrossRef]
- Pichhode, M.; Nikhil, K. Effect of heavy metals on plants: An overview. IJAIEM 2016, 3, 58–66. [Google Scholar] [CrossRef]
- Mustafa, G.; Komatsu, S. Toxicity of heavy metals and metal-containing nanoparticles on plants. Biochim. Biophys. Acta Proteins Proteomics 2016, 1864, 932–944. [Google Scholar] [CrossRef]
- Shahid, M.; Khalid, S.; Abbas, G.; Shahid, N.; Nadeem, M.; Sabir, M.; Aslam, M.; Dumat, C. Heavy metal stress and crop productivity. In Crop Production and Global Environmental Issues; Hakeem, K.R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–25. [Google Scholar] [CrossRef]
- Giller, K.E.; Witter, E.; McGrath, S. Heavy metals and soil microbes. Soil Biol. Biochem. 2009, 41, 2031–2037. [Google Scholar] [CrossRef]
- Kapri, A.; Mohammed, A.S.; Goel, R. Heavy metal pollution: Source, impact, and remedies. In Biomanagement of Metal-Contaminated Soils; Khan, M., Zaidi, A., Goel, R., Musarrat, J.E., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 1–28. [Google Scholar] [CrossRef]
- Bhat, A.S.; Hassan, T.; Majid, S. Heavy metal toxicity and their harmful effects on living organisms—A review. IJMSDR 2019, 3, 106–122. Available online: https://www.ijmsdr.com/index.php/ijmsdr/article/view/210/195 (accessed on 12 December 2024).
- Sun, Z.; Shao, Y.; Yan, K.; Yao, T. The link between trace metal elements and glucose metabolism: Evidence from Zinc, Copper, Iron, and Manganese-mediated metabolic regulation. Metabolites 2023, 13, 1048. [Google Scholar] [CrossRef]
- Achema, K.O.; Alhassan, K.J. Effect of biodegradable multiple pesticides on aquatic biospecies. In Insecticides—Impact and Benefits of Its Use for Humanity; Ranz, R.E.R., Ed.; IntechOpen: London, UK, 2022; pp. 225–244. [Google Scholar] [CrossRef]
- Mensah, A.K.; Marschner, B.; Shaheen, S.M.; Wang, J.; Wang, S.L.; Rinklebe, J. Arsenic contamination in abandoned and active gold mine spoils in Ghana: Geochemical fractionation, speciation, and assessment of the potential human health risk. Environ. Poll. 2020, 261, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy metal contamination: An alarming threat to environment and human health. In Environmental Biotechnology: For Sustainable Future; Sobti, R., Arora, N., Kothari, R., Eds.; Springer: Singapore, 2019; pp. 103–125. [Google Scholar] [CrossRef]
- Yin, N.; Wang, P.; Li, Y.; Du, H.; Chen, X.; Sun, G.; Cui, Y. Arsenic in rice bran products: In vitro oral bioaccessibility, arsenic transformation by human gut microbiota, and human health risk assessment. J. Agric. Food Chem. 2019, 67, 4987–4994. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, B.; Guo, Z.; Han, J.; Li, H.; Jin, L.; Chen, F.; Xiong, Y. Human health risk assessment of groundwater arsenic contamination in Jinghui irrigation district, China. J. Environ. Manag. 2019, 237, 163–169. [Google Scholar] [CrossRef]
- Shahriar, S.; Rahman, M.M.; Naidu, R. Geographical variation of cadmium in commercial rice brands in Bangladesh: Human health risk assessment. Sci. Total Environ. 2020, 716, 137049. [Google Scholar] [CrossRef]
- Suwatvitayakorn, P.; Ko, M.S.; Kim, K.W.; Chanpiwat, P. Human health risk assessment of cadmium exposure through rice consumption in cadmium-contaminated areas of the Mae Tao sub-district, Tak, Thailand. Environ. Geochem. Health 2020, 42, 2331–2344. [Google Scholar] [CrossRef]
- Manoj, S.; Ramya Priya, R.; Elango, L. Long-term exposure to chromium contaminated waters and the associated human health risk in a highly contaminated industrialised region. Environ. Sci. Poll. Res. 2020, 28, 4276–4288. [Google Scholar] [CrossRef]
- Yang, D.; Liu, J.; Wang, Q.; Hong, H.; Zhao, W.; Chen, S.; Yan, C.; Lu, H. Geochemical and probabilistic human health risk of chromium in mangrove sediments: A case study in Fujian, China. Chemosphere 2019, 233, 503–511. [Google Scholar] [CrossRef]
- Zeinali, T.; Salmani, F.; Naseri, K. Dietary intake of cadmium, chromium, copper, nickel, and lead through the consumption of meat, liver, and kidney and assessment of human health risk in Birjand, Southeast of Iran. Biol. Trace Elem. Res. 2019, 191, 338–347. [Google Scholar] [CrossRef]
- Julander, A.; Kettelarij, J.; Liden, C. Cobalt. Kanerva’s Occupational Dermatology; Springer: Cham, 2020; pp. 661–669. [Google Scholar] [CrossRef]
- van den Brink, S.; Kleijn, R.; Sprecher, B.; Tukker, A. Identifying supply risks by mapping the cobalt supply chain. Resour. Conserv. Recy. 2020, 156, 104743. [Google Scholar] [CrossRef]
- Radke, E.G.; Galizia, A.; Thayer, K.A.; Cooper, G.S. Phthalate exposure and metabolic effects: A systematic review of the human epidemiological evidence. Environ. Int. 2019, 132, 104768. [Google Scholar] [CrossRef]
- Hillman, R.S. Hematopoietic agents: Growth factors, minerals, and vitamins. In Goodman and Gilman’s, the Pharmacological Basis of Therapeutics; Hardman, J.G., Limbird, L.E., Gilman, A.G., Eds.; McGraw-Hill: New York, NY, USA, 2001; pp. 1487–1518. [Google Scholar]
- Santamaria, A.; Sulsky, S. Risk assessment of an essential element: Manganese. J. Toxicol. Environ. Health 2010, 73, 128–155. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi, O.; Sorenson, P.B.; Darko, G.; Ansah, E.; Bak, J.L. Human health risk assessment of exposure to indoor mercury vapour in a Ghanaian artisanal small-scale gold mining community. Chemosphere 2020, 241, 125014. [Google Scholar] [CrossRef] [PubMed]
- Begum, W.; Rai, S.; Banerjee, S.; Bhattacharjee, S.; Mondal, M.H.; Bhattarai, A.; Saha, B. A comprehensive review on the sources, essentiality and toxicological profile of nickel. RSC Adv. 2022, 12, 9139–9153. [Google Scholar] [CrossRef]
- Du, B.; Zhou, J.; Lu, B.; Zhang, C.; Li, D.; Zhou, J.; Jiao, S.; Zhao, K.; Zhang, H. Environmental and human health risks from cadmium exposure near an active lead-zinc mine and a copper smelter, China. Sci. Total Environ. 2020, 720, 137585. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhou, H.; Gu, J.; Liu, C.; Yang, W.; Liao, B.; Zhou, H. Differences in absorption of cadmium and lead among fourteen sweet potato cultivars and health risk assessment. Ecotoxicol. Environ. Saf. 2020, 203, 111012. [Google Scholar] [CrossRef]
- Cima, F. Tin: Environmental pollution and health effects. In Encyclopedia of Environmental Health; Elsevier: Padova, Italy, 2018; pp. 351–359. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Q.; Meng, F.; Chen, M.; Wang, B. Effects of long-term zinc smelting activities on the distribution and health risk of heavy metals in agricultural soils of Guizhou province, China. Environ. Geochem. Health 2023, 45, 5639–5654. [Google Scholar] [CrossRef]
- Romania on the Map. Available online: https://pe-harta.ro/judete/Iasi.jpg (accessed on 12 April 2025).
- Simulated Historical Climate and Weather Data for Iași. Available online: https://www.meteoblue.com/ro/vreme/historyclimate/climatemodelled/ia%C8%99i_rom%C3%A2nia_675810 (accessed on 4 March 2025).
- Dick, R.P.; Thomas, D.R.; Halvorson, J.J. Standardized methods, sampling, and sample pretreatment. In Methods for Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; SSSA Special Publication 49; Soil Science Society of America: Madison, WI, USA, 1997; pp. 107–121. [Google Scholar]
- Toti, M.; Dumitriu, S.; Vlad, V.; Eftene, A. Atlasul Pedologic al Podgoriilor României; Terra Nostra: Iași, Romania, 2017. [Google Scholar]
- Google Maps. Available online: https://www.google.com/maps (accessed on 4 March 2025).
- Carter, M.R. Soil sampling and methods of analysis. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2008; p. 16. [Google Scholar]
- Obrejanu, G.; Vintilă, I.; Moţoc, E.; Teodoru, O.I.; Trandafirescu, T.; Thaler, R.; Canarache, A.; Florescu, C.I.; Blănaru, V.; Stîngă, N.; et al. Metode de Cercetare a Solului; Academia R.P. Române: Bucureşti, Romania, 1964. [Google Scholar]
- ISO 11465; Soil Quality. Determination of Dry Matter and Water Content on a Mass Basis-Gravimetric Method. ISO: Geneva, Switzerland, 1993.
- ISO 10694; Soil Quality. Determination of Organic and Total Carbon after Dry Combustion. ISO: Geneva, Switzerland, 1993.
- Stoica, E.; Răuţă, C.; Florea, N. Metode de analiză Chimică a Solului; ICPA: Bucureşti, Romania, 1986. [Google Scholar]
- ISO 10390; Soil Quality. Determination of pH. ISO: Geneva, Switzerland, 2015.
- Inkson, B.J. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) for materials characterization, In Materials Characterization Using Non-Destructive Evaluation (NDE) Methods; Hübschen, G., Altpeter, I., Tschuncky, R., Herrmann, H.G., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 17–43. [Google Scholar] [CrossRef]
- Zhou, W.; Apkarian, R.P.; Wang, Z.L.; Joy, D. Fundamentals of scanning electron microscopy (SEM). In Scanning Microscopy for Nanotechnology; Zhou, W., Wang, Z.L., Eds.; Springer: New York, NY, USA, 2006; pp. 1–40. [Google Scholar] [CrossRef]
- Raghavendra, T.; Bhat, S.G. Enzyme immobilized nanomaterials. In Micro and Nano Technologies, Nanomaterials for Biocatalysis; Castro, G.R., Nadda, A.K., Nguyen, T.A., Qi, X., Yasin, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 17–65. [Google Scholar] [CrossRef]
- Wollman, D.A.; Irwin, K.D.; Hilton, G.C.; Dulcie, L.L.; Newbury, D.E.; Martinis, J.M. High-resolution, energy-dispersive microcalorimeter spectrometer for X-ray microanalysis. J. Microsc. 2003, 188, 196–223. [Google Scholar] [CrossRef]
- Shojaei, T.R.; Soltani, S.; Derakhshani, M. Synthesis, properties, and biomedical applications of inorganic bionanomaterials. In Micro and Nano Technologies, Fundamentals of Bionanomaterials; Barhoum, A., Jeevanandam, J., Danquah, M.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 139–174. [Google Scholar] [CrossRef]
- Ulmanu, M.; Anger, I.; Gamenţ, E.; Mihalache, M.; Plopeanu, G.; Ilie, L. Rapid determination of some heavy metals in soil using an X-ray fluorescence portable instrument. Res. J. Agric. Sci. 2011, 43, 235–241. [Google Scholar]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol. Meeresunters. 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Muhammad, S.; Ullah, R.; Jadoon, I.A.K. Heavy Metals contamination in soil and food and their evaluation for risk assessment in the Zhob and Loralai valleys, Baluchistan province, Pakistan. Microchem. J. 2019, 149, 103971. [Google Scholar] [CrossRef]
- Müller, G. Index of geoaccumulation in sediments of the Rhine River. Geojournal 1969, 2, 108–118. [Google Scholar]
- Förstner, U.; Ahlf, W.; Calmano, W. Sediment quality objectives and criteria development in Germany. Water Sci. Technol. 1993, 28, 307–316. [Google Scholar] [CrossRef]
- Cachada, A.; Dias, A.C.; Pato, P.; Mieiro, C.; Rocha-Santos, T.; Pereira, M.E.; Ferreira da Silva, E.; Duarte, A.C. Major inputs and mobility of potentially toxic elements contamination in urban areas. Environ. Monit. Assess. 2013, 185, 279–294. [Google Scholar] [CrossRef]
- Liu, G.D.; Hanlon, E. Soil pH Soil pH Range for Optimum Commercial Vegetable Production. Available online: https://edis.ifas.ufl.edu/hs1207 (accessed on 30 April 2025).
- USDA. Natural Resources Conservation Service. Soil Quality Indicators: pH. 1998. Available online: https://directives.nrcs.usda.gov/sites/default/files2/1719842780/Soil%20Quality%20-%20General%20190-08%2C%20Soil%20Quality%20Information%20Sheets.pdf (accessed on 9 March 2025).
- Manta, D.S.; Angelone, M.; Bellanca, A.; Neri, R.; Sprovieri, M. Heavy metals in urban soils: A case study from the city of Palermo (Sicily). Italy. Sci. Total Environ. 2002, 300, 229–243. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Szatmári, G.; Pásztor, L. Maps of heavy metals in the soils of the European Union and proposed priority areas for detailed assessment. Sci. Total Environ. 2016, 565, 1054–1062. [Google Scholar] [CrossRef]
- Sintorini, M.M.; Widyatmoko, H.; Sinaga, E.; Aliyah, N. Effect of pH on metal mobility in the soil. IOP Conf. Ser. Earth Environ. Sci. 2021, 737, 012071. [Google Scholar] [CrossRef]
- Mahmood, T. Phytoextraction of heavy metals—The process and scope for remediation of contaminated soils. Soil Syst. 2010, 29, 91–109. [Google Scholar]
- Ramakrishnaiah, H.; Somashaker, R.K. Heavy metal contamination in roadside soil and their mobility in relation to pH and organic carbon. Soil Sediment Contam. 2010, 11, 643–654. [Google Scholar] [CrossRef]
- Ettler, V.; Rohovec, J.; Navrátil, T.; Mihaljevič, M. Mercury distribution in soil profiles polluted by lead smelting. Bull. Environ. Contamin. Toxicol. 2007, 78, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Bache, B.W. Soil acidification and aluminium-mobility. Soil Use Manag. 1985, 1, 10–14. [Google Scholar] [CrossRef]
- Jonczak, E.; Vladimír Šimanský, V.; Polláková, N. characteristics of iron and aluminium forms and quantification of soil forming processes in chernozems in western Slovakia. Pol. J. Soil Sci. 2015, XLVIII, 241–251. [Google Scholar] [CrossRef]
- Baver, L.D.; Gardner, W.H.; Gardner, W.R. Soil Physics, 4th ed.; John Wiley: New York, NY, USA, 1972. [Google Scholar]
- Williams, O.H.; Rintoul-Hynes, N.L. Legacy of war: Pedogenesis divergence and heavy metal contamination of the WWI front line a century after the battle. Eur. J. Soil Sci. 2022, 73, e13297. [Google Scholar] [CrossRef]
- Heiderscheidt, D. The impact of World War One on the forests and soils of Europe. Ursidae: Undergrad. Res. J. Univ. N. Colo. 2018, 7, 3. Available online: https://digscholarship.unco.edu/urj/vol7/iss3/3 (accessed on 11 March 2025).
- Cai, K.; Duan, Y.; Luan, W.; Li, Q.; Ma, Y. Geochemical behavior of heavy metals Pb and Hg in the farmland soil of Hebei plain. Geol. China 2016, 43, 1420–1428. [Google Scholar] [CrossRef]
- Hou, D.; O’Connor, D.; Igalavithana, A.D.; Alessi, D.S.; Luo, J.; Tsang, D.C.W.; Sparks, D.L.; Yamauchi, Y.; Rinklebe, J.; Ok, Y.S. Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat. Rev. Earth Environ. 2020, 1, 366–381. [Google Scholar] [CrossRef]
- Salminen, R.; Batista, M.; Bidovec, M.; Demetriades, A.; De Vivo, B.; De Vos, W.; Duris, M.; Gilucis, A.; Gregorauskienė, V.; Halamić, J.; et al. FOREGS Geochemical Atlas of Europe, Part 1: Background Information, Methodology and Maps; EuroGeoSurveys: Brussels, Belgium, 2005. [Google Scholar]
- Micó, C.; Peris, M.; Recatalá, L.; Sánchez, J. Baseline values for heavy metals in agricultural soils in an European Mediterranean region. Sci. Total Environ. 2007, 378, 13–17. [Google Scholar] [CrossRef]
- Brus, D.J.; Lamé, F.P.J.; Nieuwenhuis, R.H. National baseline survey of soil quality in the Netherlands. Environ. Poll. 2009, 157, 2043–2052. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Order, no. 756 of 3 November 1997 Issued by the Minister of Water, Forests and Environmental Protection. 1997. Available online: https://legislatie.just.ro/Public/detaliidocument/13572 (accessed on 11 March 2025).
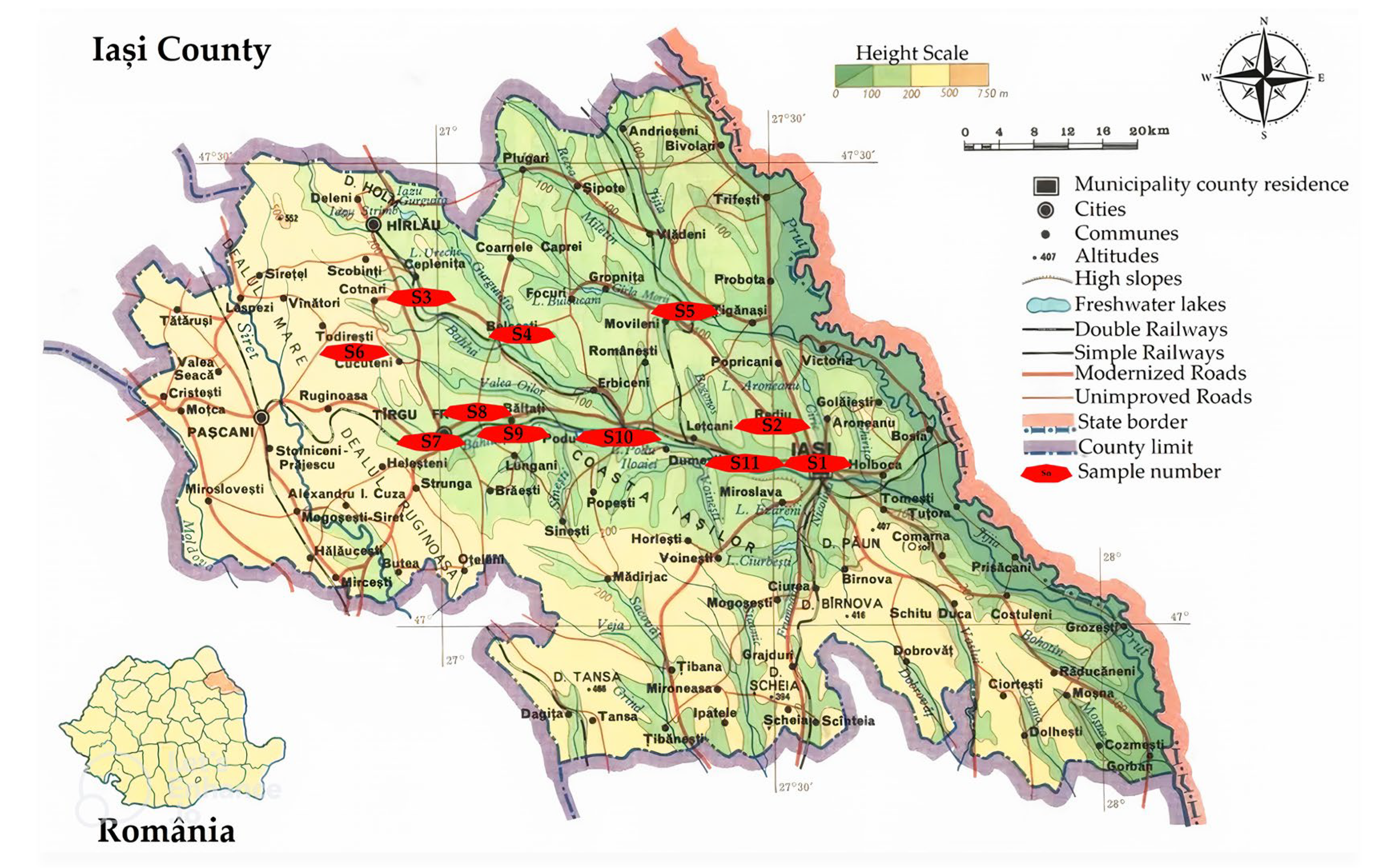
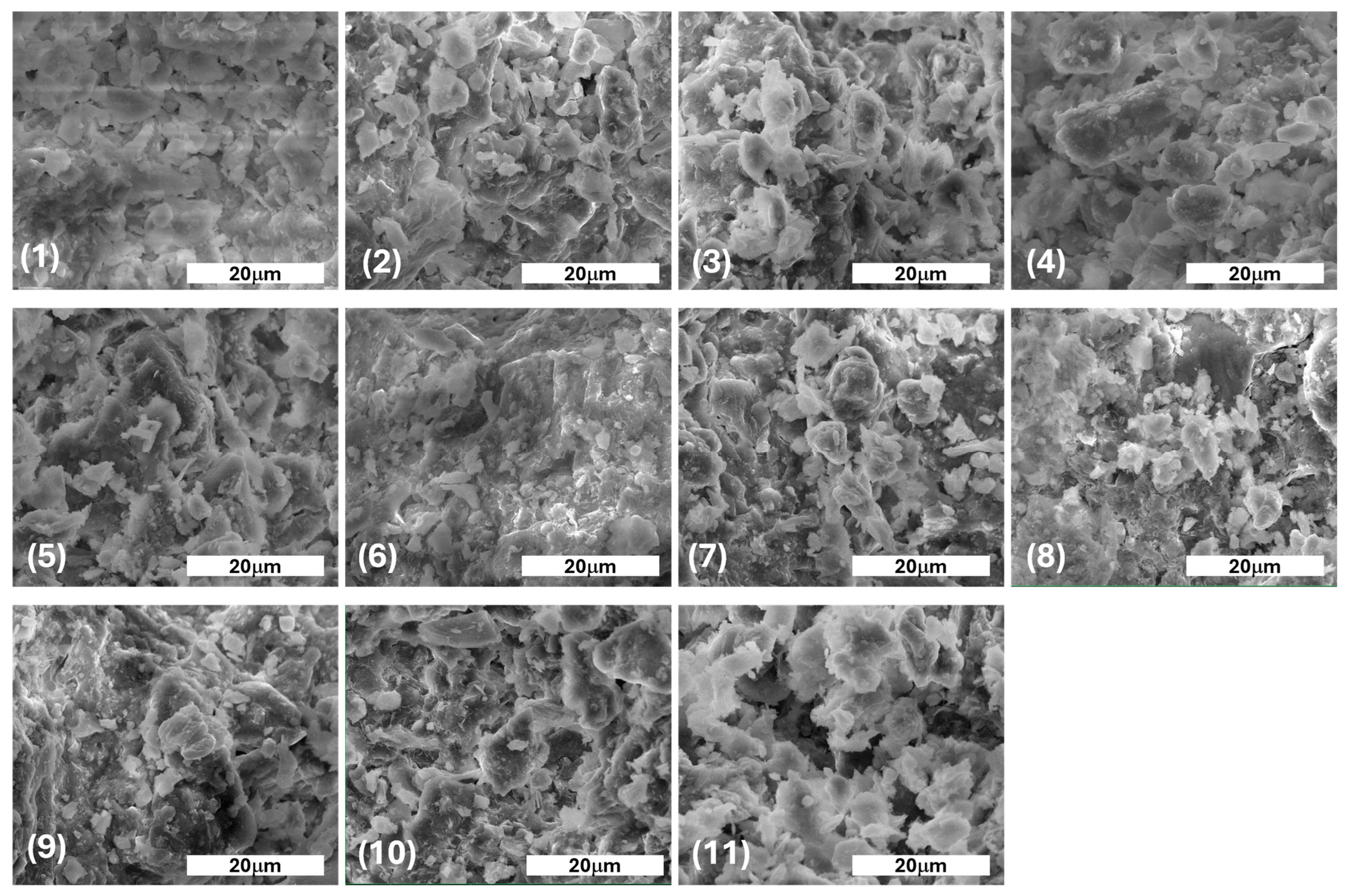
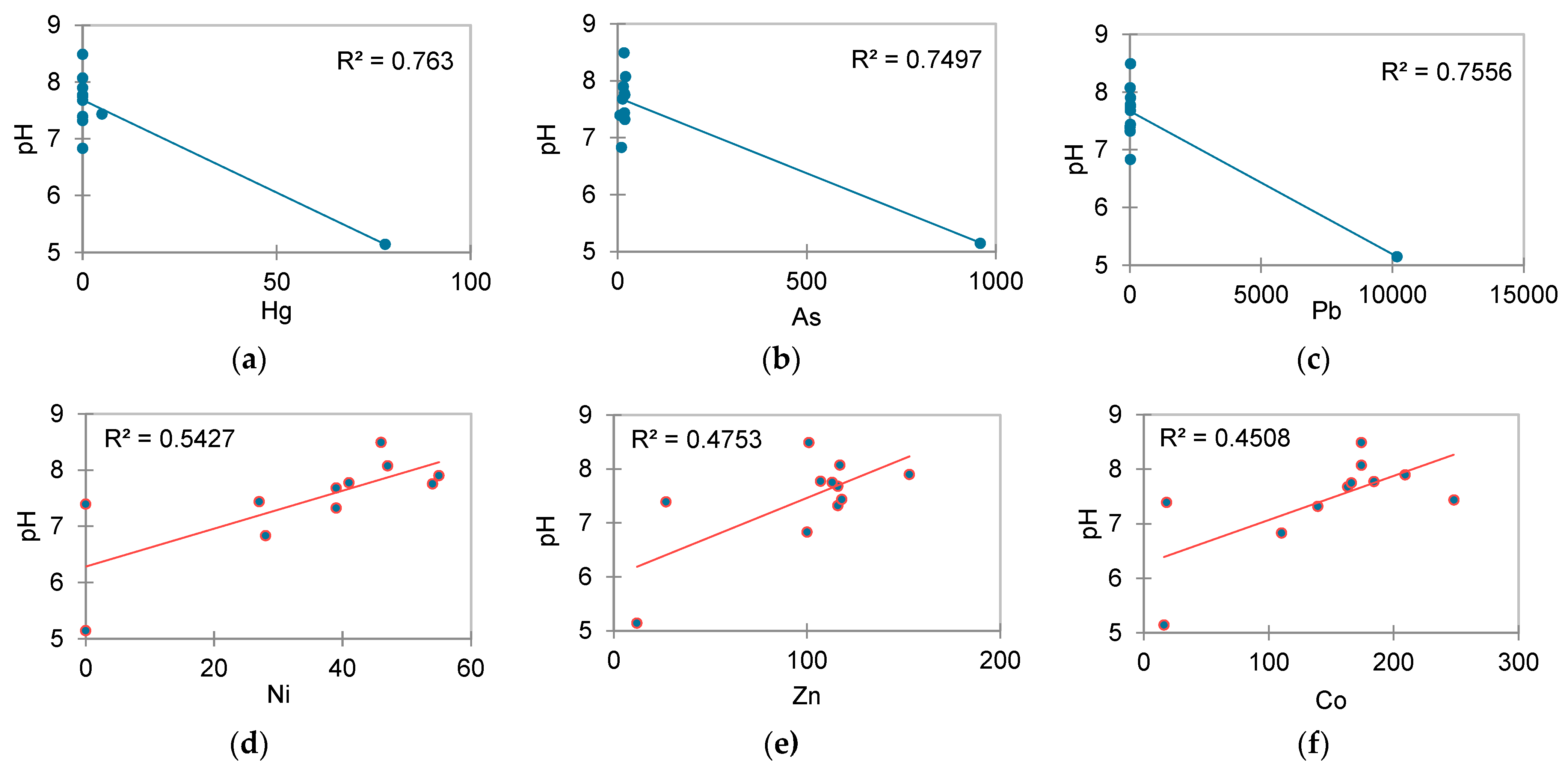

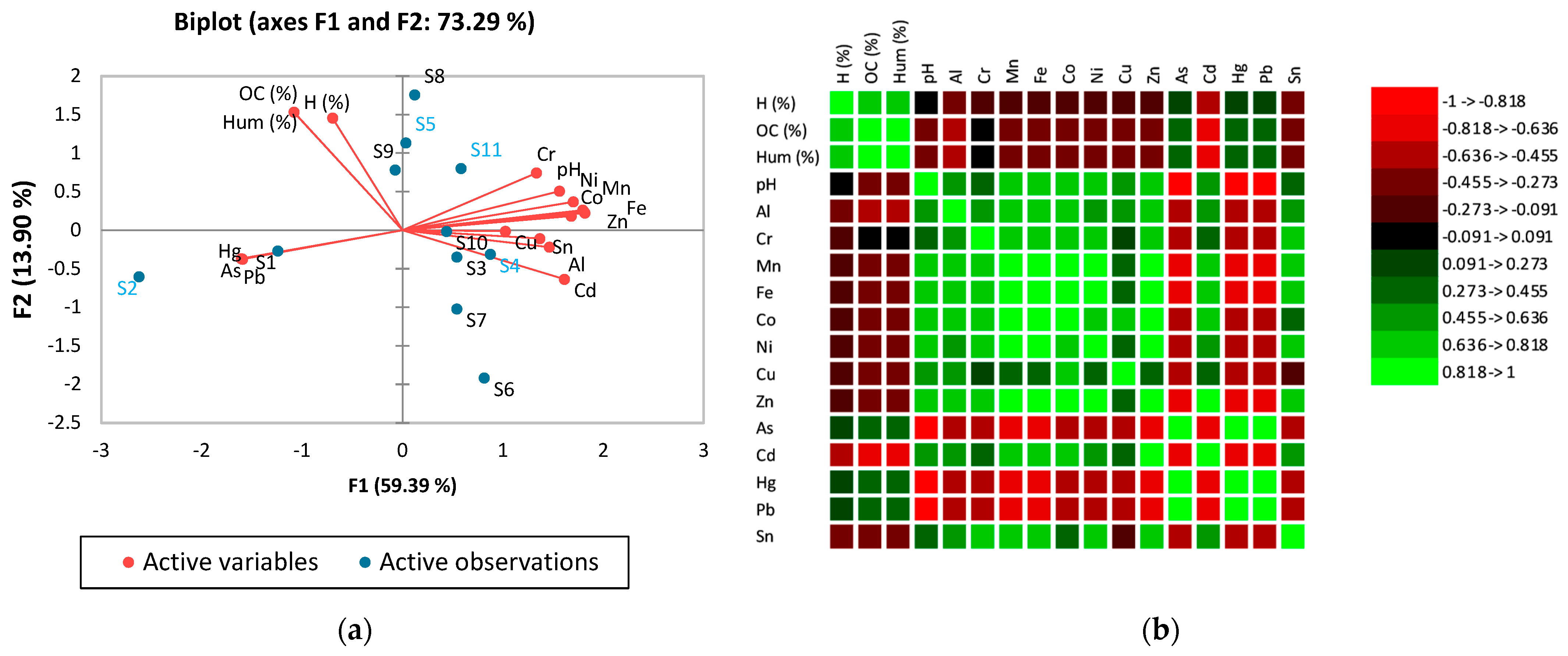
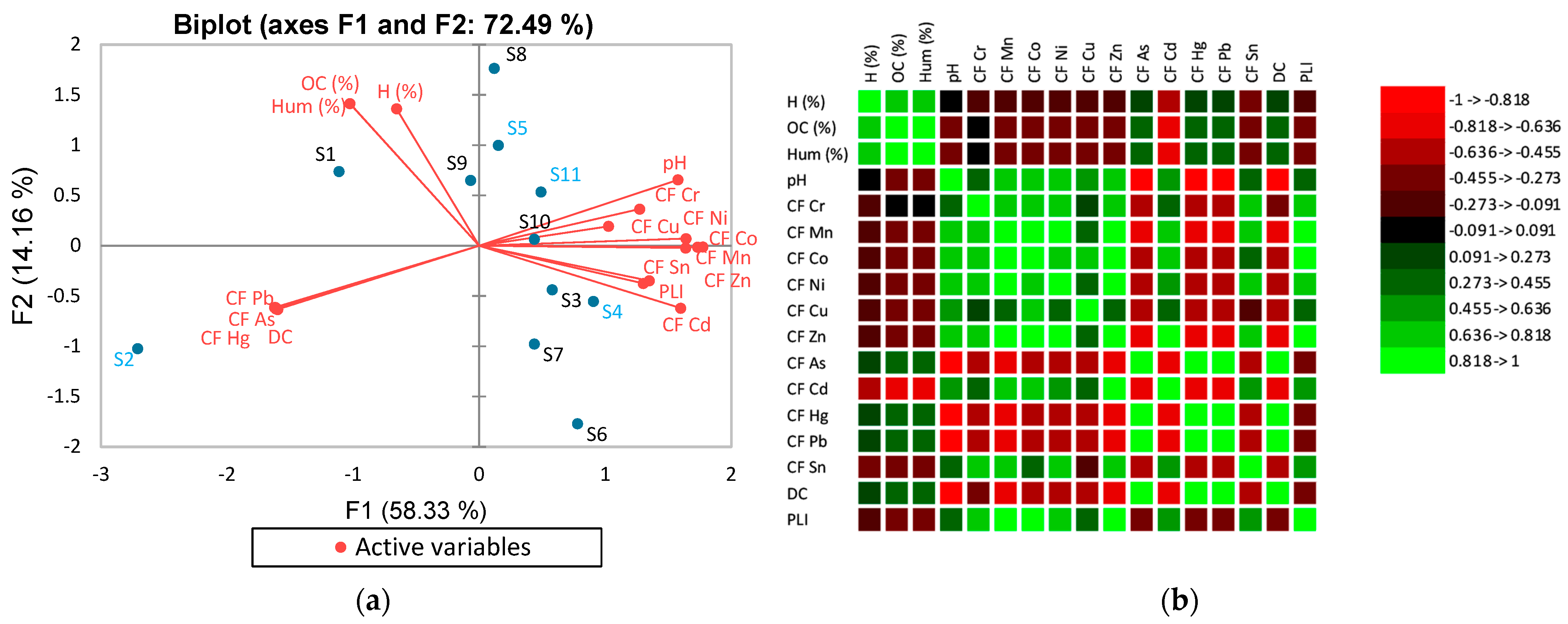
| Sample | Sampling Place | Dominant Economic Activity | Soil Classification [45] | Geographical Location [46] |
|---|---|---|---|---|
| S1 | Adamachi Farm | Agriculture | Chernozem | 47.194374518197066, 27.55311250136081 |
| S2 | Mârzești Forest | Protected area | Chernozem | 47.232779486481824, 27.497558910470836 |
| S3 | Cotnari Winery | Winemaking | Chernozem and phaeozem | 47.36383421700579, 26.945706995006784 |
| S4 | Plopi Lake, Belcești | Protected area | Chernozem | 47.325697560359906, 27.1010127547471 |
| S5 | Moldova Delta | Protected area | Chernozem | 47.35252959717897, 27.372164884260567 |
| S6 | Todirești Commune | Agriculture and animal husbandry | Chernozem and phaeozem | 47.30612296028698, 26.842769644010666 |
| S7 | Orchard, Târgu Frumos | Horticulture | Chernozem | 47.21392362480122, 26.96193925069864 |
| S8 | Meadow, Ion Neculce Commune | Agriculture | Chernozem | 47.244195859750484, 27.053936241207516 |
| S9 | Olma Orchard, Bălțați | Horticulture | Chernozem | 47.223306965075466, 27.10131728108934 |
| S10 | Podu Iloaiei | Agriculture | Chernozem | 47.218037296762404, 27.25369206666353 |
| S11 | Valea lui David | Protected area | Chernozem | 47.191101871447884, 27.468039211982386 |
| Values in Aqueous Suspension | Reaction Type |
|---|---|
| ≤3.5 | Strongly acid |
| 3.51–4.30 | Very strongly acidic |
| 4.31–5.00 | Moderately strong acid |
| 5.01–5.40 5.41–5.80 | Moderately acid |
| 5.81–6.40 6.41–6.80 | Slightly acid |
| 6.81–7.20 | Neutral |
| 7.21–7.80 7.81–8.40 | Slightly alkaline |
| 8.41–9.00 | Moderately alkaline |
| >9.01 | Strongly alkaline |
| Sample | Humidity (%) | Organic Carbon Content (%) | Humus Content (%) | pH | Reaction Type |
|---|---|---|---|---|---|
| S1 | 17.54 | 3.43 | 5.92 | 7.395 | Slightly alkaline |
| S2 | 19.24 | 4.08 | 7.04 | 5.146 | Slightly acid |
| S3 | 19.50 | 1.63 | 2.82 | 8.075 | Slightly alkaline |
| S4 | 14.49 | 2.55 | 4.40 | 7.901 | Slightly alkaline |
| S5 | 18.35 | 4.09 | 7.06 | 7.682 | Slightly alkaline |
| S6 | 11.58 | 0.59 | 1.02 | 7.777 | Slightly alkaline |
| S7 | 14.56 | 1.77 | 3.06 | 7.324 | Slightly alkaline |
| S8 | 23.30 | 4.16 | 7.18 | 8.492 | Moderately alkaline |
| S9 | 17.08 | 4.08 | 7.04 | 6.833 | Neutral |
| S10 | 18.12 | 2.58 | 4.45 | 7.439 | Slightly alkaline |
| S11 | 17.41 | 3.23 | 5.58 | 7.754 | Slightly alkaline |
| Samples | C | O | Na | Mg | Al | Si | Hg | Pb | Cd | K | Ca | Mn | Fe | Ni | Co | Cu | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 5.08 ± 0.68 | 52.86 ± 2.27 | 0.18 ± 0.07 | 1.33 ± 0.15 | 8.83 ± 0.92 | 20.87 ± 1.09 | 0.04 ± 0.01 | 0.10 ± 0.02 | 0.18 ± 0.06 | 2.34 ± 0.27 | 1.01 ± 0.14 | 0.32 ± 0.11 | 5.14 ± 0.83 | 0.29 ± 0.08 | 0.23 ± 0.08 | 0.35 ± 0.09 | 0.36 ± 0.08 |
| S2 | 7.35 ± 0.55 | 59.31 ± 0.22 | 0.09 ± 0.02 | 0.99 ± 0.04 | 6.94 ± 0.48 | 18.78 ± 1.18 | 0.02 ± 0.003 | 0.09 ± 0.004 | 0.09 ± 0.01 | 1.17 ± 0.08 | 0.52 ± 0.02 | 0.18 ± 0.02 | 3.26 ± 0.05 | 0.14 ± 0.01 | 0.22 ± 0.02 | 0.21 ± 0.02 | 0.25 ± 0.01 |
| S3 | 4.71 ± 0.58 | 50.01 ± 2.81 | 0.19 ± 0.08 | 1.53 ± 0.28 | 7.22 ± 0.66 | 18.47 ± 2.61 | 0.12 ± 0.03 | 0.19 ± 0.04 | 0.21 ± 0.04 | 2.43 ± 0.74 | 2.73 ± 0.93 | 0.49 ± 0.04 | 9.14 ± 1.84 | 0.40 ± 0.01 | 0.48 ± 0.06 | 0.55 ± 0.03 | 0.28 ± 0.07 |
| S4 | 8.45 ± 1.72 | 54.55 ± 2.47 | 0.05 ± 0.02 | 0.93 ± 0.02 | 5.63 ± 0.58 | 19.52 ± 3.10 | 0.10 ± 0.02 | 0.08 ± 0.002 | 0.15 ± 0.03 | 1.28 ± 0.33 | 2.79 ± 0.69 | 0.37 ± 0.08 | 4.23 ± 0.86 | 0.29 ± 0.04 | 0.31 ± 0.05 | 0.40 ± 0.07 | 0.54 ± 0.08 |
| S5 | 15.36 ± 2.76 | 56.38 ± 1.95 | 1.28 ± 0.58 | 0.97 ± 0.04 | 5.61 ± 0.69 | 14.24 ± 1.11 | 0.01 ± 0.001 | 0.08 ± 0.02 | 0.09 ± 0.02 | 1.00 ± 0.08 | 1.44 ± 0.17 | 0.10 ± 0.01 | 2.73 ± 0.70 | 0.09 ± 0.002 | 0.10 ± 0.01 | 0.14 ± 0.01 | 0.19 ± 0.01 |
| S6 | 3.77 ± 0.65 | 54.33 ± 3.40 | 0.67 ± 0.28 | 0.74 ± 0.09 | 9.38 ± 0.30 | 22.23 ± 2.57 | 0.07 ± 0.02 | 0.08 ± 0.02 | 0.16 ± 0.03 | 3.90 ± 0.55 | 0.64 ± 0.06 | 0.23 ± 0.02 | 2.23 ± 0.36 | 0.28 ± 0.02 | 0.23 ± 0.03 | 0.40 ± 0.06 | 0.34 ± 0.04 |
| S7 | 8.61 ± 1.71 | 57.89 ± 0.29 | 0.06 ± 0.02 | 1.78 ± 0.25 | 8.48 ± 0.29 | 15.44 ± 0.92 | 0.10 ± 0.04 | 0.07 ± 0.01 | 0.11 ± 0.02 | 1.33 ± 0.03 | 1.07 ± 0.20 | 0.22 ± 0.01 | 3.41 ± 0.34 | 0.16 ± 0.02 | 0.21 ± 0.01 | 0.29 ± 0.01 | 0.29 ± 0.02 |
| S8 | 5.41 ± 0.18 | 43.90 ± 2.93 | 0.31 ± 0.05 | 1.23 ± 0.05 | 7.88 ± 0.19 | 20.96 ± 1.04 | 0.06 ± 0.01 | 0.13 ± 0.02 | 0.21 ± 0.03 | 2.34 ± 0.17 | 6.43 ± 2.07 | 0.53 ± 0.12 | 7.30 ± 1.26 | 0.56 ± 0.12 | 0.52 ± 0.12 | 0.21 ± 0.07 | 0.28 ± 0.08 |
| S9 | 14.65 ± 3.92 | 57.93 ± 1.23 | 0.15 ± 0.04 | 1.01 ± 0.16 | 6.13 ± 0.71 | 15.62 ± 2.44 | 0.06 ± 0.2 | 0.11 ± 0.04 | 0.05 ± 0.01 | 1.00 ± 0.13 | 0.74 ± 0.21 | 0.08 ± 0.01 | 1.88 ± 0.33 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.10 ± 0.02 | 0.13 ± 0.01 |
| S10 | 5.62 ± 0.40 | 58.53 ± 0.67 | 0.05 ± 0.03 | 1.25 ± 0.11 | 8.75 ± 0.11 | 18.67 ± 0.37 | 0.05 ± 0.01 | 0.08 ± 0.02 | 0.08 ± 0.01 | 1.60 ± 0.08 | 0.73 ± 0.06 | 0.30 ± 0.05 | 3.07 ± 0.19 | 0.19 ± 0.02 | 0.20 ± 0.01 | 0.15 ± 0.02 | 0.28 ± 0.02 |
| S11 | 5.35 ± 1.59 | 52.10 ± 2.81 | 2.82 ± 1.60 | 2.77 ± 1.06 | 10.19 ± 0.62 | 20.35 ± 3.26 | 0.11 ± 0.02 | 0.08 ± 0.01 | 0.14 ± 0.02 | 0.72 ± 0.23 | 0.45 ± 0.11 | 0.22 ± 0.04 | 3.48 ± 1.09 | 0.21 ± 0.04 | 0.18 ± 0.02 | 0.25 ± 0.03 | 0.27 ± 0.07 |
| Samples | Cr | Mn | Co | Ni | Cu | Zn | As | Cd | Hg | Pb | Sn | Mean |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 0 | −5.08 | −0.32 | 0 | 0.82 | −2.47 | −0.32 | 1.74 | 0 | −3.91 | 0 | −0.86 |
| S2 | −0.97 | −5.08 | −0.49 | 0 | 0.26 | −3.64 | 7.00 | 0 | 9.02 | 8.41 | 0 | 1.32 |
| S3 | 0.92 | −0.64 | 2.95 | 0.65 | 0.62 | −0.36 | 1.49 | 2.87 | 0 | −1.10 | 1.18 | 0.78 |
| S4 | 1.40 | −0.73 | 3.22 | 0.87 | 0.90 | 0.03 | 1.00 | 3.32 | 0 | −0.32 | 1.14 | 0.99 |
| S5 | 1.44 | −0.89 | 2.86 | 0.38 | 0.79 | −0.37 | 0.79 | 2.42 | 0 | −0.91 | 0.38 | 0.63 |
| S6 | 1.09 | −0.75 | 3.03 | 0.45 | 1.07 | −0.49 | 1.26 | 3.00 | 0 | −0.58 | 0.85 | 0.81 |
| S7 | 0.79 | −0.69 | 2.63 | 0.38 | 0.68 | −0.37 | 1.34 | 2.87 | 0 | −1.00 | 1.09 | 0.70 |
| S8 | 0.98 | −0.85 | 2.95 | 0.62 | 1.02 | −0.57 | 1.18 | 2.00 | 0 | −0.05 | −0,10 | 0.65 |
| S9 | 1.61 | −0.78 | 2.29 | −0.10 | 0.42 | −0.58 | 0.42 | 2.42 | 0 | −0.45 | 0.90 | 0.56 |
| S10 | 1.24 | −0.83 | 3.46 | −0.15 | 1.30 | −0.35 | 1.26 | 2.74 | 5.06 | −0.91 | 0 | 1.17 |
| S11 | 2.05 | −0.71 | 2.88 | 0.85 | 0.79 | −0.41 | 1.34 | 1.74 | 0 | −0.58 | 1.28 | 0.84 |
| Mean | 0.96 | −1.55 | 2.31 | 0.36 | 0.79 | −0.87 | 1.52 | 2.28 | 1.29 | −0.13 | 0.61 | - |
| Max | 2.05 | −0.64 | 3.46 | 0.87 | 1.30 | 0.03 | 7.00 | 3.32 | 9.02 | 8.41 | 1.28 | - |
| Min | −0.97 | −5.08 | −0.49 | −0.15 | 0.26 | −3.64 | −0.32 | 0 | 0 | −3.91 | −0.10 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luchian, C.E.; Motrescu, I.; Dumitrașcu, A.I.; Scutarașu, E.C.; Cara, I.G.; Colibaba, L.C.; Cotea, V.V.; Jităreanu, G. Comprehensive Assessment of Soil Heavy Metal Contamination in Agricultural and Protected Areas: A Case Study from Iași County, Romania. Agriculture 2025, 15, 1070. https://doi.org/10.3390/agriculture15101070
Luchian CE, Motrescu I, Dumitrașcu AI, Scutarașu EC, Cara IG, Colibaba LC, Cotea VV, Jităreanu G. Comprehensive Assessment of Soil Heavy Metal Contamination in Agricultural and Protected Areas: A Case Study from Iași County, Romania. Agriculture. 2025; 15(10):1070. https://doi.org/10.3390/agriculture15101070
Chicago/Turabian StyleLuchian, Camelia Elena, Iuliana Motrescu, Anamaria Ioana Dumitrașcu, Elena Cristina Scutarașu, Irina Gabriela Cara, Lucia Cintia Colibaba, Valeriu V. Cotea, and Gerard Jităreanu. 2025. "Comprehensive Assessment of Soil Heavy Metal Contamination in Agricultural and Protected Areas: A Case Study from Iași County, Romania" Agriculture 15, no. 10: 1070. https://doi.org/10.3390/agriculture15101070
APA StyleLuchian, C. E., Motrescu, I., Dumitrașcu, A. I., Scutarașu, E. C., Cara, I. G., Colibaba, L. C., Cotea, V. V., & Jităreanu, G. (2025). Comprehensive Assessment of Soil Heavy Metal Contamination in Agricultural and Protected Areas: A Case Study from Iași County, Romania. Agriculture, 15(10), 1070. https://doi.org/10.3390/agriculture15101070









